ABSTRACT
Purpose
To determine if low doses of topical latrunculin B (LAT-B) will increase outflow facility and decrease intraocular pressure (IOP) without adversely affecting the cornea, and inhibit miotic and accommodative responses to pilocarpine, in ocular normotensive monkeys.
Methods
Intraocular pressure was measured by Goldmann tonometry before and after one and nine dose(s) of 0.005% and 0.01% topical LAT-B/vehicle given twice daily on successive weeks. Outflow facility was then measured by perfusion following 15 doses. Central corneal thickness was measured by ultrasonic pachymetry before and after one and nine dose(s) of 0.01% LAT-B/vehicle. Pupillary diameter (calipers) and accommodation (refractometry) before and after one dose of 0.005% and 0.02% LAT-B were determined.
Results
LAT-B dose-dependently decreased IOP, multiple doses more than a single dose. Maximal hypotension after one dose was 2.5 ± 0.3 mm Hg (0.005% LAT-B; n = 8; P < .001) or 2.7 ± 0.6 mm Hg (0.01% LAT-B; n = 8; P < .005); maximal hypotension after nine doses was 3.2 ± 0.5 mm Hg (0.005% LAT-B; n = 8; P < .001) or 4.4 ± 0.6 mm Hg (0.01% LAT-B; n = 8; P < .001). Outflow facility was increased by 75 ± 13% (n = 7; P < .005). Central corneal thickness was not changed after one or nine dose(s) of 0.01% LAT-B. The miotic and accommodative responses to intramuscular pilocarpine were dose-dependently inhibited. At 0.02% LAT-B, the inhibition of miosis was essentially complete when compared with the pre-LAT-B value, whereas the inhibition of accommodation was only about 25%. At 0.005% LAT-B, the effects were trivial.
Conclusions
In ocular normotensive monkeys, 0.005/0.01% LAT-B administered topically increases outflow facility and/or decreases IOP, but does not affect the cornea. Multiple doses reduce IOP more than a single dose. LAT-B dose-dependently relaxes the iris sphincter and ciliary muscle, with some separation of the miotic and accommodative effects.
INTRODUCTION
Latrunculins, macrolides isolated from the marine sponge Latrunculia magnifica, are specific and potent actin-disrupting agents that sequester monomeric G-actin, leading to the disassembly of actin filaments.1–3 Latrunculins A and B (LAT-A and B) are two common latrunculins, which cause reversible dose- and time-dependent destruction of actin bundles and associated proteins in varieties of cultured cells, including human trabecular meshwork cells.1–7 In living monkeys, both LAT-A and LAT-B increase outflow facility and decrease intraocular pressure (IOP).6,8,9 LAT-B also increases outflow facility in organ-cultured anterior segment of porcine eyes,5 suggesting a direct effect on outflow resistance in the conventional drainage pathway. The latter has been confirmed by a recent morphologic study of the trabecular meshwork in the live monkey eye (Tian B, et al, ARVO, 2004; abstract). Since LAT-B, compared with LAT-A, is more potent in increasing outflow facility6,8 and produces smaller transient increases in aqueous humor formation, corneal endothelial permeability, and protein concentration in the anterior chamber,9 LAT-B may be a better candidate than LAT-A as a potential antiglaucoma medication. However, a single dose of 20 μL of 500 μM (~0.02%) LAT-B administered topically, which decreases IOP in living monkeys,9 still produces a transient increase in corneal thickness when applied to the central cornea as four drops of 5 μL volume.9 Presumably, multiple treatments with the high concentration of LAT-B might induce more apparent side effects in the cornea.
We hypothesized that repetitive lower concentrations and total doses in higher-solution volumes, spread out over the entire corneal or conjunctival surface in the larger human eye, might minimize or avoid corneal toxicity induced by high concentrations of cytoskeletal drugs without attenuating their effects on outflow resistance.9,10 To test this hypothesis, we determined the effects of a single dose or multiple doses of 0.005/0.01% topical LAT-B on outflow facility, IOP, and/or central corneal thickness in normotensive monkey eyes. To learn more about the drug-induced changes in the anterior segment physiology, the pupil diameter and accommodation following 0.005/0.02% topical LAT-B were also determined.
METHODS
Animals and Anesthesia
Twenty-seven adult normal cynomolgus monkeys (Macaca fascicularis) of both sexes, weighing 3 to 8 kg, were studied. All experiments were conducted in accordance with University of Wisconsin and National Institutes of Health guidelines, and with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research. All monkeys were free of anterior chamber cells and flare by slit-lamp biomicroscopy when studied. Anesthesia for tonometry or pachymetry was induced with intramuscular ketamine (10 mg/kg) and maintained with supplemental intramuscular injections as required (usually 5 mg/kg every 30 to 45 minutes). Anesthesia for anterior chamber perfusion or refractometry was induced with intramuscular ketamine (10 mg/kg), followed by intravenous pentobarbital sodium (15 mg/kg).
Drug Preparation and Administration
LAT-B was obtained from Sigma Chemical Co (St Louis, Missouri) and stored as a 2 mM stock solution in dimethyl sulfoxide (DMSO; Sigma Chemical Co) at –20°C. LAT-B solutions for topical administration were freshly prepared in Bárány’s solution11 with 25% DMSO. Twenty microliters of 0.005% (1 μg/20 μL), 0.01% (2 μg/20 μL), or 0.02% (4 μg/20 μL) LAT-B were composed of 1.26, 2.53, or 5.00 μL of 2 mM LAT-B stock solution and 3.74, 2.47, or 0.00 μL of DMSO plus 15 μL of Bárány’s solution. The 25% DMSO served as a vehicle control. In IOP protocols, the drug or vehicle solution was administered to the central cornea of opposite eyes of either ketamine-anesthetized (day 1 and day 5; 4 × 5 μL drops) or fully conscious and manually restrained monkeys (day 2 through day 4; 2 × 10 μL drops) twice daily for 4.5 days at 8 am and 4 pm. Eye drops were administered at 30- to 60-second intervals with blinking prevented between drops. The same eyes of the same animals were treated with the drug in two IOP protocols, with the 0.01% LAT-B experiment conducted the week immediately following the 0.005% LAT-B experiment. Following the 0.01% LAT-B IOP experiment, the monkeys were treated with 0.01% drug/vehicle solution at 4 pm on day 5, and then once (days 6 and 7) or twice (day 8) daily (2 × 10 μL) for three additional days while fully conscious and manually restrained. On day 9, these monkeys were treated again with the same dose of the drug (4 × 5 μL) under ketamine anesthesia 2 hours before the anterior chamber perfusion. For pachymetry, different monkeys were treated with 0.01% LAT-B twice daily for 4.5 days under ketamine anesthesia. For refractometry and pupil-diameter measurement, monkeys were treated with 0.005/0.02% LAT-B (4 × 5 μL) one time under ketamine plus pentobarbital anesthesia. Administering the drug/vehicle solution to fully conscious and manually restrained monkeys in the IOP/outflow facility protocol was designed to reduce any potential cumulative effect of repeated ketamine administration on IOP or outflow facility during the multiple treatments (Bunch TJ, et al, ARVO, 2003; abstract).
IOP Measurement
Intraocular pressure was determined on day 1 (before and after the first dose) and day 5 (before and after the ninth dose) with a minified Goldmann applanation tonometer,12 using “Half and Half” creamer solution (Borden Inc, Columbus, Ohio) as the tear film indicator, with the monkey lying prone in a head holder. For each eye, three IOP readings were averaged as a baseline or pretreatment IOP before administration of the first or ninth dose of 0.005/0.01% LAT-B or vehicle, and single IOP readings were taken after the drug/vehicle administration hourly for 6 hours.
Outflow Facility Measurement
Total outflow facility was determined by two-level constant pressure perfusion of the anterior chamber with Bárány’s mock aqueous humor,11 using a one-needle technique and correcting for internal apparatus resistance.13 Outflow facility was measured for 90 minutes 2 hours after the 15th dose of 0.01% LAT-B or vehicle on day 9.
Central Corneal Thickness Measurement
Central corneal thickness was determined by ultrasonic pachymetry (DGH-1000 ultrasonic pachymeter, DGH Technology, Inc, Solana Beach, California) on day 1 (before and after the first dose) and day 5 (before and after the ninth dose). For each eye, three readings were averaged as a baseline or pretreatment value before administration of the first or ninth dose of 0.01% LAT-B or vehicle, and single readings were taken after the drug/vehicle administration every 30 minutes for 4 hours and then hourly for 2 hours.
Pupil and Accommodation Measurement
Accommodation (difference between baseline and post-drug refraction) was determined with a Hartinger coincidence refractometer. Pupil diameter was measured with vernier calipers under normal room light (350 lux). Baseline refraction, pupillary diameter, or both were measured, followed by topical application of 2.5% phenylephrine (stimulates the iris dilator muscle without influencing the iris sphincter and ciliary muscle,14,15 facilitating measurement of miosis and accommodation16). Refraction and/or pupillary diameter were measured again approximately 30 minutes later, after which 20 μL (4 × 5 μL) of 0.005/0.02% LAT-B was administered topically to one eye and vehicle to the other. Refraction and pupillary diameter were determined 85 minutes after LAT-B. Five minutes later, approximately 3 mL of pilocarpine solution was infused intramuscularly in the thigh (1.5 mg/kg) over 10 minutes. Refraction was determined every 5 minutes after pilocarpine infusion until stable, and final pupillary diameter was then measured.
Slit-lamp Examination
Slit-lamp biomicroscopy was performed before drug administration, during IOP measurement (1, 3, and 6 hours after drug administration), and before pachymetry and anterior chamber perfusion. The integrity of the corneal epithelium and endothelium, the presence of flare or cells in the anterior chamber, and the clarity of lens were noted. All animals were free of preexisting ocular abnormalities when studied.
Data Analysis
Data are given as mean ± SEM for n eyes or animals. Predrug or postdrug treated versus contralateral control; postdrug or postvehicle versus ipsilateral baseline; and baseline corrected postdrug treated versus control comparisons were made using the two-tailed paired t test for differences versus 0.0 or ratios versus 1.0.
RESULTS
Intraocular Pressure
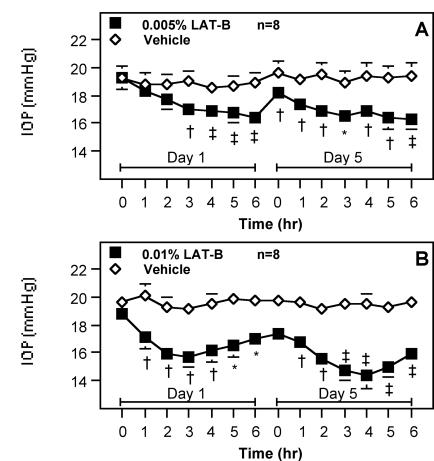
A single dose of 0.005% LAT-B lowered IOP from 19.3 ± 0.8 to 16.4 ± 0.7 mm Hg within 6 hours. After adjustment for baseline and contralateral IOP, the maximal hypotension of 2.5 ± 0.3 mm Hg (n = 8, P < .001) occurred at hour 6. Multiple doses (nine doses) of 0.005% LAT-B reduced IOP similar to a single dose, but the significant IOP reduction occurred earlier (hour 1 versus hour 3) and the maximal ocular hypotension was slightly greater (3.2 ± 0.5 mm Hg; P < .001). Intraocular pressure at 16 hours after the eighth treatment (IOP at 0 hours on day 5) in the LAT-B–treated eye was significantly lower than that in the contralateral control eye (–1.4 ± 0.3 mm Hg; P < .005) (Figure 1A). A single dose of 0.01% LAT-B lowered IOP from 18.8 ± 0.7 to 15.7 ± 0.8 mm Hg within 6 hours. After adjustment for baseline and contralateral IOP, the maximal hypotension of 2.7 ± 0.6 mm Hg (n = 8, P < .005) occurred at hour 3. Multiple doses (nine doses) of 0.01% LAT-B induced a greater IOP reduction than a single dose, with the maximal hypotension of 4.4 ± 0.6 mm Hg (P < .001) at hour 4. The pre-ninth-treatment IOP (IOP at 0 hours on day 5) in the LAT-B–treated eye tended to be lower than that in the contralateral control eye (–1.7 ± 0.7 mm Hg; P = .056). Although the monkeys had not received any treatment for 3 days after the ninth treatment with 0.005% LAT-B, the baseline IOP (IOP at 0 hours on day 1) in the LAT-B–treated eye in the 0.01% LAT-B protocol (Figure 1B) did not return to the level before the first treatment with 0.005% LAT-B (Figure 1A).
Figure 1.
Effect of latrunculin B (LAT-B) on intraocular pressure (IOP) in monkeys. 0.005% (A) or 0.01% (B) LAT-B and vehicle were administered to opposite eyes of monkeys topically twice daily for 4.5 days. IOP was measured before and after the first (on day 1) and ninth (on day 5) treatment. The same eyes of the same monkeys were treated with the drug in the two-dose studies, and the higher-dose experiment was conducted the week immediately following the lower-dose experiment, with only 2 days’ drug-free interval between studies. IOP before the first treatment in each study was used as a baseline. Data are mean ± SEM mm Hg for n animals. IOP difference between eyes corrected for baseline was tested for differences ≠ 0.0 by the two-tailed paired t test: *P < .01; †P < .005; ‡P < .001.
Outflow Facility
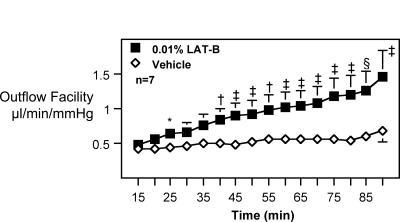
LAT-B significantly increased outflow facility by 75 ± 13% (n = 7; P < .005) during the overall 90-minute post-drug perfusion beginning 2 hours after the 15th treatment of 0.01% LAT-B. The reason n = 7 rather than 8 is that one monkey died on day 6 due to an unrelated disease. In analysis per three 30-minute perfusion periods, the drug increased outflow facility by 35 ± 14%, 69 ± 14%, and 100 ± 14% in the first, second, and third 30-minute durations, respectively (Table 1; Figure 2).
Table 1.
Effect of lat-b on outflow facility in monkeys*
| Perfusion period | Lat-b | Vehicle | Lat-b/vehicle |
|---|---|---|---|
| 90 min | 0.93 ± 0.19 | 0.51 ± 0.08 | 1.75 ± 0.13† |
| First 30 min | 0.58 ± 0.10 | 0.43 ± 0.05 | 1.35 ± 0.14‡ |
| Second 30 min | 0.89 ± 0.19 | 0.51 ± 0.08 | 1.69 ± 0.14† |
| Third 30 min | 1.19 ± 0.28 | 0.57 ± 0.11 | 2.00 ± 0.14§ |
LAT-B, latrunculin B.
Following 15 doses of 0.01% LAT-B/vehicle (Figure 2), outflow facility was measured by two-level constant pressure perfusion for 90 minutes. No baseline outflow facility was determined, but all monkeys were selected from those that had similar baseline facilities in both eyes per previous studies. Data are mean ± SEM (μL/min/mm Hg for outflow facility) for seven animals. Ratios are unitless. Difference between eyes was tested for ratios ≠ 1.0 by the two-tailed paired
t test.
P<.005.
P<.05.
P<.001.
Figure 2.
Effect of latrunculin B (LAT-B) on outflow facility in monkeys. Following the 0.01% LAT-B intraocular (IOP) protocol (Figure 1B), treatment with 0.01% LAT-B or vehicle to opposite eyes of monkeys topically once or twice daily was continued without interruption for three additional days. Outflow facility was measured by two-level constant pressure perfusion for 90 minutes on day 9 (2 hours after the 15th treatment). No baseline outflow facility was determined, but all monkeys were selected from those that had similar baseline facilities in both eyes per previous studies. Data are mean ± SEM (μL/min/mm Hg) for n animals (n = 7 rather than 8 because one monkey died of unrelated disease in its cage before perfusion). Difference between eyes was tested for differences ≠ 0.0 by the two-tailed paired t test: *P < .05; †P < .025; ‡P < .02; §P < .01.
Corneal Thickness
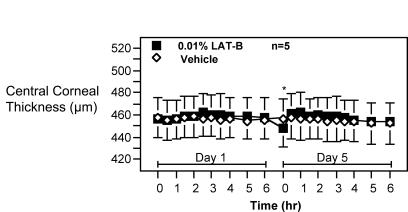
On day 1, baseline central corneal thickness was 456.3 ± 17.0 μm in the LAT-B–treated eye and 457.7 ± 18.2 μm in the contralateral control eye. Central corneal thickness after the first treatment varied between 454.6 ± 17.2 and 462.4 ± 17.0 μm in the LAT-B–treated eye, and between 453.4 ± 15.4 and 458.6 ± 18.7μm in the contralateral control eye during 6 hours pachymetry. On day 5, pre-ninth-treatment central corneal thickness was 448.4 ± 17.9 μm in the LAT-B–treated eye and 455.9 ± 18.6 μm in the contralateral control eye. The central corneal thickness after the ninth treatment varied between 454.4 ± 16.4 and 462.2 ± 18.2 μm in the LAT-B–treated eye, and between 452.2 ± 18.8 and 457.2 ± 19.6 μm in the contralateral control eye during 6 hours pachymetry. Collectively, the central corneal thickness in the LAT- B–treated eye was only 0.9 to 8.1 μm (P = .08–.82) or 3.1 to 7.1 μm (P = .07–.37) thicker than that in the vehicle-treated eye after one dose or nine doses of 0.01% LAT-B, after adjustment for ipsilateral baseline (Figure 3).
Figure 3.
Effect of latrunculin B (LAT-B) on central corneal thickness in monkeys. 0.01% LAT-B and vehicle were administered to opposite eyes of monkeys topically twice daily for 4.5 days. Central corneal thickness (CCT) was measured before and after the first (on day 1) and ninth (on day 5) treatment. CCT before the first treatment was used as a baseline. Data are mean ± SEM μm for n animals. CCT difference between eyes corrected for baseline was tested for differences ≠ 0.0 by the two-tailed paired t test: *P < .05.
Pupil and Accommodation Measurement
Pupillary Diameter
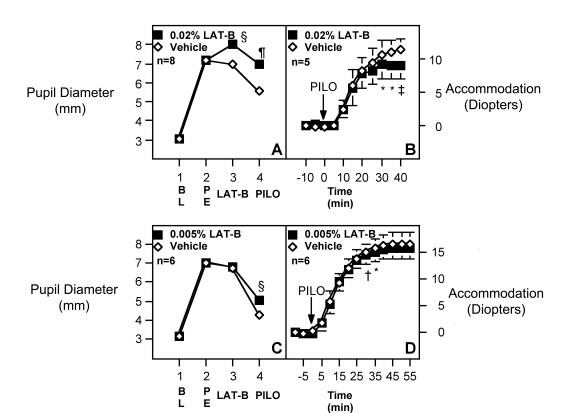
Baseline pupil diameters of both eyes in all monkeys were similar (Figure 4A and C). Twenty-five minutes after phenylephrine administration, both pupils dilated equally (in the 0.02% LAT-B protocol: 7.2 ± 0.3 mm versus 7.2 ± 0.3 mm, n = 8; P = NS, Figure 4A; in the 0.005% LAT-B protocol: 7.0 ± 0.3 mm versus 7.0 ± 0.3 mm, n = 6, P = NS, Figure 4C). Eighty-five minutes after topical administration of 20 μL of 0.02% LAT-B, the pupils in the LAT-B–treated eyes dilated further relative to the contralateral controls (to 8.0 ± 0.3 mm versus 7.0 ± 0.4 mm, P < .005, Figure 4A). However, 85 minutes after 20 μL of 0.005% LAT-B, the pupil in the LAT-B–treated eye was only slightly larger than that in the vehicle-treated eye. When pilocarpine was infused intramuscularly in the thigh, the control pupils constricted but the pupils after 0.02% LAT-B did not (5.6 ± 0.3 mm in controls versus 7.0 ± 0.4 mm in LAT-B–treated eyes, P < .001, Figure 4A). The inhibition of miosis was essentially complete when compared with the pre-LAT-B value (7.0 ± 0.4 versus 7.2 ± 0.3). This miosis was only slightly inhibited by 0.005% LAT-B (5.0 ± 0.4 mm in the LAT-B eye versus 4.3 ± 0.3 mm in the control eye).
Figure 4.
Effect of latrunculin B (LAT-B) on miotic and accommodative responses to pilocarpine in monkeys. Pupillary and accommodative responses to topical phenylephrine (PE), 0.005% (C, D) or 0.02% (A, B) topical LAT-B, and intramuscular pilocarpine (PILO, 1.5 mg/kg) in monkeys. BL, baseline. Data are mean ± SEM for n animals (accommodation data for three animals in the 0.02% LAT-B protocol are not available). Difference between eyes was tested for differences ≠ 0.0 by the two-tailed paired t test: *P < .05; †P < .02; ‡P < .01; §P < .005; ¶P < .001.
Accommodation
No significant differences between pilocarpine-induced accommodation in LAT-B–treated versus control eyes were observed initially after 20 μL of 0.02% LAT-B (Figure 4B). However, the accommodation plateau in the LAT-B–treated eye occurred earlier than that in the control eye (30 versus 40 minutes after the intramuscular pilocarpine). A statistically significant difference between eyes was observed during the period of 30 to 40 minutes after intramuscular pilocarpine, with the LAT-B–treated eyes accommodating approximately 2.5 ± 0.5 diopter (~25 ± 8%) less than the controls eventually (8.9 versus 11.4 D; n = 5; P < .01; Figure 4B). The accommodation was only slightly inhibited by 0.005% LAT-B (Figure 4D).
Slit-lamp Examination
During IOP measurement, most monkeys had mild punctate corneal epithelial defects at 3 to 6 hours after the drug administration, but the defects in LAT-B–treated eyes were similar to that in control eyes. Additionally, the punctate corneal epithelial defects seen during tonometry after the first treatment on day 1 had disappeared in both eyes of almost all monkeys at approximately 16 hours after the eighth dose (before tonometry on day 5). No other abnormality was observed in any monkey in any protocol during slit-lamp examination.
DISCUSSION
This study has shown that LAT-B administered topically decreases IOP in normotensive monkeys in a dose-dependent manner, with multiple doses producing greater IOP reduction than a single dose. This is consistent with many current clinical and experimental antiglaucoma drugs that have greater effects following multiple treatments in both normotensive17,18 and glaucomatous19,20 monkeys. Some ocular hypotensive effect of multiple administrations of LAT-B appears to last more than 16 hours, evidenced by the lower IOP in the LAT-B–treated eye than in the vehicle-treated eye at 16 hours after the eighth treatment in both the 0.005% and 0.01% LAT-B protocols (Figure 1A and B), and by the tendency toward slightly lower baseline in the drug-treated eye than in the control eye 3 days after the ninth treatment of 0.005% LAT-B (Figure 1B). In a previous study,9 a single dose of 20 μL of 500 μM (~0.02%) LAT-B maximally decreased IOP by 3.1 mm Hg, slightly greater than the maximal IOP reduction (–2.7 mm Hg) induced by a single dose of 0.01% LAT-B, and apparently smaller than the IOP reduction (–4.4 mm Hg) induced by multiple doses of 0.01% LAT-B, in the current experiments. This further indicates that LAT-B dose-dependently decreases IOP and that multiple doses of LAT-B are more effective than a single dose. In the present study, 15 treatments with 0.01% LAT-B significantly increase outflow facility in the monkey eye, which, in conjunction with our previous findings,5,8 suggests that LAT-B decreases IOP by reducing outflow resistance in the trabecular meshwork.
In the previous study,9 a single dose of 0.02% topical LAT-B also transiently increased the central corneal thickness of the monkey eye by up to 47 μm within 3 hours. Unlike the higher dose studied previously, a single and multiple dose(s) of 0.01% LAT-B administered topically in the present study do not change the central corneal thickness. This indicates that the 0.01% concentration of the drug does not significantly affect the corneal endothelium. By slit-lamp biomicroscopy, 0.01% LAT-B is also less toxic to the corneal epithelium than the higher dose studied before.9 The LAT-B doses used in this study do not produce any additional punctate corneal epithelial defects in the LAT-B–treated eye compared with the vehicle-treated eye. The mild punctate corneal epithelial defects in both eyes, occurring 3 to 6 hours after the drug administration, is a common phenomenon during tonometry in ketamine-anesthetized animals, presumably due to reduced blinking under ketamine anesthesia and frequent IOP measurements. All these seem to support our hypothesis from previous studies9,10 that repetitive lower concentrations and total doses in higher-solution volumes, spread out over the entire corneal or conjunctival surface, may minimize or avoid corneal toxicity.
A recent morphologic study (Tian B, et al, ARVO, 2004; abstract) has revealed that LAT-B induces formation of numerous cytoplasmic projections of the subcanalicular cells and massive “ballooning” of the JXT region, leading to a substantial expansion of the space between the subcanalicular cell layer and the trabecular collagen beams. Additionally, LAT-B also significantly increases the junction-to-junction distance of the inner wall cells of Schlemm’s canal (Tian B, et al, ARVO, 2004; abstract), although the increase is not as great as that after the serine-threonine kinase inhibitor H-7.21,22 All these structural changes in the trabecular meshwork may be consequent to the drug-induced cellular relaxation and account for the drug-induced decrease of outflow resistance in the trabecular meshwork. The current physiology data indicate that LAT-B dose-dependently relaxes intraocular smooth muscles. This further supports that cellular relaxation could be an important mechanism by which LAT-B decreases outflow resistance in the trabecular meshwork, since H-7, which decreases outflow resistance primarily by relaxing the trabecular meshwork,21,22 also relaxes the iris sphincter in vivo and ciliary muscle strips in vitro.23 More interestingly, although 0.02% LAT-B almost completely inhibits the miotic response of the monkey eye to pilocarpine, it inhibits the accommodative response to pilocarpine by only up to 25%. The reason for the separation is not clear, but a pharmacokinetic explanation seems plausible.23 Pilocarpine is a classic antiglaucoma medication, which indirectly increases outflow facility by contracting the ciliary muscle. However, the induced miosis, which reduces vision especially in elderly patients with incipient cataract,24 restricts its usage. The relative dissociation of miotic and accommodative responses to pilocarpine after LAT-B provides a possibility that the combination of a low but still facility-effective dose of pilocarpine with a facility-effective and cornea-safe dose of LAT-B may induce a facility increase greater than that induced by either drug alone, without damaging the cornea and constricting the pupil.
Collectively, the fact that 0.005% and 0.01% topical LAT-B increases outflow facility and/or decreases IOP without adversely affecting the cornea suggests that a low dose of topical LAT-B may have potential as a safe and trabecular meshwork–selective antiglaucoma medication.
DISCUSSION
Dr Robert Ritch
Sponges are extremely interesting creatures. The phylum Porifera consists of about 15,000 species of one of the earliest forms of metazoan development. They consist of about 10 different cell types and can be disaggregated into a single cell suspension by removing calcium from the medium. When calcium is re-added, the cells reaggregate into clumps, each of which can develop again into a mature sponge. Sponges are sessile and produce numerous toxic substances, perhaps to discourage predators, but more likely to protect the space comprising their environment from settlement by competitive species. Some of the many compounds isolated from sponges have potential beneficial effects for humans, including compounds with respiratory, cardiovascular, gastrointestinal, anti-inflammatory, antitumor, and antibiotic activities. To these, we can hopefully add the latrunculins.
Transient disruption of the cellular structure of the trabecular meshwork offers a potential new approach to increasing aqueous outflow. Inhibition of aqueous production, the basis of most of our presently used drugs, may be detrimental to trabecular function in the long term, a concept well reviewed a decade ago by Becker.1 Pilocarpine, the first drug used to treat glaucoma, is usually prescribed 4 times daily for lowering IOP, although nasolacrimal occlusion can effectively make this a twice daily drug.2 Nevertheless, its use has been largely abandoned in favor of newer agents.
A generation ago, Anders Bill et al3 perfused eyes with EDTA. This produced distention of the juxtacanalicular meshwork, washout of extracellular material, and disintegration of the denuded trabecular cores. Its toxicity prevented clinical use. In 1982, Kaufman and Erickson showed similar effects with cytochalasins.4 However, a great idea is a great idea, and Dr. Kaufman never gave up.
The authors have shown that low concentrations of topically administered latrunculin-B in single or multiple doses decrease intraocular pressure (IOP) and increase the coefficient of aqueous outflow in normotensive monkeys. Importantly, the cornea was not adversely affected, suggesting that this drug may have potential as an antiglaucoma drug which can be targeted specifically at the trabecular meshwork.
I would ask a few questions. In these experiments, the latrunculin was dissolved in a medium containing 25% DMSO? Is this necessary for solubilization and, if so, how would a formulation be prepared for human use? Have the authors performed the same studies yet in glaucomatous monkeys? In 1986, Epstein et al described acute reduction of outflow in cynomolgus monkey eyes when perfused with pigment particles isolated from the iris and ciliary body.5 Have the authors considered pretreating a group of monkeys with latrunculin-B to see if this reduction can be prevented when compared to perfusion into a control group and would they think such a study worthwhile?
From my perspective, the most important potential application of latrunculin would be in eyes with exfoliation syndrome (XFS), which is, overall, the most common identifiable cause of open-angle glaucoma worldwide, accounting for the majority of the open-angle glaucoma in some countries.6 We have estimated that there are about 60 million people with XFS, of whom 15 million have elevated IOP and 5 million have glaucoma. Elevated IOP with or without glaucomatous damage occurs in approximately 25% of persons with XFS, or about 6 to 10 times the rate in eyes without XFS.7 Glaucoma in XFS has a more serious clinical course and worse prognosis than primary open-angle glaucoma. Exfoliative glaucoma is associated with an increase in aqueous outflow resistance and elevated IOP. The most likely mechanism responsible is blockage of the meshwork by a combination of exfoliation material and liberated iris pigment.8
Increased trabecular pigmentation is prominent and is apparent in virtually all patients with clinically evident disease. In virtually all studies of patients with clinically unilateral XFS, the trabecular pigment is almost always denser in the involved eye.9,10 Eyes with exfoliative glaucoma tend to have greater pigmentation than both eyes with XFS but without glaucoma11,12 and eyes with POAG.13,14 There appears to be a highly significant correlation between elevated IOP and the degree of pigmentation of the meshwork.15
I have hypothesized that, if these eyes were perfused with latrunculin, transient disruption of the trabecular meshwork might allow release of trapped exfoliation material and pigment and, when the meshwork is reconstituted, markedly improved function and lowered IOP. Indirect evidence suggesting this possibility are reports that suctioning of the meshwork in eyes with XFS16 and trabeculotomy17 have greater success in eyes with XFS than those with primary open-angle glaucoma. It is possible that a single treatment might have a prolonged effect, needing to be repeated perhaps only at widely spaced intervals. Certainly such a breakthrough would be a welcome therapeutic advance benefiting millions of people.
I would like to commend the authors for an elegant investigation and urge them to attempt to bring this drug to fruition for clinical use.
REFERENCES
- 1.Becker B. Does hyposecretion of aqueous humor damage the trabecular meshwork? J Glaucoma. 1995;4:303–305. [PubMed] [Google Scholar]
- 2.Sharir M, Zimmerman TJ. Nasolacrimal occlusion improves the therapeutic index of antiglaucoma medications. J Assoc Acad Minor Phys. 1994;5:62–67. [PubMed] [Google Scholar]
- 3.Bill A, Lütjen-Drecoll E, Svedbergh B. Effects of intra-cameral Na2 EDTA and EGTA on aqueous outflow routes in the monkey eye. Invest Ophthalmol Vis Sci. 1980;19:492–504. [PubMed] [Google Scholar]
- 4.Kaufman PL, Erickson KA. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1982;23:646–650. [PubMed] [Google Scholar]
- 5.Epstein DL, Freddo TF, Anderson PJ, et al. Experimental obstruction to aqueous outflow by pigment particles in living monkeys. Invest Ophthalmol Vis Sci. 1986;27:387–395. [PubMed] [Google Scholar]
- 6.Ritch R. Exfoliation syndrome: The most common identifiable cause of open-angle glaucoma. J Glaucoma. 1994;3:176–178. [PubMed] [Google Scholar]
- 7.Ringvold A, Blika S, Elsås T. The Middle-Norway eye-screening study. II. Prevalence of simple and capsular glaucoma. Acta Ophthalmol. 1991;69:273–280. doi: 10.1111/j.1755-3768.1991.tb04814.x. [DOI] [PubMed] [Google Scholar]
- 8.Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 9.Ritch R. Exfoliation syndrome: Clinical findings and occurrence in patients with occludable angles. Trans Am Ophthalmol Soc. 1994;92:845–944. [PMC free article] [PubMed] [Google Scholar]
- 10.Kunishi Y, Kunishi M, Yoshino H, Nishida T. Gonioscopic features of pseudoexfoliation. Jpn J Clin Ophthalmol. 1998;52:1683–1689. [Google Scholar]
- 11.Puska P. The amount of lens exfoliation and chamber-angle pigmentation in exfoliation syndrome with or without glaucoma. Acta Ophthalmol. 1995;73:226–232. doi: 10.1111/j.1600-0420.1995.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 12.Rouhiainen H, Teräsvirta M. Pigmentation of the anterior chamber angle in normal and pseudoexfoliative eyes. Acta Ophthalmol. 1990;68:700–702. doi: 10.1111/j.1755-3768.1990.tb01698.x. [DOI] [PubMed] [Google Scholar]
- 13.Konstas AGP, Dutton GN. Gonioscopic findings in Greek patients with exfoliation glaucoma. Acta Ophthalmol. 1991;69:281–287. doi: 10.1111/j.1755-3768.1991.tb04815.x. [DOI] [PubMed] [Google Scholar]
- 14.Futa R, Shimizu T, Furuyoshi N. Clinical features of capsular glaucoma in comparison with primary open-angle glaucoma in Japan. Acta Ophthalmol. 1992;70:214–219. doi: 10.1111/j.1755-3768.1992.tb04126.x. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Montañés J, Quintero Alonso A, Alvarez Serna A, Alcolea Paredes A. Pseudoexfoliation syndrome: clinical study of the anterior chamber angle. J Fr Ophtalmol. 1990;13:183–188. [PubMed] [Google Scholar]
- 16.Jacobi PC, Krieglstein GK. Trabecular aspiration: A new mode to treat pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 1995;36:2271–2276. [PubMed] [Google Scholar]
- 17.Tanihara H, Negi A, Akimoto M, et al. Surgical effect of trabeculotomy ab externo on adult eyes with primary open angle glaucoma and pseudoexfoliation syndrome. Arch Ophthalmol. 1993;111:1653–1661. doi: 10.1001/archopht.1993.01090120075025. [DOI] [PubMed] [Google Scholar]
Dr Allan J. Flach
The only real toxicity that you mentioned was some minimal corneal thickening, and I wonder if that corneal thickening may have at least in part been an artifact of the so-called drop-dry approach to giving your drop. If you take athletic subjects and put them in space boots and hang them upside-down, you can increase the intraocular pressure significantly. Might I suggest that you take your monkeys and hang them upside-down for periods of time to see the effect on intraocular pressure?
Dr Ralph C. Eagle, Jr
Did you look at the iris and ciliary musculature by ultrastructure examination?
Dr Dan B. Jones
I know this is a short-term study, but if it becomes a long-term drug, and considering the potential action of the drug, it will be necessary to look at other things about the cornea with regard to toxicity and, particularly, corneal stem cells, which have the potential to create a variety of favorable effects. It may take time to see the adverse outcome in terms of poor epithelial stasis, static state, and epithelial repair.
Dr Paul L. Kaufman
Dr Ritch alluded to a concept that Dr Bernard Becker also had a number of decades ago about reduction of aqueous flow possibly affecting outflow facility adversely. We just published a paper in Experimental Eye Research in monkeys1 showing that chronic secretory suppression reduces aqueous outflow facility in the live, nonhuman primates. It is even more so if you use prostaglandin treatment as well because that redirects fluid away from the trabecular meshwork and out the ciliary muscle. In fact, there may be long-term downsides to these approaches.
Is DMSO necessary? These were proof-of-principle studies and also LAT-B is a relatively insoluble molecule. This is a problem for the pharmaceutical industry to solve. Whether you change the molecule or whether you change the vehicle, you can actually get away with a little bit less of a concentration of DMSO than I showed in these studies. DMSO itself, at this concentration and for this period of time, does not seem to have an adverse effect. We would certainly like to have a vehicle or perhaps an altered molecule that doesn't require an extraordinary solubilizing agent. We have not done this in glaucomatous monkeys yet but we have done it in glaucomatous monkeys with some other compounds on the contractility side. It does work. I would just remind all of you that the glaucoma monkey model that we use now, the laser glaucoma model, is a scar model so the physiology may be completely different. It would be great if we had a reliable steroid glaucoma model in the monkey, but unfortunately, we do not.
In terms of the pigment and exfoliation obstruction type of pathology that Dr Ritch alluded to, we haven't used pigment and obviously we don't have monkeys with exfoliation. We have created an excess of extracellular matrix in the monkey trabecular meshwork by chronic therapy with phospholine iodide. That goes back to another series of research projects that we’ve done and published.2,3 These compounds do work under those circumstances4 so that at least, in principle, this might work. We have not done that in this experiment. I think we would like to wash out all excess extracellular material at one time. If that’s what the pathophysiology of primary open-angle glaucoma or exfoliation and pigmentary glaucoma really is, this might be an approach. The monkeys that we’ve used so far are normotensive monkeys except for this little study with echothiophate. You do get a return of normal resistance after a period of time. If we were using glaucoma eyes, whether you’d get a return of pathological resistance, we just don’t know. We’re not going to know that until we get into clinical trials.
The more physiologic way of doing larger drop volume does not seem to give us the corneal changes. We had a clue in that, if you look at the peripheral corneal thickness, you did not see the thickening. We measured corneal thickness centrally and peripherally, and the peripheral cornea was not thickened. Therefore we knew it was a very high concentration of drug that was sitting in the center of the cornea. Gravity boots and neckties have not been done in a monkey. We could do so, but it seems inadvisable.
In terms of ultrastructure, neither the iris, the ciliary muscle, nor the ciliary epithelium as well, have shown changes in these acute experiments. These were single-dose intracameral infusions to look for acute toxicity; we didn't see any. What happens with weeks or months or years will not be known with any drug until you actually get out into clinical trials. The FDA often mandates, as it did with the prostaglandins, Phase 4 post-marketing studies, not for purposes of advertising the drug, but for purposes of following long-term toxicity. Unfortunately, at this stage I do not have answers to a lot of these questions. We will just have to see what happens, as you do with any new class of drugs.
REFERENCES
- 1.Kiland JA, Gabelt BT, Kaufman PL. Studies on the mechanism of action of timolol and on the effects of suppression and redirection of aqueous flow on outflow facility. Exp Eye Res. 2004;78:639–651. doi: 10.1016/j.exer.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Lütjen-Drecoll E, Kaufman PL. Echothiophate-induced structural alterations in the anterior chamber angle of the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1979;18:918–929. [PubMed] [Google Scholar]
- 3.Lütjen-Drecoll E, Kaufman PL. Biomechanics of echothiophate-induced anatomic changes in monkey aqueous outflow system. Graefes Arch Clin Exp Ophthalmol. 1986;224:564–575. doi: 10.1007/BF02154746. [DOI] [PubMed] [Google Scholar]
- 4.Gabelt BT, Hennes EA, Seeman JL, et al. H-7 effect on outflow facility after trabecular obstruction following long-term echothiophate treatment in monkeys. Invest Ophthalmol Vis Sci. 2004;451:2732–2736. doi: 10.1167/iovs.04-0083. [DOI] [PubMed] [Google Scholar]
Footnotes
This study was supported by grants from the US National Eye Institute (EY02698), the Glaucoma Research Foundation, Research to Prevent Blindness, the Wisconsin Alumni Research Foundation, and the Ocular Physiology Research and Education Foundation. University of Wisconsin–Wisconsin Alumni Research Foundation holds a patent related to latrunculin B; accordingly, Dr Kaufman has a proprietary interest.
REFERENCES
- 1.Coué M, Brenner SL, Spector I, et al. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 2.Lyubimova A, Bershadsky AD, Ben-Ze’ev A. Autoregulation of actin synthesis responds to monomeric actin levels. J Cell Biol. 1997;65:469–478. [PubMed] [Google Scholar]
- 3.Spector I, Shochet N, Kashman Y, et al. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- 4.Spector I, Shochet NR, Blasberger D, et al. Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth: comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 5.Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81. [PubMed] [Google Scholar]
- 6.Peterson JA, Tian B, Bershadsky AD, et al. Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci. 1999;40:931–941. [PubMed] [Google Scholar]
- 7.Cai S, Liu X, Glasser A, et al. Effect of latrunculin-A on morphology and actin-associated adhesions of cultured human trabecular meshwork cells. Mol Vision 2000;6:132–143. Available at: http://www.molvis.org/molvis/v6/a18/. Accessed May 20, 2004. [PubMed]
- 8.Peterson JA, Tian B, Geiger B, et al. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70:307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- 9.Peterson JA, Tian B, McLaren JW, et al. Latrunculin effects on intraocular pressure, aqueous humor flow and corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:1749–1758. [PubMed] [Google Scholar]
- 10.Tian B, Sabanay I, Peterson JA, et al. Acute effects of H-7 on ciliary epithelium and corneal endothelium in monkey eyes. Curr Eye Res. 2001;22:109–120. doi: 10.1076/ceyr.22.2.109.5529. [DOI] [PubMed] [Google Scholar]
- 11.Bárány EH. Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol. 1964;3:135–143. [PubMed] [Google Scholar]
- 12.Kaufman PL, Davis GE. Minified Goldmann applanating prism for tonometry in monkeys and humans. Arch Ophthalmol. 1980;98:542–546. doi: 10.1001/archopht.1980.01020030538022. [DOI] [PubMed] [Google Scholar]
- 13.Bárány EH. Relative importance of autonomic nervous tone and structure as determinants of outflow resistance in normal monkey eyes (Cercopithecus ethiops and Macaca irus). In: Rohen JW, ed. The Structure of the Eye, Second Symposium. Stuttgart: FK Schattauer Verlag; 1965:223–236.
- 14.Kaufman PL. Accommodation and presbyopia: neuromuscular and biophysical aspects. In: Hart WM Jr, ed. Adler’s Physiology of the Eye. 9th ed. St Louis: Mosby; 1992:391–411.
- 15.Thompson HS. The pupil. In: Hart WM Jr, ed. Adler’s Physiology of the Eye. 9th ed. St Louis: Mosby; 1992:412–441.
- 16.Bito LZ, DeRousseau CJ, Kaufman PL, et al. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- 17.Crawford K, Kaufman PL, Gabelt BT. Effects of topical PGF2 alpha on aqueous humor dynamics in cynomolgus monkeys. Curr Eye Res. 1987;6:1035–1044. doi: 10.3109/02713688709034874. [DOI] [PubMed] [Google Scholar]
- 18.Tian B, Gabelt BT, Crosson CE, et al. Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp Eye Res. 1997;64:979–989. doi: 10.1006/exer.1997.0296. [DOI] [PubMed] [Google Scholar]
- 19.Serle JB, Podos SM, Kitazawa Y, et al. A comparative study of latanoprost (Xalatan) and isopropyl unoprostone (Rescula) in normal and glaucomatous monkey eyes. Jpn J Ophthalmol. 1998;42:95–100. doi: 10.1016/s0021-5155(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 20.Wang RF, Camras CB, Lee PY, et al. Effects of prostaglandins F2 alpha, A2, and their esters in glaucomatous monkey eyes. Invest Ophthalmol Vis Sci. 1990;31:2466–2470. [PubMed] [Google Scholar]
- 21.Sabanay I, Gabelt BT, Tian B, et al. H-7 effects on structure and fluid conductance of monkey trabecular meshwork. Arch Ophthalmol. 2000;118:955–962. [PubMed] [Google Scholar]
- 22.Sabanay I, Tian B, Gabelt BT, et al. Functional and structural reversibility of H–7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004;78:137–150. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Tian B, Millar C, Kaufman PL, et al. H–7 effects on the iris and ciliary muscle in monkeys. Arch Ophthalmol. 1998;116:1070–1077. doi: 10.1001/archopht.116.8.1070. [DOI] [PubMed] [Google Scholar]
- 24.Nardin GF, Zimmerman TJ. Ocular cholinergic agents. In: Ritch R, Shields MB, Krupin T, eds. The Glaucomas, Glaucoma Therapy. 2nd ed. St Louis: Mosby; 1996:1399–1407.