ABSTRACT
Purpose
To present the final results of the Early Treatment for Retinopathy of Prematurity Study.
Methods
Infants with bilateral high-risk prethreshold retinopathy of prematurity (ROP) (n = 317) had one eye randomized to early retinal ablative treatment and the fellow eye managed conventionally (control eye). In asymmetric cases (n = 84), the eye with high-risk prethreshold ROP was randomized to early or to conventional management. High risk was determined using a model based on the Cryotherapy for Retinopathy of Prematurity natural history cohort. The primary outcome was visual acuity assessed by masked testers using the Teller acuity card procedure. Structural examinations were performed at 6 and 9 months.
Results
Grating acuity results showed a reduction in unfavorable visual acuity outcomes with earlier treatment, from 19.8% to 14.3% (P < .005). Unfavorable structural outcomes were reduced from 15.6% to 9.0% (P < .001) at 9 months. Further analysis supported retinal ablative therapy for eyes with type I ROP, defined as zone I, any stage ROP with plus disease; zone I, stage 3 ROP without plus disease; or zone II, stage 2 or 3 with plus disease. The analysis supported a “wait and watch” approach to type II ROP, defined as zone I, stage 1 and 2 without plus disease, or zone II, stage 3 without plus disease. These eyes should be considered for treatment only if they progress to type I ROP or threshold.
Conclusion
Early treatment of high-risk prethreshold ROP significantly reduced unfavorable outcomes in both primary and secondary (structural) measures.
INTRODUCTION
Despite the success of peripheral retinal ablation in reducing the risk of retinal detachment, vision impairment still remains common in infants with severe retinopathy of prematurity (ROP).1 In the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study, peripheral retinal ablation was administered when ocular findings indicated a risk of approximately 50% for retinal detachment.2 This degree of severity was termed the threshold for treatment of ROP, and was defined as at least five contiguous or eight cumulative sectors (clock hours) of stage 3 retinopathy of prematurity (ROP) in zone I or II in the presence of plus disease (a degree of dilation and tortuosity of the posterior retinal blood vessels meeting or exceeding that of a standard photograph).2 Treatment at threshold results in approximately a 50% reduction in the rate of retinal detachment.
With the hope of improving this rate of unfavorable outcome, the timing indications for treatment of ROP have been questioned, with some investigators advocating earlier treatment and others advocating conventionally timed treatment.3,4 One concern with earlier treatment is the expected increase in surgical intervention in eyes with ROP that would otherwise regress spontaneously. This concern has led to efforts to identify treatment selection criteria that would result in earlier treatment only in those eyes at highest risk for developing threshold ROP and/or an unfavorable visual or structural outcome in the absence of treatment.
In 1999, the National Eye Institute funded a cooperative agreement to study early treatment for ROP (ETROP).5,6 In the study, eyes of infants were randomized to early peripheral retinal ablation or standard treatment (conventional management) if they developed prethreshold ROP and if RM-ROP2, a risk analysis program based on natural history data from the CRYO-ROP study,7 indicated a high risk for an unfavorable outcome. Prethreshold ROP was defined as any ROP in zone I that was less than threshold; or in zone II stage 2 with plus disease (dilation and tortuosity of posterior pole retinal vessels in at least two quadrants, meeting or exceeding that of a standard photograph2); or zone II, stage 3 disease without plus disease; or zone II, stage 3 with plus disease but fewer than five contiguous or eight cumulative clock hours.2 This paper presents the final results of this study, including implications for the timing of retinal ablative treatment for ROP. Preliminary results were published previously.8
METHODS
Study protocols were approved by the review boards of all participating institutions, and parents provided written informed consent prior to infants’ enrollment into the study and again at randomization. Details of the study design and laser technique are described elsewhere.6
Infants with birth weights less than 1,251 g and birth dates between October 1, 2000, and September 30, 2002, were screened at 26 participating centers. If an infant developed ROP, parents were asked to consent to data collection and possibly increased frequency of examinations. Study-certified ophthalmologists conducted serial examinations to detect rate of progression of ROP, development of prethreshold ROP, and development of threshold ROP. If at least one eye reached prethreshold ROP, the infant’s demographic and ROP information was entered into the RM-ROP2 risk model to determine the likelihood of progression to an unfavorable outcome in the absence of treatment.7
The risk determination was made at the coordinating center, using the RM-ROP2 model7 to evaluate data provided by the clinical center. If the risk of progression to an unfavorable outcome in the absence of treatment was calculated to be ≥15%, consent for the randomized trial was obtained, and randomization occurred. These eyes that had a risk of ≥15% were termed “high-risk” prethreshold. Eyes with <15% risk were termed “low-risk” and were followed every 2 to 4 days for at least 2 weeks until the ROP regressed, or the risk progressed to ≥15%. If both eyes were eligible for randomization, one eye was assigned at random to earlier treatment with ablative therapy within 48 hours of the first diagnosis of high-risk prethreshold ROP. Treatment was generally laser therapy, but cryotherapy was allowed. The fellow eye served as the control and was managed conventionally, which meant that it was observed either until it reached threshold and was treated or until the ROP regressed without progressing to threshold. In cases where only one eye had reached high-risk prethreshold ROP, that eye was randomized to treatment within 48 hours or to serve as a conventionally managed control, receiving treatment only if the ROP progressed to threshold severity. Infants in whom either eye had developed threshold ROP prior to randomization were excluded from the study.
For the analyses, eyes with prethreshold ROP that remained at low risk were categorized by the lowest zone and highest stage of ROP that ever developed. Eyes in the randomized group were classified according to the zone and stage of ROP that were present at the time of randomization, as determined by the confirming examiner’s observations.
Functional Outcome
The functional outcome of each randomized eye at 9 months corrected age was evaluated by assessment of monocular grating acuity, conducted by one of two testers masked to the eye’s treatment assignment, who traveled to the study centers for testing.
The technique employed to evaluate grating acuity was the Teller acuity card procedure9,10 as used previously in the CRYO-ROP study.11,12 Acuity was scored as the spatial frequency of the finest grating to which the infant showed a consistent fixation response. Eyes in which visual acuity was too poor to be quantified in this way were categorized as having no light perception (NLP), light perception only (LP), or detection of the grating on the low vision (LV) card only. The LV card has 2.2-cm-wide black-and-white stripes covering one half of the card. It was not used to quantify vision, but only to determine whether the infant had pattern vision. The tester was permitted to move the LV card and/or to present it at any distance and at any location in the infant’s visual field.
Visual acuity data were included in analyses only if the following criteria were met: (1) an acuity result (measurable acuity, detection of the grating on the LV card, LP, or NLP) was obtained for each eye of bilateral high-risk prethreshold cases or the randomized eye of asymmetric cases; (2) treatment for amblyopia, if present, had been prescribed for at least 4 weeks prior to the acuity test; and (3) refractive error, if present in either eye of bilateral high-risk prethreshold cases or in the randomized eye in asymmetric cases, had been corrected for at least 2 weeks prior to the acuity test. The criteria for correction of refractive errors were myopia greater than −4.00 diopters (D), hyperopia greater than +5.00 D, and/or astigmatism greater than 2.50 D in one or both eyes. Correction of anisometropia greater than 1.50 D spherical equivalent or 1.50 D cylinder was required only if the examining physician found evidence of amblyopia.
The visual acuity outcome was divided into four categories of functional response: normal, defined as greater than or equal to 3.70 cycles per degree13,14; below normal, defined as 1.85 to less than 3.70 cycles per degree (from approximately 4 to ≥2 standard deviations below the mean grating acuity for a 9-month-old child14); poor, if less than 1.85 cycles per degree but measurable with one of the standard acuity cards (not the LV card); and blind/low vision (NLP, LP only, or LV only). These functional outcome categories of grating acuity results were further grouped into “favorable” and “unfavorable” designations. The favorable grouping included eyes in the normal and below normal categories. The unfavorable grouping included eyes in the poor and blind/low vision categories, which would be expected to have a poor long-term prognosis for visual function.15
Structural Outcome
Structural outcome was documented with a dilated fundus examination at 6 months and 9 months corrected age by study-certified examiners. Complete ophthalmologic examinations were performed at both of these ages; at the 9-month examination, a developmental questionnaire (DDST16; results not reported in this article) was conducted. Refractive errors were determined by cycloplegic retinoscopy after instilling 1% cyclopentolate hydrochloride. When there was a medical contraindication to this drop, either 0.5% cyclopentolate or 1% tropicamide was used. At 6 months, an unfavorable outcome was defined as (1) a posterior retinal fold involving the macula, (2) a retinal detachment involving the macula, or (3) retrolental tissue or “mass” obscuring the view of the posterior pole. If an infant required a vitrectomy or scleral buckle, the 6-month examination was conducted prior to the surgery. At the 9-month examination, eyes that had received a vitrectomy or scleral buckle were classified for study purposes as having an unfavorable structural outcome.
Statistical Analyses
The ETROP study was designed to detect a 35% reduction in the percentage of eyes having an unfavorable structural outcome with a type I error rate of 0.05 and a power of 80%.6 Using data from the CRYO-ROP study, the percentage of unfavorable eyes managed conventionally was predicted to be 20%.6 If earlier treatment produced a 35% reduction, 13% of the earlier treated eyes would have an unfavorable outcome. Taking into account that approximately 80% of infants were expected to have both eyes eligible for the study, the number of infants needed for the study was 370.6 The primary outcome for this study was visual function, for which there are limited data on which to conduct sample size calculations. Therefore, we based sample size on structural outcome. This was a conservative approach, since in the CRYO-ROP study, unfavorable functional outcome rates were approximately 50% higher than unfavorable structural outcome rates at ages at which functional outcome was tested.1,17,18
The statistical technique used to compare the eyes treated at high-risk prethreshold with the conventionally managed high-risk prethreshold eyes was developed and used in the CRYO-ROP study.19 It combines the data from infants with bilateral disease (both eyes eligible) and asymmetric disease (one eye eligible) into one overall chi-square analysis of outcome differences between the two treatment groups. Although not part of the original study design, functional and structural results are also presented by the International Classification of ROP (ICROP) and by RM-ROP2 categories to allow a more detailed examination of the data.
A Data and Safety Monitoring Committee of researchers, clinicians, and an ethicist not directly involved in the ETROP study met in person every 6 months to review adverse event and outcome data and to monitor study progress. The committee approved the protocol and monitored the performance of participating centers.
RESULTS
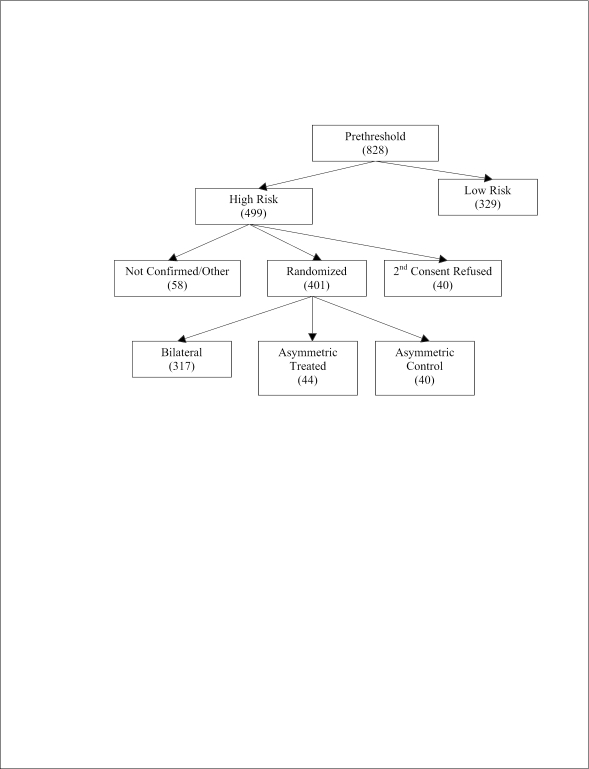
At the 26 clinical sites, 828 infants whose parents had given consent for systematic follow-up of ROP were identified as having prethreshold disease in one or both eyes. Among the 828 infants with prethreshold ROP, there were 499 (60%) whose eye or eyes were classified as high-risk and who were thereby eligible for the randomized trial (Figure 1). Among these 499 infants, consent for randomization was not obtained for 40 infants, and high-risk prethreshold ROP was not confirmed by the required second study-certified examiner or for other reasons in 58 infants. Thus, 401 infants were enrolled into the randomized trial. The remaining 329 infants, who had prethreshold ROP that was judged to be low-risk, were followed as clinically indicated and then underwent follow-up study examinations at 6 months corrected age to determine retinal outcomes.
Figure 1.
Flow chart indicating status of the 828 infants in the ETROP study who developed prethreshold retinopathy of prematurity in one or both eyes.
Table 1 shows the distribution of prethreshold eyes by RM-ROP2 risk classification7 and by severity of prethreshold ROP according to the ICROP20 characteristics. One eye per infant is represented in Table 1, and that is the eye with the higher risk, according to the RM-ROP2 model.7 Table 1 shows that the ICROP can serve as a good indicator of most of the high-risk prethreshold eyes. For prethreshold zone I eyes with plus disease, 100% were high-risk; when stage 3 was present without plus disease, 95.7% were high-risk; and with stage 1 or 2, without plus disease, 92.3% were high-risk. In zone II, 95.2% of eyes that were classified as stage 3 with plus disease and 83.3% of eyes with stage 2 with plus disease were high-risk, whereas only 2.6% of the 303 eyes that were zone II, stage 3, without plus disease were high-risk. The parallels between the RM-ROP2 model and ICROP are particularly striking even though the former takes into account a number of demographic and disease-related factors that are not part of ICROP.
Table 1.
Risk status by icrop category for the eye with higher risk of poor structural outcome for all 828 infants with prethreshold retinopathy of prematurity in one or both eyes*
| Risk in prethreshold eye |
||||||
|---|---|---|---|---|---|---|
| Icrop category |
Low risk | High risk | ||||
| Zone | Stage | Plus disease | At least one eye prethreshold no. of patients | No. of patients | No. | % |
| I | 3 | Yes | 29 | 0 | 29 | 100 |
| I | 3 | No | 23 | 1 | 22 | 95.7 |
| I | 1 or 2 | Yes | 19 | 0 | 19 | 100 |
| I | 1 or 2 | No | 117 | 9 | 108 | 92.3 |
| II | 3 | Yes | 271 | 13 | 258 | 95.2 |
| II | 3 | No | 303 | 295 | 8 | 2.6 |
| II | 2 | Yes | 66 | 11 | 55 | 83.3 |
| Total | 828 | 329 | 499 | 60.3 | ||
ICROP, International Classification of Retinopathy of Prematurity.20
Risk of poor structural outcome was based on the RM-ROP2 risk model analysis for each eye (a risk analysis program based on natural history data from the Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Study).7 Low risk was less than 0.15; high risk was 0.15 or greater. Plus disease was defined as a degree of dilation and tortuosity of the posterior retinal blood vessels meeting or exceeding that of a standard photograph.2
Table 2 provides baseline characteristics for the 401 infants who entered the randomized trial. The mean birth weight was 703 g and the mean gestational age was 25.3 weeks. At the time of randomization, 79.1% of the infants had RM-ROP2 high-risk prethreshold disease bilaterally. The remaining 20.9% of infants had asymmetric disease, with high-risk prethreshold ROP in only one eye; the fellow eye had less severe ROP.
Table 2.
Baseline characteristics of 401 randomized patients
| Patients with bilateral high-risk prethreshold ROP | 79.1 |
| Birth weight, mean ± SD, g | 703 ± 148 |
| Gestational age, mean ± SD, wk | 25.3 ± 1.4 |
| Male | 54.4 |
| Singleton births | 71.1 |
| Born in the study hospital | 80.3 |
| Race | |
| White | 63.8 |
| African American | 18.0 |
| Hispanic | 14.7 |
| Other | 3.5 |
ROP, retinopathy of prematurity.
Data are presented as percentage unless otherwise indicated.
Table 3 shows the distribution of eyes treated at high-risk prethreshold and conventionally managed (control) eyes by ICROP categories at randomization, along with the percentage of conventionally managed eyes that reached threshold ROP. Zone I disease accounted for approximately 40% of randomized eyes. The largest categories of high-risk prethreshold eyes were those with zone II, stage 3, with plus disease (42.1% of prethreshold treated eyes and 43.7% of conventionally managed eyes), and those with zone I, stage 1 or 2 with no plus disease (27.4% of prethreshold treated eyes and 26.1% and conventionally managed eyes). Table 3 also indicates that 66.4% of eyes in the conventionally managed group progressed to threshold and underwent peripheral retinal ablation at that time.
Table 3.
Distribution of severity of rop at randomization in eyes with high-risk prethreshold rop and percentage of conventionally managed eyes with high-risk prethreshold rop that reached threshold*
| Icrop category at randomization |
Eyes treated at high-risk prethreshold (n = 361) |
Conventionally managed eyes (n = 357) |
|||||
|---|---|---|---|---|---|---|---|
| Zone | Stage | Plus disease | No. | (%) | No. | (%) | Reaching threshold (%) |
| I | 3 | Yes | 19 | (5.3) | 19 | (5.3) | 94.7 |
| I | 3 | No | 17 | (4.7) | 16 | (4.5) | 68.8 |
| I | 1 or 2 | Yes | 14 | (3.9) | 16 | (4.5) | 87.5 |
| I | 1 or 2 | No | 99 | (27.4) | 93 | (26.1) | 49.5 |
| II | 3 | Yes | 152 | (42.1) | 156 | (43.7) | 71.8 |
| II | 3 | No | 5 | (1.4) | 4 | (1.1) | 25 |
| II | 2 | Yes | 55 | (15.2) | 53 | (14.8) | 66 |
| Total | 361 | (100) | 357 | (100) | 66.4 | ||
ICROP, International Classification of Retinopathy of Prematurity20; ROP, retinopathy of prematurity.
High risk was 0.15 or greater. Plus disease was defined as a degree of dilation and tortuosity of the posterior retinal blood vessels meeting or exceeding that of a standard photograph.
The average age at high-risk prethreshold treatment was 35.2 weeks postmenstrual age (SD, 2.3; range, 30.6 to 42.1 weeks) and 10.0 weeks chronological age (SD, 2.0). The average age for treatment of eyes in the conventionally managed group that went on to threshold was 37.0 weeks postmenstrual age (SD, 2.5; range, 31.9 to 46.6 weeks) and 11.9 weeks chronological age (SD, 2.2). Only one eye received cryotherapy at threshold ROP as the primary treatment for ROP, with the others receiving laser retinal ablation. Some eyes received supplementary cryotherapy at the time of the initial treatment. Retreatment was conducted in 13.9% of eyes treated at high-risk prethreshold ROP and in 11.0% of conventionally managed eyes treated at threshold ROP.
Primary Outcome
In this final report, grating visual acuity data have been obtained from 372 infants (98.2% of patients that survived). Data were not obtained from 22 infants who died prior to the 9-month examination and seven infants whose parents did not bring them for the examination. Average corrected age (age from expected date of delivery) at the time of grating acuity assessment was 10.3 months (SD, 1.8).
Table 4 presents the proportion of randomized eyes with unfavorable grating acuity outcomes at 9 months. Overall, there was a significant benefit of treatment of eyes at high-risk prethreshold ROP, with a reduction in unfavorable visual acuity outcome from 19.8% to 14.3% (P < .005). Within-subject comparison afforded a powerful opportunity to examine treatment effects while controlling for individual characteristics. Results from the 33 infants with bilateral disease in whom there were discordant outcomes in the two eyes provide even stronger evidence of a beneficial effect of treatment at high-risk prethreshold ROP (P < .005). Thirty-seven infants with bilateral high-risk prethreshold disease had an unfavorable outcome in both eyes.
Table 4.
Nine-month grating acuity outcome for randomized patients*
| Eyes treated | Eyes treated at high-risk prethreshold | Conventionally managed eyes | χ2 | p value |
|---|---|---|---|---|
| Bilateral | 292 (15.4) | 292 (21.2) | 8.76† | <.005 |
| Asymmetric | 44 (6.8) | 36 (8.3) | 0.07 | .80 |
| Total | 336 (14.3) | 328 (19.8) | 8.03 | <.005 |
Data are presented as number (percentage unfavorable) unless otherwise indicated.
Based on discordant pairs (25 infants with favorable outcomes in earlier treated eyes and unfavorable outcomes in conventionally managed eyes; eight infants with unfavorable outcomes in earlier treated eyes and favorable outcomes in conventionally managed eyes).
Table 5 provides a more detailed presentation of the grating acuity results. Although differences were not statistically significant in these smaller categories, there were more high-risk prethreshold treated eyes than conventionally managed eyes that had grating acuity in the normal range for age (P = .38). In addition, fewer eyes randomized to high-risk prethreshold treatment than conventionally managed eyes were designated as blind or low vision (P = .07).
Table 5.
Distribution of nine-month grating acuity outcomes among randomized eyes by treatment assignment*
| Outcome | Eyes treated at high-risk prethreshold (n = 336) | Conventionally managed eyes (n = 328) | ||
|---|---|---|---|---|
| Favorable | ||||
| Normal (≥3.70 cycles per degree) | 219 | (65.2) | 203 | (61.9) |
| Below normal (1.85 to <3.70 cycles per degree) | 69 | (20.5) | 60 | (18.3) |
| Unfavorable | ||||
| Poor (measurable but <1.85 cycles per degree) | 15 | (4.5) | 18 | (5.5) |
| Blind/LV (NLP, LP only, LV card only) | 33 | (9.8) | 47 | (14.3) |
| Total | 336 | (100) | 328 | (100) |
LP, light perception; LV, low vision; NLP, no light perception.
Data are presented as number (percentage).
Secondary Outcomes
Structural outcome data have been obtained from 366 infants (94.8% of patients that survived) at 6 months corrected age and 372 infants (98.2% of patients that survived) at 9 months corrected age. Six-month data were not obtained from 15 infants who died prior to the examination and 20 infants whose parents did not bring them in for the examination. Average corrected age at the 6-month examination was 5.5 months (SD, 2.2). At 9 months, structural outcome data were not obtained from 22 infants who died prior to the examination and seven infants whose parents did not bring them in for the examination. Average corrected age at the 9-month examination was 9.8 months (SD, 1.4). This is younger than the average age for acuity testing because the final acuity data were sometimes collected during retesting after the 9-month structural examination.
The results for the 9-month structural outcome are presented in Table 6. Data indicate a statistically significant benefit of treatment of eyes with high-risk prethreshold ROP, with unfavorable structural findings reduced from 15.6% in conventionally managed eyes to 9.0% in high-risk prethreshold treated eyes (P < .001). As is the case for grating acuity outcome, results from infants with bilateral disease in whom there were discordant outcomes in the two eyes provide strong evidence of a beneficial effect of treatment at high-risk prethreshold.
Table 6.
Nine-month structural outcome for randomized patients*
| Eyes treated | Eyes treated at high-risk prethreshold | Conventionally managed eyes | χ2 | P value |
|---|---|---|---|---|
| Bilateral | 290† (10.3) | 291† (17.2) | 11.7‡ | <.001 |
| Asymmetric | 42§ (0) | 36 (2.8) | 1.2 | .28 |
| Total | 332 (9.0) | 327 (15.6) | 12.6 | <.001¶ |
Data are presented as number (percentage unfavorable) unless otherwise indicated.
Less than 293 because of inability to determine the structural outcome.
Based on discordant pairs (25 infants with favorable outcomes in earlier treated eyes and unfavorable outcomes in conventionally managed eyes; 6 infants with unfavorable outcomes in earlier treated eyes and favorable outcomes in conventionally managed eyes).
Less than 43 because of inability to determine the structural outcome.
Twenty-four eyes with partial retinal detachment not including the macula (stage 4A) had vitrectomy or a scleral buckling procedure prior to the 9-month examination and are included in this Table as having an unfavorable outcome. When the analysis was based on the structural outcome of these examinations, P = .002. Stage 4B or 5 eyes were a priori considered unfavorable in this study.
Among the 30 high-risk prethreshold treated eyes that had an unfavorable structural outcome at 9 months, two had a partial retinal detachment involving the macula, 23 had undergone a vitrectomy or a scleral buckle, and five had total retinal detachment. Among the 51 conventionally managed eyes with an unfavorable outcome, four had a partial retinal detachment involving the macula, 43 had undergone a vitrectomy or a scleral buckle, and four had total retinal detachment.
Structural outcome results at the 6-month examination for eyes randomized at high-risk prethreshold ROP were similar to those at 9 months, as shown in Table 7. Six-month structural outcome data were also collected for low-risk prethreshold eyes (determined by the RM-ROP2 program to have a <15% risk for an unfavorable outcome). Among this group of 329 infants, 51 (15.5%) had at least one eye that progressed to the conventional threshold for treatment and was treated accordingly. An unfavorable outcome occurred in only 1.3% of the 302 low-risk prethreshold eyes for which 6-month structural outcome data were available.
Table 7.
Six-month structural outcome for randomized patients*
| Eyes treated | Eyes treated at high-risk prethreshold | Conventionally managed eyes | χ2 | P value |
|---|---|---|---|---|
| Bilateral | 286† (5.6) | 283† (11.0) | 8.3‡ | .004 |
| Asymmetric | 39§ (0) | 36 (2.8) | 1.1 | .29 |
| Total | 325 (4.9) | 319 (10) | 9.2 | .002 |
Data are presented as number (percentage unfavorable) unless otherwise indicated.
Less than 290 because of inability to determine the structural outcome.
Based on discordant pairs (21 infants with favorable outcomes in earlier treated eyes and unfavorable outcomes in conventionally managed eyes; 6 infants with unfavorable outcomes in earlier treated eyes and favorable outcomes in conventionally managed eyes).
Less than 40 because of inability to determine the structural outcome.
Relationship to ICROP Classification
Tables 8 and 9 present the visual acuity and structural outcomes for randomized eyes, stratified by ICROP category and by RM-ROP2 risk category. The greatest benefit of treatment at high-risk prethreshold ROP versus conventional management occurred in eyes that had zone I, stage 3 ROP, with and without plus (30.8% unfavorable versus 53.8% unfavorable). A relative benefit from intervention at high-risk prethreshold ROP for both visual acuity and structural outcomes was also seen among eyes that had zone I, stage 1 or 2 ROP without plus disease, and among eyes that had zone II, stage 3 ROP with plus disease.
Table 8.
Grating acuity at nine months for infants with bilateral high-risk prethreshold retinopathy of prematurity by icrop category and rm-rop2 risk*
| Icrop classification |
Discordant pairs |
|||||
|---|---|---|---|---|---|---|
| Zone | Stage | Plus disease | Eyes treated at high-risk prethreshold | Conventionally managed eyes | A† | B‡ |
| I | 3 | Yes or No | 26 (30.8) | 26 (53.8) | 7 | 1 |
| I | 1 or 2 | Yes | 9 (22.2) | 9 (22.2) | 0 | 0 |
| I | 1 or 2 | No | 76 (10.5) | 76 (15.8) | 4 | 0 |
| II | 3 | Yes | 111 (15.3) | 111 (18.0) | 9 | 6 |
| II | 3 | No | 3 (0) | 3 (0) | 0 | 0 |
| II | 2 | Yes | 34 (14.7) | 34 (17.6) | 2 | 1 |
| RM-ROP2 risk | ||||||
| 0.15 to <0.30 | 110 (11.8) | 110 (12.7) | 4 | 3 | ||
| 0.30 to <0.45 | 75 (13.3) | 75 (20.0) | 7 | 2 | ||
| ≥0.45 | 81 (22.2) | 81 (34.6) | 13 | 3 | ||
ICROP, International Classification of Retinopathy of Prematurity20; RM-ROP2,7 a risk analysis program based on natural history data from the Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Study.
Data are presented as number (percentage unfavorable) unless otherwise indicated. High risk was 0.15 or greater. Plus disease was defined as a degree of dilation and tortuosity of the posterior retinal blood vessels meeting or exceeding that of a standard photograph.2
For group A, earlier treated eyes had a favorable outcome, and conventionally managed eyes had an unfavorable outcome.
For group B, earlier treated eyes had an unfavorable outcome, and conventionally managed eyes had a favorable outcome.
Table 9.
Structural outcome at nine months for infants with bilateral high-risk prethreshold retinopathy of prematurity by icrop category and rm-rop2 risk*
| Icrop classification |
Discordant pairs |
|||||
|---|---|---|---|---|---|---|
| Zone | Stage | Plus disease | Eyes treated at high-risk prethreshold | Conventionally managed eyes | A† | B‡ |
| I | 3 | Yes or No | 27 (29.6) | 27 (55.6) | 8 | 1 |
| I | 1 or 2 | Yes | 9 (22.2) | 9 (22.2) | 0 | 0 |
| I | 1 or 2 | No | 75 (2.7) | 75 (9.3) | 6 | 1 |
| II | 3 | Yes | 109 (7.3) | 110 (10.9) | 6 | 3 |
| II | 3 | No | 3 (0) | 3 (0) | 0 | 0 |
| II | 2 | Yes | 34 (20.6) | 34 (20.6) | 1 | 1 |
| RM-ROP2 risk | ||||||
| 0.15 to <0.30 | 109 (5.5) | 109 (7.3) | 4 | 2 | ||
| 0.30 to <0.45 | 74 (9.5) | 74 (17.6) | 7 | 1 | ||
| 0.45 | 81 (18.5) | 82(30.5) | 12 | 3 | ||
ICROP, International Classification of Retinopathy of Prematurity20; RM-ROP2,7 a risk analysis program based on natural history data from the Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Study.
Data are presented as number (percentage unfavorable) unless otherwise indicated. High risk was 0.15 or greater. Plus disease was defined as a degree of dilation and tortuosity of the posterior retinal blood vessels meeting or exceeding that of a standard photograph. Data include only those eyes for which structural outcome was able to be graded.
For group A, earlier treated eyes had a favorable outcome, and conventionally managed eyes had an unfavorable outcome.
For group B, earlier treated eyes had an unfavorable outcome, and conventionally managed eyes had a favorable outcome.
As shown at the bottom of Tables 8 and 9, examination of outcome by RM-ROP2 risk category showed greater benefit in both grating acuity and structural outcomes for earlier treatment in high-risk prethreshold eyes with ≥30% risk than in high-risk prethreshold eyes with 15% to <30% risk.
Other Ocular and Clinical Findings
The distribution of refractive errors at the 9-month examination was similar between the high-risk prethreshold treated eyes that received early treatment and those that were conventionally managed.
Cataract/aphakia that was not associated with total retinal detachment or vitrectomy was found in four eyes (1.2%) in the group treated at high-risk prethreshold and in four eyes (1.2%) in the conventionally managed group. Nystagmus occurred in 22% of randomized infants with bilateral high-risk ROP.
Table 10 compares other ocular and systemic complications of treatment among eyes and infants treated at high-risk prethreshold versus conventionally managed high-risk prethreshold eyes that progressed and later underwent treatment at threshold ROP. Ocular complication rates were similar in the two groups. Systemic complications were higher following treatment at high-risk prethreshold. High-risk prethreshold eyes randomized to early treatment received peripheral retinal ablation at an average postmenstrual age of 35.2 weeks, compared with an average postmenstrual age at treatment of 37.0 weeks in conventionally managed eyes that underwent peripheral retinal ablation at threshold ROP.
Table 10.
Complications in eyes treated for rop early or at threshold*
| Complication | Eyes treated at high-risk prethreshold† (n = 361) | Eyes treated conventionally at threshold† (n = 236) |
|---|---|---|
| Intraoperative ocular complications | ||
| Conjunctival or subjunctival hematoma | 30 (8.3) | 16 (6.8) |
| Conjunctival laceration, unintended | 16 (4.4) | 5 (2.1) |
| Hemorrhage (retinal, preretinal, vitreous) | 14 (3.9) | 12 (5.1) |
| Closure of the central retinal artery | 0 (0.0) | 2 (0.8) |
| Inadvertent burn or freeze to area outside of target zone | 2 (0.6) | 1 (0.4) |
| Other | 2 (0.6) | 0 (0) |
| Systemic complications | ||
| Apnea, bradycardia, or arrhythmia | 31 (8.6) | 10 (4.2) |
| Acquired or increased cyanosis | 13 (3.6) | 4 (1.7) |
| Need for reintubation within 10 days of treatment after stopping artificial ventilator | 40 (11.1) | 12 (5.1) |
| Other | 1 (0.3) | 1 (0.4) |
Data are presented as number (percentage).
Mean ± SD postmenstrual age at treatment in the early treatment group was 35.2 ± 2.3 weeks and in the threshold treatment group was 37.0 ± 2.5 weeks.
DISCUSSION
This study of treatment for high-risk prethreshold ROP showed a benefit of earlier treatment compared to conventional management in the primary outcome measure of grating visual acuity at 9 months corrected age, and a much greater benefit for structural outcome at 6 and 9 months corrected age. Whereas the rates of ophthalmologic complications were similar among the two treatment arms, infants in the high-risk prethreshold treatment group were more likely to experience systemic complications of apnea, bradycardia, or reintubation following earlier treatment than with treatment at conventional threshold, perhaps due to the earlier average postmenstrual age at which treatment was conducted. There was no mortality or known permanent morbidity attributed to treatment in either group.
This report includes all the 9-month visual acuity and structural outcome examinations (98.2% of infants followed to age 9 months corrected). The beneficial effect of treatment at high-risk prethreshold ROP on structural outcome and on visual acuity outcome is even stronger than originally reported and provides further support for treatment of certain prethreshold eyes (type 1, see below) and careful observation of other prethreshold eyes (type 2).8
In the ETROP study, a novel risk model that was developed based on natural history data from the CRYO-ROP study was used to identify infants at high risk for adverse outcomes from ROP, and only those infants were randomized.5–7 The model is available at http://www.sph.uth.tmc.edu/rmrop/riskcalc/disclaimer.aspx. The model used demographic characteristics of the infant and clinical features of the ROP to classify eyes with prethreshold ROP as high-risk or low-risk. The validity of the model is demonstrated in the finding that high-risk prethreshold eyes that received conventional management showed a much higher percentage of progression to threshold disease than those at low risk (66.4% versus 15.5%, respectively) and a much higher percentage of unfavorable structural outcome (10.0% versus 1.3%, respectively, at 6 months). Eyes with low-risk prethreshold ROP were managed conventionally, with treatment administered if conventional threshold was reached. Overall, study data support treatment of only selected eyes that develop prethreshold ROP.
In the CRYO-ROP study, only about 9.6% of eyes in the natural history cohort with prethreshold ROP had zone I disease, and of these eyes, 33.3% had an unfavorable structural outcome.21 This produced strong weighting on the risk factor, presence of disease in zone I, in the RM-ROP2 model. In the ETROP study, 22.7% of eyes with prethreshold ROP had zone I disease, and because of the strong weighting of zone I disease in the risk model, 94.7% of these eyes were classified as high-risk. These eyes represented about 40% of eyes in the ETROP randomized trial.
The difference between the CRYO-ROP study and the ETROP study in frequency of zone I disease and in the more benign course of zone I disease in the ETROP study is noteworthy. It is tempting to attribute the large number of zone I cases to advances in neonatal care and improved survival rates of the smallest premature infants. However, a thorough analysis (not presented here) of the data from the two studies (CRYO-ROP and ETROP) showed that even when the effects of birth weight and gestational age are controlled, the number of zone I eyes in the ETROP cohort is still significantly higher than in the CRYO-ROP study. Perhaps other changes in the care of premature infants, as yet unrecognized, have given rise to an increase in zone I disease and a decrease in its severity.
An alternative explanation is that examiners may now be more attentive to diagnosing zone I ROP than they were previously. The CRYO-ROP study showed a clear benefit of retinal ablative therapy, but the results in zone I eyes were not impressive, since most of these eyes developed unfavorable visual and structural outcomes even after receiving treatment at threshold. After publication of the CRYO-ROP results, it is possible that eyes were more carefully monitored and observed by ophthalmologists, and that some eyes diagnosed as zone I today might have been categorized as zone II in the era before treatment was proven effective. Additionally, some prior investigators have considered posterior zone II and zone I eyes to be in the same category.4 An assignment of posterior zone II eyes to the zone I category could have the effect of increasing the number of zone I eyes in this study. These subtle factors could explain both the increased frequency and the improved outcome of zone I eyes in ETROP subjects compared to CRYO-ROP subjects.
For all groups of eyes in the ETROP study, the effect of treatment at high-risk prethreshold is more pronounced for structural outcomes than for visual acuity outcomes. A similar discrepancy between the magnitude of the difference between treatment groups for visual acuity versus structural outcomes was also observed in the CRYO-ROP study.1,17,18 The ETROP study chose visual acuity as its primary outcome because vision is the most important measure of a treatment designed to prevent visual loss from severe ROP. Also, there was a safety concern that treatment at high-risk prethreshold with laser could have some previously unrecognized deleterious effect on visual acuity. The Teller acuity card procedure was selected as the assessment tool for measurement of visual acuity at the 9-month examination because it allows quantification of visual acuity in infants and because it had been used successfully to test infants of a similar age in the CRYO-ROP study.11,17
As in the CRYO-ROP study,1,17,18 the finding of a discrepancy between the magnitude of the treatment group differences in visual acuity versus structural outcomes in the ETROP study is likely to be due, in part, to non-ROP-related ophthalmologic and neurologic problems that can occur in very premature infants. Children with severe ROP may develop visual impairment secondary to neural insult or other cerebral factors.22,23 In addition, nystagmus, which reduces visual acuity, was found in over 20% of randomized infants with bilateral disease. These non-ROP-related factors may have resulted in reduced acuity in conventionally managed eyes, as well as in eyes treated at high-risk prethreshold ROP, thereby decreasing the difference in visual acuity outcome between the two groups of eyes.
Another likely contributor to the difference between functional and structural outcomes in this study is the immaturity of the visual system at 9 months postterm. Because the visual acuity of a 9-month-old infant is well below that of a normal adult, it is possible that some visual deficits that result from structural abnormalities will not be apparent until older ages, when acuity in normal eyes has improved to near-adult levels. Follow-up testing using recognition (letter) visual acuity charts at older ages would be expected to reveal these deficits in visual acuity, as it did in the CRYO-ROP study.1,18
In evaluating the benefit of treatment at high-risk prethreshold, it is important to take into account possible adverse effects and trade-offs related to earlier treatment. These include an increased rate of systemic complications, potential long-term risks of earlier treatment, an increase in the number of eye examinations needed to detect prethreshold ROP, and an increased frequency of treatment of eyes that would otherwise have undergone spontaneous regression of ROP. In the following paragraphs, we discuss these issues.
In the ETROP study, systemic complications, including apnea, bradycardia, and reintubation, occurred more frequently when peripheral retinal ablative therapy was performed at high-risk prethreshold than at conventional threshold, probably because of the younger average postmenstrual age at which the treatment of high-risk prethreshold eyes occurred. Ophthalmic complications following retinal ablative therapy were comparable in eyes treated at high-risk prethreshold and conventionally managed eyes, as were ophthalmic complications (other than retinal detachment) when the entire group of conventionally managed eyes was compared to the group of eyes treated at high-risk prethreshold. One potential deleterious effect of earlier treatment that was not evaluated in this study is the effect of peripheral retinal ablation on peripheral vision. It is possible that ablation in zone I will result in a greater loss in visual field extent than peripheral ablation in zone II.
Another issue related to earlier treatment of ROP concerns the treatment of eyes that would have undergone spontaneous involution without treatment. The question arises: “How many eyes must receive treatment unnecessarily in order to achieve the benefit of earlier treatment for those eyes that need it?” Based on the structural outcome data at 6 months for a cohort of eyes with prethreshold ROP of all degrees of severity that were conventionally managed, it is possible to determine the number of eyes with high-risk prethreshold ROP that had a favorable outcome without peripheral retinal ablation, as illustrated in Appendix 1. Table A in Appendix 1 summarizes the results of an analysis of data from the natural history cohort of prethreshold eyes in the ETROP study. The table shows that 136 (36.6%) of the 372 high-risk prethreshold eyes in the conventionally managed group that were examined at 6 months had favorable structural outcomes and never developed threshold ROP. That is, these eyes met the criteria for early treatment, yet, without treatment, went on to a favorable outcome at 6 months.
Appendix Table A.
Structural outcomes of low-risk prethreshold eyes and conventionally managed high-risk prethreshold eyes at six months post-term
| Low-risk (<0.15) prethreshold (n = 292) |
High-risk (≥0.15) prethreshold (n = 372) |
|||
|---|---|---|---|---|
| Six-month outcome | Never developed threshold rop | Developed threshold rop | Never developed threshold rop | Developed threshold rop |
| Favorable | 245 | 44 | 136 | 205 |
| Unfavorable | 1 | 2 | 4 | 27 |
ROP, retinopathy of prematurity.
To reduce the number of eyes treated that would have had a favorable outcome without intervention, additional strategies for selecting eyes for earlier treatment were explored using this same cohort of prethreshold eyes. Table B in Appendix 1 indicates that eyes with zone I, stage 1 or 2 without plus disease, as well as eyes with zone II, stage 3 without plus disease, had lower rates of progressing to threshold or unfavorable outcome than eyes in the other ICROP categories. And, when treated at the conventional threshold, those two groups of eyes had less than 5% unfavorable structure outcomes.
Appendix Table B.
Prethreshold eyes that reached high-risk prethreshold rop and prethreshold eyes that reached threshold or unfavorable structural outcome at six months post-term
| High-risk (≥0.15) |
Threshold or uf |
||||||
|---|---|---|---|---|---|---|---|
| Zone | Icrop stage | Plus | N | n | (%) | n | (%) |
| I | 3 | Yes | 19 | 19 | (100.0) | 19 | (100.0) |
| I | 3 | No | 14 | 14 | (100.0) | 9 | (64.3) |
| I | 1 or 2 | Yes | 18 | 18 | (100.0) | 15 | (83.3) |
| I | 1 or 2 | No | 101 | 93 | (92.1) | 41 | (40.6) |
| II | 3 | Yes | 172 | 167 | (97.1) | 118 | (68.6) |
| II | 3 | No | 274 | 5 | (1.8) | 44 | (16.1) |
| II | 2 | Yes | 66 | 56 | (84.8) | 37 | (56.1) |
| Total | 664 | 372 | (56.0) | 283 | (42.6) | ||
| Type I ROP | 289 | 274 | (94.8) | 198 | (68.5) | ||
| Type II ROP (shaded above) | 375 | 98 | (26.1) | 85 | (22.7) | ||
ICROP, International Classification of Retinopathy of Prematurity; ROP, retinopathy of prematurity; UF, unfavorable.
The results of this analysis, along with the results in Tables 8 and 9, led to a clinical algorithm in which treatment should be considered for eyes with zone I, any stage ROP with plus disease; eyes with zone I, stage 3 ROP without plus disease; and eyes with zone II, stage 2 or 3 with plus disease. As shown in Table C of Appendix 1, use of this ICROP-based, limited selection algorithm would have resulted in treatment of 91 eyes that showed favorable outcomes and never reached threshold disease. This is a reduction of 33% from the 136 such eyes that would have been treated using the RM-ROP2 risk model as applied in the ETROP study (Table A in Appendix 1).
Appendix Table C.
Effect of using icrop-based criteria (type i and type ii rop) to select infants for peripheral ablative treatment
| Type II prethreshold (N = 375) |
Type I prethreshold (N = 289) |
|||
|---|---|---|---|---|
| Six-month outcome | Never developed threshold rop | Developed threshold rop | Never developed threshold rop | Developed threshold rop |
| Favorable | 290 | 77 | 91 | 172 |
| Unfavorable | 1 | 7 | 4 | 22 |
ICROP, International Classification of Retinopathy of Prematurity; ROP, retinopathy of prematurity.
If it is assumed that conventional threshold ROP continues to occur in 6% of infants weighing less than 1,251 g at birth, as in the CRYO-ROP study,24 the early treatment algorithm based on RM-ROP2 would result in treatment of 9% of infants. The ICROP-based, limited selection criteria described in the preceding paragraph would result in treatment of 8% of infants while retaining the advantage of early treatment for eyes at highest risk of adverse outcomes.
An alternative approach to using the ICROP-based, limited selection algorithm is to base treatment on the RM-ROP2 risk model using a risk of ≥30%, instead of ≥15% as the criterion for early treatment. Since the absolute risk of an unfavorable outcome is low in the risk range from 0.15 to <0.30 (Tables 8 and 9) and therefore the relative benefit is not as great as in the higher-risk categories, use of the higher-risk criterion for treatment would reduce treatment of eyes that would not progress to threshold, while maintaining the benefit of earlier treatment in higher-risk eyes. However, using such a model may be more difficult in a clinical setting than using a revised treatment algorithm based on the eye findings (ICROP) alone.
In the ETROP protocol, timely identification of high-risk prethreshold ROP was important to the successful application of an early treatment program; hence, infants were followed on a weekly basis after developing zone II, stage 2 ROP or if they had retinal vessel immaturity with vessels ending in zone I, but no ROP in zone I. Infants with low-risk prethreshold disease were followed twice weekly and managed conventionally unless a change in status caused by development of more severe ROP resulted in advancement into the high-risk category. Thus, a screening program aimed at identifying eyes for treatment prior to conventional threshold may require an increase in the number of screening examinations conducted in the neonatal nursery.
The long-term effects of earlier treatment for ROP are as yet unknown. Because there is considerable development of visual acuity that occurs between infancy and childhood, and because it is possible to measure aspects of visual function in childhood that are not assessed easily in infancy, the National Eye Institute has funded continued follow-up of randomized children in the ETROP study to the age of 6 years. At that age, recognition visual acuity will be measured with Early Treatment Diabetic Retinopathy Study charts.25 Visual field extent, contrast sensitivity, and ocular status will also be evaluated, and each child’s developmental status will be assessed. This longer follow-up will give a more detailed evaluation of the impact of earlier treatment on the visual, ophthalmologic, and general developmental status of study participants.
Clinical Implications
The results of this study show that it is possible to identify characteristics of ROP that predict which eyes are most likely to benefit from early peripheral retinal ablation. Based on study data, a clinical algorithm was developed to identify for early treatment eyes with prethreshold ROP that are at highest risk for retinal detachment and blindness, while minimizing treatment of prethreshold eyes likely to show spontaneous regression of ROP. The use of this algorithm circumvents the need for computer-based calculation of low risk or high risk, as was used in this study.
The clinical algorithm shows that, in most circumstances, peripheral retinal ablation should be considered for any eye with:
Type I ROP
Zone I, any stage ROP with plus disease or
Zone I, stage 3, with or without plus disease or
Zone II, stage 2 or 3 ROP, with plus disease
Plus disease, in this instance, requires at least two quadrants (usually six or more clock hours) of dilation and tortuosity of the posterior retinal blood vessels and, hence, the presence of significant disease. The algorithm does not take into account all of the other known risk factors (eg, extent of stage 3, birth weight), and therefore some clinical judgment is required in applying this initial step to the management of ROP.
The clinical algorithm also indicates that continued serial examinations, as opposed to peripheral retinal ablation, should be considered for any eye with:
Type II ROP
Zone I, stage 1 or 2 with no plus disease or
Zone II, stage 3 with no plus disease
Treatment should be considered for an eye with type II ROP when progression to type I status or threshold ROP occurs.
It is important to note that even with the addition of early treatment of selected eyes with prethreshold ROP, some eyes will still progress to an unfavorable visual and/or structural outcome. Thus, additional research is needed to identify better methods for the prevention and treatment of severe ROP.
DISCUSSION
Dr John T. Flynn
It is a privilege to discuss Dr Bill Good’s paper detailing the outcome of the Early Treatment for ROP (ETROP) Randomized Clinical Trial, the latest in a series of successful trials to ask and answer important questions about the disease ROP. Surely this must place such trials, CRYO-ROP, LIGHT ROP, STOP ROP, and ETROP among the most successful intervention trials supported by taxpayer monies through the medium of the National Eye Institute.
When discussing such a massive trial in a few short minutes it seems to me best to try to focus on a few questions that get to the heart of the effort involving 26 centers, 240 investigators and 823 infants studied at a cost to the taxpayer of 13 millions of dollars.
Is the disease studied important? The answer is yes. ROP once thought banished as an ophthalmic and pediatric curiosity is back with a vengeance. It is among the three top causes of blindness in infancy and is almost world-wide (first and second world) in its distribution. It is very much a paradox of medical progress: As modern neonatology has advanced in its sophistication, the result has been the salvage of many infants well under a kilogram in birth weight and 30 weeks gestational age. This has provided an almost inexhaustible pool of infants vulnerable to the most severe forms of ROP in numbers never seen before.
Is the question asked an important one? Once again the answer is yes, emphatically yes. Should the threshold for treatment be lowered to include infants previously classed as not yet at threshold but at pre- threshold? Should they be treated at this new level of severity of disease? The downside risk here would be subjecting some (an unknown number) of these infants to a treatment that might not indeed be necessary.
Is the study design adequate to provide an unequivocal answer? The Randomized Clinical Trial is the gold standard in assessing the benefit of intervention or treatment outcomes in medicine today. This is a lesson learned early and well by the cadre of investigators who have become addicted to the disease (ROP) and its treatment over the course of the last two decades.
Are the results significant? Both clinically and statistically they are. Most importantly what gives them that significance clinically? This study includes in its sample 188 eyes (23%) with Zone 1 disease in contrast to CRYO-ROP that had 33 eyes (12%) eyes with Zone 1 disease. The results of treatment of Zone 1 eyes in the CRYO study were dismal; 87% had an unfavorable response to treatment, little better than no therapy at all, where 93% were unfavorable. In the current trial, Zone 1 high risk eyes had seven more discordant pairs where the early treatment produces a favorable response in that eye compared to an unfavorable outcome in its fellow eye treated at threshold versus only one pair where the threshold treated eye had a favorable response compared to the unfavorable response in its fellow early treated eye. An added benefit is the clinical algorithm for Zone 1 disease that has arisen from the data with regard to treatment. This algorithm is one that will be useful to the hundreds of clinicians throughout the world who are and will continue to be faced with making very tough decisions about whether and when to treat these infants.
Are there any downsides we should be aware of in this otherwise well done study? Lest we come away thinking this study has no downside, it does. It is something Bill Good, as PI, and all of the investigators are well aware of. Treating these pre-threshold infants on an average of 2 weeks earlier than those treated at threshold carries levels of risk of side effects, both ocular and systemic, not encountered with infants treated at threshold. The results (of any treatment trial) can be summarized in this regard from two standpoints: the number needed to treat to see the benefit of the new treatment. It is simply the reciprocal of the absolute difference in benefit between the two treatments. In this case it is the difference between 19.8% and 14.3% or 5%. So one must treat 20 infants before a difference in outcome is seen in one infant. The same logic holds for side effects or harm as well. And here the two weeks earlier treatment plays a critical role—just 2 weeks in age between the two groups it makes a tremendous difference. How many infants must be treated until one observes a difference in the systemic side effects of therapy? The answer is, if one treats two infants, one will see a potentially serious systemic side effect in one. The systemic complication will occur twice as often in the early treated infants. This should give us a measure of circumspection in our decision-making regarding applying the results of this study in the clinic.
Dr Good and his co-investigators have brought home another in a series of successful treatment trials in the prevention of this potentially devastating disease. The effect though small is significant. As important, if not more so, they have been able to derive rules for dealing with Zone 1 disease that may prove to be the most important outcome of the study.
Dr Allan J. Flach
Could you put the quality of life of these tiny infants into a clearer perspective? How do these kids do in later life?
Dr Gerhard W. Cibis
I have a study interest in children that were treated with lasers and then developed maculopathies in situations that the laser treatment did not directly involve the posterior pole. I am concerned with reflective phototoxicity of the laser treatment itself. As we narrow the criteria to where we have to treat more and more children in order to achieve a 5 percent improvement, I have a concern that when we’re only comparing the laser-treated against the laser-untreated, we may be creating some phototoxicity maculopathies with long-range vision problems that are lost in the study because that’s not looking at that.
Dr Edward L. Raab
What you do in the study for following Zone I immature vessels with no ROP at one-week intervals? That might have been something to enhance recruitment, or it might actually have made a difference whether you followed those individuals at one week or at two weeks, particularly if you’re examining them closer to 28 days or closer to 42 days. Did you conclude that it is necessary to follow nearly incomplete vascularization in Zone II at intervals of one week?
Dr George R. Beauchamp
This might be an ideal disease to apply the tools of value-based medicine to get some context of how much this intervention is going to improve the lives of these children. This disease is perhaps the highest-value intervention in all of medicine, let alone ophthalmology. I suggest that some of us consider with you extending the evidence that you have to the value-based tools to get these answers.
Dr William V. Good
No doubt it makes an enormous difference to a child, regardless of neurocognitive or neurologic outcome, to have sight. In the CRYO-ROP Study, where many infants who had favorable retinal outcomes still have visual acuity less than 20/40, there’s no argument that it is far better to have, say, 20/100 visual acuity than to be blind.
If one looks at the overall outcome for premature infants in the birth weight category <1000 grams, about 40 percent of these children have neurocognitive problems, and about 20 percent have cerebral palsy. The mortality rates have gone down in this birthweight group, but the morbidity rates have stayed the same or increased. ROP rates appear to be approximately the same if you just look at the data that we present in this paper.
Thank you to Dr Beauchamp. I would be interested in pursuing value-based medicine further with him and others.
Any treatment intervention carries a side-effect profile that needs evaluation. We can debate the number needed to treat in the ETROP Study. The control infants in this Study were often treated, as part of their conventional management. About 66 percent of control infants went to threshold and had to have the control eye treated with laser or cryotherapy. Therefore, we’re not comparing treatment to no treatment; we’re comparing earlier treatment to conventional management and treatment. So, this number needed to treat doesn’t hold. To avoid over-treatment, we adopted the Type I/Type II algorithm, to reduce the rate of unnecessary earlier treatment. Guidelines from the ETROP Study indicate that certain eyes can avoid earlier treatment, and be observed for signs of progression of disease.
We are concerned about reflective phototoxicity and other potential side effects of treatment, particularly for Zone I eyes. From personal experience looking at some of the Zone I eyes that had treatment with resulting favorable structural and favorable visual acuity outcome, there is a lot of ablated retina. We will have the opportunity to follow these children at least to the age of 6 to learn more about what the long-term potential effects are from such extensive ablation.
Concerning the question about Zone I immature vessels and the frequency with which these children were followed, we did follow these children on a weekly basis. This was a study design issue. It is not necessarily a recommendation for clinical practice. We wanted to identify pre-threshold disease at the earliest possible time point. We are evaluating our data now to determine if we can come up with evidence-based screening guidelines. We hope to determine whether the eyes that were followed more frequently were at risk for getting pre-threshold and at what time sequence they were at risk so that we might be able to offer additional guidelines useful to clinicians.
APPENDIX 1
The results presented in this Appendix are based on data from 664 infants who had one eye identified at prethreshold ROP that was not treated unless the ROP progressed to threshold. This natural history cohort was examined to consider alternative treatment strategies for managing prethreshold eyes. Included in this natural history cohort were all control eyes in the asymmetric randomized group, the conventionally managed eyes of infants with bilateral high-risk prethreshold disease, and one eye selected at random from the infants with low-risk prethreshold disease. Table A categorizes these eyes by RM-ROP2 high-risk and low-risk classification, and by whether or not they progressed to threshold ROP for treatment and/or had an unfavorable outcome.
Table B shows this cohort of eyes classified by ICROP categorization and indicates that nearly 100% of zone I eyes were classified as high-risk. However, among eyes with zone I, stage 1 or 2 ROP with no plus disease, a much lower percentage progressed to threshold and/or an unfavorable outcome than occurred in all other categories of zone I disease. Among zone II eyes, those with zone II, stage 3 with no plus disease did much better than eyes in other categories. These data led to a proposed grouping of the eyes by the ICROP classification into type I and type II ROP. The outcome results achieved by dividing the cohort into type I and type II ROP based on the ICROP classification are shown in Table C.
APPENDIX 2 ETROP STUDY INVESTIGATORS
Writing Committee: Chair: William V. Good, MD; Robert J. Hardy, PhD; Velma Dobson, PhD; Earl A. Palmer, MD; Dale L. Phelps, MD; Michelle Quintos, BA; Betty Tung, MS.
Stanford Center (Palo Alto, California)
Lucille Packard Children’s Hospital, Stanford University. Co-principal Investigators: Ashima Madan, MD; Michael Gaynon, MD. Study Center Coordinators: M. Bethany Ball, BS; Patricia N. Hartsell, RN, BA; Dottie Inguillo, RN. Coinvestigators: Deborah Alcorn, MD; William V. Good, MD; Donna Ornitz, MD; David Stevenson, MD.
San Francisco Center (San Francisco, California)
California Pacific Medical Center, Oakland Children’s Hospital, University of California, San Francisco, Medical Center. Principal Investigator: William V. Good, MD. Study Center Coordinators: Monica Hubbard, MS, PNP; Jason Lee, MD. Coinvestigators: Daniel Brinton, MD; Susan Day, MD; David Durand, MD; Douglas Fredrick, MD; Roderic H. Phibbs, MD; Daniel Schwartz, MD; Terri Slagle, MD; Gordon Smith, MD.
Chicago Center (Chicago, Illinois)
University of Illinois at Chicago Hospital and Medical Center. Principal Investigator: Michael Shapiro, MD. Study Center Coordinators: Yesenia Garcia; Maria Genio; Jeffrey Parker; Bernadine Rupar. Coinvestigators: Herbert Becker, MD; Rama Bhat, MD; Jeffrey N. Bloom, MD; Jessica V. Corsino, MD; Lawrence Kaufman, MD; Wico Waikwan Lai, MD; Jose Pulido, MD, MS; Tonse N.K. Raju, MD; Arvid K. Shukla, MD; Benjamin Ticho, MD; Dharmapuri Vidyasagar, MD.
Indianapolis Center (Indianapolis, Indiana)
(Indiana University School of Medicine) James Whitcomb Riley Hospital for Children, Indiana University Hospital, Wishard Memorial Hospital, Methodist Hospital, Community Hospitals of Indianapolis. Principal Investigator: James Lemons, MD. Co-principal Investigator: Daniel Neely, MD. Study Center Coordinators: Dee Dee Appel, RN; Elizabeth A. Hynes, RN; Leslie Wright, RN. Coinvestigators: David Plager, MD; Naval Sondhi, MD; Derek Sprunger, MD.
Louisville Center (Louisville, Kentucky)
Kosair Children’s Hospital, University of Louisville Hospital. Principal Investigator: Charles C. Barr, MD. Study Center Coordinator: Greg K. Whittington, PsyS. Coinvestigators: Marianne Cowley, MD; Craig H. Douglas, MD; Peggy H. Fishman, MD; Tonya Robinson, MD; Paul J. Rychwalski, MD.
New Orleans Center (New Orleans, Louisiana)
Tulane University Medical Center, Medical Center of Louisiana at New Orleans. Principal Investigator: Robert A. Gordon, MD. Study Center Coordinator: Deborah S. Neff, LPN. Coinvestigators: Douglas B. Babel, OD, MD; James G. Diamond, MD; William L. Gill, MD.
Baltimore G Center (Baltimore, Maryland)
University of Maryland Medical Systems, Mercy Medical Center, Franklin Square Hospital. Principal Investigator: Ira H. Gewolb, MD. Co-principal Investigator: Kelly A. Hutcheson, MD. Study Center Coordinators: Loni Huynh, COA; Rani Kalsi, BA, COA; Xiaonong Liu; L. Jennifer Smell, RN. Coinvestigators: Susan J. Dulkerian, MD; Michael J. Elman, MD; Eric Jones, MD; Mark W. Preslan, MD; Scott M. Steidl, MD, DMA.
Baltimore R Center, Baltimore, Maryland)
Johns Hopkins Hospital, Johns Hopkins Bayview Medical Center, Howard County General Hospital, Greater Baltimore Medical Center, St Joseph Medical Center. Principal Investigator: Michael X. Repka, MD. Study Center Coordinators: Jennifer A. Shepard, NNP; Pamela Donahue, PhD. Coinvestigators: Susan W. Aucott, MD; Tuvia Blechman, MD; Mary Louise Collins, MD; Maureen M. Gilmore, MD; James T. Handa, MD; Ananth Vijay Mudgil, MD; Quan Dong Nguyen, MD; Cameron F. Parsa, MD; Dante Pieramici, MD; David Plotsky, MD; Jeffrey J. Pomerance, MD.
Boston Center (Boston, Massachusetts)
New England Medical Center, Children’s Hospital, Brigham and Women’s Hospital, Beth Israel Deaconess Medical Center, Lowell General Hospital, Lawrence General Hospital, Winchester Hospital, Newton-Wellesley Hospital, South Shore Hospital, Melrose-Wakefield Hospital, Beverly Hospital. Principal Investigator: Cynthia H. Cole, MD, MPH. Co-principal Investigator: Deborah Vanderveen, MD; Study Center Coordinators: Lacy Berman; Christy Faherty, RN, BSN; Caitlin Hurley, BS; Terry Mansfield, RN; Brenda McKinnon, RNC; Marianne Moore, RN. Coinvestigators: Caroline Baumal, MD, FRCSC; Amita Bhatt, MD; Mark Dacey, MD; Jay Duker, MD; Janine Eagle, MD; Anthony Fraioli, MD; Paul Greenberg, MD; Mark Hughes, MD; Robert Lacy, MD; O’ine McCabe, MD; Robert Peterson, MD; Elias Reichel, MD; Adam Rogers, MD; William Stinson, MD; Mitchell Strominger, MD.
Detroit Center (Detroit, Michigan)
William Beaumont Hospital, Children’s Hospital of Michigan, St John’s Hospital Detroit. Principal Investigator: John Baker, MD. Study Center Coordinators: Kristi Cumming, MSN; Michelle Kulak RN; Pat Manatrey, RN. Coinvestigators: Daniel Batton, MD; Mary Bedard, MD; Antonio Capone, MD; Renato Casabar, MD; Edward O’Malley, MD; Rajesh Rao, MD; John Roarty, MD; Michael Trese, MD; George Williams, MD.
Minneapolis Center (Minneapolis, Minnesota)
Fairview University Medical Center, Children’s Health Care, Minneapolis, Hennepin County Medical Center. Principal Investigator: Stephen P. Christiansen, MD. Study Center Coordinators: Sally Cook, BA; Ann Holleschau, BA; Molly Maxwell, RN; Marla Mills, RN, MSN; Carol Miller, RN; Kristin Rebertus, RN, NNP; Nancy Trower, RN, NNP. Coinvestigators: Steven Bennett, MD; David Brasel, MD; Robert Couser, MD; Sundeep Dev, MD; Allison Jensen, MD; Richard Lussky, MD; George Miller, MD; Robert Mittra, MD; Timothy Olsen, MD; Robert Ramsey, MD; William Rosen, MD; Edwin Ryan, MD; Shelley Springer, MD; Eric Steuer, MD; C. Gail Summers, MD; David Williams, MD.
St Louis Center (St Louis, Missouri)
Cardinal Glennon Children’s Hospital, St Mary’s Health Center. Principal Investigator: Bradley V. Davitt, MD. Study Center Coordinators: Julie Beuer, RN; Linda Breuer, LPN. Coinvestigators: Oscar Cruz, MD; Stephen Feman, MD; William Keenan, MD; Greg Mantych, MD.
North Carolina Center (Durham and Chapel Hill, North Carolina)
Duke University Medical Center, University of North Carolina Hospital. Principal Investigator: Sharon Freedman, MD. Co-principal Investigator: David Wallace, MD. Study Center Coordinators: Eileen Camp, RN; Sharon Clark, RN; Lori Hutchins, RN; Lora Lake, RN. Coinvestigators: Edward Buckley, MD; Laura Enyedi, MD; Ricki Goldstein, MD; Maurice Landers III, MD; Diane Marshall, MD; Travis Meredith, MD; Kean Oh, MD; Joan Roberts, MD.
Buffalo Center (Buffalo, New York)
Women’s and Children’s Hospital of Buffalo, Sisters of Charity Hospital. Principal Investigator: James D. Reynolds, MD. Study Center Coordinators: Dawn C. Gordon, RNC; Barbara Kuppel, RN, BSN. Coinvestigators: George P. Albert, MD; Steven Awner, MD; Rita Ryan, MD.
Long Island/Westchester Center (New York)
Stony Brook University Hospital, Westchester Medical Center. Principal Investigator: Pamela Ann Weber, MD. Study Center Coordinators: Adriann Combs, RNC; Natalie Dweck, RN. Coinvestigators: Howard Charles, MD; Tina Chou, MD; Joseph DeCristofaro, MD; Corina Gerontis, MD; Marc Horowitz, MD; Richard Koty, MD; Edmund LaGamma, MD; Maury Marmor, MD.
New York Center (New York, New York)
New York Presbyterian Hospital, Columbia Campus, New York Presbyterian Hospital, Cornell Campus. Principal Investigator: John Flynn, MD. Co-principal Investigator: Thomas Lee, MD. Study Center Coordinators: Osode Coki, RNC, BSN. Coinvestigators: Michael Chiang, MD; Steven Kane, MD; Alfred Krauss, MD; Robert Lopez, MD; Richard Polin, MD.
Rochester/Syracuse Center (New York)
University of Rochester Medical Center, Crouse-Irving Memorial Hospital. Principal Investigator: Dale L. Phelps, MD. Co-principal Investigators: Steven J. Gross, MD; David Hakanson, MD. Study Center Coordinators: Marcia Dodge, RN; Cassandra Horihan, MS; Pamela Parker, BA; Jane Phillips. Coinvestigators: Dennis Asselin, MD; Shi-Hwa W. Chang, MD; Ernest Guillet, MD; Robert Hampton, MD; Gary Markowitz, MD; Walter Merriam, MD; Leon-Paul Noel, MD; Robert Olsen, MD; Suzanne Pesce, MD; Steven Rose, MD; Bryan Rutledge, MD; Richard Simon, MD; Sam Spalding, MD; Donald Tingley, MD; Paul Torrisi, MD; Robert Vanderlinde, MD.
Columbus Center (Columbus, Ohio)
Columbus Children’s Hospital, Ohio State University Hospital, Mount Carmel Medical Center, Grant Medical Center, Riverside Methodist Hospital, Mount Carmel East Hospital, St Ann’s Hospital. Principal Investigator: Gary L. Rogers, MD. Co-principal Investigator: Don Bremer, MD. Study Center Coordinators: Rae Fellows, MEd; Sharon Klamfoth, LPN; Brenda Mann, RNC; Coinvestigators: Leandro Cordero, MD; Richard Hertle, MD; Alan Letson, MD; Richard McClead, MD; Mary Lou McGregor, MD; Patrick Wall, MD.
Oklahoma City Center (Oklahoma City, Oklahoma)
Children’s Hospital of Oklahoma. Principal Investigator: R. Michael Siatkowski, MD. Study Center Coordinators: Karen E. Corff, MS, ARNP; Melissa Fuhr, RN. Coinvestigators: Reagan H. Bradford, MD; Robert E. Leonard, MD; Mark H. Scott, MD.
Portland Center (Portland, Oregon)
Doernbecher Children’s Hospital at Oregon Health and Science University, Legacy Emanuel Children’s Hospital, Providence St Vincent’s Hospital. Principal Investigator: David T. Wheeler, MD. Study Center Coordinators: Karen Davis, RN; Nancy Dolphin, RN; Sharon Dunham, RN. Coinvestigators: Aazy Aaby, MD; Shawn Goodman, MD; Andreas Lauer, MD; Valerie Newman, MD; Earl A. Palmer, MD; De-Ann Pillers, MD, PhD; Joseph Robertson, MD; Ann Stout, MD; Tim Stout, MD; Andrea Tongue, MD.
Philadelphia Center (Philadelphia, Pennsylvania)
The Children’s Hospital of Philadelphia, The Hospital of the University of Pennsylvania, Pennsylvania Hospital. Principal Investigator: Graham E. Quinn, MD, MSCE. Study Center Coordinators: Jamie G. Koh, RN, MSN, CCRC; Marianne E. Letterio, RN, BSN; Molly McDaniel, BA. Coinvestigators: Soraya Abbasi, MD; Jane C. Edmond, MD; Brian J. Forbes, MD, PhD; Albert M. Maguire, MD; Monte D. Mills, MD; Eric A. Pierce, MD, PhD; Terri L. Young, MD.
Pittsburgh Center (Pittsburgh, Pennsylvania)
Magee-Women’s Hospital. Principal Investigator: Kenneth Cheng, MD. Study Center Coordinator: Judith Jones, RNC, BSN. Coinvestigators: Robert Bergren, MD; Beverly Brozanski, MD; Bernard Doft, MD; Mitchell Fineman, MD; Louis Lobes, MD; Karl Olsen, MD.
Charleston Center (Charleston, South Carolina)
Medical University of South Carolina. Principal Investigator: Richard A. Saunders, MD. Study Center Coordinator: Lisa Langdale, RN. Coinvestigators: Amy Hutchinson, MD; M. Millicent Peterseim, MD; Dilip Purohit, MD.
Houston Center (Houston, Texas)
Baylor College of Medicine, Texas Children’s Hospital, Texas Woman’s Hospital, Ben Taub General Hospital. Principal Investigator: David K. Coats, MD. Study Center Coordinators: Laura Gonzalez; Nataliya Kazymyrko, MD; Alma Sanchez, COT; Michele Steward, COT. Coinvestigators: Kathryn Brady-McCreery, MD; Joseph Garcia-Prats, MD; Eric Holz, MD; Scott Jarriel, MD; Karen Johnson, MD; George Mandy, MD; Evelyn A. Paysee, MD; A. Melinda Rainey, MD; Kimberly G. Yen, MD.
San Antonio Center (San Antonio, Texas)
University Hospital, Christus Santa Rosa Children’s Hospital. Principal Investigator: W.A.J. van Heuven. Co-principal Investigator: Alice K. Gong, MD. Study Center Coordinator: Melanie H. Drummond, RN. Coinvestigators: Timothy Paul Cleland, MD; James C. MacDonald, MD; Lina M. Marouf, MD; Juan Elian Rubio, MD.
Salt Lake City Center (Salt Lake City, Utah)
University of Utah Health Science Center, Primary Children’s Medical Center. Principal Investigator: Robert Hoffman, MD. Study Center Coordinator: Susan Bracken, RN. Coinvestigators: Paul Bernstein, MD; David Dries, MD; Jerald King, MD; Richard Olson, MD; Michael Teske, MD; Kimberly Yen, MD.
National Eye Institute, Bethesda, Maryland
Program Officer: Maryann Redford, DDS, MPH (June, 2001-Present); Richard L. Mowery, PhD (October, 2000-May, 2001); Donald F. Everett, MA (September, 1999-September, 2000).
Study Headquarters: Smith-Kettlewell Eye Research Institute, San Francisco, California. Principal Investigator: William V. Good, MD. Project Coordinator: Michelle Quintos, BA.
Coordinating Center: School of Public Health, Coordinating Center for Clinical Trials, University of Texas Health Science Center, Houston, Texas. Principal Investigator: Robert J. Hardy, PhD. Project Manager: Betty Tung, MS. Coordinating Center Staff: Charles Cooper, MS; Gordon Tsai, MS; Meng-Fen Wu, MS; Charles Minard, MS; Krystal Rather, BS.
Vision Testing Center: University of Arizona, School of Medicine, Tucson, Arizona. Principal Investigator: Velma Dobson, PhD. Coinvestigator: Graham E. Quinn, MD. Vision Testers: Kathleen M. Mohan, MA; Meigan B. Baldwin, BA. Vision Testing Center Coordinator: Suzanne M. Delaney, PhD.
Data and Safety Monitoring Committee: Chair:John Connett, PhD. Members: Edward F. Donovan, MD; Argye Hillis, PhD; Jonathan M. Holmes, MD; Joseph M. Miller, MD; Carol R. Taylor, RN, CSFN, PhD. Ex-officio Members: William V. Good, MD; Robert J. Hardy, PhD; Maryann Redford, DDS, MPH.
Executive Committee, Permanent Members: Chair: William V. Good, MD; Robert J. Hardy, PhD; Velma Dobson, PhD; Earl A. Palmer, MD; Dale L. Phelps, MD; Ex-officio member: Maryann Redford, DDS, MPH.
Executive Committee, Elected Members: W.A.J. van Heuven, MD (2000–2001); Charles Barr, MD (2001–2002); Michael Gaynon, MD (2002–2003); Michael Shapiro, MD (2003–2004); Rae Fellows, MEd (2000–2001); Judith Jones, RNC, BSN (2001–2002); Kristi Cumming, MSN (2002–2003); Deborah S. Neff, LPN (2003–2004).
Editorial Committee: Chair: William V. Good, MD; Robert J. Hardy, PhD; Velma Dobson, PhD; Earl A. Palmer, MD; Dale L. Phelps, MD; Michelle Quintos, BA; Betty Tung, MS.
Footnotes
A complete list of the participants in the study appears in Appendix 2.
The authors have no affiliation with or financial interest in the subject matter or materials discussed in the paper (eg, employment, consultancies, stock ownership, honoraria), with the exception of Velma Dobson, PhD, who has received royalties from the sale of Teller acuity cards.
Supported by Cooperative Agreements (5U10 EY12471 and 5U10 EY12472) with the National Eye Institute of the National Institutes of Health, US Department of Health and Human Services, Bethesda, Maryland.
Portions of this article were published in Archives of Ophthalmology (2003;121:1684-1694), copyright 2003, American Medical Association, and are reprinted with permission.
REFERENCES
- 1.Cryotherapy for Retinopathy of Prematurity Cooperative Group. . Multicenter trial of cryotherapy for retinopathy of prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119:1110–1118. doi: 10.1001/archopht.119.8.1110. [DOI] [PubMed] [Google Scholar]
- 2.Cryotherapy for Retinopathy of Prematurity Cooperative Group. . Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol. 1988;106:471–479. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 3.Rescan Z, Vamos R, Salacz G. Laser treatment of zone I prethreshold and stage 3 threshold retinopathy of prematurity. J Ophthalmol Strabismus. 2003;40:204–207. doi: 10.3928/0191-3913-20030701-06. [DOI] [PubMed] [Google Scholar]
- 4.Vander JF, Handa J, McNamara JA, et al. Early treatment of posterior retinopathy of prematurity: a controlled trial. Ophthalmology 1997;104:1731–1735; discussion 1735–1736. [DOI] [PubMed]
- 5.Good WV, Hardy RJ for the ETROP Multicenter Study Group. . The multicenter study of early treatment for retinopathy of prematurity (ETROP) [guest editorial] Ophthalmology. 2001;108:1013–1014. doi: 10.1016/s0161-6420(01)00540-1. [DOI] [PubMed] [Google Scholar]
- 6.Early Treatment for Retinopathy of Prematurity Cooperative Group. . Multicenter trial of Early Treatment for Retinopathy of Prematurity: study design. Contr Clin Trials. 2004;25:311–325. doi: 10.1016/j.cct.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Hardy RJ, Palmer EA, Dobson V, et al. Risk analysis of prethreshold ROP. Arch Ophthalmol. 2003;121:1699–1701. doi: 10.1001/archopht.121.12.1697. [DOI] [PubMed] [Google Scholar]
- 8.Early Treatment for Retinopathy of Prematurity Cooperative Group. . Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1696. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 9.McDonald MA, Dobson V, Series SL, et al. The acuity card procedure: a rapid test of infant acuity. Invest Ophthalmol Vis Sci. 1985;26:1158–1162. [PubMed] [Google Scholar]
- 10.Teller DY, McDonald MA, Preston K, et al. Assessment of visual acuity in infants and children: the acuity card procedure. Dev Med Child Neurol. 1986;28:779–789. doi: 10.1111/j.1469-8749.1986.tb03932.x. [DOI] [PubMed] [Google Scholar]
- 11.Dobson V, Quinn GE, Biglan AW, et al. Acuity card assessment of visual function in the cryotherapy for retinopathy of prematurity trial. Invest Ophthalmol Vis Sci. 1990;31:1702–1708. [PubMed] [Google Scholar]
- 12.Trueb L, Evans J, Hammel A, et al. Assessing visual acuity of visually impaired children using the Teller acuity card procedure. Am Orthopt J. 1992;42:149–154. [Google Scholar]
- 13.Salomão SR, Ventura DF. Large sample population age norms for visual acuities obtained with Vistech-Teller Acuity Cards. Invest Ophthalmol Vis Sci. 1995;36:657–670. [PubMed] [Google Scholar]
- 14.Mayer DL, Beiser AS, Warner AF, et al. Monocular acuity norms for the Teller Acuity Cards between ages one month and four years. Invest Ophthalmol Vis Sci. 1995;36:671–685. [PubMed] [Google Scholar]
- 15.Dobson V, Quinn GE, Siatkowski RM, et al. for the Cryotherapy for Retinopathy of Prematurity Cooperative Group. . Agreement between grating acuity at age 1 year and Snellen acuity at age 5. 5 years in the preterm child. Invest Ophthalmol Vis Sci. 1999;40:496–503. [PubMed] [Google Scholar]
- 16.Frankenburg WK, Dodds JB. The Denver developmental screening test. J Pediatr. 1967;71:181–191. doi: 10.1016/s0022-3476(67)80070-2. [DOI] [PubMed] [Google Scholar]
- 17.Cryotherapy for Retinopathy of Prematurity Cooperative Group. . Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome—structure and function. Arch Ophthalmol. 1990;108:1408–1416. [PubMed] [Google Scholar]
- 18.Cryotherapy for Retinopathy of Prematurity Cooperative Group. . Multicenter trial of cryotherapy for retinopathy of prematurity. Snellen visual acuity and structural outcome at 5-1/2 years after randomization. Arch Ophthalmol. 1996;114:417–424. doi: 10.1001/archopht.1996.01100130413008. [DOI] [PubMed] [Google Scholar]
- 19.Hardy RJ, Davis BR. The design, analysis, and monitoring of an ophthalmological clinical trial. ASA Proceedings of the Biopharmaceutical Section 1989;248–253.
- 20.Committee for the Classification of Retinopathy of Prematurity. . An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 21.Cryotherapy for Retinopathy of Prematurity Cooperative Group. The natural ocular outcome of premature birth and retinopathy: status at 1 year. Arch Ophthalmol. 1994;112:903–912. doi: 10.1001/archopht.1994.01090190051021. [DOI] [PubMed] [Google Scholar]
- 22.Msall ME, Phelps DL, DiGaudio KM, et al. on behalf of the Cryotherapy for Retinopathy of Prematurity Cooperative Group. . Severity of neonatal retinopathy of prematurity is predictive of neurodevelopmental functional outcome at age 5. 5 years. Pediatrics. 2000;106:998–1005. doi: 10.1542/peds.106.5.998. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson L, Fernell E, Broberger U, et al. Children with blindness due to retinopathy of prematurity: a population-based study. Perinatal data, neurological and ophthalmological outcome. Dev Med Child Neurol. 1998;40:155–159. doi: 10.1111/j.1469-8749.1998.tb15439.x. [DOI] [PubMed] [Google Scholar]
- 24.Palmer EA, Flynn JT, Hardy RJ, et al. for the Cryotherapy for Retinopathy of Prematurity Cooperative Group. . Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98:1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 25.Ferris FL, III, Kassoff A, Bresnick GH, et al. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]