ABSTRACT
Purpose
To ascertain the extent to which ophthalmologic interventions have been evaluated in value-based medicine format.
Methods
Retrospective literature review. Papers in the healthcare literature utilizing cost-utility analysis were reviewed by researchers at the Center for Value-Based Medicine, Flourtown, Pennsylvania. A literature review of papers addressing the cost-utility analysis of ophthalmologic procedures in the United States over a 12-year period from 1992 to 2003 was undertaken using the National Library of Medicine and EMBASE databases. The cost-utility of ophthalmologic interventions in inflation-adjusted (real) year 2003 US dollars expended per quality-adjusted life-year ($/QALY) was ascertained in all instances.
Results
A total of 19 papers were found, including a total of 25 interventions. The median cost-utility of ophthalmologic interventions was $5,219/QALY, with a range from $746/QALY to $6.5 million/QALY.
Conclusions
The majority of ophthalmologic interventions are especially cost-effective by conventional standards. This is because of the substantial value that ophthalmologic interventions confer to patients with eye diseases for the resources expended.
INTRODUCTION
Evidence-based medicine is the practice of medicine incorporating the highest level of scientific evidence available.1,2 Since the inception of the term in 1992,3 it has gained widespread notoriety.
Value-based medicine is the practice of medicine incorporating the highest level of evidence-based data1,2 with the patient-perceived value conferred by healthcare interventions for the resources (dollars) expended.4–8 Value-based medicine takes the best evidence-based data from clinical trials, then converts these data to value-based form using the preferences of patients who have lived with the disease or health state under study. The patient-perceived value of virtually any intervention in healthcare can then be compared to that of any other intervention using the quality-adjusted life-year (QALY) as a common outcome measure. When the associated costs are added, the dollars expended for the value ($/QALY) gained, or the cost-utility, can be ascertained and compared across all specialties, no matter how diverse. Cost-utility analysis is the instrument that allows a value-based medicine database to be created.
To date, the cost-utility of multiple ophthalmologic interventions has been studied. Because of the increasing importance of cost-utility analysis due to the awareness of the modality by policymakers,9 the authors undertook a study to ascertain the status of cost-utility analysis in the ophthalmic literature.
METHODS
A literature search was performed using the National Library of Medicine (PubMed) database and the EMBASE database. The key words used in the search were ophthalmology, ocular, cost-effectiveness analysis, and cost-utility analysis. The years included in the search were 1992 through 2003. The search was confined solely to papers dealing with reimbursement in the United States because of the considerable differences in reimbursement schema, and thus the incomparability of healthcare economic analyses, among different countries. The personal experiences and familiarity of the authors with the value-based literature were also utilized to make the search as complete as possible.
For inclusion in the analysis, each paper was required to measure the outcome of value conferred by an intervention in terms of improvement in length of life and/or quality of life, both of which are incorporated in the $/QALY (dollars expended per quality-adjusted life-year). Thus, only papers reporting the results in $/QALY were utilized. It should be noted that some authors refer to healthcare economic analyses measured in $/QALY as cost-effectiveness analyses,10 whereas those in countries other than the Unites States refer to them as cost-utility analyses.11 The authors of the present study believe that a healthcare economic analysis reporting an outcome in $/QALY should be termed a cost-utility analysis. Papers reporting outcomes in the form of life-years gained or years of vision gained were excluded from the study.
Each paper found in the search was analyzed for the following variables: (1) the health-related quality of life analysis methodology utilized, (2) the source of the preferences used in the health-related quality-of-life analysis, (3) the treatment of comorbidities, (4) the general perspective of the analysis (eg, societal, third-party insurer, patient), (5) the individual perspective of analysis (reference case or age-specific), (6) the cost basis for facility, provider, and pharmaceutical expenditures, and (7) the annual discount rate(s) employed for costs and outcomes. Depending upon the year in which the study was undertaken, the authors of the present paper adjusted the dollars for general inflation, thus converting the results to real dollar form using year 2003 US dollars.
RESULTS
A total of 19 articles were found meeting the criteria outlined.12–30 Each of the articles reported results in the form of $/QALY, or dollars spent gained per quality-adjusted life-year gained. The total number of interventions evaluated in the 19 articles found was 25.
The cost-utility of the interventions ranged from $761/QALY to $6.5 million/QALY, with a median cost of $6,470/QALY. The most cost-effective intervention was treatment of threshold retinopathy of prematurity using laser photocoagulation, with a $/QALY of $746.12 The least cost-effective treatment, at $6.5 million/QALY, was that for acute central retinal artery occlusion using anterior chamber paracentesis and in-hospital treatment with intermittent inhalation of 95% oxygen and 5% carbon dioxide.30
Among the 25 interventions, 21 (84%) had a cost-utility under $100,000/QALY. An upper limit of $100,000/QALY has been suggested as the cutoff for interventions that are cost-effective.31,32
A list of the ophthalmologic interventions studied with cost-utility analysis is shown in Table 1. The year the study was published is shown, as is the cost-utility of each intervention in year 2003 US dollars adjusted for general inflation according to the consumer price index. Twenty-two of the 25 interventions (88%) were in articles published within the 5-year period from 1999 through 2003, and three of 25 (12%) were published in the preceding 7 years from 1992 through 1998.
Table 1.
Cost-utility analyses for ophthalmologic interventions (year 2003 US dollars)
| Intervention | Year of study publication | $/QALY gained* |
|---|---|---|
| Laser therapy for threshold ROP12 | 1999 | 761 |
| Cryotherapy for threshold ROP12 | 1999 | 2,028 |
| Vitrectomy for vitreous hemorrhage in type 1 diabetes13 | 2001 | 2,038 |
| Cataract surgery, initial14 | 2002 | 2,093 |
| Amblyopia detection and therapy15 | 2002 | 2,395 |
| Cataract surgery, second eye16 | 2003 | 2,863 |
| Repair of senile ectropion17 | 2003 | 3,180 |
| Laser therapy for DME18 | 2000 | 3,309 |
| Biweekly screening of, and cryotherapy for, threshold ROP19 | 1993 | 3,623 |
| Laser therapy for extrafoveal CNVM with histplasmosis20 | 2000 | 4,528 |
| Laser therapy for subfoveal CNVM with ARMD21 | 2000 | 6,118 |
| Laser therapy for macular edema associated with BRVO22 | 2002 | 6,821 |
| Normoglycemic DM management23 | 1997 | 16,002 |
| Laser therapy to prevent neovascular glaucoma with very ischemic CRVO24 | 2000 | 16,657 |
| Laser therapy for extrafoveal CNVM with ARMD25 | 2003 | 23,640 |
| Annual screening for retinopathy (vs every 2 yr) in high-risk type 2 diabetics26 | 2000 | 43,254 |
| Surgery for PVR, C3F8 (no previous vitrectomy)27 | 2002 | 49,742 |
| Surgery for PVR, C3F8 (previous vitrectomy)27 | 2002 | 48,932 |
| Surgery for PVR, silicone oil (no previous vitrectomy)27 | 2002 | 42,667 |
| Surgery for PVR, silicone oil (previous vitrectomy)27 | 2002 | 66,126 |
| Prophylactic oral ganciclovir treatment for CMV retinitis28 | 1997 | 90,957 |
| PDT for subfoveal CNVM with ARMD29 | ||
| • 20/40 initial vision | 2001 | 94,526 |
| • 20/200 initial vision | 2001 | 189,643 |
| Treatment for acute CRAO30 | ||
| • AC paracentesis | 2000 | 366,104 |
| • AC paracentesis and CO2/O2 therapy | 2000 | 6.5 million |
AC, anterior chamber; ARMD, age-related macular degeneration; BRVO, branch retinal vein occlusion; C3F8, perfluoropropane gas; CMV, cytomegalovirus; CNVM, choroidal neovascularization; CO2/O2, 5% carbon dioxide, 95% oxygen gas mixture; CRAO, central retinal artery occlusion; CNVM, choroidal neovascular membrane; CRVO, central retinal vein occlusion; DM, diabetes mellitus; DME, diabetic macular edema; PPT, photodynamic therapy; PVR, proliferative vitreoretinopathy; ROP, retinopathy of prematurity.
$/QALY gained = dollars expended per quality-adjusted life-year gained.
Other parameter variables of the analyses are shown in Table 2. Included among these parameters are the following:
Table 2.
Parameter variables utilized in cost-utility analysis
| Intervention | Utility | Source | Perspective | Costs | Discount rate |
|---|---|---|---|---|---|
| Laser, ROP12 | TTO | Patients | Third party | Medicare | 3% |
| Cryo, ROP12 | TTO | Patients | Third party | Medicare | 3% |
| PPV, vit heme diabetes13 | TTO | Patients | Third party | Medicare | 3% |
| Cataract, first eye14 | TTO | Patients | Third party | Medicare | 3% |
| Amblyopia treatment15 | TTO | Patients | Third party | Medicare | 3% |
| Cataract, second eye16 | TTO | Patients | Third party | Medicare | 3% |
| Entropion repair17 | TTO | Patients | Third party | Medicare | 3% |
| Laser treatment, DME18 | TTO | Patients | Third party | Medicare | 3% |
| ROP, screening and cryotherapy19 | Multi-attribute | Community | Societal | Medicare | 3% |
| Laser extrafoveal CNVM, histoplasmosis20 | TTO | Patients | Third party | Medicare | 3% |
| Laser, subfoveal CNVM, ARMD21 | TTO | Patients | Third party | Medicare | 3% |
| Laser, macular edema, BRVO22 | TTO | Patients | Third party | Medicare | 3% |
| Normoglycemic DM management23 | Unspecified | Patients | Third party | Average US costs | 3% |
| Laser, ischemic CRVO24 | TTO | Patients | Third party | Medicare | 3% |
| Laser, extrafoveal CNVM, ARMD25 | TTO | Patients | Third party | Medicare | 3% |
| DR screening26 | TTO | Patients | Third party | Medicare | 3% |
| PVR, C3F8, no previous PPV27 | TTO | Patients | Third party | Medicare | 3% |
| PVR, silicone oil, no previous PPV27 | TTO | Patients | Third party | Medicare | 3% |
| PVR, C3F8, previous PPV27 | TTO | Patients | Third party | Medicare | 3% |
| PVR, silicone oil, previous PPV27 | TTO | Patients | Third party | Medicare | 3% |
| Prophylaxis, CMV retinitis28 | Unspecified | Patients | Third party | Average US costs | 3% |
| PDT, subfoveal classic CNVM | |||||
| • 20/40 vision29 | TTO | Patients | Third party | Medicare | 3% |
| •20/200 vision29 | TTO | Patients | Third party | Medicare | 3% |
| CRAO treatment30 | |||||
| •AC paracentesis | TTO | Patients | Third party | Medicare | 3% |
| •AC paracentesis + O2, CO2 | TTO | Patients | Third party | Medicare | 3% |
AC, anterior chamber; ARMD, age-related macular degeneration; BRVO, branch retinal vein occlusion; C3F8, perfluoropropane gas; CMV, cytomegalovirus; CNVM, choroidal neovascular membrane; CO2/O2, 5% carbon dioxide, 95% oxygen gas mixture; CRAO, central retinal artery occlusion; CRVO, central retinal vein occlusion; DM, diabetes mellitus; DME, diabetic macular edema; DR, diabetic retinopathy; PDT, photodynamic therapy; PPV, pars plana vitrectomy; PVR, proliferative vitreoretinopathy; ROP, retinopathy of prematurity; TTO, time tradeoff; vit heme, vitreous hemorrhage.
Utility value (patient preference) methodology. In 22 (88%) of 25 interventions, time tradeoff utility analysis was utilized. The quality of life measure used was not specified in two instances,23,28 and a multi-attribute utility value was used in one instance.19
Source of utility values. In 24 (96%) of 25 interventions, utility values obtained from patients with a health state under study were used in the analysis and in one of 25 (4%),19 the utility values were obtained from community members.
Treatment of comorbidities. In 21 (84%) of 25 of studies, a holistic approach33 (utilizing a utility value that is disease-specific, rather than a multi-attribute utility value obtained from an aggregation of symptoms and signs such as pain, anxiety, or mobility) was undertaken, and comorbidities were not accounted for in the utility values employed. In three cases,19,23,26 it was unspecified whether comorbidities were accounted for, and in one case the utility values were multi-attribute in nature.19
Cost perspective. The third-party insurer perspective was utilized in 24 (96%) of 25 of analyses. The costs included in this perspective were the direct healthcare costs for providers, facilities, and drugs. The benefits included in the third-party insurer perspective included gains in quality of life and/or length of life. One analysis was performed from the societal point of view,19 and one paper included the third-party insurer perspective, the societal perspective, and the governmental perspective.26
Value perspective. With the exception of one study,17 24 of the 25 studies were performed from the viewpoint of the reference case. The reference case is the average case, using the average age and the average clinical course, of a person with a disease.
Costs. The Medicare fee schedule for physicians, as well as the Medicare reimbursement for facilities, was utilized in 23 (92%) of 25 of interventions.34 Estimated average costs in the United States were used for two interventions. The average wholesale price for drugs was used to evaluate pharmaceutical costs.35
Discount rate. In each study, a 3% annual discount rate was used for costs and health outcomes. This is the rate recommended by the Panel for Cost-Effectiveness in Health and Medicine.10 In most studies, other discount rates were also analyzed with sensitivity analysis.
DISCUSSION
From 1992 through 2003, the cost-utility for 25 ophthalmologic interventions was reported. The number of cost-utility value analyses performed on ophthalmologic interventions has increased considerably over the past decade. Three (16%) of the 19 papers were published during the first 6 years of the 12-year period, while 16 of 19 (84%) were published during the second 6-year period. This trend of an increasing prevalence of healthcare economic analyses has also been noted for healthcare interventions overall.36 The interest of top federal policymakers in incorporating cost-utility analysis and value-based medicine into policy correlates with the increased number of publications in the literature.9
The data presented herein demonstrate that most ophthalmologic interventions in the United States studied with cost-utility analysis are cost-effective using the conventionally accepted upper limit of $100,000/QALY.31,32 It should be noted, however, that the $100,000/QALY number is arbitrary and varies from country to country, depending upon the resources available to spend on healthcare services.8 As more healthcare interventions are studied with cost-utility analysis, it is very likely that this standard will change.
The most common intraocular surgical intervention, cataract surgery, is particularly cost-effective, whether performed in the first eye ($2,093/QALY)14 or the second eye of a person who has already had cataract surgery in the first eye ($2,863/QALY).16 The majority of interventions in the posterior segment appear to be cost-effective as well. The exceptions are treatment of acute central retinal artery obstruction and photodynamic therapy for classic subfoveal choroidal neovascularization when the visual acuity at the time of initial treatment is poor (20/200). Nonetheless, when the visual acuity at the time of initial treatment is 20/40, photodynamic therapy for classic subfoveal choroidal neovascularization falls within the upper cost-effective limit of $100,000/QALY at $94,563/QALY.28
The comparability of many of the cost-utility analyses in ophthalmology results from the fact that the majority have been performed by a core group of researchers.12–30 This is very different for the rest of healthcare, in which most cost-utility analyses are not comparable to other cost-utility analyses.
Value-Based Medicine
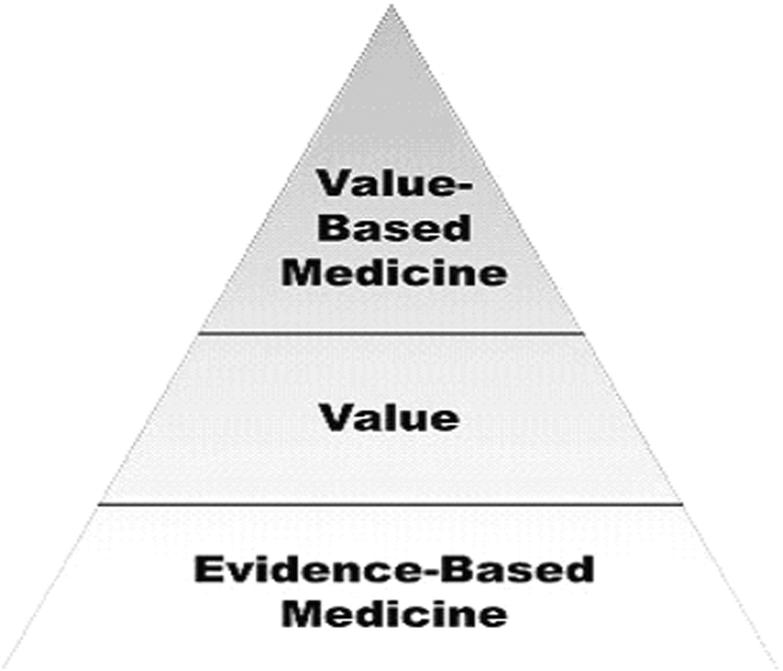
Value-based medicine, as defined in previous reports,4–8 incorporates three essential components: (1) the highest level of evidence-based data, (2) the conversion of evidence-based data to value form using patient-based quality of life preferences (utility values), and (3) the integration of the associated costs with the value conferred by an intervention to yield a final value-based medicine result measured in $/QALY (cost per quality-adjusted life-year) (Figure 1). The majority of ophthalmologic interventions studied to date are cost-effective due to the great value patients place upon their vision.7,8
Figure 1.
The value-base medicine pyramid. The best evidence-based data are initially converted to value form. This value conferred is then integrated with the associated costs of an intervention to yield a value-based medicine outcome measured in $/QALY, or cost per quality-adjusted life-year gained.
Utility value analysis measures the quality of life associated with a health state.9–24 By convention, utility values range from 1.0 (perfect health) to 0.0 (death). The better the health state, the closer the utility value is to 1.0, while the poorer the health state, the closer the utility value is to 0.0. For example, the utility value associated with treated systemic arterial hypertension is 0.98,37 while that associated with a severe stroke is 0.34.38
Utility values associated with ocular diseases most closely correlate with visual acuity in the better seeing eye, rather than vision in the poorer seeing eye or the cause of the visual loss.7,8,39,40 As the vision in the better seeing eye decreases, the corresponding utility value decreases as well. Utility values are often referred to as preferences, since patients are given a theoretical choice of whether they prefer to (1) remain in their current health state or (2) risk or lose something of value (eg, their life, a proportion of remaining life, money) to return to a normal health state.
When an intervention is undertaken, utility analysis can quantify the improvement in quality of life conferred by that intervention. Quality-adjusted life-years measure the total value gained from an intervention. The number of quality-adjusted life-years gained is calculated by multiplying the improvement in utility value conferred by the intervention by the duration of the improvement in years. For example, if an intervention raises a utility value from 0.50 to 1.00 for 12 years, the total number of QALYs gained is (1.00 – 0.50) × 12 = 6.0. When the associated costs of an intervention are added, the cost-utility ($/QALY), or dollars spent for the value conferred by the intervention, can be ascertained.
Because value-based medicine incorporates patient-perceived quality of life parameters typically not factored into the primary outcomes of evidence-based trials, the accuracy of value-based medicine in quantifying the real benefit of an intervention to a patient can supersede that of evidence-based medicine. As an example, evidence-based data from a clinical trial for cancer chemotherapy might show a primary evidence-based result such as the improvement of the average life expectancy from 12 months to 13 months (an 8.3% gain in QALYs over no treatment). In this instance, value-based data also show an improvement of the average life expectancy from 12 months to 13 months, but additionally take into account the severe vomiting during this time that decreases the overall value of remaining life (in QALYs) by 30%. Thus, value-based medicine—incorporating patient-perceived quality of life parameters—shows that during the 13 months there is an actual loss of 24.2% of value over no treatment, even with the extra month of life taken into account. Since value-based medicine provides a more accurate measure of the patient-perceived worth of a healthcare intervention than most primary, evidence-based medicine outcomes, value-based medicine allows for the practice of higher-quality medical care than value-based medicine. Succinctly, value-based medicine incorporates quality of life parameters and, most important, from the patient point of view.
It should be noted that there is confusion in the literature regarding cost-utility analysis and cost-effectiveness analysis. Some authors in the United States use the terms interchangeably,10 but authors in other countries differentiate between the two.11 We agree with the latter authors and believe that cost-utility analysis should be reserved for those interventions that measure outcome results in $/QALY. Cost-effectiveness analysis measures outcomes in terms of dollars spent per life-year gained, per year of good vision gained, or years of disability obviated, but not in terms of $/QALY. When the outcome of $/QALY is used, the study should be termed a cost-utility analysis. Despite the fact that an analysis is a cost-utility analysis, interventions measured using it are referred to as more or less cost-effective, rather than more or less cost-utilitarian.
Standardization
To date, the cost-utility literature has been largely composed of publications involving vastly different input parameters and methodologies. In regard to the quality of life measures employed, multiple utility value instruments and multiple respondents have been employed. Tengs and Wallace,41 in a comprehensive literature review, found 30 different variants of quality of life instruments used to obtain values for cost-utility analyses. These variants include time tradeoff utility analysis, standard gamble utility analysis, rating scales, and expert judgment. The respondents from whom the quality of life values were obtained included authors, experts, the general community, patients, and others.
When one includes the possible general perspectives of cost-utility analysis (third-party insurer, societal, governmental), the most common discount rates that have been utilized (0%, 3%, 5%), and the cost basis (eg, Medicare fee schedule, average US costs, regional costs) used, the number of possible variants conservatively rises to 810. When the year of the analysis and the national currency used in the analysis are factored in, there are thousands of different, possible cost-utility variants. Thus it is no small surprise that the great majority of cost-utility analyses are not comparable with other cost-utility analyses. This lack of comparability of cost-utility analyses likely accounts, in part, for the failure of value-based medicine to be incorporated into public policy in the United States at the current time. Nonetheless, it is very likely that value-based medical standards will play a role in clinical healthcare practice within the decade.7,8 The following parameters should be standardized for one cost-utility analysis to be comparable to another.
Evidence-Based Medical Data
The importance of standardization for cost-utility analyses cannot be overemphasized. The highest level of evidence-based data (α ≤ 0.05 and β ≤ 0.20) should be utilized, preferably from randomized clinical trials with level 1 evidence.2
Utility Analysis
The utility values should come from a standardized database for comparability. Time tradeoff utility analysis, the quality of life measure used in the majority of the cost-utility analyses studied herein, has demonstrated the highest reproducibility and construct validity among preference-based quality of life instruments.42,43
While some have suggested that quality of life values from the community be used for cost-utility analyses performed for healthcare resource distribution,10 the authors agree with others44–46 who believe that utility values derived from patients who have lived in a given health state should be the criterion (“gold standard”). Previous studies have shown that utility values obtained from patients often differ considerably from those obtained from physicians and the general community when the latter two groups are asked to assume they had the same health state as patients.47,48
Comorbidities
Comorbidities have been incorporated into utility values by some investigators, typically with the result of decreasing the value of an intervention. For example, the quality of life assessment methodologies of many investigators would quantify cataract surgery as less valuable in a patient with diabetes and cardiac disease than in someone who is in otherwise excellent systemic health. This runs counter to the Americans With Disabilities Act of 1990,49 which forbids discrimination against those who are disabled. Thus, utilization of any quality of life instrument or methodology that biases against those with comorbidities (disabilities) cannot be incorporated into public policy. The majority of the ophthalmologic analyses did not incorporate comorbidities into the analysis, although one used a multi-attribute technique that inherently alters utility values to account for comorbidities.19
Costs
The source of costs of a cost-utility analysis must also be standardized for a valid comparison of studies. The most standardized costs for providers and facilities in the United States are those used by Medicare for reimbursement.34 In regard to pharmaceuticals, the average wholesale price is considered the most standardized cost of a drug.35
The cost perspective must also be standardized. Direct healthcare costs include provider costs, facility costs, and drug costs. Direct nonhealthcare costs include caregiver and travel costs, and indirect costs include disability payments and failure to contribute to the gross domestic product (GDP).50 The societal perspective includes all of the preceding costs, while the governmental perspective includes the preceding costs but ignores caregiver costs and travel costs. The third-party insurer perspective, which includes only the direct healthcare costs to an insurer (or some other payer), helps simplify an already complex methodology. The third-party insurer perspective has been used in the majority of ophthalmic cost-utility analyses performed to date. While the societal viewpoint is more all-encompassing than the third-party insurer perspective, there is not uniform agreement on which costs to include in the societal perspective. The lack of conformity in this area is another reason cost-utility analysis has not been incorporated into public policy.
Discounting
It is generally agreed that both the costs and outcomes (QALYs) in cost-utility analyses be discounted at an annual rate of 3%. Each of the 25 studies analyzed herein used a 3% yearly rate, as suggested by the Panel on Cost-Effectiveness in Health and Medicine.10 Costs are discounted to account for the money they could have earned above inflation had they not been invested in healthcare services. There is some controversy in regard to the discounting of outcomes (QALYs gained),10 but the authors of the current paper believe the same concept must be applied—that good health now is worth more than good health in the future because it can be used to produce resources that can be invested to yield additional resources with time.
Table 3 shows the input parameter variables the authors believe are most appropriate for the performance of cost-utility analyses. While some may argue for other parameters, until a standardized format12–18,20–22,24,25,27 is undertaken, it is unlikely that cost-utility analysis will play a large role in healthcare delivery.
Table 3.
Standardized variables for use in cost-utility analysis
| Variables | Recommended parameters |
|---|---|
| Evidence-based data | From level 1 clinical trials2 |
| Utility analysis methodology | Time tradeoff |
| Utility respondents | Patients with a health state |
| Perspective | Third-party payer |
| Viewoint | Reference case (average person) |
| Costs | |
| • Physicians | Average CMS reimbursement |
| • Hospitals | Average CMS reimbursement |
| • Ambulatory surgical centers | Average CMS reimbursement |
| • Pharmaceuticals | Average wholesale price |
| Discount rate | 3% per year |
CMS, Centers for Medicare and Medicaid Services.
In summary, value-based medicine, the natural extension of evidence-based medicine, shows that the majority of the ophthalmologic interventions reported to date deliver excellent value for the resources expended. Value-based medicine incorporates the patient-perceived quality of life parameters associated with healthcare interventions that evidence-based medicine often ignores. In addition to providing a more accurate assessment of the overall worth of healthcare interventions than evidence-based medicine owing to the integration of patient-perceived quality of life improvement, value-based medicine provides a measure of cost-effectiveness. Interest by federal healthcare policymakers strongly suggests that value-based medicine will become an integral part of the healthcare system in the near future.9
DISCUSSION
Dr Charles P. Wilkinson
It should be obvious to all of us that we face a genuine health care crisis in the United States. The issues of increasing demand and costs combined with limited recourses mandate change in the status quo and a closer look at the genuine value of the practices and procedures that are performed. The ability to reliably measure and compare costs and value gained will become increasingly important.
The authors are genuine experts in the field of health care economic analyses. As of March 15, these authors in different combinations have published at least 40 papers devoted to this topic, and of the 19 papers cited in this current study, 15 were written by one or more of these authors. For those of you interested in learning more about this arena, I would recommend beginning with an article in the 2003 Survey of Ophthalmology,1 and I have used this in preparation of this brief discussion.
When attempting to analyze papers regarding quality and costs of health care, a neophyte such as I becomes immediately impressed and, candidly, overwhelmed with the large number of terms that are employed by various authors. These include, but are not limited to, “utility analysis,” “decision analysis,” “cost-benefit analysis,” “cost-effectiveness analysis,” “cost-minimization analysis,” and “cost utility analysis.” Looking at so-called quality of life instruments, one will discover the terms “sickness-impact profile,” “short form or SF-36,” “quality of well-being scale,” “health utility index,” and “Euro-QOL”. Ophthalmic quality of life measures include the “VF-14” and “VFQ-25.” Things become even more confusing when one is confronted with terms such as “quality-adjusted life year,” “standard gamble,” “time-tradeoff,” “Markov modeling,” and “Monte Carlo simulation”!
Value-based medicine to me represents an attempt to link a given procedure’s effectiveness, cost, and value to the patient in a standardized manner, so that one procedure can be compared to another across many lines of health care. Effectiveness and cost appear to be at least logically measurable, and the major difficulty (at least for me) lies in calculating value, a term that in this context implies a favorable change in quality of life. It is very important to note that the authors believe that patients’ opinions regarding value are more legitimate than the values assigned by payers or healthy individuals.
Values are a function of the change in so-called “utilities” and are measured, not in dollars, but in terms of so called “quality-adjusted life years” that have been described by Dr Brown. The known costs in dollars of a given procedure are then applied to the change in quality-adjusted life years to provide a figure in dollars per quality-adjusted life year. This figure can then be compared to any other procedure.
I believe I may now understand the basics of value-based medicine at an elementary level. However, I have some concerns regarding the concepts associated with assigning utilities to various states of health and therefore to some of the values associated with differing disease states. My major difficulty may be a function of my inexperience, but I wonder about the genuine “reasonableness” of some of these utility values. We all know that patients highly value vision as a very critical quality-of-life variable. Nevertheless, I am astounded by the fact that patients allegedly assign the same utility values to breast cancer requiring lumpectomy or mastectomy and subsequent radiation therapy (0.89) and to 20/25 vision in the better eye and less than or equal to 20/40 in the other eye (0.87). In addition, I had difficulty in determining precisely how costs per QALY that were below $50,000 or $100,000 per QALY came to be defined as “reasonable.” As far as I can tell, these are very arbitrary figures that have been perpetuated.
I have four questions for Dr Brown:
Can you defend these relatively low utility values associated with mild losses of vision to payers and the public?
In these days of an aging “me generation,” is it realistic and acceptable to compare one patient’s opinions of how bad his or her specific problem to those of another patient with another problem? After all, things seem worse when they happen to us than to unknown individuals.
There are substantial differences in $20,000/QALY versus $50,000/QALY versus $100,000/QALY, and yet these seem to have been very arbitrarily determined. Are the literal numbers of practical importance?
Although I agree with the importance of patients’ views of their quality of life, it remains difficult for me to believe that the assignments of utilities are similar across all socioeconomic, educational, gender, and ethnic levels. Is this really true?
REFERENCE
- 1.Brown MM, Brown GC, Sharma S, et al. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48:204–223. doi: 10.1016/s0039-6257(02)00457-5. [DOI] [PubMed] [Google Scholar]
Dr W. Banks Anderson, Jr
We had a practical application of this at our local VA hospital that has a prosthesis budget. One hip prosthesis costs about the same as 20 intraocular lenses. How would approach the administrator with that problem?
Dr George L. Spaeth
You are suggesting that your new measure is in some way more valid than old quality-of-life indicators. One of the problems that some of the studies that used old quality-of-life indicators, such as the VFQ, is that the old indicators are variable in terms of different groups. They might be reproducible, but whether or not they were valid was not determined. There’s been no validation of any quality-of-life indicator because there has been no standard against which to validate it. You have not mentioned the work that’s been done by Guralnik, and some of others, who have discussed performance-based measures in which the standard is what the person can actually do. Can the person read under different levels of light, find boxes in a room, and so forth? Why is it that you have not discussed the performance-based measures? Is not your “yardstick” at least partially arbitrary and is it not at least partially culturally dependent?
Dr David L. Guyton
There probably must be some regulation (rationing) of who gets what in this era of expensive techniques and surgeries. You suggest that value-based medicine might perform as an anti-rationing tool. How are you defining rationing?
Dr Rick Ferris
I’m very interested in outcome variables and it is the totality of the evidence that is important. I am sure you are not saying that we should throw away those outcome variables that are used as the basis of evidence-based medicine, but rather it is the combination of, here is what we have found and here is how it relates to the qualities. I wondered if you would comment about that. We have all seen data that suggest people would trade 10 percent of their life to throw away their glasses. How does this relate to your study? Many of the economic analyses that we have had to do in the past tend to be about income and age. If you’re over 65, it was virtually that your life after that point got zero value.
Dr Carol L. Shields
How does ophthalmology compare or rank with other subspecialties, like ENT or orthopedics, in value-based medicine?
Dr Gary C. Brown
The authors thank Dr Wilkinson for his scholarly review and will address his questions first.
Why do ocular utility values seem relatively low when associated with good vision?
How can a relatively good visual acuity of 20/25 in the better-seeing eye have a utility as low as 0.89? The utility values we obtained were those from people with ocular diseases. Since utilities are all encompassing, they take fear of the future into account. For example, the average person with an ocular disease and 20/20 vision in each eye does not have a utility value of 1.0, but rather one of 0.97 because of the worry about losing vision in the future. Once people start to lose vision, they worry even more. Thus, a person with 20/20 vision in one eye and 20/40 or worse vision in the fellow eye has a utility value of 0.92, while if the vision is 20/25 in the better eye and 20/40 or worse in the fellow eye, the utility value is 0.89. Most ocular diseases are bilateral, and the concern that the second eye will be involved markedly diminishes quality of life and, therefore, the utility values.
Perspective
Dr Wilkinson brings up the point that adverse events always seem more serious to us than when they happen to other people. This is specifically why we recommend using utility values derived from patients who have lived with a disease in our cost-utility analyses. It has been shown that surrogate respondents typically rate the quality of life associated with a disease better than people who have that disease.
Cost-utility standards
Regarding cost-utility (cost-effectiveness) standards, Laupacis and colleagues suggested the number of $100,000 per quality-adjusted life-year (QALY) gained as the upper cutoff for cost-effectiveness in 1992 (Can. Med. Assoc. J., Feb 1992; 146: 473–481). This number continues to be used as the upper cutoff but is somewhat irrelevant at this point. A society will ultimately have to decide how much it will pay and what the upper cutoff will be. In large part, this is dependent upon the resources a society is willing to devote to healthcare services.
Utility values and demographic variables
Utility values appear to be innate to human nature. Therefore, the person with an 8th-grade education is willing to trade the same number of years in return for perfect health as the person with an 18th-grade education. Utility values have been demonstrated repeatedly to transcend gender, age, ethnicity, education level, income bracket and nationality.
Value comparison of disparate interventions
Dr Anderson’s question regarding whether total hip arthroplasty or cataract surgery is more valuable can be answered with cost-utility analysis. It turns out that the value conferred by the intervention of total hip replacement surgery is very close to that conferred by cataract surgery. At the Center for Value-Based Medicine we have calculated that there is already more than enough money in the US healthcare system to pay for all healthcare interventions that work for everyone. However, if the only option is to perform one total hip arthroplasty or 20 cataract surgeries, putting the money into the hip surgery is not the best use of resources.
Validity
In answer to Dr Spaeth, there are two types of validity: 1) criterion validity and 2) construct validity. Criterion validity assesses how well a health-related quality of life instrument measures up to the “criterion” or “gold standard” in the field. Since there is no gold standard for health-related quality of life instruments, the criterion validity of utility values cannot be measured. Construct validity assesses how well a health-related quality of life instrument measures what it is supposed to measure—health-related quality of life.
Typically, predicting events or behaviors shows construct validity. We have demonstrated the construct of systemic utility values by showing that they correlate with worsening symptoms and clinical signs associated with diseases. Ocular utility values correlate most closely with the visual acuity in the better-seeing eye rather than the underlying cause of visual loss. With decreasing vision, the construct validity of utility values is corroborated by the fact that there are especially large utility value drops associated with visual loss that correlates with: 1) loss of driving privileges, 2) the inability to read with low-vision aids and 3) the loss of navigating vision.
We believe that utility analysis evaluates function (motor, psychological, social, etc.) very well; but function isn’t enough. As a patient with a serious health condition, there is also concern about caregiver status, the well being of family, fear of the future, the economic consequences of disease, and many additional issues. There are about 30 or 40 different parameters we’ve come up with for quality of life and we believe that utility analysis is all encompassing. It takes every single one into account, and I know of none of the other quality-of-life instruments that can do that. It’s interesting that most people who developed the quality-of-life instruments, from my point of view, were never deathly sick themselves because they certainly missed a good number of quality-of-life parameters important to people who are seriously ill.
Anti-rationing tool
In response to Dr Guyton’s question, some people label cost-utility analysis as being a rationing tool, thinking about the ill-fated Oregon plan in the early ‘90s. It is just the opposite. Two points merit discussion here.
We do not perform cost-utility analyses based on an individual patient’s age, but rather on the basis of the age of the reference case, or the case of the average patient with a disease. Age based standards would never be politically palatable since they discriminate against seniors. Reference case analyses prevent discrimination or any rationing based upon age.
Addressing the population of the entire country, we have 44 million uninsured people in the United States. For the people in that group, there is severe rationing of healthcare services. We believe that value-based medicine standards created using cost-utility analysis will identify interventions that confer negligible value, no value, or some that are even harmful. The resources saved from these value-less interventions can then be shifted to interventions that work for everybody. We therefore refer to value-based medicine as the “anti-rationing system.”
Evidence-based medicine and value-based medicine
Concerning Dr Ferris’ comment, it is impossible to have good value-based medicine standards without utilizing the highest level of evidence-based data from clinical trials. The evidence-based data are then converted to value-based (utility value) form. For example, what is the diminution in quality of life when the vision in the better-seeing eye decreases three lines from 20/20 to 20/40 on the Early Treatment Diabetic Retinopathy Study chart? And is the quality of life change the same as losing three lines of vision from 20/125 to 20/250? Furthermore, are the degrees of visual loss comparable? It turns out that the utility value decrease from 20/20 to 20/40 is –0.12, while that for 20/125 to 20/250 is –0.06. Thus the vision change from 20/20 to 20/40 causes twice the decrement in quality of life than the vision change from 20/125 to 20/250 does.
Evidence-based medicine and value-based medicine are inextricably linked. But value-based standards allow clinicians to deliver higher quality care than evidence-based standards because value-based medicine incorporates the quality of life variables often ignored in primary, evidence-based, clinical trial outcomes.
Value-based medicine and other specialties
Lastly, in regard to Dr Shields’ comments, ophthalmic interventions deliver extraordinary value to patients, often much more than we appreciate. To illustrate, we found the mean time tradeoff utility value of ophthalmologists at Wills Eye Hospital who were asked to assume they had severe macular degeneration to be 0.69. This means the average respondent was willing to trade approximately three of every 10 theoretical remaining years of life in return for permanent normal vision. In contrast, patients with macular degeneration and counting fingers or worse vision in each eye had a mean time tradeoff utility value of 0.40, meaning that the average patient would have traded six of every 10 remaining years, or twice the proportion of the ophthalmologists! Because of the great value that ophthalmic interventions deliver, we believe they will be viewed very favorably in the era of value-based medicine.
Footnotes
Supported in part by The Premier’s Award for Excellence, Ontario, Canada, and Principal’s Initiative Research Fund, Kingston, Ontario, Canada.
REFERENCES
- 1.Sackett DL, Straus SE, Richardson WS, et al. Evidence-Based Medicine. How to Practice and Teach EBM. 2nd ed. Philadelphia: Churchill Livingstone; 2000:1–12.
- 2.Sharma S. Levels of evidence. Evid Based Eye Care. 1999;1:5–6. [Google Scholar]
- 3.Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268:2420–2425. doi: 10.1001/jama.1992.03490170092032. [DOI] [PubMed] [Google Scholar]
- 4.Brown MM, Brown GC, Sharma S; Quillen DA, ed Value-based medicine. A paradigm for the 21st century. Curr Concepts Ophthalmol. 2002;10:7–11. [Google Scholar]
- 5.Brown MM, Brown GC, Sharma S. Value-based medicine. Evid Based Eye Care. 2002;3:8–9. [Google Scholar]
- 6.Brown MM, Brown GC. Outcome of corneal transplantation. Value-based health care. Br J Ophthalmol. 2002;86:2–3. doi: 10.1136/bjo.86.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MM, Brown GC, Sharma S, et al. Quality of life associated with visual loss. Time tradeoff utility analysis comparison with systemic health states. Ophthalmology. 2003;110:1076–1081. doi: 10.1016/S0161-6420(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 8.Brown MM, Brown GC, Sharma S, et al. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48:204–223. doi: 10.1016/s0039-6257(02)00457-5. [DOI] [PubMed] [Google Scholar]
- 9.Pear R. Congress weighs drug comparisons. New York Times. August 23, 2003.
- 10.Gold MR, Patrick DL, Torrance GW, et al. Identifying and valuing outcomes. In: Gold ME, Siegel JE, Russell LB, et al, eds. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996:82–134.
- 11.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 2nd ed. New York: Oxford University Press; 1999:139–199.
- 12.Brown GC, Brown MM, Sharma S, et al. Cost-effectiveness of treatment for threshold retinopathy of prematurity. Pediatrics. 1999;104:e47. doi: 10.1542/peds.104.4.e47. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Hollands H, Brown GC, et al. The cost-effectiveness of early vitrectomy for the treatment of vitreous hemorrhage in diabetic retinopathy. Curr Opin Ophthalmol. 2001;12:230–234. doi: 10.1097/00055735-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Busbee B, Brown MM, Brown GC, et al. Incremental cost-effectiveness of initial cataract surgery. Ophthalmology. 2002;109:606–612. doi: 10.1016/s0161-6420(01)00971-x. [DOI] [PubMed] [Google Scholar]
- 15.Membreno J, Brown MM, Brown GC, et al. A cost-utility analysis of therapy for amblyopia. Ophthalmology. 2002;109:2265–2271. doi: 10.1016/s0161-6420(02)01286-1. [DOI] [PubMed] [Google Scholar]
- 16.Busbee B, Brown MM, Brown GC, et al. A cost-utility analysis of cataract surgery in the second eye. Ophthalmology. 2003;110:2310–2317. doi: 10.1016/S0161-6420(03)00796-6. [DOI] [PubMed] [Google Scholar]
- 17.Brown MM, Brown GC. Cost-utility analysis. The foundation of value-based medicine. A cost-utility analysis of senile entropion repair. Evid Based Eye Care. 2003;4:114–118. [Google Scholar]
- 18.Sharma S, Brown GC, Brown MM, et al. The cost-effectiveness of grid laser photocoagulation for the treatment of diabetic macular edema: results of a patient-based cost-utility analysis. Curr Opin Ophthalmol. 2000;11:175–179. doi: 10.1097/00055735-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Javitt J, Dei Cas R, Chiang YP. Cost-effectiveness of screening and cryotherapy for threshold retinopathy of prematurity. Pediatrics. 1993;91:859–866. [PubMed] [Google Scholar]
- 20.Brown GC, Brown MM, Sharma S, et al. Incremental cost-effectiveness of laser photocoagulation for choroidal neovascularization associated with histoplasmosis. Retina. 2000;20:331–337. doi: 10.1097/00006982-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Brown GC, Brown MM, Sharma S. Incremental cost-effectiveness of laser therapy for subfoveal choroidal neovascularization. Ophthalmology. 2000;107:1374–1380. doi: 10.1016/s0161-6420(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 22.Brown GC, Brown MM, Sharma S, et al. Incremental cost-effectiveness of therapeutic interventions for branch retinal vein occlusion. Ophthalmic Epidemiol. 2002;9:1–10. doi: 10.1076/opep.9.1.1.1715. [DOI] [PubMed] [Google Scholar]
- 23.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care. 1997;20:685–686. doi: 10.2337/diacare.20.5.735. [DOI] [PubMed] [Google Scholar]
- 24.Brown GC, Brown MM. Is prophylactic PRP in ischemic CRVO a good idea? Rev Ophthalmol 2000;7:106,108,111.
- 25.Busbee B, Brown MM, Brown GC, et al. A cost-utility analysis of laser photocoagulation for extrafoveal choroidal neovascularization. Retina. 2003;23:279–287. doi: 10.1097/00006982-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000;283:889–896. doi: 10.1001/jama.283.7.889. [DOI] [PubMed] [Google Scholar]
- 27.Brown GC, Brown MM, Sharma S, et al. A cost-utility analysis of interventions for proliferative vitreoretinopathy. Am J Ophthalmol. 2002;133:365–372. doi: 10.1016/s0002-9394(01)01423-4. [DOI] [PubMed] [Google Scholar]
- 28.Moore RD, Chiasson RE. Cost-utility analysis of prophylactic treatment with oral ganciclovir for cytomegalovirus retinitis. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;1:15–21. doi: 10.1097/00042560-199709010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Sharma S, Brown GC, Brown MM, et al. The cost-effectiveness of photodynamic therapy for fellow eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2001;108:2051–2059. doi: 10.1016/s0161-6420(01)00764-3. [DOI] [PubMed] [Google Scholar]
- 30.Brown MM, Brown GC, Sharma S. Cost-effective analysis. The value component of evidence-based medicine. Evid Based Eye Care. 2000;1:243–247. [Google Scholar]
- 31.Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Can Med Assoc J. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]
- 32.Heudebert GR, Centor RM, Klapow JC, et al. What is heartburn worth? A cost-utility analysis of management strategies. J Gen Intern Med. 2000;15:175–182. doi: 10.1046/j.1525-1497.2000.02639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froberg DG, Kane RL. Methodology for measuring health-state preferences—I: Measurement strategies. J Clin Epidemiol. 1989;42:345–354. doi: 10.1016/0895-4356(89)90039-5. [DOI] [PubMed] [Google Scholar]
- 34.Medicare Fee Schedule. Public Use Files. Medicare Web site. Available at: http://www.cms.hhs.gov. Accessed September 25, 2003.
- 35.Cohen HE, ed. 2003 Drug Topics Red Book. Montvale, NJ: Medical Economics Co; 2003.
- 36.Koopmanschap MA, Touw KC, Rutten FF. Analysis of costs and cost-effectiveness in multinational trials. Health Policy. 2001;54:175–186. doi: 10.1016/s0168-8510(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 37.Stein J, Brown GC, Brown MM, et al. The quality of life of patients with hypertension. J Clin Hypertens. 2002;4:181–188. doi: 10.1111/j.1524-6175.2002.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tengs TO, Yu M, Luistro E. Health-related quality of life after stroke. A comprehensive review. Stroke. 2001;32:964–972. doi: 10.1161/01.str.32.4.964. [DOI] [PubMed] [Google Scholar]
- 39.Brown GC. Vision and quality of life. Trans Am Ophthalmol Soc. 1999;97:473–512. doi: 10.1016/s0002-9394(00)00513-4. [DOI] [PubMed] [Google Scholar]
- 40.Brown MM, Brown GC, Sharma S, et al. Quality of life with visual acuity loss from diabetic retinopathy and age-related macular degeneration. Arch Ophthalmol. 2002;120:481–484. doi: 10.1001/archopht.120.4.481. [DOI] [PubMed] [Google Scholar]
- 41.Tengs TO, Wallace MA. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Read JL, Quinn DM, Berwick DM, et al. Preferences for health outcomes. Comparisons of assessment methods. Med Decis Making. 1984;4:315–329. doi: 10.1177/0272989X8400400307. [DOI] [PubMed] [Google Scholar]
- 43.Brown MM, Brown GC, Sharma S. Value-Based Medicine. Evidence-Based Medicine and Beyond. Chicago: AMA Press. In press.
- 44.Kassirer JP. Adding insult to injury: usurping patients’ prerogatives. N Engl J Med. 1983;308:898–901. doi: 10.1056/NEJM198304143081511. [DOI] [PubMed] [Google Scholar]
- 45.Kassirer JP. Incorporating patients’ preferences into medical decisions. N Engl J Med. 1994;330:1895–1896. doi: 10.1056/NEJM199406303302611. [DOI] [PubMed] [Google Scholar]
- 46.Angell M. Patients’ preferences in randomized clinical trials. N Engl J Med. 1984;310:1385–1387. doi: 10.1056/NEJM198405243102111. [DOI] [PubMed] [Google Scholar]
- 47.Landy J, Stein JD, Brown GC, et al. Patient, community and clinician perceptions of the quality of life associated with diabetes mellitus. Med Sci Monit. 2002;8:543–548. [PubMed] [Google Scholar]
- 48.Brown GC, Brown MM, Sharma S. Difference between ophthalmologist and patient perceptions of quality-of-life associated with age-related macular degeneration. Can J Ophthalmol. 2000;35:27–32. doi: 10.1016/s0008-4182(00)80005-8. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Mejia LR, Balkin DB, Cardy RL. Managing Human Resources. Englewood Cliffs, NJ: Prentice Hall; 1995:134–136.
- 50.Smith A, Brown GC. Understanding cost-effectiveness: a detailed review. Br J Ophthalmol. 2000;54:794–798. doi: 10.1136/bjo.84.7.794. [DOI] [PMC free article] [PubMed] [Google Scholar]