Abstract
Antigen-presenting cells (APC) play a key role in orchestrating immune responses. T-cell proliferative responses are inhibited during the erythrocyte stages of malaria infection, and a number of studies have suggested that APC are responsible for this phenomenon. In the present studies we examine individual components of the T-cell-activating function of APC: expression of costimulatory and major histocompatibility complex (MHC) class II proteins, the ability to process and present antigen to T cells, and the ability to support cytokine production. We find that during the acute phases of Plasmodium yoelii erythrocyte stage infection, APC upregulate the expression of class II MHC and CD80, maintain expression of CD86, process and present antigen, and support gamma interferon production. However the CD11b+ subpopulation produces a soluble factor or factors that specifically inhibit interleukin-2 (IL-2) production by responding CD4 T cells. This factor is distinct from prostaglandin E2, NO, or transforming growth factor β. The data suggest that IL-2 suppression observed during malaria infection is not due to functional defects of APC but is triggered by production of a factor(s) that actively suppresses production of IL-2 by T cells.
Antigen-presenting cells (APC) influence the outcome of an immune response through cytokine production and display of cell surface protein such as CD80 and CD86, which are essential for T-cell activation. Different types of infection can affect APC function in distinct ways, thereby influencing the subsequent T-cell response. For example, Mycobacterium infection of macrophages may inhibit expression of CD80 and CD86, suppressing the subsequent T-cell response (14). Plasmodium berghei appears to inhibit interleukin-12 (IL-12) production by macrophages as early as 1 day postinfection (34).
Very few studies of the interaction between T cells and APC during the erythrocyte stages of malaria infection have been undertaken. To date, only two detailed reports directly address this issue. In one, the ability of purified dendritic cells to stimulate T-cell proliferation after ingesting Plasmodium falciparum was examined (29). That study found that expression of class II major histocompatibility complex (MHC), expression of costimulatory proteins, and the ability of dendritic cells to support T-cell proliferation were all impaired after ingestion of parasite. In another report, macrophages from mice infected with Plasmodium chabaudi were also found to have deficient accessory function, unrelated to antigen processing or presentation, which was similar to the deficiency induced by purified β-hematin (19). The expression of costimulatory proteins was not examined in that study.
The notion that macrophages from infected mice suppress T-cell proliferation has been a part of our understanding of malaria function for several decades (9, 33), although the underlying mechanisms have been the subject of very few investigations. A similar phenomenon has been described for trypanosome infection (21, 22). In this disease, the mediators nitric oxide (NO) and prostaglandin E2 (PGE2) produced by macrophages suppress IL-2 production, and inhibition of these mediators fully restores IL-2 production. The roles of NO and PGE2 in malaria infection have not been well defined. In one study, indomethacin, which suppresses PGE2 production, partially restored proliferation by lymphocytes from people infected with P. falciparum (13). Inhibition of both NO and PGE2 partially reversed IL-2 suppression associated with P. chabaudi (1), whereas inhibition of NO alone had no effect on the production of IL-2 (19). More studies are needed to determine the significance of each of these mediators in IL-2 inhibition.
In the experiments reported here, we investigated the function of APC from Plasmodium yoelii-infected mice with respect to their ability to support T-cell activation. In contrast to studies carried out in vitro with P. falciparum (16, 29), we find that all three APC subsets (macrophages, dendritic cells, and B cells) upregulate both class II MHC and CD80 expression during malaria infection. APC from malaria-infected mice are potent stimulators of gamma interferon (IFN-γ) production by naive T cells but actively suppress IL-2 production. We demonstrate that IL-2 suppression is mediated by a soluble factor that is distinct from NO, PGE2, or transforming growth factor β (TGF-β). The biological significance of these findings is affirmed by in vivo studies that recapitulate the in vitro results.
MATERIALS AND METHODS
Mice.
All mice (BALB/c, B10.D2, IFN-γ knockout on a B6 background, and T-cell receptor [TCR] transgenic [Tg]) were purchased from Jackson Laboratories and used after a 3-week acclimation period. B10.D2-DO11.10 and BALB-DO11.10 mice express a TCR for chicken ovalbumin (OVA) on CD4 T cells (10). These mice were purchased from Jackson Laboratories and bred in our laboratory animal facility by crossing heterozygous females with non-Tg males. Offspring were determined to be heterozygous if greater than 90% of the CD4+ cells in the peripheral blood were positive when stained with anti-Vβ8.1/8.2 (clone MR5-2; Pharmingen). Mice were housed in the Painter Center, Colorado State University, and all experiments approved by the Institutional Animal Care and Use Committee.
Infection.
For infection with P. yoelii 17X nonlethal parasites (15), a frozen stock of infected BALB/c red blood cells was thawed and injected intraperitoneally (i.p.) into several BALB/c mice (source mice). Four to seven days later, mice were bled from the tail and parasitemia was evaluated. Source mice with parasitemia close to 10% were used. The source mouse was sacrificed and bled from the heart. A total of 106 infected red blood cells diluted in PBS were given to each experimental mouse i.p. in a total volume of 0.1 ml. Female mice were used in all experiments, except male Tg mice were used as a source of CD4+ T cells in vitro. Parasitemias were determined by counting a minimum of 200 Wright-Giemsa-stained red blood cells from a tail nick on day 6 postinfection.
Antibodies.
Antibodies to CD4, Vβ8.1/8.2, CD80 (B7.1), CD86 (B7.2), MHC class II (clone 2G9 for detecting I-Ad and I-Ed), CD11b, and CD11c (clone HL3) were purchased from Pharmingen. Various conjugates were used for cell purification and flow cytometry. The anti-TGF-β antibody specific for murine TGF-β1 and TGF-β2 was purchased from R&D Systems (clone 1D11).
Cell separation.
Spleen cells were prepared after mice were sacrificed with CO2. Cells were isolated in Hanks balanced salt solution plus 2% fetal bovine serum by standard methods (3), with DNase (Sigma) added to all isolation media at a concentration of 5 U/ml to prevent the cell clumping frequently seen in spleens from infected mice.
(i) CD4 T-cell purification.
CD4 T cells were isolated by passing spleen cells over a Sephadex G-10 column (3) to deplete adherent cells, followed by sequential incubation with biotinylated anti-CD4 and streptavidin-labeled magnetic beads. The labeled cells were purified on a magnetic column under conditions recommended by the manufacturer (Miltenyi). This procedure resulted in a population of cells that were approximately 90% pure. All experiments included a control that consisted of CD4+ T cells cultured with OVA and no APC. The results of an experiment were not included if significant cytokine production was seen in this situation, because it implied that the T cells had not been sufficiently depleted of APC.
(ii) CD11b+ cell purification.
Methods used to purify the CD11b+ subset of cells were identical to those used for T-cell purification except that prepassage over a Sephadex column was not performed. The purity of the CD11b population averaged approximately 80%, whereas CD11b-depleted cell preparations contained fewer than 2% CD11b+ cells.
In experiments involving anti-CD3 as a stimulus, APC were prepared from spleen cells by depleting splenocytes of T cells through antibody-mediated cytotoxicity as described previously (3).
Cell culture.
Cell cultures were carried out either in 96-well round-bottomed plates in a total volume of 200 μl or in 48-well flat-bottomed plates in a total volume of 600 μl. APC were cultured at a concentration of 1.5 × 106/ml, and CD4+ T cells were cultured at a concentration of 0.5 × 106/ml. Unless otherwise indicated, all values represent the mean of three replicates, and the standard deviation is given. Anti-CD3 was used as tissue culture supernatant from the cell line 145-2C11 (American Type Culture Collection) at a 1:50 dilution. OVA was obtained from Sigma and used at a concentration of 1 mg/ml. All cultures were harvested at 24 h. Preliminary experiments (in which cultures were harvested at 24 and 72 h) indicated that IL-2 is difficult to measure at later time points, presumably because of utilization. IFN-γ production increased with similar kinetics in cultures from control and infected mice, so we used the 24-h harvest time point for convenience.
Parasitized erythrocytes were used as the antigen in one experiment. For this experiment, the parasitemia of this blood was determined, and it was diluted to 10% with erythrocytes from uninfected mice. A total of 107 erythrocytes were added to each well of the culture. Control wells received 107 uninfected erythrocytes.
Cultures involving the DO11.10 hybridoma, which expresses a TCR recognizing OVA (20), used this cell line at a concentration of 1 × 106/ml and whole spleen cells as APC at a concentration of 2.5 × 106/ml. In experiments where fixed splenic APC were employed, spleen cells were cultured overnight with 1 mg of OVA per ml, harvested, washed three times, and then fixed in 1% paraformaldehyde-phosphate-buffered saline (PBS) for 20 min at room temperature. They were washed again under the same conditions and placed back in culture with the hybridoma cells and no added antigen. Control unfixed cells were treated the same way except that they were incubated in PBS only.
Cytokine production varied between experiments. Some of this variation is due to the fact that early experiments were carried out in 48-well plates in a larger volume and later experiments were carried out in 96-well plates, resulting in a more concentrated supernatant.
The medium for cell culture consisted of Dulbecco modified Eagle medium supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 1 mM HEPES, 0.3 mM l-asparagine, 0.67 mM l-arginine, and penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively). Fresh 2-mercaptoethanol was added to each culture at a final concentration of 50 μM. We found that this combination of reagents, maximized for T-cell growth (8), gave results slightly superior to those with standard Dulbecco modified Eagle medium-based formulations in our system.
Proliferation assays.
CD4 T cells and T-cell-depleted spleen cells were cultured as described above. Anti-CD3-stimulated cultures were incubated for 36 h and pulsed with 1 μCi of tritiated thymidine during the last 8 h. Cells stimulated with parasitized red blood cells were cultured for 5 days in 96-well round-bottomed plates and pulsed during the last 8 h with 1 μCi of tritiated thymidine. Cells were harvested and counted with a Microbeta system (Wallac). For proliferation assays, APC were irradiated at 650 rads before culture.
Cytokine assays.
The enzyme-linked immunosorbent assays for IL-2 and IFN-γ were carried out using reagents purchased from Pharmingen according to the protocol provided by the manufacturer. The clones used for the IL-2 assay were JES6-1A12 (capture) and JES6-5H4 (detection). The clones used for the IFN-γ assay were R4-6A2 (capture) and XMG1.2 (detection).
Transwell cultures.
Transwell culture plates (6.5-mm diameter) were used to search for a soluble IL-2-suppressive factor. For these cultures, 5 × 106 cells were added to the outer well in a total volume of 0.6 ml. The inner well contained 0.3 × 106 purified CD4 T cells from TCR Tg mice (hereafter referred to as OVA-TCR Tg cells) and 1 × 106 spleen cells from uninfected mice in a total volume of 0.1 ml. The inner well was separated from the outer well by a 0.1-μm-pore-size membrane. OVA was added at a final concentration of 1 mg/ml. Medium was harvested from the inner and outer wells after 24 h and pooled for analysis. In experiments testing the effects of indomethacin and NG-monomethyl-l-arginine acetate, these compounds were added at the beginning of the culture period to the outer well.
Flow cytometry.
Cells (0.5 × 106) were incubated for 20 min in PBS with 10 μg of mouse immunoglobulin per ml and 10 μg of rat immunoglobulin per ml (Pierce) and a 1:4 dilution of tissue culture supernatant from a cell line secreting antibody to CD32 (Fc receptor [American Type Culture Collection]) to block Fc binding. Cells were spun and resuspended in 50 μl of PBS with 2 mM EDTA, 0.5% bovine serum albumin, and 0.1% sodium azide with the appropriate dilution of first-stage antibody. After 20 min, cells were washed several times in PBS and incubated with second-stage reagent (a streptavidin conjugate) if necessary in the same manner. All incubations were carried out at room temperature. Cells were then washed in PBS several more times and fixed in 1% paraformaldehyde in PBS until analysis. Flow cytometry was carried out on an Coulter XL instrument, and analysis was accomplished using Elite software (Coulter). For determining the percentage of APC subpopulations expressing CD80 or CD86, cell suspensions were stained with anti-CD11b, anti-CD11c, and either anti-CD80, anti-CD86, or a similarly conjugated isotype-matched control. Alternatively, cultures were stained with anti-CD19 and either anti-CD80, anti-CD86, or a similarly conjugated isotype-matched control. The relevant APC population was gated, first by size and side scatter and then by fluorescence, and the percentage of cells expressing the indicated costimulatory protein in the gated population was determined after setting the lower limits of positivity such that the isotype control stained fewer than 2% of the cells. A minimum of 5,000 gated cells were included in the analysis. Mean fluorescence calculations gave similarly significant results. Class II staining was carried out as described above, substituting an anti-class II antibody for anti-CD80 or anti-CD86 antibodies.
Adoptive transfer.
Spleen cells (40 × 106) from B10.D2-DO11.10 or BALB/c-DO11.10 mice were isolated and adoptively transferred to recipient mice by i.p. administration in 0.2 ml of PBS. The next day, all mice were immunized with 1 mg of OVA suspended in 0.1 ml of PBS. Half of the mice were also infected with 106 parasitized erythrocytes. Six days later the mice were sacrificed, and their spleen cells were isolated and stimulated with 1 mg of OVA per ml. Supernatants were harvested 24 h later for measurement of cytokines.
Nitric oxide and prostaglandin assays.
The assay for NO was performed using the Greiss reagent (3). The prostaglandin assays were carried out using a commercial kit (PGE2 Assay; Neogen, Lexington, Ky.) according to the manufacturer's instructions. Assays were carried out on supernatants harvested after 24 h of culture.
Statistics.
Data were analyzed using Student's t test or one-way analysis of variance (ANOVA) as appropriate. Individual comparisons following ANOVA were made using the Student-Newman-Keul method. The criterion for significance in all studies was a P value of <0.05. Unless otherwise indicated, all values were derived from the mean of three replicates and the standard deviation is reported.
RESULTS
T cells from parasitized mice can proliferate normally when APC from uninfected mice are used.
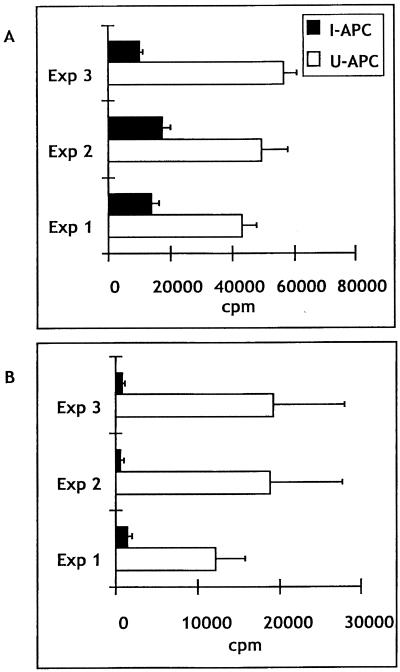
A number of studies indicate that ineffective APC function and/or active suppression by macrophages is responsible for diminished proliferative responses to malaria parasite antigen as well as mitogens and unrelated antigens during malaria infection. However, it is not clear if the APC compartment is the only factor in this suppression or if T cells are also primarily affected by the parasite. In order to test this question, we purified T cells from acutely infected mice and added them to irradiated T-cell-depleted spleen cell preparations from uninfected or infected mice. Both parasitized erythrocytes and anti-CD3 were used as stimuli. While the provision of APC from infected mice resulted in virtually no proliferative response, APC from uninfected mice supported significant proliferation in response to two separate stimuli, parasitized erythrocytes (Fig. 1A) and anti-CD3 (Fig. 1B). This experiment confirms observations by other groups that proliferative responses in acutely infected individuals are diminished, and it demonstrates that T cells from infected mice are fully capable of generating proliferative responses when they are removed from the environment of the infected spleen. Therefore, we can conclude that the T-cell compartment in these mice is functional with respect to proliferation, and it is likely that the defect in proliferation lies primarily with the APC population.
FIG. 1.
T cells from infected mice proliferate when APC from uninfected mice are used. CD4 T cells were cultured with T-cell-depleted spleen cells from uninfected (U-APC) or infected (I-APC) mice. (A) Three separate experiments in which T cells responded to parasitized erythrocytes.(B) Three separate experiments in which T cells responded to stimulation with anti-CD3. In each experiment, three mice were sacrificed and their spleen cells were pooled. Data are expressed as mean ± standard deviations.
Naive T cells exhibit diminished IL-2 production in the presence of APC from infected mice.
In order to focus on the function of APC, for the remainder of these studies we used CD4+ T cells purified from naive mice as responders. T cells came from two sources. For polyclonal stimulation with anti-CD3, CD4 T cells from uninfected mice of various strains were purified by positive selection with anti-CD4 magnetic beads. For stimulation with OVA, CD4 T cells were purified in the same way from OVA-TCR Tg mice with a B10.D2 or BALB/c background. The majority of these T cells have receptors for an OVA-derived peptide presented by class II MHC and are therefore able to generate a primary in vitro response.
Despite numerous reports that T cells from infected mice exhibit diminished proliferative responses, there are few studies that include a measurement of IL-2 production (7). Therefore, we compared IL-2 production by T cells stimulated with either anti-CD3, in the case of non-Tg responders, or OVA, in the case of OVA-TCR Tg T cells. The results shown in Table 1 indicate that the use of APC from infected mice is associated with a substantial reduction in IL-2 production by T cells. Decreased IL-2 production is independent of the mouse strain. Moreover, the degree of parasitemia does not influence the magnitude of inhibition, as BALB/c mice develop a high parasitemia when infected with 17X (average of 49% [n = 25] at day 6 postinfection) whereas B10.D2 and B6 mice develop a relatively low parasitemia (B10.D2, 13% [n = 39]; B6, 15% [n = 24]).
TABLE 1.
Splenic APC from infected mice of several strains fail to support CD4-derived IL-2 production
| Stimulusa | Expt | Background strain | IL-2 (mean pg/ml ± SD) with the following source of APCb:
|
Fold changec | |
|---|---|---|---|---|---|
| Infected spleen | Uninfected spleen | ||||
| CD3 | 1 | B6 | 5.7 ± .5b | 66.7 ± 8.2 | 12 |
| 2 | B10.D2 | 3.2 ± 1.2 | 26.6 ± 3.3 | 8 | |
| 3 | B10.D2 | 14.1 ± 2.2 | 542 ± 52 | 38 | |
| 4 | BALB/c | 17.4 ± 4.7 | 278 ± 46 | 16 | |
| OVA | 5 | BALB/c | 41 ± 4 | 164 ± 24 | 4 |
| 6 | B10.D2 | 17.5 ± 4 | 74.8 ± 15 | 4 | |
| 7 | B10.D2 | 50.5 ± 1.2 | 275 ± 90 | 5 | |
| 8 | B10.D2 | 255 ± 19 | 1,275 ± 104 | 5 | |
| 9 | B10.D2 | 300 ± 19 | 619 ± 104 | 2 | |
T cells responding to CD3 stimulation were purified from wild-type mice of the indicated strain. T cells responding to OVA stimulation were purified from OVA-TCR Tg mice on the indicated background.
All comparisons within an experiment between uninfected and infected mice were significant.
The fold change was derived by dividing the picograms of IL-2 per milliliter with uninfected spleen cells by the picograms of IL-2 per milliliter with infected spleen cells.
IL-2 responses are also decreased when using two types of stimuli, one that depends upon APC uptake, processing, and presentation (OVA) and one that does not (anti-CD3). We have noted in the experiments shown in Table 1 that the decrease in IL-2 production associated with anti-CD3 stimulation is generally greater than the decrease associated with OVA stimulation. It is not clear if this a function of the stimulus itself or of the population of responding T cells (while both are naive, one is a Tg population with more limited heterogeneity). Direct comparison of CD3 and OVA stimulation using the same group of cells would be necessary to further investigate this observation.
APC from infected mice express increased levels of class II MHC and CD80 but not CD86.
Two general mechanisms could account for the diminished IL-2 production observed in these experiments: lack of support for IL-2 production, as might be seen in the absence of costimulation or with failure to present antigen, or specific suppression of IL-2 production by a soluble or cell-associated mediator. The minimum requirements for activation of CD4 T cells are presentation of antigen on class II MHC, followed by ligation of the T-cell receptor, and expression of the appropriate costimulatory proteins CD80 and CD86 by the APC (5). Responses to anti-CD3 do not require class II MHC expression but do require the expression of costimulatory proteins. Several studies have suggested that for P. falciparum, one potential mechanism of diminished T-cell responses is decreased expression of class II MHC and costimulatory proteins (16, 29). We therefore examined the expression of each of these proteins on different APC subpopulations.
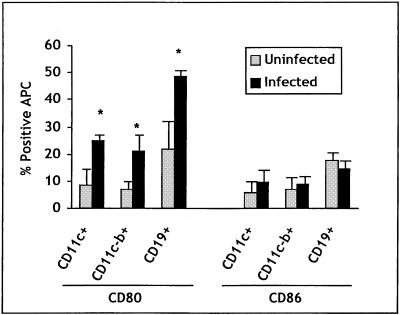
As the data in Fig. 2 indicate, infection resulted in an increase in CD80 expression on CD11c+ (dendritic), CD11c-b+ (macrophage), and CD19+ cells. These data were consistent in experiments carried out over several years and with several different strains of mice. When the data from one strain of mouse (B10.D2) were pooled (three experiments carried out over several months), the difference in CD80 expression between infected and uninfected mice was significant (P < 0.05 when comparing either mean fluorescence intensity or percentage of positive cells). CD86 expression, by contrast, was not consistently different between infected and uninfected mice.
FIG. 2.
Expression of costimulatory proteins on APC subsets. The percentage of the indicated APC subpopulation from B10.D2 mice infected 6 days previously that express CD80 or CD86 above background staining with an isotype control is shown. The values are the means for three (uninfected) and five (infected) mice from three separate experiments. Asterisks indicate values significantly different from the mean for the uninfected group. Error bars indicate standard deviations.
Class II expression was substantially higher on all APC subpopulations taken from infected mice at day 6 postinfection. An example is shown in Fig. 3, in which class II MHC expression by dendritic cells and macrophages from infected and uninfected mice is compared. On these and CD19+ cells, class II expression was consistently increased in every experiment (n ≈ 15 experiments representing three strains of mice, i.e., B6, BALB/c, and B10.D2). Together these experiments demonstrate that APC from infected mice express normal to increased levels of the proteins essential for T-cell activation. We conclude that failure of APC to activate and costimulate T cells is not an explanation for diminished IL-2 responses in our system.
FIG. 3.
Class II MHC is upregulated on APC subsets during infection. The indicated population was stained for class II MHC using two- or three-color fluorescence as described in Materials and Methods. The black histograms depict class II expression by cells from infected mice, and the grey histograms depict class II expression by cells from uninfected mice.
Diminished IL-2 responses are the result of direct suppression by a soluble factor.
The experiments described above suggest that the diminished IL-2 production is unlikely to be the result of a failure to support T-cell activation by APC, because all of the requisite machinery for stimulating T cells is present. However, the experiments do not address the question of whether APC from infected mice can process and present antigen. While this function is not necessary for anti-CD3 stimulation, it is required for stimulation of OVA-TCR Tg T cells. We addressed this issue by determining if T-cell hybridomas specific for OVA could be stimulated to produce IL-2 in the presence of APC from infected mice. Hybridomas are insensitive to the ability of APC to provide costimulatory activity; they require only the presentation of antigen by MHC for activation. When APC from infected mice were used, the hybridomas failed to produce IL-2. However, when APC from infected mice were cultured with OVA for 16 h and then fixed with paraformaldehyde, the IL-2 response was restored (Table 2). This finding demonstrates that APC from infected mice are able to process and present protein antigen and that they must be metabolically active to inhibit IL-2 production, making it likely that the cells are producing or expressing a suppressive activity.
TABLE 2.
T-cell hybridomas produce IL-2 when antigen is presented on fixed APC from infected mice
| Expt no. | Stimulus | IL-2 (mean pg/ml ± SD) with the following source of APCa:
|
|||
|---|---|---|---|---|---|
| Uninfected spleen | Infected spleen | Uninfected spleen, fixed | Infected spleen, fixed | ||
| 1 | OVA | 159.7 | 4.2 | 381.7 | 330.7 |
| None | NDb | ND | 6.8 | 6.8 | |
| 2 | OVA | 229 ± 80 | 37 ± 5 | 423.2 ± 22 | 354.5 ± 36 |
| None | 31.9 ± 5 | ND | 3.8 ± 0.4 | 2.4 ± 0.3 | |
IL-2 in tissue culture supernatants of DO11.10 T cell hybridomas cultured with indicated APC. In experiment 1, only duplicate wells were cultured, so no standard deviation is given. The DO11.10 hybridoma is derived from a BALB/c mouse; therefore BALB/c APC were used.
ND, not done.
The mechanism of IL-2 inhibition by APC was investigated by using a transwell culture system. T cells purified from OVA-TCR Tg mice were cultured with APC (spleen cells from uninfected mice) in the inner well of a two-chamber system separated by a 0.1-μm-pore-size membrane. When spleen cells from infected mice were placed in the outer well of the system, IL-2 production was inhibited compared to that in cultures with spleen cells from uninfected mice in the outer well (Fig. 4). This result indicates that IL-2-suppressive activity is soluble rather than cell associated, as infected cells were not in contact with responding T cells.
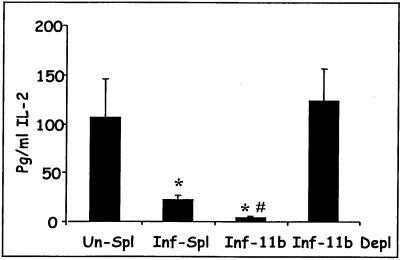
FIG. 4.
Suppression of IL-2 production is mediated by a soluble factor. Purified OVA-TCR Tg CD4 T cells were placed with spleen cells from uninfected mice in the inner well of a transwell culture system. Cells from the following sources were added to the outer well; spleen cells from uninfected mice (Un-Spl), spleen cells from infected mice (Inf-Spl), CD11b+ cells from infected mice (Inf-11b), or spleen cells depleted of CD11b+ cells (Inf-11b Depl). Cultures were stimulated with 1 mg of OVA per ml. Supernatants were collected for IL-2 measurement 24 h later and measured in triplicate. Results from two separate experiments are shown, and data are expressed as means ± standard deviations. ∗, values significantly different from that for Un-Spl; #, value significantly different from that for Inf-Spl (P < 0.05 by one-way ANOVA).
We also investigated the nature of the APC subpopulation that was responsible for IL-2 suppression. In a series of experiments by Scorza et al. (19), hemozoin pigment was found to replicate the IL-2-suppressive function of macrophages from infected mice. We reasoned that phagocytic cells capable of ingesting pigment would be more likely to produce the suppressive factor than nonphagocytic cells. Macrophages and phagocytic dendritic cells express CD11b (12). We therefore positively selected cells with anti-CD11b by using paramagnetic beads. CD11b+ cells from infected mice suppressed IL-2 production to a significantly greater extent than unfractionated spleen cells (Fig. 4). The suppressive activity was removed when CD11b+ cells were depleted from the cell preparation.
The soluble factor is not PGE2, NO, or TGF-β.
We then asked if the soluble inhibitory factor(s) is a mediator commonly implicated in the suppression of IL-2 production in this and other systems, such as PGE2, nitric oxide (NO) (see, for example references 1 and 23), or TGF-β (6). Indomethacin and l-NMMA were used to inhibit PGE2 and NO, respectively. In preliminary experiments using the transwell system, we found that cultures with spleen cells from infected or uninfected mice in the outer well both produced approximately 8 μg of PGE2 per ml. Addition of 0.6, 2.5, and 10 μg of indomethacin per ml reduced this amount to 2 μg/ml. Cultures with spleen cells from infected mice had approximately 27 μM NO, whereas cultures with spleen cells from uninfected mice had approximately 8 μM NO. Addition of 0.125, 0.25, and 0.5 mM l-NMMA reduced the levels of NO in all cultures to approximately 5 μM.
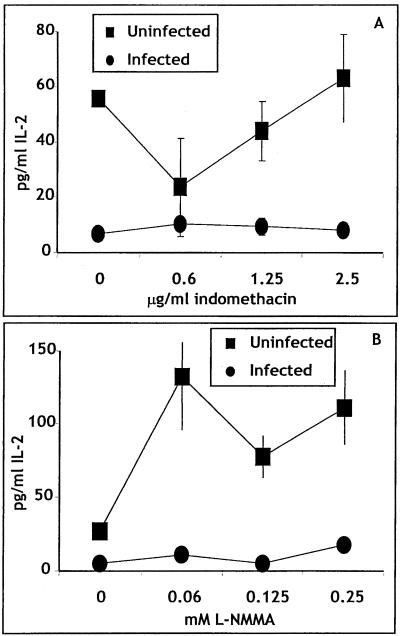
Titrations of both l-NMMA and indomethacin were added to the transwell culture system (Fig. 5). No reversal of IL-2 inhibition was noted at any dose of any inhibitor. Furthermore, in experiments not shown, both compounds were added separately and together to nontranswell cultures stimulated with anti-CD3, and similar results were obtained.
FIG. 5.
The IL-2-suppressive factor is not PGE2α or nitric oxide. Purified OVA-TCR Tg cells and spleen cells from uninfected mice were placed in the inner well of a two-chamber culture plate. Spleen cells from uninfected or infected mice were placed in the outer well. OVA (1 mg/ml) was added, and the indicated inhibitor was also added. Supernatants were collected 24 h later. Error bars indicate standard deviations.
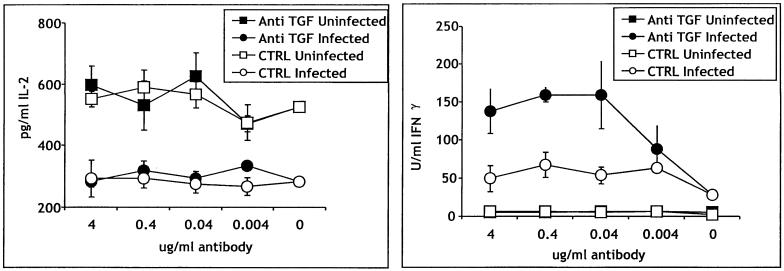
To evaluate the possibility that TGF-β might be inhibiting IL-2 production, we cultured APC from infected and uninfected mice with OVA-TCR Tg T cells in the presence of neutralizing antibody to TGF-β1 and TGF-β2 or an isotype-matched control (Fig. 6). The amount of IL-2 was not affected by this treatment; T cells stimulated with OVA in the presence of infected APC made significantly less IL-2 than T cells stimulated in the presence of uninfected APC at all doses of anti-TGF-β. By contrast, in the same cultures, the addition of TGF-β almost tripled the amount of IFN-γ produced in the presence of infected spleen cells, demonstrating the efficacy of the anti-TGF-β antibody.
FIG. 6.
The suppressive factor is not TGF-β. APC (purified CD11b+ cells) from uninfected or infected B10.D2 mice were cultured with TCR Tg cells and OVA in the presence of anti-TGF-β or an isotype-matched control antibody at the indicated concentrations. The antibody was added at the beginning of the culture period. Supernatants were analyzed for IL-2 or IFN-γ 24 h later. Error bars indicate standard deviations.
In preliminary experiments using IFN-γ and IL-10 knockout mice (not shown), we found that even in the absence of either cytokine, IL-2 production by T cells stimulated with anti-CD3 was still diminished. Stimulation with OVA has not yet been carried out using these strains. The nature of the suppressive activity is the subject of ongoing studies.
APC from infected mice support high levels of IFN-γ production.
We also asked if suppression of IL-2 production was the result of a general suppression of T-cell function by determining if T cells could produce another cytokine, IFN-γ, when cultured with infected APC. As shown in Table 3, when splenic APC from infected mice were used, the production of IL-2 was suppressed to various degrees. Simultaneously, the production if IFN-γ in those same cultures was dramatically increased. In the absence of added OVA, the production of IL-2 and IFN-γ was at the lower limits of detection of the assay, indicating that in this system, the production of both cytokines is an antigen-specific response. This observation indicates that the cytokines are being elaborated by T cells rather than NK cells. These findings show that the suppressive factor does not induce a global T-cell suppression, for example, by inducing death of responding T cells. Rather, the factor appears to target IL-2 production by T cells.
TABLE 3.
Splenic APC support high levels of IFN-γ but fail to support IL-2 production
| Mouseb | Concn (mean ± SD)a
|
|
|---|---|---|
| IL-2 | IFN-γ | |
| Uninfected | 1,164.5 ± 107 | 6.0 ± 1 |
| Inf-1 | 224.5 ± 5 | 119.3 ± 68 |
| Inf-2 | 337.3 ± 57 | 188.4 ± 83 |
| Inf-3 | 584.0 ± 48 | 248.0 ± 13 |
| Inf-4 | 353.7 ± 74 | 119.5 ± 65 |
Units are picograms per milliliter for IL-2 and units per milliliter for IFN-γ. In the absence of added OVA, IFN-γ production was less than 3 U/ml, and IL-2 production was less than 25 pg/ml.
Each entry represents an individual mouse.
T cells that develop in vivo exhibit similar differential cytokine production.
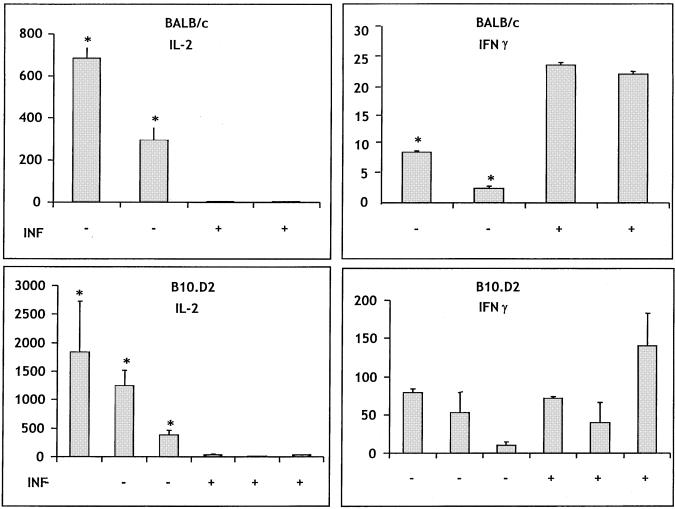
In order to validate our in vitro cell culture model as relevant to events unfolding in vivo during malaria infection, we carried out two adoptive transfer experiments. OVA-TCR Tg cells were transferred to uninfected recipients and then stimulated by immunization with OVA. Half of the recipients were simultaneously infected, allowing the OVA-TCR Tg cells to mature in the environment of an infected spleen. An adoptive transfer system was chosen over direct infection of Tg mice because the latter do not have a complete repertoire of T cells that would allow a normal immune response to the parasite.
For these experiments we first asked if the chosen immunization protocol (a single dose of OVA administered i.p. without adjuvant) would activate resident T cells to produce cytokines, thereby masking cytokine production by transferred OVA-TCR Tg T cells. We found that OVA immunization using this protocol did not result in any detectable IL-2 responses and resulted in an IFN-γ response of less than 5 U/ml when measured 6 days after immunization. This finding demonstrates that cytokine production after adoptive transfer can be attributed to the transferred T cells. These cells are identical to those we previously studied in vitro, thereby allowing us to directly correlate the results of the in vitro and in vivo experiments.
We then compared the cytokines produced by OVA-TCR Tg T cells when they were activated by antigen in vivo in an uninfected and an infected environment. Figure 7 shows the results of two similar experiments. Both experiments demonstrate that if the transferred T cells matured for 6 days in the presence of malaria infection, they did not produce IL-2 in response to restimulation with OVA in vitro. This was not due to changes in splenic architecture preventing the cells from localizing in the spleen, since spleen cells from infected mice produce significantly higher (experiment 1) or similar (experiment 2) amounts of IFN-γ in response to OVA than spleen cells from uninfected mice. Furthermore, IL-2 inhibition was seen in mice of both BALB/c and B10.D2 backgrounds. These findings exactly parallel the in vitro data. We should note that the number of OVA-TCR Tg T cells in the spleens of recipient mice was less than 1% in all cases. We could not, therefore, evaluate the degree to which OVA-specific T cells had expanded in each treatment group. Experiments to further address this issue are under way.
FIG. 7.
Cytokine profiles elaborated by T cells that develop in vivo resemble those generated in vitro. Two groups of mice were studied in each experiment, and results from two experiments are shown. Both groups received 40 × 106 spleen cells from naive OVA-TCR Tg mice of the indicated background. The following day, both groups of mice were given 1 mg of OVA i.p. One group of these mice were infected (INF), and the other group were not. Six days later the mice were sacrificed, and unfractionated spleen cells were cultured with OVA. Supernatants harvested 24 h later were measured for the indicated cytokine. The units for IL-2 are picograms per milliliter, and those for IFN-γ are units per milliliter. In experiment 1, two mice were studied in each group, and in experiment 2, three mice were in each group. Asterisks indicate values statistically different from that for the infected group by the nonpaired Student t test. In the absence of added OVA, IFN-γ production was less than 7 U/ml, and IL-2 production was less than 2 pg/ml. Error bars indicate standard deviations.
DISCUSSION
Our studies were designed to investigate the nature of the diminished proliferative responses that have been observed during acute malaria infection both in naturally occurring disease and in rodent models. We find that, in contrast to in vitro studies with P. falciparum, APC from P. yoelii-infected mice express increased levels of the costimulatory protein CD80 and increased levels of class II MHC. APC from infected mice process and present protein antigen to T cells and, rather than causing a generalized immunosuppression, support high levels of IFN-γ in responding T cells. APC from infected mice do, however, suppress IL-2 production through a soluble mediator that is distinct from PGE2, NO, or TGF-β. Finally, we show that all of the responses observed in vitro are recapitulated in vivo.
Using a similar experimental design and P. chabaudi infection, Scorza et al. (19) reached some conclusions paralleling our own. For example, they found that a hen egg white lysozyme-specific T-cell hybridoma produced IL-2 in the presence of macrophages from uninfected but not infected mice. Furthermore, as in our experiments, when macrophages from infected mice were fixed, IL-2 production was restored. Finally, that group also found that neither NO nor PGE2 played a role in suppression of IL-2 production.
We have extended these observations in several ways. Very few studies have examined the expression of costimulatory proteins on APC during malaria infection. Our findings are particularly important because of recent attention focused on the outcome of interactions between APC and P. falciparum (16, 29). In particular, earlier studies examined the expression of class II MHC proteins on macrophages and dendritic cells, respectively, after exposure of these cells to the parasite. P. falciparum inhibited the upregulation of class II MHC and, where examined, of costimulatory proteins as well. The relative paucity of studies addressing the APC function of cells from infected individuals seems to be a remarkable gap in our understanding of this important disease.
After approximately 1 week of infection, a time during which a marked decrease in T-cell responses is noted, APC from P. yoelii-infected mice express increased levels of class II MHC and CD80. There are several explanations for the differences between our finding of increased expression of costimulatory and MHC molecules and those of previous reports in which interaction with P. falciparum inhibited expression of these proteins (16, 29). P. falciparum may affect APC through its cytoadherent properties (29), possibly through binding to CD36 (30). Murine Plasmodium species are not believed to share this property. However, an alternative explanation is that in the P. falciparum studies, APC were purified before being exposed to the parasite. Under such conditions, the interaction between APC and parasite took place in the absence of T-cell interaction and an inflammatory environment that can activate APC. In our system, APC encountered the parasite in vivo before we examined their accessory function. Our findings suggest that the in vivo environment may overcome the ability of the parasite or parasite-derived factors to downregulate the expression of costimulatory proteins and class II MHC.
Our results support and extend the suggestion of Scorza et al. (19) that a soluble suppressive factor downregulates IL-2 production by directly demonstrating that suppression of IL-2 production occurs across a porous membrane. Furthermore, we show that this activity is contained within the CD11b+ subpopulation of spleen cells. This group of cells contains both macrophages and dendritic cells with increased phagocytic capacity (12). Phagocytosis of hemozoin pigment has often been implicated in malaria-associated immunosuppression (9, 16-19, 25), so it follows that phagocytic cells would be responsible for producing the observed soluble factor. While the nature of the soluble suppressive factor is not yet clear, our results and those of Scorza et al. demonstrate that it is neither NO nor PGE2, two factors often implicated in suppression of IL-2 production (1, 2, 22-24). Furthermore, TGF-β does not mediate IL-2 inhibition, because anti-TGF-β failed to enhance IL-2 production. As Tsutsui and Kamiyama (28) observed in vitro in P. chabaudi-infected mice, anti-TGF-β enhanced IFN-γ production, which is strongly stimulated by APC from infected mice. The observation that TGF-β is produced during P. yoelii infection is consistent with the in vivo observations of Omer and Riley (11). However the significance of this production is unclear, since neutralizing TGF-β in vivo did not change the course of P. yoelii infection in BALB/c mice. Finally, we found in preliminary experiments using CD3 stimulation that IL-10 and IFN-γ knockout mice exhibit the same level of inhibition of IL-2 production (data not shown). The nature of the soluble factor is the subject of ongoing experiments.
APC isolated during acute infection are capable of stimulating high levels of IFN-γ production from naive CD4+ T lymphocytes. Many studies have shown that IFN-γ is produced at high levels during the acute stages of murine malaria infection (4, 31, 32) and that the source of this IFN-γ is both T cells and NK cells. In the experiments described here, we demonstrate that APC from infected mice stimulate significantly higher levels of IFN-γ in responding CD4 T cells than APC from uninfected mice. In our system IFN-γ production is antigen driven, since we do not detect any in the absence of OVA. This finding suggests that the IFN-γ is T-cell derived. Studies are under way to determine if this observation is the result of higher IL-12 production by APC and which APC subsets are responsible for the stimulation.
The finding that APC from infected mice selectively inhibit IL-2 production but not elaboration of IFN-γ contributes to our understanding of how cytokine responses evolve during malaria infection. A well-described phenomenon associated with murine malaria infection is the observation that the cytokine pattern changes during infection from one dominated by type 1 cytokines, typified by IFN-γ, early in infection to one dominated by type 2 cytokines late in infection (4, 26, 27, 32). This observation has been made with P. chabaudi infection, but we have confirmed the finding with P. yoelii infection in some, but not all, strains of mice (unpublished findings). The cytokine switch relies on B lymphocytes, because it is not observed in B-cell knockout mice (26, 32) and the provision of B cells to B-cell-deficient animals restores the progression to type 2 cytokine production (27).
One way to couple our findings with those of other investigators is to suggest a model in which phagocytic cells initiate early immune responses which are characterized by high levels of IFN-γ production but IL-2 suppression, thereby limiting the expansion of IFN-γ-producing cells. Subsequent immune responses may be guided by antigen-specific B cells acting as APC and supporting a dominant type 2 cytokine response.
In summary, we have undertaken a detailed examination of the T-cell accessory function of APC from malaria-infected mice. Our results help illuminate phenomena associated with the immune response to malaria. In particular, we show that infection does not disable APC function with respect to T-cell activation. Rather, APC become focused on stimulating IFN-γ production by T lymphocytes, which may result in a differentiated lineage with limited abilities to expand. Future studies will address the mechanism of IL-2 suppression as well as the role of APC subsets in the control of cytokine expression.
Acknowledgments
We thank Joe Smith, Paul Avery, and Christine Olver for critical reading of the manuscript.
This work was supported by Public Health Service grant AI-42354 to A.C.A. from the National Institute of Allergy and Infectious Diseases.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Ahvazi, B. C., P. Jacobs, and M. M. Stevenson. 1995. Role of macrophage-derived nitric oxide in suppression of lymphocyte proliferation during blood-stage malaria. J. Leukoc. Biol. 58:23-31. [DOI] [PubMed] [Google Scholar]
- 2.Betz, M., and B. S. Fox. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146:108-113. [PubMed] [Google Scholar]
- 3.Coligan, J. E., A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.). 1996. Current protocols in immunology, vol. 1. John Wiley and Sons, New York, N.Y.
- 4.Langhorne, J., S. Gillard, B. Simon, S. Slade, and K. Eichmann. 1989. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int. Immunol. 1:416-424. [DOI] [PubMed] [Google Scholar]
- 5.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 6.Letterio, J. J., and A. B. Roberts. 1998. Regulation of immune responses by TGFβ. Annu. Rev. Immunol. 16:137-161. [DOI] [PubMed] [Google Scholar]
- 7.Lucas, B., L. H. Kasper, K. Smith, and A. Haque. 1996. In vivo treatment with interleukin 2 reduces parasitemia and restores IFN-γ gene expression and T-cell proliferation during acute murine malaria. C. R. Acad. Sci. 319:705-710. [PubMed] [Google Scholar]
- 8.Maryanski, J. L., J. Van Snick, J. C. Cerottini, and T. Boon. 1982. Immunogenic variants obtained by mutagenesis of mouse mastocytoma P815. III. Clonal analysis of the syngeneic cytolytic T lymphocyte response. J. Immunol. 12:401-406. [DOI] [PubMed] [Google Scholar]
- 9.Morakote, N., and D. E. Justus. 1988. Immunosuppression in malaria: effect of hemozoin produced by Plasmodium berghei and Plasmodium falciparum. Int. Arch. Allergy Appl. Immunol. 86:28-34. [DOI] [PubMed] [Google Scholar]
- 10.Murphy, K. M., A. B. Heimberger, and D. Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250:1720-1723. [DOI] [PubMed] [Google Scholar]
- 11.Omer, F. M., and E. M. Riley. 1998. Transforming growth factor b production is inversely correlated with severity of murine malaria infection J. Exp. Med. 188:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulendran, B., J. Lingappa, M. K. Kennedy, J. Smith, M. Teepe, A. Rudensky, and C. R. Maliszewski. 1997. Development pathways of dendritic cells in vivo. J. Immunol. 159:2222-2231. [PubMed] [Google Scholar]
- 13.Riley, E. M., C. Maclennan, D. Kwiatkowski, and B. M. Greenwood. 1989. Suppression of in-vitro lymphoproliferative responses in acute malaria patients can be partially reversed by indomethacin. Parasite Immunol. 11:509-517. [DOI] [PubMed] [Google Scholar]
- 14.Saha, B., G. Das, H. Vohra, N. K. Ganguly, and G. C. Mishra. 1994. Macrophage-T cell interaction in experimental mycobacterial infection. Selective regulation of costimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur. J. Immunol. 24:2618-2624. [DOI] [PubMed] [Google Scholar]
- 15.Sayles, P. C., and D. L. Wassom. 1988. Immunoregulation in murine malaria: susceptibility of inbred mice to infection with Plasmodium yoelii depends on the dynamic interplay of host and parasite genes. J. Immunol. 14:241-248. [PubMed] [Google Scholar]
- 16.Schwarzer, E., M. Alessio, D. Ulliers, and P. Arese. 1998. Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibiltity complex class II antigen, CD54, and CD11c in human monocytes. Infect. Immun. 66:1601-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarzer, E., and P. Arese. 1996. Phagocytosis of malarial pigment hemozoin inhibits NADPH-oxidase activity in human monocyte-derived macrophages. Biochim. Biophys. Acta 1316:169-175. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzer, E., F. Turrini, D. Ulliers, G. Giribaldi, H. Ginsburg, and P. Arese. 1992. Impairment of macrophage function after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J. Exp. Med. 176:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scorza, T., S. Magez, L. Brys, and P. De Baetselier. 1999. Hemozoin is a key factor in the induction of malaria-associated immunosuppression. Parasite Immunol. 21:545-554. [DOI] [PubMed] [Google Scholar]
- 20.Shimonkevitz, R., J. Kappler, P. Marrack, and H. Grey. 1983. Antigen recognition by H-2-restricted T cells. J. Exp. Med. 158:303-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sileghem, M., A. Darji, R. Hamers, M. Van De Winkel, and P. De Baetselier. 1989. Dual role of macrophages in the suppression of interleukin 2 production and interleukin 2 receptor expression in trypanosome-infected mice. Eur. J. Immunol. 19:829-835. [DOI] [PubMed] [Google Scholar]
- 22.Sileghem, M., A. Darji, L. Remels, R. Hamers, and P. De Baetselier. 1989. Different mechanisms account for the suppression of interleukin 2 production and the suppression of interleukin 2 receptor expression in Trypanosoma brucei-infected mice. Eur. J. Immunol. 19:119-124. [DOI] [PubMed] [Google Scholar]
- 23.Sileghem, M., and J. N. Flynn. 1992. Suppression of interleukin 2 secretion and interleukin 2 receptor expression during tsetse-transmitted trypanosomiasis in cattle. Eur. J. Immunol. 22:767-773. [DOI] [PubMed] [Google Scholar]
- 24.Sternberg, J. M., and N. A. Mabbott. 1996. Nitric oxide-mediated suppression of T cell responses during Trypanosoma brucei infection: soluble trypanosome products and interferon-γ are synergistic inducers of nitric oxide synthase. Eur. J. Immunol. 26:539-543. [DOI] [PubMed] [Google Scholar]
- 25.Taramelli, D., N. Basilico, E. Pagani, R. Grande, D. Monti, M. Ghione, and P. Olliaro. 1995. The heme moiety of malaria pigment (β-hematin) mediates the inhibition of nitric oxide and tumor necrosis factor-α production by lipopolysaccharide-stimulated macrophages. Exp. Parasitol. 81:501-511. [DOI] [PubMed] [Google Scholar]
- 26.Taylor-Robinson, A. W., and R. S. Phillips. 1994. B cells are required for the switch from Th1- to Th2-regulated immune response to Plasmodium chabaudi chabaudi infection. Infect. Immun. 62:2490-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor-Robinson, A. W., and R. S. Phillips. 1996. Reconstitution of B-cell-depleted mice with B cells restores Th2-type immune responses during Plasmodium chabaudi chabaudi infection. Infect. Immun. 64:366-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsutsui, N., and T. Kamiyama. 1999. Transforming growth factor beta-induced failure of resistance to infection with blood-stage Plasmodium chabaudi in mice. Infect. Immun. 67:2306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 30.Urban, B. C., N. Willcox, and, D. J. Roberts. 2001. A role for CD36 in the regulation of dendritic cell function. J. Immunol. 98:8750-8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Heyde, H. C., B. Pepper, J. Batchelder, F. Cigel, and W. P. Weidanz. 1997. The time course of selected malarial infection in cytokine-deficient mice. Exp. Parasitol. 85:206-213. [DOI] [PubMed] [Google Scholar]
- 32.von der Weid, T., and J. Langhorne. 1993. Altered response of CD4+ T cell subsets to Plasmodium chabaudi in B cell deficient mice. Int. Immunol. 5:1343-1348. [DOI] [PubMed] [Google Scholar]
- 33.Warren, H. S., and W. P. Weidanz. 1976. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur. J. Immunol. 6:816-819. [DOI] [PubMed] [Google Scholar]
- 34.Xu, X., K. Sumita, C. Feng, X. Xiong, H. Shen, S. Maruyama, M. Kanoh, and, Y. Asano. 2001. Down-regulation of IL-12 p40 gene in Plasmodium berghei-infected mice. J. Immunol. 167:235-241. [DOI] [PubMed] [Google Scholar]