ABSTRACT
Objective
To report the clinical and histopathologic findings of retinal astrocytic tumors that showed progressive growth in four patients with tuberous sclerosis complex (TSC).
Methods
Four young children each developed an enlarging retinal neoplasm that eventually necessitated enucleation of the affected eye. The systemic findings, clinical course, and histopathologic findings were reviewed.
Results
Each patient had a progressively enlarging retinal mass associated with a total exudative retinal detachment and neovascular glaucoma. Enucleation was necessary in each case because the affected eye became blind and painful. The mean patient age at enucleation was 7 years, and the median age was 3 years. At the time of enucleation the tumors ranged from 10 to 20 mm in basal diameter and from 10 to 25 mm in thickness. Histopathologic studies of each eye revealed a giant cell astrocytoma that had produced a total exudative retinal detachment. The tumor cells showed positive immunoreactivity to neuron-specific enolase and glial fibrillary acidic protein. The retinal neoplasms in these cases were identical histopathologically to the subependymal giant cell astrocytoma that typifies TSC in the brain. One tumor filled the entire eye and perforated the globe. Although the lesions simulated retinoblastoma clinically, each patient had ocular and systemic findings of TSC, supporting the diagnosis of astrocytic hamartoma.
Conclusions
Although retinal astrocytic lesions of TSC generally are stationary, they can sometimes grow relentlessly and cause severe ocular complications. Patients with retinal astrocytic hamartomas should have serial ophthalmic evaluations because of this possibility.
INTRODUCTION
Retinal astrocytic hamartoma is the best-known ocular manifestation of tuberous sclerosis complex (TSC).1,2 It is generally a sessile or slightly elevated lesion in the nerve fiber layer of the retina, but it can have several clinical variations. It can be unilateral, bilateral, solitary, multifocal, transparent, opaque, noncalcified, or calcified.1,2 Retinal astrocytic hamartoma in association with TSC generally is considered to be a relatively stationary lesion that has little potential for aggressive behavior.1–5 In rare instances, however, a retinal astrocytic hamartoma can show progressive growth and cause severe local complications. We report the clinical course and histopathologic findings in four patients with TSC, each of whom developed progressive growth of a juxtapapillary astrocytic hamartoma that caused secondary retinal detachment and neovascular glaucoma, necessitating enucleation of the affected eye.
METHODS
The clinical records and histopathologic findings were reviewed and summarized for four patients with TSC who underwent enucleation of one eye because of tumor growth and neovascular glaucoma. Clinical findings evaluated included patient age at enucleation, patient’s sex, tumor dimensions, and frequency of retinal exudation, retinal detachment, neovascular glaucoma, and extraocular extension. Assessment of pathologic findings included review of grossly sectioned eyes, histopathologic sections, and immunohistochemical preparations. The literature on aggressive retinal astrocytic neoplasms that came to enucleation was reviewed, and a comparison was made between those associated with TSC and those unassociated with TSC.
RESULTS
In the computerized files of the Ocular Oncology Service at Wills Eye Hospital, we identified four cases of aggressive astrocytic retinal tumors associated with TSC that required enucleation. The TSC in each patient was characterized by hypopigmented cutaneous macules, facial angiofibromas, and subependymal or cortical lesions typical of TSC seen with computed tomography or magnetic resonance imaging. One patient had renal cysts and none had cardiac, lung, or other lesions of TSC.
Concerning ocular manifestations, each patient had a similar clinical course, characterized by progressive enlargement of a previously recognized yellow retinal juxtapapillary mass (Figure 1) that ultimately caused a blind, painful eye and necessitated enucleation. In addition, three of the four patients had multiple retinal astrocytic tumors in both eyes and only one patient had a solitary retinal tumor.
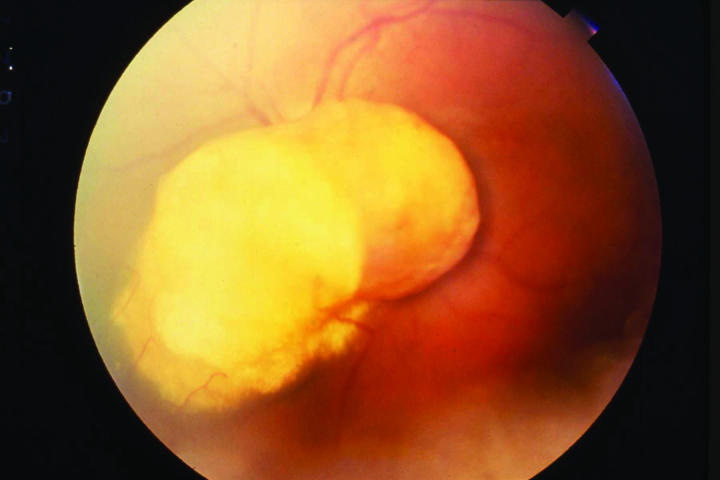
Figure 1.
Case 2. Fundus photograph showing bilobed nodular mass arising from the retina and overlying the optic nerve.
The pertinent clinical information on our four cases is summarized in Table 1. There were two boys and two girls. Each tumor was surrounded by yellow intraretinal exudation that was documented to slowly progress to a total exudative retinal detachment and neovascular glaucoma. This progressive exudation and retinal detachment appeared to be directly related to gradual enlargement of the tumor. The intervals from the initial diagnosis of retinal tumor to enucleation ranged from 6 months to 13 years. In the patient with the 13-year interval, enucleation had been advised at age 7 years, but the parents had refused and only consented to enucleation when the tumor caused perforation of the globe 6 years later. Based on clinical estimation and ultrasonography, the tumor sizes at the time of enucleation ranged from 12×8×9 mm to 20×20×25 mm, with the latter (case 4) being the tumor that filled the entire globe and perforated the cornea. Seven years earlier that tumor measured 8×8×4 mm, attesting to the relentless, slow growth that characterized all of the tumors.
Table 1.
Clinical information on four patients with tuberous sclerosis complex and aggressive retinal astrocytoma
| Case | Age at enuc | Sex | Retinal exudation | RD | NVG | Months from initial diagnosis to enuc | Tumor size at enuc (mm) | EOE |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | M | Yes | Yes | Yes | 20 | 12×8×9 | No |
| 2 | 3 | M | Yes | Yes | Yes | 24 | 14×14×7 | No |
| 3 | 1 | F | Yes | Yes | Yes | 6 | 10×10×10 | No |
| 4 | 14 | F | Yes | Yes | Yes | 156 (13 yr) | 20×20×25 | Yes |
Enuc, enucleation; EOE, extraocular extension; NVG, neovascular glaucoma; RD, retinal detachment.
It is of interest that the smaller, more peripheral astrocytomas in the ipsilateral and contralateral eyes of three patients did not exhibit growth during the course of follow-up, ranging from 3 to 12 years. In every case, only one larger tumor near the optic disk showed progressive growth and retinal detachment, whereas all other tumors remained stationary.
Two of our patients (cases 2 and 3) had surgical attempts elsewhere to control the growing tumor and the retinal detachment by laser, vitrectomy, and retinal reattachment. In each case, the attempts failed and there was slow, relentless growth of the tumor.
Pathological Findings
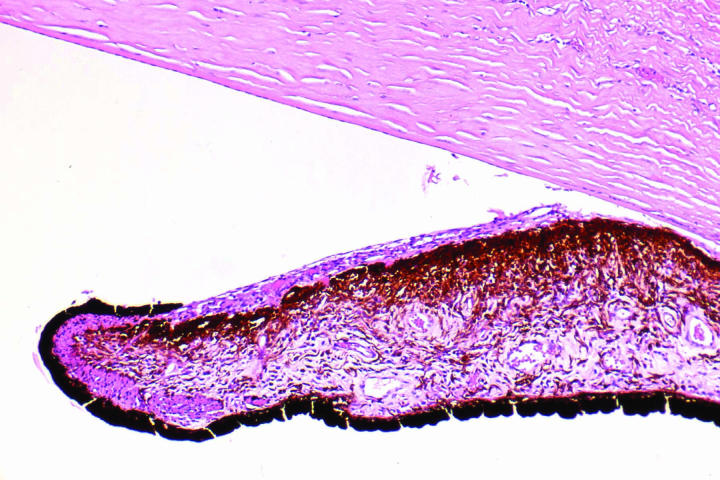
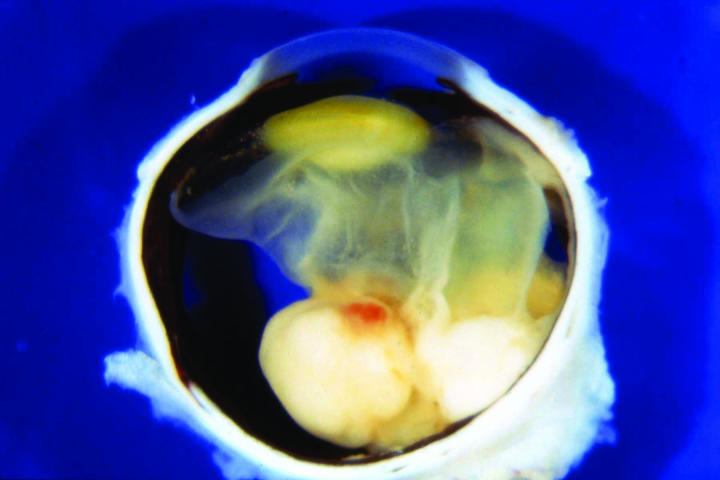
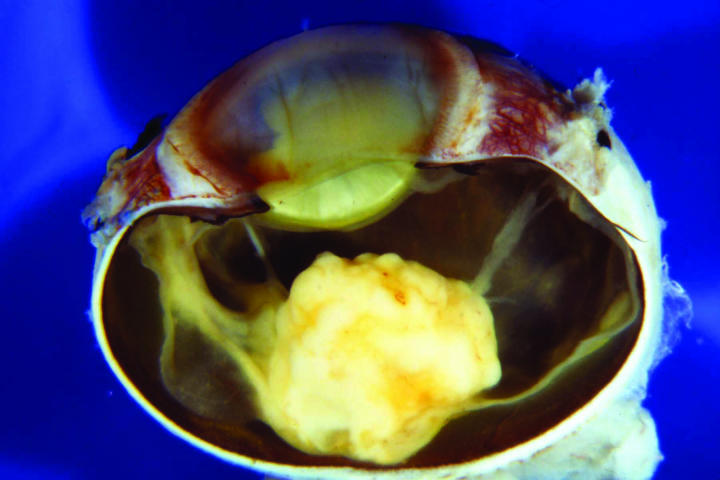
The macroscopic appearance of the sectioned enucleated eyes is illustrated in Figures 2 through 5. In cases 1 through 3, yellow-white tumors arose from the posterior retina in the region of the optic disk. These tumors measured 7, 10, and 14 mm in largest diameter and were associated with total secondary retinal detachments. Two were predominantly exophytic, and the third had a combined exophytic-endophytic growth pattern. In case 4, the neoplasm filled the entire globe and perforated the sclera.
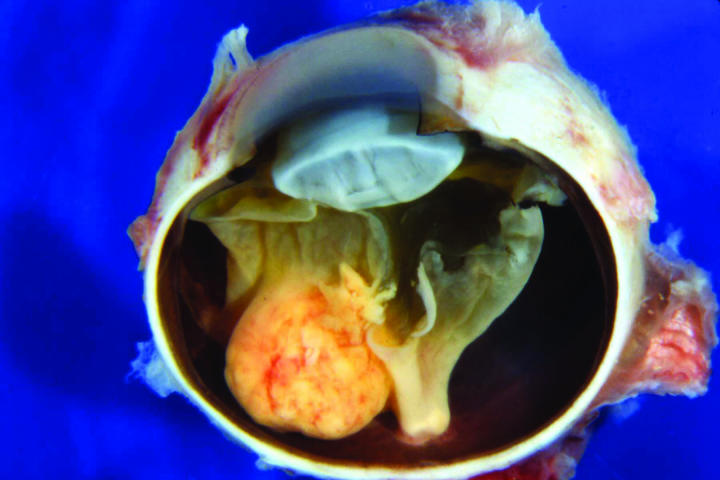
Figure 2.
Case 2. Gross appearance of sectioned globe showing bilobed epipapillary retinal mass and total retinal detachment.
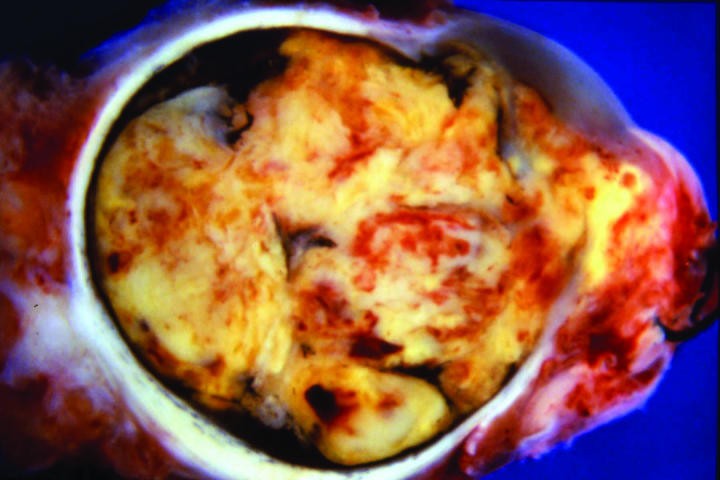
Figure 5.
Case 4. Gross appearance of sectioned globe showing neoplasm totally filling interior of eye and extending anteriorly through corneoscleral perforation.
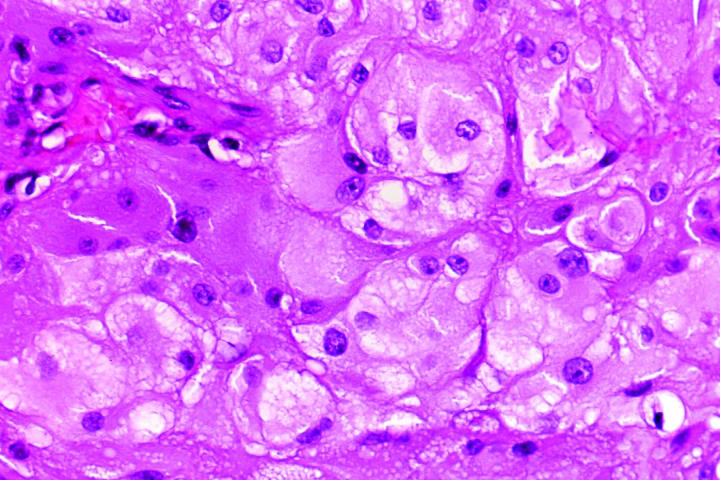
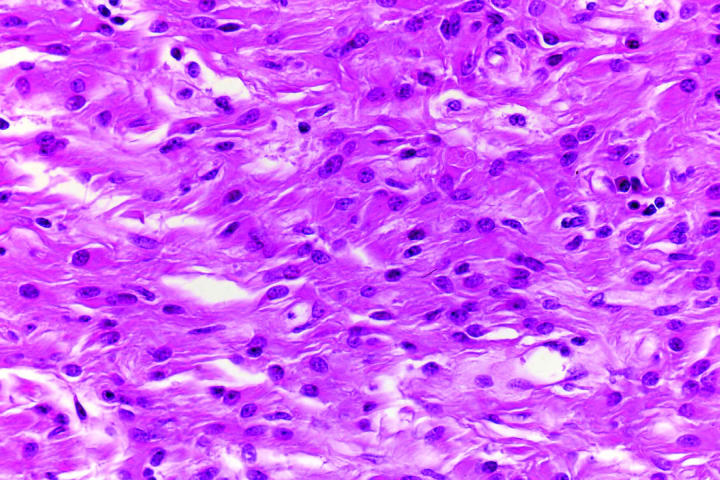
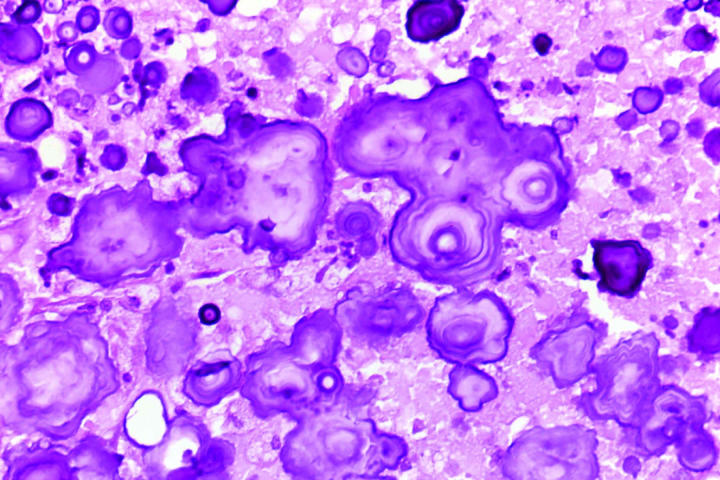
Pertinent histopathologic features are summarized in Table 2. All four eyes had florid iris neovascularization and secondary angle closure (Figure 6), and all had total nonrhegmatogenous exudative retinal detachments. The retina in case 4 was largely destroyed. All four neoplasms contained extensive areas of necrosis, comprising 50% to 95% of the tumor (Figure 7). Each of the retinal neoplasms was composed of two types of cells that were present in varying proportions. One group of cells had copious quantities of pale eosinophilic cytoplasm and resembled those found in subependymal giant cell astrocytoma (SEGA) of the brain in TSC (Figure 8). These giant cells typically were large and round or oval in profile, and the periphery of their cytoplasm occasionally was vacuolated. Many of the giant cells had large round or oval nuclei with prominent nucleoli. The cells in the second group were more elongated and fusiform in shape and had more intensely eosinophilic cytoplasm and smaller, darker nuclei (Figure 9). In several cases the retina and the parenchyma of the optic nerve contained large numbers of these plump spindle cells. The large, aggressive neoplasm in case 4 had invaded the choroid and optic nerve; it had a moderate degree of nuclear pleomorphism and atypia and occasional mitotic figures. Glial cells with abundant cytoplasm were found in the retrolaminar part of the atrophic optic nerve in all cases. All of the astrocytic tumors contained foci of basophilic, multilaminated calcospherites (Figure 10), and two contained metaplastic bone. Hamartomas of the iris and ciliary epithelia were present in two eyes. The latter finding has been reported previously.6
Table 2.
Histopathologic findings in four patients with tuberous sclerosis complex and aggressive retinal astrocytoma
| Case | Growth pattern | % necrosis | Giant cells | Plump spindle cells | Calcospherites | Bone | RD | NVI | EOE | Optic nerve invasion |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Exo | 95% | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
| 2 | Exo | 70% | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
| 3 | End-exo | 50% | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 4 | End-exo | 60% | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
End, endophytic; EOE, extraocular extension; Exo, exophytic; NVI, iris neovascularization; RD, retinal detachment.
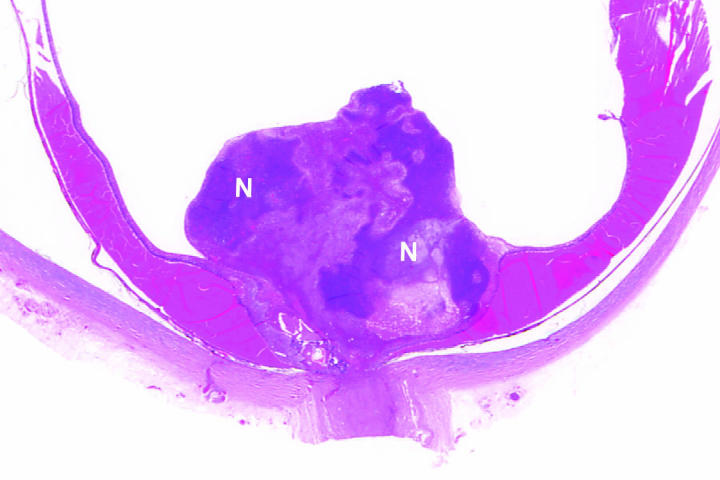
Figure 6.
Case 2. Neovascular glaucoma. Florid fibrovascular membrane flattens anterior iridic surface central to wide peripheral anterior synechia. Ectropion iridis is present (hematoxylin-eosin, original magnification ×50). N indicates area of necrosis.
Figure 7.
Case 3. Low magnification photomicrograph showing largely endophytic epipapillary tumor with extensive sheets of basophilic necrosis. The neighboring retina is detached by densely proteinaceous subretinal fluid (hematoxylin-eosin, original magnification ×10).
Figure 8.
Case 2. Giant cells. Round or oval cells have abundant pale eosinophilic cytoplasm with peripheral vacuolization and round nuclei with nucleoli. They resemble cells found in subependymal giant cell astrocytoma (hematoxylin-eosin, original magnification ×100).
Figure 9.
Case 1. Plump spindle cells in retinal stalk. Plump fusiform cells have intensely eosinophilic cytoplasm and oval nuclei that are smaller and darker (hematoxylin-eosin, original magnification ×100).
Figure 10.
Case 2. Calcospherites. Tumor contains multilaminated, basophilic calcium deposits (hematoxylin-eosin, original magnification ×100).
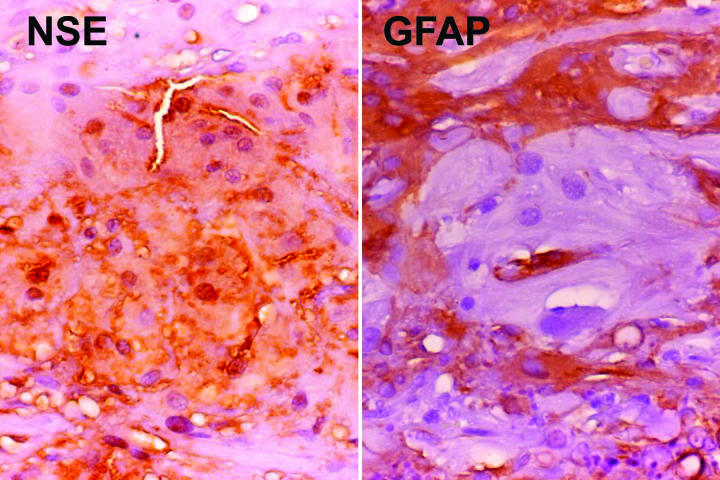
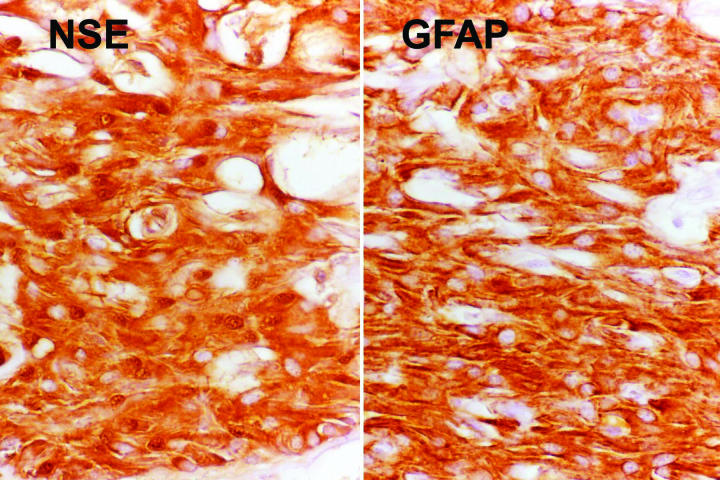
The results of special immunohistochemical studies are summarized in Table 3. All tumors coexpressed neuronal marker neuron-specific enolase (NSE) and glial marker glial fibrillary acidic protein (GFAP). In general, the giant cells were immunoreactive for NSE but were negative or only minimally reactive for GFAP (Figure 11). In contrast, the spindle cells expressed both NSE and GFAP (Figure 12). The tumor cells also were strongly immunoreactive for S-100 protein and vimentin. Melanoma marker HMB-45 was uniformly negative.
Table 3.
Summary of immunohistochemical reactivity*
| Case | NSE-GC | NSE-SP | GFAP-GC | GFAP-SP | S-100 | Vimentin | HMB-45 |
|---|---|---|---|---|---|---|---|
| 1 | +2 | +3 | – | +3 | +3 | +4 | Not done |
| 2 | +2 | +2 | Trace or − | +2 | +2 | +3 | – |
| 3 | +1 | Trace | Trace or − | +2 | +2 | +4 | – |
| 4 | +4 | +4 | – | +2 focal | +3 | Trace | – |
GC, giant cell component; GFAP, glial fibrillary acidic protein; HMB-45, melanoma-specific antigen; NSE, neuron-specific enolase; S-100, S-100 protein; SP, spindle cell component.
Staining was interpreted as trace to intense (+4) as graded on a scale of +1 to +4. Lack of staining was indicated with a minus (−).
Figure 11.
Case 2. Immunoreactivity of giant cells for neuron-specific enolase (NSE) and glial fibrillary acidic protein (GFAP). Giant cells are immunoreactive for NSE, but do not stain for GFAP. Neighboring spindle cells are strongly GFAP-positive (original magnification ×100).
Figure 12.
Case 1. Immunoreactivity of giant cells for neuron-specific enolase (NSE) and glial fibrillary acidic protein (GFAP). Spindle cells show intense immunoreactivity for both NSE and GFAP (original magnification ×100).
Reported cases of aggressive retinal astrocytomas without clinical evidence of TSC are summarized in Table 4. We were able to identify 12 reported cases that we believe are acceptable based on a review of the clinical description and the illustrations.7–17 Reported cases of aggressive retinal astrocytomas in patients with TSC, including our four cases, are summarized in Table 5. Our literature search revealed five previously reported acceptable cases of aggressive retinal astrocytic hamartomas that were clearly associated with TSC,18–22 one of which is included in our series of four cases.22
Table 4.
Reports of enlarging retinal astrocytomas with no evidence of tuberous sclerosis complex, managed by enucleation*
| First author | Year | Age (yr) at enuc | Sex | Solitary | Multifocal | Unilateral | Bilateral | Tumor size (base x thickness)† | Yellow exudation | Neovascular glaucoma | Reason for enuc | Predominantly spindle type | Predominantly round cell | Necrosis | Calcification | Optic nerve extension |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boles7 | 1958 | 6 | x | |||||||||||||
| Foos8 | 1965 | 31 | F | x | x | 8×6 | x | BPE | x | Yes | Yes | Yes | ||||
| Jordano9 | 1974 | 9 | F | x | x | 14×12 | BPE | x | Yes | Yes | ||||||
| Reeser10 | 1978 | 6 | ||||||||||||||
| Ramsay11 | 1979 | 41 | M | x | x | 8×6 | R/O MM | x | No | No | ||||||
| Wolter12 | 1982 | 26 | M | x | x | 22×19 | BPE | x | No | No | Yes | |||||
| Jakobiec13 | 1983 | <1 | M | x | x | 10×10 | BPE | x | Yes | No | No | |||||
| Ulbright14 | 1984 | 1 | F | x | x | 13×7 | R/O RB | x | No | No | No | |||||
| Arnold15 | 1985 | 16 | F | x | x | 11×9 | x | x | BPE | x | x | Yes | ||||
| Bornfeld16 | 1987 | 56 | M | x | x | x | x | BPE | x | Yes | ||||||
| Shields C17 | 2004 | 16 | F | x | x | 10×6 | x | Growth | x | No | No | No | ||||
| Shields C17 | 2004 | 33 | F | x | x | 9×7 | x | Growth | x | No | No | No |
BPE, blind, painful eye; ENUC, enucleation; R/O MM, rule out melanoma; R/O RB, rule out retinoblastoma.
Incomplete list of reported cases. A few reported cases were not included because of poor documentation.
Largest basal diameter and thickness. Some sizes are estimated, based on review of published illustrations.
Table 5.
Reports of enlarging retinal astrocytomas with evidence of tuberous sclerosis complex, managed by enucleation
| First author | Year | Age (yr) at enuc | Sex | Solitary | Multifocal | Unilateral | Bilateral | Tumor size (base x thickness)† | Yellow exudation | Neovascular glaucoma | Reason for enuc | Predominantly spindle type | Predominantly round cell | Necrosis | Calcification | Optic nerve extenstion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wolter18 | 1969 | 10 | M | x | x | 10×10 | ? | Yes | BPE | x | Yes | No | ||||
| Garron19 | 1964 | 6 | F | x | x | 9×6 | ? | Yes | BPE | x | ? | Yes | No | |||
| Coppeto20 | 1982 | 9 | F | x | x | 10×4 | Yes | Yes | BPE | x | Yes | Yes | Yes | |||
| Margo21 | 1993 | 27 | F | x | x | 9×5 | Yes | No | R/O MM | x | No | Yes | No | |||
| Gunduz22 | 1999 | |||||||||||||||
| Shields J (this series) (case 1) | 2003 | 3 | M | x | x | 12×9 | Yes | Yes | BPE | x | Yes | Yes | No | |||
| Shields J (this series) (case 12) | 2003 | 3 | M | ? | ? | 14×7 | Yes | Yes | BPE | x | Yes | Yes | Yes | |||
| Shields J (this series) (case 3) | 2003 | 1 | F | x | x | 10×10 | Yes | Yes | BPE | x | Yes | Yes | Yes | |||
| Shields J (this series) (case 4)* | 2003 | 14 | F | x | x | 20×10 | Yes | Yes | BPE | x | Yes | Yes | Yes |
BPE, blind, painful eye; Enuc, enucleation; R/O MM, rule out melanoma; R/O RB, rule out retinoblastoma.
This is same case reported by Gunduz et al.22
Largest basal diameter and thickness. Some sizes are estimated, based on review of published illustrations.
A comparison of Tables 3 and 4 suggests that there are many similarities between aggressive retinal astrocytomas unassociated with overt manifestations of TSC and those associated with TSC. However, those unassociated with TSC tended to require enucleation at an older age, and choroidal melanoma was often a diagnostic consideration prior to enucleation. All were solitary lesions that occurred in patients who had no other fundus tumors or other abnormalities. There appeared to be a greater component of spindle cells in tumors unassociated with TSC, but this was difficult to assess accurately in our literature review. Otherwise, there were no important clinical or histopathologic differences between the two groups. Histopathologically, the tumors in each group were similar to the subependymal giant cell astrocytoma that characterizes TSC.23,24
DISCUSSION
Tuberous sclerosis complex is a heritable disorder characterized by a variety of hamartias and hamartomas, including congenital hypopigmented cutaneous macules (“ash-leaf sign”), facial angiofibromas (“adenoma sebaceum”), intracranial paraventricular or subependymal astrocytomas, cardiac rhabdomyoma, renal angiomyolipoma, and retinal astrocytic hamartomas. About 60% of cases are sporadic, and 40% are familial and appear to be inherited in an autosomal dominant fashion.25 However, the disorder is believed to be recessive at a molecular level, similar to retinoblastoma. About half of the cases with TSC show linkage to chromosome 9q34 and about half to chromosome 16p13.23.26 The clinical manifestations of both types seem to be very similar.
Each of our patients had clinical findings compatible with TSC. The main ocular finding of TSC is the astrocytic hamartoma of the retina.1–5 Less frequent ocular manifestations include patches of iris hypopigmentation and atypical inferior iris colobomas. Another recently described intraocular lesion in patients with TSC is hamartoma of the iris and ciliary body epithelium.6 This lesion usually is not appreciated clinically but has been found on histopathologic examination of enucleated eyes. It was present in two of our patients and possibly is more common, but it is overlooked clinically and histopathologically.
Retinal astrocytic hamartomas occur in about 50% of patients with TSC, about 50% of whom have bilateral retinal involvement.4,5 This tumor can manifest as a small, sessile noncalcified lesion in the nerve fiber layer, as a multinodular yellow-white calcified lesion, or as a combination of the two.4,5 Most lesions are relatively small, ranging from 0.5 to 5.0 mm in diameter, and larger lesions are exceptional.
There have been a number of reports in the literature on retinal astrocytomas associated with TSC that remained fairly stable or, rarely, produced mild vitreous tumor seeding or vitreous hemorrhage, but did not require enucleation. Those reports were not cited in this review, which addressed only lesions that were aggressive enough to require enucleation and undergo histopathologic studies.
It has been stressed that astrocytic hamartoma of TSC is generally a fairly stationary lesion that shows little or no tendency to grow.3–5 The four cases reported here are clearly exceptions to that rule. Each showed slowly progressive enlargement that eventually caused total retinal detachment and neovascular glaucoma that required enucleation of the affected blind, painful eye.
The retinal tumors described in this report are similar to those that occur in the brain in patients with TSC. The characteristic brain lesion of TSC is the subependymal astrocytoma.23,24 In both the brain and the retina, the astrocytic hamartoma can be an isolated finding without other clinical evidence of TSC. Those in the brain, like those in the retina, are generally asymptomatic and are detected on radiographic evaluation. However, the subependymal astrocytoma of TSC sometimes can behave aggressively and cause a sudden increase in intracranial pressure, visual loss, and death.23,24,27 However, the prognosis for life is good when tumors are surgically removed or decompressed.27 It is of interest that such aggressive behavior in both the brain and the retina is more likely to occur in very young children, often before the age of 5 years. In our review, the retinal astrocytic neoplasm appeared to require enucleation at a somewhat earlier age in children with TSC (mean, 9 years; Table 5) than those patients without TSC (mean, 20 years; Table 1).
The histopathologic findings of retinal astrocytic hamartoma can vary from case to case. The typical small, stationary astrocytic hamartoma usually is confined to the nerve fiber layer of the retina and is composed of elongated fibrous astrocytes with interlacing cytoplasmic processes.1,2,28 The more aggressive tumors described in this report are composed predominantly of large, plump cells with abundant eosinophilic cytoplasm, nuclear pleomorphism, and some mitotic activity. The tumors characteristically show extensive necrosis and calcospherites. These findings, present in our four cases, are virtually identical to those that characterize the subependymal giant cell astrocytoma of TSC.22,23
Immunohistochemical analysis of the retinal giant cell astrocytoma characteristically shows a mixed glioneuronal phenotype. Despite the fact that the tumor has an overtly astrocytic appearance, the giant cells are often nonreactive or weakly positive for GFAP, but typically stain strongly for NSE. Most tumors are also immunoreactive for S-100 protein. This mixed immunophenotype suggests that the tumor is heterogeneous or hybrid in nature, expressing proteins of both glial and neuronal cells.22 Our cases tended to show a biphasic pattern of immunoreactivity; the larger giant cells were positive for NSE and negative or only weakly positive for GFAP, whereas the neighboring plump spindle cells were positive for NSE and GFAP. These immunohistochemical properties are also similar to those of the giant cell astrocytomas of the central nervous system.22,23
An intriguing aspect of our cases was that three patients had multiple, bilateral retinal astrocytomas, but only one of the tumors demonstrated progressive growth and complications. Each tumor that showed growth was located adjacent to the optic disk, and all four tumors invaded the optic nerve. It is of interest that neuropathologists still classify the giant cell astrocytoma of TSC as a benign neoplasm, despite the fact that it can slowly enlarge and demonstrate local invasion.23,24 The more peripheral tumors in the ipsilateral and contralateral eyes remained stable and had no tendency to proliferate.
There have been several case reports of tumors that seem identical histopathologically to the cases reported here but occurred as unilateral solitary lesions in somewhat older patients who had no clinical findings of TSC.7–17 We have chosen to call such lesions acquired retinal astrocytomas in order to differentiate them from the congenital astrocytic lesions that classically are associated with TSC.1,2 However, it is not possible to categorically state that the acquired retinal astrocytoma is not a forme fruste of TSC in which the disease is expressed only as a solitary retinal lesion that may cause ocular complications somewhat later in life. It was not possible to perform genetic studies on our patients, but in the future, genetic studies may answer the question as to whether the solitary acquired retinal astrocytoma is related to TSC.
The clinical differential diagnostic considerations in the progressive retinal astrocytic hamartoma include retinoblastoma, choroidal melanoma, and several other fundus conditions. In an infant or young child, systemic and ocular manifestations of TSC should suggest astrocytic hamartoma rather than retinoblastoma. Ophthalmoscopically, retinal astrocytic hamartoma characteristically shows an exudative retinal detachment with yellow, lipoproteinaceous exudation in the sensory retina and subretinal space. Such exudation is not seen in untreated retinoblastoma. Even though the retinal blood vessels that supply retinal astrocytic tumors are slightly dilated, they typically are not markedly dilated and tortuous, as seen in a comparable-sized retinoblastoma. In rare instances, a carefully planned fine-needle aspiration biopsy has been employed to differentiate retinal astrocytic hamartoma from retinoblastoma.29 However, that technique should be reserved for exceptional cases where the diagnosis has not been established by other methods.
In contrast to choroidal melanoma, progressive retinal astrocytic hamartoma is entirely amelanotic, produces yellow exudation, and is located in the sensory retina rather than the choroid.30 In one reported case, the eye was enucleated with the diagnosis of melanoma.21 In retrospect, the typical fundus features and the finding of subependymal astrocytomas should have suggested the diagnosis of astrocytic hamartoma. A review of other reported cases that clinically simulated melanoma also shows features more suggestive of retinal astrocytoma.9,10
Other fundus tumors such as choroidal hemangioma, choroidal osteoma, and choroidal metastasis should not be confused with astrocytoma, because they have distinguishing features and lack appreciable yellow retinal and subretinal exudation.30 In addition, conditions that produce yellow exudation, like retinal capillary hemangioma and Coats’ disease, have typical features that should differentiate them from retinal astrocytoma.31,32
In summary, we report here on four patients with TSC who developed progressive growth of a retinal astrocytic hamartoma, each of whom eventually required enucleation because of complications of neovascular glaucoma. The histopathologic features of these unusual tumors are identical to those seen with the subependymal giant cell astrocytoma of TSC. Similar lesions have been reported in patients without clinical manifestations of TSC and may represent a forme fruste of that syndrome. Progressive retinal astrocytic hamartoma has rather distinctive features that serve to differentiate it from retinoblastoma, choroidal melanoma, and other related fundus conditions.
DISCUSSION
Dr Dennis M. Robertson
The paper by Shields reports four cases of singularly unusual giant cell astrocytomas that developed in the retinas of patients who had features of tuberous sclerosis; in each case, the tumors demonstrated aggressive growth with destruction of the eye, and in each case enucleation was required. The tumors in these cases are unusual in that they do not represent the type of spindle cell astrocytic retinal tumor usually seen in cases with tuberous sclerosis. The cell type in the four cases reported by the authors has two major cell components: one a spindle cell and the other a larger neuronal cell. These retinal tumors appear similar to the subependymal giant cell astrocytomas that occur in the brain in patients with tuberous sclerosis. The question arises as to whether or not these retinal tumors can actually become malignant and metastasize. The issue of malignancy in the giant cell astrocytomas in the brain has largely been settled. In a large, systematically studied series of giant cell astrocytomas reviewed at the Mayo Clinic among patients with tuberous sclerosis, although cytologic atypia was common occurring in approximately 80 percent of the cases and mitotic figures were recognized in one to slightly more than five mitotic cells per high-power field, these histologic findings appeared not to affect the prognosis and none of the tumors have been known to metastasize.1,2
This is a very rare tumor. The incidence of tuberous sclerosis in population studies has been estimated to be between 1 in 15,0003 and 1 in 150,000.4 The likelihood of finding retinal tumors in patients with tuberous sclerosis is approximately 50 percent. I have been fortunate to work with Dr. Manfred Gomez at the Mayo Clinic, who developed an in-depth interest in tuberous sclerosis and who attracted a large referral practice of patients with tuberous sclerosis. In 1976, I published a study based on 116 patients that we had seen at the Mayo Clinic with tuberous sclerosis.5 Since then, I have continued to see patients with tuberous sclerosis, totaling now over 160 patients, and I have never seen the type of lesion that has been reported by the authors of this report.
The authors acknowledge that the retinal lesions in tuberous sclerosis generally do not grow. This well-known fact has been supported by numerous reports, including a study published by Zimmer-Galler and Robertson in 1995.6 Doctor Zimmer-Galler and I published a series of cases with long-term follow-up showing the relative stability of these tumors over intervals beyond two decades. Although the development of intratumoral calcifications over follow-up of one to two decades was illustrated in that publication, we also showed that a typical translucent velvety hamartoma could remain unchanged without developing calcification over follow-up of nearly two decades.
It appears that the diagnosis of the retinal tumors, such as reported by the authors, even in the setting of tuberous sclerosis, may be very difficult. To absolutely differentiate these from retinoblastoma may not be possible without histology. In all four cases reported by the authors, the tumors were first seen in infancy, were parapapillary, contained calcium, and developed exudative components coincident with their growth. Are the authors aware of any patients who have developed coexisting retinoblastoma in the setting of tuberous sclerosis?
Finally, when faced with another similar case, have the authors formulated a strategy for some form of therapeutic intervention that might conceivably save the eye. If these tumors were recognized at an early age when their growth was just beginning and if histology confirmed the nature of the lesion, and if the optic nerves were not invaded, would the authors consider transvitreal endoresection with vitrectomy, removal of the tumor, and retinal repair? The latter is a formidable procedure, but since the tumors themselves appear not to be comprised of malignant cells, it is not likely that the surgical procedure itself would be associated with a systemic problem. Also, since enucleation appears otherwise inevitable, such a procedure might be a reasonable consideration.
REFERENCES
- 1.Shepherd CW, Scheithauer BW, Gomez MR, et al. Subependymal giant cell astrocytoma: a clinical, pathological, and flow cytometric study. Neurosurgery. 1991;28:864–868. [PubMed] [Google Scholar]
- 2.Scheithauer BW and Reagan TJ. Neuropathology, Chapter 9. In: Gomez MR, Sampson JR, Whittemore VH, ed. Tuberous Sclerosis Complex, 3rd Edition. New York: Oxford University Press; 1999:101–144.
- 3.Shepherd CW, Beard CM, Gomez MR, et al. Tuberous sclerosis complex in Olmsted County, Minnesota 1950–1989. Arch Neurol. 1991;48:400–401. doi: 10.1001/archneur.1991.00530160068015. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson AC, Fischer OD. Frequency of epiloia in Northern Ireland. Br J Prev Soc Med. 1956;10:134–135. doi: 10.1136/jech.10.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyboer JH, Robertson DM, Gomez MR. Retinal lesions in tuberous sclerosis. Arch Ophthalmol. 1976;94:1277–1980. doi: 10.1001/archopht.1976.03910040149003. [DOI] [PubMed] [Google Scholar]
- 6.Zimmer-Galler IE, Robertson DM. Long-term observation of retinal lesions in tuberous sclerosis. Am J Ophthalmol. 1995;119:318–324. doi: 10.1016/s0002-9394(14)71174-2. [DOI] [PubMed] [Google Scholar]
Dr Victor M. Elner
Did any of the other hamartomas in the brain grow in these patients? In other words, is this only something that was isolated to the retina or did the same event occur within the CNS lesions? How is this distinguished histopathologically from massive gliosis or could this be a massive gliotic response to tumor?
Dr Jerry A. Shields
Concerning Dr Robertson’s comments, I can make the following comments and responses. From our literature review and personal experience, we are not aware of metastasis from retinal astrocytoma. This parallels the lack of metastasis from the similar neoplasm in the brain. The co-existence of retinoblastoma and tuberous sclerosis must be extremely rare. We are aware of one report of presumed retinoblastoma and astrocytoma in the same eye of a patient who apparently did not have tuberous sclerosis.1
I believe there is a strong argument for early management of an aggressive retinal astrocytic hamartoma. If we detect a small astrocytic hamartoma that shows either progressive growth or exudation and retinal detachment, early treatment would seem justified. However, transvitreal endoresection would probably not be my first choice. If the tumor were still small, one could consider laser treatment. If it were medium-sized, one could consider plaque brachytherapy. If it were a large tumor with extensive retinal detachment, one might consider vitrectomy, intraocular gas, and endolaser or cryotherapy or subsequent plaque brachytherapy. If the patient has total retinal detachment and neovascular glaucoma, as in our cases, then enucleation is justified.
With regard to the questions of Dr Elner, the similar tumor in the brain can also rarely show aggressive local behavior and produce hydrocephalus or other complications. To our knowledge, none of the brain tumors have progressed in the patients reported in our series or in other reported cases of aggressive retinal astrocytoma. The solitary tumor reported here can be clinically similar to some cases of massive gliosis of the retina, which can occasionally present as a distinct mass. However, massive gliosis generally occurs as extensive, diffuse gliosis in eyes that have had trauma or chronic retinal detachment from Coats disease, retinal capillary hemangiomatosis and other such insults.
REFERENCE
- 1.Imhof SM, Moll AC, Van Der Valk P, Schouten-Van Meeteren AY. Retinoblastoma and retinal astrocytoma: unusual double tumour in one eye. Br J Ophthalmol. 2002;86:1441–1442. doi: 10.1136/bjo.86.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figure 3.
Case 1. Gross appearance of sectioned globe showing exophytic retinal mass and total retinal detachment.
Figure 4.
Case 3. Gross appearance of sectioned globe showing yellowish white epipapillary mass and total retinal detachment.
ACKNOWLEDGMENT
The authors thank Drs Stephen Sinclair, Abdel Hadj, Marwan Zeidan, David Doka, and Mark Wood for referral of the patients in this study.
Footnotes
Supported in part by the Eye Tumor Research Foundation, Philadelphia, Pennsylvania (Dr J. Shields and Dr C. Shields); the Award of Merit in Retina Research, Houston, Texas (Dr J. Shields); the Macula Foundation, New York, New York (Dr C. Shields); and the Noel T. and Sara L. Simmonds Endowment for Ophthalmic Pathology, Wills Eye Hospital, Philadelphia, Pennsylvania (Dr Eagle).
REFERENCES
- 1.Shields JA, Shields CL. Glial tumors of the retina and optic disc. In: Intraocular Tumors. A Text and Atlas. Philadelphia: WB Saunders; 1992:422–431.
- 2.Shields JA, Shields CL. Glial tumors of the retina and optic disc. In: Atlas of Intraocular Tumors. Philadelphia: Lippincott, Williams & Wilkins; 1999:272–283.
- 3.Nyboer JH, Robertson DM, Gomez MR. Retinal lesions in tuberous sclerosis. Arch Ophthalmol. 1976;94:1277–1280. doi: 10.1001/archopht.1976.03910040149003. [DOI] [PubMed] [Google Scholar]
- 4.Robertson DM. Ophthalmic manifestations of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:17–25. doi: 10.1111/j.1749-6632.1991.tb37744.x. [DOI] [PubMed] [Google Scholar]
- 5.Zimmer-Galler IE, Robertson DM. Long-term observation of retinal lesions in tuberous sclerosis. Am J Ophthalmol. 1995;119:318–324. doi: 10.1016/s0002-9394(14)71174-2. [DOI] [PubMed] [Google Scholar]
- 6.Eagle RC, Jr, Shields JA, Shields CL, et al. Hamartomas of the iris and ciliary epithelium in tuberous sclerosis complex. Arch Ophthalmol. 2000;118:711–715. doi: 10.1001/archopht.118.5.711. [DOI] [PubMed] [Google Scholar]
- 7.Boles WM, Naugle TC, Samson CIM. Glioma of the optic nerve. Arch Ophthalmol. 1958;59:229–231. doi: 10.1001/archopht.1958.00940030097008. [DOI] [PubMed] [Google Scholar]
- 8.Foos RY, Straatsma BR, Allen RA. Astrocytoma of the optic nerve head. Arch Ophthalmol. 1965;74:319–326. doi: 10.1001/archopht.1965.00970040321006. [DOI] [PubMed] [Google Scholar]
- 9.Jordano J, Galera H, Toro M, et al. Astrocytoma of the retina. Report of a case. Br J Ophthalmol. 1974;58:555–559. doi: 10.1136/bjo.58.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeser FH, Aaberg TM, Van Horn DL. Astrocytic hamartoma of the retina not associated with tuberous sclerosis. Am J Ophthalmol. 1978;86:688–698. doi: 10.1016/0002-9394(78)90192-7. [DOI] [PubMed] [Google Scholar]
- 11.Ramsay RC, Kinyoun JL, Hill CW. Retinal astrocytoma. Am J Ophthalmol. 1979;88:32–36. doi: 10.1016/0002-9394(79)90748-7. [DOI] [PubMed] [Google Scholar]
- 12.Wolter JR, Schut AL, Dorovini-Zis K. Massive ocular astrocytoma in a congenitally deformed eye. Br J Ophthalmol. 1982;66:583–588. doi: 10.1136/bjo.66.9.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakobiec FA, Brodie SE, Haik B, et al. Giant cell astrocytoma of the retina. A tumor of possible Mueller cell origin. Ophthalmology. 1983;90:1565–1576. doi: 10.1016/s0161-6420(83)34348-7. [DOI] [PubMed] [Google Scholar]
- 14.Ulbright TM, Fulling KH, Helveston EM. Astrocytic tumors of the retina. Differentiation of sporadic tumors from phakomatosis-associated tumors. Arch Pathol Lab Med. 1984;108:160–163. [PubMed] [Google Scholar]
- 15.Arnold AC, Hepler RS, Yee RW, et al. Solitary retinal astrocytoma. Surv Ophthalmol. 1985;30:173–181. doi: 10.1016/0039-6257(85)90061-x. [DOI] [PubMed] [Google Scholar]
- 16.Bornfeld N, Messmer EP, Theodossiadis G, et al. Giant cell astrocytoma of the retina. Clinicopathologic report of a case not associated with Bourneville’s disease. Retina. 1987;7:183–189. [PubMed] [Google Scholar]
- 17.Shields CL, Shields JA, Eagle RC, Jr, et al. Progressive enlargement of acquired retinal astrocytoma in two cases. Ophthalmology. 2004;111:363–368. doi: 10.1016/j.ophtha.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Wolter JR, Mertus JM. Exophytic retinal astrocytoma in tuberous sclerosis. J Pediatr Ophthalmol. 1969;6:186–191. [Google Scholar]
- 19.Garron LK, Spencer WH. Retinal glioneuroma associated with tuberous sclerosis. Trans Am Acad Ophthalmol Otolaryngol. 1964;68:1018–1021. [PubMed] [Google Scholar]
- 20.Coppeto JR, Lubin JR, Albert DM. Astrocytic hamartoma in tuberous sclerosis mimicking necrotizing retinochoroiditis. J Pediatr Ophthalmol Strabismus. 1982;19:306–313. doi: 10.3928/0191-3913-19821101-07. [DOI] [PubMed] [Google Scholar]
- 21.Margo CE, Barletta JP, Staman JA. Giant cell astrocytoma of the retina in tuberous sclerosis. Retina. 1993;13:155–159. doi: 10.1097/00006982-199313020-00013. [DOI] [PubMed] [Google Scholar]
- 22.Gunduz K, Eagle RC, Jr, Shields CL, et al. Invasive giant cell astrocytoma of the retina in a patient with tuberous sclerosis. Ophthalmology. 1999;106:639–642. doi: 10.1016/S0161-6420(99)90133-1. [DOI] [PubMed] [Google Scholar]
- 23.Burger PC, Scheithauer BW. Subependymal giant cell astrocytoma (tuberous sclerosis). In: Burger PC, Scheithauer BW, eds. Tumors of the Central Nervous System. Atlas of Tumor Pathology, Third series, Fascicle 10. Washington, DC: Armed Forces Institute of Pathology; 1994:383–385.
- 24.Kleihues P, Cavenee WK. Tuberous sclerosis complex and subependymal giant cell astrocytoma. In: Kleihues P, Cavenee WK, eds. Pathology and Genetics of Tumours of the Nervous System. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2000:227–230.
- 25.Kwiatkowski DJ, Short MP. Tuberous sclerosis. Arch Dermatol. 1994;130:348–354. [PubMed] [Google Scholar]
- 26.Sampson JR, Harris PC. The molecular genetics of tuberous sclerosis. Hum Mol Genet. 1994;3:1477–1480. doi: 10.1093/hmg/3.suppl_1.1477. [DOI] [PubMed] [Google Scholar]
- 27.Boesel CP, Paulson GW, Kosnik EJ, et al. Brain hamartomas and tumors associated with tuberous sclerosis. Neurosurgery. 1979;4:410–417. doi: 10.1227/00006123-197905000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Green WR. Retina. Astrocytic (glial) hamartomas. In: Spencer WH, et al, eds. Ophthalmic Pathology. An Atlas and Textbook. Vol 2, ed 4. Philadelphia: WB Saunders; 1996:695–696.
- 29.Shields JA, Shields CL, Ehya H, et al. Atypical retinal astrocytic hamartoma diagnosed by fine needle biopsy. Ophthalmology. 1996;103:949–952. doi: 10.1016/s0161-6420(96)30581-2. [DOI] [PubMed] [Google Scholar]
- 30.Shields JA, Shields CL. Clinical features of posterior uveal melanoma. In: Atlas of Intraocular Tumors. Philadelphia: Lippincott, Williams & Wilkins; 1999:74–93.
- 31.Shields JA, Shields CL, Honavar S, et al. Coats’ disease. Clinical variations and complications of Coats’ disease in 150 cases. The 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol. 2001;131:561–571. doi: 10.1016/s0002-9394(00)00883-7. [DOI] [PubMed] [Google Scholar]
- 32.Shields JA, Shields CL, Honavar SG, et al. Classification and management of Coats’ disease. The 2000 Proctor lecture. Am J Ophthalmol. 2001;131:572–583. doi: 10.1016/s0002-9394(01)00896-0. [DOI] [PubMed] [Google Scholar]