ABSTRACT
Purpose
To assess the safety and efficacy of photorefractive keratectomy (PRK) in children with anisometropic amblyopia and to define the characteristics of children who may be candidates for PRK.
Methods
This thesis comprises four parts: (1) a retrospective analysis of risk factors predictive of amblyopia treatment failure in 104 children, (2) a prospective study of pachymetry in 198 eyes of 108 children, (3) development and implementation of a protocol to perform PRK under general anesthesia, and (4) a prospective interventional case-comparison study of PRK in 11 noncompliant children with anisometropic amblyopia to evaluate safety and long-term outcomes. Compliant and noncompliant children with anisometropic amblyopia were analyzed as controls.
Results
Factors associated with conventional anisometropic amblyopia treatment failure were poor compliance (P = .004), age 6 years or older (P = .01), astigmatism ≥1.5 diopters (P = .0002), and initial visual acuity of 20/200 or worse (P = .02). Central and paracentral pachymetry measurements were similar to published adult values. The general anesthesia protocol was efficient, and the laser functioned properly in all cases. All children did well with no anesthesia-related or treatment-related complications. Two years following PRK, the mean reduction in refractive error was 9.7 ± 2.6 diopters for myopes (P = .0001) and 3.4 ± 1.3 diopters for hyperopes (P = .001). The cycloplegic refractive error in 9 of 11 treated eyes was within 3 diopters of that in the fellow eye. Uncorrected visual acuity in the amblyopic eye improved by ≥2 lines in seven of nine children; best-corrected visual acuity improved by ≥2 lines in six of nine children. Stereopsis improved in five of nine children. The mean visual acuity of the PRK patients at last follow-up was significantly better than that of noncompliant controls (P = .003). The safety and efficacy indices for PRK in this study were 1.24 and 1.12, respectively.
Conclusions
Photorefractive keratectomy can be safely performed in children with anisometropic amblyopia. Visual acuity and stereopsis improved in most eyes, even in older children. Photorefractive keratectomy may have an important role in the management of anisometropic amblyopia in noncompliant children.
HYPOTHESIS
Photorefractive keratectomy for anisometropic amblyopia in children can be safely performed and results in better uncorrected and best-corrected visual acuity and stereopsis in children who are poorly compliant with standard refractive correction and other amblyopia treatment measures.
INTRODUCTION
Amblyopia
The word “amblyopia,” derived from Greek, literally means “dullness of vision.” Ophthalmologic examination demonstrates reduced visual acuity that is not fully explained by obvious aberrations of the retina or optic nerve. Von Graefe stated over a century ago that amblyopia was the condition in which the observer sees nothing and the patient sees very little.1
Amblyopia affects approximately 2% to 5% of the American population2–6 and is the most frequent cause of unilateral visual impairment in children and young adults in the United States and Western Europe.7–13 Vision screening is recommended between the ages of 3 and 5 years and is usually done in schools or by primary care physicians.14 Amblyopia is most often detected during this routine vision screening.14 Despite these facts, adequate screening is believed to occur in only 21% of preschool children in the United States.15,16 Treatment of amblyopia is less likely to be successful in children older than 6 years of age.17–20
Anisometropia is the most common cause of amblyopia and occurs because of uncorrected unequal refractive error between fellow eyes.12 Uncorrected anisometropia produces image blur in one eye (form vision deprivation) and/or abnormal binocular interaction by producing dissimilar images on the fovea of each eye. Anisometropic amblyopia is often detected later than other forms of amblyopia because vision is generally good in the fellow eye, the eyes are typically orthotropic, and the child functions well with the use of the sound eye. The level of anisometropia required to cause amblyopia has been well studied. In general, anisomyopia of more than 2 diopters, anisohyperopia of more than 1 diopter, and anisoastigmatism of more than 1.5 diopters may result in amblyopia.21,22 A direct relationship between the degree of anisometropia and the severity of amblyopia has been reported.18,23,24 Studies of anisometropic amblyopia indicate a prevalence of amblyopia of 100% in hyperopes with 4.0 diopters of uncorrected anisometropia and in myopes with 6.0 diopters of uncorrected anisometropia.23,25 Anisometropia of more than about 4 diopters is also believed to portend a worse prognosis for successful visual outcome with traditional amblyopia therapy.18
Treatment of Anisometropic Amblyopia
Traditional therapy for anisometropic amblyopia includes refractive correction with spectacles or contact lenses, minimization of aniseikonia with contact lenses, and amblyopia management with occlusion therapy and/or pharmacologic and/or optical penalization of the sound eye.23,26–30 Despite this seemingly simple treatment strategy, traditional treatment is often problematic and unsuccessful.
Spectacle correction of significant anisometropia produces aniseikonia. Aniseikonia of more than 5% to 6% (typically present with 3 or more diopters of anisometropia) cannot be readily fused.31 Suppression of the amblyopic eye occurs, often limiting the effectiveness of the amblyopia therapy.31 An occasional child will experience diplopia due to the aniseikonia.32,33 Thus, glasses for moderate to severe anisometropia are commonly not well tolerated. Spectacles for anisometropia of more than 2 to 3 diopters are also cosmetically problematic because of the differential magnification or minification effect of the hyperopic or myopic lens, respectively. Parents and children often complain of a noticeable size difference in the appearance of the eyes through such spectacles.
Contact lenses are an alternative treatment for anisometropia. Contact lenses essentially eliminate the issue of aniseikonia for most patients. Unfortunately, contact lens use in children is difficult for other reasons. Contact lenses are often difficult for parents to insert and remove, loss is frequent, and the costs are relatively high. Significant lapses of time without proper refractive correction in place are common following lens loss. And though uncommon, the risk of microbial keratitis, higher in contact lens wearers, may put the sound eye at risk.34–37 Children, who are usually less hygienic than adults, may be at higher risk for this complication than adult contact lens wearers.38,39
Although refractive correction is sometimes all that is needed to correct anisometropic amblyopia, additional amblyopia treatment is frequently required. Occlusion therapy, pharmacologic penalization with atropine or other cycloplegic agents, optical penalization, or all of these in combination are used in cases where refractive correction alone fails to normalize the visual acuity. Noncompliance with these treatment measures is common, especially with occlusion therapy.40 Disadvantages of atropine penalization include photosensitivity, anticholinergic side effects, and inability to rapidly titrate treatment.41 Optical penalization using a lens to blur the vision in the sound eye is an accepted treatment alternative.26,27,42,43 However, it is successful only in willing patients; uncooperative children simply remove or look around their spectacles to avoid the penalizing lens.
Significant psychosocial stress related to amblyopia therapy has been reported by amblyopic children and the families of amblyopic children during the treatment period.40 Even adults with a history of amblyopia treatment in childhood continue to have psychosocial difficulties related to the previous amblyopia therapy that adversely affect self-image, work, school, and friendships.44
Certain neurotransmitters have been implicated in neuronal plasticity. Based on this finding, levodopa/carbidopa and citicoline, which act to enhance dopaminergic neurotransmission in the brain, have been experimentally used to treat amblyopia in adults and children.45–51 Both have been associated with some mild improvement of visual acuity that unfortunately was not sustained after discontinuing the medication.46–48,50,52,53
Successful treatment of anisometropic amblyopia with traditional therapy has been reported in 48% to 82% of children.12,23,27,29,54–58 The success rate varies widely among studies, depending on the definition of success, parameters at initiation of treatment, and other factors. Flynn and associates18 conducted a meta-analysis of 23 studies of therapy for amblyopia that were published from 1965 to 1994; the investigators calculated an overall success rate of 67% (defined as visual acuity of 20/40 or better) for the anisometropic amblyopia subgroup treated with traditional therapy. They also found an inverse relationship between the degree of anisometropia and the final visual acuity. The greater the anisometropia, the more likely a poor visual outcome was the result. Successfully treated patients typically had less than 4 diopters of anisometropia. A direct relationship between initial and final visual acuity has also been reported.18,23
Amblyopia will remain a major public health problem until new and improved treatment modalities are developed. Despite all efforts to date to treat anisometropic amblyopia, up to one third of treated children with this condition will not achieve a visual acuity of 20/40 or better (the level of acuity required to obtain an unrestricted driver’s license in most states (www.lowvisioncare.com) with available treatment. A report from the United Kingdom even questioned the efficacy of amblyopia therapy, because no controlled studies had been done in which the control group did not receive treatment.59 In response to this report, a recent study that included a “no treatment” control group reported that amblyopia treatment is worthwhile in children with visual acuity of less than 20/40 in the amblyopic eye.60 Additionally, there is a higher incidence of traumatic vision loss in the sound eye of individuals who have only one normally sighted eye,61 putting amblyopic patients at higher risk for bilateral visual impairment.
Amblyopia treatment is economically sound. Membreno and coworkers62 reported on the incremental cost-effectiveness of therapy for amblyopia and calculated a savings of $2,281 per quality-adjusted life year with amblyopia treatment. They concluded that when compared to healthcare interventions for other medical conditions, amblyopia care is highly cost-effective.
Poor compliance with treatment is commonly associated with amblyopia treatment failure.17,63 The Pediatric Eye Disease Investigator Group58 recently reported better compliance with atropine penalization than with occlusion therapy, though compliance remained a problem for both treatment groups. Patient compliance with any medical treatment is notoriously suboptimal. Even patients with life-threatening disorders such as asthma and organ transplant frequently fail to comply with treatment recommendations.64–67 Poor compliance may be even more frequent when the patient is a child who cannot comprehend the reason for the treatment, as is the case with amblyopia.
Given the known problems with treatment compliance, the long-lasting psychosocial issues associated with standard amblyopia therapy, and the high percentage of treatment failures with standard therapy, consideration of nontraditional treatment options for anisometropic amblyopia that are less dependent on long-term compliance is justified. Refractive surgery is a reasonable alternative to consider. Photorefractive keratectomy (PRK) and laser in situ keratomileusis (LASIK) have both been well received by adults with refractive errors.68–70 Refractive procedures that may have utility in children include PRK, LASIK, laser epithelial keratomileusis (LASEK), and possibly others.
Refractive Surgery
Excimer laser refractive surgery has been successfully used in the treatment of myopia, hyperopia, and astigmatism in adults.71–79 Most adult patients who undergo PRK or LASIK are satisfied with the outcome.68–70 PRK and LASIK have been the most extensively studied of the excimer laser procedures. Photorefractive keratectomy involves removing the corneal epithelium, either with the excimer laser or manually, followed by computer-guided ablation of the underlying Bowman’s membrane and anterior corneal stroma. Laser in situ keratomileusis involves creating a central corneal flap composed of epithelium, Bowman’s membrane, and anterior stroma. Computer-guided excimer laser ablation of the posterior corneal stroma is then performed, followed by repositioning of the corneal flap.
Advantages of LASIK over PRK include less postoperative discomfort, faster visual recovery, and maintenance of an intact Bowman’s membrane.80,81 Advantages of PRK include avoidance of several serious potential complications associated with LASIK, including corneal flap loss, tear or striae, and keratectasia.81–91 An important risk of PRK reported in adult patients is temporary or permanent corneal haze.91–93 The implications of persistent or even temporary corneal haze for a child are vastly different from those for the adult because of the child’s immature visual system and the risk of worsening the amblyopia from form vision deprivation. Fortunately, postoperative corneal haze typically has been mild in the few children treated with PRK thus far, provided the recommended postoperative topical steroid regimen was followed.
Refractive Surgery in Children
Refractive surgery in children, to date, has been applied in a haphazard fashion, without preliminary work to establish which children are most likely to benefit from treatment and to determine if there are unique characteristics of the pediatric cornea that could alter PRK treatment nomograms, intraoperative techniques, postoperative management, or all of these. Experience with other pediatric ophthalmic surgical procedures dictates that children cannot be treated merely as small adults. For example, experience with pediatric corneal transplantation, cataract surgery, and intraocular lens implantation has revealed important, often vision-threatening, differences in pediatric response to surgery compared with adult patients undergoing the same procedures.78,94–99 Surgical techniques for children have often required modification due to issues such as differences in corneal and scleral rigidity, elasticity of the lens capsule, and lens/vitreous characteristics. Anticipation of future eye growth must also be considered when planning and implementing eye surgery in young children. Additionally, postoperative care of the child does not usually parallel that of the adult because of differences in healing time, inflammatory response, cooperation, and childhood behaviors that may place the newly operated eye at increased risk for trauma. To avoid repeating serious mistakes of the past when attempting to translate accepted adult procedures to children, careful scientific evaluation of refractive surgery in children is of paramount importance.
When considering performing a procedure on a child that has been performed only on adults, one must be ever cognizant of potential complications that could occur immediately or many years after the procedure. Informed consent for pediatric PRK from the parent and assent of the child (if old enough) must include discussion and understanding of the fact that no data are available on extremely long-term outcomes in excimer laser–treated eyes. This is particularly important for a child who has potentially 70 to 80 more years to live.
Several uncontrolled studies have been published regarding PRK and LASIK in children.86,100–109 In total, 118 children have been included in publications of pediatric excimer refractive procedures. Most studies had fewer than seven children in them; the largest study had 27. Only one study has reported long-term results,108 and most studies were conducted outside of the United States.86,100–103,105–108 With the exception of three children in one study,106 all previous studies86,100–103,105,108,109 have reported only on PRK or LASIK for the treatment of anisometropic myopia or bilateral high myopia. Most studies have included only children older than 7 years, an age often considered to be less responsive to amblyopia treatment because of closure of the sensitive period of visual development.110–113 Only one study has provided data on stereopsis,108 and none have included a control group. More important, all previous studies have apparently been conducted without preliminary investigation of potential issues related to the pediatric eye that might alter or even eliminate refractive procedures as an option for children.
Anisometropic Amblyopia Failure Risk Factors
Knowledge about risk factors for anisometropic amblyopia treatment failure could be useful in the early identification of children who are most likely to fail conventional amblyopia therapy. More aggressive treatment and closer follow-up might be warranted to improve the chance of a successful outcome in these children. Early utilization of nonconventional treatments, including refractive surgery, might also be warranted in selected children with identifiable risk factors for failure.
Central and Paracentral Corneal Thickness in Children
Both PRK and LASIK are subtraction refractive procedures, resulting in permanent reduction in the thickness of the cornea. Current US Food and Drug Administration guidelines for LASIK limit treatment parameters to ensure that the cornea maintains a minimum thickness of at least 410 to 430 μm (250 μm in posterior stromal bed plus 160 to 180 μm in cap) to protect against potential keratectasia.90,114–117 Very little is known about normative values for corneal thickness (pachymetry) in the pediatric population.
Corneal thickness in premature and neonatal subjects has been reported.118–121 In addition to the age-limited information available from these studies, minimal ethnically diverse information has been included.118–121 Variation in adult corneal thickness by race has been well documented, with the central corneal thickness in African Americans being significantly thinner than in Caucasians.122 The previous studies on infant and newborn corneal thickness have reported only central and limbal corneal thickness measurements, which are thicker than those of adults.118–121 Paracentral pachymetry data are unavailable for pediatric patients. Both PRK and LASIK ablate tissue in the paracentral region of the cornea; thus knowledge about corneal thickness in this region is important. Only one study to date has evaluated corneal thickness in children older than the neonatal age group.123 The investigators reported only central measurements and used optical pachymetry, an older technology that is known to be less accurate than modern ultrasound pachymetry.124 Establishing normative corneal thickness values for children is essential if refractive surgery is to play a role in pediatric ophthalmology. If corneal thickness in children is found to be significantly different than in adults, treatment nomograms may need to be altered for best visual and refractive outcomes.
Practical Issues Regarding Refractive Surgery in Children
Refractive surgery is often considered impractical in young children because of poor cooperation, the need for general anesthesia, and the need for postanesthesia monitoring. Unfortunately, anisometropic amblyopia is best managed early in life during the time the visual system is most responsive to treatment. Photorefractive keratectomy in adults is performed under topical anesthesia in an office setting. Voluntary immobilization of the eye is required during the procedure. Young children, however, are usually not cooperative, even for a detailed biomicroscopy examination, much less ophthalmic surgery. Therefore, general anesthesia will be required in most cases if refractive surgery is to be done in children under 10 or 11 years of age. Most of the published literature on pediatric refractive surgery for anisometropia has included only children old enough to cooperate for surgery under topical anesthesia.86,100,102–107 In theory and probably in practice, serious application of pediatric refractive surgery for anisometropic amblyopia must include younger children well within the sensitive period of visual development if it is to be maximally effective.
The requirement for general anesthesia creates a host of important practical problems. The excimer laser is not typically housed in a site that is safe for administration of general anesthesia, and most lasers are not easily portable. Inhalational anesthetic agents can alter excimer laser function and even cause laser shutdown.125 Operational, procedural, and organizational hurdles must be overcome to safely and reliably apply refractive surgery under general anesthesia.
Healing of the corneal epithelial defect following PRK in adults typically occurs over a period of approximately 5 days.126 Postoperative pain is an important drawback of PRK in adult patients.99,127–130 No published reports have described the rate of corneal healing and the degree of postoperative pain in children treated with PRK. These are important practical issues that pertain to the feasibility and public acceptance of this procedure for children.
Children with severe anisometropic amblyopia who are noncompliant with traditional therapy typically will have permanent, significant visual impairment.17,18,23,57,63,131 Refractive surgery could play an important role in treating this difficult subset of patients. The purpose of this series of studies was to systematically investigate the mechanics, safety, efficacy, and appropriate application of PRK in children with anisometropic amblyopia noncompliant with traditional therapy.
METHODS
This study on patient selection, mechanics, safety, and efficacy of PRK in children with anisometropia consists of four parts: (1) retrospective evaluation of the records of children with anisometropic amblyopia to identify characteristics of children most likely to fail standard treatment, (2) prospective evaluation of central and paracentral corneal thickness in a pediatric population to ensure the feasibility of refractive surgery in children and to make initial judgments regarding the potential need to modify PRK treatment parameters for children, (3) development and implementation of a standardized general anesthesia protocol for PRK in children, and (4) performance of PRK and follow-up of a group of children with anisometropic amblyopia who were noncompliant with conventional anisometropic amblyopia therapy. In this group, we analyzed corneal healing, postoperative discomfort, visual acuity, refractive response, stereopsis, corneal clarity, and complications over a 2-year period. Visual acuity gains and refractive errors were compared to those of two control groups: (1) children with anisometropic amblyopia who were either diagnosed late (after 6 years of age) or were noncompliant with amblyopia therapy (noncompliant group), and (2) children with anisometropic amblyopia who were diagnosed before 6 years of age and were compliant with amblyopia therapy (compliant group). The studies that make up this report were all approved by our institutional review board.
Anisometropic Amblyopia Treatment Failure Risk Factors
In an effort to identify characteristics of children most likely to fail standard therapy for anisometropic amblyopia, a retrospective review was performed of the records of 104 children with anisometropic amblyopia we had treated with refractive correction and occlusion and/or atropine penalization of the sound eye. Inclusion criteria included (1) age 3 to 8 years at the time of treatment initiation, (2) ability to perform Snellen or HOTV visual acuity testing, (3) an initial difference in visual acuity between fellow eyes of at least 3 lines of logMAR acuity, (4) anisometropia of at least 1 diopter, (5) visual acuity in the amblyopic eye of 20/50 or worse, (6) absence of structural ocular abnormalities in either eye, and (7) at least 1 year follow-up or follow-up to successful “functional outcome” (visual acuity of at least 20/40 in the amblyopic eye), whichever came first.
The data analyzed included age at initiation of treatment, male or female sex, initial and final best-corrected visual acuity, initial cycloplegic refraction, presence of manifest strabismus, treatment modality, and treatment compliance by parental report at the first follow-up examination. Visual acuity was obtained using either Snellen or HOTV charts. Compliance was determined from the physician’s assessment in the medical record based on the parental report. Lack of response to treatment was defined prior to data collection in two ways: (1) relative failure was defined as failure of visual acuity to improve by at least 3 lines of logMAR visual acuity, regardless of the final vision, and (2) functional failure was defined as a final visual acuity of less than 20/40 in the amblyopic eye. This level of visual acuity was chosen as the definition of functional failure because 20/40 is the minimum monocular visual acuity required to obtain an unrestricted driver’s license in most states (www.lowvisioncare.com, Vision and Driving: State Rules/ Regulations/ Policies). Visual acuities were converted to logMAR acuities for analysis. They were then converted back to the more familiar Snellen values to facilitate review of the data.
For analysis of age at presentation, we grouped our patients into two groups: 3 to 5 years and 6 years or older. For analysis of the effect of the degree of anisometropia, we grouped the children into those with less than 4 diopters of anisometropia and those with anisometropia of 4 diopters or more. To test the effect of compliance with treatment, we categorized the children into two groups, those with good compliance and those with suboptimal compliance by parental report at first follow-up examination. For analysis of the effect of refractive error in the amblyopic eye, we divided patients into those with spherical equivalent refractive error of greater than or equal to 3 diopters and those with spherical equivalent refractive error of less than 3 diopters. We also categorized patients having astigmatism into those with astigmatic error of 1.5 diopters or more and those with astigmatic error of less than 1.5 diopters.
Statistical analysis was performed using Intercooled Stata, version 7.0 (Stata Corp, College Station, Texas). Logistic regression models were constructed for each of the outcomes to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for each characteristic. An OR greater than 1 indicates an increased effect of the characteristic on treatment failure. The Hosmer-Lemeshow goodness-of-fit statistic was computed for visual acuity. A P value of .05 was chosen for significance.
Central and Paracentral Corneal Thickness
A prospective investigation to determine the normative values for corneal thickness in children aged 6 months to 14 years was conducted. Written parental informed consent was obtained for all participants. Pachymetry measurements were performed on 198 eyes of 108 children undergoing routine strabismus surgery under general anesthesia, using an ultrasound pachymeter (DGH-2000, DGH Technology, Inc, Frazer, Pennsylvania) with a sound velocity of 1,640 meters per second. Any patient with history of a corneal anomaly, cataract, or glaucoma was excluded.
Following induction of general anesthesia, a wire eyelid speculum was placed in the eye. A pre-inked, standard, 6 mm single-ended ring marker with cross hairs (Duckworth & Kent, St Louis, Missouri) was applied to identify the center and four standard paracentral sites 3 mm from the center of the cornea at the 3-, 6-, 9-, and 12-o’clock positions. Next, three pachymetry measurements were recorded at each of these five sites. If a value was greater than 5% different from the other recordings at that site, an additional measurement was taken. The lowest (thinnest) value at each site was used for analysis, because this represented the most perpendicular path through the cornea. The cornea was moistened during the procedure with balanced salt solution.
Statistical analysis was conducted in this part of the study using Microsoft Excel 2000 (Microsoft Corp, Redmond, Washington). The subjects were stratified into the following age groups prior to data collection: less than 2 years, 2 to 4 years, 5 to 9 years, and 10 to 18 years. The two-tailed t test was used for comparison of the continuous means for values of corneal thickness. Analysis of variance (ANOVA) was performed to determine differences among age groups and among different ethnic groups (Caucasian, Hispanic, African American, other). Values are reported as the mean corneal thickness in microns (± standard deviation). Right and left eyes of each patient were analyzed separately.
General Anesthesia Photorefractive Keratectomy Protocol
This general anesthesia PRK protocol has been previously published as briefly reviewed below.132 Nine children (aged 2 to 9 years) treated with PRK in this study required general anesthesia because of inability to cooperate for the procedure under local anesthesia. Idiosyncrasies of the excimer laser were addressed prior to performing an excimer laser procedure under general anesthesia to reduce the risk of unexpected refractive results and/or malfunction of the laser during treatment. The purpose of this component of the study was to develop and implement a standardized, reproducible, effective, and efficient means of conducting excimer laser surgery on children under general anesthesia and to report on the efficiency of the procedure and intraoperative and postoperative complications.
The anesthesia procedure from induction to anesthesia recovery was as follows. General anesthesia was induced in a separate induction room using halothane and nitrous oxide by mask inhalation. An intravenous line was placed after the child was asleep, and a laryngeal mask airway was inserted into the posterior pharynx. Several patients also received small doses of propofol to deepen anesthesia. An adhesive, nonporous drape was placed over the laryngeal mask airway to minimize escape of the inhalational anesthetic agents. The child was then transported to a nearby operating room fully monitored and breathing oxygen and halothane through a Jackson-Rees circuit. Before entering the operating room, the halothane was discontinued. In the operating room, the laryngeal mask airway was connected to a standard semiclosed-circle system through which the patient received 70% nitrous oxide in oxygen. Nitrous oxide given through the semiclosed circuit was administered throughout the remainder of the case. Additional boluses of propofol were administered as needed. The PRK then proceeded as described in the next section.
The time intervals between cases, intraoperative laser function, and intraoperative and postoperative complications were analyzed.
Photorefractive Keratectomy: Safety and Impact on Refractive Error, Visual Acuity, and Stereopsis
A prospective case-comparison study was conducted of PRK in children. Written parental informed consent (and verbal assent from the children old enough to understand) was obtained for all participants. Eleven children between 2 and 11 years of age were treated with PRK for severe anisometropia with amblyopia. In this study, PRK was investigated rather than LASIK because we felt PRK had a better risk profile for children, with less risk of serious postoperative complications, such as flap loss and keratectasia.81,83,90 Inclusion criteria were (1) anisomyopia of at least 6 diopters or anisohyperopia of at least 4 diopters, (2) poor compliance with spectacles and/or contact lenses and occlusion therapy based on parental report, and (3) moderate to severe amblyopia of the eye with the highest refractive error, defined as a best-corrected visual acuity in the amblyopic eye that was at least 3 logMAR lines lower than the sound eye or a strong fixation preference for the fellow eye in preverbal children. Children with an abnormality of the cornea, lens, or fovea were excluded.
Each child underwent a comprehensive ophthalmologic examination that included uncorrected and best spectacle-corrected visual acuity, stereoacuity testing (Titmus stereo fly test, Stereo Optical Co, Chicago, Illinois), pupillary examination, ocular motility, tactile tonometry, biomicroscopy, funduscopy, and cycloplegic refraction. Visual acuity testing was done with the most sophisticated standard visual acuity test the child could comprehend and perform. Visual behavior was tested in younger children using the fixation and following response and the vertical prism test for fixation preference.133 Quantitative visual acuity testing was done as soon as patient comprehension permitted. The Titmus stereo fly test was chosen to test stereoacuity because of its ease of use and reproducibility in young children. Ultrasound pachymetry and keratometry were performed during the preoperative examination in cooperative children and under general anesthesia prior to the procedure in uncooperative children.
The refractive goal for each child was to reduce the anisometropia to 3 diopters or less, up to a maximum myopic treatment of 11.50 diopters and a maximum hyperopic treatment of 5.25 diopters. Reducing anisometropia to less than or equal to 3 diopters eliminates or greatly reduces the spectacle-induced aniseikonia to the point where fusion is possible, making the condition more amenable to treatment with spectacles. Myopic treatment was limited to no more than 11.50 diopters even though some of our patients had higher levels of myopia, because extensive corneal haze with PRK for higher levels of myopia has been reported in adults.134–137
Photorefractive keratectomy was performed as follows. The supine child’s head was fixated in the desired position with the plane of the iris perpendicular to the laser beam. For cooperative children, topical anesthesia was used for the PRK. These cooperative children then fixated on the fixation light of the excimer laser machine (Visx Star S2, San Jose, California), and the PRK proceeded in the standard fashion. For the children requiring general anesthesia, the surgeon fixated the eye manually with forceps, taking care to avoid globe compression. The laser aiming beam was centered on the entrance pupil. For myopic PRK, laser scrape was used to remove the epithelium, with any residual epithelium being removed manually with a spatula. For hyperopic PRK, the entire epithelium was removed manually. The desired refractive correction was then programmed into the excimer laser, and the PRK was performed. During the entire procedure under general anesthesia, two observers positioned on either side of the patient continually monitored eye position to ensure that the iris plane remained perpendicular to the laser beam. The size of the optical zone was 6.5 mm for all myopic PRKs and 9.0 mm for all hyperopic PRKs.
After the procedure was completed, topical atropine 1%, ketorolac 0.5% (Acular, Allergan, Irvine, California), and gentamicin were placed in the treated eye and a disposable contact lens (SureVue, Johnson and Johnson, Jacksonville, Florida) was placed on the cornea. Collagen plugs were inserted into the upper and lower puncta to maximize the tear film during the initial healing phase, and a soft patch was placed over the eye. Since escape of the inhalational anesthetic in the operating room could potentially affect the function of the excimer laser on subsequent patients, removal of the laryngeal mask airway was deferred until the patient was in the recovery room. The eye patch was removed when the patient was awake in the recovery room.
Postoperative medications included topical ofloxacin (Ocuflox, Allergan, Irvine, California) and loteprednol 0.5% (Lotemax, Bausch and Lomb, Rochester, New York), four times a day in the treated eye until the corneal epithelium healed. Topical ketorolac was prescribed up to four times a day as needed for discomfort for the first 2 postoperative days. Hydrocodone oral elixir was also prescribed as needed for severe discomfort for the first few days. Ofloxacin and loteprednol were discontinued after 1 week, and fluoromethalone 0.25% (FML Forte, Allergan, Irvine, California) was prescribed four times a day for 1 month, followed by a slow taper over the next 5 months.
The children were examined postoperatively at the same time each day until the corneal epithelial defect had healed, at which time the contact lens was removed. The size of the corneal epithelial defect was measured horizontally and followed to determine the rate of corneal healing. The residual epithelial defect size was recorded as the ratio between the diameter of the defect and the horizontal diameter of the cornea.
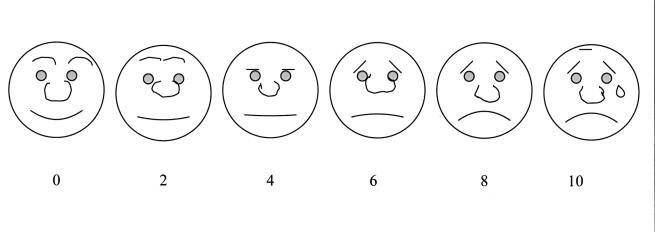
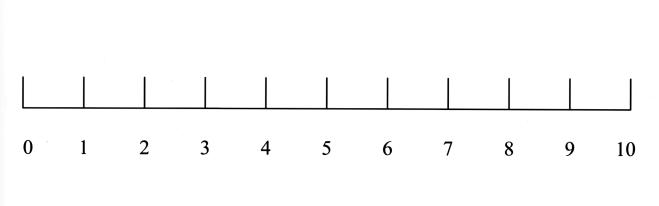
Each day, including the day of surgery, ocular discomfort was assessed using a two-part pain assessment index consisting of a facial expression scale138 and a digital analog scale.138,139 These findings on corneal healing and discomfort following PRK in children have been previously published.139 For the facial expression scale, a sheet of paper with six faces was presented to the parent and child. The six faces had different facial expressions with the happiest face rated “0” and the saddest rated “10” (Figure 1A). The parent and all children who could cooperate were asked to identify the face that they felt best represented the degree of discomfort felt by the child. On the digital analog scale, a line with the numbers 0 to 10 was presented to all parents and to children 5 years and older (Figure 1B). The parent alone for the younger children or the parent and child together for the children 5 years and older were asked to choose the number that best described the child’s discomfort. The number “0” represented no pain and the number “10” represented the worst pain imaginable. The child was examined daily until the corneal epithelium was fully healed and both scales were rated as “0.”
Figure 1a.
Facial expression scale. Note the gradual change in emotion in each face progressing from left to right. The parent and children who could cooperate chose the face that best represented how the child felt.138
Figure 1b.
Digital analog scale. “0” represented no pain and “10” represented the worst pain imaginable. The parent and children who could cooperate were asked to choose the number that most accurately represented the child’s discomfort.
Thereafter, the children were examined 1 month after the procedure and then every 3 months for 12 months and again at 24 months following the surgery. Cycloplegic refractive correction was prescribed as needed at the 1-month examination and updated as needed thereafter. Occlusion therapy was recommended up to 8 hours per day for the sound eye based on the child’s age and visual deficit. Compliance was assessed at each follow-up visit. “Excellent” compliance was defined as parental reporting of compliance with treatment recommendations 76% or more of the time. “Good” compliance meant that the parent reported compliance 51% to 75% of the recommended time, “fair” that parent reported compliance 25% to 50% of the recommended time, and “poor” that the parent reported compliance less than 25% of the recommended time.58
Data analyzed from each comprehensive follow-up examination included uncorrected and best spectacle-corrected visual acuity, stereoacuity, ocular motility, degree of corneal haze, and cycloplegic refraction. Postoperative subepithelial corneal haze was graded on a scale of 0 to 4+ (0 = clear cornea; 1+ = trace haze, only detectable with tangential illumination; 2+ = mild, discrete haze visible with difficulty by focal illumination; 3+ = moderately dense opacity partially obscuring iris detail; 4+ = dense opacity obscuring details of intraocular structures).103,140
Postoperative corneal topography (Humphrey Atlas, version A11.2, Dublin, California) was performed as patient cooperation allowed to assess for centration. Using tangential maps (standardized scale) from the Humphrey Atlas, centration was determined according to the method previously described by Lin and coworkers.141 The edges of the ablation in the X-axis and Y-axis were marked, and the center of the ablation was estimated to be the intersection of the X and Y axes. With the computer cursor positioned at this point, the legend on the topographic map indicated the distance to the nearest 0.01 mm and the angle (semimeridian in degrees) of the ablation zone relative to the pupillary center.
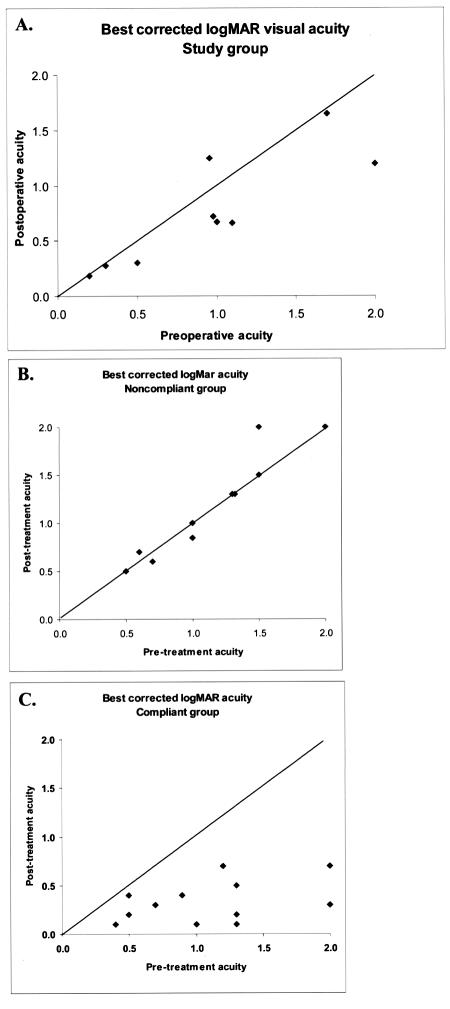
The best spectacle-corrected visual acuities for the PRK study group at the 24-month examination (or last follow-up visit for one child who was lost to follow-up after 6 months) were compared to those of two control groups: (1) anisometropic children who either were diagnosed after age 6 years or were noncompliant with amblyopia therapy (noncompliant control group), and (2) anisometropic amblyopic children who were diagnosed before age 6 and were compliant with therapy (compliant control group). The best-corrected visual acuity at the last visit in the control group was used for comparison. Control group patients were identified retrospectively by medical records review because we felt it would have been unethical to randomize children prospectively to a “no treatment” group in order to obtain these comparison visual acuity data. Control patients came from my practice and from a database of amblyopia patients from multiple pediatric ophthalmology practices. The control children from my practice were consecutively identified using a computer search for “anisometropia.” All control patients had at least 4 diopters of anisometropia and at least 1 year follow-up. Strabismus was the only other eye abnormality the control patients were allowed to have. We believe that the visual acuities in the noncompliant control group were comparable to visual acuities that our treated children might have had had PRK not been performed, and the visual acuities in the compliant group were comparable to visual acuities that our treated children might have had had they been compliant with standard amblyopia therapy.
Visual acuities in the PRK group and the control groups were converted to logMAR acuities for the analyses because of linearity. They were then converted back to the more familiar Snellen values to facilitate review of the data. Statistical calculations were performed using Intercooled Stata, version 7.0 (Stata Corp, College Station, Texas). Continuous data were compared between PRK cases and control groups using the Student t test. Ordinal data were analyzed using logistic regression. Refractive and corneal haze results were analyzed throughout the 24-month follow-up period. Visual acuity outcomes were analyzed at the 12- and 24-month follow-up visits.
Safety of PRK in children with anisometropic amblyopia was assessed using a previously published refractive surgery safety index (safety index = postoperative best-corrected visual acuity ÷ preoperative best-corrected visual acuity).107,108 Efficacy was assessed using a previously published refractive surgery efficacy index (efficacy index = postoperative uncorrected visual acuity ÷ preoperative best-corrected visual acuity).107,108
RESULTS
Anisometropic Amblyopia Treatment Failure Risk Factors
One hundred and four children were included. The mean age at initiation of amblyopia treatment was 4.8 ± 1.5 years. Thirty children (29%) were more than 6 years old, and 59 (57%) were male. Seventyone (68%) were Caucasian, 16 (15%) were Hispanic, 9 (9%) were African American, and 8 (8%) were of mixed origin. Amblyopia affected the right eye of 46 patients (44%), and strabismus was present in 66 (64%). The mean duration of follow-up was 17 months (range, 3 to 95 months).
The absolute value of the mean difference in spherical equivalent refraction between the two eyes was 5.00 diopters (range, 1.00 to 13.00). The mean spherical equivalent refraction in the amblyopic eye was +4.30 diopters (range, +0.75 to +11.00 D) in the hyperopic group and −5.40 diopters (range, −1.50 to −13.00) in the myopic group. The initial best-corrected visual acuity of the amblyopic eye was 20/60 or better in 27 (26%), 20/70 to 20/100 in 31 (30%), 20/125 to 20/200 in 18 (17%), and worse than 20/200 in 27 (26%). The mean best-corrected logMAR acuity in the amblyopic eye was 0.9 (20/160) (range, 0.4 to 2 [20/50 to 20/2000]). The mean logMAR visual acuity in the sound eye was 0.2 (20/30). The mean difference in the logMAR acuity between fellow eyes was 5 lines (range, 3 to 8). Eighty-six patients (83%) were treated with occlusion, and 18 (17%) used atropine penalization of the sound eye.
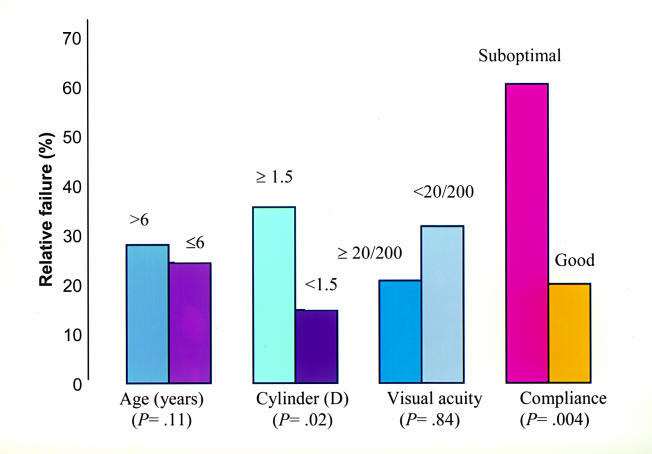
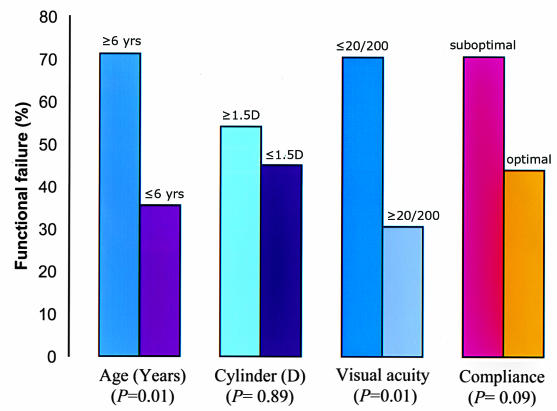
Table 1 summarizes the patient demographics. Table 2 summarizes the relative and functional failure rates for each suspected risk factor. The unadjusted and adjusted ORs and P values for each risk factor for relative and functional failures are presented in Tables 3 and 4, respectively. The correlations of the outcome of treatment to age at initiation of treatment, compliance with the treatment regimen, and amount of astigmatism in the amblyopic eye are presented in Table 5. Overall, 78 patients (75%) experienced relative success (improvement by at least 3 lines of logMAR acuity in the amblyopic eye), and 57 patients (55%) experienced functional success (20/40 or better visual acuity of the amblyopic eye). Each suspected risk factor is explored in detail below.
Table 1.
Anisometropic amblyopia treatment failure analysis: demographics
| Characteristic | Metric |
|---|---|
| Mean age ± SD | 4.8 ± 1.5 years |
| Male:female | 59:45 |
| Median duration of follow-up (range) | 17 months (3 to 95 months) |
| Absolute value of the mean difference in SERE (range) | 5.00 D (1.00 to 13.00 D) |
| Mean best-corrected logMAR acuity in the amblyopic eye (range)[Snellen equivalents] | 0.9 (0.4 to 2) [20/160 (20/50 to 20/2000)] |
| Mean difference in the logMAR acuity between the two eyes (range) | 5 lines (3 to 8 lines) |
SD, standard deviation; SERE, spherical equivalent refractive error.
Table 2.
Suspected risk factors for failure of treatment for anisometropic amblyopia among children aged 3 to 8 years of age
| Characteristic | No. (%) of patients | No. (%) relative failure* | No. (%) functional failure† |
|---|---|---|---|
| Age ≥6 years | 25 (24) | 8 (32) | 18 (72) |
| Concurrent strabismus | 66 (63 ) | 20 (30) | 34 (51) |
| SERE amblyopic eye ≥3.00 D | 70 (67) | 18 (26) | 30 (43) |
| Cylinder of amblyopic eye ≥ 1.50 D | 30 (29) | 13 (43) | 19 (63) |
| Interocular SERE difference ≥4.00 D | 22 (21) | 5 (23) | 12 (54) |
| Initial visual acuity of amblyopic eye of 20/200 or worse | 35 (34) | 7 (20) | 25 (71) |
| Suboptimal treatment compliance | 23 (22) | 12 (52) | 14 (72) |
| Myopia | 23 (22) | 8 (35) | 12 (52) |
D, diopters; SERE, spherical equivalent refractive error.
Relative failure means failure of visual acuity to improve by at least 3 logMAR lines in the amblyopic eye.
Functional failure means failure to achieve a final visual acuity of 20/40 or better in the amblyopic eye.
Table 3.
Multivariate regression analysis of suspected risk factors for relative failure of anisometropic amblyopia treatment (failure of final visual acuity to improve by at least 3 logMAR lines)
| Characteristic | Unadjusted or (95% ci) | P value | Adjusted or (95% ci) | P value |
|---|---|---|---|---|
| Age ≥6 years | 1.68 (0.66, 4.26) | .28 | 2.80 (0.80, 9.84) | .11 |
| Concurrent strabismus | 3.30 (1.13, 9.63) | .03 | 3.96 (0.95, 16.6) | .06 |
| SERE amblyopic eye ≥ 3.00 D | 0.37 (0.15, 0.92) | .031 | 0.41 (0.12, 1.41) | .16 |
| Cylinder of amblyopic eye ≥1.50 D | 3.00 (1.08, 8.35) | .04 | 5.78 (1.27, 26.5) | .02 |
| Interocular SERE difference ≥4.00 D | 0.85 (0.24, 2.89) | .78 | 1.19 (0.68, 2.06) | .58 |
| Initial visual acuity in amblyopic eye of 20/200 or worse | 0.98 (0.36, 2.67) | .97 | 1.15 (0.30, 4.33) | .84 |
| Suboptimal amblyopia treatment compliance | 5.48 (2.00, 15.03) | .001 | 5.47 (1.70, 17.6) | .004 |
| Myopia | 1.87 (0.18, 1.65) | .21 | 1.65 (0.53, 3.75) | .31 |
D, diopter; CI, confidence interval; OR, odds ratio; SERE, spherical equivalent refractive error.
Table 4.
Multivariate regression analysis of suspected risk factors for functional failure of anisometropic amblyopia treatment (final visual acuity of less than 20/40 in the amblyopic eye)
| Characteristic | Unadjusted or (95% ci) | P value | Adjusted or (95% ci) | P value |
|---|---|---|---|---|
| Age ≥6 years | 2.84 (1.18, 6.83) | .02 | 4.69 (1.55, 14.2) | .01 |
| Concurrent strabismus | 2.45 (1.06, 5.65) | .04 | 2.41 (0.79, 7.31) | .12 |
| SERE amblyopic eye ≥3.00 D | 0.88 (0.39, 1.98) | .76 | 1.08(0.37, 3.20) | .89 |
| Cylinder of amblyopic eye ≥1.50 D | 1.63 (0.61, 4.35) | .33 | 1.10 (0.29, 4.21) | .89 |
| Interocular SERE difference of ≥4.00 D | 1.61 (0.57, 4.60) | .32 | 1.40 (0.78, 2.50) | .29 |
| Initial visual acuity in amblyopic eye of 20/200 or worse | 2.61 (1.05, 6.46) | .04 | 3.79 (1.28, 11.2) | .02 |
| Suboptimal amblyopia treatment compliance | 2.07 (0.84, 5.09) | .11 | 2.43 (0.86, 6.85) | .09 |
| Myopia | 1.29 (0.47, 3.53) | .58 | 1.11 (0.7, 2.75) | .76 |
D, diopters; CI, confidence interval; OR, odds ratio; SERE, spherical equivalent refractive error.
Table 5.
Dose-response relationship between (a) age, (B) compliance with the treatment, and (c) cylinder in the amblyopic eye at the onset of treatment and the outcome of anisometropic amblyopia therapy
|
A. | ||||
|
Relative failure* |
Functional failure |
|||
|
Age at onset of treatment |
Unadjusted or (95% CI) |
P value |
Unadjusted or (95% CI) |
P value |
| ≤4 years | 1.00 (referent) | – | 1.00 (referent) | – |
| 4–5 years | 1.74 (0.40, 7.46) | .40 | 0.94 (0.32, 2.73) | .90 |
| ≥6 years
|
2.82 (0.72, 11.20)
|
.08
|
3.02 (1.02, 9.12)
|
.02
|
|
B. | ||||
|
Relative failure |
Functional failure* |
|||
|
Amblyopia treatment compliance |
Adjusted or (95% CI) |
P value |
Unadjusted or (95% CI) |
P value |
| Good | 1.00 (referent) | – | 1.00 (referent) | – |
| Fair | 6.65 (1.58, 28.0) | .01 | 2.02 (0.52, 7.79) | .31 |
| Poor
|
12.0 (2.16, 66.2)
|
.004
|
6.86 (1.36, 34.6)
|
.02
|
|
C. | ||||
|
Relative failure |
Functional failure |
|||
|
Cylinder of amblyopic eye |
Adjusted or (95% CI) |
P value |
Unadjusted or (95% CI) |
P value |
| <1.00 D | 1.00 (referent) | – | 1.00 (referent) | – |
| 1.00 to 1.50 D | 2.2 (0.53, 9.06) | .20 | 2.05 (0.65, 6.47) | .16 |
| ≥1.5 D | 6.6 (2.00, 22.3) | .0002 | 4.6 (1.55, 14.00) | .002 |
Adjusted for age, concurrent strabismus, high cylinder, and poor initial vision.
D, diopters; CI, confidence interval; OR, odds ratio.
Age
Twenty-five children (24%) were 6 years of age or older. Of these, 17 (68%) achieved relative success and 7 (28%) achieved functional success. Of the 79 patients below 6 years of age, 60 (76%) achieved relative success and 49 (62%) achieved functional success. Table 5 shows the dose-response relationship between the age and the risk of amblyopia treatment failure. The risk of relative and functional failure increased as age increased. Age of 6 years or more at the onset of treatment was a statistically significant risk factor for functional failure (OR = 4.69 [1.55, 14.2]; P = .01) (Tables 4 and 5) (Figures 2 and 3).
Figure 2.
Analysis of suspected risk factors for relative failure (failure to achieve at least 3 lines improvement of logMAR visual acuity) of treatment of anisometropic amblyopia.
Figure 3.
Analysis of suspected risk factors for functional failure (failure achieve at least 20/40 visual acuity in the amblyopic eye) of treatment of anisometropic amblyopia.
Degree of Anisometropia
Twenty-two patients (21%) had anisometropia of 4 diopters or more. Of these, 17 (77%) achieved relative success and 10 (45%) achieved functional success. Of the 82 patients who had anisometropia of less than 4 diopters, 59 (72%) achieved relative success and 44 (54%) achieved functional success. The degree of anisometropia was not found to be a statistically significant risk factor for treatment failure (Tables 3 and 4).
Compliance With Treatment
Suboptimal compliance with treatment was reported in 23 patients (22%). Of these, 11 (48%) achieved relative success and 9 (39%) achieved functional success. Among the 81 patients with good compliance, 67 (83%) achieved relative success and 48 (59%) achieved functional success. Table 5 shows the dose-response relationship between compliance and the risk of amblyopia treatment failure. The risk of relative and functional failure increased as compliance with therapy decreased. Poor compliance with treatment was found to be a statistically significant risk factor for relative failure (OR = 5.47 [2.00, 15.03]; P = .004) (Table 5, Figures 2 and 3).
Visual Acuity
Thirty-five patients (34%) had initial acuity of 20/200 or worse. Of these, 28 (80%) achieved relative success and 10 (29%) achieved functional success. Of 69 patients with visual acuity better than 20/200, 50 (73%) achieved relative success and 47 (68%) achieved functional success. The Hosmer-Lemeshow goodness-of-fit statistic showed good fit for the models for lines of acuity gained (P = .84) and for best visual acuity obtained (P = .29) (Figures 2 and 3). Visual acuity of 20/200 or worse in the amblyopic eye was found to be a statistically significant risk factor for functional failure (OR = 3.79 [1.28, 11.2]; P = .01).
Concurrent Strabismus
Sixty-six patients (63%) had concurrent strabismus. Of these, 46 (70%) achieved relative success and 32 (49%) achieved functional success. Of the 38 patients who did not have strabismus, 32 (84%) achieved relative success and 25 (66%) achieved functional success. The association of strabismus with anisometropia was a risk factor for both relative failure (OR = 3.30 [1.13, 9.63]; P = .03) and functional failure (OR = 2.45 [1.06, 5.65]; P = .04). When the results were adjusted for the other risk factors, however, strabismus was not found to be a statistically significant independent risk factor for treatment failure.
Type of Refractive Error
Twenty-three (22%) of the patients were myopic. Of these, 14 (61%) achieved relative success and 11 (48%) achieved functional success. Of the 81 hyperopic patients, 63 (78%) had relative success and 46 (57%) had functional success. Although the risk for failure was slightly higher in myopes, this difference was not statistically significant (Tables 3 and 4).
Spherical Equivalent
Seventy patients (67%) had a spherical equivalent refractive error of more than 3.00 diopters. Of these, 52 (74%) achieved relative success and 40 (57%) achieved functional success. Of the 34 patients with spherical equivalent refractive error of less than 3.00 diopters, 26 (77%) achieved relative success and 17 (50%) achieved functional success. Spherical equivalent refractive error of more than 3.00 diopters in the amblyopic eye was not found to be a statistically significant risk factor for treatment failure (Tables 3 and 4).
Astigmatism
Thirty patients (29%) had astigmatism of 1.5 diopters or more in the amblyopic eye. Of these, 17 (57%) achieved relative success and 11 (37%) achieved functional success. Of the 74 patients with astigmatism of less than 1.5 diopters, 61 (83%) achieved relative success and 46 (62%) achieved functional success. Table 5 shows the dose-response relationship between the amount of astigmatism in the amblyopic eye and the risk of amblyopia treatment failure. The risk of failure increased as the degree of astigmatism in the amblyopic eye increased. Astigmatism of 1.5 diopters or more in the amblyopic eye was found to be a statistically significant risk factor for relative failure (OR = 5.78 [1.27, 26.5]; P = .02) (Figure 2).
Summary of Risk Factors for Anisometropic Amblyopia Treatment
The following risk factors were significantly associated with conventional treatment failure of anisometropic amblyopia: (1) poor compliance with treatment recommendations (relative failure), (2) age 6 years or greater at initiation of treatment (relative failure), (3) astigmatism of 1.5 diopters or more (functional failure), and (4) initial visual acuity of 20/200 or worse (functional failure).
Corneal Thickness
We prospectively examined 198 eyes of 108 children. Fifty-seven patients (53%) were male. The eyes examined were divided equally between the right and left eyes (99 eyes each). One hundred ten eyes (56%) belonged to Caucasian patients, 64 (32%) to Hispanic patients, 12 (6%) to African Americans, and 12 (6%) eyes to patients of multiracial origin.
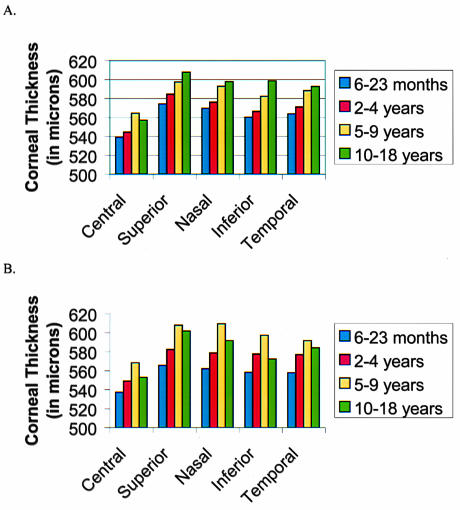
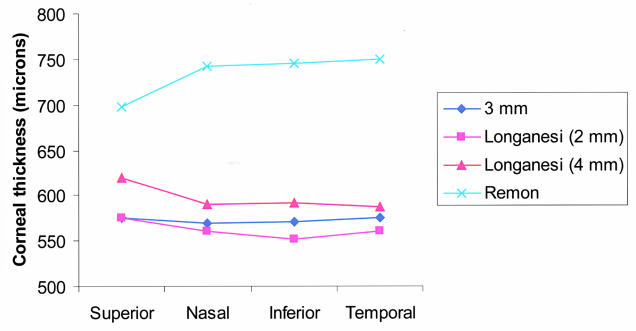
The mean central corneal thickness ± standard deviation (SD) was 544 ± 46 μm. The mean paracentral corneal thickness values ± SD measured at 3 mm from the corneal center were as follows: superior, 575 ± 52 μm; nasal, 568 ± 50 μm; inferior, 568 ± 51 μm; and temporal, 574 ± 47 μm. The mean central corneal thickness values were significantly thinner than at each of the mean paracentral thicknesses (P < .05 for each comparison, paired t test). The paracentral corneal thickness measurements demonstrated no significant differences between locations (P > .05, ANOVA). The mean central corneal thickness values for the right and the left eyes were 548 μm and 550 μm, respectively, which were not significantly different.
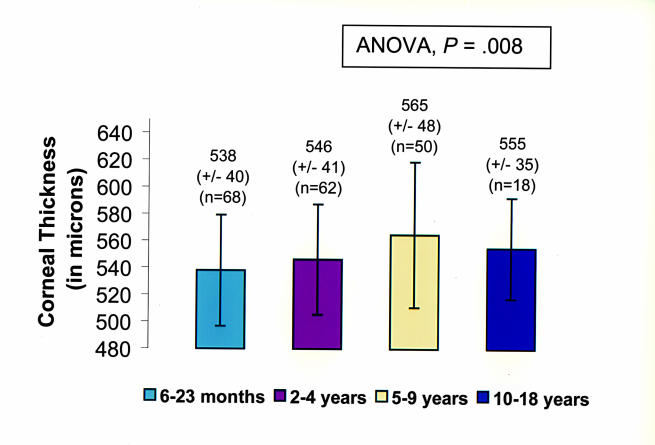
Patients ranged in age from 7 months to 14.7 years old. The number of eyes in each age group was as follows: younger than 2 years old, 68; 2 to 4 years, 62; 5 to 9 years, 50; and 10 to 18 years, 18. The mean central corneal thickness ± SD for each age group was as follows: 6 to 23 months, 538 ± 40 μm; 2 to 4 years, 546 ± 41 μm; 5 to 9 years, 565 ± 48 μm; and 10 to 18 years, 555 ± 35 μm (Figure 4). ANOVA performed on the central pachymetry measurement yielded a significant difference between age groups (P = .008). The two-tailed t test performed in the different age subgroups showed that the central cornea was significantly thicker in the group of children aged 5 to 9 years when compared with either the younger-than-2-years age group or the 2- to 4-year-old age group. The difference in the mean central corneal thickness in the other age groups was not statistically significant. Trends of the central corneal thickness among age groups were similar to those of the paracentral locations (Figure 5).
Figure 4.
Mean central corneal thickness compared by age for both right and left eyes.
Figure 5.
Mean corneal thickness by age and location for right eyes (A) and left eyes (B).
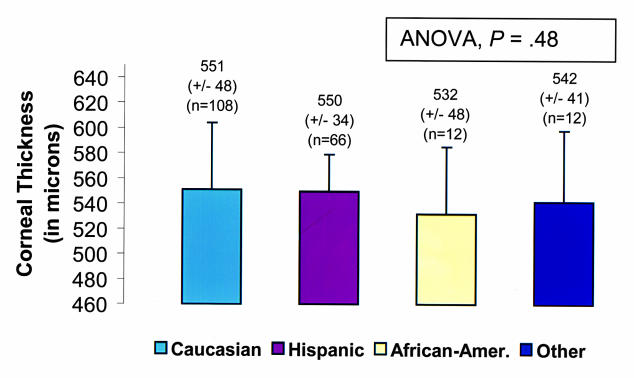
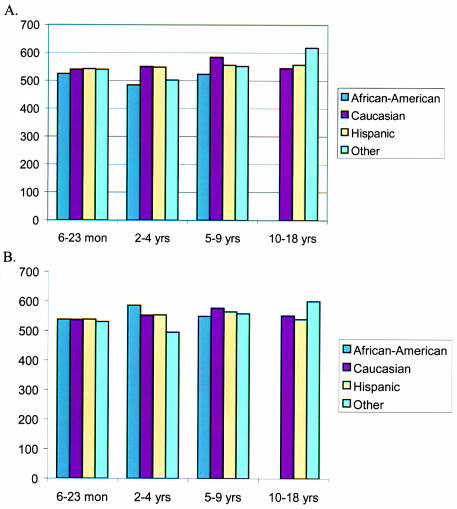
The data were subdivided by ethnic group. Mean central corneal thickness measurements ± SD for each ethnic group were as follows: Caucasian, 551 ± 48 μm; Hispanic, 550 ± 34 μm; African American, 532 ± 48 μm; and other, 542 ± 41 μm (Figure 6). ANOVA performed on central pachymetry values demonstrated no significant differences among racial subgroups overall (ANOVA, P =.48) and when divided into the different age subgroups (ANOVA, P = .79) (Figure 7).
Figure 6.
Mean central corneal thickness measurements by race.
Figure 7.
Mean pachymetry by age and race for right eyes (A) and left eyes (B). There was no significant difference between ethnic groups divided by age.
General Anesthesia Protocol
Nine (82%) of the 11 children who underwent PRK in this study required general anesthesia for the procedure. The mean age of this subgroup was 5.5 years (range, 2 to 9 years). Two were female. None suffered anesthesia-related or treatment-related complications. The mean duration from induction of one case to induction of the next was 31 minutes (22 to 44 minutes). The excimer laser functioned normally with no unexpected refractive results. All patients were discharged home after the standard recovery room observation period of 1 hour. No postoperative complications occurred.
Photorefractive Keratectomy: Safety and Impact on Refractive Error, Visual Acuity, and Stereopsis
The mean age of the 11 treated children was 6.1 years (range, 2 to 11 years). Nine children (82%) were male and 10 (91%) of the treated eyes were right eyes. Eight children were treated for anisomyopia and three for anisohyperopia. Eight children (73%) were Caucasian, one (10%) was Hispanic, and two (18%) were African American. Mean follow-up time was 22 ± 9.4 months (Table 6).
Table 6.
Patient demographics and refractive results of the children who underwent photorefractive keratectomy for anisometropia
| Characteristic | Myopic group | Hyperopic group |
|---|---|---|
| No. of patients | 8 | 3 |
| Mean age in years (range) | 4 (2 to 8) | 9 (8 to 11) |
| Mean preop K readings ± SD (D) | 44.80 ± 1.54 | 42.30 ± 1.06 |
| Mean preop corneal thickness ± SD (μm) | 521 ± 43.4 | 536 ± 42.4 |
| Mean preop SERE ± SD (D) | −13.70 ± 3.77 | +4.75 ± 0.50 |
| Mean interocular SERE difference ± SD (D) | 11.07 ± 4.02 | 4.38 ± 0.45 |
| Maximum refractive SERE dose (D) | −11.50 | +5.25 |
| Mean target SERE ± SD (D) | −3.50 ± 3.70 | plano |
| Mean target SERE reduction ± SD (D) | 10.10 ± 1.39 | 4.75 ± 0.5 |
| Mean 12-mo SERE reduction ± SD (D) | 10.56 ± 3.0 | 4.08 ± 0.80 |
| Mean 24-mo SERE reduction ± SD (D) | 9.70 ± 2.80 | 2.80 ± 1.00 |
| Mean 12-mo postop SERE ± SD (D) | −3.20 ± 2.50 | +0.67 ± 0.50 |
| Mean 24-mo postop SERE ± SD (D) | −3.30 ± 2.54 | +1.78 ± 0.40 |
| Mean SERE 12-mo regression ± SD (D) | 2.50 ± 2.23 | 1.10 ± 1.60 |
| Mean SERE 12- to 24-mo regression ± SD (D) | 0.8 ± 1.27 | 0.90 ± 0.84 |
| No. of pts within 1 D of target at 24 months | 3 of 8 | 1 of 2 |
| No. of pts within 2 D of target at 24 months | 6 of 8 | 2 of 2 |
| % reduction in RE at 24 months | 76% | 63% |
D, diopters; K, keratometry; preop, preoperative; postop, postoperative; mo, month; SD, standard deviation; SERE, spherical equivalent refractive error.
Corneal Healing and Discomfort
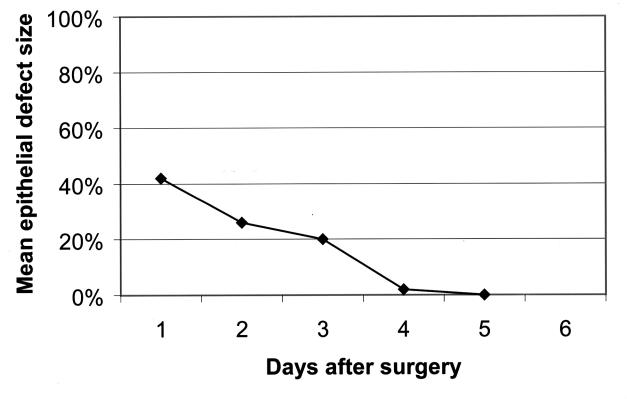
The corneal epithelial defect healed steadily each day in all patients. The mean epithelial defect size (mean percentage of the horizontal corneal diameter) was 43 ± 19% on the first postoperative day, 26 ± 15% on the second postoperative day, 20 ± 6% on the third postoperative day, and 2 ± 2% on the fourth postoperative day (Figure 8). All were healed by the fifth postoperative day. The mean time for complete healing of the corneal defect was 3.5 days (range, 3 to 5 days). The corneal epithelium healed completely in 3 days in six patients (60%), in 4 days in three patients (30%), and in 5 days in one patient (10%). The mean healing time for myopic PRK was 2.8 days and for hyperopic PRK was 4.5 days.
Figure 8.
Mean healing of the corneal epithelial defect following photorefractive keratectomy. The defect size is expressed as a percentage of the cornea size. Note the rapid decrease in the size of the epithelial defect.
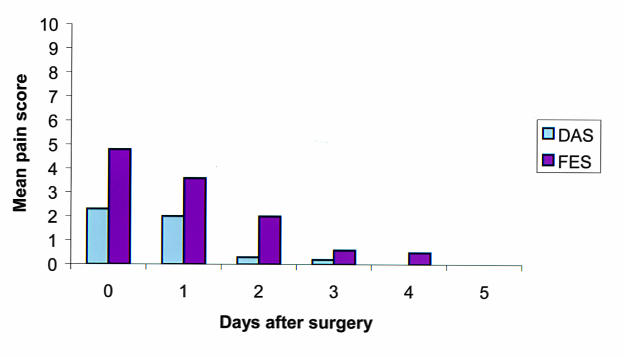
Seven (70%) of the children, aged 6 to 10 years, were able to understand and were willing to evaluate their own discomfort using the facial expression and digital analog scales. The parents of three other children, aged 2 to 5 years, solely evaluated their children’s discomfort. Postoperatively, patients/parents reported mild to moderate discomfort on the day of surgery with a mean facial expression rating of 4.8 (range, 2 to 10) and a mean digital analog rating of 2.3 (range, 1 to 7) (Figure 9). On the first postoperative day, patients/parents reported mild postoperative discomfort, with a mean facial expression score of 3.6 (range, 2 to 10) and a mean digital analog score of 2.0 (range, 0 to 7). On the second postoperative day, the patients/parents reported minimal discomfort, with a mean facial expression score of 2.0 (range, 0 to 4) and a mean digital score of 0.3 (range, 0 to 2). After the second postoperative day, all reported no pain or other discomfort. Five children (50%) used topical ketorolac for discomfort once or twice on the first postoperative day and none thereafter. Three children (30%) used the hydrocodone oral elixir analgesic on the first postoperative day, and none used it thereafter.
Figure 9.
The mean degree of discomfort after PRK as graded using the digital analog scale (DAS) and the facial expression scale (FES). Note the rapid decrease in discomfort in the first 2 days.
Refractive Error
Myopia Group
Table 6 demonstrates complete refractive results. Table 7 shows the preoperative and 24-month postoperative results of the individual patients. The mean preoperative spherical equivalent in the myopic group was −13.70 ± 3.77 diopters; the mean interocular spherical equivalent difference was 11.07 ± 4.02 diopters. The maximum refractive spherical equivalent treatment dose was 11.50 diopters. The mean final target spherical equivalent was −3.50 ± 3.70 diopters. The mean target refractive error reduction was 10.10 ± 1.39 diopters of myopia. The mean spherical equivalent refractive error reductions at 12 and 24 months were 10.56 ± 3.00 diopters and 9.70 ± 2.80 diopters, respectively. The mean 12-month and 24-month postoperative myopic spherical equivalents were −3.20 ± 2.50 diopters and −3.30 ± 2.54 diopters, respectively (Table 6, Figure 10).
Table 7.
Preoperative and postoperative results of all patients with photorefractive keratectomy
| Preoperative data |
2-year postoperative data |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Age (yr) | Se (d) | Interocular se diff (d) | Ucva | Bscva | Stereo (secs of arc) | Ocular alignment (pd) | Prk dose (d) | Se (d) | Interocular se diff (d) | Ucva | Bscva | Stereo (secs of arc) | Ocular alignment (pd) | Corneal haze (0-4+) |
| 1 | 3 | −15.75 | 12.87 | F&F | F&F | Unable | Ortho | −11.5 | −5.9 | 3.00 | F&F | F&F | Unable | Ortho | 1+ |
| 2 | 8 | −10.00 | 8.13 | 20/300 | 20/200 | Nil | Ortho | −10 | 1 | 2.75 | 20/200 | 20/100 | Nil | Ortho | 0.5+ |
| 3 | 2 | −13.75 | 11.62 | F&F | F&F | Unable | Ortho | −10 | −2.5 | 1.50 | 20/60 | 20/60 | Unable | Ortho | 0 |
| 4* | 6 | −15.75 | 14.00 | 3/400 | 20/200 | Nil | Ortho | −10 | −7 | 5.50 | 20/100 | 20/100 | 140 | Ortho | 0.5+ |
| 5 | 10 | +4.25 | 3.88 | 20/60 | 20/30 | 800 | Ortho | +4.25 | 0.8 | 0.60 | 20/60 | 20/30 | 50 | Ortho | 0 |
| 6 | 4 | −11.50 | 11.25 | 20/200 | 20/200 | Nil | Ortho | −11.5 | −5 | 5.00 | 20/600 | 20/400 | 400 | Ortho | 2+ |
| 7 | 7 | −21.00 | 18.35 | 20/100 | 20/800 | Nil | ET 25 | −10 | −4.9 | 1.75 | 20/800 | 20/800 | Nil | ET 16 | 0.5+ |
| 8 | 4 | −9.75 | 9.25 | 20/250 | 20/200 | Nil | ET 20 | −7 | −2.5 | 3.00 | 20/400 | 20/100 | Nil | X(T) 16 | 0.5+ |
| 9 | 4 | −11.75 | 10.25 | 5/400 | 5/400 | Nil | Ortho | −10.5 | −2.75 | 2.75 | 10/300 | 10/300 | Nil | Ortho | 0 |
| 10* | 13 | +5.25 | 4.75 | 20/300 | 20/40 | 400 | Ortho | +5.25 | 0.25 | 0.50 | 20/40 | 20/40 | 100 | Ortho | 0.5+ |
| 11 | 8 | +4.75 | 4.50 | 20/200 | 20/60 | 400 | Ortho | +4.75 | 2.75 | 1.75 | 20/60 | 20/50 | 3000 | Ortho | 1+ |
Follow-up 12 months or less.
BSCVA, best spectacle-corrected visual acuity; diff, difference; F&F, fix and follow; NA, not able; PD, prism diopters; SE, spherical equivalent; UCVA, uncorrected visual acuity.
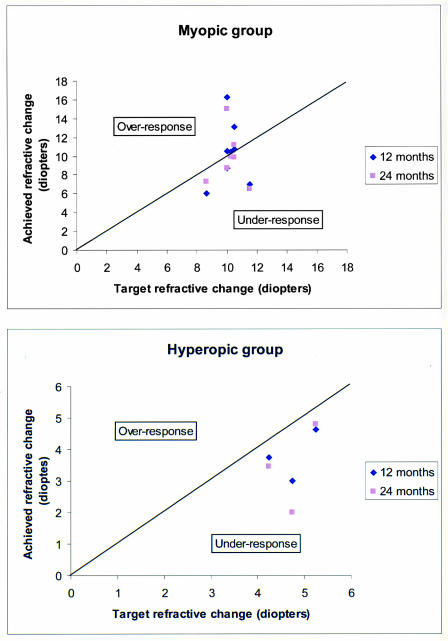
Figure 10.
Target refractive treatment change compared to the 12-month and 24-month results in the myopic and hyperopic groups treated with PRK. Note that the points above the line represent overresponse from target and those below the line represent underresponse from target.
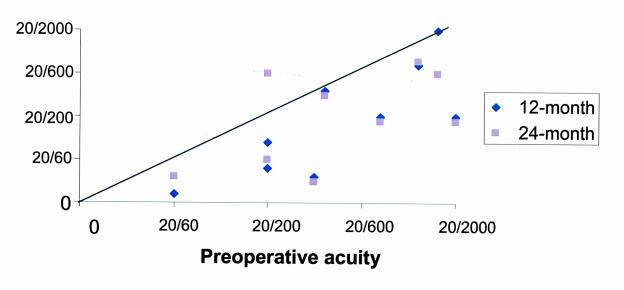
The mean spherical equivalent difference between the 12-month target and 12-month achieved refractive change after myopic PRK was 0.20 ± 2.67 diopters of overresponse. No patient had an overresponse producing hyperopia. At the 24-month follow-up visit, the cycloplegic refractive error of the treated eye was within 3 diopters of that of the fellow eye in six of eight eyes. At this same visit, three of eight myopes were within 1 diopter of target refractive spherical equivalent and six of eight were within 2 diopters (Table 6 and Figure 10). Two patients who were highly myopic preoperatively achieved a greater degree of correction than targeted. Patient 7, with a preoperative spherical equivalent refractive error of −21.00 diopters, had a refractive target reduction of 11.50 diopters but at 12 months achieved a refractive reduction of 16.75 diopters and a spherical equivalent result of −4.75 diopters. Patient 3, with a preoperative spherical equivalent refractive error of −13.75 diopters, had a refractive target reduction of 10.50 diopters and at 12 months achieved a final refractive reduction of 13.25 diopters and a spherical equivalent result of −0.50 diopters. The spherical equivalent refractive errors on these two patients at 24 months were –5.9 diopters and –2.50 diopters, respectively, demonstrating some regression of effect.
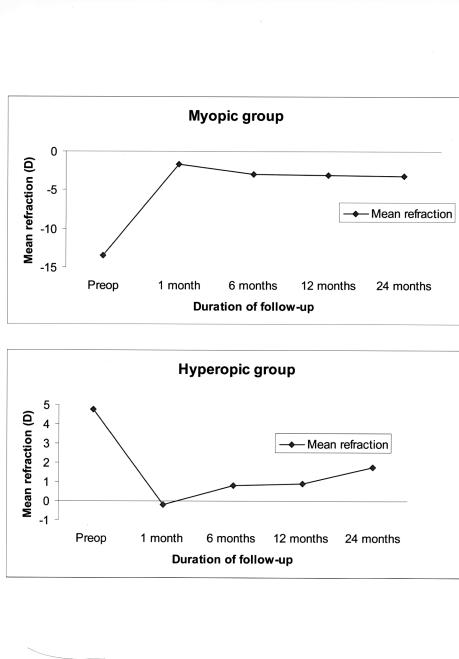
Refractive error stability over the 24-month follow-up period is illustrated in Figure 11. Our myopic group had moderate refractive regression over the first 12-month follow-up period with a mean spherical equivalent regression of 2.50 ± 2.23 diopters, which stabilized over the next 12 months with minimal further regression of 0.50 ± 1.07 diopters.
Figure 11.
Refractive error stability over time in the myopic and hyperopic subgroups of children treated with PRK. The mean refraction is the spherical equivalent refraction.
Hyperopia Group
Table 6 demonstrates complete refractive results. Table 7 shows the preoperative and 24-month postoperative results of the individual patients. The mean preoperative spherical equivalent in the hyperopic group was +4.75 ± 0.50 diopters; the mean interocular spherical equivalent difference was 4.38 ± 0.45 diopters. The maximum refractive spherical equivalent treatment dose was 5.25 diopters. The mean final target spherical equivalent was plano, and the mean target refractive error reduction was 4.75 diopters ± 0.50 diopters. The mean refractive error reductions at 12 and 24 months were +4.08 ± 0.80 diopters and 2.80 ± 1.00 diopters, and the mean 12-month and 24-month postoperative hyperopic spherical equivalent refractive errors were +0.67 ± 0.50 diopters and +1.78 ± 1.40 diopters, respectively (Table 6, Figure 10). The mean spherical equivalent difference between the 12-month target and 12-month achieved refractive change after hyperopic PRK was 0.96 ± 0.68 diopters of underresponse. At the 24-month follow-up visit, the cycloplegic refractive error of the treated eye in both children who returned for follow-up was within 3 diopters of the fellow eye. At this same visit, one hyperope was within 1 diopter of target spherical equivalent. The other, who had developed late-onset peripheral anterior corneal stromal haze, was within 2 diopters of target. The last child did not return for follow-up (Figure 10).
Refractive error stability over the 24-month follow-up period is demonstrated in Figure 11. Over the first 12-month follow-up interval, our hyperopic group showed mild refractive regression with a mean spherical equivalent regression of 1.10 ± 1.6 diopters. Between 12 and 24 months follow-up, further regression of 0.9 ± 0.8 diopters occurred.
Corneal Haze and Topography
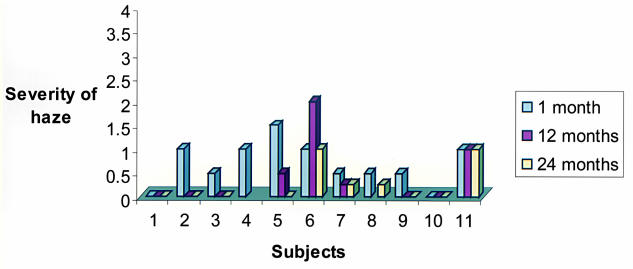
The mean postoperative corneal haze measurement at 12 months was 0.5+ (range, 0 to 2+) (Figure 12). All but one child with residual corneal haze were myopic. Only one patient (age 4 at treatment) had mild to moderate corneal haze (2+) at 12 months. This child had not been compliant with the postoperative treatment protocol. He did not return for follow-up after the 1-month examination until the 12-month examination and discontinued the fluoromethalone drops 1 month after the surgery. At 24 months after treatment, the haze in this child has decreased to 1+. The remainder of the patients had only minimal or no haze throughout the entire follow-up period. At the 24-month follow-up visit, the mean corneal haze measurement was 0.25+.
Figure 12.
Corneal haze at 1, 12, and 24 months after PRK in 11 children.
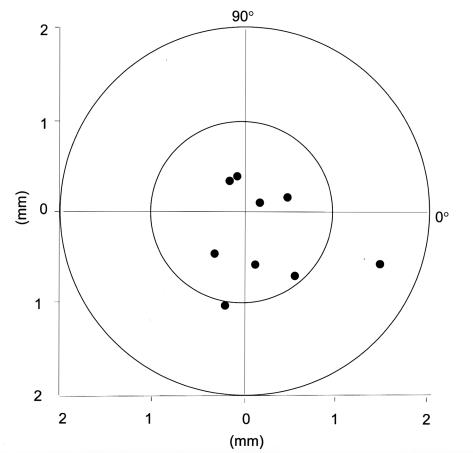
The mean treatment decentration on the cornea of the nine patients cooperative enough to undergo corneal topography was 0.68 ± 0.43 mm (Table 8, Figure 13). The child with the largest decentration was 7 years old at the time of the procedure, had a preoperative spherical equivalent refractive error of –21.00, and had a visual acuity of 5/200 preoperatively and postoperatively with eccentric fixation in this eye. The other outlier with 1.05 mm of decentration was 8 years old at the time of the procedure and had undergone hyperopic PRK under general anesthesia. Her postoperative uncorrected and best spectacle-corrected visual acuities in this eye at the 24-month examination were 20/60 and 20/40, respectively, compared with 20/200 and 20/60 preoperatively.
Table 8.
Corneal topography for decentration of photorefractive keratectomy (prk) treatment
| Patient | Prk type | Decentration distance (mm) | Semimeridian (degree) |
|---|---|---|---|
| 2 | Myopic | 0.59 | 281 |
| 4 | Myopic | 0.57 | 234 |
| 5 | Hyperopic | 0.38 | 117 |
| 6 | Myopic | 0.19 | 32 |
| 7 | Myopic | 1.59 | 339 |
| 8 | Myopic | 0.49 | 19 |
| 9 | Myopic | 0.89 | 308 |
| 10 | Hyperopic | 0.40 | 103 |
| 11 | Hyperopic | 1.05 | 258 |
Mean decentration was 0.68 ± 0.43 mm (SD).
Figure 13.
Decentration measurements of the children treated with PRK who were cooperative enough for corneal topography. Mean decentration was 0.68 ± 0.43 mm. The one extreme outlier had a preoperative spherical equivalent refractive error of −21.00 and visual acuity of 5/200 preoperatively and postoperatively with eccentric fixation in this eye. The other outlier with decentration of more than 1 mm had had a hyperopic PRK.
Visual Acuity
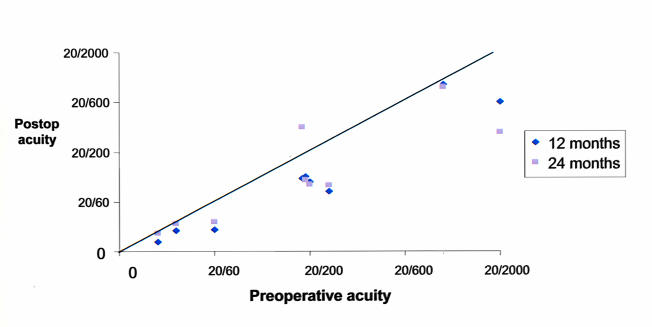
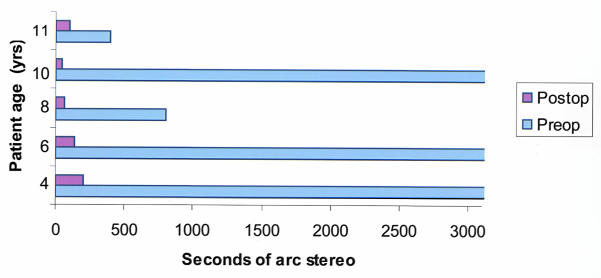
Nine patients were able to perform quantitative acuity tests preoperatively and postoperatively. At last follow-up (mean, 22 months), the uncorrected visual acuity at the 24-month visit had improved by 2 or more Snellen lines from the preoperative acuity in seven of nine eyes, with the maximum improvement of 7 lines (Figure 14A). In this same group, best spectacle-corrected visual acuity improved at 24 months by 2 or more logMAR lines in six of nine eyes and remained within 1 line of the preoperative visual acuity in two eyes (Figure 14B). Three children who experienced an improvement in visual acuity improved to the point that the amblyopic eye was no longer considered legally blind.
Figure 14a.
Comparison of preoperative, 12-month, and 24-month postoperative uncorrected visual acuities. Points below the line represent improved postoperative visual acuity, and points above the line represent reduced postoperative acuity. Seven of nine children able to perform psychophysical visual acuity testing preoperatively and postoperatively had at least 2 lines of improved uncorrected visual acuity.
Figure 14b.
Comparison of preoperative, 12-month, and 24-month postoperative best spectacle-corrected visual acuities. Points below the line represent improved postoperative visual acuity, and points above the line represent reduced postoperative acuity. Six of nine children able to perform psychophysical visual acuity testing preoperatively and postoperatively had at least 2 lines of improved best spectacle-corrected visual acuity.
Case-Control Refractive and Visual Acuity Comparison
Tables 9 and 10 show data comparing our PRK cases to the control groups of compliant children (compliant group, n = 13) and noncompliant/late diagnosis children (noncompliant group, n = 10). The two control groups had similar initial best spectacle-corrected visual acuity, and all control patients had anisometropia of at least 4 diopters. The mean spherical equivalent interocular differences in the myopic PRK and control groups were 12.1 ± 3.2 diopters and 11.1 ± 4.0 diopters, respectively (P = .58). The mean spherical equivalent interocular differences in the hyperopic PRK and control groups were 4.4 ± 0.4 diopters and 5.5 ± 1.2 diopters, respectively (P = .15).
Table 9.
Summary comparisons of prk case and control baseline and final refractive and visual outcomes
| Myopic group |
Hyperopic group |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Cases (n = 9) | Controls (n = 9) | p value | Cases (n = 3) | Controls (n = 15) | p value |
| Age (years) | 4.8 ± 2.1 | 5.1 ± 1.9 | .74 | 9.7 ± 1.5 | 5.6 ± 2.6 | .01 |
| Mean follow-up ± SD (months) | 24 ± 10.2 | 19 ± 5.6 | .09 | 20 ± 6.9 | 15 ± 11.9 | .22 |
| Interocular SERE difference ± SD (D) | 12.1 ± 3.2 | 11.1 ± 4.0 | .58 | 4.4 ± 0.4 | 5.5 ± 1.2 | .15 |
| Final SERE ± SD (D) | −3.3 ± 2.5 | −11.3 ± 4.3 | .0007 | 1.78 ± 1.4 | 6.5 ± 1.4 | <.00001 |
| Difference between initial and final SERE ± SD (D) | −9.9 ± 2.7 | +0.2 ± 0.8 | <.00001 | 3.86 ± 1.8 | 0.6 ± 1.2 | .001 |
| Initial BCVA (Snellen) | 20/400 | 20/350 | .87 | 20/40 | 20/200 | |
BCVA, best corrected visual acuity; D, diopters; SD, standard deviation; SERE, spherical equivalent refractive error; UCVA, uncorrected visual acuity.
Table 10.
Comparison of improvement of visual acuity of photorefractive keratectomy cases and noncompliant and compliant controls
| Characteristic | Cases | Controls | p value |
|---|---|---|---|
| No. of patients with ≥2 lines BCVA after treatment | 6 of 9 | 0 of 10 (noncompliant group) | .003 |
| 12 of 13 (compliant group) | .26 |
BCVA, best-corrected visual acuity.
For the myopic subgroup, comparing our PRK patients (cases) to controls, final spherical equivalent refractive error (P = .007) and difference between initial and final spherical equivalent refractive error (P = .0001) were significantly different. For the hyperopic subgroup, comparing our PRK cases to controls, initial best spectacle-corrected visual acuity (P = .02), final spherical equivalent refractive error (P < .0001), and final difference between spherical equivalent refractive error (P =.001) were significantly different. The mean posttreatment best spectacle-corrected visual acuity of the compliant control group (both myopes and hyperopes) was 20/40, whereas that of the noncompliant control group was 20/270 (P = .002). Six of nine PRK children experienced improved best spectacle-corrected visual acuity by 2 or more logMAR acuity lines with a maximum improvement of 7 lines. In contrast, none of the 10 noncompliant control patients achieved ≥2 logMAR lines of acuity improvement (P = .003) (Table 10, Figure 15).
Figure 15.
Comparison of best spectacle-corrected logMAR visual acuities in our PRK study group (A) compared with a group of noncompliant or late diagnosis anisometropic amblyopic children (P = .003, Fisher’s exact test) (B) and a group of compliant anisometropic amblyopic children (P = .26) (C). Improved visual acuity after treatment is demonstrated as a point below the line. Note how the compliant group uniformly experienced improved visual acuity and the noncompliant group almost uniformly experienced no change in the visual acuity. Most children in the PRK group experienced some improvement in visual acuity, though not as marked as the compliant group.
The safety index was 1.24 (>1 means the best-corrected visual acuity improved postoperatively and vice versa), and the efficacy index was 1.12 (>1 means the postoperative uncorrected visual acuity was better than the preoperative best-corrected visual acuity and vice versa).
Stereoacuity, Ocular Alignment, and Amblyopia Therapy Compliance
Stereopsis was testable preoperatively and postoperatively in nine orthotropic children. Four (aged 4, 4, 7, and 8 years at treatment) had no measurable stereoacuity before or after the treatment. Five patients realized an improvement in stereopsis. These children were 4, 6, 8, 10, and 11 years old at the time of the PRK procedure. The best response was in a 10-year old child at the time of PRK, who improved from no measurable stereopsis to 60 seconds of arc (Figure 16).
Figure 16.
Stereopsis improvement in the five subjects treated with PRK who demonstrated improved postoperative stereopsis. The graph is arranged by age with the youngest child on the bottom. The four other testable nonstrabismic children had no stereopsis preoperatively or postoperatively. Preop, preoperative level; postop, postoperative level.
Ocular alignment did not change postoperatively in most subjects. One patient had a small decrease in his esotropia. Another changed from a small esotropia to a small exotropia (Table 7). Compliance with amblyopia occlusion therapy did not improve postoperatively in any patient. All continued to have poor compliance. Patching attempts were discontinued after 6 to 9 months of effort.
DISCUSSION
The prevalence of amblyopia in the American population is estimated to be 2% to 5%, affecting more than 14 million people.2–6 Amblyopia is the most frequent cause of unilateral visual impairment in children and young adults in the United States and Western Europe.7–11,142,143 It is even more prevalent elsewhere in the world.144 If the amblyopia is severe and bilateral, an affected person can be functionally blind from the condition. Tommila and Tarkkanen145 reported that the risk of vision loss in the fellow eye is markedly higher in people with amblyopia. The incidence of loss of vision in the healthy eye of amblyopes was 1.75 per 1,000 compared to an incidence of 0.11 per 1,000 in the general population. In more than 50% of the cases of visual loss in this study, the cause was traumatic.
Anisometropic amblyopia is the most common form of amblyopia, and because the affected child is usually asymptomatic, late diagnosis is common.7–9,11 Problems with traditional treatments for anisometropia amblyopia are frequent and include poor compliance and long-lasting psychosocial stress for families and patients that continues even into adulthood.40,44 Treatment success for anisometropic amblyopia is achieved in only about two thirds of cases.10–12,18,23,27,29,55–58 Consideration of alternative treatments less dependent on patient/family compliance, such as refractive surgery, is a reasonable consideration.
Currently, most refractive surgeons use the following as upper limits of treatment dose for both PRK and LASIK: 12 diopters for myopia, 4 to 5 diopters for hyperopia, and 4 to 5 diopters for astigmatism.78 Although treating extremely high myopia (greater than 12 diopters) with PRK and LASIK has been reported in adults, the results have been less predictable and the risk for postoperative corneal haze higher.137,146,147 We chose to study PRK in children rather than LASIK because refractive outcomes are equivalent and we felt PRK had a better risk profile for children.
Anisometropic Amblyopia Treatment Failure Risk Factors
Several studies have reported on factors adversely affecting the outcome of treatment for anisometropic amblyopia.17,19,23,57,131,148,149 The severity of the amblyopia has been found to be the most important factor. We explored risk factors that we believed could potentially predict failure of amblyopia treatment in patients with anisometropic amblyopia with or without concurrent strabismus in an attempt to better identify a clinical profile of children who were most likely to fail treatment. Identification of such children might alter the treatment approach, including the potential future application of refractive surgery. Although functional success (visual acuity of at least 20/40) is the more important outcome because of its implications for driving and other activities of daily living, we elected to use a secondary outcome measure (relative success) of at least 3 logMAR lines of acuity improvement as well. This was done because children with extremely poor vision in the amblyopic eye were not excluded. A secondary outcome measure was felt to be necessary to detect useful response to therapy in these children because it was unlikely that they would achieve a visual acuity of 20/40 or better.
Suboptimal compliance with treatment by parental report at the first follow-up examination was the most important predictive factor for both relative and functional failure in our cohort. The worse the reported treatment compliance, the higher was the risk for failure. These results are consistent with other studies showing that compliance with treatment was a factor predicting the success or failure of treatment.19,23,63,131
Older children with amblyopia generally respond less favorably to treatment.7 There are, however, contradictory reports in the literature regarding the role of age at treatment initiation to treatment failure. Oliver and Nawratzki150 found that good therapeutic results were obtained in all age groups up to age 6 years. Lithander and Sjostrand17 also found that age was not critical for a successful treatment up to age 7 years. The Pediatric Eye Disease Investigator Group demonstrated a favorable response to treatment at 4 months in children with moderate and severe anisometropic amblyopia in children up to 6 years of age with no substantial differences in response from 3 to 6 years.10,11,142 In contrast, Flynn and Cassady20 demonstrated a lower success rate and the need for longer duration of therapy with initiation of therapy after 5 years of age. A clear threshold age above which treatment began to fail could not be demonstrated in this retrospective review, though a statistically significant increasing risk for functional failure after 6 years of age was demonstrated.
The risk of functional failure (acuity worse than 20/40) was higher when astigmatism greater than or equal to 1.5 diopters was present in the amblyopic eye. We also demonstrated that the amount of astigmatism was directly proportional to the risk of failure. Weakley21,22 showed that 1.5 diopters of cylindrical anisometropia, regardless of whether the eyes were myopic or hyperopic, was amblyogenic and resulted in decreased binocular function. Somer and coworkers151 concluded that against-the-rule astigmatism had an unfavorable impact on the outcome of amblyopia treatment. A possible explanation for this lack of treatment response in the face of astigmatism is that these children experience image blur at all distances,152 unlike myopic patients who can see clearly at their anterior focal point and hyperopic patients who can partially or completely focus an image through accommodation, leading to more time without a focused image with anisoastigmatism. An alternative explanation is that cylindrical refraction can be more difficult to accurately determine in children, who are often uncooperative for examination, potentially reducing the beneficial effect of spectacle treatment.
It has been reported that initial visual acuity was worse in amblyopic children with both strabismus and anisometropia as compared to those with pure anisometropic amblyopia.17,18,23,56,57 The combination of anisometropia and strabismus has also been reported to have a lower treatment success rate when compared with pure anisometropia.18,29,148,153 In a large, well-controlled prospective study, however, the Pediatric Eye Disease Investigator Group10,11,142 did not find a difference in the initial visual acuity or response to amblyopia treatment in children with concurrent strabismus and anisometropic amblyopia. We, likewise, found that concurrent strabismus was not an independent risk factor for treatment failure in anisometropic amblyopic children.
The relationship between the degree of anisometropia and the depth of the amblyopia is controversial. Although some clinicians have reported no correlation between the degree of anisometropia and the depth of amblyopia,154 others found that higher degrees of anisometropia were associated with more severe amblyopia.18,155,156 Flynn,18 in his meta-analysis of 23 studies, found that successful treatment was associated with less than 4 diopters of anisometropia. Prior to analysis of our data, our clinical gestalt was that we would also find a positive association between the level of anisometropia and the severity of amblyopia. Interestingly, this was not the case. The number of children, however, in our cohort with extreme anisometropia (ie, 6 diopters or more) was small, comprising only 11% of the retrospective cohort. Perhaps our results would have been different if this subgroup had been larger.
Previous studies have demonstrated that poor initial visual acuity in the amblyopic eye was more often associated with a poor outcome.18,23,57 The Pediatric Eye Disease Investigator Group demonstrated, in three well-controlled studies, a good response to treatment in children with moderate or severe amblyopia up to 20/400 acuity, though visual acuity did not reach 20/40 in 22% to 25%.10,11,58 In this study, initial visual acuity of 20/200 or worse was not a risk factor for relative failure but was a risk factor for functional failure, the more important outcome, because this level of acuity (20/40) is required to procure an unrestricted driver’s license in most states in the United States. Patients with very poor initial vision in the amblyopic eye can experience improvement of their vision with treatment, though they may not achieve a final visual acuity of 20/40 or better.
We have demonstrated in this study that the major risk factors predicting failure of traditional therapy for anisometropic amblyopia include poor reported treatment compliance (relative failure), age of 6 years or more at treatment initiation (relative failure), the presence of 1.5 diopters or more of astigmatism (functional failure), and an initial visual acuity of 20/200 or worse (functional failure). It is our belief that such patients have the greatest potential to benefit from refractive surgery.
Corneal Thickness
Knowledge about the thickness of the cornea in children is critical for surgical planning and results of subtraction refractive surgery, such as PRK. The thickness of the cornea limits the degree of correction of refractive errors, because there is a relatively fixed amount of refractive correction that occurs for each micron of cornea ablated. Moreover, it is generally accepted that at least 250 mm of posterior corneal stroma should be left intact following subtraction refractive procedures to protect against the occurrence of keratectasia.157
Both central and peripheral corneal thicknesses have been well studied and reported in adults.158–162 In contrast, very little information has been reported regarding corneal thickness in children, especially children older than 1 year of age. This is particularly true regarding the paracentral cornea, an area included in the treatment zone of most refractive surgical procedures. Such information is essential before embarking on excimer refractive procedures in children. If the pediatric corneal thickness measurement were found to be significantly different from the corneal thickness measurement in adults, PRK treatment nomogram modification may be necessary for best visual and refractive outcomes.
Autzen and Bjornstrom119 found that the mean central corneal thickness in premature infants was 654 mm, significantly thicker than that of the adult. Other studies on corneal thickness in the full-term newborn have likewise reported that the central cornea was thicker than that of the adult, averaging between 573 mm and 583 mm.118,120,124 Ehlers and coworkers123 published the only study that evaluated the central corneal thickness in the age range between birth and 14 years, even though only 60% of the 61 subjects were older than 1 year of age. They also used optical pachymetry, a technology that is less accurate than modern ultrasound pachymetry. The mean thickness of the central cornea from Ehlers’ study was 541 mm for infants and toddlers between 0 and 2 years of age and 520 mm for children in all three groups, 2 to 4 years, 5 to 9 years, and 10 to 14 years of age. Ehlers and coworkers believed that adult corneal thickness was attained by about 3 years of age. Although the report of Ehlers and coworkers gives some good information, more accurate data using modern ultrasound pachymetry on more children between the ages of 2 and 10 years are desirable.
The mean pediatric central and paracentral corneal thicknesses in our pediatric cohort measured with ultrasonic pachymetry were 544 mm and 571 mm, respectively. This central corneal thickness result was not different from the mean adult central corneal thickness.158–161 Furthermore, the trend of a thicker cornea paracentrally in our cohort was similar to that reported in adults.122,158,163,164 The thickness of the paracentral cornea 3 mm from the center in our children fell midway between the mean thicknesses of the paracentral cornea in adults as measured at 2 mm and 4 mm from the center163 (Figure 17).
Figure 17.
Comparison of paracentral corneal measurements from our study and peripheral corneal thickness measurements from several other studies by Longanesi and coworkers163 and Remon and coworkers.121 Our study group (3 mm line) measurements fall between the 2 mm and 4 mm measurements from the Longanesi study on adult corneal thickness.
Combining our data with the previously published information from premature babies and full-term infants, it appears that the central cornea is thicker at birth.118–120,123,124 From the data from our cohort and Ehler and coworkers,123 it then rapidly decreases during the first few months of life followed by a slow increase over the next 9 years. The rapid decrease in the thickness of the cornea occurring in the first few months of life may be explained by regulatory mechanisms that control hydration, evaporation, and transparency.118,123,165
Contrary to what was reported by Ehlers and coworkers,123 who found no difference in the thickness of the cornea in different age groups of children older than 2 years, our results showed a slight increase in the thickness of the cornea with age during the first 9 years. This might be explained by the slight increase in the thickness of Descemet’s membrane with age.166
The central corneal thickness in the adult African American population has been reported to be thinner than that in the Caucasian population.122 The average thickness of the cornea in our African American subgroup was slightly thinner than in our Hispanic and Caucasian subgroups; however, the number of African American children in our study was too small to yield reliable statistical results.
In summary, pediatric central and paracentral corneal thicknesses in the 2- to 14-year-old age groups were consistent with those of the adult. This information is important when planning pediatric subtraction refractive surgery such as PRK. Based on these findings, special surgical considerations regarding corneal thickness are probably not warranted for PRK in children 2 years and older.
General Anesthesia Protocol
Photorefractive keratectomy in adults is performed under topical anesthesia in an office setting. Use of topical or local anesthesia with facial block has been reported in children.106 Children younger than 10 years of age, however, typically cannot be treated with either of these approaches because of inability to adequately cooperate. Unfortunately, waiting until children are old enough to cooperate for the PRK under topical anesthesia is less likely to produce favorable visual outcomes because the sensitive period of visual development will have passed. Therefore, a protocol for general anesthesia was developed to enable PRK treatment of younger children.
This protocol included induction of general anesthesia in an induction room, using the laryngeal mask airway with transfer of the child to the operating room, where nitrous oxide was then administered through a semiclosed circuit. This was done to minimize the risk of leakage of volatile gases into the operating room. The 193-nm wavelength of the argon fluoride excimer laser lies within the absorption spectrum of many anesthetic gases, including nitrous oxide. If a volatile gas escapes, attenuation of the laser beam may occur and the laser will increase voltage in an effort to maintain fluence until the laser malfunctions and an error message appears.125 Using my protocol, no laser malfunctions occurred, implying no or insignificant levels of leakage of nitrous oxide, the only volatile gas used during the PRK procedure.
Other reported protocols to anesthetize children for refractive surgery have included sedation with induction using nitrous oxide and halothane without intubation,102 sevoflurane 50% inhalation with a laryngeal mask,86 and sedation using intravenous ketamine.107 Cook and coworkers125 reported laser shutdown during anesthesia induction using nitrous oxide. They, however, performed anesthesia induction in the same room as the laser procedure, which predisposed to leakage of the nitrous oxide and/or halothane into the operating room. This probable leakage of nitrous oxide most likely caused secondary attenuation of the laser beam and an increase in laser voltage until the laser malfunctioned. An important potential problem with the use of ketamine is its tendency to induce nystagmus, which could render treatment difficult and inaccurate.167,168
Because of the inability of anesthetized children to maintain gaze on a fixation target, another issue of concern with use of general anesthesia was the possibility of a decentered ablation. Mild decentration has not been shown to be a significant problem in any published study.141 It is generally assumed that decentration of greater than 1 mm is needed in order to cause clinically significant adverse visual effects.169 In one pediatric PRK study, one child among the six operated on under general anesthesia had decentration of more than 0.5 mm.102 These results are comparable with optical zone centration analysis following PRK in adults with a mean decentration of 0.5 mm.170 Deitz and coworkers171 reported that 9% of his adult PRK patients had decentration of 1 mm or more from the pupillary center. Importantly, they noted that this amount of decentration did not adversely affect best-corrected visual acuity or contrast sensitivity. In our series, using manual centration and two observers to monitor eye position, the mean decentration (0.68 mm) was slightly higher than that reported for adults but was still within an acceptable range in seven of nine children able to be tested. The cause of the decentration in my group could certainly have been intraoperative difficulty in detecting tilt of the iris plane; however, it is also possible that some of the error was artifactual because of suboptimal fixation of the children during the topographic measurement, especially in those with significant residual postoperative amblyopia. Furthermore, it is noteworthy that the child with the decentration of 1.05 mm experienced a large improvement in uncorrected and best spectacle-corrected visual acuity postoperatively, with the uncorrected visual acuity improving from 20/200 to 20/60 and the best spectacle-corrected visual acuity improving from 20/60 to 20/40.
Photorefractive Keratectomy: Safety and Efficacy
Both PRK and LASIK have been performed in a small number of children.86,100–103,105–109,132,139 Previous studies have reported good short- and moderate-term refractive and vision results. Most were conducted outside of the United States and have predominantly included children aged 7 years and older.86,100,102,103,105–108 By the time a child is 7 years of age, amblyopia is less likely to respond to therapy because the period of visual cortical plasticity (ie, the sensitive period) has passed. Almost all reports in the literature on refractive surgery in children have reported only on myopic PRK in children; only three children treated with hyperopia PRK have been reported.106 None of the previous studies of refractive surgery in children have compared their results with those in a control group.
Ideally, amblyopia should be treated as early in life as possible. If refractive surgery is to play an active role in the treatment of anisometropic amblyopia, it is likely that it, too, will need to be applied early in life, during the sensitive period of visual development. Very little was known about the pediatric response to refractive surgery, namely, the corneal healing rate, postoperative discomfort, refractive response, long-term corneal status, visual acuity, and stereopsis.
We have now followed 11 children, aged 2 to 11 years, for 2 years who were treated with PRK for anisometropic amblyopia, of which six (55%) were less than 6 years of age at treatment. These are some of the youngest children reported to date to have undergone an excimer laser refractive procedure. We have also compared these PRK patients’ visual gains to a control group. I chose to include only children with extreme amounts of anisometropia (≥6 diopters of myopia or ≥4 diopters of hyperopia) who were noncompliant with traditional therapy in order to be as conservative as possible while exploring the potential benefits or complications of PRK in children. In fact, our study group had a mean spherical equivalent interocular difference of 9.90 diopters, well above the entry criteria minimum. We believe, from our own clinical experience and from my noncompliant control group data, that our study group children were likely to have had worse visual outcomes if they had not undergone the PRK.
Corneal epithelial wound healing following PRK in adults treated for myopia has been shown to take approximately 3 to 5 days.126,172 Postoperative epithelial healing is affected by several parameters, with a major factor being the size of the initial epithelial defect. It is generally thought that the corneal epithelium in younger individuals heals faster than in older patients following injury.172 This proved to be the case in our surgically induced epithelial defects for myopia; all but one healed within 3 days. The one who did not heal within 3 days had sustained a postoperative traumatic enlargement of the epithelial defect after being hit in the eye with a ball on the second postoperative day. This postoperative event further supports our decision to study PRK in children because of concerns for postoperative flap disruption from trauma or eye rubbing that could occur with LASIK. The epithelial defect that is created from hyperopic PRK is larger than that created to treat myopia, and healing time is prolonged in adults.173 This was true in our hyperopic study patients as well, with a mean healing time of 4.5 days in the hyperopic group versus 2.8 days for the myopic group.
It has been demonstrated that after PRK, the status of the corneal epithelium can influence collagen synthesis.174 An unhealthy epithelium may result in anterior stromal haze and regression of the refractive correction. The rapid and smooth healing of the corneal epithelium after PRK that occurred in our study patients may imply the presence of healthy epithelium, which may in turn have translated to the minimal corneal haze most of these treated children experienced.
Conflicting reports exist regarding the effect of bandage contact lenses on the rate of epithelial healing after PRK.172,175,176 The disposable soft contact lens used in our study children may have decreased postoperative discomfort and possibly sped up healing. This, however, may not necessarily be true, because two study children lost their contact lenses at 1 and 2 days after PRK and did not appear to experience increased discomfort. Because they were comfortable, the contact lens was not replaced, and the corneal epithelium healed at the same rate as in those children who retained their contact lenses. Insertion of collagen punctal plugs in the upper and lower puncta of the treated eye may also have contributed to rapid epithelial healing by keeping the corneal surface well hydrated during the early healing period. This may have also aided in reducing the postoperative discomfort.
The refractive goal in our study was to reduce the amount of anisometropia to less than or equal to 3 diopters. The aniseikonia would then be reduced to the point where fusion was possible and amblyopia potentially more amenable to therapy.177 The full amount of myopia in the patients with more than −11.50 diopters of spherical equivalent myopia was not treated because PRK for these higher levels of myopia has been associated with severe corneal haze,134–137 an undesirable situation in an adult and an even more undesirable situation in a child whose visual system is immature. Six of eight myopes and all hyperopes had their anisometropia reduced to 3 diopters or less. Interestingly, two patients with extremely high levels of myopia (spherical equivalent of −21.00 diopters and −13.75 diopters) had larger-than-expected responses to their PRK treatment dose of 11.50 diopters, reducing their final refractive errors at 12 months to −4.75 diopters and −0.50 diopters, respectively. Both results were within 3 diopters of the fellow eye. The basis for this overresponse is not known. Williams178 reported a similar result in adults with refractive errors over −10.00 diopters. Astle and coworkers101 also noted this same response in their series of myopic children treated with PRK. Reduced scleral rigidity and/or a difference in corneal remodeling in the highly myopic eye may play a role in children and adults with extremely high levels of myopia treated with PRK.
In the myopic subgroup, four of eight had refractions within 1 diopter of the refractive target and five of eight within 2 diopters at the 12-month postoperative visit. At the 24-month follow-up visit (mean, 22 months), three of eight were within 1 diopter of the target and six of eight were within 2 diopters. The mean preoperative refractive error of −13.70 diopters was reduced to a 24-month mean postoperative refractive error of −3.30 diopters. These results are in agreement with those found in previous studies of PRK in myopic children,100,102,108 though our patients were generally younger. These results are also similar to results reported from adults with similar levels of extremely high myopia treated with PRK.137,179–181 In our hyperopic group, two of the three patients were within 1 diopter of the refractive target and all were within 2 diopters at the 12-month postoperative visit. At the 24-month follow-up visit, only two returned for follow-up. One was within 1 diopter and the other was within 2 diopters of target. There are not enough hyperopic children in this study to make any conclusions, but these results are similar to those of the only study that included pediatric hyperopic PRK.106
The myopic group had moderate refractive regression over the first 12 months following PRK with a mean spherical equivalent regression of 2.50 diopters, though there was wide variation (Figure 12). This early regression was likely due to corneal healing and is similar to refractive regression reported in extremely high myopic adult patients treated with PRK. Regression thereafter in our myopic subgroup appeared to have stabilized with minimal further change. The small change in refractive error after 12 months was probably due to continued eye growth in this pediatric population.182–184 The myopic regression in adults treated with PRK appears to be associated with increased corneal haze and fibrosis. Significant corneal haze was not an issue in our compliant PRK children, so the mechanism for regression in children may be different. Other studies in children treated with PRK for myopia have demonstrated a myopic shift (ie, regression) ranging from 0.8 diopters to 1.7 diopters over a 12-month follow-up period, though the levels of treated myopia tended to be lower overall than in the current study.100–103,108
The hyperopic group showed mild refractive regression over the initial 12-month follow-up interval, with a mean spherical equivalent regression of 1.10 diopters. This amount of regression is similar to that found in adult hyperopic PRK for similar levels of preoperative hyperopia.73,185–187 At the 24-month follow-up, one was stable and the other had experienced further regression. There are too few hyperopic subjects to draw any further conclusions.
Corneal haze in our cohort during the early preoperative period was minimal and not visually important, even in the one child who was noncompliant with the postoperative topical steroid regimen. The haze was at its maximum at the 1-month follow-up visit and was never worse than mild in the group who followed postoperative treatment recommendations. The corneal haze never impacted the retinoscopic reflex during retinoscopy and thus was not felt to be visually important in any patient. Four (36%) of the study patients had a minimal non–visually significant degree of residual corneal haze, and one child had mild to moderate corneal haze 1 year after surgery. All but one of these children were high myopes. All children had no or minimal haze at the 24-month visit.
Higher degrees of corneal haze have been reported in adults with extremely high myopia following PRK.134–137,182 Alio and coworkers102 reported that corneal haze was the main optical complication following pediatric PRK, but noted that it decreased by 1 year. In the only study that included hyperopic PRK in children, corneal haze was not mentioned.106 The low incidence of corneal haze in our study group may have been due to the longer use of topical corticosteroids, good hydration during the early postoperative period, or improvements in excimer laser technology. The VISX Star S2 laser, a third-generation excimer laser, was used in this study. Older studies of pediatric refractive surgery used first-generation broad-beam excimer lasers.102,106
Most children treated with PRK in our study enjoyed mild to moderate improvement in uncorrected and best spectacle-corrected visual acuity at 12 months and 24 months after PRK, despite many having severe amblyopia at presentation. When compared with the noncompliant control group, my PRK group experienced a statistically significant improvement in best-corrected visual acuity. This is the first study of refractive surgery in children that has compared visual outcomes to a control group of comparable children. Efficacy has been demonstrated even in the face of severe preexisting amblyopia in many of the study children. We feel that if refractive surgery were performed at an earlier age, before severe amblyopia has been established, long-term visual outcomes might improve more than was seen in this study group. As an example, the youngest child in this study, who preoperatively at age 2 years could be assessed only by comparing fixation behavior, had a best-corrected visual acuity of 20/50 at the 12-month and the 24-month visits, despite his continued postoperative lack of compliance with spectacle use and amblyopia therapy. Compared with our noncom-pliant control group, his vision was markedly better than what we might have anticipated otherwise (Table 10). For example, there were two noncompliant control patients with spherical equivalent refractive errors on either side of his spherical equivalent refractive error of −13.75 diopters. One, with a spherical equivalent refractive error of −10.50 diopters, had no visual acuity improvement with a pretreatment and posttreatment visual acuity of 20/200. The other child, with a spherical equivalent refractive error of −17.00 diopters, also had no change in visual acuity with a pretreatment and posttreatment visual acuity of 20/300.
Prior to commencing this study, we were concerned first about the safety and second about efficacy of PRK in children with anisometropic amblyopia. The safety index (1.24) found in our study demonstrated that PRK in noncompliant children with anisometropic amblyopia appears to be safe through 24 months follow-up, though this safety index only evaluates visual acuity as an indicator for safety. The efficacy index (1.12) in our study showed some efficacy, even in this group of children with marked anisometropia and profound amblyopia who had already failed traditional amblyopia therapy. We postulate that performing PRK on younger children identified as having a high risk for amblyopia treatment failure would be significantly more efficacious. Stereoacuity improved in five of nine testable children. This was an encouraging but unexpected finding, since most of our patients were well beyond 2 years of age, the age at which most ophthalmologists feel stereopsis development is largely complete. Longer-term follow-up is planned to evaluate for any late complications.
This study, although important, has some limitations. First and foremost, our case-comparison study is limited by a small sample size. However, this small sample size was intentional. Because refractive surgery in children is a new area for research, with little previously published information, we elected to treat only a small group of noncompliant children with severe anisometropia and follow them for a significant period of time to detect any adverse long-term complications, before subjecting other children to this procedure. Because of small sample size in some of the parts of this study, categorical variables were sometimes used for the analysis. Next, when performing the statistical analysis, we assumed that the control groups were representative of severe anisometropic amblyopes in the population at large. Selection bias is a potential weakness of all studies; however, we controlled for this bias as much as possible by including all patients that met the inclusion criteria in our practice and the amblyopia database.
At 24 months post-PRK for anisometropic amblyopia in children, the refractive error response appears to be similar to that of adults with comparable refractive errors. More experience is needed with larger numbers of children with extremely high myopia in order to adequately address the possible need to modify treatment nomograms for this subgroup of patients. Postoperative corneal haze was minimal. Improvements in uncorrected and best spectacle-corrected visual acuities and stereopsis were seen in most children. Photorefractive keratectomy, indeed, appears to have potential as a treatment option for anisometropic amblyopia in children noncompliant with traditional therapy.
SUMMARY
We set out to systematically investigate and demonstrate the potential use of PRK for children with anisometropic amblyopia who were noncompliant with conventional therapy. The predictive risk factors for failure of traditional amblyopia therapy were determined and included (1) suboptimal compliance, (2) age 6 years or older, (3) astigmatism of 1.5 diopters or more, and (4) initial visual acuity of 20/200 or worse. We investigated corneal thickness and found that children between the ages of 2 and 14 years have corneal thickness parameters similar to those of adults, suggesting that PRK treatment nomograms probably do not require adjustment for children or adolescents. A protocol to perform PRK under general anesthesia was designed and successfully implemented, with no intraoperative or postoperative complications or laser malfunctions. Finally, we demonstrated that PRK for anisometropic amblyopia in children was safe, well tolerated, and associated with no significant complications through 2 years of follow-up. Photorefractive keratectomy resulted in statistically significant improvement of uncorrected and best spectacle-corrected visual acuity when compared to a noncompliant control group. Lastly, stereopsis improved in a majority of patients, and this improvement did not seem limited by age. Treating children with anisometropic amblyopia well within the sensitive period of visual development, soon after identification of failure risk factors, appears to be a reasonable consideration and may result in better long-term visual outcomes.
ACKNOWLEDGMENTS
I thank Dr Mohamed A.W. Hussein of Baylor College of Medicine, Department of Ophthalmology, for assisting me in the data collection and graphics. I thank Dr Kirk Wilhelmus of Baylor College of Medicine, Department of Ophthalmology, for assisting me in the statistical analysis of the data.
Footnotes
Supported in part by a grant from the Knights Templar Eye Foundation.
REFERENCES
- 1.Revell MJ. Strabismus: a history of orthoptic techniques. In: Revell M, ed. Strabismus: A History of Orthoptic Techniques. London: Barrie & Jenkins; 1971:164.
- 2.Preslan MW, Novak A. Baltimore Vision Screening Project. Ophthalmology. 1996;103:105–109. doi: 10.1016/s0161-6420(96)30753-7. [DOI] [PubMed] [Google Scholar]
- 3.Preslan MW, Novak A. Baltimore Vision Screening Project. Phase 2. Ophthalmology. 1998;105:150–153. doi: 10.1016/s0161-6420(98)91813-9. [DOI] [PubMed] [Google Scholar]
- 4.McNeil NL. Patterns on visual defects in children. Br J Ophthalmol. 1955;39:688–701. doi: 10.1136/bjo.39.11.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downing AH. Ocular defects in sixty thousand selectees. Arch Ophthalmol. 1947;33:137. [Google Scholar]
- 6.Russell EL, Kada JM, Hufhines DM. Orange County vision screening project. II. Ophthalmologic evaluation. Sight Sav Rev. 1961;31:215. [Google Scholar]
- 7.Greenwald MS. Amblyopia. In: Tasman W, Jaeger EA, eds. Duane’s Clnical Ophthalmology. Philadelphia: JB Lippincott; 1994:1–22.
- 8.Mittelman D. Amblyopia. Pediatr Clin North Am. 2003;50:189–196. doi: 10.1016/s0031-3955(02)00107-4. [DOI] [PubMed] [Google Scholar]
- 9.LaRoche GR. Amblyopia: detection, prevention, and rehabilitation. Curr Opin Ophthalmol. 2001;12:363–367. doi: 10.1097/00055735-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Holmes JM, Kraker RT, Beck RW, et al. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2075–2087. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Repka MX, Beck RW, Holmes JM, et al. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol. 2003;121:603–611. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- 12.de Vries J. Anisometropia in children: analysis of a hospital population. Br J Ophthalmol. 1985;69:504–507. doi: 10.1136/bjo.69.7.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Eye Institute Office of Biometry and Epidemiology Report on the National Eye Institute Visual Acuity Impairment Survey Pilot Study. Washington, DC: National Eye Institute; 1984:81–84.
- 14.Committee on Practice and Ambulatory Medicine Section on Ophthalmology; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Eye examination in infants, children, and young adults by pediatricians: organizational principles to guide and define the child health care system and/or improve the health of all children. Ophthalmology. 2003;110:860–865. doi: 10.1016/S0161-6420(03)00414-7. [DOI] [PubMed] [Google Scholar]
- 15.Committee on Practice and Ambulatory Medicine and Section on Ophthalmology; American Academy of Pediatrics. Use of photoscreening for children’s vision screening. Pediatrics. 2002;109:524–525. doi: 10.1542/peds.109.3.524. [DOI] [PubMed] [Google Scholar]
- 16.Policy Statement on the Use of Photorefraction for Children’s Vision Screening. Schaumburg, Ill: Prevent Blindness America; 1994.
- 17.Lithander J, Sjostrand J. Anisometropic and strabismic amblyopia in the age group 2 years and above: a prospective study of the results of treatment. Br J Ophthalmol. 1991;75:111–116. doi: 10.1136/bjo.75.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn JT, Schiffman J, Feuer W, et al. The therapy of amblyopia: an analysis of the results of amblyopia therapy utilizing the pooled data of published studies. Trans Am Ophthalmol Soc. 1998;96:431–450. [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver M, Neumann R, Chaimovitch Y, et al. Compliance and results of treatment for amblyopia in children more than 8 years old. Am J Ophthalmol. 1986;102:340–345. doi: 10.1016/0002-9394(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 20.Flynn JT, Cassady JC. Current trends in amblyopia therapy. Ophthalmology. 1978;85:428–450. doi: 10.1016/s0161-6420(78)35651-7. [DOI] [PubMed] [Google Scholar]
- 21.Weakley DR., Jr The association between anisometropia, amblyopia, and binocularity in the absence of strabismus. Trans Am Ophthalmol Soc. 1999;97:987–1021. [PMC free article] [PubMed] [Google Scholar]
- 22.Weakley DR., Jr The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108:163–171. doi: 10.1016/s0161-6420(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 23.Kivlin JD, Flynn JT. Therapy of anisometropic amblyopia. J Pediatr Ophthalmol Strabismus. 1981;18:47–56. doi: 10.3928/0191-3913-19810901-12. [DOI] [PubMed] [Google Scholar]
- 24.Cobb CJ, Russell K, Cox A, et al. Factors influencing visual outcome in anisometropic amblyopes. Br J Ophthalmol. 2002;86:1278–1281. doi: 10.1136/bjo.86.11.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanlamai T, Goss DA. Prevalence of monocular amblyopia among anisometropes. Am J Optom Physiol Opt. 1979;56:704–715. doi: 10.1097/00006324-197911000-00006. [DOI] [PubMed] [Google Scholar]
- 26.France TD, France LW. Optical penalization can improve vision after occlusion treatment. J AAPOS. 1999;3:341–343. doi: 10.1016/s1091-8531(99)70042-x. [DOI] [PubMed] [Google Scholar]
- 27.Kaye SB, Chen SI, Price G, et al. Combined optical and atropine penalization for the treatment of strabismic and anisometropic amblyopia. J AAPOS. 2002;6:289–293. doi: 10.1067/mpa.2002.127920. [DOI] [PubMed] [Google Scholar]
- 28.Roberts CJ, Adams GG. Contact lenses in the management of high anisometropic amblyopia. Eye. 2002;16:577–579. doi: 10.1038/sj.eye.6700159. [DOI] [PubMed] [Google Scholar]
- 29.Hiscox F, Strong N, Thompson JR, et al. Occlusion for amblyopia: a comprehensive survey of outcome. Eye. 1992;6:300–304. doi: 10.1038/eye.1992.59. [DOI] [PubMed] [Google Scholar]
- 30.von Noorden GK, Campos EC. Binocular Vision and Ocular Motility. St Louis: Mosby; 2002:538–558.
- 31.Campos EC, Enoch JM. Amount of aniseikonia compatible with fine binocular vision: some old and new concepts. J Pediatr Ophthalmol Strabismus. 1980;17:44–47. doi: 10.3928/0191-3913-19800101-11. [DOI] [PubMed] [Google Scholar]
- 32.Nordlow W. Anisometropia, amblyopia, induced aniseikonia and estimated correction with iseikonic lenses in 4 year-olds. Acta Ophthalmol (Copenh) 1970;48:959–970. doi: 10.1111/j.1755-3768.1970.tb08217.x. [DOI] [PubMed] [Google Scholar]
- 33.van der Torren K. Treatment of amblyopia in strongly anisometropic eyes. Doc Ophthalmol. 1985;59:99–104. doi: 10.1007/BF00162017. [DOI] [PubMed] [Google Scholar]
- 34.Suchecki JK, Donshik P, Ehlers WH. Contact lens complications. Ophthalmol Clin North Am. 2003;16:471–484. doi: 10.1016/s0896-1549(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 35.Wilhelmus KR, Robinson NM, Font RA, et al. Fungal keratitis in contact lens wearers. Am J Ophthalmol. 1988;106:708–714. doi: 10.1016/0002-9394(88)90705-2. [DOI] [PubMed] [Google Scholar]
- 36.Wilhelmus KR. Review of clinical experience with microbial keratitis associated with contact lenses. CLAO J. 1987;13:211–214. [PubMed] [Google Scholar]
- 37.Dart JK. Predisposing factors in microbial keratitis: the significance of contact lens wear. Br J Ophthalmol. 1988;72:926–930. doi: 10.1136/bjo.72.12.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinch TE, Palmon FE, Robinson MJ, et al. Microbial keratitis in children. Am J Ophthalmol. 1994;117:65–71. doi: 10.1016/s0002-9394(14)73016-8. [DOI] [PubMed] [Google Scholar]
- 39.Cruz OA, Sabir SM, Capo H, et al. Microbial keratitis in childhood. Ophthalmology. 1993;100:192–196. doi: 10.1016/s0161-6420(93)31671-4. [DOI] [PubMed] [Google Scholar]
- 40.Holmes JM, Beck RW, Kraker RT, et al. Impact of patching and atropine treatment on the child and family in the amblyopia treatment study. Arch Ophthalmol. 2003;121:1625–1632. doi: 10.1001/archopht.121.11.1625. [DOI] [PubMed] [Google Scholar]
- 41.von Noorden GK. Amblyopia caused by unilateral atropinization. Ophthalmology. 1981;88:131–133. doi: 10.1016/s0161-6420(81)35063-5. [DOI] [PubMed] [Google Scholar]
- 42.Simons K, Gotzler KC, Vitale S. Penalization versus part-time occlusion and binocular outcome in treatment of strabismic amblyopia. Ophthalmology. 1997;104:2156–2160. doi: 10.1016/s0161-6420(97)30047-5. [DOI] [PubMed] [Google Scholar]
- 43.Simons K, Stein L, Sener EC, et al. Full-time atropine, intermittent atropine, and optical penalization and binocular outcome in treatment of strabismic amblyopia. Ophthalmology. 1997;104:2143–2155. doi: 10.1016/s0161-6420(97)30048-7. [DOI] [PubMed] [Google Scholar]
- 44.Packwood EA, Cruz OA, Rychwalski PJ, et al. The psychosocial effects of amblyopia study. J AAPOS. 1999;3:15–17. doi: 10.1016/s1091-8531(99)70089-3. [DOI] [PubMed] [Google Scholar]
- 45.Bhartiya P, Sharma P, Biswas NR, et al. Levodopacarbidopa with occlusion in older children with amblyopia. J AAPOS. 2002;6:368–372. doi: 10.1067/mpa.2002.129043. [DOI] [PubMed] [Google Scholar]
- 46.Campos EC, Schiavi C, Benedetti P, et al. Effect of citicoline on visual acuity in amblyopia: preliminary results. Graefes Arch Clin Exp Ophthalmol. 1995;233:307–312. doi: 10.1007/BF00177654. [DOI] [PubMed] [Google Scholar]
- 47.Leguire LE, Walson PD, Rogers GL, et al. Levodopa/carbidopa treatment for amblyopia in older children. J Pediatr Ophthalmol Strabismus. 1995;32:143–151. doi: 10.3928/0191-3913-19950501-05. [DOI] [PubMed] [Google Scholar]
- 48.Leguire LE, Walson PD, Rogers GL, et al. Longitudinal study of levodopa/carbidopa for childhood amblyopia. J Pediatr Ophthalmol Strabismus. 1993;30:354–360. doi: 10.3928/0191-3913-19931101-04. [DOI] [PubMed] [Google Scholar]
- 49.Leguire LE, Rogers GL, Bremer DL, et al. Levodopa and childhood amblyopia. J Pediatr Ophthalmol Strabismus. 1992;29:290–298. doi: 10.3928/0191-3913-19920901-08. [DOI] [PubMed] [Google Scholar]
- 50.Pandey PK, Chaudhuri Z, Kumar M, et al. Effect of levodopa and carbidopa in human amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39:81–89. doi: 10.3928/0191-3913-20020301-07. [DOI] [PubMed] [Google Scholar]
- 51.Gottlob I, Wizov SS, Reinecke RD. Visual acuities and scotomas after 3 weeks’ levodopa administration in adult amblyopia. Graefes Arch Clin Exp Ophthalmol. 1995;233:407–413. doi: 10.1007/BF00180943. [DOI] [PubMed] [Google Scholar]
- 52.Leguire LE, Komaromy KL, Nairus TM, et al. Long-term follow-up of l-dopa treatment in children with amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39:326–330. doi: 10.3928/0191-3913-20021101-05. [DOI] [PubMed] [Google Scholar]
- 53.Mohan K, Dhankar V, Sharma A. Visual acuities after levodopa administration in amblyopia. J Pediatr Ophthalmol Strabismus. 2001;38:62–67. doi: 10.3928/0191-3913-20010301-05. [DOI] [PubMed] [Google Scholar]
- 54.Flynn JT, Schiffman J, Feuer W, et al. The therapy of amblyopia: an analysis of the results of amblyopia therapy utilizing the pooled data of published studies. Trans Am Ophthalmol Soc. 1998;96:431–450. [PMC free article] [PubMed] [Google Scholar]
- 55.Hardman Lea SJ, Snead MP, Loades J, et al. Microtropia versus bifoveal fixation in anisometropic amblyopia. Eye. 1991;5:576–584. doi: 10.1038/eye.1991.100. [DOI] [PubMed] [Google Scholar]
- 56.Kutschke PJ, Scott WE, Keech RV. Anisometropic amblyopia. Ophthalmology. 1991;98:258–263. doi: 10.1016/s0161-6420(91)32307-8. [DOI] [PubMed] [Google Scholar]
- 57.Woodruff G, Hiscox F, Thompson JR, et al. Factors affecting the outcome of children treated for amblyopia. Eye. 1994;8:627–631. doi: 10.1038/eye.1994.157. [DOI] [PubMed] [Google Scholar]
- 58.Pediatric Eye Disease Investigator Group. . A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002;120:268–278. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 59.Snowdon SK, Stewart-Brown SL. Preschool vision screening: results of a systematic review. York, England: NHS Centre for Review and Dissemination, University of York; 1997:1–92.
- 60.Clarke MP, Wright CM, Hrisos S, et al. Randomised controlled trial of treatment of unilateral visual impairment detected at preschool vision screening. BMJ. 2003;327:1251. doi: 10.1136/bmj.327.7426.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rahi J, Logan S, Timms C, et al. Risk, causes, and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population-based study. Lancet. 2002;360:597–602. doi: 10.1016/s0140-6736(02)09782-9. [DOI] [PubMed] [Google Scholar]
- 62.Membreno JH, Brown MM, Brown GC, et al. A cost-utility analysis of therapy for amblyopia. Ophthalmology. 2002;109:2265–2271. doi: 10.1016/s0161-6420(02)01286-1. [DOI] [PubMed] [Google Scholar]
- 63.Loudon SE, Polling JR, Simonsz HJ. Electronically measured compliance with occlusion therapy for amblyopia is related to visual acuity increase. Graefes Arch Clin Exp Ophthalmol. 2003;241:176–180. doi: 10.1007/s00417-002-0570-z. [DOI] [PubMed] [Google Scholar]
- 64.Loghman-Adham M. Medication noncompliance in patients with chronic disease: issues in dialysis and renal transplantation. Am J Manag Care. 2003;9:155–171. [PubMed] [Google Scholar]
- 65.Vasquez EM, Tanzi M, Benedetti E, et al. Medication noncompliance after kidney transplantation. Am J Health Syst Pharm. 2003;60:266–269. doi: 10.1093/ajhp/60.3.266. [DOI] [PubMed] [Google Scholar]
- 66.Zerwic JJ, Sherry DC, Simmons B, et al. Noncompliance in heart transplantation: a role for the advanced practice nurse. Prog Cardiovasc Nurs. 2003;18:141–146. doi: 10.1111/j.0889-7204.2003.02003.x. [DOI] [PubMed] [Google Scholar]
- 67.Cabana MD, Bruckman D, Bratton SL, et al. Association between outpatient follow-up and pediatric emergency department asthma visits. J Asthma. 2003;40:741–749. doi: 10.1081/jas-120023499. [DOI] [PubMed] [Google Scholar]
- 68.Schein OD, Vitale S, Cassard SD, et al. Patient outcomes of refractive surgery. The refractive status and vision profile. J Cataract Refract Surg. 2001;27:665–673. doi: 10.1016/s0886-3350(01)00844-6. [DOI] [PubMed] [Google Scholar]
- 69.Miller AE, McCulley JP, Bowman RW, et al. Patient satisfaction after LASIK for myopia. CLAO J. 2001;27:84–88. [PubMed] [Google Scholar]
- 70.Wright KW, Guemes A, Kapadia MS, et al. Binocular function and patient satisfaction after monovision induced by myopic photorefractive keratectomy. J Cataract Refract Surg. 1999;25:177–182. doi: 10.1016/s0886-3350(99)80123-0. [DOI] [PubMed] [Google Scholar]
- 71.Kim JH, Hahn TW, Lee YC, et al. Photorefractive keratectomy in 202 myopic eyes: one year results. Refract Corneal Surg. 1993;9:S11–16. [PubMed] [Google Scholar]
- 72.Nagy ZZ, Fekete O, Suveges I. Photorefractive keratectomy for myopia with the Meditec MEL 70G-Scan flying spot laser. J Refract Surg. 2001;17:319–326. doi: 10.3928/1081-597X-20010501-05. [DOI] [PubMed] [Google Scholar]
- 73.Nagy ZZ, Krueger RR, Hamberg-Nystrom H, et al. Photorefractive keratectomy for hyperopia in 800 eyes with the Meditec MEL 60 laser. J Refract Surg. 2001;17:525–533. doi: 10.3928/1081-597X-20010901-05. [DOI] [PubMed] [Google Scholar]
- 74.MacRae SM, Peterson JS, Koch DD, et al. Photoastigmatic refractive keratectomy in myopes. Nidek US Investigators Group. J Refract Surg. 2000;16:122–132. doi: 10.3928/1081-597X-20000301-04. [DOI] [PubMed] [Google Scholar]
- 75.Hugger P, Kohnen T, La Rosa FA, et al. Comparison of changes in manifest refraction and corneal power after photorefractive keratectomy. Am J Ophthalmol. 2000;129:68–75. doi: 10.1016/s0002-9394(99)00268-8. [DOI] [PubMed] [Google Scholar]
- 76.Pop M, Payette Y. Results of bilateral photorefractive keratectomy. Ophthalmology. 2000;107:472–479. doi: 10.1016/s0161-6420(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 77.Pop M, Payette Y. Photorefractive keratectomy versus laser in situ keratomileusis: a control-matched study. Ophthalmology. 2000;107:251–257. doi: 10.1016/s0161-6420(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 78.Stein R. Photorefractive keratectomy. Int Ophthalmol Clin. 2000;40:35–56. doi: 10.1097/00004397-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 79.el Danasoury MA, el Maghraby A, Klyce SD, et al. Comparison of photorefractive keratectomy with excimer laser in situ keratomileusis in correcting low myopia (from −2.00 to −5.50 diopters). A randomized study. Ophthalmology. 1999;106:411–420. doi: 10.1016/S0161-6420(99)90084-2. [DOI] [PubMed] [Google Scholar]
- 80.Gimbel HV, Penno EE, van Westenbrugge JA, et al. Incidence and management of intraoperative and early postoperative complications in 1000 consecutive laser in situ keratomileusis cases. Ophthalmology. 1998;105:1839–1847. doi: 10.1016/s0161-6420(98)91026-0. [DOI] [PubMed] [Google Scholar]
- 81.Gimbel HV, Levy SG. Indications, results, and complications of LASIK. Curr Opin Ophthalmol. 1998;9:3–8. doi: 10.1097/00055735-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 82.Davidorf JM, Zaldivar R, Oscherow S. Results and complications of laser in situ keratomileusis by experienced surgeons. J Refract Surg. 1998;14:114–122. doi: 10.3928/1081-597X-19980301-09. [DOI] [PubMed] [Google Scholar]
- 83.Davis EA, Hardten DR, Lindstrom RL. LASIK complications. Int Ophthalmol Clin. 2000;40:67–75. doi: 10.1097/00004397-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Epstein D, Frueh BE. Indications, results, and complications of refractive corneal surgery with lasers. Curr Opin Ophthalmol. 1995;6:73–78. doi: 10.1097/00055735-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 85.Knorz MC. Flap and interface complications in LASIK. Curr Opin Ophthalmol. 2002;13:242–245. doi: 10.1097/00055735-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 86.Rashad KM. Laser in situ keratomileusis for myopic anisometropia in children. J Refract Surg. 1999;15:429–435. doi: 10.3928/1081-597X-19990701-07. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz GS, Park DH, Schloff S, et al. Traumatic flap displacement and subsequent diffuse lamellar keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:781–783. doi: 10.1016/s0886-3350(00)00744-6. [DOI] [PubMed] [Google Scholar]
- 88.Smith RJ, Maloney RK. Diffuse lamellar keratitis. A new syndrome in lamellar refractive surgery. Ophthalmology. 1998;105:1721–1726. doi: 10.1016/S0161-6420(98)99044-3. [DOI] [PubMed] [Google Scholar]
- 89.Tabbara KF, El-Sheikh HF, Vera-Cristo CL. Complications of laser in situ keratomileusis (LASIK) Eur J Ophthalmol. 2003;13:139–146. doi: 10.1177/112067210301300204. [DOI] [PubMed] [Google Scholar]
- 90.Piccoli PM, Gomes AA, Piccoli FV. Corneal ectasia detected 32 months after LASIK for correction of myopia and asymmetric astigmatism. J Cataract Refract Surg. 2003;29:1222–1225. doi: 10.1016/s0886-3350(02)01914-4. [DOI] [PubMed] [Google Scholar]
- 91.Alio JL, Artola A, Claramonte PJ, et al. Complications of photorefractive keratectomy for myopia: two year follow-up of 3000 cases. J Cataract Refract Surg. 1998;24:619–626. doi: 10.1016/s0886-3350(98)80256-3. [DOI] [PubMed] [Google Scholar]
- 92.Moller-Pedersen T, Cavanagh HD, Petroll WM, et al. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 93.van de Pol C, Soya K, Hwang DG. Objective assessment of transient corneal haze and its relation to visual performance after photorefractive keratectomy. Am J Ophthalmol. 2001;132:204–210. doi: 10.1016/s0002-9394(01)01003-0. [DOI] [PubMed] [Google Scholar]
- 94.Pandey SK, Wilson ME, Trivedi RH, et al. Pediatric cataract surgery and intraocular lens implantation: current techniques, complications, and management. Int Ophthalmol Clin. 2001;41:175–196. doi: 10.1097/00004397-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 95.Rozenman Y, Folberg R, Nelson LB, et al. Painful bullous keratopathy following pediatric cataract surgery with intraocular lens implantation. Ophthalmic Surg. 1985;16:372–374. [PubMed] [Google Scholar]
- 96.Dana MR, Moyes AL, Gomes JA, et al. The indications for and outcome in pediatric keratoplasty. A multicenter study. Ophthalmology. 1995;102:1129–1138. doi: 10.1016/s0161-6420(95)30900-1. [DOI] [PubMed] [Google Scholar]
- 97.Sharma N, Pushker N, Dada T, et al. Complications of pediatric cataract surgery and intraocular lens implantation. J Cataract Refract Surg. 1999;25:1585–1588. doi: 10.1016/s0886-3350(99)00296-5. [DOI] [PubMed] [Google Scholar]
- 98.Wilson ME., Jr The challenge of pediatric cataract surgery. J AAPOS. 2001;5:265–266. doi: 10.1067/mpa.2001.117095. [DOI] [PubMed] [Google Scholar]
- 99.Stein R, Stein HA, Cheskes A, et al. Photorefractive keratectomy and postoperative pain. Am J Ophthalmol. 1994;117:403–405. doi: 10.1016/s0002-9394(14)73155-1. [DOI] [PubMed] [Google Scholar]
- 100.Nucci P, Drack AV. Refractive surgery for unilateral high myopia in children. J AAPOS. 2001;5:348–351. doi: 10.1067/mpa.2001.119787. [DOI] [PubMed] [Google Scholar]
- 101.Astle WF, Huang PT, Ells AL, et al. Photorefractive keratectomy in children. J Cataract Refract Surg. 2002;28:932–941. doi: 10.1016/s0886-3350(02)01304-4. [DOI] [PubMed] [Google Scholar]
- 102.Alio JL, Artola A, Claramonte P, et al. Photorefractive keratectomy for pediatric myopic anisometropia. J Cataract Refract Surg. 1998;24:327–330. doi: 10.1016/s0886-3350(98)80319-2. [DOI] [PubMed] [Google Scholar]
- 103.Nano HD, Jr, Muzzin S, Irigaray F. Excimer laser photorefractive keratectomy in pediatric patients. J Cataract Refract Surg. 1997;23:736–739. doi: 10.1016/s0886-3350(97)80283-0. [DOI] [PubMed] [Google Scholar]
- 104.Autrata R, Rehurek J, Holousova M. [Photorefractive keratectomy in high myopic anisometropia in children] Cesk Slov Oftalmol. 1999;55:216–221. [PubMed] [Google Scholar]
- 105.Nassaralla BR, Nassaralla JJ., Jr Laser in situ keratomileusis in children 8 to 15 years old. J Refract Surg. 2001;17:519–524. doi: 10.3928/1081-597X-20010901-04. [DOI] [PubMed] [Google Scholar]
- 106.Singh D. Photorefractive keratectomy in pediatric patients. J Cataract Refract Surg. 1995;21:630–632. doi: 10.1016/s0886-3350(13)80558-5. [DOI] [PubMed] [Google Scholar]
- 107.Agarwal A, Agarwal T, Siraj AA, et al. Results of pediatric laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:684–689. doi: 10.1016/s0886-3350(00)00299-6. [DOI] [PubMed] [Google Scholar]
- 108.Autrata R, Rehurek J. Clinical results of excimer laser photorefractive keratectomy for high myopic anisometropia in children: four-year follow-up. J Cataract Refract Surg. 2003;29:694–702. doi: 10.1016/s0886-3350(02)01896-5. [DOI] [PubMed] [Google Scholar]
- 109.O’Keefe M, Nolan L. LASIK surgery in children. Br J Ophthalmol. 2004;88:19–21. doi: 10.1136/bjo.88.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Faber JT, Kingma-Wilschut C. Amblyopia. Curr Opin Ophthalmol. 1996;7:8–12. doi: 10.1097/00055735-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 111.Campos E. Amblyopia. Surv Ophthalmol. 1995;40:23–39. doi: 10.1016/s0039-6257(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 112.Billson FA, Fitzgerald BA, Provis JM. Visual deprivation in infancy and childhood: clinical aspects. Aust N Z J Ophthalmol. 1985;13:279–286. doi: 10.1111/j.1442-9071.1985.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 113.Grigg J, Thomas R, Billson F. Neuronal basis of amblyopia: a review. Indian J Ophthalmol. 1996;44:69–76. [PubMed] [Google Scholar]
- 114.Ou RJ, Shaw EL, Glasgow BJ. Keratectasia after laser in situ keratomileusis (LASIK): evaluation of the calculated residual stromal bed thickness. Am J Ophthalmol. 2002;134:771–773. doi: 10.1016/s0002-9394(02)01656-2. [DOI] [PubMed] [Google Scholar]
- 115.Muravchik J. Keratectasia after LASIK. J Cataract Refract Surg. 2000;26:629–630. doi: 10.1016/s0886-3350(00)00456-9. [DOI] [PubMed] [Google Scholar]
- 116.Randleman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267–275. doi: 10.1016/S0161-6420(02)01727-X. [DOI] [PubMed] [Google Scholar]
- 117.Argento C, Cosentino MJ, Tytiun A, et al. Corneal ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:1440–1448. doi: 10.1016/s0886-3350(01)00799-4. [DOI] [PubMed] [Google Scholar]
- 118.Autzen T, Bjornstrom L. Central corneal thickness in full-term newborns. Acta Ophthalmol (Copenh) 1989;67:719–720. doi: 10.1111/j.1755-3768.1989.tb04409.x. [DOI] [PubMed] [Google Scholar]
- 119.Autzen T, Bjornstrom L. Central corneal thickness in premature babies. Acta Ophthalmol (Copenh) 1991;69:251–252. doi: 10.1111/j.1755-3768.1991.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 120.Portellinha W, Belfort R., Jr Central and peripheral corneal thickness in newborns. Acta Ophthalmol (Copenh) 1991;69:247–250. doi: 10.1111/j.1755-3768.1991.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 121.Remon L, Cristobal JA, Castillo J, et al. Central and peripheral corneal thickness in full-term newborns by ultrasonic pachymetry. Invest Ophthalmol Vis Sci. 1992;33:3080–3083. [PubMed] [Google Scholar]
- 122.La Rosa FA, Gross RL, Orengo-Nania S. Central corneal thickness of Caucasians and African Americans in glaucomatous and nonglaucomatous populations. Arch Ophthalmol. 2001;119:23–27. [PubMed] [Google Scholar]
- 123.Ehlers N, Sorensen T, Bramsen T, et al. Central corneal thickness in newborns and children. Acta Ophthalmol (Copenh) 1976;54:285–290. doi: 10.1111/j.1755-3768.1976.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 124.Salz JJ, Azen SP, Berstein J, et al. Evaluation and comparison of sources of variability in the measurement of corneal thickness with ultrasonic and optical pachymeters. Ophthalmic Surg. 1983;14:750–754. [PubMed] [Google Scholar]
- 125.Cook DR, Dhaliwal DK, Davis PJ, et al. Anesthetic interference with laser function during excimer laser procedures in children. Anesth Analg. 2001;92:1444–1445. doi: 10.1097/00000539-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 126.Patel GM, Chuang AZ, Kiang E, et al. Epithelial healing rates with topical ciprofloxacin, ofloxacin, and ofloxacin with artificial tears after photorefractive keratectomy. J Cataract Refract Surg. 2000;26:690–694. doi: 10.1016/s0886-3350(00)00411-9. [DOI] [PubMed] [Google Scholar]
- 127.Brilakis HS, Deutsch TA. Topical tetracaine with bandage soft contact lens pain control after photorefractive keratectomy. J Refract Surg. 2000;16:444–447. doi: 10.3928/1081-597X-20000701-07. [DOI] [PubMed] [Google Scholar]
- 128.Cherry PM. The treatment of pain following excimer laser photorefractive keratectomy: additive effect of local anesthetic drops, topical diclofenac, and bandage soft contact. Ophthalmic Surg Lasers. 1996;27:S477–480. [PubMed] [Google Scholar]
- 129.Frangouli A, Shah S, Chatterjee A, et al. Efficacy of topical nonsteroidal drops as pain relief after excimer laser photorefractive keratectomy. J Refract Surg. 1998;14:S207–208. doi: 10.3928/1081-597X-19980401-14. [DOI] [PubMed] [Google Scholar]
- 130.Verma S, Marshall J. Control of pain after photorefractive keratectomy. J Refract Surg. 1996;12:358–364. doi: 10.3928/1081-597X-19960301-10. [DOI] [PubMed] [Google Scholar]
- 131.Noda S, Hayasaka S, Setogawa T. Occlusion therapy of Japanese children with anisometropic amblyopia without strabismus. Ann Ophthalmol. 1993;25:145–147. [PubMed] [Google Scholar]
- 132.Paysse EA, Hussein MA, Koch DD, et al. Successful implementation of a protocol for photorefractive keratectomy in children requiring anesthesia. J Cataract Refract Surg. 2003;29:1744–1747. doi: 10.1016/s0886-3350(03)00592-3. [DOI] [PubMed] [Google Scholar]
- 133.Wright KW, Walonker F, Edelman P. 10-Diopter fixation test for amblyopia. Arch Ophthalmol. 1981;99:1242–1246. doi: 10.1001/archopht.1981.03930020116012. [DOI] [PubMed] [Google Scholar]
- 134.Piovella M, Camesasca FI, Fattori C. Excimer laser photorefractive keratectomy for high myopia: four-year experience with a multiple zone technique. Ophthalmology. 1997;104:1554–1565. doi: 10.1016/s0161-6420(97)30096-7. [DOI] [PubMed] [Google Scholar]
- 135.Lipshitz I, Loewenstein A, Varssano D, et al. Late onset corneal haze after photorefractive keratectomy for moderate and high myopia. Ophthalmology. 1997;104:369–373. doi: 10.1016/s0161-6420(97)30306-6. [DOI] [PubMed] [Google Scholar]
- 136.Zato MA, Matilla A, Gomez T, et al. Multizone versus monozone in the treatment of high and moderate myopia with an excimer laser. Ophthalmic Surg Lasers. 1996;27:S466–470. [PubMed] [Google Scholar]
- 137.Menezo JL, Martinez-Costa R, Navea A, et al. Excimer laser photorefractive keratectomy for high myopia. J Cataract Refract Surg. 1995;21:393–397. doi: 10.1016/s0886-3350(13)80526-3. [DOI] [PubMed] [Google Scholar]
- 138.Hicks CL, von Baeyer CL, Spafford PA, et al. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 139.Paysse EA, Hamill MB, Koch DD, et al. Epithelial healing and ocular discomfort after photorefractive keratectomy in children. J Cataract Refract Surg. 2003;29:478–481. doi: 10.1016/s0886-3350(03)00010-5. [DOI] [PubMed] [Google Scholar]
- 140.Maguen E, Salz JJ, Nesburn AB, et al. Results of excimer laser photorefractive keratectomy for the correction of myopia. Ophthalmology. 1994;101:1548–1556. doi: 10.1016/s0161-6420(94)31137-7. [DOI] [PubMed] [Google Scholar]
- 141.Lin DT, Sutton HF, Berman M. Corneal topography following excimer photorefractive keratectomy for myopia. J Cataract Refract Surg. 1993;19 (Suppl):149–154. doi: 10.1016/s0886-3350(13)80399-9. [DOI] [PubMed] [Google Scholar]
- 142.Pediatric Eye Disease Investigator Group. . A comparison of atropine and patching treatments for moderate amblyopia by patient age, cause of amblyopia, depth of amblyopia, and other factors. Ophthalmology. 2003;110:1632–1637. doi: 10.1016/S0161-6420(03)00500-1. [DOI] [PubMed] [Google Scholar]
- 143.The course of moderate amblyopia treated with patching in children: experience of the amblyopia treatment study. Am J Ophthalmol 2003;136:620–629 [DOI] [PubMed]
- 144.Saw SM, Husain R, Gazzard GM, et al. Causes of low vision and blindness in rural Indonesia. Br J Ophthalmol. 2003;87:1075–1078. doi: 10.1136/bjo.87.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tommila V, Tarkkanen A. Incidence of loss of vision in the healthy eye in amblyopia. Br J Ophthalmol. 1981;65:575–577. doi: 10.1136/bjo.65.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guell JL, Muller A. Laser in situ keratomileusis (LASIK) for myopia from −7 to −18 diopters. J Refract Surg. 1996;12:222–228. doi: 10.3928/1081-597X-19960201-03. [DOI] [PubMed] [Google Scholar]
- 147.Kim HM, Jung HR. Laser assisted in situ keratomileusis for high myopia. Ophthalmic Surg Lasers. 1996;27:S508–511. [PubMed] [Google Scholar]
- 148.Levartovsky S, Oliver M, Gottesman N, et al. Factors affecting long term results of successfully treated amblyopia: initial visual acuity and type of amblyopia. Br J Ophthalmol. 1995;79:225–228. doi: 10.1136/bjo.79.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Levartovsky S, Gottesman N, Shimshoni M, et al. Factors affecting long-term results of successfully treated amblyopia: age at beginning of treatment and age at cessation of monitoring. J Pediatr Ophthalmol Strabismus. 1992;29:219–223. doi: 10.3928/0191-3913-19920701-08. [DOI] [PubMed] [Google Scholar]
- 150.Oliver M, Nawratzki I. Screening of pre-school children for ocular anomalies. II. Amblyopia. Prevalence and therapeutic results at different ages. Br J Ophthalmol. 1971;55:467–471. doi: 10.1136/bjo.55.7.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Somer D, Budak K, Demirci S, et al. Against-the-rule (ATR) astigmatism as a predicting factor for the outcome of amblyopia treatment. Am J Ophthalmol. 2002;133:741–745. doi: 10.1016/s0002-9394(02)01345-4. [DOI] [PubMed] [Google Scholar]
- 152.Jampolsky A, Flom BC, Weymouth FW, et al. Unequal corrected visual acuity as related to anisometropia. Arch Ophthalmol. 1955;54:893–905. doi: 10.1001/archopht.1955.00930020899013. [DOI] [PubMed] [Google Scholar]
- 153.Ohlsson J, Baumann M, Sjostrand J, et al. Long term visual outcome in amblyopia treatment. Br J Ophthalmol. 2002;86:1148–1151. doi: 10.1136/bjo.86.10.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Helveston EM. Relationship between degree of anisometropia and depth of amblyopia. Am J Ophthalmol. 1966;62:757–759. doi: 10.1016/0002-9394(66)92207-0. [DOI] [PubMed] [Google Scholar]
- 155.Sen DK. Anisometropic amblyopia. J Pediatr Ophthalmol Strabismus. 1980;17:180–184. doi: 10.3928/0191-3913-19800501-13. [DOI] [PubMed] [Google Scholar]
- 156.Townshend AM, Holmes JM, Evans LS. Depth of anisometropic amblyopia and difference in refraction. Am J Ophthalmol. 1993;116:431–436. doi: 10.1016/s0002-9394(14)71400-x. [DOI] [PubMed] [Google Scholar]
- 157.Speicher L, Gottinger W. [Progressive corneal ectasia after laser in situ keratomileusis (LASIK)] Klin Monatsbl Augenheilkd. 1998;213:247–251. doi: 10.1055/s-2008-1034982. [DOI] [PubMed] [Google Scholar]
- 158.Tomlinson A. A clinical study of the central and peripheral thickness and curvature of the human cornea. Acta Ophthalmol (Copenh) 1972;50:73–82. doi: 10.1111/j.1755-3768.1972.tb05643.x. [DOI] [PubMed] [Google Scholar]
- 159.Herndon LW, Choudhri SA, Cox T, et al. Central corneal thickness in normal, glaucomatous, and ocular hypertensive eyes. Arch Ophthalmol. 1997;115:1137–1141. doi: 10.1001/archopht.1997.01100160307007. [DOI] [PubMed] [Google Scholar]
- 160.Hansen FK. A clinical study of the normal human central corneal thickness. Acta Ophthalmol (Copenh) 1971;49:82–89. [PubMed] [Google Scholar]
- 161.Price FW, Jr, Koller DL, Price MO. Central corneal pachymetry in patients undergoing laser in situ keratomileusis. Ophthalmology. 1999;106:2216–2220. doi: 10.1016/S0161-6420(99)90508-0. [DOI] [PubMed] [Google Scholar]
- 162.Martola EL, Baum JL. Central and peripheral corneal thickness. A clinical study. Arch Ophthalmol. 1968;79:28–30. doi: 10.1001/archopht.1968.03850040030009. [DOI] [PubMed] [Google Scholar]
- 163.Longanesi L, Cavallini GM, Toni R. Quantitative clinical anatomy of the human cornea in vivo. A morphometric study by ultrasonic pachymetry and computer-assisted topographic videokeratoscopy. Acta Anat (Basel) 1996;157:73–79. [PubMed] [Google Scholar]
- 164.Hitzenberger CK, Baumgartner A, Drexler W, et al. Interferometric measurement of corneal thickness with micrometer precision. Am J Ophthalmol. 1994;118:468–476. doi: 10.1016/s0002-9394(14)75798-8. [DOI] [PubMed] [Google Scholar]
- 165.Weinreb RN, Lu A, Beeson C. Maternal corneal thickness during pregnancy. Am J Ophthalmol. 1988;105:258–260. doi: 10.1016/0002-9394(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 166.Rodrigues MM. Cornea. In: Jakobiec FA, ed. Ocular Anatomy, Embryology, and Teratology. Philadelphia: Harper & Row, 1982:153–165.
- 167.Sekerci C, Donmez A, Ates Y, et al. Oral ketamine premedication in children (placebo controlled double-blind study) Eur J Anaesthesiol. 1996;13:606–611. doi: 10.1046/j.1365-2346.1996.00058.x. [DOI] [PubMed] [Google Scholar]
- 168.Onoe H, Inoue O, Suzuki K, et al. Ketamine increases the striatal N-[11C]methylspiperone binding in vivo: positron emission tomography study using conscious rhesus monkey. Brain Res. 1994;663:191–198. doi: 10.1016/0006-8993(94)91263-7. [DOI] [PubMed] [Google Scholar]
- 169.Uozato H, Guyton DL. Centering corneal surgical procedures. Am J Ophthalmol. 1987;103:264–275. [PubMed] [Google Scholar]
- 170.Schwartz-Goldstein BH, Hersh PS. Corneal topography of phase III excimer laser photorefractive keratectomy. Optical zone centration analysis. Summit Photorefractive Keratectomy Topography Study Group. Ophthalmology. 1995;102:951–962. doi: 10.1016/s0161-6420(95)30928-1. [DOI] [PubMed] [Google Scholar]
- 171.Deitz MR, Piebenga LW, Matta CS, et al. Ablation zone centration after photorefractive keratectomy and its effect on visual outcome. J Cataract Refract Surg. 1996;22:696–701. doi: 10.1016/s0886-3350(96)80305-1. [DOI] [PubMed] [Google Scholar]
- 172.Demers P, Thompson P, Bernier RG, et al. Effect of occlusive pressure patching on the rate of epithelial wound healing after photorefractive keratectomy. J Cataract Refract Surg. 1996;22:59–62. doi: 10.1016/s0886-3350(96)80271-9. [DOI] [PubMed] [Google Scholar]
- 173.Griffith M, Jackson WB, Lafontaine MD, et al. Evaluation of current techniques of corneal epithelial removal in hyperopic photorefractive keratectomy. J Cataract Refract Surg. 1998;24:1070–1078. doi: 10.1016/s0886-3350(98)80100-4. [DOI] [PubMed] [Google Scholar]
- 174.Del Pero RA, Gigstad JE, Roberts AD, et al. A refractive and histopathologic study of excimer laser keratectomy in primates. Am J Ophthalmol. 1990;109:419–429. doi: 10.1016/s0002-9394(14)74608-2. [DOI] [PubMed] [Google Scholar]
- 175.Robin JB, Keys CL, Kaminski LA, et al. The effect of collagen shields on rabbit corneal reepithelialization after chemical debridement. Invest Ophthalmol Vis Sci. 1990;31:1294–1300. [PubMed] [Google Scholar]
- 176.Acheson JF, Joseph J, Spalton DJ. Use of soft contact lenses in an eye casualty department for the primary treatment of traumatic corneal abrasions. Br J Ophthalmol. 1987;71:285–289. doi: 10.1136/bjo.71.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bateman JB, Christmann LM, Dankner SR, et al. Preferred Practice Pattern: Amblyopia. San Francisco: American Academy of Ophthalmology; 2003:2–25.
- 178.Williams DK. Multizone photorefractive keratectomy for high and very high myopia: long-term results. J Cataract Refract Surg. 1997;23:1034–1041. doi: 10.1016/s0886-3350(97)80077-6. [DOI] [PubMed] [Google Scholar]
- 179.Shah S, Chatterjee A, Smith RJ. Predictability of spherical photorefractive keratectomy for myopia. Ophthalmology 1998;105:2178–2184; discussion 2184–2175. [DOI] [PubMed]
- 180.Heitzmann J, Binder PS, Kassar BS, et al. The correction of high myopia using the excimer laser. Arch Ophthalmol. 1993;111:1627–1634. doi: 10.1001/archopht.1993.01090120049021. [DOI] [PubMed] [Google Scholar]
- 181.Sher NA, Hardten DR, DeMarchi J, et al. Excimer photorefractive keratectomy in very high myopia. Semin Ophthalmol. 1994;9:97–101. doi: 10.3109/08820539409060001. [DOI] [PubMed] [Google Scholar]
- 182.Tabbara KF, El-Sheikh HF, Sharara NA, et al. Corneal haze among blue eyes and brown eyes after photorefractive keratectomy. Ophthalmology. 1999;106:2210–2215. doi: 10.1016/S0161-6420(99)90507-9. [DOI] [PubMed] [Google Scholar]
- 183.Kim JH, Kim MS, Hahn TW, et al. Five years results of photorefractive keratectomy for myopia. J Cataract Refract Surg. 1997;23:731–735. doi: 10.1016/s0886-3350(97)80282-9. [DOI] [PubMed] [Google Scholar]
- 184.Hersh PS, Stulting RD, Steinert RF, et al. Results of phase III excimer laser photorefractive keratectomy for myopia. The Summit PRK Study Group. Ophthalmology. 1997;104:1535–1553. doi: 10.1016/s0161-6420(97)30073-6. [DOI] [PubMed] [Google Scholar]
- 185.Jackson WB, Casson E, Hodge WG, et al. Laser vision correction for low hyperopia. An 18-month assessment of safety and efficacy. Ophthalmology. 1998;105:1727–1738. doi: 10.1016/S0161-6420(98)99045-5. [DOI] [PubMed] [Google Scholar]
- 186.Autrata R, Rehurek J. Laser-assisted subepithelial keratectomy and photorefractive keratectomy for the correction of hyperopia. Results of a 2-year follow-up. J Cataract Refract Surg. 2003;29:2105–2114. doi: 10.1016/s0886-3350(03)00415-2. [DOI] [PubMed] [Google Scholar]
- 187.Vinciguerra P, Epstein D, Radice P, et al. Long-term results of photorefractive keratectomy for hyperopia and hyperopic astigmatism. J Refract Surg. 1998;14:S183–185. doi: 10.3928/1081-597X-19980401-08. [DOI] [PubMed] [Google Scholar]