ABSTRACT
Purpose
To validate a computerized expert system evaluating visual fields in a prospective clinical trial, the Ischemic Optic Neuropathy Decompression Trial (IONDT). To identify the pattern and within-pattern severity of field defects for study eyes at baseline and 6-month follow-up.
Design
Humphrey visual field (HVF) change was used as the outcome measure for a prospective, randomized, multi-center trial to test the null hypothesis that optic nerve sheath decompression was ineffective in treating nonarteritic anterior ischemic optic neuropathy and to ascertain the natural history of the disease.
Methods
An expert panel established criteria for the type and severity of visual field defects. Using these criteria, a rule-based computerized expert system interpreted HVF from baseline and 6-month visits for patients randomized to surgery or careful follow-up and for patients who were not randomized.
Results
A computerized expert system was devised and validated. The system was then used to analyze HVFs. The pattern of defects found at baseline for patients randomized to surgery did not differ from that of patients randomized to careful follow-up. The most common pattern of defect was a superior and inferior arcuate with central scotoma for randomized eyes (19.2%) and a superior and inferior arcuate for nonrandomized eyes (30.6%). Field patterns at 6 months and baseline were not different. For randomized study eyes, the superior altitudinal defects improved (P = .03), as did the inferior altitudinal defects (P = .01). For nonrandomized study eyes, only the inferior altitudinal defects improved (P = .02). No treatment effect was noted.
Conclusions
A novel rule-based expert system successfully interpreted visual field defects at baseline of eyes enrolled in the IONDT.
INTRODUCTION
The Ischemic Optic Neuropathy Decompression Trial (IONDT) was a randomized prospective study designed to establish the safety and efficacy of optic nerve sheath decompression as a treatment for nonarteritic anterior ischemic optic neuropathy (NAION), as well as to document the natural history of NAION.1 Based upon visual acuity as the primary outcome measure, the IONDT demonstrated that optic nerve sheath decompression is not effective and may be harmful.2
For NAION, characterized clinically as causing visual field loss, basing conclusions about efficacy of treatment and natural history solely on visual acuity may be inadequate. For this reason, the Humphrey visual field (HVF) was included in the study as a secondary outcome measure. Quantitative assessment of visual field function has been aided considerably by the advent of computerized automated static perimeters such as the HVF analyzer (Humphrey Instruments, Dublin, California). These instruments provide a standardized testing environment, quantitative assessment of threshold sensitivity to spots of light at fixed points throughout the visual field, and some data regarding reliability of patients’ responses.
Initial visual field evaluation based only on a readily computed global measure, mean deviation, failed to distinguish any difference between surgical and observational management. However, mean deviation alone may not be an adequate measure for assessment of eyes with NAION. The classic patterns of defect encountered in this disease may shift without changing average loss. Furthermore, there may be important changes in sensitivity within small areas of the visual field corresponding to nerve fiber bundle defects. These may not be detected when averaged into the mean deviation calculation. Therefore, a more detailed analysis of the quantitative visual field testing is important, even though such an analysis was not originally part of the IONDT methodology.
Whereas visual acuity based upon the logMAR charts developed for the Early Treatment Diabetic Retinopathy Study is a simple, well-tested measure of visual function, visual field assessment is complex and well beyond the scope of the original analysis. Prospective glaucoma trials have utilized a number of approaches for evaluating progression, but the algorithms seldom include classifications based upon the type of defect. The Optic Neuritis Treatment Trial categorized visual field defects, but the methodology did not involve strict definitions for classification. Furthermore, patterns of field loss were qualitatively rather than quantitatively determined.3 In the context of a prospective clinical trial, an automated, objective classification may be preferable.
For the current study, a Visual Field Analysis Committee was formed in 1998, consisting of neuro-ophthalmologists from six of the 26 participating Clinical Centers. As an initial step, each member of this group classified the visual fields of patients with NAION but not enrolled in the study. Using an iterative process to achieve consensus, a set of rules was devised to categorize the visual defects encountered in the disease. These rules were then incorporated into a computerized expert system to analyze the study fields in a consistent, reproducible manner.
The validated computerized expert system, described in detail herein, was used to determine the pattern of defect present as well as the density of defect within each pattern for both enrolled and fellow eyes. Based upon the categorization from the computerized expert system, a detailed evaluation of baseline visual field defects noted by HVF for patients enrolled in the IONDT was performed. The 6-month visual field for each patient was then compared quantitatively to the visual field at baseline, allowing for short-term fluctuations in each data set. The following questions were addressed:
Are the pattern and severity of visual field defects found in eyes randomized to treatment similar to those randomized to careful follow-up?
Is the pattern or severity of visual field defects found in eyes with better than 20/64 visual acuity substantially different from those with worse acuity who were eligible for randomization?
Are there preexisting conditions (eg, diabetes, hypertension) that affect the pattern and severity of visual field defects?
What is the relationship between global indices of HVF performance and the pattern or severity of visual field defects?
What is the relationship between visual acuity and pattern or severity of visual field defect?
Are visual field defects present in fellow eyes without known optic neuropathy that may indicate the presence of subclinical optic disk ischemia?
Are there changes in the visual fields between baseline and 6-month visits for randomized eyes, nonrandomized study eyes, and fellow eyes?
Are there changes in the visual fields for eyes randomized to surgery as compared to careful follow-up?
METHODS
Study Protocol
The eligibility criteria, randomization procedure, and visit protocols are extensively described in prior publications.1 Briefly, patients aged 50 or above were eligible for randomization into surgical and careful observation groups if they had symptoms and signs characteristic of NAION and their visual acuity was 20/64 or less. A Late Entry subset of the randomized patients included eyes for which acuity in the study eye was better than 20/64 at baseline but lost acuity to below this level within 30 days. Patients with acuity better than 20/64 were followed but not randomized. Fellow eyes were tested, and the results were recorded. At the time of examination, the clinician determined whether the fellow eye had optic neuropathy (of any type) or not. Visual fields were obtained at baseline, 6 months, 12 months, and at closeout of the study. Although multiple replications of the fields at some or all evaluation visits might have been helpful in minimizing training effects, fatigue effects may have increased because only time-consuming standard threshold strategies were available at the outset of the study. All centers utilized protocols approved by investigational review boards at their respective institutions. Patients were enrolled between 1992 and 1994. At that time the Data Safety and Monitoring Committee halted further recruitment, because surgery was found not to be effective.2
Organizational Structure
The Data Coordinating Center maintained the IONDT database that included visual field results from HVFs (both hard copies and diskettes). The Center masked and forwarded hard copies of visual fields to the visual field committees. The Center performed statistical analyses on the results of visual field analyses as well as other measures.
A Visual Field Steering Committee was formed at the outset of the study, evolving by 1994 to consist of the Director of the Visual Field Analysis Center (VFAC), the Director and key members of the Data Coordinating Center, and a consultant expert in visual field analysis. The VFAC developed the visual field assessment protocol and ensured that all visual fields were of good quality, masked as to patient and group identities, and handled appropriately. The committee reviewed and approved the procedures for conduct of the study, resolved technical issues arising during the course of the study, and reviewed study progress, acting when necessary to correct deficiencies. It also set the priorities for the visual field portion of the study and provided oversight for study analysis and publications.
An expert panel was formed in 1996, consisting of the Director of the VFAC and five other neuro-ophthalmologists with expertise in the interpretation of visual fields. It was responsible for developing and validating a computerized expert system, based on an agreed-upon set of rules for identification of the various types of field defects. The VFAC maintained the digital file inventory of all analyzed fields that were then forwarded to the Data Coordinating Center.
Determination of Quality
Visual fields were evaluated for compliance with the protocol,1 that is, that the field was a 24-2 performed on a HVF using test stimulus size III with the foveal sensitivity switch “on.” The IONDT did not utilize 30-2 visual fields because the additional peripheral test points were considered too variable. A few 30-2 visual fields that otherwise corresponded with the protocol were analyzed by utilizing only those points also represented on the 24-2 HVF test. The number and percentages of false-positive responses, false-negative responses, and fixation losses were recorded. Although unreliable fields in severely affected patients might have contained useful information, the interpretation would not be meaningful; thus, they were excluded from analysis. Visual fields were included for analysis if they were deemed reliable, using the four basic criteria of the Collaborative Initial Glaucoma Treatment Study (CIGTS) intra-test reliability rating.4 If fixation losses were greater than 20% for more than 20 trials, one point was added. If false-positives for eight or more trials were 33% or greater, one point was added. A similar criterion was applied for false-negatives. Short-term fluctuation (dB) was rated at zero for less than or equal to 4.0, one if greater than 4.0 but less than or equal to 6.0, two if greater than 6.0 but less than or equal to 7.0, and three if greater than 7.0. A rating of less than four was considered reliable.
Classification of Visual Fields
Validation of a system for classifying visual fields is complex. Given that there is no “gold standard,” experts will likely disagree on interpretation. This problem is well known in medicine. For instance, studies validating the use of computer-assisted diagnosis tools5–7 suggest that the differences between computer diagnosis and human expert diagnosis vary to about the same extent as human experts disagree among themselves. Given that computerized diagnosis may be no better than that of an expert panel, the principal reason for utilizing a computerized expert system in the context of a clinical trial is to reduce inconsistency by eliminating intraobserver and interobserver variability.
In the absence of a “gold standard,” validation of a classification system for visual fields requires several steps.5–7 First, an expert panel needs to achieve consensus on a set of rules. Second, the experts should be able to apply these rules in such a manner that the rate of disagreement is not different from that reported for similar classifications in other medical contexts. Third, the consistent application of the rules by a computerized expert system should produce classifications that do not disagree with the panel more than the expert panel disagrees with itself. Finally, the computerized expert system should reach reasonable clinical interpretations, such that major disagreements with the expert panel are rare.
The number of experts required on the panel was determined after a statistical computation determined that the chance of all six experts agreeing on ten patterns by guessing would be 0.00001. A majority of the experts needed to agree to categorize a field defect as a specific pattern. The chance of this degree of concordance occurring by guessing alone was .01215. For any field in which the agreement among panelists was not significantly better than guessing, the field was called “nonclassifiable.”
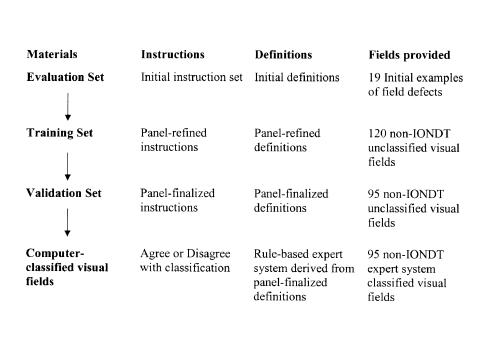
A sequence similar to that used by Molino and associates5 was developed to facilitate derivation of the rules, as shown in Figure 1. The information associated with this sequence was formulated into “sets” and included an evaluation set, a training set, and a validation set.
Figure 1.
Sequence developed to facilitate derivation of the rules used for classification system for visual fields.
Evaluation Set
The Visual Field Steering Committee developed an evaluation set for each of the six expert panelists. It consisted of instructions to the panelists, a set of proposed definitions for 13 types of defects and for severity accompanied by a series of 19 examples thought to correspond to the proposed definitions, a grading form, and a set of sample visual fields from nonstudy patients with AION for analysis.
Using preliminary definitions as modified by the expert panel, an initial classification was made for the 19 visual fields provided in the evaluation set. Many of these fields contained more than one type of pattern defect. The panelists then independently reported the degree to which they agreed with the classification. In addition, the panelists were instructed to categorize the density of the defect as mild, moderate, severe, or absolute. Results are shown in Table 1. Based upon this exercise, additional revisions of the definitions were deemed necessary. Of importance, three separate categories of scotoma were identified: peripheral, paracentral, and central. Also, an admonition was added to the instructions that the category of “other” be utilized only for visual fields that were impossible to fit into a specific category.
Table 1.
Expert panelists’ degree of agreement with classification of 19 examples of visual fields
| Pattern | Excellent | Good | Uncertain | Poor | Bad |
|---|---|---|---|---|---|
| Normal | 83% | 17% | |||
| Absolute defect | 100% | ||||
| Mild diffuse depression | 50% | 17% | 33% | ||
| Severe diffuse depression | 17% | 66% | 17% | ||
| Mild superior altitudinal | 50% | 50% | |||
| Moderate superior and inferior altitudinal | 66% | 17% | 17% | ||
| Severe superior altitudinal | 100% | ||||
| Mild inferior altitudinal | 33% | 67% | |||
| Moderate inferior altitudinal | 17% | 50% | 33% | ||
| Severe inferior altitudinal and moderate superior arcuate | 83% | 17% | |||
| Moderate superior arcuate | 67% | 17% | 17% | ||
| Severe superior and inferior arcuates | 33% | 33% | 17% | 17% | |
| Mild inferior arcuate | 100% | ||||
| Moderate inferior arcuate | 67% | 17% | 17% | ||
| Severe inferior arcuate | 67% | 33% | |||
| Moderate inferior nasal step | 83% | 17% | |||
| Mild paracentral scotoma | 33% | 17% | 50% | ||
| Moderate central scotoma | 67% | 33% | |||
| Severe central scotoma | 50% | 50% |
Training Set
Using the final instructions and forms derived from the evaluation set, the expert panel analyzed 120 masked, representative nonstudy NAION fields. IONDT fields were not utilized for training in order to preserve the integrity of the classification tool. To assess the ability of the panelists to accurately apply the rules, 10 of these fields were duplicates and 10 were example fields from the evaluation set. For 55 of the analyzed fields, at least 83% agreed on the categorization without any collaboration among experts. Agreement on classification of the remaining 65 fields was achieved through a series of four interactive reconciliation meetings of the expert panel, held either by teleconference or face-to-face. These resulted in refinement of the pattern definitions and consensus on interpretation of the fields in the training set. These rules were then programmed into the computerized expert system.
The consensus derived from the training set included identification of 14 different field types, shown in Table 2. Severity was restricted to mild, moderate, or severe. General rules for classification included the following:
If a field is noted as normal or as an absolute defect, no other notations can be made.
A depressed point is defined as equal to or greater than 4-dB loss.
An attempt should be made to classify fields even though they may appear unreliable from the indices.
Severity is based upon subjective judgment. Only the arcuate/altitudinal category can have more than one severity with a separate severity assignable to the arcuate and the altitudinal components.
Table 2.
Types of field defects based on consensus of interpretation
| Type | Definition |
|---|---|
| Normal | No quadrants depressed or only a few points in no specific pattern. One depressed point in a location surrounding the blind spot is normal unless it is part of another defined field defect. |
| Absolute defect | No response (sensitivity = zero) was recorded for all points in all quadrants or if only one point is less than or equal to 9 dB sensitivity and all other points are zero. If the retest is zero, then the point sensitivity is zero. Foveal sensitivity must be equal to zero. |
| Diffuse depression | Entire visual field equally depressed, including fixation as defined as presence of both a superior and an inferior altitudinal defect that are equally depressed and a central scotoma. |
| Superior altitudinal | Upper half of field equally depressed as defined as all points in the superior two quadrants approximately equally depressed, excluding those nasal to the blind spot (ie, points 11 and 19 on the visual field map). Depression should extend down to horizontal meridian including approximate equal involvement of the superior paracentral points (points 21 and 22 on the visual field map). |
| Inferior altitudinal | Lower half of field equally depressed as defined as all points in the inferior two quadrants approximately equally depressed, excluding those nasal to the blind spot (ie, points 27 and 35 on the visual field map). Depression should extend up to horizontal meridian, including approximate equal involvement of the superior paracentral points (points 29 and 30 on the visual field map). |
| Superior arcuate | Peripheral defect (at least four peripheral points must be depressed within one quadrant) that appears in either or both superior quadrants with relative sparing of either one or both of the superior paracentral points, or either one of the superior paracentral points is less depressed in comparison to the superior periphery in either quadrant and it is not a nasal step. Superior periphery is defined as all points in the superior two quadrants except points 21 and 22. |
| Inferior arcuate | Peripheral defect (at least four peripheral points must be depressed within one quadrant) that appears in either or both inferior quadrants with relative sparing of either one or both of the inferior paracentral points, or either one of the inferior paracentral points is less depressed in comparison to the inferior periphery in either quadrant and it is not a nasal step. Inferior periphery is defined as all points in the inferior two quadrants except points 29 and 30. |
| Superior nasal step | An isolated superior nasal quadrant defect which preferentially involves the peripheral points (points 18, 25, and 26) adjacent to the horizontal meridian. Cannot be part of a superior arcuate defect and there cannot be an arcuate defect in the superior temporal quadrant. Superior nasal points adjacent to the vertical meridian (points 3, 8, 15, and 22) are relatively spared. |
| Inferior nasal step | An isolated inferior nasal quadrant defect which preferentially involves the peripheral points (points 33, 34, and 42) adjacent to the horizontal meridian. Cannot be part of an inferior arcuate defect and there cannot be an arcuate defect in the inferior temporal quadrant. Inferior nasal points adjacent to the vertical meridian (points 30, 39, 46, and 51) are relatively spared. |
| Central scotoma | Decreased sensitivity of the fovea by 5 dB relative to the least depressed point in the rest of the field or the foveal sensitivity is less than 10 dB. |
| Paracentral scotoma | Focal depression of the visual field not corresponding to any other pattern and located within the paracentral region (points 20, 21, 22, 28, 29, 30) adjacent to the blind spot, but sparing fixation (ie, no central scotoma). One isolated, depressed paracentral point next to the blind spot (point 20 or 28) is not a paracentral scotoma. If there is a central scotoma and, as defined, a paracentral scotoma, then the defect is categorized as a central scotoma. |
| Superior arcuate/altitudinal | Both superior paracentral points (points 21 and 22) are equally depressed, but the superior periphery is more depressed than the paracentral. Superior paracentral points must differ substantially from the inferior paracentral points (points 29 and 30), ie, no central or paracentral scotoma involving these points. |
| Inferior arcuate/altitudinal | Both inferior paracentral points (points 21 and 22) are equally depressed, but the inferior periphery is more depressed than the paracentral. Inferior paracentral points must differ substantially from the superior paracentral points (points 29 and 30), ie, no central or paracentral scotoma involving these points. |
| Other | Pattern defect that does not fit any of the above definitions, eg, shifted field. Use this category only if you are certain that you cannot categorize the defect using the other 13 categories. |
Validation Set
Having determined the rules for classification, another set of 95 non-IONDT visual fields was sent to the expert panel as a validation set. The classification results from each panelist were determined (Table 3). The agreement obtained from the panel was then compared with the classifications obtained from the computer program. The level of agreement is shown in Table 4. There was a large percentage of internal disagreement among the panelists as to classification of fields within the validation set, despite a common set of rules derived by consensus. In turn, there was disagreement between the panel and the computer program.
Table 3.
Agreement in classification of 95 visual fields among readers
| Amount of agreement | No. of fields | % of total fields |
|---|---|---|
| 6 of 6 readers agree | 7 | 7 |
| 5 of 6 readers agree | 14 | 15 |
| 4 of 6 readers agree | 23 | 24 |
| 3 of 6 readers agree | 22 | 23 |
| 2 of 6 readers agree | 25 | 26 |
| None agree | 4 | 4 |
| Total | 95 | 100 |
Table 4.
Agreement by computer in classification of 66 fields where there was agreement of at least 3 of 6 readers in defect classification
| No. of fields for stated level of agreement between experts | No. of fields for which computer and panel agreed | % of fields for which computer and panel agreed |
|---|---|---|
| In 7 fields where 6 of 6 readers agreed | 7 | 100 |
| In 14 fields where 5 of 6 readers agreed | 10 | 71 |
| In 23 fields where 4 of 6 readers agreed | 16 | 70 |
| In 22 fields where 3 of 6 readers agreed | 16 | 73 |
| Total (in 66 visual fields with agreement) | 50 | 66 |
The inability of the experts to independently agree with each other or the computer is consistent and of the same order of magnitude as reported in the literature for instances for which no gold standard exists.5 This result reinforces the need, within the context of a large clinical trial, to have a computerized system for consistent application of clinically meaningful rules.
Due to the lack of consensus, a second method of validation was performed. In this method, the computer program evaluated all the fields, and the panelists were asked to agree or disagree with the computer results. This method changed the question asked of the experts from “How would you try to apply rules to classify a defect?” to “Does the consistent application of consensus-derived rules applied by the computer result in a classification that is clinically acceptable?”
With the new method of validation, in only four instances was there initial disagreement with the computer by half or more of the panelists, as shown in Table 5. These were determined to be secondary to data entry errors in two instances and due to computer criteria differentiating altitudinal from arcuate defects in two instances. In the latter instances, investigation revealed that further manipulation of the computer algorithm to allow concordance with the panel would result in other classification errors; therefore, these discrepancies were allowed to stand. There was majority agreement between the computer and the expert panel in 91 (94%) of 95 fields, indicating that incorporation of the rules into a computer program unanimously agreed upon by the expert panel was a valid method for analysis of the patterns and severities of the visual field data collected by the IONDT.
Table 5.
Defect pattern agreement between computer and expert panel
| Agree/total | No. of fields | % of fields |
|---|---|---|
| 6/6 | 59 | 62 |
| 5/6 | 24 | 25 |
| 4/6 | 8 | 8 |
| 3/6 | 2 | 2 |
| 2/6 | 2 | 2 |
| Total | 95 | 100 |
The Computer-Based Expert System
Data Entry
Humphrey visual field data consisted of five components: (1) visual field identification (patient number, visit number, and eye examined), (2) reliability indices (fixation losses, false-positives, and false-negatives), (3) foveal sensitivity (dB) and highest point of sensitivity for the whole field (dB), (4) dB loss at each of the 52 data points on the total deviation plot, and (5) short-term fluctuation. For each visual field, the location and dB loss (if any) at each of the 52-point locations is entered into an Excel database on a personal computer (Figure 2). In addition, the foveal sensitivity was recorded, and in instances of diffuse depression, the absolute sensitivity of each point was entered. Although digitized data in the form of floppy disks and flat files were available, changes in computer hardware and software precluded their use in constructing the database. Therefore, the printed visual fields were transcribed into a Microsoft 97/00 Excel compatible database, using double-entry verification.
Figure 2.
Location and decibel loss at each of 52 data points on the total deviation plot.
Software Structure
The computer-based expert system was constructed as a rule-based system on an Excel platform, running under Windows 98, evaluating each field quadrant-by-quadrant. Each rule consisted of a logical statement that could be found true or false, taking the form “if…then.” A truth table was utilized to define specific types of field defects, based upon definitions of the expert panel. Two forms of logical statements were used to identify pattern defects. The first form was based upon average dB loss within a quadrant corresponding to a particular pattern. If no average depression was present, then the number of disturbed points within a quadrant was used to determine the presence of pattern defects. Thus, the numbers of disturbed points were used primarily to find mild defects that were missed by averaging. A listing of the algorithms utilized by the expert system is included in the Appendix.
For instance, if the average dB loss is greater in the periphery than in the central field by 5 dB, then an arcuate defect is present in that quadrant. If the central dB loss is greater by 5 dB than the periphery, then a central or paracentral defect is present. If no pattern defect can be found by averaging, then disturbed point algorithms are used to find mild or smaller pattern defects within a quadrant. A disturbed point is defined as >3-dB loss. If there are a predetermined number of disturbed points within the boundary of a pattern, then the defect is detected. Some pattern defects are determined by the presence or absence of other defects. For example, if there is a superior and an inferior altitudinal defect and a central scotoma, then the pattern is a diffuse depression. If there is a paracentral scotoma and a central scotoma, then there is just a central scotoma. Average dB loss within a pattern defect is used to determine severity, as shown in Table 6. In the instance of determining absolute defect, the expert computer system operator reviews all fields noted to have diffuse depression; then, actual sensitivity rather than relative sensitivity loss is used to determine whether or not the field has an absolute defect.
Table 6.
Determination of severity of field defects, based upon a non-iondt field test analysis
| Severity | n | Average dB loss | 95% CI | Average no. points | 95% CI |
|---|---|---|---|---|---|
| Mild | 1 | 6.2 | 5 | ||
| Moderate | 5 | 18.2 | 12.9–23.5 | 25 | 24.3–25.7 |
| Severe | 17 | 27.43 | 26.3–28.5 | 26 | 25.8–26.2 |
CI, confidence interval; IONDT, Ischemic Optic Neuropathy Decompression Trial.
Calculation of Pattern Changes Between 6-month and Baseline Visits
Longitudinal evaluation of changes in the visual fields for individual patients between baseline and the 6-month follow-up visit was determined by modification of the computerized expert system to correct for short-term fluctuation. The joint short-term fluctuation (SF) was calculated as follows: SF = Sqrt (SFbaseline2 + SF6-month2). The baseline visit HVF sensitivities were not altered. The HVFs from the 6-month visit were altered ±1.96 SF at test loci that differentiate arcuate from altitudinal (points 21 and 22 or points 29 and 30 in Figure 2), central from paracentral defects (foveal sensitivity), and absence of defect from arcuate, altitudinal, central, and paracentral defects. For instance, let us assume an eye has a superior arcuate scotoma at baseline but an altitudinal defect at the 6-month visit. Since relative sparing of paracentral points 21 and 22 for the upper visual field distinguishes an arcuate from an altitudinal defect, the two paracentral points from the 6-month visit were increased in sensitivity by 1.96 times the SF for that field. If the difference in field pattern persisted despite this manipulation, then the follow-up field was determined as “changed.” If the difference in field pattern disappeared due to this manipulation, then the follow-up visual field was determined as “not changed.”
Statistical Methods
Data analysis was carried out using Stata statistical software for Windows (StataCorp 2001, Stata Statistical Software: Release 7.0; Stata Corporation, College Station, Texas). SPSS software for Windows was used to produce some of the tables (SPSS Inc 1998, SPSS: Release 8.0.1; SPSS, Chicago, Illinois). All statistical tests were two-tailed. Techniques included paired and unpaired Student t tests, the Fisher exact test, Kendall tau-b test for association of ordinal variables, Mantel-Haenszel extension test, and Stuart-Maxwell test of marginal homogeneity producing a chi-square test with 2 degrees of freedom, as appropriate.
RESULTS
Baseline Visual Fields
Visual fields were available on 247 randomized eyes, 158 nonrandomized study eyes, 79 fellow eyes with optic neuropathy at baseline, and 326 fellow eyes with no optic neuropathy at baseline (total 810). A total of 13 fields could not be scored for reliability, three fields were determined to be unreliable, and four fields were 30-2 programs for which data were unavailable for analysis. The frequency of unreliable visual fields was similar to the 1% found by Katz and associates,4 using the same CIGTS criteria. Of the remaining reliable fields, 38 had no foveal sensitivity. Baseline visual fields were available for analysis on 229 study eyes randomized to either surgery or careful observation. Baseline visual fields were also available on an additional 147 eyes with vision better than 20/64 followed with careful observation. Fellow eyes were also evaluated at baseline. Of the 376 fellow eyes, 75 were identified at baseline as having optic neuropathy and 301 were identified at baseline as having no optic neuropathy. These data included four 30-2 visual fields for which points not covered by the 24-2 field were not analyzed and 16 Fast-Pac program fields.
Distribution of Field Defect Patterns by Category
The distribution of defect patterns for the various categories of eyes is summarized in Tables 7A and 7B. There was no detectable difference between the frequency of various patterns of field defect comparing eyes randomized to surgery and careful follow-up. Therefore, all randomized eyes are shown as a single group. Differences were observed between the randomized and the observation groups. Central scotomas were much more frequent in the randomized study eyes than in the observation study eyes. The most commonly observed defect in the randomized group of 229 eyes was a superior and inferior arcuate defect with central scotoma in 44 eyes (19.2%), followed by superior arcuate defect and inferior altitudinal defect with central scotoma in 39 eyes (17.0%).
Table 7a.
Categorization of field defects at baseline, by patient group and eye
| Study eye:randomized | Study eye:observation | Fellow eye: with optic neuropathy | Fellow eye: no optic neuropathy | |||||
|---|---|---|---|---|---|---|---|---|
| Pattern | No. | Col % | No. | Col % | No. | Col % | No. | Col % |
| Normal | 1 | 0.4 | 1 | 0.7 | 0 | 0.0 | 47 | 15.6 |
| Superior arcuate isolated | 0 | 0.0 | 3 | 2.0 | 0 | 0.0 | 35 | 11.6 |
| Inferior arcuate isolated | 1 | 0.4 | 16 | 10.9 | 1 | 1.3 | 20 | 6.6 |
| Superior + inferior arcuates | 6 | 2.6 | 45 | 30.6 | 10 | 13.3 | 169 | 56.1 |
| Superior + inferior arcuates +paracentral | 11 | 4.8 | 10 | 6.8 | 2 | 2.7 | 3 | 1.0 |
| Superior + inferior arcuates +central scotoma | 44 | 19.2 | 10 | 6.8 | 6 | 8.0 | 7 | 2.3 |
| Superior arcuate + inferior altitudinal | 3 | 1.3 | 17 | 11.6 | 4 | 5.3 | 1 | 0.3 |
| Superior arcuate + inferior altitudinal + central scotoma | 39 | 17.0 | 8 | 5.4 | 14 | 18.7 | 0 | 0.0 |
| Superior altitudinal + inferior arcuate | 5 | 2.2 | 6 | 4.1 | 8 | 10.7 | 3 | 1.0 |
| Superior altitudinal + inferior arcuate + central scotoma | 19 | 8.3 | 3 | 2.0 | 5 | 6.7 | 1 | 0.3 |
| Superior + inferior altitudinal | 13 | 5.7 | 5 | 3.4 | 3 | 4.0 | 2 | 0.7 |
| Diffuse depression | 30 | 13.1 | 1 | 0.7 | 10 | 13.3 | 1 | 0.3 |
| Absolute defect | 30 | 13.1 | 0 | 0.0 | 6 | 8.0 | 0 | 0.0 |
| Other: isolated defect | 3 | 1.3 | 4 | 2.7 | 0 | 0.0 | 5 | 1.7 |
| Other: 2 or more defects | 24 | 10.5 | 18 | 12.2 | 6 | 8.0 | 7 | 2.3 |
| Total | 229 | 100 | 147 | 100 | 75 | 100 | 301 | 100 |
Table 7b.
Further characterization of field defects at baseline by patient group and eye
| Study eye: randomized | Study eye: observation | Fellow eye: no optic neuropathy | |||||
|---|---|---|---|---|---|---|---|
| Pattern (isolated or in combination) | No. | Col % | No. | Col % | No. | Col % | |
| Any superior defect | No | 73 | 31.9 | 30 | 20.4 | 79 | 26.2 |
| Yes | 156 | 68.1 | 117 | 79.6 | 222 | 73.8 | |
| Any inferior defect | No | 67 | 29.3 | 9 | 6.1 | 92 | 30.6 |
| Yes | 162 | 70.7 | 138 | 93.9 | 209 | 69.4 | |
| Any superior or inferior defect | No | 63 | 27.5 | 5 | 3.4 | 57 | 18.9 |
| Yes | 166 | 72.5 | 142 | 96.6 | 244 | 81.1 | |
| Any scotoma | No | 91 | 39.7 | 99 | 67.3 | 283 | 94.0 |
| Yes | 138 | 60.3 | 48 | 32.7 | 18 | 6.0 | |
| Any diffuse defect | No | 169 | 73.8 | 146 | 99.3 | 296 | 98.3 |
| Yes | 60 | 26.2 | 1 | 0.7 | 5 | 1.7 | |
Central scotoma is part of the definition for both diffuse depression in 30 randomized eyes (13.1%) and absolute defect in 30 randomized eyes (13.1%). Excluding diffuse defects, central or paracentral scotoma was present, either isolated or in combination, in 138 eyes (60.3%) (Table 7B). By comparison, the most commonly observed visual field in the 147 nonrandomized study eyes was a combined superior and inferior arcuate defect in 45 eyes (30.6%) followed by superior arcuate and inferior altitudinal defect in 17 eyes (11.6%) and isolated inferior arcuate defect in 16 eyes (10.9%), none of which included central scotoma. Central or paracentral scotoma, isolated or in combination, occurred in only 48 of the nonrandomized study eyes (32.7%). The difference in distribution is not surprising, because central scotoma includes foveal sensitivity as part of its definition. Another notable difference was that there was only a single instance of diffuse defect for the study observation group.
Fellow eyes with optic neuropathy were more widely distributed regarding the patterns of field loss encountered, possibly because these 75 eyes were not segregated according to visual acuity (Table 7A). Using the established criteria, the computerized expert system identified only 47 (15.6%) of 301 fellow eyes without optic neuropathy as having normal visual fields. The most commonly encountered abnormalities were isolated superior arcuate defects in 35 eyes (11.6%), isolated inferior arcuate defects in 20 eyes (6.6%), and, especially, combined superior and inferior arcuate defects in 169 eyes (56.1%). Severity of 35 superior arcuate defects in nonoptic neuropathy fellow eyes was mild in 33 (94.3%), and severity of 169 combined superior and inferior arcuate defects was mild in 100 eyes (59.2%) (Table 8). A severe defect was included in only 9 (5.3%) of 169 fellow eyes that had superior and inferior arcuate defects with no optic neuropathy. Scotomas were noted in only 18 eyes in this group (6%). Diffuse defects were even more rare, present in only 5 eyes (1.7%) (Table 7B).
Table 8a.
Severity of superior arcuate isolated defects in fellow eyes
| Defect | n | Col % |
|---|---|---|
| Mild only | 33 | 94.3 |
| Moderate only | 2 | 5.7 |
| Total | 35 | 100.0 |
Characteristics of Visual Fields for Late-Entry Randomized Eyes
The patterns of visual field defects for eyes randomized at regular entry (n = 175) were compared to those randomized at late entry due to progression of acuity loss (n = 54). Results are summarized in Table 9. Diffuse depression was present in 27 (15.4%) of the eyes randomized to regular entry, and an absolute defect was present in 24 (13.7%) of these eyes. By comparison, diffuse depression was present in only 3 (5.6%) of the eyes randomized to late entry, and an absolute defect was present in 6 (11.1%) of these eyes. The combination of superior altitudinal and inferior arcuate defects with central scotoma was noted in 11 eyes (6.3%) randomized to regular entry compared to 8 eyes (14.8%) of those randomized in the late-entry group. Although there was a trend for the patterns of defects seen in the regular-entry and late-entry groups to be different, this difference did not reach statistical significance (Fisher’s exact test, P = .078).
Table 9a.
Categorization of field defects at baseline, by randomized entry and eye
| Categorization by time of randomization |
||||
|---|---|---|---|---|
| Randomized regular entry | Randomized late entry | |||
| Pattern | No. | Col % | No. | Col % |
| Normal | 1 | 0.6 | 0 | 0.0 |
| Inferior arcuate isolated | 1 | 0.6 | 0 | 0.0 |
| Superior + inferior arcuate | 2 | 1.1 | 4 | 7.4 |
| Superior + inferior arcuate + paracentral scotoma | 11 | 6.3 | 0 | 0.0 |
| Superior + inferior arcuate + central scotoma | 36 | 20.6 | 8 | 14.8 |
| Superior arcuate + inferior altitudinal | 3 | 1.7 | 0 | 0.0 |
| Superior arcuate + inferior altitudinal + central scotoma | 27 | 15.4 | 12 | 22.2 |
| Superior altitudinal + inferior arcuate | 3 | 1.7 | 2 | 3.7 |
| Superior altitudinal + inferior arcuate + central scotoma | 11 | 6.3 | 8 | 14.8 |
| Superior + inferior altitudinal | 12 | 6.9 | 1 | 1.9 |
| Diffuse depression | 27 | 15.4 | 3 | 5.6 |
| Absolute defect | 24 | 13.7 | 6 | 11.1 |
| Other: isolated defect | 2 | 1.1 | 1 | 1.9 |
| Other: 2 or more defects | 15 | 8.6 | 9 | 16.7 |
| Total | 175 | 100 | 54 | 100 |
Relationship Between Severity of Defect and Global Indices of Visual Function
The severity of field defects for the study eye was compared to global indices of visual field abnormality provided by the HVF. Both the mean deviation and the corrected pattern standard deviation (CPSD) were included in the evaluation (Table 10). Fields may have had more than one defect with differing severity (eg, mild superior arcuate and severe inferior altitudinal). Therefore, visual fields were divided into normal, mild only, mild and moderate, moderate only, mild and severe, mild-moderate and severe, moderate and severe, and severe only. The average mean deviation for randomized study eyes was −21.47, compared to −14.51 for nonrandomized study eyes, and −4.70 for fellow eyes without optic neuropathy. Corrected pattern standard deviation was largest for the observation group, averaging 10.18, compared to 7.03 for the randomized group and 3.50 for the fellow eye group without optic neuropathy. Across all categories, the mean deviations tended to decline with increasing severity of field defect. However, the decline was not monotonic for any of the categories. Corrected pattern standard deviation tended to be small for mild-only and severe-only disease but did not differ systematically for the other categories of severity.
Table 10.
Global indices at baseline by severity of defect and patient group and eye
| Categorization of eyes for baseline paper |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study eye: randomized |
Study eye: observation |
Fellow eye: no optic neuropathy |
|||||||||||||
| Mean deviation |
Corrected pattern SD |
Mean deviation |
Corrected pattern SD |
Mean deviation |
Corrected pattern SD |
||||||||||
| Defect | n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD |
| Normal field | 1 | −0.56 | . | 0.41 | . | 1 | −0.60 | . | 2.55 | 47 | −0.05 | 0.77 | 1.06 | 0.77 | |
| Mild only | 3 | −7.93 | 5.74 | 4.01 | 2.82 | 15 | −3.83 | 2.57 | 3.80 | 2.39 | 162 | −3.19 | 2.26 | 2.54 | 1.65 |
| Mild and moderate | 22 | −9.64 | 4.31 | 8.51 | 3.12 | 23 | −10.08 | 3.93 | 8.53 | 3.22 | 39 | −6.60 | 2.38 | 5.17 | 2.12 |
| Moderate only | 9 | −14.79 | 3.69 | 9.78 | 2.63 | 14 | −9.99 | 4.64 | 9.24 | 3.72 | 35 | −11.39 | 3.96 | 7.39 | 2.42 |
| Mild and severe | 62 | −22.29 | 3.85 | 8.91 | 2.84 | 34 | −19.19 | 4.36 | 11.38 | 3.07 | 9 | −17.16 | 6.01 | 10.05 | 1.97 |
| Mild, moderate, and severe | 53 | −23.69 | 8.51 | 6.06 | 5.10 | 6 | −14.37 | 4.86 | 11.58 | 2.78 | 1 | −5.17 | - | 4.12 | - |
| Moderate and severe | 18 | −14.40 | 6.23 | 11.89 | 2.79 | 34 | −16.15 | 3.79 | 13.53 | 2.36 | 1 | −13.00 | - | 13.11 | - |
| Severe only | 61 | −27.06 | 5.68 | 3.83 | 3.38 | 20 | −20.80 | 7.90 | 9.72 | 5.15 | 3 | −16.24 | 14.59 | 3.39 | 3.52 |
| Total | 229 | −21.47 | 8.22 | 7.03 | 4.41 | 147 | −14.51 | 7.11 | 10.18 | 4.31 | 297* | −4.70 | 4.96 | 3.50 | 2.87 |
*Total is not 301 because four patients have missing severity.
Relationship Between Visual Acuity and Category of Visual Field Defect
Analysis was performed to evaluate the visual acuity at baseline for the various patterns of field defects. For the 374 study eyes (Tables 11A and B), visual acuity was almost equally distributed for visual acuity 20/10 to <20/64 , 133 eyes; 20/64 to <20/200, 108 eyes; and 20/200 or worse, 133 eyes. Visual acuity better than 20/64 was associated with isolated superior arcuate in three eyes (100% of all superior arcuate defects), inferior arcuate defects in 15 eyes (88.2%), combined superior and inferior arcuate defects in 41 eyes (80.4%), and combined superior arcuate with inferior altitudinal defects in 13 eyes (65%). Visual acuity 20/200 or worse was found most frequently for 24 eyes with diffuse depression (77.4%) or 29 eyes with absolute defects (96.7%). Other patterns of field defects, including those with central scotomas, were divided between the 20/64 to 20/200 and the 20/200 and worse categories. Intermediate visual loss of 20/64 to 20/200 was most characteristic of the following visual field patterns: superior and inferior arcuate defects with paracentral scotoma in 10 eyes (50.0%) and central scotoma in 29 eyes (53.7%). For the fellow eye without optic neuropathy (Table 11C), 284 eyes (95.6%) had visual acuity of 20/64 or better. Acuity of 20/64 to 20/200 was noted in eight eyes (2.7%), and acuity worse than 20/200 in four eyes (1.4%).
Table 11a.
Visual acuity at baseline by category of field defect, study eye
| Visual acuity at baseline |
||||||||
|---|---|---|---|---|---|---|---|---|
| 20/10 to <20/64 | 20/64 to <20/200 | 20/200 and worse | total | |||||
| Pattern | n | Row % | n | Row % | n | Row % | n | Row % |
| Normal | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 2 | 100.0 |
| Superior arcuate isolated | 3 | 100.0 | 0 | 0.0 | 0 | 0.0 | 3 | 100.0 |
| Inferior arcuate isolated | 15 | 88.2 | 0 | 0.0 | 2 | 11.8 | 17 | 100.0 |
| Superior + inferior arcuate | 41 | 80.4 | 8 | 15.7 | 2 | 3.9 | 51 | 100.0 |
| Superior + inferior arcuate + paracentral scotoma | 9 | 45.0 | 10 | 50.0 | 1 | 5.0 | 20 | 100.0 |
| Superior + inferior arcuate + central scotoma | 10 | 18.5 | 29 | 53.7 | 15 | 27.8 | 54 | 100.0 |
| Superior arcuate + inferior altitudinal | 13 | 65.0 | 4 | 20.0 | 3 | 15.0 | 20 | 100.0 |
| Superior arcuate + inferior altitudinal + central scotoma | 6 | 13.0 | 18 | 39.1 | 22 | 47.8 | 46 | 100.0 |
| Superior altitudinal + inferior arcuate | 6 | 54.5 | 5 | 45.5 | 0 | 0.0 | 11 | 100.0 |
| Superior altitudinal + inferior arcuate + central scotoma | 3 | 13.6 | 6 | 27.3 | 13 | 59.1 | 22 | 100.0 |
| Superior + inferior altitudinal | 4 | 22.2 | 5 | 27.8 | 9 | 50.0 | 18 | 100.0 |
| Diffuse depression | 1 | 3.2 | 6 | 19.4 | 24 | 77.4 | 31 | 100.0 |
| Absolute defect | 0 | 0.0 | 1 | 3.3 | 29 | 96.7 | 30 | 100.0 |
| Other: isolated defect | 4 | 57.1 | 1 | 14.3 | 2 | 28.6 | 7 | 100.0 |
| Other: 2 or more defects | 17 | 40.5 | 14 | 33.3 | 11 | 26.2 | 42 | 100.0 |
| Total | 133 | 35.6 | 108 | 28.9 | 133 | 35.6 | 374 | 100.0 |
Table 11b.
Visual acuity at baseline by further characterization of field defects, study eye
| Visual acuity at baseline |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20/10 to <20/64 | 20/64 to <20/200 | 20/200 and worse | Total | ||||||
| Pattern (isolated or in combination) | n | Row % | n | Row % | n | Row % | n | Row % | |
| Any superior defect | No | 29 | 28.2 | 15 | 14.6 | 59 | 57.3 | 103 | 100.0 |
| Yes | 111 | 38.5 | 94 | 32.6 | 83 | 28.8 | 288 | 100.0 | |
| Any inferior defect | No | 9 | 11.8 | 11 | 14.5 | 56 | 73.7 | 76 | 100.0 |
| Yes | 133 | 42.0 | 98 | 30.9 | 86 | 27.1 | 317 | 100.0 | |
| Any superior or inferior defect | No | 5 | 7.4 | 8 | 11.8 | 55 | 80.9 | 68 | 100.0 |
| Yes | 137 | 42.2 | 101 | 31.1 | 87 | 26.8 | 325 | 100.0 | |
| Any scotoma | No | 89 | 46.8 | 32 | 16.8 | 69 | 36.3 | 190 | 100.0 |
| Yes | 44 | 23.9 | 76 | 41.3 | 64 | 34.8 | 184 | 100.0 | |
| Any diffuse defect | No | 132 | 42.2 | 101 | 32.3 | 80 | 25.6 | 313 | 100.0 |
| Yes | 1 | 1.6 | 7 | 11.5 | 53 | 86.9 | 61 | 100.0 | |
Table 11c.
Visual acuity at baseline by further characterization of field defects, fellow eye: no optic neuropathy
| Visual acuity at baseline |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20/10 to <20/64 | 20/64 to <20/200 | 20/200 and worse | Total | ||||||
| Pattern (isolated or in combination) | n | Row % | n | Row % | n | Row % | n | Row % | |
| Any superior defect | No | 78 | 98.7 | 0 | 0.0 | 1 | 1.3 | 79 | 100.0 |
| Yes | 219 | 95.2 | 8 | 3.5 | 3 | 1.3 | 230 | 100.0 | |
| Any inferior defect | No | 91 | 98.9 | 0 | 0.0 | 1 | 1.1 | 92 | 100.0 |
| Yes | 210 | 95.0 | 8 | 3.6 | 3 | 1.4 | 221 | 100.0 | |
| Any superior or | No | 56 | 98.2 | 0 | 0.0 | 1 | 1.8 | 57 | 100.0 |
| inferior defect | Yes | 246 | 95.7 | 8 | 3.1 | 3 | 1.2 | 257 | 100.0 |
| Any scotoma | No | 273 | 96.8 | 6 | 2.1 | 3 | 1.1 | 282 | 100.0 |
| Yes | 15 | 83.3 | 2 | 11.1 | 1 | 5.6 | 18 | 100.0 | |
| Any diffuse defect | No | 284 | 96.3 | 8 | 2.7 | 3 | 1.0 | 295 | 100.0 |
| Yes | 4 | 80.0 | 0 | 0.0 | 1 | 20.0 | 5 | 100.0 | |
As shown in Table 12A, for the study eye mild defects were associated with good acuity in 15 of 18 eyes (83.3%), whereas severe defects were associated with good acuity in 81 (28.3%) of 286 eyes. In the fellow eye without optic neuropathy (Table 12B), there were only 14 eyes with severe defects. However, of the 14 cases for which a severe field defect was present, visual acuity was still between 20/10 and 20/64 in 11 (78.6%) and 20/200 or worse in the remainder.
Table 12a.
Visual acuity at baseline by worst defect severity, study eye
| Visual acuity at baseline |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20/10 to <20/64 | 20/64 to <20/200 | 20/200 and worse | Total | ||||||
| Severity | n | Row % | n | Row % | n | Row % | n | Row % | |
| Worst | Normal field | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 2 | 100.0 |
| Mild | 15 | 83.3 | 1 | 5.6 | 2 | 11.1 | 18 | 100.0 | |
| Moderate | 36 | 52.9 | 22 | 32.4 | 10 | 14.7 | 68 | 100.0 | |
| Severe | 81 | 28.3 | 84 | 29.4 | 121 | 42.3 | 286 | 100.0 | |
| Total | 133 | 35.6 | 108 | 28.9 | 133 | 35.6 | 374 | 100.0 | |
Table 12b.
Visual acuity at baseline by worst defect severity, fellow eye: no optic neuropathy
| Visual acuity at baseline |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20/10 to <20/64 | 20/64 to <20/200 | 20/200 and worse | Total | ||||||
| Severity | n | Row % | n | Row % | n | Row % | n | Row % | |
| Worst | Normal field | 47 | 100.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| Mild | 157 | 97.5 | 4 | 2.5 | 0 | 0.0 | 161 | 100.0 | |
| Moderate | 69 | 93.2 | 4 | 5.4 | 1 | 1.4 | 74 | 100.0 | |
| Severe | 11 | 78.6 | 0 | 0.0 | 3 | 21.4 | 14 | 100.0 | |
| Total | 284 | 95.9 | 8 | 2.7 | 4 | 1.4 | 296 | 100.0 | |
Relationship of Age to Pattern and Severity of Visual Field Defect
The relationship of age to the pattern and severity of visual field defect was evaluated for patients over 65 years and compared to those 65 years or under. Results are summarized in Table 13.
Table 13a.
Category of field defect at baseline by risk factor, study eye
| Age |
Hx of 1+ vascular conditions at baseline |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≥65 | > 65 | No | Yes | |||||
| Pattern | n | Col % | n | Col % | n | Col % | n | Col % |
| Normal | 2 | 1.3 | 0 | 0.0 | 1 | 0.6 | 1 | 0.5 |
| Superior arcuate isolated | 2 | 1.3 | 1 | 0.5 | 1 | 0.6 | 2 | 0.9 |
| Inferior arcuate isolated | 11 | 7.1 | 6 | 2.7 | 9 | 5.8 | 8 | 3.6 |
| Superior + inferior arcuate | 28 | 17.9 | 23 | 10.5 | 23 | 14.9 | 28 | 12.6 |
| Superior + inferior arcuate + paracentral scotoma | 7 | 4.5 | 14 | 6.4 | 10 | 6.5 | 11 | 5.0 |
| Superior + inferior arcuate + central scotoma | 21 | 13.5 | 33 | 15.0 | 23 | 14.9 | 31 | 14.0 |
| Superior arcuate + inferior altitudinal | 8 | 5.1 | 12 | 5.5 | 11 | 7.1 | 9 | 4.1 |
| Superior arcuate + inferior altitudinal + central scotoma | 12 | 7.7 | 35 | 15.9 | 17 | 11.0 | 30 | 13.5 |
| Superior altitudinal + inferior arcuate | 5 | 3.2 | 6 | 2.7 | 5 | 3.2 | 6 | 2.7 |
| Superior altitudinal + inferior arcuate + central scotoma | 12 | 7.7 | 10 | 4.5 | 11 | 7.1 | 11 | 5.0 |
| Superior + inferior altitudinal | 3 | 1.9 | 15 | 6.8 | 4 | 2.6 | 14 | 6.3 |
| Diffuse depression | 9 | 5.8 | 22 | 10.0 | 10 | 6.5 | 21 | 9.5 |
| Absolute defect | 7 | 4.5 | 23 | 10.5 | 9 | 5.8 | 21 | 9.5 |
| Other: isolated defect | 6 | 3.8 | 1 | 0.5 | 2 | 1.3 | 5 | 2.3 |
| Other: 2 or more defects | 23 | 14.7 | 19 | 8.6 | 18 | 11.7 | 24 | 10.8 |
| Total | 156 | 100 | 220 | 100 | 154 | 100 | 222 | 100 |
Based on a total of 220 patients aged 65 or over and 156 patients under age 65, combined superior and inferior arcuate defects were the most frequently encountered (n = 28) in the under 65 age group for the study eye (17.9%). They were less common (n = 23) in the 65 or over age group (10.5%). Second most common in the younger age group were the 21 eyes with superior and inferior arcuate defects with central scotoma (13.5%), but this did not differ in frequency much from the 33 eyes in the older age group (15%). The most frequently encountered pattern of defect in the older age group was a superior arcuate and inferior altitudinal defect with central scotoma in 35 eyes (15.9%). Only 12 eyes (7.7%) of the under age 65 group demonstrated a similar defect. As shown in Table 13B, evaluating study eyes based upon the worst severity of field defects encountered within a single visual field demonstrated that only four eyes in patients 65 or over (1.8%) had mild defects compared to 14 eyes in the under 65 group (9%). Conversely, there were 108 eyes (69.2%) in the under age 65 group manifesting severe field defect compared to 180 eyes (81.8%) in the 65 or over age group. This pattern was statistically significant (Kendall’s tau-b = 3.041, P = .002).
Table 13b.
Worst defect severity at baseline by risk factors, study eye
| Age |
Hx of 1+ vascular conditions at baseline |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥65 | > 65 | No | Yes | ||||||
| Severity | n | Col % | n | Col % | n | Col % | n | Col % | |
| Worst | Normal field | 2 | 1.3 | 0 | 0.0 | 1 | 0.6 | 1 | 0.5 |
| Mild | 14 | 9.0 | 4 | 1.8 | 5 | 3.2 | 13 | 5.9 | |
| Moderate | 32 | 20.5 | 36 | 16.4 | 29 | 18.8 | 39 | 17.6 | |
| Severe | 108 | 69.2 | 180 | 81.8 | 119 | 77.3 | 169 | 76.1 | |
| Total | 156 | 100 | 220 | 100 | 154 | 100 | 222 | 100 | |
In the fellow eye without optic neuropathy (Table 13C), a few differences in the frequency of pattern defect were noted related to age. Normal fields were found in 29 eyes (21.6%) in the younger age group compared to 18 eyes in the 65 or over age group (10.8%). The frequency of other types of field defects did not vary based upon the age range in which they fell. Greatest severity at the moderate level (Table 13D) was present in 49 eyes in the older age group (29.7%) but in only 25 eyes in the younger age group (18.9%). The differences in severity of field defects based upon age were statistically significant (Kendall’s tau-b = 3.313, P = .001).
Table 13c.
Category of field defect at baseline by risk factor, fellow eye: optic neuropathy, no optic neuropathy
| Age |
Hx of 1+ vascular conditions at baseline |
|||||||
|---|---|---|---|---|---|---|---|---|
| <65 | > 65 | No | Yes | |||||
| Pattern | n | Col % | n | Col % | n | Col % | n | Col % |
| Normal | 29 | 21.6 | 18 | 10.8 | 25 | 20.7 | 22 | 12.2 |
| Superior arcuate isolated | 15 | 11.2 | 20 | 12.0 | 14 | 11.6 | 21 | 11.7 |
| Inferior arcuate isolated | 12 | 9.0 | 8 | 4.8 | 10 | 8.3 | 10 | 5.6 |
| Superior + inferior arcuate | 67 | 50.0 | 102 | 61.1 | 61 | 50.4 | 108 | 60.0 |
| Superior + inferior arcuate + paracentral scotoma | 0 | 0.0 | 3 | 1.8 | 1 | 0.8 | 2 | 1.1 |
| Superior + inferior arcuate + central scotoma | 2 | 1.5 | 5 | 3.0 | 2 | 1.7 | 5 | 2.8 |
| Superior arcuate + inferior altitudinal | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 1 | 0.6 |
| Superior altitudinal + inferior arcuate | 2 | 1.5 | 1 | 0.6 | 2 | 1.7 | 1 | 0.6 |
| Superior altitudinal + inferior arcuate + central scotoma | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 1 | 0.6 |
| Superior + inferior altitudinal | 0 | 0.0 | 2 | 1.2 | 0 | 0.0 | 2 | 1.1 |
| Diffuse depression | 0 | 0.0 | 1 | 0.6 | 1 | 0.8 | 0 | 0.0 |
| Other: isolated defect | 2 | 1.5 | 3 | 1.8 | 3 | 2.5 | 2 | 1.1 |
| Other: 2 or more defects | 5 | 3.7 | 2 | 1.2 | 2 | 1.7 | 5 | 2.8 |
| Total | 134 | 100 | 167 | 100 | 121 | 100 | 180 | 100 |
Table 13d.
Worst defect severity baseline by risk factors, fellow eye: optic neuropathy, no optic neuropathy
| Age |
Hx of 1+ vascular conditions at baseline |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| <65 | > 65 | No | Yes | ||||||
| Severity | n | Col % | n | Col % | n | Col % | n | Col % | |
| Worst | Normal field | 29 | 22.0 | 18 | 10.9 | 25 | 20.8 | 22 | 12.4 |
| Mild | 74 | 56.1 | 88 | 53.3 | 65 | 54.2 | 97 | 54.8 | |
| Moderate | 25 | 18.9 | 49 | 29.7 | 21 | 17.5 | 53 | 29.9 | |
| Severe | 4 | 3.0 | 10 | 6.1 | 9 | 7.5 | 5 | 2.8 | |
| Total | 132 | 100 | 165 | 100 | 120 | 100 | 177 | 100 | |
Relationship of Vascular Conditions to Pattern and Severity of Visual Field Defect
Several risk factors were evaluated, including history of hypertension, stroke, myocardial infarction, angina, and transient ischemic attack. No differences in pattern were noted among the various vascular risk factors, and the numbers for some categories were small; therefore, these risks were combined. Results are summarized in Table 13.
The effect of vascular conditions at baseline on pattern of visual field in the study eye was assessed (Table 13A). No important differences were found. Patients with vascular conditions at baseline numbered 222, and those without numbered 154. The most commonly encountered defects for eyes with or without vascular conditions were superior and inferior arcuate defects (n = 28 with, 23 without), superior and inferior arcuate defects with central scotoma (n = 31 with, 23 without), and superior arcuate and inferior altitudinal defects with central scotoma (n = 30 with, 17 without). The percentage of total field patterns for any one pattern ranged from 11.0% to 14.9%. In evaluating greatest magnitude of visual field defect (Table 13B), the distribution did not appear to differ between the group with or without vascular conditions at baseline (Kendall’s tau-b = −.367, P = .714). In the vascular category, 169 of 222 eyes (76.1%) had a greatest magnitude of visual field defect that was severe, similar to the same severity group in the nonvascular category of 119 of 154 eyes (77.3%).
The effect of vascular conditions at baseline on pattern of visual field in the nonstudy eye without optic neuropathy was assessed as well (Table 13C). In the vasculopathy group of 180 patients, normal fields were found in 22 (12.2%). In the nonvasculopathy group of 121 patients, normal fields were found in 25 (20.7%). The frequency of other types of field defects did not vary based upon the presence or absence of vasculopathic risk factors. Of the 177 eyes with vascular conditions at baseline, 53 (29.9%) had a greatest magnitude of visual field defect that was moderate. In contrast, of the 120 eyes with no vascular conditions at baseline, 21 (17.5%) had a greatest magnitude of visual field defect that was moderate (Table 13D). In the category of greatest magnitude of field defect of severe, only five eyes (2.8%) were in the vascular condition group compared to nine eyes (7.5%) in the no vascular condition group. However, there was no statistically significant difference in overall distribution of field defect magnitude between the two groups ( Kendall’s tau-b = .095, P = .087).
Longitudinal Follow-up: Comparing 6-Month to Baseline Visual Fields
Overall Description
There were 605 eyes with visual fields performed at both the baseline and the 6-month visit. Of these, 571 had data at both time points and had foveal sensitivity. After unreliable fields were excluded, 559 eyes were available for longitudinal analysis. Of these, 203 (36.3%) were randomized study eyes, 75 (13.4%) were nonrandomized study eyes, 57 (10.2%) were fellow eyes with optic neuropathy, and 224 (40.1%) were fellow eyes without optic neuropathy.
The SFs at baseline and at the 6-month follow-up field were compared. For all 559 eyes, the mean SF at baseline was 2.43 (1.45 SD) compared to 2.27 (1.49 SD) at 6 months. For the randomized eyes, mean SF at baseline was 2.58 (1.80 SD), and at 6 months it was 2.57 (1.78 SD). For the nonrandomized study eyes, the mean SF was 2.56 (1.24 SD) at baseline and 2.60 (1.35 SD) at 6 months. For fellow eyes without optic neuropathy, the mean SF at baseline was 2.16 (0.89 SD) and at 6 months, 1.81 (0.78 SD).
Visual Field Changes Between 6-Month and Baseline Visits for Randomized Study Eyes
In evaluating the 203 randomized study eyes, the pattern of defect in the superior and inferior visual fields did not change significantly between baseline and 6-month follow-up (Table 14A). For superior visual fields, the Stuart-Maxwell test of marginal homogeneity had a chi-square of 3.40, P = .18; for inferior visual fields the chi-square was 0.88, P = .64. A statistically significant change in central field was noted for randomized eyes with chi-square of 11.43, P = .003. However, there was not a consistent direction of change. For instance, of 160 central field defects at baseline, 29 (18.1%) changed to neither a central nor a paracentral defect and 14 (8.8%) changed to paracentral defects. Of 28 eyes with neither central nor paracentral defects at baseline, 14 (50.0%) developed central defects.
Table 14a.
Frequency of visual field defects at baseline and at 6-month follow-up: randomized eyes
| Superior visual field |
Inferior visual field |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6-month follow-up field | 6-month follow-up field | ||||||||
| Baseline field | No defect | Arcuate | Altitudinal | Total | Baseline field | No defect | Arcuate | Altitudinal | Total |
| No defect (n) | 3 | 9 | 1 | 13 | No defect (n) | 3 | 3 | 1 | 7 |
| Row % | 23.08 | 69.23 | 7.69 | 100 | Row % | 42.86 | 42.86 | 14.29 | 100 |
| Column % | 27.27 | 9.38 | 1.04 | 6.40 | Column % | 42.86 | 3.37 | 0.93 | 3.45 |
| Arcuate | 8 | 83 | 10 | 101 | Arcuate | 3 | 70 | 11 | 84 |
| Row % | 7.92 | 82.18 | 9.90 | 100 | Row % | 3.57 | 83.33 | 13.10 | 100 |
| Column % | 72.73 | 86.46 | 10.42 | 49.75 | Column % | 42.86 | 78.65 | 10.28 | 41.38 |
| Altitudinal | 0 | 4 | 85 | 89 | Altitudinal | 1 | 16 | 95 | 112 |
| Row % | 0.00 | 4.49 | 95.51 | 100 | Row % | 0.89 | 14.29 | 84.82 | 100 |
| Column % | 0.00 | 4.17 | 88.54 | 43.84 | Column % | 14.29 | 17.98 | 88.79 | 55.17 |
| Total | 11 | 96 | 96 | 203 | Total | 7 | 89 | 107 | 203 |
| Row % | 5.42 | 47.29 | 47.29 | 100 | Row % | 3.45 | 43.84 | 52.71 | 100 |
| Column % | 100 | 100 | 100 | 100 | Column % | 100 | 100 | 100 | 100 |
| Marginal homogeneity (Stuart-Maxwell) | chi-square | df | P | Marginal homogeneity (Stuart-Maxwell) | chi-square | df | P | ||
| 3.40 | 2 | .18 | 0.88 | 2 | .64 | ||||
For those randomized eyes that maintained the same pattern of defect at 6 months and at baseline, the severity of defect was compared (Table 15A). Superior arcuate defect severity did not vary (t = −0.098, P = .92) in the interval with a mean loss for 83 eyes of 14.71 dB (7.86 SD) at baseline and of 14.63 dB (8.01 SD) at 6 months. However, superior altitudinal defects for 85 eyes did improve significantly (t = −2.24, P = .028).
Table 15a.
Severity of visual field defect at baseline and 6 months, for eyes with same defect at both visits: randomized eyes
| Superior arcuate | Inferior arcuate | ||||||||
| Examination | n | mean | SE | SD | Examination | n | mean | SE | SD |
| 6 month | 83 | 14.63 | 0.88 | 8.01 | 6 month | 70 | 19.20 | 1.01 | 8.46 |
| baseline | 83 | 14.71 | 0.86 | 7.86 | baseline | 70 | 18.42 | 1.03 | 8.66 |
| difference | 83 | −0.79 | 0.81 | 7.38 | difference | 70 | 0.78 | 0.72 | 6.00 |
| Paired t test | t | P | Paired t test | t | P | ||||
| −0.098 | .92 | 1.09 | .28 | ||||||
| Superior altitudinal | Inferior altitudinal | ||||||||
| Examination | n | mean | SE | SD | Examination | n | mean | SE | SD |
| 6 month | 85 | 25.49 | 0.62 | 5.67 | 6 month | 95 | 27.85 | 0.44 | 4.28 |
| baseline | 85 | 26.63 | 0.55 | 5.03 | baseline | 95 | 28.84 | 0.34 | 3.35 |
| difference | 85 | −1.14 | 0.51 | 4.72 | difference | 95 | −0.99 | 0.38 | 3.68 |
| Paired t test | t | P | Paired t test | t | P | ||||
| −2.24 | .028 | −2.63 | .01 | ||||||
| Paracentral scotoma | Central scotoma | ||||||||
| Examination | n | mean | SE | SD | Examination | n | mean | SE | SD |
| 6 month | 9 | 16.72 | 2.17 | 6.51 | 6 month | 117 | 5.37 | 0.79 | 8.56 |
| baseline | 9 | 18.81 | 1.54 | 4.62 | baseline | 117 | 6.17 | 0.77 | 8.34 |
| difference | 9 | −2.09 | 1.99 | 5.97 | difference | 117 | −0.80 | 0.97 | 10.54 |
| Paired t test | t | P | Paired t test | t | P | ||||
| −1.05 | .32 | −0.82 | .41 | ||||||
Inferior field changes mirrored the superior field changes. For inferior arcuate defects of 70 eyes, the mean depression at baseline was 18.42 dB (8.66 SD) compared to a mean sensitivity loss of 19.20 (8.26 SD) at 6 months. The difference was not significant (t = 1.09, P = .28). For inferior altitudinal defects, the baseline mean sensitivity loss was 28.84 dB (3.35 SD) compared to a 6-month sensitivity loss of 27.84 (4.28.SD). The mean difference of −.99 (3.68 SD) was statistically significant (t = −2.63, P = .01). Paracentral visual field defects did not differ in severity between baseline and 6 months for randomized study eyes (t = −1.05, P = .32), nor did central defects (t = −0.82, P = .41). Of nine paracentral scotomas, the mean depression was 18.81 dB (4.62 SD) at baseline compared to 16.72 (6.51 SD) at 6 months. Of 117 central scotomas, the mean depression was 6.17 dB (8.34 SD) at baseline compared to 5.37 (8.56 SD) at 6 months.
Visual Field Changes Between 6-Month and Baseline Visits for Nonrandomized Study Eyes
In evaluating the 75 nonrandomized study eyes, the pattern of defect in the superior and inferior visual fields did not change significantly between baseline and 6-month follow-up (Table 14B). For superior visual fields, the Stuart-Maxwell test of marginal homogeneity had a chi-square of 0.55, P =.76; for inferior visual fields the chi-square was 3.67, P = .16. A statistically significant change in central field was noted for nonrandomized eyes with chi-square of 6.03, P = .05. The numbers within each cell of the 3×3 table were too small to consider the direction of change.
Table 14b.
Frequency of visual field defects at baseline and at 6-month follow-up: nonrandomized eyes
| Superior visual field |
Inferior visual field |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6-month follow-up field | 6-month follow-up field | ||||||||
| Vaseline field | No defect | Arcuate | Altitudinal | Total | Baseline field | No defect | Arcuate | Altitudinal | Total |
| No defect (n) | 8 | 5 | 2 | 15 | No defect (n) | 3 | 0 | 0 | 3 |
| Row % | 53.33 | 33.33 | 13.33 | 100 | Row % | 100.00 | 0.00 | 0.00 | 100 |
| Column % | 44.44 | 10.87 | 18.18 | 20.00 | Column % | 50.00 | 0.00 | 0.00 | 4.00 |
| Arcuate | 9 | 36 | 3 | 48 | Arcuate | 3 | 51 | 4 | 58 |
| Row % | 18.75 | 75.00 | 6.25 | 100 | Row % | 5.17 | 87.93 | 6.90 | 100 |
| Column % | 50.00 | 78.26 | 27.27 | 64.00 | Column % | 50.00 | 96.23 | 25.00 | 77.33 |
| Altitudinal | 1 | 5 | 6 | 12 | Altitudinal | 0 | 2 | 12 | 14 |
| Row % | 8.33 | 41.67 | 50.00 | 100 | Row % | 0.00 | 14.29 | 85.71 | 100 |
| Column % | 5.56 | 10.87 | 54.55 | 16.00 | Column % | 0.00 | 3.77 | 75.00 | 18.67 |
| Total | 18 | 46 | 11 | 75 | Total | 6 | 53 | 16 | 75 |
| Row % | 24.00 | 61.33 | 14.67 | 100 | Row % | 8.00 | 70.67 | 21.33 | 100 |
| Column % | 100 | 100 | 100 | 100 | Column % | 100 | 100 | 100 | 100 |
| Marginal homogeneity (Stuart-Maxwell) | chi-square | df | P | Marginal homogeneity (Stuart-Maxwell) | chi-square | df | P | ||
| 0.55 | 2 | .76 | 3.67 | 2 | .16 | ||||
For those nonrandomized eyes that maintained the same pattern of defect at 6 months and at baseline, the severity of defect was compared (Table 15B). Superior arcuate defect severity worsened (t = 2.98, P = .005) in the interval with a mean loss for 36 eyes at baseline of 11.85 dB (6.73 SD) and at 6 months of 15.68 dB (8.05 SD). However, superior altitudinal defects did not change significantly (t = −2.07, P = .093), with a mean at baseline of 23.89 dB (7.75 SD) and at 6 months of 22.02 dB (7.63 SD). Inferior altitudinal defects improved (t = −2.83, P = .017). For inferior arcuate defects of 51 eyes, the mean depression at baseline was 18.29 dB (7.81 SD) compared to a mean sensitivity loss of 18.41 (7.97 SD) at 6 months. The difference was not significant (t = 0.13, P = .90). For inferior altitudinal defects, the baseline mean sensitivity loss for 12 eyes was 26.69 dB (5.50 SD) compared to a 6-month sensitivity loss of 25.13 (5.89 SD). The mean difference of −1.57 (1.92 SD) was statistically significant (P = −2.83, P = .016). Paracentral visual field defects improved in severity between baseline and 6 months for 10 nonrandomized study eyes (t = −2.40, P = .04), but 4 central defects did not (t = 1.98, P = .14). Of 10 paracentral scotomas, the mean depression was 14.55 dB (7.08 SD) at baseline compared to 12.98 (6.95 SD) at 6 months. Of four central scotomas the mean depression was 12.25 dB (14.43 SD) at baseline compared to 21.00 (12.06 SD) at 6 months.
Table 15b.
Severity of visual field defect at baseline and 6 months, for eyes with same defect at both visits: nonrandomized eyes
| Superior arcuate | Inferior arcuate | ||||||||
| Examination | n | mean | SE | SD | Examination | n | mean | SE | SD |
| 6 month | 36 | 15.68 | 1.34 | 8.05 | 6 month | 51 | 18.41 | 1.12 | 7.97 |
| Baseline | 36 | 11.85 | 1.12 | 6.73 | Baseline | 51 | 18.29 | 1.09 | 7.81 |
| Difference | 36 | 3.83 | 1.28 | 7.7 | Difference | 51 | 0.12 | 1.00 | 7.12 |
| Paired t test | t | P | Paired t test | t | P | ||||
| 2.98 | 0.005 | 0.13 | 0.90 | ||||||
| Superior altitudinal | Inferior altitudinal | ||||||||
| Examination | n | mean | SE | SD | Examination | n | mean | SE | SD |
| 6 month | 6 | 22.02 | 3.11 | 7.63 | 6 month | 12 | 25.13 | 1.70 | 5.89 |
| Baseline | 6 | 23.89 | 3.17 | 7.75 | Baseline | 12 | 26.69 | 1.59 | 5.50 |
| Difference | 6 | −1.87 | 0.9 | 2.21 | Difference | 12 | −1.57 | 0.55 | 1.92 |
| Paired t test | t | P | Paired t test | t | P | ||||
| −2.07 | 0.093 | −2.83 | 0.016 | ||||||
| Paracentral scotoma | Central scotoma | ||||||||
| Examination | n | mean | SE | SD | Examination | n | mean | SE | SD |
| 6 month | 10 | 12.98 | 2.2 | 6.95 | 6 month | 4 | 21 | 6.03 | 12.06 |
| Baseline | 10 | 14.55 | 2.24 | 7.08 | Baseline | 4 | 12.25 | 7.22 | 14.43 |
| Difference | 10 | −1.57 | 0.65 | 2.06 | Difference | 4 | 8.75 | 4.42 | 8.85 |
| Paired t test | t | P | Paired t test | t | P | ||||
| −2.4 | 0.04 | 1.98 | 0.14 | ||||||
Visual Field Changes Between 6-Month and Baseline Visits for Fellow Eyes Without Optic Neuropathy
In evaluating the 224 fellow eyes without optic neuropathy, there was a significant change in pattern of defect in the superior and inferior visual fields between baseline and 6-month follow-up (Table 14C). For superior visual fields, the Stuart-Maxwell test of marginal homogeneity had a chi-square of 27.02, P < .0001; for inferior visual fields the chi-square was 27.60, P < .0001. Central fields were too few to be analyzed. The direction of change for both upper and lower fields was primarily that of an arcuate defect at baseline changing to no defect at the 6-month visit, seen for 27.4% of 164 eyes with a superior arucate defect at baseline and for 29.9% of 154 eyes with an inferior arcuate defect at baseline.
Table 14c.
Frequency of visual field defects at baseline and at 6-month follow-up: fellow eyes without optic neuropathy
| Superior visual field |
Inferior visual field |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6-month follow-up field | 6-month follow-up field | ||||||||||
| Baseline field | No defect | Arcuate | Altitudinal | Other | Total | Baseline field | No defect | Arcuate | Altitudinal | Other | Total |
| No defect (n) | 44 | 8 | 0 | 1 | 53 | No defect (n) | 51 | 9 | 1 | 3 | 64 |
| Row % | 83.02 | 15.09 | 0.00 | 1.89 | 100 | Row % | 79.69 | 14.06 | 1.56 | 4.69 | 100 |
| Column % | 48.89 | 6.45 | 0.00 | 16.67 | 23.66 | Column % | 51.00 | 7.89 | 25.00 | 50.00 | 28.57 |
| Arcuate | 45 | 113 | 1 | 5 | 164 | Arcuate | 46 | 104 | 1 | 3 | 154 |
| Row % | 27.44 | 68.9 | 0.61 | 3.05 | 100 | Row % | 29.87 | 67.53 | 0.65 | 1.95 | 100 |
| Column % | 50 | 91.13 | 25.00 | 83.33 | 73.21 | Column % | 46.00 | 91.23 | 25.00 | 50.00 | 68.75 |
| Altitudinal | 0 | 0 | 3 | 0 | 3 | Altitudinal | 0 | 0 | 2 | 0 | 2 |
| Row % | 0.00 | 0.00 | 100.00 | 0.00 | 100 | Row % | 0.00 | 0.00 | 100.00 | 0.00 | 100 |
| Column % | 0.00 | 0.00 | 75.00 | 0.00 | 1.34 | Column % | 0.00 | 0.00 | 50.00 | 0.00 | 0.89 |
| Other defect | 1 | 3 | 0 | 0 | 4 | Other defect | 3 | 1 | 0 | 0 | 4 |
| Row % | 25 | 75 | 0.00 | 0.00 | 100 | Row % | 75.00 | 25.00 | 0.00 | 0.00 | 100 |
| Column % | 1.11 | 2.42 | 0.00 | 0.00 | 1.79 | Column % | 3.00 | 0.88 | 0.00 | 0.00 | 1.79 |
| Total | 90 | 124 | 4 | 6 | 224 | Total | 100 | 114 | 4 | 6 | 224 |
| Row % | 40.18 | 55.36 | 1.79 | 2.68 | 100 | Row % | 44.64 | 50.89 | 1.79 | 2.68 | 100 |
| Column % | 100 | 100 | 100 | 100 | 100 | Column % | 100 | 100 | 100 | 100 | 100 |
| Marginal homogeneity (Stuart-Maxwell) | chi-square | df | P | Marginal homogeneity(Stuart-Maxwell) | chi-square | df | P | ||||
| 27.02 | 3 | <.0001 | 27.6 | 3 | <.0001 | ||||||
For those fellow eyes that maintained the same pattern of defect at 6 months and at baseline, the severity of defect was compared (Table 15C). Superior arcuate defect severity improved (t = −4.08, P = .0001) in the interval with a mean loss for 113 eyes at baseline of 9.63 dB (5.98 SD) and at 6 months of 7.68 dB (5.13 SD). For inferior arcuate defects of 104 eyes, the mean depression at baseline was 9.66 dB (5.85 SD) compared to a mean sensitivity loss of 7.24 (5.36 SD) at 6 months. The difference was highly significant (t = −4.78, P < .0001). Altitudinal defects, paracentral scotomas, and central scotomas were few.
Table 15c.
Severity of visual field defect at baseline and 6 months, for eyes with same defect at both visits: fellow eyes without optic neuropathy
| Superior arcuate | Inferior arcuate | ||||||||
| Examination | n | mean | SE | SD | Examination | n | mean | SE | SD |
| 6 month | 113 | 7.68 | 0.48 | 5.13 | 6 month | 104 | 7.24 | 0.53 | 5.36 |
| Baseline | 113 | 9.63 | 0.56 | 5.98 | Baseline | 104 | 9.66 | 0.57 | 5.85 |
| Difference | 113 | −1.95 | 0.48 | 5.08 | Difference | 104 | −2.42 | 0.51 | 5.16 |
| Paired t test | t | P | Paired t test | t | P | ||||
| −4.08 | <.0001 | −4.78 | <.0001 | ||||||
| Superior altitudinal | Inferior altitudinal | ||||||||
| Examination | n | mean | SE | SD | Examination | n | mean | SE | SD |
| 6 month | 3 | 8.31 | 3.44 | 5.96 | 6 month | 2 | 9.48 | 3.66 | 5.18 |
| Baseline | 3 | 9.99 | 1.45 | 2.51 | Baseline | 2 | 9.87 | 1.97 | 2.79 |
| Difference | 3 | −1.68 | 2.19 | 3.79 | Difference | 2 | −0.39 | 1.69 | 2.39 |
| Paracentral scotoma | Central scotoma | ||||||||
| Examination | n | mean | Examination | n | mean | SE | SD | ||
| 6 month | 1 | 11.17 | 6 month | 2 | 23.5 | 0.5 | 0.71 | ||
| Baseline | 1 | 11.33 | Baseline | 2 | 14.5 | 1.5 | 2.12 | ||
| Difference | 1 | −0.17 | Difference | 2 | 9 | 2 | 2.83 | ||
Comparison of Careful Follow-up and Surgery Treatments for Randomized Patients
Visual Field Patterns
The pattern of defects in the superior visual fields for the study eyes randomized to careful follow-up showed no difference between baseline and the 6-month visit using the Stuart-Maxwell test of marginal homogeneity (χ2 df = 1.43, P = .49). Similarly, the pattern of defects in the superior fields for the study eyes randomized to surgery showed no difference between baseline and the 6-month visit (χ2 df = 2.57, P = .28). However, seven of the eight patients who received surgery in their study eye and who had no superior defect at baseline developed either an arcuate or an altitudinal defect at 6 months. Table 16 compares the proportion of eyes that declined, stayed the same, and improved, wherein improvement is defined as changing from an altitudinal defect to an arcuate defect and decline is defined as the opposite. Using the Mantel-Haenzel extension test (M-H), no treatment effect was noted when comparing the pattern changes for the superior field described for careful follow-up and for surgery to each other (χ2 = 0.49, P = .48).
Table 16.
Comparison of the proportion of eyes that declined, stayed the same, and improved: careful follow-up versus surgery
| Status | Careful follow-up | Surgery | Odds | 95% ci | |
|---|---|---|---|---|---|
| Superior portion of the eye* | |||||
| Decline | 8 | 12 | 1.50† | 0.61 | 3.67 |
| Same | 87 | 84 | 0.97† | 0.72 | 1.30 |
| Improve | 6 | 6 | 1.00† | 0.32 | 3.10 |
| Inferior portion of the eye‡ | |||||
| Decline | 8 | 7 | 0.88† | 0.32 | 2.41 |
| Same | 81 | 87 | 1.07† | 0.79 | 1.45 |
| Improve | 12 | 8 | 0.67† | 0.27 | 1.63 |
| Central portion of the eye§ | |||||
| Decline | 10 | 8 | 0.80† | 0.32 | 2.03 |
| Same | 70 | 69 | 0.99† | 0.71 | 1.37 |
| Improve | 21 | 25 | 1.19† | 0.67 | 2.13 |
*Extension of M-H test: chi square, 1 df = 0.49, P = .48.
†For surgery group compared with careful follow-up.
‡Extension of M-H test: chi square, 1 df = 0.26 , P = .61.
§Extension of M-H test: chi square, 1 df = 0.57, P = .45.
The pattern of defects in the inferior visual fields for the study eyes randomized to careful follow-up showed no difference between baseline and the 6-month visit (χ2 = 1.47, P = .48), and neither did the study eyes randomized to surgery (χ2 = 0.70, P = .71). As shown in Table 16, no treatment effect was noted when comparing the pattern changes for the inferior field described for careful follow-up and for surgery to each other, (M-H test,χ2 = 0.26, P = .61).
There was no significant shift in the patterns of central defects assigned to careful follow-up (χ2 = 4.19, P = .12), whereas there was a significant shift for study eyes assigned to surgery (χ2 = 7.41, P = .025), with 24.51% of eyes improving and only 7.84% of eyes worsening. On the other hand, comparing the patterns of change in central visual fields of patients in the careful follow-up and surgical treatment groups, no treatment difference was noted, wherein improvement in Table 16 is defined as a change from a central to a paracentral defect and decline as the opposite (M-H test, χ2 = 0.57, P = .45).
Visual Field Severity
For those visual fields that showed no change in pattern of defect over time, the severity of visual field loss at baseline and at the 6-month visit were compared using Student’s t tests (Table 17). In the careful follow-up group, only inferior altitudinal defects showed a significant change in mean defect, with an improvement of −1.13 dB (P = .02). In the surgery group, only the superior altitudinal defects showed a significant change in mean defect, with an improvement of −1.69 dB (P = .007). It is worth noting, however, that all mean differences between the baseline and the 6-month visit in the careful follow-up group showed some improvement, whereas in the surgery group the superior arcuate and inferior arcuate mean severity of defect actually worsened slightly. However, in evaluating the changes in severity between the two groups (Table 18), the differences were not significant.
Table 17.
Comparison of changes in severity of visual fields for various defects, for eyes with same defect at both visits
| Baseline | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Defect type | n | Mean | Se | Mean | Se | Mean diff* | P value | |
| Careful follow-up group | ||||||||
| Superior arcuate | 41 | 16.62 | 1.21 | 15.14 | 1.24 | −1.48 | 0.2 | |
| Superior altitudinal | 44 | 25.59 | 0.95 | 24.96 | 0.95 | −0.63 | 0.44 | |
| Inferior arcuate | 33 | 18.94 | 1.53 | 18.50 | 1.59 | −0.44 | 0.57 | |
| Inferior altitudinal | 46 | 28.44 | 0.58 | 27.31 | 0.65 | −1.13 | 0.02 | |
| Paracentral | 3 | 22.33 | 2.62 | 20.83 | 4.02 | −1.50 | 0.76 | |
| Central | 62 | 5.89 | 1.04 | 5.68 | 1.08 | −0.21 | 0.88 | |
| Surgery group | ||||||||
| Superior arcuate | 42 | 12.84 | 1.18 | 14.13 | 1.26 | 1.29 | 0.26 | |
| Superior altitudinal | 41 | 27.75 | 0.43 | 26.06 | 0.76 | −1.69 | 0.007 | |
| Inferior arcuate | 37 | 17.95 | 1.42 | 19.83 | 1.30 | 1.88 | 0.11 | |
| Inferior altitudinal | 49 | 29.21 | 0.39 | 28.35 | 0.59 | −0.86 | 0.15 | |
| Paracentral | 6 | 17.06 | 1.57 | 14.67 | 2.35 | −2.39 | 0.37 | |
| Central | 55 | 6.49 | 1.15 | 5.02 | 1.17 | −1.47 | 0.30 | |
* Mean difference = mean at 6 months minus mean at baseline.
Table 18.
Comparison of differences in severity of visual field defects, for eyes with same defect at both visits: careful follow-up versus surgery
| Careful follow-up |
Surgery |
|||||||
|---|---|---|---|---|---|---|---|---|
| Defect type | n | Mean | Sd | n | Mean | Sd | Difference* | P value |
| Superior arcuate defects | 41 | −1.48 | 7.25 | 42 | 1.29 | 7.38 | −2.78 | 0.09 |
| Superior altitudinal defects | 44 | −0.63 | 5.43 | 41 | −1.69 | 3.79 | 1.06 | 0.30 |
| Inferior arcuate defects | 33 | −0.44 | 4.46 | 37 | 1.88 | 7.00 | −2.32 | 0.11 |
| Inferior altitudinal defects | 46 | −1.13 | 3.24 | 49 | −0.86 | 4.09 | −0.27 | 0.72 |
| Paracentral scotomas | 3 | −1.50 | 7.42 | 6 | −2.39 | 5.89 | 0.89 | 0.85 |
| Central scotomas | 62 | −0.21 | 10.71 | 55 | −1.47 | 10.39 | 1.26 | 0.52 |
*Difference is baseline to 6-month change for careful follow-up minus baseline to 6-month change for surgery.
DISCUSSION
Discussion of Methods
Automated perimetry facilitates the collection of quantitative data on the pattern and severity of visual field defects. A standard for automated interpretation of these defects requires development of decision criteria. There are three evaluations of importance in the interpretation of visual fields—detection, progression, and characterization. Difficulties in detection relate primarily to distinguishing appropriately between short-term and long-term fluctuation. This problem is further compounded in various disease states, such as glaucoma, wherein the pathologic process itself produces fluctuation in sensitivity.8 The Ocular Hypertension Treatment Study, wherein multiple confirmation fields were required to diagnose the presence or absence of a defect, provides an example of a method to deal with detection of visual field defects.9
Progression of field defects is a common end point for glaucoma studies. The issue, once again, is determining change, but from an abnormal as opposed to a normal baseline. Katz10 has reviewed scoring methods employed by two multicenter clinical trials, the Advanced Glaucoma Intervention Study and the CIGTS. These studies utilize a cumulative score (0 to 20), based on depression of adjacent points occurring within specified regions of the visual field. Depression is defined by total deviation plot on the HVF printout in the Advanced Glaucoma Intervention Study and by probability values in the CIGTS.
More advanced models of visual field perturbations have also been investigated. De la Rosa and colleagues11 utilized an approach for rapid assessment of glaucomatous field defects based on multiple correlations. McNaught and coworkers12 developed a linear model of pointwise sensitivity values against time to identify progression in normal tension glaucoma. By any of these methods, detection and progression can be determined operationally, based upon the sensitivity and reliability to be required in any particular study.
In contrast to detection and progression of visual field defects, characterization is a more complex task. It requires pattern recognition open to multiple interpretations, depending upon “lumping” versus “splitting” biases, as well as conformity to established biases formed from nonrigorous clinical observations. In one of the few clinical trials to utilize pattern recognition as an outcome for visual field testing, the Optic Neuritis Treatment Trial established 15 monocular types of field defects (14 local and diffuse) of three different severities occurring in optic neuritis.13 After an initial review by the Director and Associate Director of the Visual Field Reading Center separately, they then reviewed them together to “reach a consensus on the final classification for each visual field.” Initial agreement was noted for 76.3% of the HVF, 81.5% on the location, and 74% on the shape. Complete agreement in every category was achieved in only 47.4% of the 309 affected eyes. In a masked retest, the shapes were in agreement in 76.2% of the 42 cases.3,13
Neural networks have been touted as providing a means for allowing computers to “learn” how to correctly categorize visual fields, even in the absence of specified rules. In the supervised class of artificial neural networks, the systems require a training set of “correctly” categorized visual fields to allow learning to occur.14–17 Thus, there is a tautology in that, in the absence of rules, how is such a training set derived? Henson and associates18 suggest that unsupervised neural networks can be used to resolve this dilemma, as they are self-classifying. However, the patterns correspond to the number of nodes used in the neural network and do not necessarily correspond to clinically identified field defects.
As demonstrated by the experts impaneled on the VFAC for the IONDT, rule-based criteria are not easily divined. There is considerable interobserver as well as intraobserver variability, even when there is agreement on the rules for decision making. The diagnostic variability in this study is similar to performance of humans and computers in validations of other expert systems, ranging from 50% to 70%.5–7 Important in the present study, experts recognize that more than one interpretation is possible for a given distribution of disturbed points on a visual field, so that discarding a computerized determination as inconsistent with clinical interpretation is an easier end point upon which to achieve consensus than independently derived agreement. Once incorporated into an expert computer system, the criteria for categorizing the pattern and severity of visual field defects are, according to Hirsbrunner and colleagues,19 “explicit, obvious, and standardized.” Such attributes are essential within the context of prospective clinical trials.
One of the potential limitations of a highly deterministic computerized expert system is that it may not allow for changes in pattern that occur solely on the basis of short-term fluctuation. We addressed this issue of pattern changes over time by determining the joint SF for visual fields of an individual at baseline and 6-month visits. The ±2 SF was then applied to those individual test points in the visual field that determined the pattern of defect.
Pattern of Visual Loss
Visual field defects are noted in 63% to 100% of NAION patients.20–23 The classic presentation of NAION often involves the sudden loss of lower or, less commonly, upper visual field. Central scotomas, arcuate defects, and quadrantic defects may also occur.24,25 Repka and colleagues24 tabulated the location and type of visual field defect by Goldmann perimetry and found that 46% of NAION patients had an inferior altitudinal defect and 20% had isolated central scotomas. Hayreh and Podhajsky25 reported inferior nasal or inferior altitudinal defects in 57% of NAION patients and central scotoma in 25%. Traustason and colleagues26 quantitatively classified field defects performed by Octopus perimetry and found that, although 55% of AION patients demonstrated a significant altitudinal field loss, the “spared” hemifields routinely showed some loss of sensitivity.
In the present study, a pure field defect confined to the upper or lower hemisphere was relatively unusual. This is consistent with prior observations that automated perimetry frequently demonstrates defects, even in asymptomatic hemifields.26 The clinical perception that field involvement is primarily altitudinal is likely derived from differential severity of involvement in the upper and lower hemifield and by ignoring the presence of central scotoma. In the present study, field defects were evaluated separately as to pattern and severity rather than together. The simultaneous evaluation of 12 identified types of defects with three levels of severity would result in insufficient data points for meaningful analysis.
Comparability of Data for Eyes Randomized to Surgery or to Careful Follow-up
The complexity of the data set, the interaction between disease state and test reliability, and the effects of learning on responses impact visual field data. Consistency and comparability at the baseline visit are important for classifying the visual fields seen with the onset of NAION, for interpreting subsequent changes, and for assessing the status of the fellow eye. Within the group randomized either to surgery or to careful follow-up, there was no difference noted in either the pattern or the severity of visual field defects. Also, there were no differences within the randomized group of eyes related to regular versus late entry (progressive NAION). Thus, any changes in subsequent visual field examinations can be reasonably attributed to treatment effect.
Differences Between the Randomized and Careful Observation Groups
The distribution of field defects in the nonrandomized patients, having visual acuity better than 20/64, demonstrates a pattern of defect substantially different from randomized eyes. The frequency and density of central scotomas are markedly reduced in this observational group. Because decreased foveal sensitivity is a major criterion in the expert system definition of central scotoma, a poor central acuity is likely to be associated with proportional decrease in foveal sensitivity. The rarity of diffuse depression of the visual field in the observational group is explained by the necessity of having equal loss of sensitivity throughout the visual field. Spared foveal sensitivity in eyes with better acuity precludes designation as diffuse depression except when the depression is mild.
Effect of Systemic Processes
Numerous studies have identified vasculopathic, anatomic, and pharmacologic risk factors associated with the onset of AION.27–29 In the IONDT, diabetes was significantly associated with NAION in the fellow eye.30 Also, prior evaluation of the IONDT cohort based upon visual acuity demonstrated that poorer acuity was associated with diabetes and hypertension. In a previous study of visual field loss in AION, diabetics were especially prone to severe diffuse depression.26 However, the results described in the present study showed that no differences were encountered in pattern or severity of pattern defect for the study eye related to systemic processes. Younger patients did tend to have milder defects and less involvement of the central visual field.
Comparison of Pattern Defects and Severity With Global Indices
Global measures of visual field function such as the mean deviation and the CPSD offer information regarding the average severity and localization of visual field loss, respectively. They are frequently utilized in prospective studies, because they are simple, quantitative measures that lend themselves to an analysis analogous to visual acuity. In the Ocular Hypertension Treatment Study, visual field end points included an abnormal CPSD at the P < .05 level.31,32 The mean deviation was utilized as a secondary outcome measure in reporting the results of the IONDT.2 CPSD was not evaluated initially but has been utilized as a measure of uneven distribution of disturbed points within the visual field.
In this study, the mean deviation was more depressed in the randomized than in the careful observation group. Thus, patients with worse visual acuity loss also tended to have more overall visual field disturbance. However, the mean deviation did not necessarily progress with progressively worse categories of field depression within specific patterns of defect. For instance, for the randomized eyes, the “moderate only” category had about the same mean deviation as the ”moderate and severe category.” Therefore, mean deviation may not be a good overall indicator of severity of specific field defects.
The CPSD did not correspond to the overall severity of field defects encountered, especially for the randomized eyes. The CPSD was highest when multiple defects with differing severities were encountered. The frequency of diffuse field defects in the randomized eyes was likely responsible for the lower CPSD noted in that group compared with the observation group. Thus, CPSD was, as might be expected, more an indicator of nonhomogeneity within the visual fields than of severity per se.
The inconsistent results for both mean deviation and for CPSD related to the severity of pattern defects suggest that such global measures have limited value for comparing visual fields in a longitudinal study.
Relationship of Visual Field Defects to Visual Acuity
In interpreting the relationship between visual fields and visual acuity, it is important to keep in mind that depressed visual acuity and the presence of a central scotoma are likely to be highly correlated, but are not always coincident. Each is determined through different testing methods, based upon different psychophysical principles. Furthermore, with many defects splitting or otherwise only partially affecting foveal function, scanning strategies used by patients for assessment of acuity might differ from fixation strategies (eg, eccentric fixation) used for assessment of visual fields. Thus, some patients with good acuity had a central scotoma, and other patients with poor acuity had no central scotoma.
As expected, the presence of a central scotoma as part of the overall visual field pattern corresponded with loss of visual acuity. Poor acuity was almost routinely observed for those fields with diffuse or absolute field defects. Classic arcuate and altitudinal defects were found primarily in eyes with good acuity. However, in about one fourth of cases, severe peripheral field defects were detected in eyes with relatively preserved acuity. The results do suggest, however, that visual acuity may be a useful, though coarse, surrogate for overall severity of visual field involvement in AION.
Late Entry
Early studies promoting the benefit of optic nerve sheath fenestration for the treatment of NAION suggested that patients with a progressive course might be better candidates for the procedure than those who showed no progression.33 As defined by the IONDT, the late entry group constituted a subset of the randomized patients whose visual acuity in the study eye was better than 20/64 at baseline but lost acuity to below this level within 30 days. The late entry group did not benefit from optic nerve sheath fenestration, based upon visual acuity. That progressive AION is no different from the more common, nonprogressive form is supported by the similarity in pattern and pattern-specific severity between these two subgroups of randomized patients.
The Fellow Eye
Two retrospective studies have compared the visual fields in patients with bilateral NAION, but with conflicting conclusions. WuDunn and associates34 found that the pattern of visual field loss in the second eye correlated poorly with that of the first eye. They also reported that mean deviation tended to be less in the second eye that in the first eye for older patients. On the other hand, Boone and associates35 found that in 75% of 16 patients with bilateral NAION, the mean deviation on HVF testing did not differ by more than 5 dB. There were 75 fellow eyes with optic neuropathy included in the cohort. However, a direct comparison of visual field involvement in the study eye and fellow eye with optic neuropathy for the same patient was not performed. This controversy in the literature will be addressed in a future analysis of IONDT data.
One surprising result of this study was the high percentage of abnormal patterns of visual fields in eyes without optic neuropathy. This finding was also reflected in the depressed mean deviation for the group. The vast majority of anomalous patterns were mild superior or inferior arcuate defects. These may have been artifactual, owing to learning effects, or secondary to non-optic nerve–related eye disease that differed from the age-matched normal population included in the HVF normative data set. Artifacts and learning effects should tend to normalize in subsequent visual field examinations, whereas field defects related to other eye diseases should be stable or progress. Analysis of the results at 6 months suggests that almost 30% of defects present at baseline normalize in follow-up; furthermore, the remaining 113 superior and 104 inferior arcuate defects become significantly less severe.
The possibility that “normal” HVFs are being classified as “abnormal” by a flawed computerized expert system should be considered. However, these criteria were set and the software algorithms validated by the expert panel. At a minimum, these criteria were consistent with the appearance of statistically significant pattern deviations being present on the HVF printout, based upon age-matched normal controls. Therefore, if the range of normal in the expert analysis is inaccurate, the HVF normal values would similarly have to be considered as inaccurate. An explanation based upon truncation of “tails” in the distribution of normal values provided by the manufacturer would not explain the frequency with which HVF sensitivities were depressed in fellow eyes.
In clinical practice, mild defects that do not correspond with clinical impressions based upon other parameters (eg, disk appearance, media opacity, retinal findings) are common and often discounted. The design of the expert system utilized in the study did not include such additional information in the analysis. Molino and associates,5 in their validation of a knowledge-based expert system, emphasize the impact of such “hidden” information.
The possibility exists, however, that some of the mild and more severe field defects are real and, therefore, suggestive of a subclinical form of NAION. This impression is supported by the preservation of good visual acuity even in those few eyes with more advanced field defects, so media-related diffuse depression is less likely. Low-tension glaucoma is known to produce visual field defects similar to those found in NAION. However, the classification of the fellow eye without optic neuropathy suggests that none of these eyes had typical disk changes associated with any alternative disease process.
Since second episodes of NAION in the same eye are rare,36 one important test of subclinical disease would be a lower frequency of clinically apparent NAION in those fellow eyes having visual field defects than in those that do not. Such an assessment would require follow-up beyond the scope of the currently available data set. If validated in future studies, the presence of subclinical NAION might support a hypothesis that the crowding associated with a disk at risk is capable of producing minimal, clinically unapparent, acute or chronic axonal loss. Such subclinical infarction might, in turn, provide more space for remaining axons and associated vascular supply, thereby precluding profound infarction of the nerve head.
Changes in Visual Fields Between 6-Month Visit and Baseline Visit
Quantitative assessment of longitudinal changes in automated visual fields is complex. For example, Katz and colleagues4 compared the proportion of glaucoma visual fields identified as progressing, using the methods of the Advanced Glaucoma Intervention Study, the CIGTS, and the Early Manifest Glaucoma Treatment study on 67 eyes of 56 patients followed with Humphrey 30–2 visual fields. Rate of progression was 11% for the Advanced Glaucoma Intervention Study, 22% for the CIGTS, and 23% for the Early Manifest Glaucoma Treatment study methods. Even for the comparable rates of progression of the CIGTS and Early Manifest Glaucoma Treatment study, the patients identified were the same only 50% of the time.
In the Optic Neuritis Treatment, 383 subjects underwent Humphrey 30–2 visual field testing. Sensitivities of each of 76 test locations at 6 months were compared with those at baseline, using the normal fellow eye sensitivity at each location as a control for learning effect.37 The percentage improvement was calculated for each test point. Fields were then divided into concentric rings with radii of 3, 9, 15, 21, and 27 degrees from fixation, and the average improvement was computed for each eccentricity. There was no attempt to compare patterns or changes within patterns.
Little is published regarding the natural history of visual field changes in NAION. Arnold and Hepler38 performed acute and convalescent visual fields in 27 patients with NAION, using the Octopus perimeter program 32. Mean visual field performance worsened in 22.2% of visual fields and improved in 24% of visual fields. Of 21 “stable” patients, 31.6% showed late improvement in visual field. The IONDT previously reported no change in mean deviation of visual fields at 6 months between surgical and careful follow-up groups.2
The pattern of visual loss does not change very much in individuals over time. One shift in distribution for randomized eyes seems to be the appearance of arcuate and altitudinal defects in areas that had no defect at baseline. This pattern shift did not alter the overall frequency of defects sufficient to reach statistical significance. The superior visual field of the nonrandomized study eyes showed a similar tendency for appearance of new arcuate and altitudinal defects. At the same time, of the 12 superior altitudinal defects, five changed to arcuate defects and one resolved by 6 months. Onset of new visual field defects may have been the result of progressive disease associated with increasing ischemia. Improvement in pattern of field may have reflected recovery of visual functions in areas of borderline ischemia or a training effect, such as that identified for the fellow eyes without optic neuropathy.
For visual fields of randomized and nonrandomized study eyes that did not change in pattern, there was a significant improvement in the severity of inferior altitudinal defects, as well as significant improvement in superior altitudinal defect for randomized patients only. One may speculate whether the eyes showing improvement of 3 lines or more in visual acuity, noted in 42.7% of patients with AION,2 also showed improvement in field severity; but testing this association was beyond the scope of the current study.
No change in pattern of field defect was found, based upon treatment category for randomized patients. In both the surgical and the careful follow-up groups there was a trend toward visual field recovery at 6 months compared to baseline. This reached statistical significance for inferior altitudinal defects in the careful follow-up group and for superior altitudinal defects in the surgery group. However, in looking at the overall changes in severity by type of defect (Table 18), no differences were noted between the treatment groups. The trend for superior and inferior arcuate defects to improve with careful follow-up and to progress with surgery did not reach statistical significance (P= .09 and .11).
Overall, the data suggested that changes over 6 months in the pattern and severity of visual field defects found in NAION were small. Furthermore, the effect of surgical intervention, if any, is probably toward less improvement over time rather than more. This conclusion is not dissimilar from the findings reported for visual acuity.2
SUMMARY
The IONDT developed a rule-based expert system capable of consistently defining valid patterns and severity of visual field defects encountered in NAION, essential for the purposes of a multicenter prospective clinical trial. Expansion of this rule-based model to account for short-term fluctuations facilitated meaningful interpretation of change over time in individual patients.
Baseline visual fields obtained from the IONDT demonstrated comparability between eyes randomized to surgery or to careful follow-up. This result supported the validity of testing visual field outcomes between treatment groups in the longitudinal analysis. The visual fields in nonrandomized eyes with better than 20/64 visual acuity were less likely to demonstrate diffuse depression and central scotomas, compared with randomized eyes with worse acuity. Classic arcuate and altitudinal defects were also more common in these eyes. The severity and pattern of visual field defects did not vary in relationship to vasculopathic risk factors, but eyes of younger patients had milder defects than older patients.
There was no constant relationship between global indices of HVF performance (mean deviation and CPSD) and the pattern or severity of visual field defects. Thus, such indices were not utilized in the longitudinal analysis. On the other hand, more severe and more diffuse visual field defects were associated with poor acuity, suggesting that acuity may be a reasonable surrogate for visual field defects due to NAION.
One surprising finding was that fellow eyes without known optic neuropathy frequently demonstrated mild, arcuate field defects. Although testing and other artifactual causes must be excluded, subclinical NAION may exist in some fellow eyes. If so, longitudinal studies should demonstrate persistence of the defect and, perhaps, protection from future clinical involvement with NAION. If not, longitudinal studies will demonstrate that these defects disappear or become highly variable. Further studies utilizing the full 5-year dataset of the IONDT are planned.
Table 8b.
Severity of superior and inferior arcuate defects in fellow eyes
| Defect | n | Col % |
|---|---|---|
| Mild only | 100 | 59.2 |
| Mild and moderate | 29 | 17.2 |
| Moderate only | 31 | 18.3 |
| Mild and severe | 7 | 4.1 |
| Moderate and severe | 1 | 0.6 |
| Severe only | 1 | 0.6 |
| Total | 169 | 100.0 |
Table 9b.
Further characterization of field defects at baseline by randomized entry and eye
| Categorization by time of randomization |
|||||
|---|---|---|---|---|---|
| Randomized regular entry | Randomized late entry | ||||
| Pattern (isolated or in combination) | No. | Col % | No. | Col % | |
| Any superior defect | No | 58 | 31.7 | 15 | 26.8 |
| Yes | 125 | 68.3 | 41 | 73.2 | |
| Any inferior defect | No | 57 | 31.1 | 10 | 17.9 |
| Yes | 126 | 68.9 | 46 | 82.1 | |
| Any superior or inferior defect | No | 54 | 29.5 | 9 | 16.1 |
| Yes | 129 | 70.5 | 47 | 83.9 | |
| Any scotoma | No | 73 | 41.7 | 18 | 33.3 |
| Yes | 102 | 58.3 | 36 | 66.7 | |
| Any diffuse defect | No | 124 | 70.9 | 45 | 83.3 |
| Yes | 51 | 29.1 | 9 | 16.7 | |
APPENDIX. EXPERT SYSTEM SOFTWARE ALGORITHMS
The ultimate defect is coded as a 0 (Mot present) or 1 (Present). Defect severity is not classified as Severe, Moderate or Mild in this program. Instead, a series of variables, each starting with “Pt” (e.g. PtSup_Arc, PtInf_Arc, and PtSup_Alt) are used to store the average dB loss over the relevant area for that defect. The thresholds associated with classifications of Severe, Moderate, and Mild are noted as comments.
First define several variables that are used later to classify the defects
gen Quad1_Avg_loss = (pt1+pt2+pt5+pt6+pt7+pt11+pt12+pt13+pt14+pt19+pt20+pt21)/12
gen Quad2_Avg_loss = (pt3+pt4+pt8+pt9+pt10+pt15+pt16+pt17+pt18+pt22+pt23+pt24+pt25+pt26)/14
gen Quad3_Avg_loss = (pt27+pt28+pt29+pt35+pt36+pt37+pt38+pt43+pt44+pt45+pt49+pt50)/12
gen Quad4_Avg_loss = (pt30+pt31+pt32+pt33+pt34+pt39+pt40+pt41+pt42+pt46+pt47+pt48+pt51+pt52)/14
gen Total_Avg_Quad_loss = (pt1+pt2+pt5+pt6+pt7+pt11+pt12+pt13+pt14+pt19+pt20+pt21+pt3+pt4+pt8+pt9+pt10+pt15+pt16+pt17+pt18+pt22 +pt23+pt24+pt25+pt26+pt27+pt28+pt29+pt35+pt36+pt37+pt38+pt43+pt44+pt45+pt49+pt50+pt30+pt31+pt32+pt33 +pt34+pt39+pt40+pt41+pt42+pt46+pt47+pt48+pt51+pt52)/52 gen AvgParaCenLoss = (pt20+pt21+pt22+pt28+pt29+pt30)/6
gen str9 Quad1 = “”
gen str9 Quad2 = “”
gen str9 Quad3 = “”
gen str9 Quad4 = “”
replace Quad1 = “Depressed” if (pt1>3 & pt2>3 & pt5>3 & pt6>3 & pt7>3 & pt12>3 & pt13>3 & pt14>3 & pt20>3 & pt21>3)
replace Quad2 = “Depressed” if (pt3>3 & pt4>3 & pt8>3 & pt9>3 & pt10>3 & pt15>3 & pt16>3 & pt17>3 & pt18>3 & pt22>3 & pt23>3 & pt24>3 & pt25>3 & pt26>3)
replace Quad4 = “Depressed” if (pt30>3 & pt31>3 & pt32>3 & pt33>3 & pt34>3 & pt39>3 & pt40>3 & pt41>3 & pt42>3 & pt46>3 &pt47>3 & pt48>3 & pt51>3 & pt52>3)
replace Quad3 = “Depressed” if (pt28>3 & pt29>3 & pt36>3 & pt37>3 & pt38>3 & pt43>3 & pt44>3 & pt45>3 & pt49>3 & pt50>3)
gen Peripheral1 = (pt1+pt2+pt5+pt6+pt7+pt11+pt12+pt13+pt14+pt19+pt20)/11
gen Peripheral2 = (pt3+pt4+pt8+pt9+pt10+pt15+pt16+pt17+pt18+pt23+pt24+pt25+pt26)/13
gen Peripheral3 = (pt27+pt28+pt35+pt36+pt37+pt38+pt43+pt44+pt45+pt49+pt50)/11
gen Peripheral4 = (pt31+pt32+pt33+pt34+pt39+pt40+pt41+pt42+pt46+pt47+pt48+pt51+pt52)/13
gen AvgSupPeriphery = (Peripheral1 + Peripheral2)/2
gen AvgInfPeriphery = (Peripheral3 + Peripheral4)/2
gen Quad1_Diff = Peripheral1 - pt21
gen Quad2_Diff = Peripheral2 - pt22
gen Quad3_Diff = Peripheral3 - pt29
gen Quad4_Diff = Peripheral4 - pt30
gen str9 Quad1_Sup_Arc = “”
gen str9 Quad2_Sup_Arc = “”
gen str9 Quad3_Inf_Arc = “”
gen str9 Quad4_Inf_Arc = “”
replace Quad1_Sup_Arc = “Sup.Arc.” if Quad1_Diff >=5
replace Quad2_Sup_Arc = “Sup.Arc.” if Quad2_Diff >=5
replace Quad3_Inf_Arc = “Inf.Arc.” if Quad3_Diff >=5
replace Quad4_Inf_Arc = “Inf.Arc.” if Quad4_Diff >=5
gen str9 SupBiquadArc = “”
gen str9 InfBiquadArc = “”
replace SupBiquadArc = “SupBiquadArc” if (Quad1_Sup_Arc==”Sup.Arc.” & Quad2_Sup_Arc==”Sup.Arc.”)
replace InfBiquadArc = “InfBiquadArc” if (Quad3_Inf_Arc==”Inf.Arc.” & Quad4_Inf_Arc==”Inf.Arc.”)
gen str9 DepQuad1 = “”
gen str9 DepQuad2 = “”
gen str9 DepQuad4 = “”
gen str9 DepQuad3 = “”
replace DepQuad1 = “SupBiquadArc” if (Quad1==”Depressed” & Quad2_Sup_Arc==”Sup.Arc.” & Quad1_Sup_Arc==””)
replace DepQuad2 = “SupBiquadArc” if (Quad2==”Depressed” & Quad1_Sup_Arc==”Sup.Arc.” & Quad2_Sup_Arc==””)
replace DepQuad3 = “InfBiquadArc” if (Quad3==”Depressed” & Quad4_Inf_Arc==”Inf.Arc.” & Quad3_Inf_Arc==””)
replace DepQuad4 = “InfBiquadArc” if (Quad4==”Depressed” & Quad3_Inf_Arc==”Inf.Arc.” & Quad4_Inf_Arc==””)
gen Quad1_Per_Scot = (pt1+pt2+pt5+pt6+pt7+pt11+pt12+pt13+pt14+pt19)/10
gen Quad3_Per_Scot = (pt27+pt35+pt36+pt37+pt38+pt43+pt44+pt45+pt49+pt50)/10
gen Quad1_Paracent = pt21
gen Quad3_Paracent = pt29
gen Quad1_Diff_Paracentral = Quad1_Per_Scot - Quad1_Paracent
gen Quad3_Diff_Paracentral = Quad3_Per_Scot - Quad3_Paracent
gen Sup_Avg_loss = (Quad1_Avg_loss + Quad2_Avg_loss)/2
gen Inf_Avg_loss = (Quad3_Avg_loss + Quad4_Avg_loss)/2
gen Sup_Quad_Diff = abs(Quad1_Avg_loss - Quad2_Avg_loss)
gen Inf_Quad_Diff = abs(Quad3_Avg_loss - Quad4_Avg_loss)
gen Sup_Inf_Diff = abs(Sup_Avg_loss - Inf_Avg_loss)
gen Sup_Nasal_Pts = (pt18+pt25+pt26)/3
gen Inf_Nasal_Pts = (pt33+pt34+pt42)/3
gen SupNasQuad = (pt4+pt9+pt10+pt16+pt17+pt18+pt23+pt24+pt25+pt26)/10
gen InfNasQuad = (pt52+pt47+pt48+pt40+pt41+pt42+pt31+pt32+pt33+pt34)/10
gen SupNasVert = (pt3+pt8+pt15+pt22)/4
gen InfNasVert = (pt51+pt46+pt39+pt30)/4
gen SupNasDiff = SupNasQuad - SupNasVert
gen InfNasDiff = InfNasQuad - InfNasVert
gen InfNasDefect = 0
gen SupNasDefect = 0
replace InfNasDefect =1 if (pt30<4 & pt31<4 & pt32<4 & pt39<4 & pt40<4 & pt41<4 & pt46<4 & pt47<4 & pt48<4 & pt51<4 & pt52<4 & pt33>=4 & pt34>=4 & pt42>=4)
replace SupNasDefect =1 if (pt3<4 & pt4<4 & pt8<4 & pt9<4 & pt10<4 & pt15<4 & pt16<4 & pt17<4 & pt22<4 & pt23<4 & pt24<4 & pt18>=4 & pt25>=4 & pt26>=4)
gen PtsAboveHorz = (pt20+pt21+pt22+pt23+pt24+pt25+pt26)/7
gen PtsBelowHorz = (pt28+pt29+pt30+pt31+pt32+pt33+pt34)/7
gen SupPtsRemainder = (pt12+pt13+pt14+pt15+pt16+pt17+pt18)/7
gen InfPtsRemainder = (pt36+pt37+pt38+pt39+pt40+pt41+pt42)/7
gen SupShiftDiff = PtsAboveHorz - SupPtsRemainder
gen InfShiftDiff = PtsBelowHorz - InfPtsRemainder
gen AboveBelowDiff = abs(PtsAboveHorz - PtsBelowHorz)
gen Sup_ParaPt_Diff = abs(pt21-pt22)
gen Inf_ParaPt_Diff = abs(pt29-pt30)
gen TempParacenDiff = abs(pt29-pt21)
gen NasalParacenDiff = abs(pt30-pt22)
/*
Test for Central Scotoma and Altitudinal defects before finalizing definitions for disturbed points and Paracentral Scotoma
gen CentScot = 0
gen Fovea_Diff = (Foveal_Sens - Point_Sens)
replace CentScot = 1 if (Fovea_Diff < -5 | Foveal_Sens < 10)
Altitudinal Defects
gen Sup_Alt=0
gen Inf_Alt=0
replace Sup_Alt = 1 if (Quad1==”Depressed” & Quad2==”Depressed” & Quad1_Diff<5 & Quad2_Diff<5 & Sup_Quad_Diff <=11.4 & Sup_ParaPt_Diff<=13)
replace Inf_Alt = 1 if (Quad3==”Depressed” & Quad4==”Depressed” & Quad3_Diff<5 & Quad4_Diff<5 & Inf_Quad_Diff <=11.4 & Inf_ParaPt_Diff<=13)
Paracentral Scotoma Elements
gen str9 Quad1_ParaScot = “”
gen str9 Quad2_ParaScot = “”
gen str9 Quad3_ParaScot = “”
gen str9 Quad4_ParaScot = “”
replace Quad1_ParaScot = “ParaScot” if (Quad1_Diff_Paracentral <= -5 & CentScot==0 & Sup_Alt==0)
replace Quad2_ParaScot = “ParaScot” if (Quad2_Diff<=-5 & CentScot==0 & Sup_Alt==0)
replace Quad3_ParaScot = “ParaScot” if (Quad3_Diff_Paracentral <=-5 & CentScot==0 & Inf_Alt==0)
replace Quad4_ParaScot = “ParaScot” if (Quad4_Diff<=-5 & CentScot==0 & Inf_Alt==0)
Disturbed Points Definitions
gen Quad1_Arcuate_DistPt = 0
gen Quad2_Arcuate_DistPt = 0
gen Quad3_Arcuate_DistPt = 0
gen Quad4_Arcuate_DistPt = 0
gen ParaCentral_DistPt = 0
Order of variables in file is as follows:
pt1 pt2 pt5 pt6 pt7 pt11 pt12 pt13 pt14 pt19 pt20 pt21
pt3 pt4 pt8 pt9 pt10 pt15 pt16 pt17 pt18 pt22 pt23 pt24 pt25 pt26
pt27 pt28 pt29 pt35 pt36 pt37 pt38 pt43 pt44 pt45 pt49 pt50
pt30 pt31 pt32 pt33 pt34 pt39 pt40 pt41 pt42 pt46 pt47 pt48 pt51 pt52
Number of points in each quadrant that are disturbed, excluding the central point. Point is considered disturbed if dB >= 4
for var pt1-pt20: replace Quad1_Arcuate_DistPt = Quad1_Arcuate_DistPt+1 if X >= 4
for var pt3-pt18 pt23-pt26: replace Quad2_Arcuate_DistPt = Quad2_Arcuate_DistPt+1 if X >= 4
for var pt27-pt28 pt35-pt50: replace Quad3_Arcuate_DistPt = Quad3_Arcuate_DistPt+1 if X >= 4
for var pt31-pt52: replace Quad4_Arcuate_DistPt = Quad4_Arcuate_DistPt+1 if X >= 4
for var pt20 pt21 pt22 pt28 pt29 pt30:replace ParaCentral_DistPt = ParaCentral_DistPt +1 if X >= 4
gen str9 Quad1_DistPt_SupArc = “”
gen str9 Quad2_DistPt_SupArc = “”
gen str9 Quad3_DistPt_InfArc = “”
gen str9 Quad4_DistPt_InfArc = “”
replace Quad1_DistPt_SupArc = “Sup.Arc.” if (Quad1_Arcuate_DistPt>=4 & Sup_Alt==0 & Quad1_Sup_Arc==”” & Quad2_Sup_Arc==””)
replace Quad2_DistPt_SupArc = “Sup.Arc.” if (Quad2_Arcuate_DistPt>=4 & Sup_Alt==0 & Quad1_Sup_Arc==”” & Quad2_Sup_Arc==””)
replace Quad3_DistPt_InfArc = “Inf.Arc.” if (Quad3_Arcuate_DistPt>=4 & Inf_Alt==0 & Quad3_Inf_Arc==”” & Quad4_Inf_Arc==””)
replace Quad4_DistPt_InfArc = “Inf.Arc.” if (Quad4_Arcuate_DistPt>=4 & Inf_Alt==0 & Quad3_Inf_Arc==”” & Quad4_Inf_Arc==””)
gen str9 DistPt_SupBiquadArc = “”
gen str9 DistPt_InfBiquadArc = “”
replace DistPt_SupBiquadArc = “SupBiquadArc” if (Quad1_DistPt_SupArc==”Sup.Arc.” & Quad2_DistPt_SupArc==”Sup.Arc.”)
replace DistPt_InfBiquadArc = “InfBiquadArc” if (Quad3_DistPt_InfArc==”Inf.Arc.” & Quad4_DistPt_InfArc==”Inf.Arc.”)
gen str9 DistPt_ParaScot = “”
replace DistPt_ParaScot = “ParaScot” if (ParaCentral_DistPt>=3 & Quad1_ParaScot==”” & Quad2_ParaScot==”” & Quad3_ParaScot==”” & Quad4_ParaScot==”” & CentScot==0 & Inf_Alt==0 & Sup_Alt==0 & Quad1_Sup_Arc==”” & Quad2_Sup_Arc==”” & Quad3_Inf_Arc==”” & Quad4_Inf_Arc==”” & Quad1_DistPt_SupArc==”” & Quad2_DistPt_SupArc==”” & Quad3_DistPt_InfArc==””& Quad4_DistPt_InfArc==””)
ArcAlt Defects, including defect based on disturbed points
The separate disturbed point ArcAlt variables are needed for severity calculations
gen Inf_arcalt = 0
gen Sup_arcalt = 0
gen DistPt_SuperiorArcAlt = 0
gen DistPt_InferiorArcAlt = 0
replace Sup_arcalt = 1 if (Quad1_Sup_Arc==”Sup.Arc.” & Quad2_Sup_Arc==”Sup.Arc.” & pt21>=4 & pt22>=4 & Sup_ParaPt_Diff<=5 & NasalParacenDiff>=5 & TempParacenDiff>=5 & Quad1_ParaScot==”” & Quad2_ParaScot==”” & Quad3_ParaScot==”” & Quad4_ParaScot==”” & CentScot==0)
replace Inf_arcalt = 1 if (Quad3_Inf_Arc==”Inf.Arc.” & Quad4_Inf_Arc==”Inf.Arc.” & pt29>=4 & pt30>=4 & Inf_ParaPt_Diff<=5 & NasalParacenDiff>=5 & TempParacenDiff>=5 & Quad1_ParaScot==”” & Quad2_ParaScot==”” & Quad3_ParaScot==”” & Quad4_ParaScot==”” & CentScot==0)
replace DistPt_SuperiorArcAlt = 1 if (Quad1_DistPt_SupArc==”Sup.Arc.” & Quad2_DistPt_SupArc==”Sup.Arc.” & pt21>=4 & pt22>=4 & Sup_ParaPt_Diff<=5 & NasalParacenDiff>=5 & TempParacenDiff>=5 & Quad1_ParaScot==”” & Quad2_ParaScot==”” & Quad3_ParaScot==”” & Quad4_ParaScot==”” & CentScot==0)
replace DistPt_InferiorArcAlt = 1 if (Quad3_DistPt_InfArc==”Inf.Arc.” & Quad4_DistPt_InfArc==”Inf.Arc.” & pt29>=4 & pt30>=4 & Inf_ParaPt_Diff<=5 & NasalParacenDiff>=5 & TempParacenDiff>=5 & Quad1_ParaScot==”” & Quad2_ParaScot==”” & Quad3_ParaScot==”” & Quad4_ParaScot==”” & CentScot==0)
replace Sup_arcalt = 1 if DistPt_SuperiorArcAlt == 1
replace Inf_arcalt = 1 if DistPt_InferiorArcAlt == 1
Arcuate Defects. Checks for BiQuad Arcs included in VF program are not included here since they are redundant after checking for individual arcs
gen Sup_Arc = 0
gen Inf_Arc = 0
replace Sup_Arc =1 if (Quad1_Sup_Arc==”Sup.Arc.”| Quad2_Sup_Arc==”Sup.Arc.” | Quad1_DistPt_SupArc==”Sup.Arc.” | Quad2_DistPt_SupArc==”Sup.Arc.” | DepQuad1==”SupBiquadArc” | DepQuad2==”SupBiquadArc”)
replace Inf_Arc =1 if (Quad3_Inf_Arc==”Inf.Arc.” | Quad4_Inf_Arc==”Inf.Arc.” | Quad3_DistPt_InfArc==”Inf.Arc.” | Quad4_DistPt_InfArc==”Inf.Arc.” | DepQuad3==”InfBiquadArc” | DepQuad4==”InfBiquadArc”)
Nasal Step defect, including defect based on disturbed points
The separate disturbed point nasal step variables are needed for severity calculations
gen Sup_Nasal_Step = 0
gen Inf_Nasal_Step = 0
gen DistPt_SupNasStep = 0
gen DistPt_InfNasStep = 0
replace Sup_Nasal_Step = 1 if (SupNasDefect==1 & Quad1_Sup_Arc==”” & Quad1_DistPt_SupArc==””)
replace Inf_Nasal_Step = 1 if (InfNasDefect==1 & Quad3_Inf_Arc==”” & Quad3_DistPt_InfArc==””)
replace DistPt_SupNasStep = 1 if (SupNasDiff >=14.5 & pt18 >=4 & pt25 >=4 & pt26>=4 & Quad1_Sup_Arc==”” &
Quad1_DistPt_SupArc==””)
replace DistPt_InfNasStep = 1 if (InfNasDiff >=14.5 & pt33 >=4 & pt34 >=4 & pt42>=4 & Quad3_Inf_Arc==”” & Quad3_DistPt_InfArc==””)
replace Sup_Nasal_Step = 1 if DistPt_SupNasStep ==1
replace Inf_Nasal_Step = 1 if DistPt_InfNasStep ==1
Summary Paracentral Scotoma /
gen ParaScot = 0
for var Quad1_ParaScot Quad2_ParaScot Quad3_ParaScot Quad4_ParaScot DistPt_ParaScot: replace ParaScot = 1 if X==”ParaScot”
Diffuse Depression
gen DiffuseDepression= 0
gen deprflag = 0
replace deprflag = 1 if (Sup_Alt == 1 & Inf_Alt ==1 & CentScot ==1 & Sup_Inf_Diff <=10)
replace DiffuseDepression = 1 if deprflag==1
replace Sup_Alt = 0 if deprflag==1
replace Inf_Alt = 0 if deprflag==1
replace CentScot = 0 if deprflag==1
drop deprflag
gen Absolute_defect = 0
gen Check_Absolute = 0
replace Check_Absolute = 1 if (DiffuseDepression==1 & Total_Avg_Quad_loss >=21.5 & Foveal_Sens==0 )
Cannot do check for Absolute without dB data from sensitivity plot, pt_1 thru pt_52
At present the sensitivity plot data is not inlcuded in the Stata file response = pt > 0
Count how many points have any response (i.e pts >0)
If no responses code as absolute
If only one response and no points are >9 code as absolute
If only one response but there is one or more point >9 keep as Diff Depression
If more than one response keep as diff Depression
gen response = 0
gen pts_above_9 = 0
replace response = . if Check_Absolute == 0
replace pts_above_9 = . if Check_Absolute == 0
for var pt_1-pt_52: replace response = response + 1 if X > 0 & Check_Absolute == 1
for var pt_1-pt_52: replace pts_above_9 = pts_above_9 + 1 if X > 9 & Check_Absolute == 1
replace Absolute_defect = 1 if Check_Absolute ==1 & response == 0
replace Absolute_defect = 1 if Check_Absolute ==1 & response == 1 & pts_above_9 <= 0
replace DiffuseDepression = 0 if Absolute_defect == 1
Normal Field
gen Normal = 1
for var Sup_Nasal_Step Inf_Nasal_Step: replace Normal=0 if X==1
for var DiffuseDepression Sup_Alt Inf_Alt Sup_Arc Inf_Arc Inf_arcalt Sup_arcalt ParaScot CentScot : replace
Normal=0 if X==1
replace Normal = 0 if Total_Avg_Quad_loss >1.55
gen Oth_Sup = 0
gen Oth_Inf = 0
gen Oth_Whole = 0
replace Oth_Sup =1 if (Sup_Alt== 1 & AboveBelowDiff<=4 & InfShiftDiff>=10)
replace Oth_Inf =1 if (Inf_Alt== 1 & AboveBelowDiff<=4 & SupShiftDiff>=10)
replace Oth_Whole =1 if (Total_Avg_Quad_loss>1.55 & ParaScot==0 & CentScot==0 & Sup_Alt==0 & Inf_Alt==0 & Sup_Arc==0 & Inf_Arc==0 & Sup_Nasal_Step==0 & Inf_Nasal_Step==0 & Sup_arcalt==0 & Inf_arcalt==0 & Absolute_defect==0 & DiffuseDepression==0 & Normal==0)
Code for missing foveal sensitivity
for var Absolute_defect DiffuseDepression Sup_Alt Inf_Alt Sup_Arc Inf_Arc Inf_arcalt Sup_arcalt ParaScot CentScot Sup_Nasal_Step Inf_Nasal_Step Oth_Sup Oth_Inf Oth_Whole : replace X=9 if Fov_sens_string == “Missing”
Severity measures Store average loss over appropriate area for paired t-test
Do not code into Severe, Moderate and Mild at this time
/*
Arcs
Thresholds were: Severe >=22.5; Moderate <22.5 & >10 ; Mild <10
gen PtSup_Arc = .
replace PtSup_Arc = AvgSupPeriphery if DepQuad1==”SupBiquadArc” | DepQuad2==”SupBiquadArc”|
SupBiquadArc==”SupBiquadArc”| DistPt_SupBiquadArc==”SupBiquadArc”
replace PtSup_Arc = Peripheral1 if PtSup_Arc == . & Quad1_Sup_Arc==”Sup.Arc.”
replace PtSup_Arc = Peripheral2 if PtSup_Arc == . & Quad2_Sup_Arc==”Sup.Arc.”
replace PtSup_Arc = Peripheral1 if PtSup_Arc == . & Quad1_DistPt_SupArc==”Sup.Arc.”
replace PtSup_Arc = Peripheral2 if PtSup_Arc == . & Quad2_DistPt_SupArc==”Sup.Arc.”
gen PtInf_Arc = .
replace PtInf_Arc = AvgInfPeriphery if DepQuad3==”InfBiquadArc” |DepQuad4==”InfBiquadArc” |InfBiquadArc==”InfBiquadArc” | DistPt_InfBiquadArc==”InfBiquadArc”
replace PtInf_Arc = Peripheral3 if PtInf_Arc == . & Quad3_Inf_Arc==”Inf.Arc.”
replace PtInf_Arc = Peripheral4 if PtInf_Arc == . & Quad4_Inf_Arc==”Inf.Arc.”
replace PtInf_Arc = Peripheral3 if PtInf_Arc == . & Quad3_DistPt_InfArc==”Inf.Arc.”
replace PtInf_Arc = Peripheral4 if PtInf_Arc == . & Quad4_DistPt_InfArc==”Inf.Arc.”
/*
Severity measures for nasal steps
Thresholds were: Severe >=20; Mod < 20 & >10; Mild <=10
gen Pt_Sup_NasStep = .
gen Pt_Inf_NasStep = .
replace Pt_Sup_NasStep = SupNasQuad if Sup_Nasal_Step == 1 & DistPt_SupNasStep == 0
replace Pt_Sup_NasStep = Sup_Nasal_Pts if Pt_Sup_NasStep == . & DistPt_SupNasStep == 1
replace Pt_Inf_NasStep = InfNasQuad if Inf_Nasal_Step == 1 & DistPt_InfNasStep == 0
replace Pt_Inf_NasStep = Inf_Nasal_Pts if Pt_Inf_NasStep == . & DistPt_InfNasStep == 1
/*
Severity for paracentral scotomas were previously by quadrant. (Columns CD,CE,CF,CG,CH)
Thresholds were: Severe >=20; Mod < 20 & >10; Mild <=10
For paired t-test between 2 time points use avg ParaCenloss, since the quadrant involved could be different each time
gen Pt_ParaScot = AvgParaCenLoss
/*
Central scotoma
Thresholds were: Severe Foveal sensitivity <=10; Mod Foveal sensitivity >10 & <=25 ; Mild Foveal sensitivity >25
gen Pt_CentralScot = Foveal_Sens
/*
Severity for Diffuse depression (column K)
Thresholds are >=21.5 ; < 21.5 & >15; <=15
gen PtDiffuseDepr = Total_Avg_Quad_loss
/*
SupAlt and InfAlt
Thresholds are >=24.5 ; <24.5 & >15 ; <=15
gen PtSup_Alt= Sup_Avg_loss
gen PtInf_Alt= Inf_Avg_loss
/*
SupArcAlt and InfArcAlt thresholds are >=24.5 ; <24.5 & >15 ; <=15
gen PtSup_arcalt = Sup_Avg_loss
gen PrInf_arcalt = Inf_Avg_loss
drop Quad1_Avg_loss - NasalParacenDiff Quad1_ParaScot- DistPt_ParaScot
drop DistPt_SuperiorArcAlt DistPt_InferiorArcAlt DistPt_SupNasStep DistPt_InfNasStep
Footnotes
A list of the members of the Ischemic Optic Neuropathy Decompression Trial and participating study centers was published in JAMA (1995;273:625-632).
Supported by grants U10-EY09557 and U10-EY09608 from the National Institutes of Health and an award from Research to Prevent Blindness, Inc.
REFERENCES
- 1.Ischemic Optic Nerve Decompression Trial Group. The Ischemic Optic Neuropathy Decompression Trial (IONDT): design and methods. Control Clin Trials. 1998;19:276–296. doi: 10.1016/s0197-2456(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 2.Ischemic Optic Nerve Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273:625–632. [PubMed] [Google Scholar]
- 3.Keltner JL, Johnson CA, Beck RW, et al. Quality control functions of the Visual Field Reading Center (VFRC) for the Optic Neuritis Treatment Trial (ONTT) Control Clin Trials. 1995;14:143–159. doi: 10.1016/0197-2456(93)90016-7. [DOI] [PubMed] [Google Scholar]
- 4.Katz J, Congdon N, Friedman D. Methodological variations in estimating apparent progressive visual field loss in clinical trials of glaucoma treatment. Arch Ophthalmol. 1999;117:1137–1142. doi: 10.1001/archopht.117.9.1137. [DOI] [PubMed] [Google Scholar]
- 5.Molino G, Marzuoli M, Molino F, et al. Validation of ICTERUS, a knowledge-based expert system for jaundice diagnosis. Methods Inf Med. 2000;39:311–318. [PubMed] [Google Scholar]
- 6.Hernandez C, Sancho JJ, Belmonte MA, et al. Validation of the medical expert system RENOIR. Comput Biomed Res. 1994;27:456–471. doi: 10.1006/cbmr.1994.1034. [DOI] [PubMed] [Google Scholar]
- 7.Sutton GC. How accurate is computer-aided diagnosis? Lancet. 1989;2:905–908. doi: 10.1016/s0140-6736(89)91560-2. [DOI] [PubMed] [Google Scholar]
- 8.Werner EB, Saheb N, Thomas D. Variability of static visual threshold responses in patients with elevated IOPs. Arch Ophthalmol. 1982;100:1627–1631. doi: 10.1001/archopht.1982.01030040605010. [DOI] [PubMed] [Google Scholar]
- 9.Keltner JL, Johnson CA, Quigg JM, et al. for the Ocular Hypertension Treatment Study Group. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2000;118:1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 10.Katz J. Scoring systems for measuring progression of visual field loss in clinical trials of glaucoma treatment. Ophthalmology. 1999;106:391–395. doi: 10.1016/S0161-6420(99)90052-0. [DOI] [PubMed] [Google Scholar]
- 11.de la Rosa MG, Reyes JAA, Sierra MAG. Rapid assessment of the visual field in glaucoma using an analysis based on multiple correlations. Graefes Arch Clin Exp Ophthalmol. 1990;228:387–391. doi: 10.1007/BF00927247. [DOI] [PubMed] [Google Scholar]
- 12.McNaught AI, Crabb DP, Fitzke FW, et al. Modelling series of visual fields to detect progression in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 1995;233:750–755. doi: 10.1007/BF00184085. [DOI] [PubMed] [Google Scholar]
- 13.Keltner JL, Johnson CA, Spurr JO, et al. Optic Neuritis Study Group. Baseline visual field profile of optic neuritis; the experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1993;111:231–234. doi: 10.1001/archopht.1993.01090020085029. [DOI] [PubMed] [Google Scholar]
- 14.Keating D, Mutlukan E, Evans A, et al. A back propagation neural network for the classification of visual field data. Phys Med Biol. 1993;38:1263–1270. [Google Scholar]
- 15.Brigatti L, Hoffman D, Caprioli J. Neural networks to identify glaucoma with structural and functional measurements. Am J Ophthalmol. 1996;121:511–521. doi: 10.1016/s0002-9394(14)75425-x. [DOI] [PubMed] [Google Scholar]
- 16.Goldbaum MH, Sample PA, Chan K, et al. Comparing machine learning classifiers for diagnosing glaucoma from standard automated perimetry. Invest Ophthalmol Vis Sci. 2002;43:162–169. [PubMed] [Google Scholar]
- 17.Kelman SE, Perell HF, D’Autrechy L, et al. A neural network can differentiate glaucoma and optic neuropathy visual fields through pattern recognition. In: Mills RP, Heijl A, ed. Perimetry Update 1990/91. Proceedings of the Sixth International Perimetric Society Meeting, Malmo, Sweden, June 17–20, 1990. Amsterdam/New York: Kugler Publications; 1991:287–290.
- 18.Henson DB, Spenceley SE, Bull DR. Spatial classification of glaucomatous visual field loss. Br J Ophthalmol. 1996;80:526–531. doi: 10.1136/bjo.80.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsbrunner H-P, Fankhauser F, Jenni A, et al. Evaluating a perimetric expert system: experience with Octosmart. Graefes Arch Clin Exp Ophthalmol. 1990;228:237–241. doi: 10.1007/BF00920027. [DOI] [PubMed] [Google Scholar]
- 20.Ellenberger C, Keltner JL, Burde RM. Acute optic neuropathy in older patients. Arch Ophthalmol. 1973;28:182–185. doi: 10.1001/archneur.1973.00490210062008. [DOI] [PubMed] [Google Scholar]
- 21.Eagling EM, Sanders MD, Miller SJH. Ischemic papillopathy: clinical and fluorescein angiographic review of forty cases. Br J Ophthalmol. 1974;58:990–1008. doi: 10.1136/bjo.58.12.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller GR, Smith JL. Ischemic optic neuropathy. Am J Ophthalmol. 1966;62:103–115. doi: 10.1016/0002-9394(66)91685-0. [DOI] [PubMed] [Google Scholar]
- 23.Cullen JF. Ischemic optic neuropathy. Trans Ophthalmol Soc U K. 1967;87:759–774. [PubMed] [Google Scholar]
- 24.Repka MX, Savino PJ, Schatz NJ, et al. Clinical profile and long-term implications of anterior ischemic optic neuropathy. Am J Ophthalmol. 1983;96:478–483. doi: 10.1016/s0002-9394(14)77911-5. [DOI] [PubMed] [Google Scholar]
- 25.Hayreh SS, Podhajsky P. Visual field defects in anterior ischemic optic neuropathy. Doc Ophthalmol Proc Ser. 1979;19:53–71. [Google Scholar]
- 26.Traustason OI, Feldon SE, Leemaster JE, et al. Anterior ischemic optic neuropathy: classification of field defects by Octopus automated static perimetry. Graefes Arch Clin Exp Ophthalmol. 1988;226:206–212. doi: 10.1007/BF02181182. [DOI] [PubMed] [Google Scholar]
- 27.Guyer DR, Miller NR, Auer CL, et al. The risk of cerebrovascular and cardiovascular disease in patients with anterior ischemic optic neuropathy. Arch Ophthalmol. 1985;103:1136–1142. doi: 10.1001/archopht.1985.01050080048018. [DOI] [PubMed] [Google Scholar]
- 28.Hayreh SS, Joos KM, Podhajsky PA, et al. Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1994;118:766–780. doi: 10.1016/s0002-9394(14)72557-7. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson DM, Vierkant RA, Belongia EA. Nonarteritic anterior ischemic optic neuropathy. A case-control study of potential risk factors. Arch Ophthalmol. 1997;115:1403–1407. doi: 10.1001/archopht.1997.01100160573008. [DOI] [PubMed] [Google Scholar]
- 30.Newman NJ, Scherer R, Langenberg P, et al. Ischemic Optic Neuropathy Decompression Trial Research Group. The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol. 2002;134:317–328. doi: 10.1016/s0002-9394(02)01639-2. [DOI] [PubMed] [Google Scholar]
- 31.Johnson C. Ocular Hypertension Treatment Study—determination of progression. In: Anderson DR, Drance SM, ed. Encounters in Glaucoma Research 3: How to Ascertain Progression and Outcome. Amsterdam/New York: Kugler Publications; 1996:165–182.
- 32.Keltner JL, Johnson CA, Quigg JM, et al. for the Ocular Hypertension Treatment Study Group. Confirmation of visual field abnormalities in the ocular hypertension treatment study. Arch Ophthalmol. 2000;118:1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 33.Sergott RC, Cohen MS, Bosley TM, et al. Optic nerve sheath decompression may improve the progressive form of ischemic optic neuropathy. Arch Ophthalmol. 1989;107:1743–1754. doi: 10.1001/archopht.1989.01070020825022. [DOI] [PubMed] [Google Scholar]
- 34.WuDunn D, Zimmerman K, Sadun AA, et al. Comparison of visual function in fellow eyes after bilateral nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 1997;104:104–111. doi: 10.1016/s0161-6420(97)30354-6. [DOI] [PubMed] [Google Scholar]
- 35.Boone MI, Massry GG, Frankel RA, et al. Visual outcome in bilateral nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 1996;103:1223–1228. doi: 10.1016/s0161-6420(96)30519-8. [DOI] [PubMed] [Google Scholar]
- 36.Hayreh SS, Podhajsky PA, Zimmerman B. Ipsilateral recurrence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2001;132:734–742. doi: 10.1016/s0002-9394(01)01192-8. [DOI] [PubMed] [Google Scholar]
- 37.Fang JP, Lin RH, Donahue SP. Recovery of visual field function in the optic neuritis treatment trial. Am J Ophthalmol. 1999;128:566–572. doi: 10.1016/s0002-9394(99)00297-4. [DOI] [PubMed] [Google Scholar]
- 38.Arnold AC, Hepler RS. Natural history of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 1991;14:66–69. [PubMed] [Google Scholar]