ABSTRACT
Purpose
To describe and analyze pediatric anterior capsulotomy techniques and make recommendations.
Methods
Five anterior capsulotomy techniques were compared using a porcine model. Extensibility was measured by calculating the mean stretch-to-rupture circumference of each capsulotomy (20 eyes per technique) as a percentage of its circumference at rest. Edge characteristics were reviewed using scanning electron microscopy. A 10-year review of consecutive pediatric cataract surgeries performed by the author focused on the anterior capsulotomy results. A worldwide survey was used to determine current practice patterns.
Results
Manual continuous curvilinear capsulorrhexis (CCC) produced the most extensible porcine capsulotomy (185%) with the most regular edge and is preferred by surgeons for patients aged 2 years and older. In the pseudophakic clinical cases reviewed, a radial tear developed in 3 (6.5%) of 46 manual CCC cases. Vitrectorhexis (porcine extensibility, 161%) is preferred by surgeons during the first 2 years of life. A radial tear developed in 16 (7.7%) of 208 vitrectorhexis pseudophakic eyes (29 tears in 284 pseudophakic eyes [10.2%] overall). The Kloti diathermy unit, Fugo plasma blade, and “can-opener” technique produced porcine capsulotomies of 145%, 170%, and 149% extensibility, respectively, and radial tears numbering 4 (21%) of 19, 5 of 8, and 1 of 2, respectively, in the clinical series.
Conclusions
All five capsulotomy techniques are recommendable for children. Only the vitrectorhexis and manual CCC are commonly used today. Vitrectorhexis is well suited for use in infants and young children; manual CCC is best used beyond infancy, and it produces the most stable edge.
INTRODUCTION
Childhood blindness occurs at a rate of one child every minute.1,2 It is estimated that there are 1.5 million blind (defined as best-corrected visual acuity less than 20/400 in the better eye) children in the world.3 Of those, 75% have treatable or preventable causes of blindness. The control of blindness in children was given a high priority within the World Health Organization (WHO) VISION 2020—The Right To Sight program.4,5
Globally, an estimated 200,000 children are bilaterally blind from cataract.3 This is despite the many technical advances that have been made in the surgical removal of adult cataracts. The overall incidence of clinically significant cataract (unilateral or bilateral) in childhood is unknown, but it has been estimated to be as high as 1 in 250 (0.4%).6 WHO VISION 2020 targets for the control of blindness in children contained the following as a specific disease-control measure: “Provide appropriate surgery to all children with cataract, with immediate and effective optical correction, in suitably equipped specialist centers.”4(p231),7(p54)
Achieving this goal has been a challenge even within the developed world. However, dramatic advances have occurred in the treatment of childhood cataracts in the last 10 years. Primary implantation of an intraocular lens (IOL) after cataract removal in children has now become commonplace.8,9 Unique features of the child’s eye, however, have created the need for variations in adult procedures, and at times, completely different techniques have been needed to ensure safe and effective cataract removal and stable central fixation of the IOL in the child.10,11
One of the most marked differences between adult and pediatric cataract surgery is the behavior of the anterior lens capsule during the procedure. The anterior capsulotomy shape, size, and edge integrity are now recognized as very important to the long-term centration of a capsular-fixated IOL.12 A surgeon who applies adult cataract surgery maneuvers to the pediatric anterior capsule may be surprised and disappointed at the outcome. Furthermore, the vast majority of surgeons operating on children perform less than 10 pediatric cataract procedures per year.9 For these reasons, an analysis of the pediatric lens capsule and an investigation of the currently available methods for performing an anterior capsulotomy in young eyes will help guide surgeons faced with the important task of removing a cataract from a child.
Opening the anterior lens capsule (anterior capsulotomy) allows access to the lens nucleus and cortex. After cataract removal, it allows for the placement of an IOL within the capsule remnant. The anterior capsulotomy must be done in a manner that does not compromise the structure and stability of the remaining lens capsule. A compromise in the capsule may affect the centration of an IOL and its separation from uveal tissue. An ideal anterior capsulotomy technique for children would be one that is both easy to perform and predictable in size and centration. In addition, it would create a strong edge that would not tear during removal of the lens substance or during implantation of the IOL. Manual continuous curvilinear capsulorrhexis (CCC) is a popular anterior capsulotomy technique for adult cataract surgery because it is relatively easy to perform and yields a low rate of radial tears. However, the anterior lens capsule in children is very elastic and requires the application of more force before tearing begins. Manual CCC is, therefore, more difficult to perform and control in young eyes. This has led researchers and surgeons to search for alternative methods to open the anterior capsule in children.12 The alternatives currently available include the use of the vitrector handpiece, a bent needle for multipuncture, diathermy, and plasma energy. Also, variations in the adult manual CCC technique have been suggested to help avoid the “runaway rhexis” that can occur when standard CCC methodology is applied to young elastic capsules.12
Therefore, the goal of this thesis is to describe and analyze the techniques currently being utilized to open the anterior lens capsule in a child and establish which techniques are recommendable.
To establish this goal, the anatomic and physiologic differences between the pediatric and adult anterior lens capsule will be described. A historical account of various pediatric capsulotomy techniques that have been utilized will be provided. A laboratory comparison of those techniques will be profiled using an animal lens capsule model that has been shown by our laboratory to exhibit similar elastic characteristics to the pediatric human lens capsule.13 Additionally, a clinical review of the anterior capsulotomy outcomes in a 10-year consecutive series of pediatric surgical cataract cases performed by the author will be reported. Cases with complications will be reviewed in limited detail to help shape the recommendations at the summation of the thesis. Finally, the results of a worldwide survey of pediatric ophthalmic surgeons will be reported to show the 2003 practice patterns and preferences of surgeons when they perform pediatric anterior capsulotomy.
The hypothesis of this thesis is as follows: The manual tear CCC provides the highest-quality edge but is harder to perform and control in children than in adults. Technique alterations based on the anatomy and physiology of the child’s lens capsule can make CCC in children more successful. Vitrectorhexis is uniquely suited to be used when cataracts are removed from those infants and children where the vitrector handpiece will also be used to perform a posterior capsulectomy and anterior vitrectomy. These children are usually aged 6 years or younger. The vitrectorhexis technique provides excellent control and reproducibility, while retaining enough edge strength to withstand the manipulation that occurs during pediatric cataract removal and IOL implantation. Alternative capsulotomy methods utilizing the Kloti radiofrequency diathermy handpiece, the Fugo plasma blade, or a bent needle for the multipuncture “can-opener” technique are also suitable for use in children if the surgeon prefers. For most surgeons, use of these techniques will be restricted to fibrotic anterior capsules from trauma or as an alternative to applying capsular dyes in those eyes with total white cataracts.
Characteristics of the Pediatric Anterior Capsule
The development of the human lens begins at the 4-mm embryonic stage when neural ectoderm making up the optic vesicles comes in close contact with surface ectoderm, initiating the formation of the lens placode.14 Because the lens is formed as an invagination of surface ectoderm, the basal portion of its cells is oriented externally and the apical portion faces the lumen. Because of this arrangement, a basement membrane secreted by lens epithelial cells is deposited externally and encases the tissue in a membranous envelope called the lens capsule. It is a true periodic acid–Schiff (PAS)-positive basement membrane. It appears homogeneous at the light-microscope magnification level, but the electron microscope reveals multiple lamellae and a three-dimensional molecular meshwork made up primarily of type IV collagen.15–18 Other extracellular matrix constituents, such as collagen types I and III,19 laminin,19,20 and fibronectin,20 have also been identified and may be important to the biomechanical properties of the capsule.
The anterior capsule is thinnest at birth, increasing in thickness with age until approximately age 75 years.21 Seland22 described the neonatal anterior lens capsule as uniform and approximately 4 mm in thickness. By old age, the capsule had increased to 20 to 25 mm centrally and 30 mm near the lens equator.22 Krag and coworkers21 analyzed 67 human eyes from age 7 months to 98 years and found anterior capsule thickness increased from 11 to 33 mm. The association of thickness with age fit a straight line from birth to age 75 years, after which it changed slope and thinned slightly.21
The neonatal anterior capsule surface contains threadlike fibers about 100 Å in thickness and 1.5 Å long.22 They penetrate about 0.6 μm into the capsular matrix. These become more numerous near the lens equator and disappear completely between ages 6 and 17 years. Numerous parallel laminae are repeated regularly at intervals of about 700 Å. Capsular laminations are lost with age, most markedly after age 50 years. Electron-dense formed elements or inclusions, thought to be excretion products from the most metabolically active epithelial cells, are found only after age 17 years and increase markedly with old age. Although the inclusions are most common near the equatorial anterior capsule, they are found to occur closer and closer to the anterior pole of the lens as aging progresses.22 In conjunction with these anatomic changes, the anterior capsule’s biomechanical properties are also altered with age. The young anterior lens capsule is strong and very elastic. The elderly anterior lens capsule is, by comparison, weak and inelastic. Krag and coworkers21 found that anterior lens capsule extensibility was maximal in infancy and decreased about 0.5% per year throughout life (measured range, 108% to 40%).
In summary, aging of the human anterior lens capsule leads to a progressive loss of mechanical strength. Overall tensile strength decreases by a factor of five during the life span, and the extensibility decreases by at least a factor of two. In surgery, the young capsule is highly elastic and difficult to puncture. Much more force is required before tearing begins. In contrast, the capsule of the elderly is much less extensible, easier to open, and tears with much less force.
Techniques for Pediatric Anterior Capsulotomy
For purposes of this introduction, the techniques will be presented in the order they will appear in the “Methods” and “Results” sections. Later (in the “Discussion” section), a more detailed historical account of the development of these techniques will be presented using a chronologic order.
Vitrector-Cut Capsulotomy
Mechanical suction and cutting instruments began to be utilized for pediatric cataract surgery in the late 1970s and early 1980s. Parks23 and Taylor24 were among the first to advocate for the performance of a primary mechanized posterior capsulectomy and anterior vitrectomy during pediatric cataract surgery. Taylor also described removal of the anterior capsule mechanically.24
In 1994, Wilson and coworkers25 performed a laboratory comparison of mechanized anterior capsulectomy (later named vitrectorhexis) and manual CCC. Because of difficulty with manual CCC on the elastic capsules of young children, Wilson began utilizing the vitrector hand-piece to perform the anterior capsulectomy during pediatric cataract surgery and IOL implantation. In the laboratory, 18 pairs of pediatric eyes obtained postmortem were operated on by Wilson within 24 hours of enucleation. The age of the donors ranged from 4 days to 16 years. A mechanized anterior capsulectomy was performed on one eye of each pair, and a CCC was performed on the fellow eye. The integrity of the anterior capsulotomy edge was assessed after capsulotomy completion, after lens removal, and again after IOL implantation. Radial tears were noted and described.
A radial tear developed in only one of the 18 pediatric eyes in which a mechanized anterior capsulectomy was performed.25 This occurred in one of the oldest pediatric eyes (age 16 years), where a single radial tear extended from an angled capsulectomy edge during IOL insertion. In contrast, no radial tears occurred in any of the fellow eyes in which a manual CCC was performed. However, in six of the fellow eyes, the manual CCC edge extended out to the lens equator rather than continuing in a circular fashion. All six of these errant capsulotomies were from eyes of children younger than 5 years of age. Wilson and coworkers26 subsequently published a prospective clinical series containing data of 20 eyes from 17 children after performance of a mechanized anterior capsulectomy at the time of cataract surgery with IOL implantation. In all patients the capsulotomy was round, centered, and accurately sized. Two patients, both aged 11 years, were noted to develop radial tears in the anterior capsule during IOL insertion. None of the children younger than age 11 years developed radial tears.
Andreo, Wilson, and Apple13 subsequently reported that whereas the CCC offered greater resistance to capsule tearing in a laboratory study utilizing a porcine model, the mechanized anterior capsulectomy displayed more than adequate resistance to unwanted capsule tears when used for IOL insertion through capsulotomy sizes currently used in clinical practice.
The original name, “mechanized anterior capsulectomy,”25,26 emphasized the mechanized nature of the vitrector handpiece and the fact that a portion of the capsule was removed (capsulectomy) rather than merely opened (capsulotomy). Later, the term “vitrectorhexis” came into common use.12,13 The vitrectorhexis name emphasizes the fact that it is a substitute for capsulorrhexis performed using the vitrector. However, the term “vitrectorhexis” is, in reality, a misnomer because “rhexis” means to tear rather than to cut.27 Nonetheless, the commonly used “vitrectorhexis” label will be used in this thesis.
Manual Continuous Curvilinear Capsulorrhexis Capsulotomy
Gimbel and Neuhann,27 developers of the manual CCC capsulotomy method, have stated that without this technique, the potential of IOL implantation in children might not have been realized. Ideally, the technique provides for any size of smooth, circular, capsular opening with a strong capsular rim that resists tearing even when stretched during lens material removal or IOL implantation. The development of CCC occurred simultaneously in Canada (by Gimbel) and in Germany (by Neuhann).27 Both investigators presented their new technique in 1985. By September of 1987, Gimbel had used CCC in 33 children older than age 2 years with good results.27 However, Gimbel noted a greater tendency in children for the tear to extend peripherally. When this occurred, he recommended releasing some of the anterior zonular fibers in the area of the tear and using an elongated forceps to pull the capsulotomy edge toward the center.27
The increased elasticity of the pediatric capsule had created the tendency for the capsulotomy edge to extend peripherally. In addition, reduced scleral rigidity in children produces posterior vitreous “upthrust” when the eye is entered. This vitreous “pressure” pushes the lens anteriorly and keeps the anterior capsule domed, convex, and taut. This also contributes to the tendency for a so-called runaway rhexis. Performing a CCC in infants proved to be even more challenging than in older children. Vasavada and Chauhan28 reported a success rate of less than 20% in 21 infant eyes.
Despite the difficulty of performing a CCC in a child, once completed, the edge integrity was reported to be excellent, easily withstanding the manipulations of lens substance removal and subsequent IOL implantation.29
Multipuncture (Can-opener) Capsulotomy
To avoid the difficulties with CCC in children, some surgeons have returned to the can-opener style capsulotomy when operating on children.30 Others advocate using the can-opener technique in children with an intumescent lens.31 Wood and Schelonka30 compared the strength and safety of a CCC with a can-opener capsulotomy in a porcine model that closely resembles the high elasticity of the human pediatric lens capsule. A CCC or can-opener capsulotomy was performed inside the anterior chamber of fresh pig eyes. Any uncontrolled tears were noted. According to these authors, the porcine capsule is more reliably opened with fewer uncontrolled tears by a can-opener capsulotomy than by a CCC.
Kloti Bipolar Radiofrequency Diathermy Capsulotomy
Radiofrequency diathermy capsulotomy, developed by Kloti and colleagues in 1984,32 has been used as an alternative to CCC for cataract surgery in children as well as intumescent adult cataracts. The Kloti device (Oertli Instruments, Berneck, Switzerland) consists of a curved cannula housing an active electrode tip. It cuts the anterior capsule with a platinum-alloy-tipped probe using high-frequency current (500 kHz). The probe tip is heated to about 160°C and produces a thermal capsulotomy as it is moved in a circular path across the anterior capsule. Small gas bubbles are formed while the tip is active. Gentle pressure must be maintained on the capsule as the tip moves either clockwise or counterclockwise. Even when performed perfectly, a diathermy-cut capsulotomy can be seen to have coagulated capsular debris along the circular edge. In addition, this edge has been shown experimentally to be less elastic than a comparable CCC edge.33,34 Since the stretching force needed to break the edge of a diathermy-cut capsulotomy is reduced compared to a CCC edge, surgical manipulations needed to remove a cataract and place an IOL may result in more radial tears when the diathermy is used. However, these observations were made using adult autopsy globes. It is well known that the pediatric capsule responds differently than the adult lens capsule. In fact, Comer and coworkers35 reported no radial tears when using the Kloti radiofrequency diathermy instrument to perform the anterior capsulotomy in 14 eyes of seven children whose mean age was 23 months.
Fugo Plasma Blade Capsulotomy
The Fugo blade has also been recently introduced as a plasma knife that can be used to perform an anterior capsulotomy.36 The Fugo blade unit is a portable electronic system that operates on rechargeable batteries. It requires no red reflex for visualization of the capsulotomy edge during the cutting procedure. The unit also allows the surgeon to easily revise the size of the capsulotomy opening. This new instrument has not yet been tested in children. It is hoped that it will work well with the elastic capsule found in children.37
METHODS
Porcine Ocular Model
Abattoir globes were purchased (Visiontech, Inc, Mesquite, Texas) from two porcine age groups: young pigs approximately 6 months of age and weighing 200 to 250 pounds, and adult pigs 2 to 3 years of age and weighing 1,000 to 1,500 pounds. Globes were collected within 4 hours following death, packed on ice, and transported to the laboratory for use. Globes were rinsed with normal saline, trimmed, and stored at 5ºC until used (24 to 48 hours). Only globes with a clear cornea were used for each closed chamber technique. Anterior lamellar corneal dissection or lidocaine injection was used as needed, to aid corneal clarity.36
Regardless of the intended surgical technique, each globe was placed on a gauze pad in a Styrofoam head-model to approximate the customary positioning of the eye for surgery.
Five anterior capsulotomy techniques were completed using the porcine model described above. Analysis was made of 20 eyes per group (ie, 10 young eyes, 10 adult eyes). Anterior capsulotomy techniques were assigned to the following groups:
Group 1: Vitrectorhexis, using an Accurus vitrector (Alcon Laboratories, Fort Worth, Texas)
Group 2: Manual CCC, using a 26-gauge cystotome (Alcon Laboratories, Fort Worth, Texas) and Utrata capsulorrhexis forceps (Ellis Ophthalmic Technologies, Inc, Jamaica, New York)
Group 3: Multipuncture can-opener, using a 26-gauge cystotome (Alcon Laboratories, Fort Worth, Texas)
Group 4: Kloti radiofrequency diathermy, using a Kloti unit (Oertli Instruments, Berneck, Switzerland)
Group 5: Fugo plasma blade, using the electrosurgical base unit (Medisurg Research and Management Co, Norristown, Pennsylvania) attached to a Fugo blade tip (Medisurg Research and Management Co, Norristown, Pennsylvania)
Two additional porcine globes were included in each group: one served as a procedure “pilot” and was not analyzed; the other was implanted with a poly(methyl-methacrylate) MC52BM intraocular lens (Alcon Laboratories, Fort Worth, Texas) following lens removal and submitted for scanning electron microscopy (SEM) evaluation.
Eight eyes were excluded from analysis of stretching capacity due to tears in the capsule edge that occurred either during the capsulotomy procedure (manual CCC, 1; Kloti radiofrequency, 3; Fugo plasma blade, 2) or during lens substance removal (manual CCC, 2). Additional eyes were operated on until 20 completed and intact capsulotomy openings were available for analysis of extensibility from each capsulotomy technique group.
The project was registered with the institutional animal care and use committee. Additional peer-reviewed approval was not required for use of these abattoir globes.
Lens Substance Removal
Hydrodissection followed each anterior capsulotomy in all groups. Lens substance of young pig eyes was aspirated with a syringe, and the lens substance of adult pig eyes was removed using phacoemulsification. Following removal of the lens substance, Healon-GV (Pharmacia, Inc, Peapack, New Jersey) was used to fill the empty capsular bag.
Anterior Capsule Opening Measurements—Unstretched and Stretched
For all capsulotomy techniques, unstretched and stretched measurements were made after the lens substance had been completely removed.
Prior to recording any ocular measurements, three eyes were used to determine that the caliper edge itself would not cause a capsule tear. Unstretched and stretched measurements of the capsule opening were then performed in each group using electronic digital vernier calipers (Chicago Brand Industrial, Inc, Fremont, California). The caliper jaws were placed inside the capsule opening, and the unstretched diameter of the capsule opening was measured (in millimeters) three times in the vertical direction and three times in the horizontal direction; the mean of these six measurements determined the unstretched diameter of each eye. Stretching of the anterior capsule opening was measured next. The caliper jaws were again placed inside the capsule opening whereupon each capsule was slowly stretched until it tore. When the capsule tore, the caliper reading was recorded in millimeters as the length of the stretched capsule opening.
Data regarding the five anterior capsulotomy techniques were compiled using the following equations13:
The unstretched capsulotomy diameter (Dun) was recorded as the average of six measurements per eye.
The circumference of the unstretched (Cun) capsulotomy opening was calculated for each eye using the formula: Cun = π(Dun)
The stretched length to breaking (Ls) of the capsule opening was recorded per eye, and the diameter of the caliper tips (Dtips) was considered to be negligible or zero.
The circumference of the stretched (Cs) capsulotomy opening was calculated for each eye using the formula: Cs = π(Dtips) + 2(Ls)
The percentage comparison (R) of the stretched to unstretched circumference was calculated for each eye using the formula: R = (Cs/Cun) × 100%
Statistical Evaluation
Compilations were stored and analyzed using Microsoft Excel 2000 (Redmond, Washington) spreadsheets and analytical tools, and SPSS for Windows 2001 (Chicago, Illinois). The five anterior capsulotomy techniques were analyzed per technique and compared. Statistical analyses included the Student t test and analysis of variance (ANOVA). The level of probability accepted as significant was P < .05.
Limitations
The number of eyes required for this study negated the use of pediatric autopsy globes. Therefore, porcine necropsy globes were chosen.
Scanning Electron Micrography of the Capsulotomy
Eyes prepared for SEM were placed in freshly prepared 2% cacodylate glutaraldehyde for 24 hours. The eyes were then rinsed in 0.1 mol/L cacodylate buffer with 7% sucrose, postfixed in 2% aqueous osmium tetroxide, and dehydrated through a series of graded ethyl alcohol concentrations (50%, 70%, 95%, and 100%). Each sample was dried using the EMS850 Critical Point Dryer (Fort Washington, Pennsylvania) then mounted onto a stub and coated with 20 nm of gold/palladium using the Polaron SC7640 Sputter Coater (Agawam, Massachusetts). The sample was viewed in the JEOL 5410LV Scanning Electron Microscope (Peabody, Massachusetts). The microscope was operated at 5 kV.
Comparative Evaluation
Representative digital images from each of the five capsulotomy techniques were collected for comparison of the capsulotomy opening and the edge.
Limitations
Scanning electron microscopy was chosen for surface comparison. Intracellular detail using transmission electron micrography was not pursued.
Pediatric Cataract Surgery Database
Between January 1, 1994, and December 31, 2003, all pediatric cataract surgery operative dictations from the practice of the author contained a description of the anterior capsulotomy and an assessment of whether any radial tears were present at the completion of each major step in the procedure (ie, anterior capsulotomy, cataractous lens removal, IOL insertion, hydrodissection, or ocular visco-surgical device removal). When radial tears occurred, the stage of the procedure and any other pertinent details about the causation of the tear were dictated as well. These consecutive patient data were entered into a computerized database, which continues to be maintained. The data from each case were entered by a trained physician after each dictated operative note was completed and signed. For purposes of this project, all surgeries performed by a clinical fellow or resident-in-training were excluded.
Retrospective database analysis of consecutive pediatric cataract surgery cases performed by the author revealed 379 consecutive surgeries on patients between the ages of birth and 18 years of age between January 1, 1994, and December 31, 2003. Surgeries to perform secondary IOL implantation (94 eyes) were not included in the surgery total listed above, since simultaneous cataract extraction was not performed and thus an anterior capsulotomy was not always needed at the time of surgery. Additionally, surgeries to remove various forms of opacification of the visual axis after cataract surgery, such as lens cortex proliferation, posterior capsule opacification not treatable with laser, or pupillary membranes, were not included in the above total. Likewise, surgeries to reposition, exchange, or remove an IOL were also not a part of the surgery total.
Consecutive pediatric cataract surgery cases to be analyzed were divided into the following postsurgery categories: (1) pseudophakic eyes (eyes undergoing cataract extraction with placement of an IOL at the primary surgery); (2) aphakic eyes (eyes undergoing cataract extraction without placement of an IOL at the primary surgery); and (3) eyes with traumatic or spontaneous anterior capsule rupture prior to surgery.
The following data were collected: (1) date of birth; (2) date of surgery; (3) eye location; (4) capsulotomy technique used; (5) IOL placement (ie, primary and where); (6) preoperative anterior capsule rupture; and (7) anterior capsule radial tear occurring during cataract surgery, when the tear occurred, and if the tear interfered with capsular fixation of an IOL.
The project received peer-reviewed approval by the institutional human investigation review board for the collection of these data and their analysis for purposes of research publication.
Statistical Evaluation
Compilations were stored and analyzed using Microsoft Excel 2000 (Redmond, Washington) spreadsheets and analytical tools and SPSS for Windows 2001 (Chicago, Illinois). Statistical analyses utilized the Student t test and ANOVA. The level of probability accepted as significant was P < .05.
Limitations
Data from consecutive cases were entered into a database. Therefore, no effort could be made to control the number of cases per age or surgical technique.
Worldwide Survey of Pediatric Ophthalmic Surgeons
In July 2003, a four-question survey of demographics and anterior capsulotomy preferences was mailed to 996 physicians representing the membership of the American Association for Pediatric Ophthalmology and Strabismus (AAPOS) (816 domestic and 180 international mailings) (see Appendix). Respondents were asked to mail or fax their completed survey to the indicated Service Center. Responses were accepted for analysis through September 2003.
Statistical Evaluation
Responses were compiled using Microsoft Excel 2000 (Redmond, Washington) spreadsheets and analytical tools and SPSS for Windows 2001 (Chicago, Illinois). Statistical analyses utilized the Student t test and ANOVA. The level of probability accepted as significant was P < .05.
Limitations
A single reminder e-mail was sent to all member physicians 2 months after the initial survey distribution. No additional reminder was sent.
RESULTS
Capsulotomy Techniques
Standardization of the surgical techniques included the following criteria: (1) all capsulotomies were performed by the author; (2) a 5-mm central, circular anterior capsule opening was the surgical goal in each eye; (3) closed chamber technique was used for manual CCC (group 2), and the cornea and iris were removed before performing the anterior capsulotomy in groups 1 and 3 through 5. The laboratory accommodations for these techniques are shown in Figure 1. Healon GV was the ophthalmic viscoelastic device (OVD) for each technique.
Figure 1.
The laboratory surgical suite utilized for the porcine capsulotomy experiments.
Each anterior capsulotomy technique is described per group:
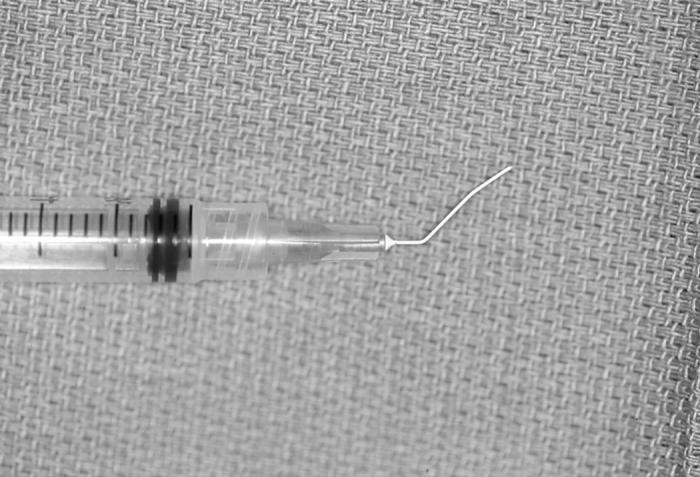
Group 1: Vitrectorhexis capsulotomy was performed using an Accurus vitrector and a cutting rate of 150 cuts per minute (Figure 2). A central capsular puncture was made with the vitrector. The vitrector probe was then positioned in contact with the center of the anterior capsule, its cutting port positioned posteriorly. The cutter was turned on and suction was gradually increased using the foot pedal, until the capsule edge was engaged. The cutting port was then moved in a spiral fashion with the cutting edge positioned posteriorly, until the desired size and shape were achieved. Care was taken to avoid leaving any right-angled edges.
Figure 2.

Vitrector equipment needed for the vitrectorhexis capsulotomy technique.
Group 2: Manual CCC was performed using a closed-chamber technique (Figure 3). Healon GV was used. Capsulorrhexis was initiated using a 26-gauge cystotome and completed with Utrata capsulorrhexis forceps. Centripetal force and frequent regrasping maneuvers were used.
Figure 3.
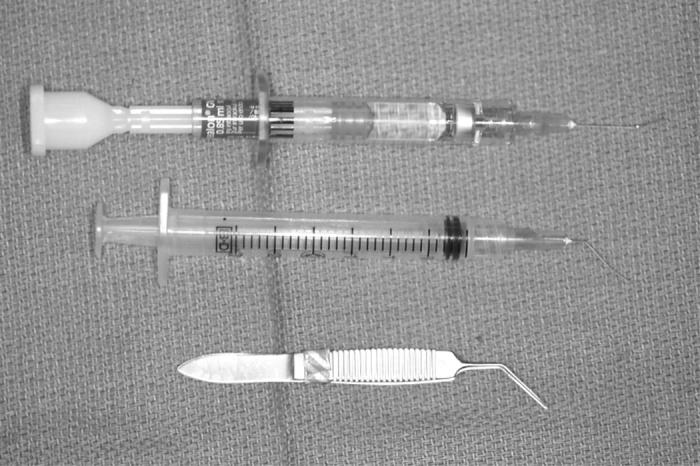
Surgical equipment needed for the manual continuous curvilinear capsulorrhexis (CCC) capsulotomy technique.
Group 3: Multipuncture can-opener capsulotomy was accomplished with a 26-gauge cystotome (Figure 4). An initial puncture was made at the 6-o’clock position, 2.5 mm from the center of the capsule. The next puncture was made 1 mm from the initial puncture in a counterclockwise direction. The cystotome punctured the capsule with a downward (posterior) movement. At the moment of puncture the cystotome was pulled toward the center of the pupil. Additional punctures were made within the 1-mm spacing, if needed, to connect the capsular opening with the one made from the adjacent puncture. This was continued until a completed circular capsulotomy of approximately 5-mm diameter was made.
Figure 4.
Surgical equipment needed for the manual multipuncture “can-opener” capsulotomy technique.
Group 4: Radiofrequency diathermy utilized a Kloti unit to perform the capsulotomy (Figure 5). The upper limits of current intensity (amperes) and potential (volts) were preselected and fixed by the manufacturer. The diathermy capsulotomy was performed using a continuous, circular, uninterrupted movement. Gentle pressure was maintained on the capsule by the instrument tip as it moved in a circular fashion, beginning in the center of the capsule.
Figure 5.
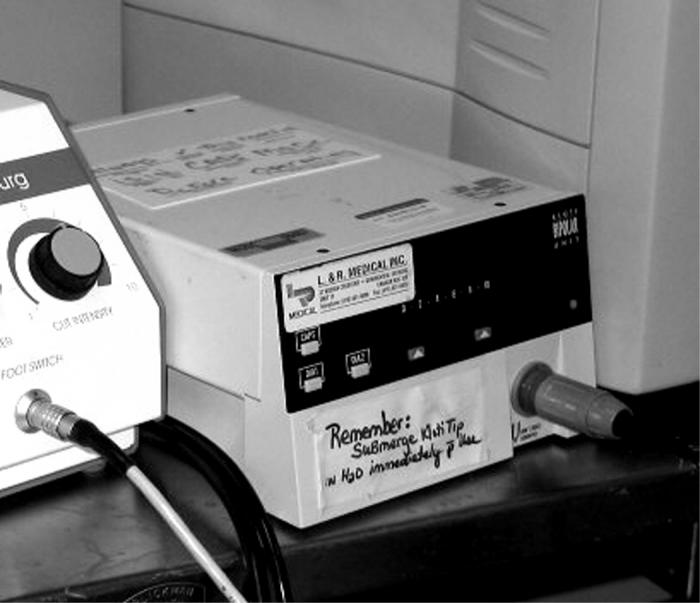

Kloti equipment needed for the radiofrequency diathermy capsulotomy technique.
Group 5: Fugo plasma-blade capsulotomy was accomplished using the electrosurgical base unit attached to a Fugo blade tip (Figure 6). The “cut power” was set to “medium” and the “cut intensity” was set to “level 5,” as per the instruction of the manufacturer. With the foot-petal engaged, the Fugo blade filament was placed in contact with the capsule and the tip was moved in a circle while maintaining contact with the capsule. An attempt was made to avoid creating any right-angled edges.
Figure 6.

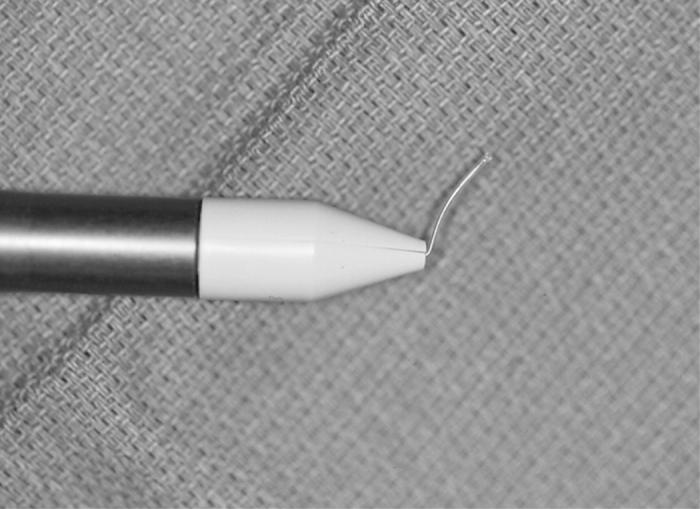
Fugo equipment needed for the plasma-blade capsulotomy technique.
Biomechanical Characteristics of the Porcine Anterior Capsule
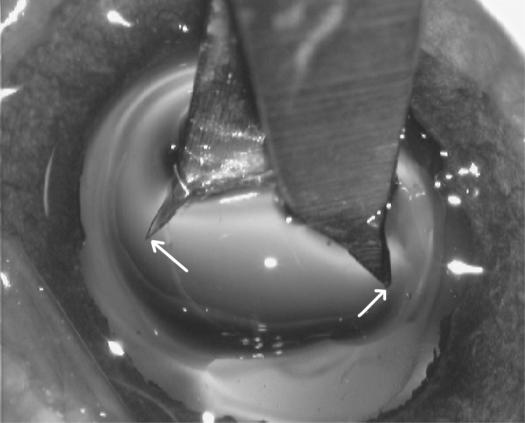
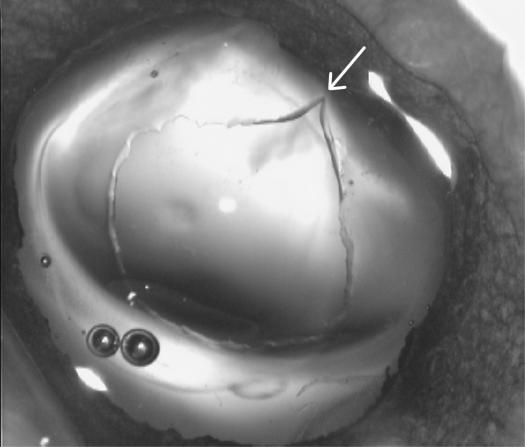
The calipers used to measure the capsulotomy opening are shown in Figure 7A. Panel B shows the caliper tips inserted in the unstretched capsulotomy, and panel C shows that the stretch-to-break occurred elsewhere (arrow) and not at the point where the caliper tips met the edge of the capsulotomy opening; in this case the tear occurred at about the 2-o’clock position.
Figure 7.
Calipers were used to measure the unstretched and stretched capsulotomy opening. Left, the caliper tips are placed in the unstretched capsulotomy opening (white arrows indicate the caliper tips). Right, The same stretched capsulotomy opening as appears at left, showing where this opening was torn when stretched (white arrow).
The unstretched capsulotomy diameter (Dun) per capsulotomy technique is shown in Table 1 (young pigs) and Table 2 (adult pigs). No significant difference (P > .05) was shown between these measurements per procedure, or between the young and adult eyes (P > .05) of each procedure. Likewise, there was no significant difference between the stretched length-to-breaking (Ls) measurements (Tables 1 and 2) per procedure (P > .05), or between the young and adult pig eyes (P > .05).
Table 1.
Unstretched diameter (Dun) mean of the young porcine anterior capsulotomy per technique, and the stretched length-to-break (Ls)
| Young pig eyes (n = 10) |
||||||
|---|---|---|---|---|---|---|
| Capsulotomy technique | Dun mean (±sd) | Dun range | Dun median | Ls mean (±sd) | Ls range | Ls median |
| Vitrectorhexis | 4.8 (±0.3) mm | (4.2 – 5.1 mm) | 4.9 mm | 11.7 (±1.5) mm | (9.4 – 14.0 mm) | 11.8 mm |
| Manual CCC | 5.1 (±0.7) mm | (3.8 – 6.2 mm) | 5.2 mm | 13.5 (±2.6) mm | (9.4 – 17.2 mm) | 13.0 mm |
| Can-opener | 4.7 (±0.6) mm | (3.9 – 6.1 mm) | 4.7 mm | 11.3 (±1.8) mm | (9.8 – 15.9 mm) | 10.7 mm |
| Kloti radiofrequency | 4.8 (±0.6) mm | (4.0 – 5.8 mm) | 4.8 mm | 11.5 (±1.9) mm | (9.2 – 14.4 mm) | 11.4 mm |
| Fugo plasma blade | 4.8 (±0.4) mm | (3.9 – 5.3 mm) | 4.8 mm | 13.1 (±3.0) mm | (9.4 – 17.7 mm) | 12.1 mm |
CCC, continuous curvilinear capsulorrhexis.
Table 2.
Unstretched diameter (Dun) mean of the adult porcine anterior capsulotomy per technique, and the stretched length-to-break (Ls)
| Adult pig eyes (n = 10) |
||||||
|---|---|---|---|---|---|---|
| Capsulotomy technique | Dun mean (±sd) | Dun range | Dun median | Ls mean (±sd) | Ls range | Ls median |
| Vitrectorhexis | 4.9 (±0.5) mm | (4.1 – 5.7 mm) | 5.0 mm | 13.0 (±1.3) mm | (10.9 – 14.5 mm) | 13.0 mm |
| Manual CCC | 5.1 (±0.5) mm | (4.4 – 6.2 mm) | 5.1 mm | 16.1 (±1.8) mm | (14.0 – 19.0 mm) | 15.7 mm |
| Can-opener | 4.9 (±0.5) mm | (4.4 – 6.1 mm) | 4.8 mm | 11.1 (±1.9) mm | (9.1 – 15.8 mm) | 10.6 mm |
| Kloti radiofrequency | 4.8 (±0.6) mm | (3.6 – 5.6 mm) | 5.0 mm | 10.3 (±1.1) mm | (8.9 – 12.2 mm) | 10.3 mm |
| Fugo plasma blade | 4.7 (±0.3) mm | (4.3 – 5.2 mm) | 4.7 mm | 12.4 (±2.3) mm | (9.2 – 15.7 mm) | 11.8 mm |
CCC, continuous curvilinear capsulorrhexis.
The unstretched and stretched circumference of the young and adult pig eyes are presented in Tables 3 and 4, respectively. The stretched to unstretched circumference per anterior capsulotomy technique is shown in Figure 8.
Table 3.
Unstretched (Cun) and stretched circumference (Cs) mean of the young porcine anterior capsulotomy per technique
| Young pig eyes (n = 10) |
|||||||
|---|---|---|---|---|---|---|---|
| Capsulotomy technique | Cun mean (±sd) | Cun range | Cun median | Cs mean (±sd) | Cs range | Cs median | Cs to Cun mean % |
| Vitrectorhexis | 15.1 (±1.0) mm | (13.1 – 16.1 mm) | 15.4 mm | 23.3 (±3.0) mm | (18.9 – 28.0 mm) | 23.7 mm | 153.7 % |
| Manual CCC | 15.9 (±2.2) mm | (11.8 – 19.5 mm) | 16.2 mm | 27.1 (±5.2) mm | (18.7 – 34.4 mm) | 26.1 mm | 171.1 % |
| Can-opener | 14.7 (±1.9) mm | (12.2 – 19.2 mm) | 14.8 mm | 22.7 (±3.6) mm | (19.5 – 31.9 mm) | 21.4 mm | 153.8 % |
| Kloti radiofrequency | 15.0 (±2.0) mm | (12.6 – 18.1 mm) | 15.2 mm | 22.9 (±3.7) mm | (18.5 – 28.8 mm) | 22.8 mm | 152.7 % |
| Fugo plasma blade | 15.2 (±1.3) mm | (12.1 – 16.6 mm) | 15.2 mm | 26.1 (±6.1) mm | (18.8 – 35.5 mm) | 24.2 mm | 172.5 % |
CCC, continuous curvilinear capsulorrhexis.
Table 4.
Unstretched (Cun) and stretched circumference (Cs) mean of the adult porcine anterior capsulotomy per technique
| Adult pig eyes (n = 10) |
|||||||
|---|---|---|---|---|---|---|---|
| Capsulotomy technique | Cun mean (±sd) | Cun range | Cun median | Cs mean (±sd) | Cs range | Cs median | Cs to Cun mean % |
| Vitrectorhexis | 15.4 (±1.7) mm | (12.8 – 17.9 mm) | 15.8 mm | 26.0 (±2.5) mm | (21.7 – 29.1 mm) | 25.9 mm | 169.2 % |
| Manual CCC | 16.1 (±1.6) mm | (13.8 – 19.3 mm) | 15.9 mm | 32.1 (±3.5) mm | (27.9 – 38.0 mm) | 31.3 mm | 199.8 % |
| Can-opener | 15.4 (±1.7) mm | (13.7 – 19.2 mm) | 14.9 mm | 22.3 (±3.7) mm | (18.2 – 31.6 mm) | 21.2 mm | 143.8 % |
| Kloti radiofrequency | 15.2 (±1.9) mm | (11.2 – 17.6 mm) | 15.6 mm | 20.7 (±2.3) mm | (17.9 – 24.4 mm) | 20.6 mm | 137.2 % |
| Fugo plasma blade | 14.8 (±0.8) mm | (13.4 – 16.2 mm) | 14.8 mm | 24.8 (±4.6) mm | (18.3 – 31.3 mm) | 23.5 mm | 168.3 % |
CCC, continuous curvilinear capsulorrhexis.
Figure 8.
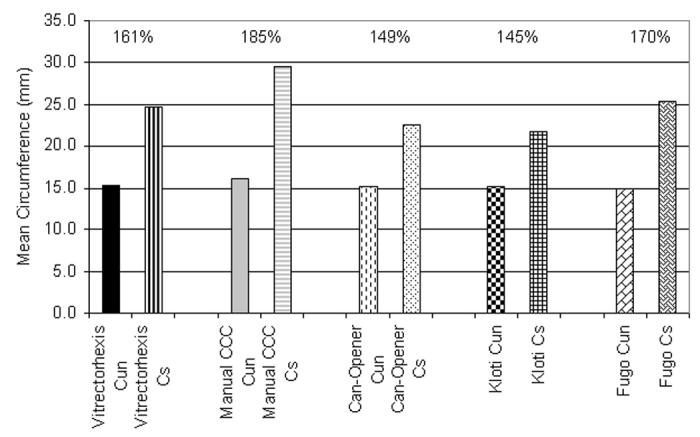
Percentage comparison of the mean stretched (Cs) to unstretched (Cus) circumference per capsulotomy technique (n = 20 eyes per technique).
The mean percentage of stretched to unstretched circumference of young lens capsules, each presented as a mean value in the order of animal used for each of the five anterior capsulotomy technologies, and similarly for the adult lens capsules, showed that in this population there was no significant difference (P > .05) between the anterior capsule stretch of the young eyes compared to the adult eyes.
Scanning Electron Micrography of Porcine Lens Capsule
One porcine globe per each of the five anterior capsulotomy techniques was implanted with a PMMA MC52BM intraocular lens (Alcon Laboratories, Fort Worth, Texas) following lens removal and submitted for SEM evaluation.
Group 1, using the vitrectorhexis anterior capsulotomy technique, is shown in Figure 9. Scanning electron microscopy images showed the capsular edge of the vitrectorhexis cut was a typical scalloped edge with the whole edge rolled-over, presenting a smooth surface toward the inside of the capsulotomy (panel A). High-magnification SEM shows a slightly less regular edge (panel B) than that achieved with the manual technique (Figure 10).
Figure 9.
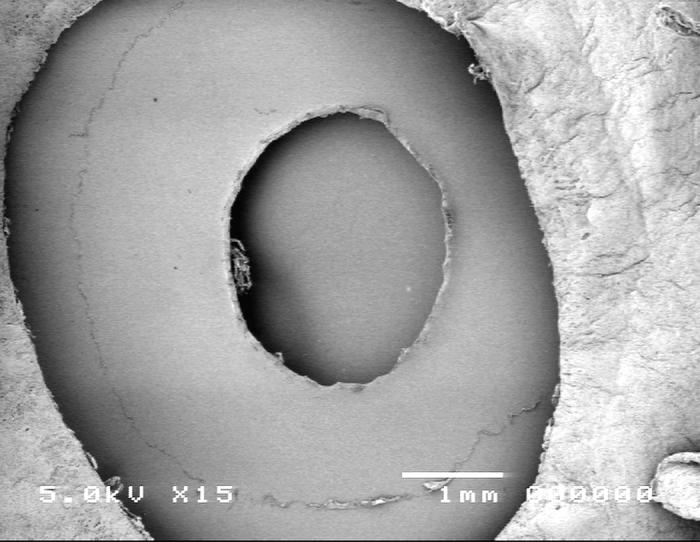

Scanning electron micrograph overview of the vitrector capsulotomy (top) and the cut edge of the anterior lens capsule (bottom).
Figure 10.
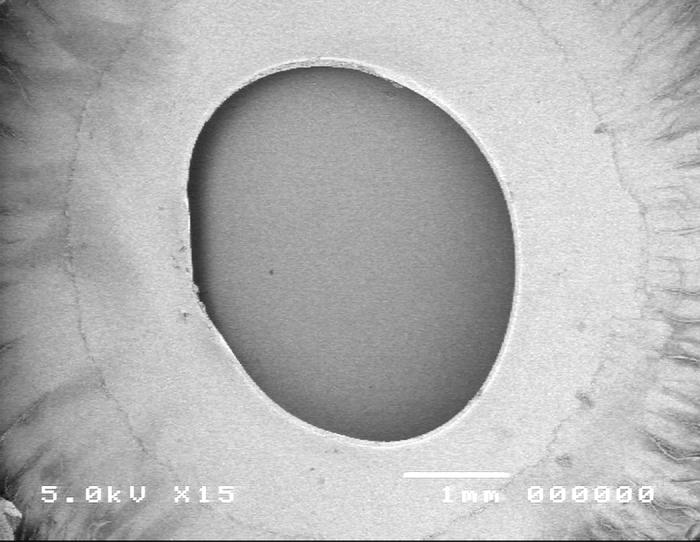
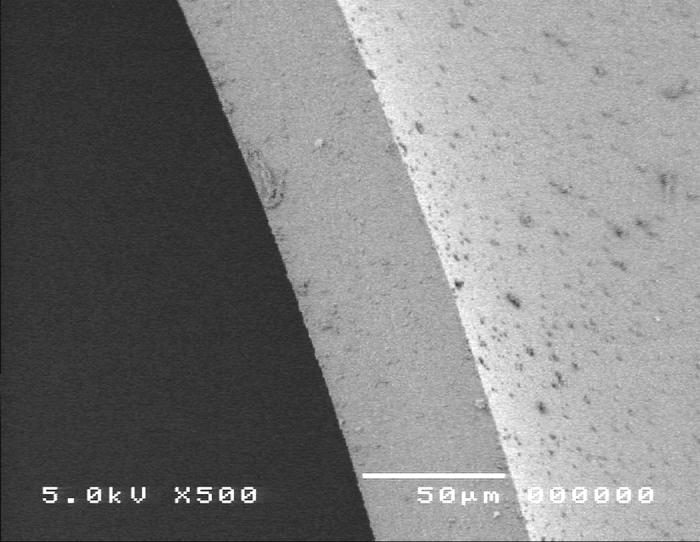
Scanning electron micrograph overview of the continuous curvilinear capsulotomy (top) and the cut edge of the anterior lens capsule (bottom).
Group 2, using the manual CCC anterior capsulo-tomy technique, is shown in Figure 10. Scanning electron microscopy images revealed that the edge of the manual capsulorrhexis is smooth and exhibits no obvious defects (panel A). High-power SEM shows a smooth, regular edge (panel B).
Group 3, multipuncture can-opener anterior capsulotomy technique, is shown in Figure 11.
Figure 11.
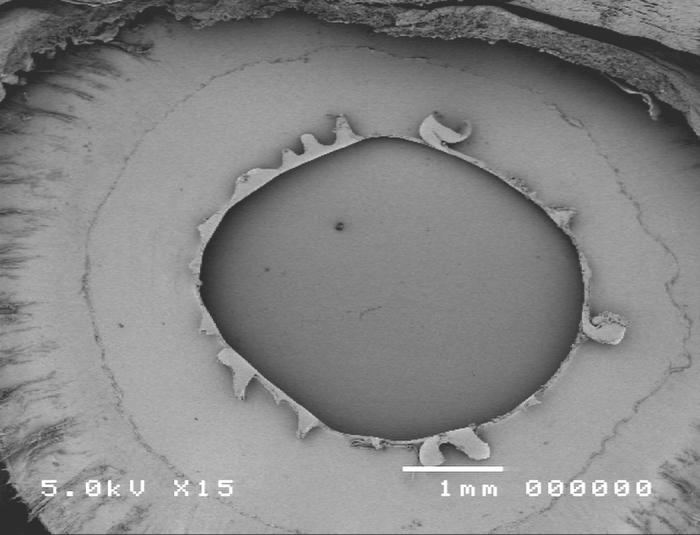
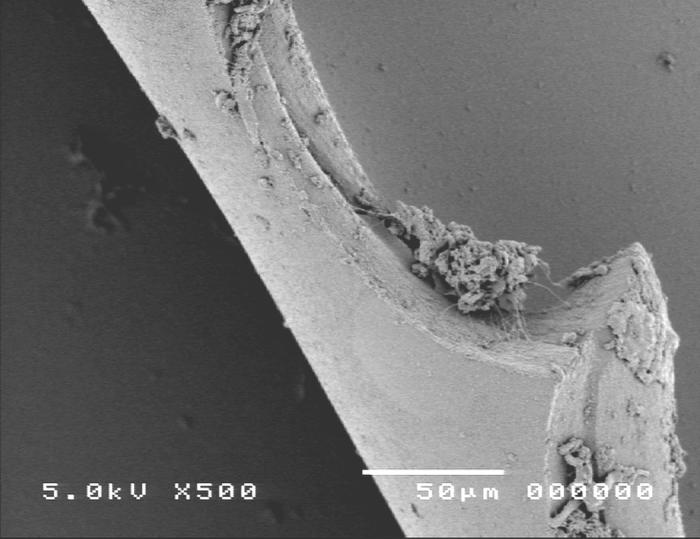
Scanning electron micrograph overview of the multipuncture can-opener capsulotomy (top) and the cut edge of the anterior lens capsule (bottom).
Group 4, using the Kloti radiofrequency diathermy anterior capsulotomy technique, is shown in Figure 12. Scanning electron microscopy images showed the ragged capsular edge of the diathermy cut (panel A). High-magnification SEM shows a rough irregular edge (panel B) when compared to the manual technique (Figure 10).
Figure 12.
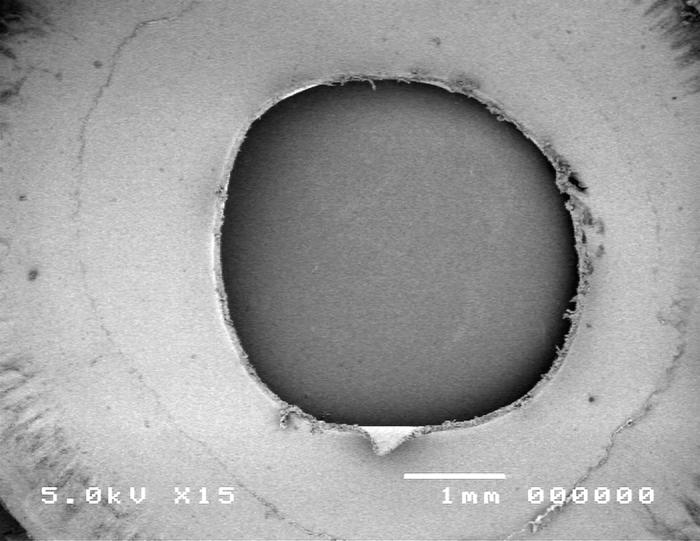
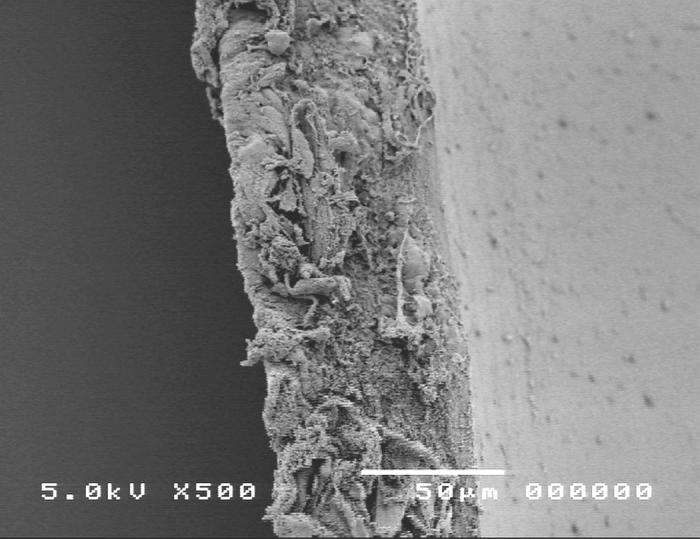
Scanning electron micrograph overview of the Kloti radiofrequency diathermy capsulotomy (top) and the cut edge of the anterior lens capsule (bottom).
Group 5, using the Fugo plasma-blade anterior capsulotomy technique, is shown in Figure 13. Scanning electron microscopy images showed the capsular edge of the plasma blade cut (panel A). High-magnification SEM shows a slightly rough edge (panel B) when compared to the manual technique (Figure 10).
Figure 13.
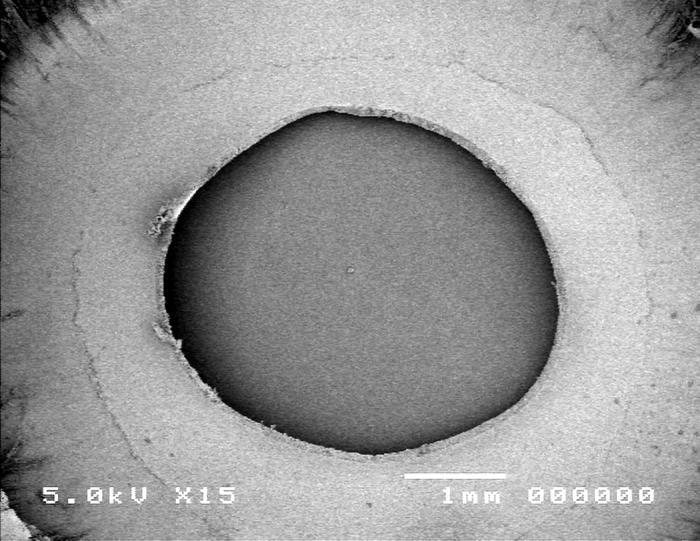
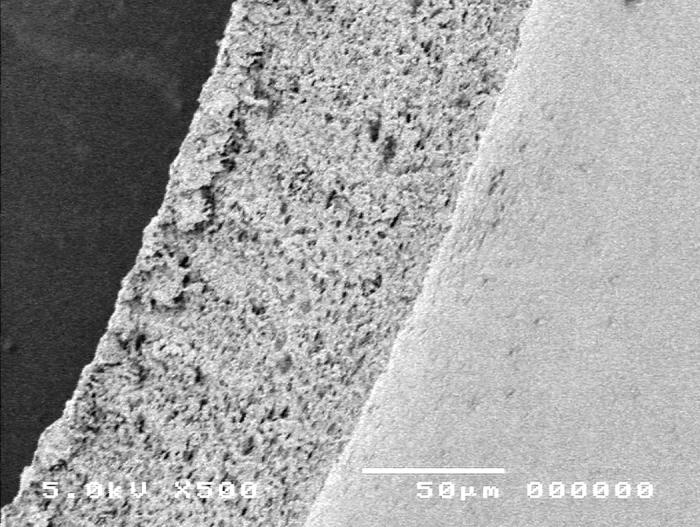
Scanning electron micrograph overview of the Fugo plasma-blade capsulotomy (top) and the cut edge of the anterior lens capsule (bottom).
Ten-Year Review of Pediatric Cataract Surgery Cases
Retrospective analysis of 379 consecutive pediatric cataract surgeries performed between January 1, 1994, and December 31, 2003, was undertaken (Figure 14). All surgery was performed by the same surgeon. Emphasis was placed on the integrity of the edge of the anterior capsulotomy during cataract surgery. The analysis revealed that the anterior capsulotomy withstood the stresses of surgical manipulation without tearing in 91.9% (329 of 358) of cases. These cases included 74 eyes left aphakic and 284 pseudophakic eyes. The remaining 21 cataract cases had preoperative rupture of the anterior capsule from trauma (19 eyes) or spontaneously (2 eyes with Alport syndrome). These groups were analyzed separately. The five different anterior capsulotomy techniques used in these surgical cases were vitrectorhexis, manual CCC, multipuncture can-opener, Kloti radiofrequency diathermy, and the Fugo plasma blade.
Figure 14.
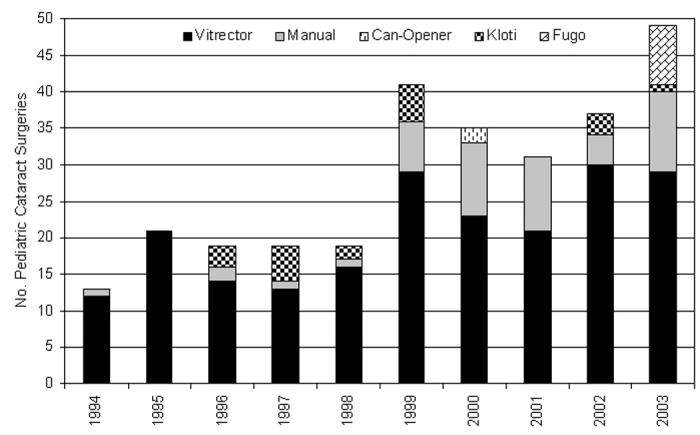
Distribution of 284 pediatric cataract surgeries in which an intraocular lens was inserted (pseudophakic group), including anterior capsulotomy technique used and year performed by one surgeon.
Twenty-nine anterior capsular tears were recorded during the 284 pediatric cataract surgeries where an IOL was implanted (Figure 15). Distribution of the capsular tears was not associated with placement in time (P > .05); that is, there were statistically no more tears occurring between 1994 and 1998 (91 cases; 10 tears) than between 1999 and 2003 (193 cases; 19 tears). The surgical step during which each tear occurred is also shown in Figure 15: 4 (13.8%) of 29 tears occurred during the capsulotomy, 7 (24.1%) during cataract removal, 13 (44.8%) during IOL insertion, 4 (13.8%) during hydrodissection, and 1 (3.4%) during OVD removal. The tears occurring during IOL insertion were not statistically different from the incidence of the other tears (P > .05).
Figure 15.
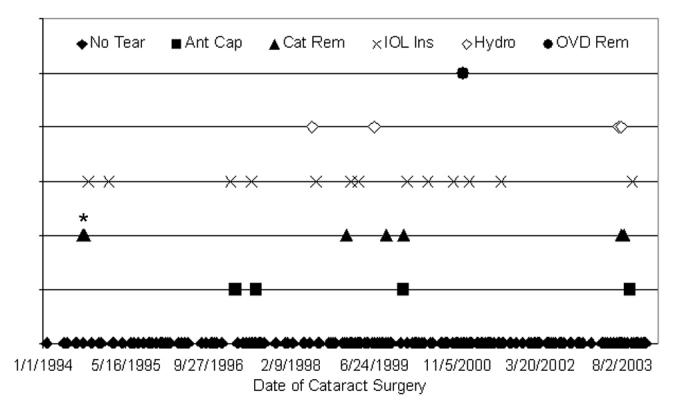
Distribution of tears per one surgeon’s experience. Twenty-nine anterior capsule tears (10.2%) occurred during 284 pediatric cataract surgeries. The incidence of each tear location was not statistically different (P > .05) from any other tear location during the 10-year period. Asterisk indicates 2 data points separated by 8 days; Ant Cap, anterior capsulotomy; Cat Rem, cataract removal; IOL Ins, intraocular lens insertion; Hydro, hydrodissection; OVD Rem, ocular viscosurgical device removal.
The age of the patients ranged from birth to 234 months. The mean age of all patients was 53.5 months ± 50.4 (median, 44 months). Capsular tears in those aged 0 to 72 months were not statistically different from those 72 to 234 months (P > .05). Of all pediatric cataract surgeries where an IOL was implanted (284), 198 (69.7%) were from patients ≤72 months of age; tears occurred in 19 (9.6%) of these 198 eyes. Table 5 presents the mean distribution of patient age, with or without tear, per capsulotomy technique. Age was not related to tear incidence (P > .05). The surgical step during which the tear occurred is shown in Figure 16, but was not statistically limited to any age group (P > .05).
Table 5.
Mean ages of 284 patients receiving various capsulotomy techniques during pediatric cataract surgery without complication, or with an anterior capsule tear during surgery
| Eyes without capsular tears |
Eyes with capsular tears |
|||||||
|---|---|---|---|---|---|---|---|---|
| Capsulotomy technique | Mean age (±sd) | Age range | Median | No. of eyes | Mean age (±sd) | Age range | Median | No. of eyes |
| Vitrectorhexis | 38.0 (±38.7) mo | (0 – 181 mo) | 22.0 mo | 192 | 67.1 (±47.9) mo | (6 – 135 mo) | 61.0 mo | 16 |
| Manual CCC | 108.9 (±57.5) mo | (7 – 234 mo) | 104.0 mo | 44 | 66.7 (±54.2) mo | (25 – 128 mo) | 47.0 mo | 3 |
| Can-opener | 1.0 mo | NA | NA | 1 | 1.0 mo | NA | NA | 1 |
| Kloti radiofrequency | 77.0 (±55.3) mo | (6 – 156 mo) | 61.7 mo | 15 | 57.0 (±26.7) mo | (25 – 86 mo) | 58.5 mo | 4 |
| Fugo plasma blade | 54.2 (±33.0) mo | (21 – 87 mo) | 54.5 mo | 3 | 58.3 (±23.9) mo | (21 – 87 mo) | 59.0 mo | 5 |
CCC, continuous curvilinear capsulorrhexis.
Figure 16.
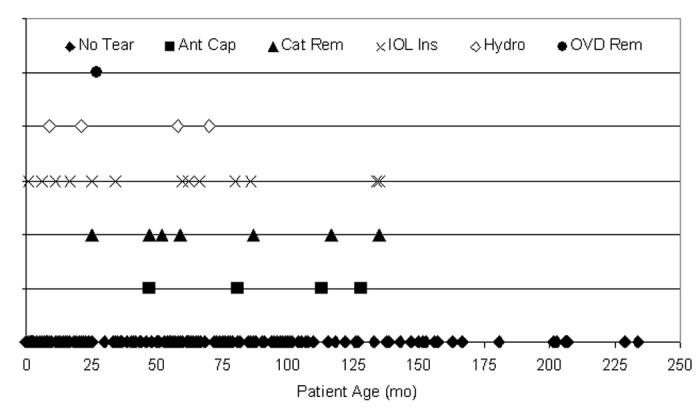
Distribution of tears per patient age. Twenty-nine anterior capsule tears (10.2%) occurred during 284 pediatric cataract surgeries, 65.5% (19 of 29) in patients 72 months of age or younger. These tears were not statistically attributable to any single year of life (P > .05). No tears occurred in patients aged 136 months or older. Ant Cap, anterior capsulotomy; Cat Rem, cataract removal; IOL Ins, intraocular lens insertion; Hydro, hydrodissection; OVD Rem, ocular viscosurgical device removal.
Each of the capsulotomy techniques is presented per patient age, beginning with the vitrectorhexis technique. Vitrectorhexis was the capsulotomy technique of choice for younger patients (Figure 17). There were 16 capsule tears (7.7%) during the 208 cases using the vitrectorhexis technique. One hundred sixty-four (78.8%) of the 208 cases were ≤72 months of age, and 9 (56.3%) of the tears were from patients ≤72 months of age. The surgical step during which the tear occurred is also shown in Figure 17.
Figure 17.
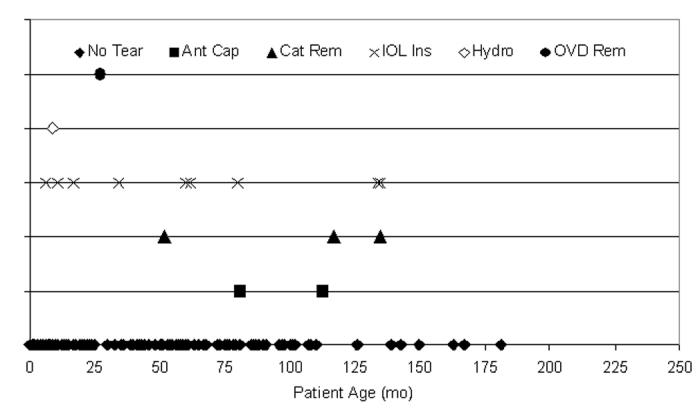
Distribution of tears per vitrector anterior capsulotomy technique. Sixteen anterior capsule tears (7.7%) occurred during 208 pediatric cataract surgeries using the vitrector for the anterior capsulotomy; 56.3% of tears (9 of 16) occurred in patients 72 months of age or younger. These tears were not statistically attributable to any single year of life (P > .05).
There were 3 capsule tears (6.4%) during the 47 cases using the manual CCC technique (Figure 18). Fourteen (29.8%) of the 47 cases were ≤72 months of age, and 2 (66.7%) of the tears were from patients who were ≤72 months of age. The surgical step during which the tear occurred is also shown in Figure 18.
Figure 18.
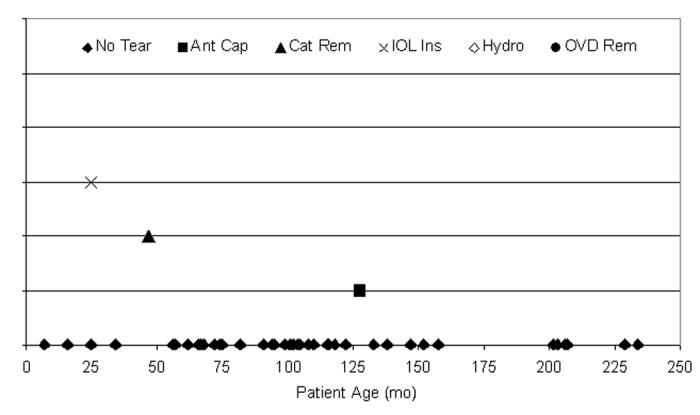
Distribution of tears per manual continuous curvilinear capsulorrhexis anterior capsulotomy technique. Three anterior capsule tears (6.4%) occurred during 47 pediatric cataract surgeries using manual CCC for the anterior capsulotomy; 66.7% of tears (2 of 3) occurred in patients 72 months of age or younger. These tears were not statistically attributable to any single year of life (P > .05).
The multipuncture can-opener capsulotomy technique did not have sufficient use in this clinical series to be statistically compared to the other techniques (Figure 19). There was one capsule tear during the two cases using the can-opener capsulotomy technique. Both patients were ≤72 months of age. The surgical step during which the tear occurred is also shown in Figure 19.
Figure 19.
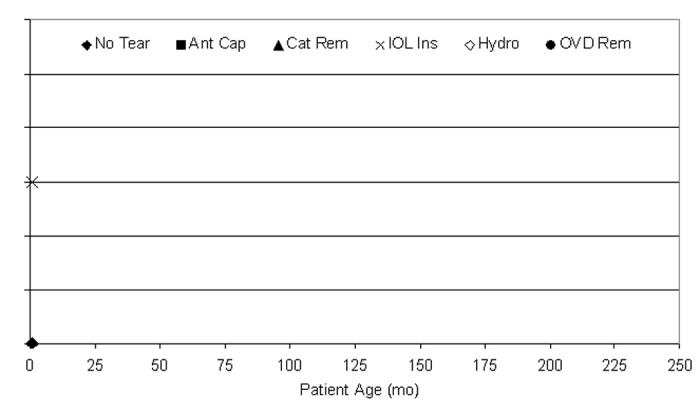
Distribution of tears per multipuncture can-opener technique. One anterior capsule tear occurred during two pediatric cataract surgeries using multipuncture can-opener for the anterior capsulotomy; this patient was younger than 72 months of age. This tear could not be statistically analyzed because of the small sample size.
Kloti radiofrequency diathermy was used less frequently in this series when compared to manual CCC (Figure 18) and vitrectorhexis (Figure 20). There were 4 capsule tears (21.1%) during the 19 cases using the Kloti radiofrequency diathermy technique. Twelve (63.2%) of the 19 cases were ≤72 months of age, and 3 (75.0%) of the tears were from patients who were ≤72 months of age. The surgical step during which the tear occurred is also shown in Figure 20.
Figure 20.
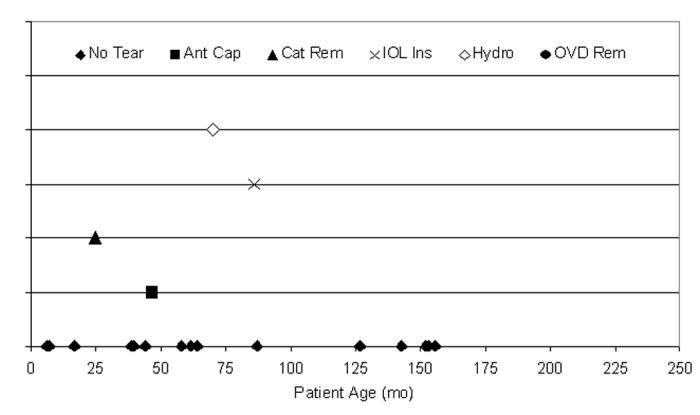
Distribution of tears per Kloti radiofrequency diathermy anterior capsulotomy technique. Four anterior capsule tears (21.1%) occurred during 19 pediatric cataract surgeries using Kloti technique for the anterior capsulotomy; 75.0% of tears (3 of 4) occurred in patients 72 months of age or younger. These tears were not statistically attributable to any single year of life (P > .05).
The Fugo plasma blade capsulotomy technique did not have sufficient use in this clinical series to be statistically compared to the other techniques (Figure 21). There were 5 capsule tears (62.5%) during the 8 cases using the Fugo plasma blade capsulotomy technique. Six (75.0%) of the 8 cases were ≤72 months of age, and 4 (80%) of the tears were from patients who were ≤72 months of age. The surgical step during which the tear occurred is also shown in Figure 21.
Figure 21.
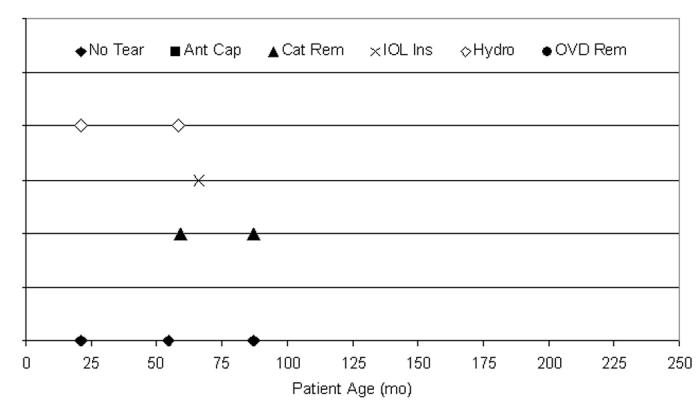
Distribution of tears per Fugo plasma blade anterior capsulotomy technique. Five anterior capsule tears (62.5%) occurred during eight pediatric cataract surgeries using Fugo technique for the anterior capsulotomy; 80.0% of tears (4 of 5) occurred in patients 72 months of age or younger. These tears could not be statistically analyzed due to the small sample size.
Overall (Figure 22), 15 capsule tears (10.1%) occurred in the 148 cataractous right eyes (mean age, 53.2 months ± 50.1; range, birth to 234 months; median, 44 months). One hundred and two (68.9%) of the 148 cases were ≤72 months of age, and 11 (73.3%) of the tears were from these 102 patients. There were 14 capsule tears (10.3%) in left eyes during these 136 surgeries (mean age, 53.9 months ± 50.8; range, birth to 229 months; median, 43.7 months). Ninety-six (70.6%) of the 136 cases were ≤72 months of age, and 8 (57.1%) of the tears were from these 96 patients. The surgical step during which the tear occurred is also shown in Figure 22 but was not statistically attributable to either eye (P > .05).
Figure 22.
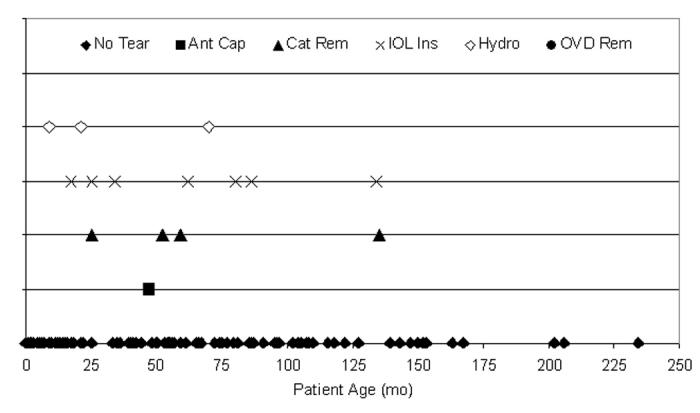
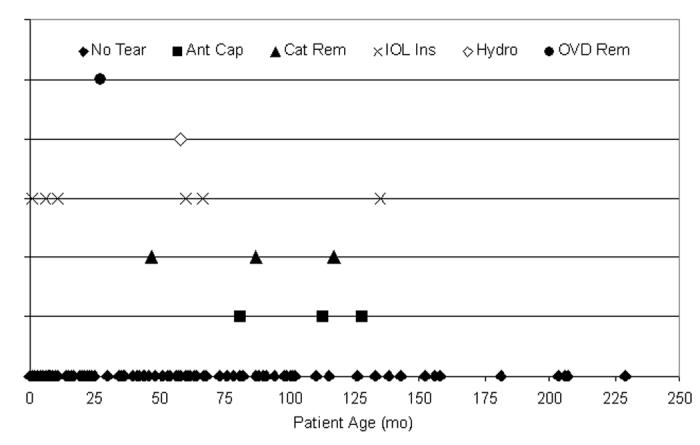
Distribution of tears per eye. Twenty-nine anterior capsule tears (10.2%) occurred during 284 pediatric cataract surgeries: 15 tears (10.1%) in the 148 right eyes (top) and 14 tears (10.3%) in the 136 left eyes (bottom). Eleven of 15 right eye tears (73.3%) and 8 of 14 left eye tears (57.1%) occurred in patients 72 months of age or younger. These tears were not statistically attributable to any single year of life or surgical ocular location (P > .05, respectively).
Seventy-four cases were left aphakic. The vitrectorhexis technique was utilized 73 times and the Kloti radiofrequency diathermy used once. No radial tears developed in this group. These were young patients (mean age, 16.2 months; range, birth to 57 months; median, 2 months).
Twenty-one eyes had preoperative rupture of the anterior capsule from trauma (19), or spontaneously (2; in association with Alport syndrome) (mean age, 85.2 months; range, 16.8 to 165.6 months; median, 84 months). In these eyes, the vitrectorhexis technique was used to complete the capsulotomy in 16 eyes, the Fugo plasma blade capsulotomy technique was used in 1, manual CCC was used in 3, and in 1 eye the ruptured anterior capsule was not enlarged at all. No additional tears occurred during these surgeries.
Pediatric Case Details For Eyes With Radial Tears
Vitrectorhexis
There were 16 anterior capsule tears in the vitrectorhexis group. Nine of these occurred during IOL insertion. Six capsule tears occurred nasally in the right eye or temporally in the left eye at the location where the trailing haptic of the IOL was exerting pressure as it was being dialed into the capsular bag. Of the remaining 3 tears, 2 occurred when three-piece acrylic lenses were unfolded too anteriorly, with the leading haptic inadvertently in the ciliary sulcus. Extensive dialing of the lens was needed to secure the IOL completely in the capsular bag. During this maneuver, a tear formed nasally in a left eye and temporally in a right eye. The remaining IOL insertion-related tear occurred superiorly as forceps were being used to place the trailing haptic. All 9 of these tears occurred during manipulation of either a PMMA (3 of 9) or a three-piece foldable IOL (6 of 9).
In 5 of 9 tears, the terminal edge of the tear was easily seen and the tear did not continue to extend toward the lens equator. In all 9, the IOL was successfully positioned completely within the capsular bag with the IOL haptics 90 degrees away from the radial tear.
Three of the vitrectorhexis tears (3 of 16, 18.7%) occurred during removal of lens cortex. Two were superior (subincisional) tears and one was an inferior tear, all at recognized right-angled edges in the capsulotomy. Two vitrectorhexis tears (2 of 16, 12.5%) occurred while the capsulotomy was being performed. In both cases, the wounds were too large and fluid leak caused anterior chamber instability. One vitrectorhexis tear occurred during hydrodissection as the lens substance forcibly moved anteriorly. This tear, however, did not extend after the fluid was decompressed from the capsular bag. It remained stable for the remainder of the case. One vitrectorhexis tear occurred after Healon GV removal as the bimanual irrigation and aspiration instruments were withdrawn from the eye. The anterior chamber became shallow and the IOL pressed against the edge of the capsulotomy. The tear occurred at a previously recognized right-angled edge, nasally.
Manual CCC
There were 3 tears in the manual continuous curvilinear capsulorrhexis group. One tear occurred during lens cortex removal, one tear occurred during IOL insertion, and one peripheral extension occurred during the capsulotomy itself. Additionally, two manual CCC capsulotomies that were extending toward the lens equator were aborted before radial tears formed. These were converted into a circular capsulotomy using the radiofrequency diathermy unit and are thus not classified as radial tears.
The manual CCC that tore during lens cortex removal had been complicated by poor visualization from liquefied cortex that escaped the capsular bag upon initiation of the capsulotomy. A capsular flap superiorly was left and not recognized until after the tear had developed in this same area. Another manual CCC radial tear occurred at a site where the keratome used to enter the eye had inadvertently pierced the capsule. Despite manipulating the CCC to incorporate this keratome puncture site, a tear emanated from this area when stressed by IOL insertion. The third manual CCC tear occurred as a peripheral extension during the capsulotomy procedure itself. This capsulotomy was completed using the vitrector. In all three of these cases, the IOL was successfully implanted into the capsular bag with the IOL haptics placed 90 degrees from the tear.
Multipuncture Can-opener
There was one capsule tear in the multipuncture can-opener group. It was recorded during the IOL insertion. Two eyes of one infant were operated on using the can-opener technique. The capsule was difficult to puncture using the needle tip. Although the capsulotomy was reasonably round, one of the two capsulotomies tore when the unfolding haptic of a three-piece acrylic IOL came in contact with the capsulotomy edge.
Kloti Radiofrequency Diathermy
There were four capsule tears in the Kloti radiofrequency diathermy group. One tear was recorded each during anterior capsulotomy, hydrodissection, lens cortex removal, and IOL insertion. In one case, a tear occurred at the completion of the capsulotomy when the instrument tip remained adherent to the capsular edge as it was withdrawn from the eye. In another case, at the completion of the capsulotomy, the wound was enlarged slightly to facilitate removal of the capsular cap. Bimanual irrigation and aspiration handpieces were used to remove the lens substance. The enlarged opening allowed fluid leakage around the instruments, making the anterior chamber unstable. Coincident with the shallowing of the anterior chamber, a radial tear occurred in the capsulotomy edge beneath the superior wound. The edge remained visible, however, and did not extend to the lens equator. In all of the radiofrequency diathermy tear cases, the IOL was successfully placed into the capsular bag with the IOL haptics placed 90 degrees from the tear.
Fugo Plasma Blade
This group had five anterior capsule tears when using the Fugo plasma blade capsulotomy technique. Among the nine eyes (8 in pseudophakic group) where the Fugo plasma blade was used, a tear developed in 5 (55.5%). One eye had a preoperative rupture of the capsule from trauma. This capsule opening was completed using the Fugo blade without additional tears occurring. Among the primary IOL cases without preoperative trauma, radial tears occurred in 5 of 8 eyes (62.5%) where the Fugo blade was used to perform the anterior capsulotomy. The tears occurred during hydrodissection (2 eyes), IOL insertion (1 eye), or cataract removal (2 eyes).
Worldwide Anterior Capsulotomy Survey of Pediatric Surgeons
Responses to the 2003 worldwide anterior capsulotomy survey of pediatric surgeon preferences were received from 563 of 996 mailed questionnaires for an overall response rate of 56.5%. The response rate from US surgeons was 57.2% (467 of 816), and the international surgeon response rate was 53.3% (96 of 180). Three retired US respondents and 134 US respondents not practicing pediatric cataract surgery and not implanting intraocular lenses were omitted from further review, leaving 330 returned questionnaires for review and preference analysis. Twenty-eight international respondents not practicing pediatric cataract surgery and not implanting intraocular lenses were omitted from further review, leaving 68 returned questionnaires for review and preference analysis.
Overall, 99.5% (396 of 398) of the respondents whose questionnaires were analyzable were performing pediatric cataract surgery. Thirty-six of the 328 respondents performing pediatric cataract surgery in the United States were not implanting IOLs (11.0%). Four of the 68 international respondents performing pediatric cataract surgery were not implanting IOLs (5.9%).
Of the overall analyzed questionnaires, 82.9% of the respondents (330 of 398) were implanting IOLs in pediatric patients. Two of the 294 US respondents (0.7%), but none of the international respondents that were implanting IOLs, were not also performing pediatric cataract surgery.
Figure 23 shows the number of responses attributed to each of five anterior capsulotomy categories and the pediatric ages each applies to. Vitrectorhexis and manual capsulotomies were the most popular choices, where preference appears to inversely relate to patient age, particularly during the first 6 years of life, between these two techniques. As would be expected, the preferences per age group for vitrectomy are significantly different from those of manual capsulotomy (P < .05). The numbers of responses to the remaining three categories of Figure 23 are too few to compare. Tables 6 and 7 show the same data redistributed as US and international preferences of the five capsulotomy techniques.
Figure 23.
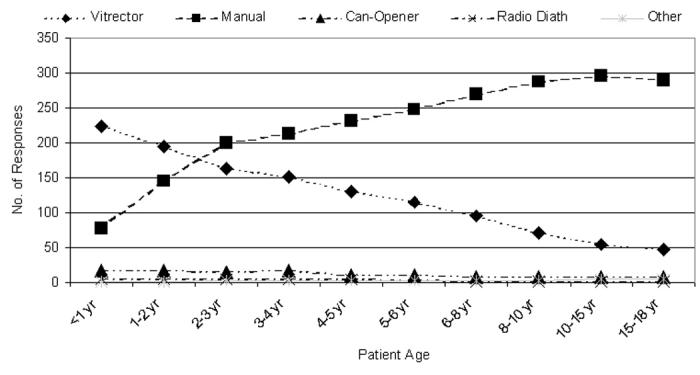
Number of questionnaire responses per patient age group per anterior capsulotomy technique (n = 398). Radio Diath, radiofrequency diathermy.
Table 6.
Percentage of total responses from us pediatric surgeons per patient age group (percentage) per anterior capsulotomy technique*
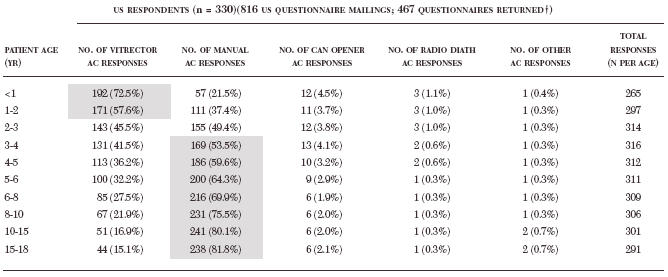 |
AC, anterior capsulotomy; NA, not applicable; radio diath, radiofrequency diathermy.* Technique preferences of greater than 50% participation are highlighted in grey.
Three US respondents were retired and 134 US respondents were not practicing pediatric cataract surgery and not implanting intraocular lenses, and thus were omitted from analyses. Multiple responses allowed.
Table 7.
Percentage of total responses from non-us pediatric surgeons per patient age group (percentage) per anterior capsulotomy technique*
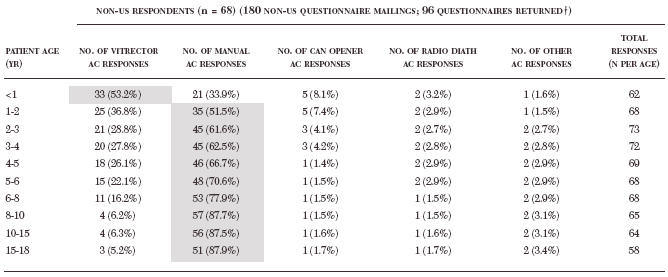 |
AC, anterior capsulotomy; NA, not applicable; radio diath, radiofrequency diathermy. * Technique preferences of greater than 50% participation are highlighted in grey.
Twenty-eight non-US respondents were not practicing pediatric cataract surgery and not implanting intraocular lenses, and thus were omitted from analyses. Multiple responses allowed.
Figure 24 shows the preference percentage per age group attributed to each of the five anterior capsulotomy techniques. The vitrectomy and manual capsulotomy preferences representing >50% of the responses are highlighted in Tables 6 and 7, where the data of Figure 24 is redistributed as the US and international preferences of these five capsulotomy techniques.
Figure 24.
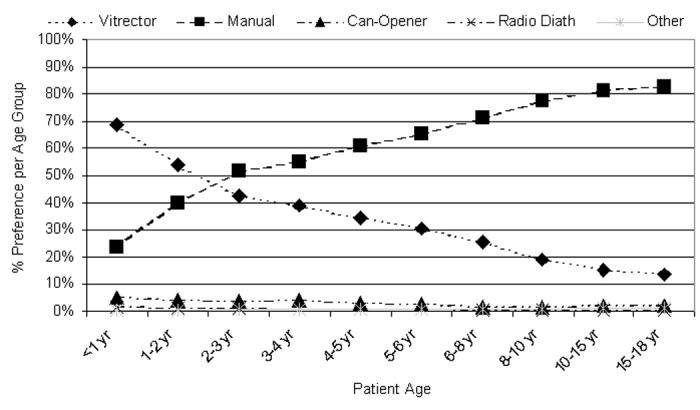
Percentage of preferences per age group per anterior capsulotomy technique (n is available in Tables 6 and 7). Radio Diath, radiofrequency diathermy.
DISCUSSION
The human lens forms from an invagination of surface ectoderm. It is because of this invagination that the lens capsule, a true PAS-positive basement membrane, comes to lie external to the cells that secreted it. The lens capsule continues to grow throughout life in conjunction with the volume increases of the crystalline lens. The lens capsule changes physiologically as well. The tensile strength of the anterior lens capsule is five times greater in infancy as compared to old age.21 The anterior lens capsule of the young eye also has double the extensibility of the aged capsule.21 These differences between the adult and child must be understood and accounted for when planning for surgery on the pediatric lens. Because modern cataract surgery at all ages takes place almost entirely within the capsule of the lens, knowledge of the mechanical properties of the lens capsule is needed to help the surgeon achieve the goal of safe and complete cataract removal followed by implantation of an IOL that will remain stable and well supported. Openings into the anterior capsule of the crystalline lens are made to gain access to the nuclear and cortical lens material during cataract surgery. In addition, they allow an IOL to be placed within the capsular bag after cataract removal. The fact that modern cataract surgery has been called “capsular surgery”38 attests to the importance of the lens capsule and the capsulotomy to the outcome of the procedure.
For purposes of this discussion, anterior capsulotomy techniques will be presented in the chronologic order in which they were developed and first utilized. This will allow a better historical perspective of the evolution of these techniques. The findings of the current study and the recommendations of this author will then be incorporated within this chronologic context.
Forceps Capsulotomy
Sir Harold Ridley39,40 described the first anterior capsulotomy technique designed to be followed by insertion of an IOL. A complete anterior capsule removal was advocated, using toothed forceps. If anterior capsular flaps or remnants were seen after lens cortex removal, they were grasped with smooth-bladed capsule forceps and cut with scissors. Tearing was avoided for fear of removing the posterior capsule as well. Drews41 described the classic technique this way: “With the pupil maximally dilated, the forceps was opened and its teeth rested against the lens capsule on either side. The exquisitely sharp teeth were then gently pressed through the capsule and the jaws of the forceps closed. Hopefully and usually, this resulted in the tearing away of an appropriate size flap.”)41(p However, if the teeth were not sharp enough, delayed puncture of the capsule would sometimes result in splitting of the posterior capsule when the forceps teeth were brought together. Drews also wrote: “To keep the … surgeon humble, the anterior capsule is relatively thick and resistant to the surgeon’s attempts to produce a uniform and controlled aperture while the posterior lens capsule is thin and easily ruptured.”41(p Nowhere is this statement truer than in pediatric cataract surgery.
Triangular or “V”-shaped Capsulotomy
Kelman42 recommended a triangular anterior capsulotomy for use with his new phacoemulsification technique in 1975. This so-called Christmas tree41 or fir tree38 capsulotomy was performed by placing an irrigating cystotome on the capsule 4 mm inferior to the optical center of the lens. The cystotome engaged the capsule gently so as not to puncture it, but merely to tent it. As the cystotome was withdrawn toward the incision, the capsule was opened in a triangular shape. Additional slits were made in the capsule to open it more widely. This technique was used for children as well as adults. However, the capsule did not always tear to produce a triangular flap. The dimensions of the capsulotomy were not always consistent or reproducible. Also, the more elastic pediatric capsule increased the stress that the technique placed on the capsule and the zonules. At times, the capsule would not tear, resulting in zonular rupture. Or, the capsule would tear too far into the lens equator.41 The V-shaped capsulotomy, popularized for developing world extracapsular adult cataract surgery by the Fred Hollows Foundation, is a variation of the Kelman capsulotomy. It utilizes the side of a sharp straight needle to do the cutting. In this way, the size and shape of the capsulotomy can be more easily controlled. This technique has been utilized for children as well as adults by the Himalayan Cataract Project.43,44 The V-shaped capsulotomy has the advantage of not requiring an OVD, thus reducing the cost of the surgery. The straight needle can be manipulated within the anterior chamber without the loss of aqueous humor. The disadvantage is that the angled edges of the “V” shape can tear out to the lens equator or even onto the posterior capsule. To test the stability of a slit or V-shaped capsulotomy in an animal model of the human pediatric anterior lens capsule, we stretched 20 adult pig eye capsulotomies made with a needle and scissors. Each capsulotomy tore very quickly as soon as even minimal stretch was placed perpendicular to one of the ends of the slit (unpublished data, 2003). These data were not part of the formal porcine study described in detail herein.
Can-opener Capsulotomy
To reduce stress placed on the zonular fibers and produce a more rounded capsulotomy with fewer tags, the multi-puncture or can-opener capsulotomy came into common use. This technique has been ascribed to Pearce38 and, alternatively, to Little.45 This technique was performed using a bent 27-gauge needle. A jagged but circular opening in the capsule was formed. Frequently, however, radial extensions of the capsulotomy occurred during lens removal or IOL insertion.38,45 Wasserman and coworkers46 analyzed 250 consecutive postmortem adult eyes operated on for cataracts between September 1978 and June 1989. More than 90% had been operated on using the can-opener anterior capsulotomy technique. Eighty-six percent of the eyes had one to five radial tears. Asymmetric IOL loop fixation and resulting decentration were more common when radial tears were present. Each of the early methods of capsulotomy discussed above allowed access to the contents of the lens, but they often resulted in numerous radial tears emanating from the capsular openings. These tears resulted in uncertainty regarding the stability of the capsular bag and the position of the IOL after implantation. Rosen38 lamented in 1990 that the can-opener technique remained the most popular anterior capsulotomy worldwide, in spite of “progressive developments which should confine the technique to history.”
Recently, Wood and Schelonka30 recommended a return to the can-opener capsulotomy technique for pediatric cataracts. Using a porcine model, Wood and Schelonka30 compared can-opener capsulotomy to manual CCC capsulotomies. A total of 47 can-opener capsulotomies were compared with 102 manual CCC capsulotomies. The first author, an experienced cataract surgeon, compared his capsulotomy performance to that of the study’s second author, a medical student with only a single capsulotomy training session prior to the study. The first author of the paper performed 20 can-opener capsulotomies using the porcine model without any radial tears. The second author had one radial tear in 27 consecutive can-opener capsulotomies (2.1%). In contrast, the overall failure rate for manual CCC in their study was 22.5%. The experienced surgeon encountered uncontrolled tears in 17 (21.3%) of 80 manual CCC eyes. The inexperienced surgeon experienced uncontrolled tears in 6 (27.3%) of 22 manual CCC capsulotomies. The number of tears compared to the experience of the surgeon was not statistically different (P > .05). The relative risk of an uncontrolled radial tear during the manual CCC in the Wood and Schelonka30 study was 10.58, as compared to the can-opener capsulotomy. Even more surprising was the finding that the mean maximum strain (stretch-to-rupture) of the can-opener capsulotomies (46.7%) was not statistically different from the mean maximum strain of the manual CCC (47.7%) in their porcine model.30
For the animal model portion of the present study, the porcine eye was also utilized. We found that the can-opener capsulotomy stretched to a mean diameter of 149% of the unstretched diameter before breaking (Figure 8). This is very similar to the findings of Wood and Schelonka30 discussed above. Extensibility in the current study is reported as the percentage of stretched compared to unstretched capsulotomy circumference. Wood and Schelonka30 reported the percentage of stretch beyond the baseline resting circumference. Thus the two figures, 149% and 46.7%, represent very similar stretch characteristics.
The manual CCC capsulotomy in our porcine study stretched to a mean of 185% compared to the unstretched circumference (Figure 8). This is in contrast to the 47.7% mean stretch-to-rupture reported by Wood and Schelonka.30 In the current porcine study, the manual CCC (P < .001, one-way ANOVA) and the Fugo plasma blade capsulotomy (P = .004, one-way ANOVA) were significantly more extensible than the can-opener capsulotomy. However, no statistical difference was detected when the mean stretch-to-break percentage of the can-opener capsulotomy was compared to the Kloti radiofrequency diathermy capsulotomy (P > .05) or the vitrectorhexis (P > .05).
The adult porcine capsule has been shown to be a valid model for the pediatric human capsule.13 For the current study, porcine eyes from young (6 months of age) as well as adult (2 to 3 years of age) pigs were studied. However, there was no significant difference (P > .05) between the percentage stretched to unstretched circumference of the young pig eyes when compared to the adult pig eyes.
As in human pediatric eyes, the capsule of the pig is very elastic and difficult to puncture. These characteristics create a very different opening after multipuncture can-opener capsulotomy in children when compared to adults. Observations during the current study revealed that each puncture of the needle created a small CCC as the needle tip was pulled toward the center of the capsule, not unlike the tearing that occurs when the Auffarth47 technique for CCC is used in rabbits. The further the needle tip is pulled toward the center of the pupil, the more extensive the small CCC arc-tear will be. When the needle is then reengaged into the capsule adjacent to the previous puncture, another small CCC arc tear occurs. A scalloped edge results, but the tips of each pointed capsular tag are directed toward the center of the pupil and tend to roll outward when OVD is placed in the capsule, thus appearing as a smooth edge. This is in contrast to a can-opener capsulotomy performed on the adult human capsule. With less elasticity and less resistance to tearing, each puncture of the needle results in a small radial tear with the capsular tags pointing outward. These outward-pointing tags have been shown by computer models and a mathematical tool called the finite element method to be areas of high stress that tear toward the lens periphery when minimal stretch is applied.48 In contrast, inward-pointing capsular flaps, as seen in the can-opener capsulotomy on the pediatric capsule, show no increased stress and no increase in tearing tendency when compared with the remainder of the capsulotomy edge.48
Prior to the development of the manual CCC, the can-opener capsulotomy had a long and favorable track record in pediatric cataract surgery. Helveston and Ellis,6 in their 1984 book entitled Pediatric Ophthalmic Practice, illustrated the use of a can-opener capsulotomy, which had been the standard of practice for pediatric cataract surgery for some time, and remained so in their hands even after they adopted the vitrector handpiece for performing the posterior capsulotomy and anterior vitrectomy. A meta-analysis of published cases from 1983 to 1995 revealed a total of 509 can-opener capsulotomies in children30,49–57 with follow-up ranging from 4 to 18 years.30 A reoperation was documented in only seven eyes (1.4%). None of the repeated surgeries were attributed to defects or tears in the capsulotomy. However, 26 IOLs had iris/papillary capture, and 324 IOLs (of 509) were believed to have one or both haptics outside of the capsular bag. Basti and coworkers54 reported a total of 169 operated eyes with all “except the last few” having had a can-opener capsulotomy. A radial tear was reported in only four eyes, and in each of these the IOL was still placed easily into the capsular bag.
These rather dramatic differences between the pediatric and adult can-opener capsulotomy make the technique more recommendable for children than for adults. However, it appears that for the past 10 years the can-opener technique has not been utilized often by pediatric surgeons. In a 1994 survey, only 15.0% of the responding surgeons reported using a can-opener capsulotomy when operating on children.25 The 2003 surgeon preference survey included in this thesis showed an even lower utilization rate of 1.9% to 5.2% (Figure 24), depending on the age of the child. For US surgeons, the can-opener capsulotomy technique utilization rate ranged from a high of 4.5% of surgeons when operating on patients less than 1 year of age to 1.9% of surgeons when operating on children older than age 6 to 8 years (Table 6). Multiple responses were allowed, so this represents the total number of responding surgeons who reported the technique as one of their preferred options utilized at the indicated ages between <1 and 18 years.
In the porcine model of this study, the capsules were difficult to puncture using even a fresh sharp cystotome. Force was directed posteriorly until the needle tip punctured the capsule, creating a sudden surge of the needle into the substance of the lens. A variable amount of capsular opening would then result. Also, additional capsular punctures were sometimes needed when the adjacent punctures did not connect with one another, leaving a gap of uncut capsule. This created strands of capsule that were frequently engaged by the aspiration handpiece, interfering with the lens removal that followed the capsulotomy. Only two eyes were operated on using the can-opener technique in the clinical series reported herein. These were a pair of infant eyes from one patient. One of the two developed a radial tear during IOL insertion when the unfolding haptic came in contact with the capsulotomy edge. Despite the fact that the movement of the needle was toward the center of the pupil, more zonular stress seemed to be created than with the other techniques. The tear occurred at a right-angled edge that had been inadvertently created.
Manual CCC
The manual CCC technique is an outgrowth of the can-opener style capsulotomy developed to address the tendency of the adult can-opener edge to develop numerous radial tears.45 The widespread recognition of the need for a better technique resulted in the near simultaneous development of a continuous tear capsulotomy on three different continents. The technique known today as manual CCC was developed simultaneously in North America by Gimbel and in Europe by Neuhann.27 A third surgeon, Shimizu, in Asia, also developed a similar technique, which was reported less than 2 years after Gimbel’s first presentation. Since Shimizu had no prior knowledge of the North American or European work (neither had yet been reported in print), he also shares credit for the CCC development.27
Gimbel27 recalls watching Gills use a combination of scissor cuts and tears to make capsular openings in 1983. Gimbel then began making a continuous tear superiorly to avoid capsular flaps that would interfere with efficient cortical removal, and gradually began tearing the capsule in a complete circle. After more than 1,000 cases of what he called “continuous tear capsulotomy,” Gimbel made a video presentation for the American Intraocular Implant Society (April 1985, Boston, Massachusetts). This video was also shown at the American Academy of Ophthalmology annual meeting in 1985 (IOLAB booth, San Fransisco, California). Ocular Surgery News reported on Gimbel’s technique in its July 1, 1985, issue (pages 20–21). The first peer-reviewed publication on this new type of capsulotomy was authored by Neuhann in 1987.58
In 1990,27 a name was suggested for the new capsulotomy technique that would incorporate terms used to describe it by all three of the originators. From Gimbel’s continuous tear capsulotomy, Neuhann’s capsulorrhexis, and Shimizu’s circular capsulotomy came the name “continuous circular capsulorrhexis” and therefore the abbreviation CCC. Later, the term “continuous curvilinear capsulorrhexis” was adopted, because the word “curvilinear” was generally more correct than “circular.”29 Gimbel and Neuhann27 also stated that CCC makes possible safe and effective IOL implantation in children, because secure posterior chamber in-the-bag implants generally cause no problems during physical maturation. The authors even stated that without the development of CCC, lens implantation in children might not have been realized. Although this appears to be an overstatement, it does emphasize that the development of CCC was important to the evolution of cataract surgery in children.
In the porcine eyes studied herein, the CCC edge was the most resistant to tearing (Figure 8) and also showed the cleanest edge microscopically with no debris or irregularities (Figure 10). The CCC method clearly creates the “gold standard” capsulotomy edge. However, as Gimbel and Neuhann27 first noted, it is much more difficult to perform this method in children as compared to adults. The increased fracture toughness and extensibility of the child’s capsule increases the tendency for the capsulotomy to extend peripherally. Also, more force is required before tearing begins. The considerable distention that occurs before propagating the tear has been called “tractional preload” and has been noted to create a pronounced outward-pulling vector force.29 In addition, reduced scleral rigidity results in posterior vitreous upthrust when the eye is entered. The vitreous “pressure” pushes the lens contents anteriorly, leading to a taut and domed anterior lens capsule.12 These difficulties are more pronounced with decreasing age of the child. Illustrative is a case series of infantile cataract surgeries reporting inadvertent peripheral extensions in 19 (90.5%) of 21 eyes when a manual CCC was attempted.28 In a cadaveric study, Wilson and coworkers25 reported inadvertent peripheral extensions in 6 of 18 pediatric eyes when a CCC was performed. All peripheral extensions were in eyes from children younger than age 5 years.25 This ex vivo study was criticized for not simulating the closed chamber conditions found in a clinical setting at cataract surgery.59 As a result, the porcine CCCs in the current study were made in a closed chamber setting without removing the cornea. Only one CCC extended peripherally. Andreo, Wilson, and Apple13 validated the porcine eye as an appropriate model for the human pediatric eye. The lower rate of peripheral extensions in the current porcine study as compared to the previous cadaveric study may have been due to the closed chamber technique in the current study, which allowed OVD to be utilized to flatten the capsule and prevent vitreous upthrust. It is interesting to note that inadvertent peripheral extensions during the capsulotomy procedure in the current porcine study occurred less often with manual CCC than with the Kloti radiofrequency diathermy, or the Fugo plasma blade technique.
Modifications in adult techniques to facilitate CCC in children are now more widely known and practiced. These include the use of a high-molecular-weight OVD, pulling more toward the center, aiming for a smaller-than-desired capsulotomy opening, and frequent regrasping near the site of the advancing tear so that the direction of pull can be readjusted more easily. Some surgeons have altered the adult technique even more substantially in order to facilitate CCC in elastic capsules. Auffarth and colleagues47 developed a modified CCC technique for use in experiments on eyes of young albino rabbits and suggested that it be used for young human capsules as well. Auffarth and coworkers47 had discovered that rabbits have very elastic lens capsules reminiscent of the pediatric lens capsule. The technique begins with a puncture of the lens capsule at the superior border of the intended capsulotomy using a 27-gauge needle. Capsulorrhexis forceps are then used to grasp the anterior capsule centrally. The capsular flap is torn toward the 6-o’clock position until a half-circle is completed. The force is then reversed toward 12 o’clock, pulling with equal force to both tearing edges. This technique was used in an experimental study with 16 rabbits (32 eyes). The authors reported a radial tear in only 2 of the 32 eyes.
Nischal60 described a modification of the Auffarth technique in which two stab incisions are made in the anterior capsule, outlining the desired diameter of the capsulorrhexis. Capsulorrhexis forceps are used to grasp one end of the distal edge of the proximal anterior capsule stab incision. The grasped edge is gently pushed toward the corresponding point of the distal stab incision until the edge reaches halfway to the distal stab. The corresponding end of the proximal edge of the distal stab incision is similarly grasped, but with the capsule pulled gently toward the proximal stab incision. The two tears meet to form the CCC. This is repeated for the other end of each stab incision to complete the entire CCC. Nischal60 has named this technique the two-incision push-pull, or “TIPP,” capsulorrhexis. The tearing force, using this technique, is always directed toward the center of the pupil.
In the clinical cases reviewed herein, the manual CCC was very stable with only 3 of 47 eyes (6.4%) having a radial tear. No eye with a continuous uninterrupted capsulotomy edge developed a tear during surgery. In one eye, from a child 3 years old with a white complete cataract, liquefied cortex escaped the capsular bag upon initiation of the capsulotomy and obscured the view of a capsular flap. It was not recognized that the capsulotomy had not been completed. The tear occurred at the site of this flap. If liquefied cortex escapes the capsular bag at the beginning of the capsulotomy, it should be gently aspirated before completing and inspecting the capsulotomy. Alternatively, the vitrectorhexis technique can be used. Since the vitrector handpiece has aspiration capability, the escaping cortex can be removed coincident with the making of the capsulotomy. Visualization is, therefore, not compromised.
Another manual CCC that developed a radial tear was in a 2-year-old child. The keratome used to enter the eye had inadvertently pierced the capsule. Despite attempting to manipulate the CCC to incorporate the puncture site, a radial tear formed at this site during IOL insertion. The in-the-bag positioning of the IOL was not compromised. The haptics were oriented 90 degrees away from the tear. The third manual CCC tear occurred during the capsulotomy itself. A peripheral extension occurred in the superior temporal quadrant of the eye of a 10-year-old child. The capsulotomy was then completed using the vitrector. The IOL was placed into the capsular bag with the haptics oriented along the 11-o’clock to 5-o’clock axis. The IOL centered well despite the tear. Two additional manual CCC openings extended toward the lens equator but were rescued (before radial tears formed) by switching to the Kloti radiofrequency diathermy technique. If these two errant capsulotomies are added to the other three, the percentage of unsuccessful manual CCC technique capsulotomies is 10.6%. The vitrectorhexis tear rate, for comparison, was 7.7% (16 of 208) when an IOL was placed and 5.7% (16 of 282) when aphakic patients were also included. Still, no manual CCC edge tore during surgery if it was a completed circular opening. The same cannot be said for any other technique utilized in this study.
A manual CCC that begins to extend peripherally can be rescued by the vitrector or the Kloti radiofrequency diathermy device, as in the cases above, or the Fugo plasma blade, or even the can-opener technique. Alternatively, the capsulotomy can be regrasped near the leading edge of the capsulotomy and redirected by pulling toward the center of the pupil. Neuhann29 has pointed out that these errant capsulotomies often encounter zonular fibers and tend to extend directly toward the lens periphery, like tearing paper alongside a ruler. He suggests increasing the microscope magnification to identify the responsible zonular fiber or fibers and removing their insertions with a needle or forceps tip. The edge can then usually be brought back toward the center of the pupil. Bluestein and colleagues61 have documented the zonular-free zone of the anterior lens capsule in children to average 6.1 mm in infancy and enlarging to 8.0 mm by age 16 years.
The manual CCC was the most commonly utilized anterior capsulotomy technique when operating on children aged 2 years and older (Tables 6 and 7) based on the survey herein of the AAPOS membership. This survey received a 57.2% (467 of 816) response rate from pediatric ophthalmologists in the United States and a 53.3% (96 of 180) response rate from pediatric ophthalmologists internationally. Practice preferences were remarkably similar between the domestic and international respondents. Although more challenging to perform well, the manual CCC remained the 2003 “gold standard” capsulotomy technique for toddlers and up.
Kloti Radiofrequency Diathermy Capsulotomy
The Kloti radiofrequency diathermy tip is a specialized instrument designed for anterior capsulotomy. It has been used in children35,37 and was tested herein. Kloti developed a miniature bipolar diathermy unit in 1984 that featured bipolar forceps for use in conjunctival, strabismus, or oculoplastic surgery, as well as a bipolar anterior capsulotomy tip.32 In 1988, Gassmann and colleagues62 stated that the anterior capsulotomy was a crucial step in cataract surgery and that the tense capsule was inherently capricious. They advocated using the bipolar radiofrequency endodiathermy probe because it was more precise. Four years later, experimental and clinical data were published on a more refined version of the Kloti radiofrequency capsulotomy instrument. The electronic control device had been improved, and the results of 21 patients compared with 21 controls were reported.32,63 Several advantages were listed for the diathermy capsulotomy over other techniques, including a precisely controlled capsulotomy size, an edge that is resistant to mechanical forces (reducing the risk of radial tears), and a well-defined grayish coagulation line that helps define the border of the capsulotomy (making implantation easier).63
Coincident with the initial 1984 Kloti publication, Berger64 (also in 1984) introduced a bipolar loop device and showed that the loop could cut a round capsular opening in animal and human cadaver eyes. However, the temperature under the anterior capsule reached 73.5ºC during the capsulotomy procedure. The temperature was 36.2ºC adjoining the capsulotomy and 27.7ºC in the anterior chamber. Hausmann and Richard65 noted that the Berger loop could not be placed through the smaller incision that was being used for phacoemulsification and therefore tested the Kloti bipolar tip instead. The Kloti tip was modified to include a concentric cannula ending near the hot tip to be used for irrigation (cooling) and maintenance of the anterior chamber. The overall external diameter of the modified tip was 1.25 mm. Results from 25 adult patients were reported. The capsulotomy procedure took no longer than 20 seconds. Temperature readings were taken using a probe placed inside the anterior chamber at the time of the capsulotomy. Corneal endothelial cell analysis was also done before and after surgery and compared to the fellow eye that had undergone traditional can-opener capsulotomy. These authors showed that room-temperature irrigation fluid provided sufficient cooling of the tip of the instrument to prevent any apparent endothelial cell damage. This lack of corneal endothelial damage was later verified by a prospective randomized study of 55 patients published in 1997.66 Other experiments also showed that no electrolysis occurs as a result of the Kloti tip.65 The Kloti tip used high-frequency current at 500 kHz. The international norm, set by the International Electrical Commission for medical applications, had defined 300 kHz as the lower limit for high-frequency current without causing electrolysis.65
Hausmann and Richard65 pointed out, in 1991, that the two commonly used capsulotomy techniques for adult cataract surgery each had disadvantages. The can-opener technique frequently led to radial tears out to the lens equator, and manual capsulorrhexis required both “specialized surgical skill” and a good red reflex for effective control. In their patients, the modified Kloti radiofrequency bipolar tip had created a capsulotomy edge that was smooth and did not tear during surgery. Kloti63 recommended using a visco-surgical device rather than continuous irrigation to ensure that the anterior chamber was maintained and that the corneal endothelium was protected from heat. Using this technique, other investigators reported, in 1993, 48 consecutive cases with more than 15 months of follow-up. No clinically observable differences were noted between Kloti radiofrequency bipolar capsulotomy and manual capsulorrhexis in adult patients in the categories of rigidity or elasticity of the capsulotomy edge, corneal decompensation, posterior synechiae, or IOL decentration.67
However, laboratory analyses utilizing adult autopsy eyes (published in 1994, 1996, and 1997) demonstrated that the diathermy capsulotomy edge was mechanically inferior to the edge produced by the manual CCC.33,34,68 Morgan and coworkers33 found that the mean increase in edge length before breakage was 53% using the manual CCC and only 18% in the diathermy group. Luck and colleagues34 also noted that on SEM analysis the diathermy caused a loss of the normal lamellar architecture of the capsule with distorted and unrecognizable collagen fibrils and adherent lens fibers. In contrast, the manual CCC edge was completely smooth and free from irregularity with a preserved lamellar organization. Despite these differences, the ability to perform a controlled size capsulotomy independent of the elasticity of the capsule was noted as a potential advantage of diathermy over manual CCC for pediatric use.33,34,68 It was noted that despite the reduction from that seen with the manual CCC, the diathermy capsulotomy edge retained considerable elasticity.34 Without the need to deliver an intact hard nucleus through the capsulotomy opening, the retained elasticity of the diathermy capsulotomy may be sufficient to meet the needs of pediatric cataract surgery. In fact, in 1997, Comer and colleagues35 reported no radial tears in 14 eyes of 7 children aged 2 weeks to 6 years (mean, 23 months; median, 16 months), where O’Keefe had utilized the Kloti radiofrequency diathermy unit to perform the anterior capsulotomy. All but four eyes were implanted with an IOL.
The human laboratory studies of the Kloti radiofrequency diathermy capsulotomy to date have utilized only adult eyes. However, Krag and colleagues68 did compare adult human eyes to adult (5- to 6-month-old) porcine eyes. Others have reported data that validate the adult porcine eye as a model of the human pediatric eye for studies of anterior lens capsule elasticity.13 Krag and coworkers68 found that the mean extensibility (percentage elongation above the resting circumference at break point) of the diathermy capsulotomy was markedly greater in the porcine eyes (62%) than in the human adult eyes (38%). Also, the difference in mean extensibility between the manual CCC and the diathermy capsulotomy (68% versus 38%, P < .001) in human adult eyes was not present in the porcine eyes (62% versus 62%, P > .05). This implies that the diathermy capsulotomy may have a maximum extensibility more like a manual CCC when performed on an elastic capsule like the pediatric human lens capsule. In the porcine study reported herein, the Kloti radiofrequency diathermy capsulotomy showed a mean stretch of 145% (Figure 8) compared to the resting capsulotomy circumference. This mean was lower than the other techniques studied. Statistically, this mean stretch percentage was not significantly different from the can-opener capsulotomy (P > .05), but was significantly less extensible than the vitrectorhexis (P = .025, one-way ANOVA), manual CCC (P < .001, one-way ANOVA), and Fugo plasma blade (P = .001, one-way ANOVA) capsulotomies.
Interestingly, Krag and coworkers68 found that the force required to reach the maximum extensibility (the break point) was much less in the diathermy capsulotomy compared to the manual CCC in both the adult human and the porcine eyes (P < .001). This implies that the heat from the diathermy denatures the collagen and causes it to lose its stiffness. Still, the model 811 CeeOn IOL (Pharmacia, Peapack, New Jersey), used in some of the eyes of the current study before 1997, generates a force of only 15 mN when its haptic springs open into the capsule.68 This is far below the breaking strength of the porcine diathermy capsulotomy (mean, 61 mN ± 15) as determined by Krag and coworkers.68 The single-piece AcrySof (Alcon, Fort Worth, Texas) currently in use is more flexible and probably generates much less force when the haptics open.
In the clinical portion of the current study, the radial tear rate with the Kloti radiofrequency diathermy capsulotomy technique was 21% (4 of 19). The radial tears were divided among the various steps in the cataract procedure, including the anterior capsulotomy, hydrodissection, lens cortex removal, and IOL insertion. It is my clinical impression that the edge produced by this capsulotomy technique expands to the break point with less force than that needed by other capsulotomy techniques. If force applied to the edge of the capsulotomy can be minimized, the radial tear rate will be small. Care must be taken to make the capsulotomy large enough so that subincisional cortex removal and IOL insertion do not place much force on the capsulotomy edge. Compared to the manual CCC, the diathermy capsulotomy edge does not seem to be as resistant to the force applied by the aspiration handpiece when the surgeon reaches deep into the capsular bag equator to remove cortex. In other words, it feels less stiff and, therefore, may reach its maximum extensibility even when relatively little force is applied. In our cases with tears, however, the tears did not extend out to the lens equator. In each case, the edge was visible and the IOL was successfully placed in-the-bag. This lack of extension into the equator or onto the posterior capsule was true of many of the anterior capsule tears in children regardless of technique used. This implies that the pediatric capsule behaves differently in this regard than the adult capsule.
Vitrectorhexis
Despite the fact that anterior capsulectomy using the vitrector handpiece had been reported as part of the “lensectomy” (with no IOL) approach to pediatric cataracts as early as 1981,24 a mechanized (vitrector-cut) anterior capsulotomy technique combined with IOL insertion in children was not reported until the 1990s.25,26 Wilson performed a mechanized anterior capsulectomy combined with IOL insertion in eight children initially.25 All of the capsular openings were curvilinear and continuous, and no radial tears were seen even after the IOL insertion was complete. The original name, “mechanized anterior capsulectomy,” emphasized the mechanized nature of the vitrector handpiece and the fact that a portion of the capsule was removed (capsulectomy) rather than merely opened (capsulotomy). As stated in the “Introduction,” the commonly used term “vitrectorhexis”12,13 emphasizes the fact that it is a substitute for capsulorrhexis performed using the vitrector. It is, in reality, a misnomer, because “rhexis” means to tear rather than to cut.27
In 1994, the new technique (called mechanized anterior capsulectomy) was compared to manual CCC using 18 pairs of human pediatric eyes and two pairs of human adult eyes obtained at autopsy.25 The age of the pediatric donors ranged from 4 days to 16 years. The adult eyes were from donors aged 65 years or older. The globes were prepared for study using a modification of a technique devised by Assia and coworkers.69 The integrity of the anterior capsule opening following lens nucleus and cortex removal and several IOL insertions was assessed by direct visual observation. A radial tear developed in only one of the 18 pediatric eyes in which a mechanized anterior capsulectomy was created. This occurred in one of two eyes from a 16-year-old child. The radial tear extended from a “squared-off” or angled capsulectomy edge during IOL insertion. No radial tears occurred in any of the manual CCC eyes. However, in six eyes (33%), all less than age 5 years, the leading edge of the manual CCC extended out to the lens equator rather than continuing in a circular path. Interestingly, a radial tear occurred in both of the elderly adult eyes (during phacoemulsification of the lens contents) in whom the mechanized anterior capsulectomy was performed. The mechanized technique seemed to work best in the youngest eyes, whereas the manual CCC seemed most suited for the oldest eyes.
Subsequently, an additional comparative study between manual CCC and mechanized anterior capsulectomy (referred to as “vitrectorhexis”) using adult pig eyes was performed.13 To validate the use of the pig eyes, CCC and vitrectorhexis made in pig eyes were also compared to those made in pediatric human eyes. The mean percentage stretch at the break point for human infant eyes aged 1 to 8 months was 165% for CCC and 138% for vitrectorhexis, which correlated well with the data from the adult porcine model (mean stretch = 157% for CCC and 135% for vitrectorhexis).13 The percentage of stretch prior to rupture was higher for CCC than for vitrectorhexis (P < .001), but all capsules stretched adequately for IOL insertion.13
The porcine eye portion of the current study was designed to include all five of the alternative techniques currently in use. The mean vitrectorhexis extensibility of 161% was significantly less than the manual CCC (P = .001, one-way ANOVA), significantly greater than the Kloti radiofrequency diathermy (P = .025, one-way ANOVA), but statistically similar to the capsulotomies produced by the Fugo plasma blade (P > .05) and the can-opener technique (P > .05).
Data collection for the clinical portion of the current study began in 1994 for all anterior capsulotomy procedures. Since then, the vitrectorhexis has been the most common capsulotomy technique used in my practice for pediatric cataract surgery when an IOL is implanted at the primary surgery (208 of 284 eyes, 73.2%) and especially when the eye is left aphakic (73 of 74 eyes, 98.6%). The radial tear rate for vitrectorhexis when an IOL was inserted was 7.7% (16 of 208 eyes) (Figure 17). If the aphakic eyes are added to the tally, the tear rate for vitrectorhexis decreased to 5.7% (16 of 281 eyes). This is less than the tear rate for the entire series (10.2% [29 of 284] for IOL cases, and 8.1% [29 of 358 eyes] when aphakic eyes are included). Furthermore, only one radial tear has been experienced in the last 80 eyes operated on using a vitrectorhexis. This success is attributed to a change in IOL preference, case selection, and technique experience. The vitrectorhexis edge is not as stable as the manual CCC edge, and extra care must be taken to avoid leaving right-angled edges. With experience in using the vitrector handpiece, a more round capsulotomy can be made each time. In addition, avoiding chamber instability throughout the case also helps avoid radial tears even when a right-angled edge is inadvertently made. This is done by creating entry wounds that maintain a tight fit around the instruments while they are in the eye. Also, the majority of the radial tears in the vitrectorhexis group have occurred during IOL insertion (56%, 9 of 16 eyes). Six of these tears occurred as a result of the trailing IOL haptic being dialed into the capsular bag. Children have low scleral rigidity and vitreous upthrust. These factors make IOL dialing more difficult. Extensive manipulation of the IOL was sometimes needed and resulted in radial tears. Placing the haptic, rather than dialing it, into the capsular bag worked better. However, one tear was caused by the forceps during a difficult haptic placement in a small eye. Utilizing the softer single-piece AcrySof IOL (Alcon, Fort Worth, Texas) and the Monarch II injector system (Alcon, Forth Worth, Texas) has helped to reduce the stress placed on the capsulotomy edge by the unfolding IOL haptics. Using a high-viscosity OVD also helps combat vitreous upthrust. Adding additional OVD just before placing the trailing haptic into the capsular bag may further reduce the stress on the capsulotomy edge.
Case selection is an additional factor that has lead to a reduction in radial tears in the last 2 years. The vitrectorhexis technique is ideally suited to the very young child with a highly elastic anterior capsule. In these children, the manual CCC is more difficult to complete even with liberal use of a high-molecular-weight OVD. In the current study, when the vitrectorhexis technique was used, the median patient age of these pseudophakic eyes was 24 months. However, the median patient age of those vitrectorhexis eyes that suffered a radial tear was 61 months. Compare this to the manual CCC, where the median age for these pseudophakic eyes was 104 months and the median patient age of eyes with tears was 47 months. The vitrectorhexis edge is more stable and resistant to tearing in the very young elastic capsules but does not behave as well in the older eyes. This was also evident from the initial vitrectorhexis laboratory study published in 1994.25 Radial tears developed in a 16-year-old eye and both adult eyes, but in none of the young eyes. The current (2003) practice pattern survey (Figures 23 and 24; Tables 6 and 7) showed that vitrectorhexis is the most commonly utilized capsulotomy technique for the first 2 years of life around the world. As the child gets older, the manual CCC becomes somewhat easier to complete and control. It produces the best edge characteristics and can withstand most of the surgical manipulations needed when operating on small soft eyes.
The other advantage of the vitrectorhexis when operating on small eyes is that the anterior capsulotomy and the lens aspiration can be done sequentially without taking the instruments from the eye. Since the vitrector is most efficient when placed through a tight-fitting wound, small-incision capsulorrhexis forceps are recommended for manual CCC in young eyes. The smaller wound (20-gauge or smaller) allows easy conversion to a vitrectorhexis if the manual CCC begins to tear peripherally.
Fugo Plasma Blade Capsulotomy
The Fugo plasma blade was recently developed and has been approved by the Food and Drug Administration for capsulotomy use. The Fugo blade uses plasma technology to create a nearly resistance-free incision into the anterior capsule. The anterior capsule is gently applanated with the tip activated, and a continuous circular movement is made to create the capsulotomy. Alternatively, several arcuate incisions on the capsule can be made and connected to form a circular capsulotomy. The capsulotomy is performed under an OVD. Cavitation bubbles are created along the path of the capsulotomy as the Fugo blade cuts it.
In the current porcine study, the Fugo plasma blade made a round capsulotomy with a mean stretch-to-rupture circumference of 170% compared to the circumference at rest. This extensibility was significantly less than that of the capsulotomy made by the manual CCC (P = .039, one-way ANOVA), but greater than that of the capsulotomy made using the can-opener (P = .004, one-way ANOVA) and the Kloti radiofrequency diathermy (P = .001, one-way ANOVA) techniques. The Fugo plasma blade anterior capsulotomy extensibility was not significantly different from the vitrectorhexis anterior capsulotomy extensibility (P > .05).
The porcine capsules were easily cut using the Fugo plasma blade. However, making a complete circular capsulotomy with a single continuous movement was not always achieved. Cutting the first 180 degrees of the capsulotomy was easy due to the stretch and tension of the capsule. However, finishing the remaining 180 degrees was sometimes complicated by folding of the capsular flap that was no longer on stretch. Nonetheless, a circular capsulotomy of the intended size usually resulted. The SEM analysis of the Fugo plasma blade capsulotomy edge revealed a rough edge that did not resemble the manual tear CCC edge.
While no clinical studies have been published using the Fugo plasma blade for pediatric anterior capsulotomy, Singh37 has reported that he now uses the instrument to perform pediatric capsulotomies. Nine children in the current clinical study had an anterior capsulotomy using the Fugo plasma blade technique. One of these cases had a traumatic rupture of the anterior capsule preoperatively. The rupture was successfully rounded using the Fugo plasma blade. Of the eight eyes in the pseudophakic group, a radial tear developed in five. While the technique did not have sufficient use in this clinical series to be statistically compared to the other techniques, the tear rate was disappointing. Two tears developed during hydrodissection, two during lens aspiration, and one during IOL insertion. In each case, the stress on the capsular edge did not seem excessive. The clinical impression was of an unstable edge that tore easily with minimal stress. This is in contrast to the mean extensibility of 170% recorded for the porcine capsulotomies made with the Fugo plasma blade. Although the force needed to reach the stretch-to-rupture circumference was not measured in the current study, my clinical impression is that, like the Kloti radiofrequency diathermy capsulotomy, the Fugo plasma blade capsulotomy has reduced elastic stiffness. Whether the collagen at the cut edge of the Fugo plasma blade capsulotomy is denatured in some way remains unknown. However, it appears that although the Fugo plasma blade creates a capsulotomy with good extensibility, it may lack the elastic stiffness present in the manual CCC. Additional clinical experience is needed before a meaningful statistical comparison can be made with other techniques used to create a pediatric anterior capsulotomy.
SUMMARY
Cataract surgery is more complex when performed on children as compared to adults. The behavior of the anterior lens capsule during surgery is an example of that complexity. The anterior lens capsule in young children will be approximately three times thinner than the capsule in an elderly adult, but with five times the tensile strength and double the extensibility.21 An intact and appropriately sized anterior capsulotomy is needed during pediatric cataract surgery to help ensure the structure and stability of the remaining lens capsule. Capsular fixation of an IOL in children at the time of cataract surgery has become the standard of care. Therefore, more emphasis is being placed on the anterior capsulotomy procedure by pediatric ophthalmic surgeons today as compared to when primary IOL implantation in children was less common.
With a response rate well over 50%, the 2003 practice preference survey indicated that manual CCC was the most common technique for anterior capsulotomy in children above age 2 years. Vitrectorhexis was the most commonly performed technique for infants. The Kloti radiofrequency diathermy, Fugo plasma blade, and can-opener style techniques were used for anterior capsulotomy in children by only a small number of surgeons.
A porcine model was used to compare the five capsulotomy techniques discussed above. All produced a round capsulotomy that was near the size intended. There was no significant difference in the mean unstretched circumference among the five groups (P > .05), and the capsulotomy produced by the techniques studied showed no difference between young pig eyes and adult pig eyes (P > .05). A previous study validated the adult pig eye as a good model for the pediatric human eye for studies on anterior capsule extensibility.13
In the current porcine study, extensibility was determined by stretching each capsulotomy until it ruptured. Each group demonstrated a mean expansion of more than 140% of its resting circumference before rupture. This degree of extensibility appears to be sufficient to allow a surgeon to place a foldable IOL through an appropriately sized (approximately 5 mm) capsulotomy created by any of these techniques. Therefore, they are all viable options for the pediatric cataract surgeon to choose from. When the mean change in circumference from unstretched to stretched was analyzed for the five groups, a significant difference was found (P < .001, one-way ANOVA). A post-hoc analysis was done to see which groups were different. The manual CCC technique created the most extensible capsulotomy. The manual CCC extensibility was significantly greater than in each of the other techniques (vitrectorhexis, P = .001; Kloti, P < .001; can-opener, P < .001; Fugo, P = .039; one-way ANOVA). The vitrectorhexis extensibility was significantly less than the manual CCC (P = .001, one-way ANOVA), but greater than the Kloti radiofrequency diathermy (P = .025, one-way ANOVA). The Fugo plasma blade capsulotomy extensibility was significantly less than the manual CCC (P = .039, one-way ANOVA), significantly greater than the can-opener (P = .004, one-way ANOVA) and the Kloti radiofrequency diathermy (P = .001, one-way ANOVA), and showed no difference from the vitrectorhexis (P > .05). The can-opener technique produced a capsulotomy that showed less extensibility than the manual CCC (P < .001, one-way ANOVA) and the Fugo plasma blade capsulotomy (P = .004, one-way ANOVA), but was not different from the vitrectorhexis (P > .05) or the Kloti radiofrequency diathermy capsulotomy (P > .05). Finally, the Kloti radiofrequency diathermy capsulotomy was less extensible than the manual CCC (P < .001, one-way ANOVA), can-opener (P = .004, one-way ANOVA), and Fugo plasma blade (P = .001, one-way ANOVA) but was not different from the vitrectorhexis (P > .05).
A retrospective analysis of 379 consecutive pediatric cataract surgeries done by one surgeon over a 10-year period was conducted. When cases with a ruptured lens capsule prior to surgery were excluded, the anterior capsulotomy was round and intact with no radial tears in 91.9% of the cases (329 of 358 eyes). Twenty-one additional cases had preoperative rupture of the capsule, primarily from trauma. These ruptured capsule openings were rounded out using the vitrector handpiece, the Kloti radiofrequency diathermy unit, or the Fugo plasma blade.
Aphakic eyes had no radial tears (0%, 0 of 74 eyes). Most were very young, with a median age of 2 months. All but one eye had a vitrectorhexis (98.6%, 73 of 74 eyes). Pseudophakic eyes had 29 radial tears (10.2%, of 284 eyes). Thirteen of these tears (44.8%, of 29) occurred during IOL insertion. Seven tears occurred during cataract removal (24.1%, of 29). Four tears occurred during the anterior capsulotomy itself (13.8%, of 29). Four tears occurred during hydrodissection (13.8%, of 29). One tear occurred during removal of the OVD at the conclusion of surgery (3.4%, of 29 tears).
The vitrectorhexis technique was more commonly used in younger eyes (mean, 40.2 months; range, 0 to 181 months; median, 23.5 months). The radial tear rate was 7.7% (16 of 208 eyes) over the course of 10 years. However, only one tear has occurred in the last 80 vitrectorhexis capsulotomies. This recent success is attributed to the use of the softer single-piece AcrySof IOL (Alcon, Fort Worth, Texas), which can be injected into the capsular bag with no dialing of the haptics, and thus less stress on the capsulotomy, and to experience with the technique. Avoiding right-angled edges in the capsulotomy and selecting younger patients with more elastic capsules have helped to minimize peripheral tears when using the vitrectorhexis.
The manual CCC capsulotomy technique was more commonly used in older pediatric eyes (mean, 106.2 months; range, 7 to 234 months; median, 104.0 months). The radial tear rate was 6.4% (3 of 47 eyes) over the course of 10 years. Two additional manual CCC capsulotomies developed peripheral extensions but were salvaged by converting to a Kloti radiofrequency diathermy capsulotomy. If these errant capsulotomies are added to the previous total, the rate of unsatisfactory capsulotomies increases to 10.6% (5 of 47 eyes). Unlike the vitrectorhexis, the manual CCC technique was easier to perform and control when it was done on older children. For manual CCC, the mean age of eyes with no tear was 108.9 months (range, 7 to 234 months; median, 104.0 months) compared to a mean age of 66.7 months for eyes with tears (range, 25 to 128 months; median, 47.0 months).
For children in the first 72 months of life, eyes in which a vitrector was used for the anterior capsulotomy had 0.53 times the risk of developing a radial tear compared to when the manual CCC technique was used. In contrast, in patients who were older than 72 months of age, eyes in which a vitrector was utilized for the anterior capsulotomy had a 3.75 times greater risk of developing a radial tear compared to when a manual CCC was performed.
The can-opener, Kloti radiofrequency diathermy, and Fugo plasma blade techniques were used in the current clinical series on 2, 19, and 8 eyes, respectively, within the pseudophakic group. Radial tears were seen in 1, 4, and 5 of these eyes, respectively. The can-opener technique produces a more stable and extensible capsulotomy in children than it does in adults. However, the can-opener technique puts more stress on the zonular fibers and is more likely to leave capsule tags or strands that can interfere with lens aspiration. The Kloti radiofrequency diathermy and Fugo plasma blade techniques can be useful for cutting through fibrotic anterior capsules and do not require a red-reflex for visualization during the capsulotomy. However, these two techniques leave a thin rim of coagulum at the capsulotomy edge, which also appears less smooth on SEM compared to the other techniques. While extensibility remains, elastic stiffness can be reduced if the collagen at the capsulotomy edge has been altered.
RECOMMENDATIONS
The development of capsulotomy techniques that consistently preserve the structure and stability of the remaining lens capsule has helped lead to safer pediatric cataract surgery and the emergence of IOL implantation for children of all ages. The thin, strong, and elastic anterior capsule of children requires a unique approach to the anterior capsulotomy. The following recommendations are offered.
Manual CCC produces the most stable capsulotomy edge and should be utilized whenever possible. However, the risk of peripheral extensions is greater when manual CCC is attempted in the very young child. The vitrectorhexis technique has become the most popular anterior capsulotomy for children in the first two years of life and remains a popular choice for patients up through age 6 to 8 years. The vitrectorhexis technique is recommended as the anterior capsulotomy of choice until the age at which the surgeon decides to leave the posterior capsule intact and not perform an anterior vitrectomy at the time of surgery. For many surgeons, a primary posterior capsulotomy and anterior vitrectomy are performed at the time of cataract surgery until the patient is aged 6 to 8 years. Others begin to leave the posterior capsule intact at an earlier age.
When the vitrectomy equipment and the vitrector handpiece are to be used later in the procedure to remove vitreous, it is recommended that it also be used for the anterior capsulotomy. When performing a vitrectorhexis anterior capsulotomy, the following caveats are offered: (1) Use a vitrector supported by a Venturi pump. Peristaltic pump systems will not cut the anterior capsule as easily. (2) Use a separate infusion cannula that matches the gauge of the vitrector handpiece, thus assuring that the incisions into the eye provide for a tight fit while the instruments are in the eye. The anterior chamber of these soft eyes will collapse readily if leakage occurs around the instruments. (3) Separate the incisions for the infusion and vitrector handpieces by at least 4 clock hours. This will enable easy access to lens capsule and lens cortex throughout the anterior chamber. If the infusion cannula and the vitrector handpiece are the same gauge, the instrument positions can be switched to gain access to subincisional lens cortex during aspiration without producing leakage at either wound site. (4) Do not begin the capsuolotomy with a bent-needle cystitome. The increased intralenticular pressure and vitreous upthrust of the pediatric eye may cause lens material to spontaneously prolapse through this initial opening and create a radial tear. Instead, place the vitrector, with its cutting port positioned posteriorly, in contact with the intact anterior capsule. Turn the cutter on and increase the suction using the foot pedal until the capsule is engaged and opened. A slow cutting rate of 150 cuts per minute with high infusion rate (raise the bottle to the maximum height for gravity-fed infusion, or use a fluid setting of 50 mm Hg for active fluid pump systems) is recommended when cutting the anterior capsule (as opposed to the high cutting rate with lower infusion used to cut the vitreous). With the cutting port facing down against the capsule, enlarge the round capsulotomy in a spiral fashion until the desired size and shape are achieved. (5) Any lens cortex that escapes into the anterior chamber during the capsulotomy can be easily aspirated without interfering with the capsulotomy technique. (6) Care should be taken to avoid right-angled edges, which are predisposed to radial tear formation. With experience, a more rounded capsulotomy is made and fewer angled edges are formed. If a right-angled edge is seen during the capsulotomy, it should be rounded out using the vitrector before completion of the capsulotomy. If the capsulotomy needs to be enlarged, the vitrector can be used for this purpose even after the IOL is in place (often coincident with removal of the OVD). (7) The vitrectorhexis technique is not recommended for older children (above age 8 years) unless the anterior capsule is fibrotic or already ruptured preoperatively.
The manual CCC is easier to control and complete in older children as opposed to the very young. The manual CCC is the “gold standard” capsulotomy and produces the most stable edge. It is recommended for children above the age of 2 years who will have the posterior capsule left intact and will undergo no anterior vitrectomy. This transition from vitrectorhexis anterior capsulotomy, lens aspiration, IOL implantation, vitrectorhexis posterior capsulotomy, and anterior vitrectomy to manual CCC, lens aspiration, IOL implantation, and an intact posterior capsule will occur with patients aged 6 to 8 years for many surgeons, but will occur at an earlier age for others.
When performing a manual CCC capsulotomy in children, the following caveats are offered. (1) Use a high-molecular-weight OVD to flatten the anterior capsule and deepen the anterior chamber. This will create laxity in the anterior capsule and combat the effects of low scleral rigidity, which leads to vitreous upthrust. (2) Aim for a slightly smaller-than-desired capsulotomy. With the stretch in the anterior capsule, the opening is usually larger at completion than it appears to be during the active tearing. (3) When creating the manual CCC capsulotomy, frequently release the capsular flap and inspect the size, shape, and direction of the tear. Regrasp near the site of the continuous tear and readjust the direction of pull if needed to keep the capsulotomy on the planned course. Often, more pull is needed toward the center of the pupil to avoid an extension of the manual CCC out toward the lens equator. (4) Additional OVD should be added as needed to keep the capsule lax during the tearing. (5) Modifications of the manual CCC technique designed for elastic capsules such as the TIPP technique will be preferred by some surgeons.60 This technique is recommended if the surgeon finds it easier to control than the standard method described above. The caveats offered above apply to the TIPP technique as well.
The Kloti radiofrequency diathermy and Fugo plasma blade techniques are recommended when fibrotic capsules are encounted, especially if a vitrector handpiece is not available. They are also useful for white cataracts with absence of the red reflex. With practice, a smooth continuous motion can be made with these instruments to create a round capsulotomy in one motion. The Fugo plasma blade is easier to master because it does not require tip contact with the capsule throughout the capsulotomy. Still, visualization of the advancing edge can be difficult as a result of the tip design with an overhanging silicone sleeve and the cavitation bubbles that become suspended within the OVD. The current Fugo plasma blade tip does not fit well through the 20-gauge opening used for the vitrector handpiece. The Kloti radiofrequency diathermy tip fits easily through a 20-gauge opening, which allows the vitrector to be utilized later in the case without sacrificing the tight fit of the wound around the vitrector handpiece. The Kloti radiofrequency diathermy handpiece is reusable but must be placed in fluid immediately after use to avoid coagulated capsule remnants from interfering with the function of the tip on the next application. Gentle but consistent contact with the capsule must be maintained when using the Kloti radiofrequency diathermy handpiece. If contact is too light or movement is too fast, skipped areas will result. If contact is too firm or movement too slow, the tip will burn through the capsule and enter the lens cortex. Subsequent tip movement drags the capsulotomy edge rather than cutting it, which can cause radial tearing.
The can-opener capsulotomy method is seldom used by pediatric surgeons, but when needed, it is safer and more effective in children than in adults. The elastic nature of the pediatric capsule causes each can-opener puncture to convert to a small arc tear similar to a mini manual CCC. For intumescent lenses in children, the use of a can-opener anterior capsulotomy, which is later converted to a manual CCC, as described by Gimbel and DeBroff,31 is recommended for those surgeons experienced with the technique. An errant manual CCC can sometimes be rescued using a can-opener maneuver, although the vitrectorhexis will be preferred by many for this salvage of a so-called runaway rhexis.
Because the pediatric anterior lens capsule is thinner, stronger, and more elastic that in adults, unique surgical maneuvers are needed when performing an anterior capsulotomy during cataract surgery on a child. The elasticity of the porcine anterior lens capsule closely approximates that of the human childhood anterior lens capsule. The porcine model is recommended as a valuable training aid to help the surgeon master the various techniques discussed in this thesis.
ACKNOWLEDGMENTS
Luanna R. Bartholomew, PhD, and Rupal H. Trivedi, MD, provided data management and statistical analysis. The author gratefully acknowledges their help.
Appendix: 2003 Survey of Anterior Capsulotomy in Pediatric Cataract Surgery
(Results to be submitted as part of the American Ophthalmology Society thesis of M. Edward Wilson, Jr., M.D.)
| 1. Location of your current country of clinical practice. | ||
| □ USA | □ Canada | □ Other (specify) _____________________ |
| 2. Are you currently performing pediatric cataract surgery in the <18-year-old patient? | ||
| □ Yes | □ No | |
| 3. Are you currently implanting IOLs in children? | ||
| □ Yes | □ No | |
| If you answered "No" to Questions 2 AND 3, please stop here and return your survey to the Service Center. | ||
| 4. Which anterior capsulotomy do you usually perform in children at each of the following ages? (Check only one per age group). Please check NA if you do NOT implant IOLs in an age group. | ||
| Age (yrs) | Vitrectorhexis | Manual CCC* | Other (specify) | Not Applicable |
|---|---|---|---|---|
| <1 | □ | □ | __________ | □ |
| 1–2 | □ | □ | __________ | □ |
| 2–3 | □ | □ | __________ | □ |
| 3–4 | □ | □ | __________ | □ |
| 4–5 | □ | □ | __________ | □ |
| 5–6 | □ | □ | __________ | □ |
| 6–8 | □ | □ | __________ | □ |
| 8–10 | □ | □ | __________ | □ |
| 10–15 | □ | □ | __________ | □ |
| 15–18 | □ | □ | __________ | □ |
continuous curvilinear capsulotomy
Thank you for completing this survey.
Please FAX (843-792-1166) or Mail the completed survey to:
M. Edward Wilson, Jr., M.D.
MUSC – Storm Eye Institute
167 Ashley Avenue
Charleston, SC 29425-5536
Footnotes
This research was supported in part by grant EY-014793 from the National Institutes of Health; an unrestricted grant to Storm Eye Institute (M.E.W.) from Research to Prevent Blindness, New York, New York; and the Grady Lyman Fund, MUSC Health Science Foundation, Charleston, South Carolina.
REFERENCES
- 1.Resnikoff S, Kocur I. World sight day: 10 October. World Health Organ 2002. Available at: <http://www.who.int/mediacentre/news/releases/pr79/en/>.
- 2.Wilson ME, Pandey SK, Thakur J. Paediatric cataract blindness in the developing world: surgical techniques and intraocular lenses in the new millennium. Br J Ophthalmol. 2003;87:14–19. doi: 10.1136/bjo.87.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster A, Gilbert C, Rahi J. Epidemiology of cataract in childhood: a global perspective. J Cataract Refract Surg. 1997;23:601–604. doi: 10.1016/s0886-3350(97)80040-5. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020—the right to sight. Bull World Health Organ. 2001;79:227–232. [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad K. WHO launches international programme to combat childhood blindness. Lancet. 2002;359:2258. doi: 10.1016/S0140-6736(02)09322-4. [DOI] [PubMed] [Google Scholar]
- 6.Helveston EM, Ellis FD. Pediatric ophthalmic practice. In: Helveston EM, Ellis FD, eds. Pediatric Ophthalmic Practice. St Louis: Mosby; 1984:93–128.
- 7.Gilbert C. New issues in childhood blindness. Community Eye Health. 2001;14:53–56. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson ME. Intraocular lens implantation. Has it become the standard of care for children? Ophthalmology. 1996;103:1719–1720. doi: 10.1016/s0161-6420(96)30436-3. [DOI] [PubMed] [Google Scholar]
- 9.Wilson ME, Jr, Bartholomew LR, Trivedi RH. Pediatric cataract surgery and intraocular lens implantation: practice preferences of the 2001 ASCRS and AAPOS memberships. J Cataract Refract Surg. 2003;29:1811–1820. doi: 10.1016/s0886-3350(03)00220-7. [DOI] [PubMed] [Google Scholar]
- 10.Wilson ME. Pediatric cataract surgery. In: Spaeth GL, ed. Ophthalmic Surgery, Principles and Practices. Philadelphia: Saunders; 2003:103–107.
- 11.Wilson ME, Pandey SK, Werner L, et al. Pediatric cataract surgery: current techniques, complications and management. In: Agarwal S, et al, eds. Textbook of Ophthalmology. New Delhi, India: Jaypee Brothers; 2002;1861–1879.
- 12.Wilson ME. Anterior capsule management for pediatric intraocular lens implantation. J Pediatr Ophthalmol Strabismus. 1999;36:314–319. doi: 10.3928/0191-3913-19991101-05. [DOI] [PubMed] [Google Scholar]
- 13.Andreo LK, Wilson ME, Apple DJ. Elastic properties and scanning electron microscopic appearance of manual continuous curvilinear capsulorhexis and vitrectorhexis in an animal model of pediatric cataract. J Cataract Refract Surg. 1999;25:534–539. doi: 10.1016/s0886-3350(99)80051-0. [DOI] [PubMed] [Google Scholar]
- 14.Kleiman NJ, Worgul BV. Lens. In: Tasman W, Jaeger EA, eds. Duane’s Foundations of Ophthalmology. Vol 1. Philadelphia: Lippincott; 1994;1–39.
- 15.Brinkler JM, Pegg MT, Howard PS, et al. Immunochemical characterization of type IV procollagen from the anterior lens capsule. Collagen Relat Res. 1985;5:233–244. doi: 10.1016/s0174-173x(85)80013-3. [DOI] [PubMed] [Google Scholar]
- 16.Cammarata PR, Cantu-Crouch D, Oakford L, et al. Macromolecular organization of bovine lens capsule. Tissue Cell. 1986;18:83–97. doi: 10.1016/0040-8166(86)90009-1. [DOI] [PubMed] [Google Scholar]
- 17.Hettlich H-J, Wenzel M, Janssen M, et al. Immunhistochemische Untersuchungen der menschlichen Linsenkapsel. Fortschr Ophthalmol. 1990;87:147–149. [PubMed] [Google Scholar]
- 18.Barnard K, Burgess SA, Carter DA, et al. Three-dimensional structure of type IV collagen in the mammalian lens capsule. J Struct Biol. 1992;108:6–13. doi: 10.1016/1047-8477(92)90002-r. [DOI] [PubMed] [Google Scholar]
- 19.Marshall GE, Konstas AG, Bechrakis NE, et al. An immunoelectron microscope study of the aged human lens capsule. Exp Eye Res. 1992;54:393–401. doi: 10.1016/0014-4835(92)90051-s. [DOI] [PubMed] [Google Scholar]
- 20.Kohno T, Sorgente N, Ishibashi T, et al. Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci. 1987;28:506–514. [PubMed] [Google Scholar]
- 21.Krag S, Olsen T, Andreassen TT. Biomechanical characteristics of the human anterior lens capsule in relation to age. Invest Ophthalmol Vis Sci. 1997;38:357–363. [PubMed] [Google Scholar]
- 22.Seland JH. Ultrastructural changes in the normal human lens capsule from birth to old age. Acta Ophthalmol. 1974;52:688–706. doi: 10.1111/j.1755-3768.1974.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 23.Parks MM. Posterior lens capsulotomy during primary cataract surgery in children. Ophthalmology. 1983;90:344–345. doi: 10.1016/s0161-6420(83)34549-8. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DSI. Choice of surgical technique in the management of congenital cataract. Trans Ophthalmol Soc U K. 1981;101:114–117. [PubMed] [Google Scholar]
- 25.Wilson ME, Bluestein EC, Wang XH, et al. Comparison of mechanized anterior capsulectomy and manual continuous capsulorhexis in pediatric eyes. J Cataract Refract Surg. 1994;20:602–606. doi: 10.1016/s0886-3350(13)80646-3. [DOI] [PubMed] [Google Scholar]
- 26.Wilson ME, Saunders RA, Roberts EL, et al. Mechanized anterior capsulectomy as an alternative to manual capsulorhexis in children undergoing intraocular lens implantation. J Pediatr Ophthalmol Strabismus. 1996;33:237–240. doi: 10.3928/0191-3913-19960701-07. [DOI] [PubMed] [Google Scholar]
- 27.Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16:31–37. doi: 10.1016/s0886-3350(13)80870-x. [DOI] [PubMed] [Google Scholar]
- 28.Vasavada A, Chauhan H. Intraocular lens implantation in infants with congenital cataracts. J Cataract Refract Surg. 1994;20:592–598. doi: 10.1016/s0886-3350(13)80644-x. [DOI] [PubMed] [Google Scholar]
- 29.Neuhann T. Capsulorrhexis. In: Steinert RF, ed. Cataract Surgery—Technique, Complications, Management. St Louis: Saunders; 2004;137–145.
- 30.Wood MG, Schelonka LP. A porcine model predicts that a can-opener capsulotomy can be done safely in pediatric patients. J AAPOS. 1999;3:356–362. doi: 10.1016/s1091-8531(99)70045-5. [DOI] [PubMed] [Google Scholar]
- 31.Gimbel HV, DeBroff BM. Surgical management of pediatric cataracts. In: Steinert RF, ed. Cataract Surgery—Technique, Complications, Management. St Louis: Saunders; 2004;273–293.
- 32.Kloti R. Bipolar wet-field diathermy in microsurgery. Klin Monatsbl Augenheilkd. 1984;184:442–444. doi: 10.1055/s-2008-1054517. [DOI] [PubMed] [Google Scholar]
- 33.Morgan JF, Ellingham RB, Young RD, et al. The mechanical properties of the human lens capsule following capsulorhexis or radiofrequency diathermy capsulotomy. Arch Ophthalmol. 1996;114:1110–1115. doi: 10.1001/archopht.1996.01100140312010. [DOI] [PubMed] [Google Scholar]
- 34.Luck J, Brahma AK, Noble BA. A comparative study of the elastic properties of continuous tear curvilinear capsulorhexis versus capsulorhexis produced by radiofrequency endodiathermy. Br J Ophthalmol. 1994;78:392–396. doi: 10.1136/bjo.78.5.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comer RM, Abdulla N, O’Keefe M. Radiofrequency diathermy capsulorhexis of the anterior and posterior capsules in pediatric cataract surgery: preliminary results. J Cataract Refract Surg. 1997;23:641–644. doi: 10.1016/s0886-3350(97)80047-8. [DOI] [PubMed] [Google Scholar]
- 36.Fugo RJ, Singh D, DelCampo D, et al. Preservation and restoration of corneal clarity with lidocaine in pig eyes. Ann Ophthalmol. 2002;34:96–99. [Google Scholar]
- 37.Singh D. Use of the Fugo blade in complicated cases. J Cataract Refract Surg. 2002;28:573–574. doi: 10.1016/s0886-3350(02)01314-7. [DOI] [PubMed] [Google Scholar]
- 38.Rosen E. Capsular surgery. Eur J Implant Refract Surg 1. 1990;2:1–4. [Google Scholar]
- 39.Ridley H. Intra-ocular acrylic lenses: a recent development in the surgery of cataract. Br J Ophthalmol. 1952;36:113–122. doi: 10.1136/bjo.36.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridley H. Intra-ocular acrylic lenses: 10 years’ development. Br J Ophthalmol. 1960;44:705–712. doi: 10.1136/bjo.44.12.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drews RC. The Mary Louise Prentice Montgomery Lecture. The lens capsule: a lens implant surgeon’s understanding. Trans Ophthalmol Soc U K. 1986;105:265–272. [PubMed] [Google Scholar]
- 42.Kelman CD. Phacoemulsification and aspiration: the Kelman technique of cataract removal. In: Tasman W, Jaeger EA, eds. Duane’s Clinical Ophthalmology. Vol 5. Philadelphia: Lippincott; 1994:6–9.
- 43.Ruit S, Tabin GC, Nissman SA, et al. Low-cost high-volume extracapsular cataract extraction with posterior chamber intraocular lens implantation in Nepal. Ophthalmology. 1999;106:1887–1892. doi: 10.1016/S0161-6420(99)90397-4. [DOI] [PubMed] [Google Scholar]
- 44.Thakur J, Reddy H, Wilson ME, Jr, et al. Pediatric cataract surgery in Nepal. J Cataract Refract Surg. 2004;30:1629–1635. doi: 10.1016/j.jcrs.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 45.Gimbel HV. Advanced capsulotomy. In: Gills JP, Fenzl R, Martin RG, eds. Cataract Surgery: State of the Art. Thorofare, NJ: Slack; 1998:69–74.
- 46.Wasserman D, Apple DJ, Castaneda VE, et al. Anterior capsular tears and loop fixation of posterior chamber intraocular lenses. Ophthalmology. 1991;98:425–431. doi: 10.1016/s0161-6420(91)32274-7. [DOI] [PubMed] [Google Scholar]
- 47.Auffarth GU, Wesendahl TA, Newland TJ, et al. Capsulorhexis in the rabbit eye as a model for pediatric capsulectomy. J Cataract Refract Surg. 1994;20:188–191. doi: 10.1016/s0886-3350(13)80164-2. [DOI] [PubMed] [Google Scholar]
- 48.Krag S, Thim K, Corydon L, et al. Biomechanical aspects of the anterior capsulotomy. J Cataract Refract Surg. 1994;20:410–416. doi: 10.1016/s0886-3350(13)80176-9. [DOI] [PubMed] [Google Scholar]
- 49.Hemo Y, BenEzra D. Traumatic cataracts in young children. Ophthalmic Paediatr Genet. 1987;8:203–207. doi: 10.3109/13816818709031471. [DOI] [PubMed] [Google Scholar]
- 50.Hiles DA. Visual rehabilitation of aphakic children. III: Intraocular lenses. Surv Ophthalmol. 1990;34:371–379. doi: 10.1016/0039-6257(90)90114-b. [DOI] [PubMed] [Google Scholar]
- 51.Dahan E, Salmenson BD. Pseudophakia in children; precautions, technique, and feasibility. J Cataract Refract Surg. 1990;16:75–82. doi: 10.1016/s0886-3350(13)80878-4. [DOI] [PubMed] [Google Scholar]
- 52.Kora Y, Inatomi M, Fukado Y, et al. Long-term study of children with implanted intraocular lenses. J Cataract Refract Surg. 1992;18:485–488. doi: 10.1016/s0886-3350(13)80103-4. [DOI] [PubMed] [Google Scholar]
- 53.Sinskey RM, Stoppel JO, Amin P. Long-term results of intraocular lens implantation in pediatric patients. J Cataract Refract Surg. 1993;19:405–408. doi: 10.1016/s0886-3350(13)80314-8. [DOI] [PubMed] [Google Scholar]
- 54.Basti S, Ravishankar U, Gupta S. Results of a prospective evaluation of three methods of management of pediatric cataracts. Ophthalmology. 1996;103:713–720. doi: 10.1016/s0161-6420(96)30624-6. [DOI] [PubMed] [Google Scholar]
- 55.Ainsworth JR, Cohen S, Levin AV, et al. Pediatric cataract management with variations in surgical technique and aphakic optical correction. Ophthalmology. 1997;104:1096–1101. doi: 10.1016/s0161-6420(97)30179-1. [DOI] [PubMed] [Google Scholar]
- 56.Gimbel HV, Basti S, Ferensowicz M, et al. Results of bilateral cataract extraction with posterior chamber intraocular lens implantation in children. Ophthalmology. 1997;104:1737–1743. doi: 10.1016/s0161-6420(97)30033-5. [DOI] [PubMed] [Google Scholar]
- 57.Dahan E, Drusedau MV. Choice of lens and dioptric power in pediatric pseudophakia. J Cataract Refract Surg. 1997;23:618–623. doi: 10.1016/s0886-3350(97)80043-0. [DOI] [PubMed] [Google Scholar]
- 58.Neuhann T. Theory and surgical technique of capsulorhexis. Klin Monatsbl Augenheilkd. 1987;190:542–545. doi: 10.1055/s-2008-1050454. [DOI] [PubMed] [Google Scholar]
- 59.Gimbel HV, Basti S. Optimal capsulorhexis technique in pediatric eyes. J Cataract Refract Surg. 1996;22:3–4. doi: 10.1016/s0886-3350(96)80258-6. [DOI] [PubMed] [Google Scholar]
- 60.Nischal KK. Two-incision push-pull capsulorhexis for pediatric cataract surgery. J Cataract Refract Surg. 2002;28:593–595. doi: 10.1016/s0886-3350(01)01125-7. [DOI] [PubMed] [Google Scholar]
- 61.Bluestein EC, Wilson ME, Wang XH, et al. Dimensions of the pediatric crystalline lens: implications for intraocular lenses in children. J Pediatr Ophthalmol Strabismus. 1996;33:18–20. doi: 10.3928/0191-3913-19960101-06. [DOI] [PubMed] [Google Scholar]
- 62.Gassmann F, Schimmelpfennig B, Kloti R. Anterior capsulotomy by means of bipolar radio-frequency endo-diathermy. J Cataract Refract Surg. 1988;14:673–676. doi: 10.1016/s0886-3350(88)80040-3. [DOI] [PubMed] [Google Scholar]
- 63.Kloti R. Anterior high frequency capsulotomy. I. Experimental study. Klin Monatsbl Augenheilkd. 1992;200:507–510. doi: 10.1055/s-2008-1045809. [DOI] [PubMed] [Google Scholar]
- 64.Berger G. Anterior capsulotomy using a diathermy cutter. Experimental principles (1) Klin Monatsbl Augenheilkd. 1984;184:410–414. doi: 10.1055/s-2008-1054507. [DOI] [PubMed] [Google Scholar]
- 65.Hausmann N, Richard G. Investigations on diathermy for anterior capsulotomy. Invest Ophthalmol Vis Sci. 1991;32:2155–2159. [PubMed] [Google Scholar]
- 66.Liekfeld A, Pham DT, Schweig F, et al. Use of the high frequency capsulotomy instrument in cataract surgery: effect of coagulation on corneal endothelium. Klin Monatsbl Augenheilkd. 1997;210:352–354. doi: 10.1055/s-2008-1035073. [DOI] [PubMed] [Google Scholar]
- 67.Delcoigne CD, Hennekes R. Circular continuous anterior capsulotomy with high frequency diathermy. Bull Soc Belge Ophtalmol. 1993;249:67–72. [PubMed] [Google Scholar]
- 68.Krag S, Thim K, Corydon L. Diathermic capsulotomy versus capsulorhexis: a biomechanical study. J Cataract Refract Surg. 1997;23:86–90. doi: 10.1016/s0886-3350(97)80156-3. [DOI] [PubMed] [Google Scholar]
- 69.Assia EI, Apple DJ, Barden A, et al. An experimental study comparing various anterior capsulectomy techniques. Arch Ophthalmol. 1991;109:642–647. doi: 10.1001/archopht.1991.01080050056028. [DOI] [PubMed] [Google Scholar]