Abstract
Maraviroc (UK-427,857) is a selective CCR5 antagonist with potent anti-human immunodeficiency virus type 1 (HIV-1) activity and favorable pharmacological properties. Maraviroc is the product of a medicinal chemistry effort initiated following identification of an imidazopyridine CCR5 ligand from a high-throughput screen of the Pfizer compound file. Maraviroc demonstrated potent antiviral activity against all CCR5-tropic HIV-1 viruses tested, including 43 primary isolates from various clades and diverse geographic origin (geometric mean 90% inhibitory concentration of 2.0 nM). Maraviroc was active against 200 clinically derived HIV-1 envelope-recombinant pseudoviruses, 100 of which were derived from viruses resistant to existing drug classes. There was little difference in the sensitivity of the 200 viruses to maraviroc, as illustrated by the biological cutoff in this assay (= geometric mean plus two standard deviations [SD] of 1.7-fold). The mechanism of action of maraviroc was established using cell-based assays, where it blocked binding of viral envelope, gp120, to CCR5 to prevent the membrane fusion events necessary for viral entry. Maraviroc did not affect CCR5 cell surface levels or associated intracellular signaling, confirming it as a functional antagonist of CCR5. Maraviroc has no detectable in vitro cytotoxicity and is highly selective for CCR5, as confirmed against a wide range of receptors and enzymes, including the hERG ion channel (50% inhibitory concentration, >10 μM), indicating potential for an excellent clinical safety profile. Studies in preclinical in vitro and in vivo models predicted maraviroc to have human pharmacokinetics consistent with once- or twice-daily dosing following oral administration. Clinical trials are ongoing to further investigate the potential of using maraviroc for the treatment of HIV-1 infection and AIDS.
Human immunodeficiency virus type 1 (HIV-1) therapy has made significant progress in recent years through the discovery, development, and prescription of HIV-1 protease and reverse transcriptase inhibitors. The combination of three or more of these inhibitors into multidrug regimens, often termed highly active antiretroviral therapy, can efficiently inhibit replication of virus in the body to achieve low or undetectable circulatory HIV-1 levels. While highly active antiretroviral therapy regimens have transformed the face of the disease for those patients on treatment, there remains a significant unmet medical need. High pill burdens, inconvenient dosing, and significant long-term toxicities contribute to poor compliance and emergence of drug-resistant virus in many patients. For those patients who harbor resistant virus, treatment options become limited and more complicated regimens are necessary to prevent further disease progression. As the incidence of drug-resistant variants in the treated HIV-infected population has increased, so has the transmission of drug-resistant virus to treatment-naive individuals (17, 20, 22, 52, 53).
Inhibition of HIV-1 entry has become a compelling target for drug discovery. Enfuvirtide (Fuzeon) is an injectable, peptidic anti-HIV drug which prevents HIV entry by blocking gp41-mediated fusion, was recently licensed for HIV infection, and has validated entry blockade as a viable approach (7, 13). A considerable research effort is now focused on the discovery and development of orally available inhibitors of HIV-1 entry (2, 11, 19, 27, 41, 42). These include inhibitors of gp120 binding to CD4 (16, 21), CXCR4 antagonists with antiviral properties (11, 32), and CCR5 antagonists (J. Lalezari, M. Thompson, P. Kumar, P. Piliero, R. Davey, T. Murtaugh, K. Patterson, A. Shachoy-Clark, J. Adkison, J. Demarest, S, Sparks, L. Fang, Y. Lou, M. Berrey, and S. Piscitelli, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., late-breaker abstr. H-11376, 2004; D. Schurmann, R. Rouzier, R. Nougerede, J. Reyes, G. Fatkenheuer, F. Raffi, C. Michelet, A. Tarral, C. Hoffmann, J. Kiunke, H. Sprenger, J. vanLier, A. Sansone, M. Jackson, and M. Laughlin, Abstr. 11th Conf. Retrovir. Opportun. Infect., abstr. 140LB, 2004.). The HIV-1 coreceptors, CXCR4 and CCR5, are inherently compelling targets for therapeutic intervention. They belong to the G protein-coupled receptor superfamily, which has historically been tractable to discovery of potent, selective, low-dose small-molecule drugs. CCR5 is an especially attractive target, since the natural genetic absence of surface-expressed CCR5 in Δ32 homozygous genotype populations has little apparent impact on their immune status or general health. Furthermore, this population is highly protected against HIV-1 infection (12, 15, 23, 40), and the reduced cell surface expression of CCR5 in CCR5Δ32 heterozygotes is associated with a slower rate of disease progression (34, 46). Such epidemiological evidence together with experimental results from CCR5-ablated mice showing no discernible phenotype other than subtle changes in immune function (55) support the hypothesis that antagonism of CCR5 may impose an antiviral effect without causing mechanism-related side effects. From an antiviral perspective, CCR5 is the coreceptor for the most commonly transmitted HIV-1 strains which predominate during the early stages of infection and remain the dominant form in >50% of late stage HIV-1-infected patients (4, 31, 32). These factors have encouraged researchers towards developing CCR5 ligands as a means to treating HIV-1 infection.
Various CCR5 ligands with antiviral properties have been described, including modified chemokines and monoclonal antibodies (6, 24) and more importantly small-molecule inhibitors with potential for oral administration (25, 43, 44). There have been many challenges faced by previously reported CCR5 antagonists, including selectivity with respect to CCR2 and other receptors, variable anti-HIV-1 activity, inhibition of the hERG ion channel leading to QT (interval of electrocardiogram) prolongation, and limited oral bioavailability (2, 35, 41, 42, 43, 54). The pharmacology, antiviral properties, selectivity, and preclinical pharmacokinetics of maraviroc are described here in the context of addressing these diverse challenges; these qualities have enabled its progression to an advanced stage of clinical development for the treatment of HIV-1 infection and AIDS.
MATERIALS AND METHODS
Viruses.
All lab-adapted HIV-1 strains, primary isolates of HIV-1, and the MT-2 and PM-1 cell lines were obtained from the AIDS Reagent Project, National Institute of Biological Standards and Control (NIBSC), Potters Bar, Herts, United Kingdom. The viruses used are listed in Table 1. Coreceptor usage of viruses was confirmed by passaging virus through MT-2 cells and monitoring for evidence of syncytium formation as previously described (3). Stocks of viruses were prepared by limited passage through phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC). Stocks of primary-isolate viruses were harvested when reverse transcriptase (RT) levels in the supernatants were >100 cpm/μl. For the studies involving the lab-adapted strain Ba-L, a stock with a 50% tissue culture infective dose of 5.76 × 104/ml was used to infect PM-1 cells (125 μl per 106 cells) and PBMC (250 μl per 106 cells).
TABLE 1.
HIV-1 primary and laboratory-adapted isolates used in this study and geometric mean antiviral potencies of maraviroc against the isolates as assessed in PBMCa
| Isolate | Codeb | Origin | Coreceptor | Clade | IC50 (nM) | IC90 (nM) | No. of assays |
|---|---|---|---|---|---|---|---|
| Ba-L | ARP 118 | United States | CCR5 | B | 1.2 | 5.5 | 20 |
| B117 | ARP 198 | Sweden | Dual-tropic | B | Inactivec | Inactive | 2 |
| BCFO1 | ARP 191 | Cameroon | CCR5 | O | 1.7 | 4.4 | 6 |
| BCF02 | ARP 192 | Cameroon | CCR5 | O | 0.7 | 1.6 | 6 |
| BCF07 | ARP 194 | Cameroon | CCR5 | O | 0.6 | 1.8 | 4 |
| BR92003 | ARP 179.1 | Brazil | CCR5 | B | 0.4 | 1.5 | 4 |
| BR92004 | ARP 179.2 | Brazil | CCR5 | B | 0.2 | 0.6 | 4 |
| BR92014 | ARP 179.3 | Brazil | CXCR4 | B | Inactivec | Inactive | 4 |
| BR92017 | ARP 179.4 | Brazil | CCR5 | B | 0.3 | 1.4 | 6 |
| BR92018 | ARP 179.5 | Brazil | CCR5 | B | 0.3 | 1.3 | 4 |
| BR92019 | ARP 179.6 | Brazil | CCR5 | B | 0.3 | 1.2 | 4 |
| BR92020 | ARP 179.7 | Brazil | CCR5 | B | 0.2 | 0.9 | 4 |
| BR92021 | ARP 179.8 | Brazil | CCR5 | B | 0.3 | 1.1 | 4 |
| BR92023 | ARP 179.9 | Brazil | CCR5 | B | 0.7 | 4.1 | 8 |
| BR92024 | ARP 179.10 | Brazil | CCR5 | B | 0.4 | 4.8 | 6 |
| BR92025 | ARP 179.11 | Brazil | CCR5 | C | 0.1 | 0.6 | 6 |
| BR92026 | ARP 179.12 | Brazil | CCR5 | B | 0.2 | 1.2 | 4 |
| BR92030 | ARP 179.14 | Brazil | CCR5 | B | 0.5 | 2.3 | 6 |
| BR93006 | ARP 179.15 | Brazil | CCR5 | B | 0.5 | 3.4 | 4 |
| BR93007 | ARP 179.16 | Brazil | CCR5 | B | 1.3 | 3.4 | 4 |
| BR93019 | ARP 179.24 | Brazil | CCR5 | B | 0.3 | 1.2 | 4 |
| BR93020 | ARP 179.25 | Brazil | CXCR4 | F | Inactivec | Inactive | 6 |
| BR93022 | ARP 179.27 | Brazil | CCR5 | B | 0.3 | 1.0 | 4 |
| BR93026 | ARP 179.31 | Brazil | CCR5 | B | 0.3 | 1.0 | 4 |
| BR93028 | ARP 179.33 | Brazil | CCR5 | B | 0.4 | 1.8 | 4 |
| BR93029 | ARP 179.34 | Brazil | CCR5 | F | 1.1 | 2.9 | 2 |
| Ca-9 | EVA 168 | Cameroon | CCR5 | O | 0.3 | 0.6 | 4 |
| EL1 | EVA 117 | Zaire | CXCR4 | D | Inactivec | Inactive | 4 |
| IN98022 | ARP 199.9 | India | CCR5 | C | 1.6 | 6.5 | 6 |
| RU132 | ARP 173 | Russia | CCR5 | G | 0.4 | 2.2 | 8 |
| RU570 | ARP 174 | Russia | CCR5 | G | 4.5 | 11.3 | 6 |
| RW92009 | ARP 178.2 | Rwanda | CCR5 | A | 1.0 | 2.0 | 6 |
| RW92016 | ARP 178.3 | Rwanda | CCR5 | A | 0.2 | 0.6 | 4 |
| RW92021 | ARP 178.5 | Rwanda | CCR5 | A | 0.4 | 1.6 | 2 |
| SE9173 | ARP 1017.1 | Zaire | CCR5 | J | 0.2 | 1.4 | 6 |
| SE9280 | ARP 1017.2 | Zaire | CCR5 | J | 1.9 | 13.4 | 4 |
| SF162 | ARP 114 | United States | CCR5 | B | 1.8 | 7.3 | 6 |
| THA92014 | ARP 180.8 | Thailand | CCR5 | B | 0.2 | 0.7 | 4 |
| THA92026 | ARP 180.15 | Thailand | CCR5 | B | 0.5 | 2.4 | 2 |
| THA93073 | ARP 180.27 | Thailand | CCR5 | E | 0.1 | 0.5 | 4 |
| THA93074 | ARP 180.28 | Thailand | CCR5 | B | 0.5 | 1.8 | 4 |
| UG92001 | ARP 177.1 | Uganda | CXCR4 | D | Inactivec | Inactive | 4 |
| UG92037 | ARP 177.8 | Uganda | CCR5 | A | 0.2 | 0.6 | 4 |
| UG93082 | ARP 177.18 | Uganda | CCR5 | D | 1.3 | 3.3 | 2 |
| UG94108 | ARP 177.23 | Uganda | CCR5 | D | 0.3 | 1.5 | 2 |
| UG94114 | ARP 177.24 | Uganda | CCR5 | D | 0.4 | 1.7 | 2 |
| UG94118 | CXCR4 | Uganda | CCR5 | D | 1.0 | 4.0 | 4 |
| ZA97003 | ARP 199.1 | Zambia | CCR5 | C | 1.0 | 5.8 | 4 |
| ZA97013 | ARP 199.3 | Zambia | CCR5 | C | 2.9 | 5.9 | 2 |
| 89.6 | ARP 189 | United States | Dual-tropic | B | Inactivec | Inactive | 4 |
All isolates were obtained from the NIBSC, Potters Bar, United Kingdom. The coreceptor usage and clade were assigned by NIBSC; the coreceptor usage was confirmed once stocks had been prepared by comparing growth of the virus in PBMC and MT-2 cells.
Code given to each isolate by NIBSC.
Inactive, IC50 of >10 nM.
Chemokines and inhibitors.
Human recombinant chemokines, MIP-1α, MIP-1β, RANTES, and SDF-1α, were obtained from R&D Systems, except for 125I-radiolabeled chemokines, which were from NEN Life Sciences. Radiolabeled chemokines were diluted to 25 μCi/ml (12.5 nM) in assay buffer prior to use in ligand binding assays. All inhibitors were synthesized at Pfizer Global R&D, Sandwich Laboratories. Inhibitors were dissolved in dimethyl sulfoxide (DMSO) and serially diluted in assay media (DMSO concentration, ≤1% [vol/vol], no-effect level in each assay) for dose-response inhibition experiments over an appropriate concentration range to determine 50% and 90% inhibitory concentrations (IC50s and IC90s).
Maintenance of cell line cultures.
All tissue culture reagents were obtained from Gibco or Sigma unless otherwise indicated. The human embyronic kidney (HEK-293) cell line expressing human recombinant CCR5 was constructed at Pfizer Global R&D and maintained in Dulbecco's modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum (FCS) (PAA Laboratories), 2 mM l-glutamine, 0.6 mg/ml geneticin, 0.4% (wt/vol) NaHCO3, 0.5 U/ml penicillin, and 0.05 mg/ml streptomycin. The HeLa-P4 cell line expressing recombinant human CD4 and CCR5 was obtained from Ned Landau (Aaron Diamond AIDS Research Centre, NY) and maintained in Dulbecco's modified Eagle medium-1640, supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 0.4% (wt/vol) NaHCO3, and antibiotics (1 U/ml penicillin and 0.1 mg/ml streptomycin). The CHO cell line expressing recombinant HIV-1 gp160 (strain JR-FL) was obtained from Mark Goldsmith (Gladstone Institute, San Francisco) and maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 1 U/ml penicillin, 0.1 mg/ml streptomycin, 0.65 mg/ml geneticin, 0.5 mg/ml hygromycin B, and 12.5 μg/ml puromycin. CHO cells expressing HIV-1 (strain Ba-L) gp120 were maintained in Gibco’s modified Eagle’s medium (GMEM-S) medium containing 10% FCS. 300.19/R5 cells (mouse pre-B-cell line, recombinantly expressing human CCR5) were maintained in RPMI-1640 medium supplemented with 10% FCS, 2 mM l-glutamine, 0.4% (wt/vol) NaHCO3, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.04% β-mercaptoethanol.
Preparation and assay of soluble recombinant gp120.
CHO cells stably transfected with the HIV-1 gp120 expression vector pEE14.1 (Ba-L strain; Lonza Biologics) were cultured in 200-ml roller bottles for 4 days, which was replaced by 200 ml GMEM-S medium with 1% FCS for 3 days prior to supernatant harvest for gp120 preparation. The cell supernatant was concentrated by ultrafiltration and empirically quantified (with or without human soluble CD4; Immunodiagnostics) using a europium-labeled anti-HIV-1 gp120 immunoglobulin G (IgG) antibody (AALTO) in the time-resolved fluorescence immunoassay as described below.
Inhibition of chemokine binding to CCR5.
Binding of 125I-labeled MIP-1α, MIP-1β, and RANTES to CCR5 was measured essentially as described previously (10) using intact HEK-293 cells stably expressing the receptor or membrane preparations thereof. Briefly, cells were resuspended in binding buffer (50 mM HEPES containing 1 mM CaCl2, 5 mM MgCl2, and 0.5% bovine serum albumin [BSA] and adjusted to pH 7.4) to a density of 2 × 106 cells/ml. For membrane preparations, phosphate-buffered saline (PBS)-washed cells were resuspended in lysis buffer (20 mM HEPES, 1 mM CaCl2, 1 tablet COMPLETE per 50 ml, pH 7.4; Boehringer) prior to homogenization in a Polytron hand-held homogenizer, ultracentrifugation (40,000 × g for 30 min), and resuspension in binding buffer to a protein concentration of 0.25 mg/ml (12.5 μg of membrane protein was used in each well of a 96-well plate). 125I-radiolabeled MIP-1α, MIP-1β, and RANTES were prepared and diluted in binding buffer to a final concentration of 400 pM in the assay. Appropriate maraviroc dilutions were added to each well to a final volume of 100 μl, the assay plates incubated for 1 h, and the contents filtered through preblocked and washed Unifilter plates (Packard) which were counted following overnight drying.
Inhibition of CCR5 signaling: calcium flux.
Maraviroc-dependent inhibition of CCR5-mediated signaling was investigated by measuring chemokine-dependent intracellular calcium redistribution (flux) by fluorescence assay using a calcium-sensitive dye, essentially as previously reported (10). Briefly, CCR5-stable transfected HEK-293 cells were washed in PBS and then incubated at 37°C for 1 h in cell culture medium containing fluo-3 dye (5 μg/ml; Molecular Probes). The dye-loaded cells were washed in PBS and resuspended in flux buffer (10 mM HEPES buffer, pH 7.4, containing 1.6 mM CaCl2 and 1 bottle of Hanks' balanced salts powder) to 5 × 105 cells/ml for the assay. The cell suspension (160 μl) was divided into aliquots, placed in each well of a black-walled clear-base 96-well plate, and centrifuged (400 × g) for 5 min. Dilutions of maraviroc and solutions of chemokines were divided into aliquots and placed in separate 96-well plates to enable their sequential addition to the HEK-293 cells and subsequent measurement of intracellular calcium redistribution effects in a fluorescent laser imaging plate reader (FLIPR). The FLIPR added maraviroc dilutions (20 μl) after 30 s, followed 4 min later by the addition of the RANTES chemokine (20 μl) to a final concentration of 20 nM in situ. Fluorescence (488-nm and 530-nm excitation and emission wavelengths, respectively) was measured over 8 min to investigate the direct effects of maraviroc on cell signaling and inhibitory effects of maraviroc on chemokine-mediated signaling.
Inhibition of CCR5 internalization.
The effects of maraviroc on CCR5 internalization were measured by flow cytometry, using a FACSCalibur instrument. Aliquots of 300.19 cells (100 μl at 5 × 106/ml) were incubated for 45 min at 37°C with maraviroc, RANTES, or SDF-1α (all 100 nM in situ) to enable CCR5 internalization. The samples were washed twice, resuspended in 0.5% BSA-PBS (40 μl), and incubated for 45 min at 4°C with 10 μl 2D7 (anti-human CCR5 mouse monoclonal antibody; Pharmingen) or isotype control antibody (mouse IgG2a). The samples were washed (0.5% BSA-PBS) and incubated with phycoerythrin-goat antimouse secondary antibody (75 μl) at 4°C for 45 min. The samples were washed and fixed with 1% (vol/vol) formaldehyde-PBS (1000 μl), and expression of CCR5 was evaluated using excitation/emission wavelengths of 488 nm and 530 nm, respectively.
Inhibition of soluble recombinant HIV-1 gp120 (Ba-L strain) binding to CCR5.
This assay was performed as described previously (14). Briefly, HEK-293 cell aliquots (100 μl at 1 × 106 cells/ml) were plated into poly-d-lysine-coated plates (Becton Dickinson) and incubated at 37°C overnight. A 1:1 mix of soluble recombinant human CD4 (sCD4) (diluted to 4.5 nM in culture medium) and HIV-1 gp120 (Ba-L strain) was incubated at room temperature for 15 min prior to its addition to PBS-washed cells in the presence of dilutions of maraviroc to enable IC50 determination. The assay plates were incubated at 37°C for 1 h and washed. Eu3+-labeled anti-gp120 antibody (1/500 dilution in assay buffer) was added to each well (50 μl) and incubated for 1 h. The plate was washed three times with wash buffer prior to the addition of enhancement solution (200 μl/well; EG&G Wallac) and measurement of Eu3+ fluorescence (Victor2 multilabel counter; “Europium” protocol). Nonspecific binding was taken as the fluorescence measured for gp120 incubated with cells in the absence of preincubation with sCD4.
Inhibition of HIV-1 gp160-CCR5-mediated cell-cell fusion.
The assay was performed as described previously (5). Briefly, HeLa-P4 cells (expressing recombinant human CD4 and CCR5 at the cell surface and encoding an HIV-1-long-terminal-repeat-regulated β-galactosidase reporter gene) were coincubated with CHO cells (expressing cell surface HIV-1 gp160 and Tat). Fusion was measured by HIV-1 Tat-induced expression and subsequent catalytic activity of HIV-1-long-terminal-repeat-regulated β-galactosidase.
Effects on hERG ion channel activity.
Effects of maraviroc on the human cardiac potassium channel human ether-a-go-go-related gene (hERG) were measured as described previously (49), except the assay was performed at room temperature.
Inhibition of HIV-1 replication in PBMC and PM-1 cells.
All antiviral drug susceptibility assays were performed in RPMI 1640 medium containing 10% (vol/vol) heat-inactivated FCS, 2 mM l-glutamine, and antibiotics (1 U/ml penicillin and 0.1 mg/ml streptomycin). PBMC were isolated from buffy coats (obtained from the North London blood transfusion service; Collingdale) from HIV-1- and hepatitis B virus-seronegative donors, using a Ficoll gradient (Amersham Pharmacia Biotech). PBMC were pooled from three to four donors and incubated at a density of 1 × 106 cells/ml for 3 days in culture medium containing PHA (1.5 μg/ml; Murex, Abbott Laboratories). PHA-activated PBMC were washed and resuspended in medium containing human recombinant interleukin-2 (IL-2) (10 ng/ml; R&D Systems) immediately prior to use in an antiviral assay.
Drug susceptibility assays were performed in 24-well tissue culture plates. Duplicate eight-point dilution series of maraviroc were prepared in DMSO and medium to yield a final DMSO concentration of 0.1% (vol/vol) in the assay. PHA-stimulated PBMC or PM-1 cells were infected with virus for 1 h at 37°C. Cells were subsequently washed once, and 3.6 × 105 PBMC or 2.0 × 105 PM-1 cells were added to each well of assay plates containing diluted compound. Plates were incubated for 5 days (lab-adapted strains) or 7 days (primary isolates) at 37°C in a humidified 5% CO2 (vol/vol) atmosphere. Control compounds, saquinavir (an HIV-1 protease inhibitor) and RANTES, were included in all assays.
Virus replication was quantified in each well by direct measurement of RT activity in the culture supernatant, using a commercially available scintillation proximity assay (Amersham). To ensure that RT levels from the supernatants were in the linear range for the assay, a standard curve was included in each assay using purified recombinant RT (0 to 20 mU/μl; Sigma). The percent inhibition of RT activity for each concentration of maraviroc was calculated to determine the anti-HIV-1 IC50 and IC90. Any compound-specific cytotoxicity was assessed by incubating uninfected PBMC or PM-1 cells with serially diluted compound for 5 to 7 days before determination of cell viability with MTS reagent (Promega).
HIV-1 envelope-recombinant pseudotyped virus assays.
The antiviral activity of maraviroc was assessed against Env-recombinant pseudotyped viruses derived from 200 HIV-1 clinical isolates using a method described previously (39). The panel of viruses comprised the following: 160 viruses were of subtype (clade) B and 40 were non-subtype-B HIV-1; 100 viruses were from patient samples which contained no known RT inhibitor (RTI)- or protease inhibitor (PI)-specific resistance mutations, the remaining 100 viruses were derived from those in which there were 1 or more RTI/PI mutations (80 viruses were subtype B in each case). Briefly, HIV-1 envelope (Env) genes were amplified from patient sera and cloned into an expression vector. Recombinant virus stocks expressing virus envelope proteins from patient samples were prepared by cotransfecting HEK-293 cells with the appropriate envelope expression vector and a replication-defective HIV-1 genomic viral vector, in which a region of the HIV-1 envelope gene has been replaced with a luciferase expression cassette. The recombinant virus particles were harvested from the supernatant and inoculated into two U87 cell lines expressing either CD4/CCR5 or CD4/CXCR4. Successful virus entry followed by a single round of viral replication results in the production of large amounts of luciferase activity in infected cells. Susceptibility to maraviroc was measured by comparing the amount of luciferase activity produced in the presence of the compound to the amount of luciferase activity produced in the absence of the compound.
Pharmacokinetic studies with rats and dogs.
Preclinical pharmacokinetic studies were carried out with maraviroc following a single intravenous and oral administration to both male Sprague-Dawley rats (1 mg/kg of body weight given intravenously [i.v.] and 10 mg/kg given orally [p.o.]; n = 2) and male beagle dogs (0.5 mg/kg i.v. and 2 mg/kg p.o; n = 4). Plasma samples were taken for up to 24 h postdose, and the concentrations of unchanged maraviroc were determined using a specific high-performance liquid chromatography-tandem mass spectrum assay.
RESULTS
Discovery of maraviroc.
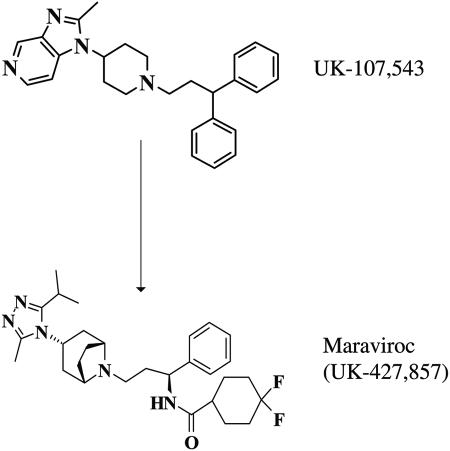
The Pfizer compound file was screened using a chemokine radioligand-binding assay to identify a small-molecule CCR5 ligand. The imidazopyridine UK-107,543 was one of the most potent and ligand-efficient lead compounds identified and was the starting point of an intensive medicinal chemistry program. Parallel screening was employed to optimize the following parameters: binding potency against the receptor, antiviral activity, and absorption and pharmacokinetics, as well as selectivity against key human targets, such as the hERG channel (Fig. 1). In the process, we developed and optimized two bespoke assays, one measuring envelope binding to the cell surface receptors (14) and a second modeling the subsequent membrane fusion events (5). The program synthesized and profiled nearly 1,000 analogues, from which maraviroc (UK-427,857) was selected.
FIG. 1.
Discovery of maraviroc from the high-throughput screening hit UK-107,453.
Maraviroc activity in CCR5 binding and functional assays.
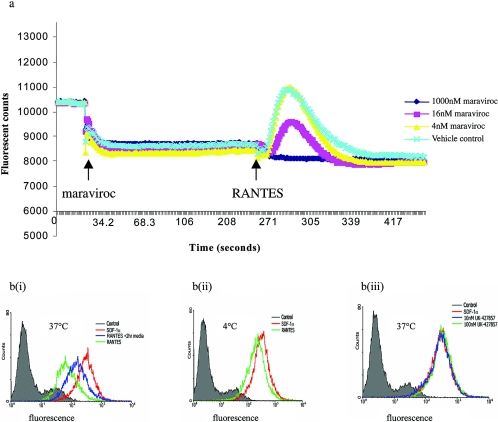
The activity of maraviroc against cognate β-chemokine binding to CCR5 was quantified using radioligand binding competition assays. Maraviroc inhibited MIP-1α (IC50, 3.3 nM; 95% confidence interval [CI], 1.9 to 5.7 nM; n = 3), MIP-1β (IC50, 7.2 nM; 95% CI, 5.5 to 9.5 nM; n = 17), and RANTES (IC50, 5.2 nM; 95% CI, 2.1 to 13 nM; n = 3) binding to cell membrane preparations of CCR5-expressing HEK-293. The ability of maraviroc to inhibit downstream CCR5 signaling events following cognate chemokine binding was also assessed. Maraviroc inhibited MIP-1β-stimulated γ-S-GTP binding to HEK-293 cell membranes, indicating its ability to inhibit chemokine-dependent stimulation of GDP-GTP exchange at the CCR5/G protein complex (18, 29, 36). Maraviroc also inhibited the downstream event of chemokine-induced intracellular calcium redistribution, with IC50s ranging from 7 to 30 nM obtained against MIP-1β, MIP-1α and RANTES (Fig. 2a). In the same experiments, maraviroc did not trigger release of intracellular calcium at concentrations up to 10 μM, indicating that it is devoid of CCR5 agonist activity. Consistent with this, maraviroc failed to induce CCR5 internalization, as shown by cytometry experiments (Fig. 2b). These results demonstrate that maraviroc is an inhibitor (functional antagonist or inverse agonist) of the CCR5 receptor.
FIG. 2.
Effects of maraviroc on CCR5-mediated signaling. a: Maraviroc-dependent inhibition of RANTES-induced calcium redistribution. Fluorescence was measured in real time by FLIPR following addition of maraviroc and subsequent addition of 20 nM RANTES (both marked). b: effect of maraviroc on 300.19 cell surface CCR5 levels (anti-CCR5 antibody-dependent cell population fluorescence). Isotype control fluorescence is depicted in gray. RANTES (100 nM)-induced reduction of CCR5 is shown by a reduction in fluorescence [green line in panel b(i)] relative to parallel experiments using the negative control ligand SDF-1α [red line in panel b(i)]. Reemergence of CCR5 at the cell surface was apparent following a 2-h incubation period after addition of RANTES [blue line in panel b(i)]. RANTES-induced reduction in cell population fluorescence was reduced at 4°C [panel b(ii)], highlighting internalization to be an active biological process. Maraviroc did not affect cell population fluorescence at 10 nM or 100 nM [blue and green lines, respectively; panel b(iii)], as also seen for the negative control SDF-1α (red line).
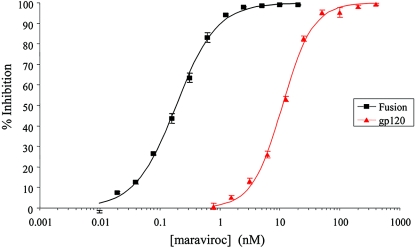
Activity against HIV-1 envelope binding to CCR5 and fusion.
The ability of maraviroc to inhibit virus attachment to CCR5 was measured in binding competition experiments against the soluble subunit of the HIV-1 (Ba-L) envelope glycoprotein gp120, following complex formation with a soluble CD4 preparation (gp120-sCD4 complex). The IC50 for inhibition in this system was determined to be 11 nM (95% CI, 10.4 to 11.6 nM; n = 18) (Fig. 3). The ability of the compound to inhibit HIV-1 envelope protein-CCR5-dependent cell-cell fusion was also investigated. This cell-based assay was designed to mimic HIV-1 entry into host cells by virtue of the fusion process being mediated by HIV-1 gp120 (strain JRFL) on CHO cells binding CD4 on HeLa-P4 cells, to enable conformational change in gp120 to promote binding of this glycoprotein to CCR5 on the HeLa P4 cells and subsequent gp41-mediated cell-cell fusion. In this assay, maraviroc gave an IC50 of 0.22 nM (95% CI, 0.13 to 0.39 nM; n = 4) (Fig. 3).
FIG. 3.
Maraviroc-dependent inhibition of gp120 binding to CCR5 (red) and gp160-CCR5-mediated cell-cell fusion (black). Each data point represents the mean percent inhibition (relative to vehicle control) ± the standard error of the mean (bars).
Antiviral activity of maraviroc against lab-adapted HIV-1 strains and primary HIV-1 isolates.
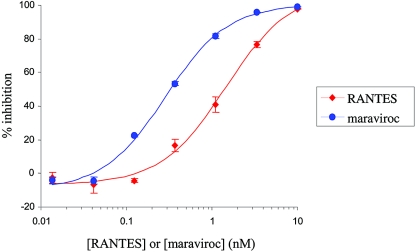
Maraviroc was active (IC90) at low nanomolar concentrations against HIV-1 Ba-L (a lab-adapted R5 strain) when measured in a 5-day antiviral assay using either isolated multiple (pooled) donor PBMC (IC90, 3.1 nM; 95% CI, 2.0 to 4.9 nM; n = 33) (Fig. 4), single-donor PBMC (IC90, 1.8 nM; 95%, CI 1.2 to 2.6 nM; n = 39) or PM-1 cells (IC90, 1.1 nM; 95% CI, 0.74 to 1.7 nM; n = 45). Under similar conditions, maraviroc had no effect on cell proliferation at concentrations up to 10 μM (the highest concentration tested), as determined using a commercially available methyl tetrazolium salt cytotoxicity assay. Similarly, no cytotoxicity was observed in any of the recombinant cell lines reported in this study at any compound test concentration, as judged by visual microscopic analysis. This confirmed that the antiviral activity of maraviroc is not due to any cytopathic properties of the compound. Furthermore, maraviroc was also inactive against laboratory-adapted HIV-1 isolates that utilize CXCR4 as a coreceptor on PBMC (IC50, >10 μM; data not shown), thereby highlighting that the antiviral mechanism of maraviroc is exclusively CCR5 mediated. Saquinavir and RANTES were included as positive controls in all assays and gave dose responses in good agreement with those previously published (8, 51).
FIG. 4.
Representative dose response for maraviroc-dependent inhibition of HIV strain Ba-L replication in pooled isolated peripheral blood lymphocytes (blue line and data points) compared to that with RANTES (red line and data points). Maraviroc yielded a geometric mean IC50 of 0.56 nM (95% CI, 0.36 to 0.9 nM; n = 33) and RANTES a value of 2.2 nM (95% CI, 1.7 to 2.8 nM; n = 14). Data points represent the mean percent inhibition ± standard error of the mean for a representative assay.
To establish the activity spectrum of maraviroc, a diverse cross-clade panel of primary HIV-1 isolates was assayed for susceptibilities to maraviroc in 7-day virus replication assays in PBMC. Maraviroc showed potent antiviral activity against the 43 primary CCR5-tropic HIV-1 isolates tested, as reflected by a geometric mean IC90 of 2.0 nM (95% CI, 1.8 to 2.4 nM [Table 2 ]). The IC90s ranged from 0.5 nM to 13.4 nM (Table 1), with the weakest activity found against the G-clade isolate RU570, previously reported to be relatively insensitive to another CCR5 antagonist, SCH-C (43). Maraviroc showed no activity when assayed against CXCR4-tropic or dual-tropic primary HIV-1 isolates under similar conditions, consistent with its mechanism of action as a CCR5-specific antagonist.
TABLE 2.
Antiviral activities of maraviroc against primary CCR5-tropic HIV-1 isolates in 7-day virus replication assays with PBMC
| Virus subtypea | No. of isolates | No. of assays | Geometric mean IC50, nM (95% CI) | Geometric mean IC90, nM (95% CI) |
|---|---|---|---|---|
| A | 4 | 16 | 0.40 (0.23-0.70) | 1.1 (0.66-1.8) |
| B | 21 | 94 | 0.42 (0.36-0.50) | 1.8 (1.5-2.2) |
| C | 4 | 20 | 0.69 (0.37-1.28) | 3.0 (1.7-5.5) |
| D | 4 | 10 | 0.68 (0.39-1.2) | 2.7 (1.7-4.1) |
| E | 1 | 4 | 0.10 (0.08-0.12) | 0.51 (0.37-0.71) |
| F | 1 | 2 | 1.1 (0.50-2.4) | 3.0 (0.92-9.5) |
| G | 2 | 14 | 1.1 (0.50-2.5) | 4.4 (2.2-9.1) |
| J | 2 | 10 | 0.52 (0.18-1.5) | 3.5 (1.3-9.9) |
| O | 4 | 20 | 0.73 (0.47-1.1) | 1.8 (1.1-2.9) |
| All | 44 | 190 | 0.51 (0.44-0.60) | 2.0 (1.8-2.4) |
Genetic subtypes were as assigned by the NIBSC based on amino acid sequence alignments of Env. Subtypes A to J comprise the main group; group O viruses form an outlying group.
Activity of maraviroc against clinically derived HIV-1 envelopes from patients resistant to existing antiretrovirals.
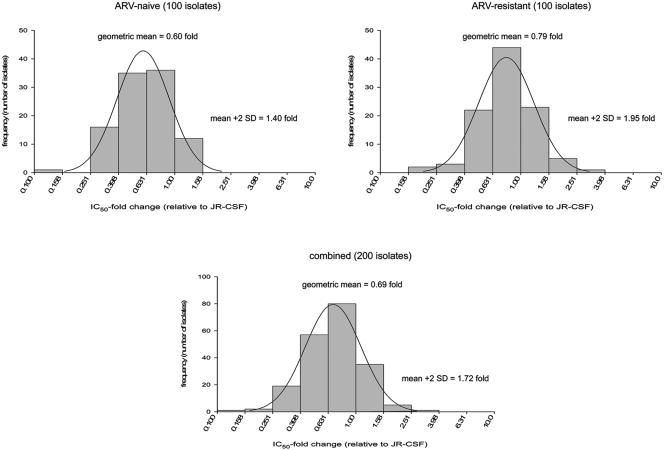
The antiviral activity of maraviroc was also assessed in a pseudovirus assay using clinically derived HIV-1 envelopes from drug-naive and drug-experienced patients. The virus panel comprised 100 viruses derived from HIV-1 clinical samples with no genotypic mutations associated with RTI/PI resistance (“drug naive”) and 100 viruses from samples containing RTI/PI-associated resistance mutations (“drug experienced”). The panel was also constructed to include 160 viruses of subtype-B origin and 40 viruses of non-subtype-B origin. Maraviroc inhibited all 200 pseudotyped viruses with a geometric mean IC90 of 13.7 nM (95% CI, 12.3 to 15.1 nM) and a geometric mean IC50 n-fold change (defined as the clinical isolate IC50/JRCSF IC50) of 0.69-fold (95% CI, 0.64 to 0.73). The range of susceptibilities to maraviroc was narrow, as illustrated by an estimated biological cutoff (= geometric mean plus 2 standard deviations [SD]) of 1.72-fold (33). There was no difference in the susceptibilities of subtype B viruses and non-B viruses to maraviroc. There was a small but statistically significant difference between the drug-naive and drug-experienced group, with geometric mean n-fold differences of 0.60-fold (95% CI, 0.55 to 0.65) and 0.79-fold (95% CI, 0.72 to 0.86), respectively (P < 0.001) (see also Fig. 5). This difference was not considered biologically significant, since it was less than the assay-to-assay variation previously reported. Importantly, there was no correlation between the susceptibility to maraviroc and the number of drug-associated mutations in the viruses from which the envelopes were derived, indicating that the compound should be effective in patients resistant to existing antiretrovirals.
FIG. 5.
Activity of maraviroc against a panel of 200 Env-recombinant pseudoviruses derived from clinical HIV-1, showing the n-fold change in IC50 relative to that for strain JR-CSF for envelopes derived from normal and drug-resistant strains.
Antiviral activity of maraviroc in combination with other antiretroviral agents.
The anti-HIV-1 activity of maraviroc in combination with licensed antiretroviral agents was investigated both in PBMCs and in the PM-1 cell line. As expected for a compound that has a novel mechanism of action, additive interactions were observed when maraviroc was combined with most of the licensed drugs (Table 3). Moderate synergy was observed in single experiments with atazanavir, indinavir, and enfuvirtide, with additive effects seen in repeat experiments. In addition to the minor synergy observed in single experiments of maraviroc combined with atazanavir, indinavir, or enfuvirtide in PM-1 cells, minor synergy was observed when maraviroc was tested in combination with efavirenz or nelfinavir in PHA-stimulated PBL (Table 3). The combination of maraviroc and efavirenz demonstrated an additive interaction when assessed in the PM-1 cell line.
TABLE 3.
Antiviral effects of maraviroc in combination with different classes of antiviral agent
| Maraviroc in combination witha: | No. of assays | Volume (μM2%)d
|
Combined effect | |
|---|---|---|---|---|
| Synergy | Antagonism | |||
| Lamivudine (NRTI)b | 2 | 41 | −34 | Additive |
| Efavirenz (NNRTI)b | 2 | 63 | −18 | Minor synergy |
| Nelfinavir (PI)b | 2 | 74 | −23 | Minor synergy |
| Abacavir (NRTI)c | 2 | 10.4 ± 14.6 | −0.2 ± 0.3 | Additive |
| Didanosine (NRTI)c | 2 | 4.0 ± 4.6 | −0.9 ± 1.3 | Additive |
| Emtricitabine (NRTI)c | 2 | 0.1 ± 0.2 | −1.2 ± 1.7 | Additive |
| Stavudine (NRTI)c | 2 | 1.6 ± 1.5 | 0.0 | Additive |
| Tenofovir (NRTI)c | 3 | 5.2 ± 7.2 | −8.0 ± 13.9 | Additive |
| Zalcitabine (NRTI)c | 2 | 0.0 | −4.3 ± 1.2 | Additive |
| Zidovudine (NRTI)c | 2 | 0.1 ± 0.1 | −0.1 ± 0.1 | Additive |
| Delavirdine (NNRTI)c | 2 | 7.1 ± 3.4 | −0.2 ± 0.3 | Additive |
| Efavirenz (NNRTI)c | 3 | 0.0 | −4.1 ± 4.5 | Additive |
| Nevirapine (NNRTI)c | 2 | 5.7 ± 8.0 | −3.2 ± 2.8 | Additive |
| Amprenavir (PI)c | 2 | 3.5 ± 2.4 | −1.6 ± 1.4 | Additive |
| Atazanavir (PI)c | 2 | 35.4 ± 43.2e | −0.2 ± 0.3e | Minor synergy |
| Indinavir (PI)c | 2 | 19.6 ± 27.7f | −6.5 ± 4.0f | Additive |
| Lopinavir (PI)c | 2 | 12.5 ± 17.6 | −11.0 ± 15.2 | Additive |
| Ritonavir (PI)c | 2 | 0.0 | −0.3 ± 0.4 | Additive |
| Saquinavir (PI)c | 3 | 10.7 ± 12.6 | −1.0 ± 0.2 | Additive |
| Enfuvirtide (fusion)c | 2, 1 | 14.4 ± 12.2g | −0.9 ± 1.5g | Additive |
NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Experiments performed using HIV-1 Ba-L strain in mitogen-stimulated PBL at an independent laboratory. For these studies, synergy (assigned a positive value) or antagonism (assigned a negative value) was defined as drug combinations yielding mean volumes in excess of 50 μM2%, and minor synergistic/antagonistic activity and strong synergistic/antagonistic activity were defined as mean volumes of 50 to 100 μM2% and in excess of 100 μM2%, respectively. Additive drug interactions were defined by mean volumes of 0 to 50 μM2%.
Experiments performed using HIV-1 Ba-L strain in PM-1 cell line. For these studies, synergy (assigned a positive value) or antagonism (assigned a negative value) was defined as drug combinations yielding mean volumes in excess of 25 μM2%, and moderate synergistic/antagonistic activity and strong synergistic/antagonistic activity were defined as mean volumes of 50 to 100 μM2% and in excess of 100 μM2%, respectively. Additive drug interactions were defined by mean volumes of 0 to 25 μM2%.
Volumes of synergy (μM2%) were calculated at 95% confidence intervals using drug combination data from three to five replicate plates per assay, with the aid of MacSynergy II software as previously reported (38). Volumes are expressed as means from two or three independent experiments (±standard deviation for the PM-1 studies).
Individual MacSynergy II volumes for atazanavir: 65.9, 4.9, synergy; −0.4, −0.0, antagonism.
Individual MacSynergy II volumes for indinavir: 39.2, 0.0, synergy; −9.3, −3.6, antagonism.
Individual MacSynergy II volumes for enfuvirtide; 27.9, 4.2, 11.2, synergy; −2.5, −0.0, −0.0, antagonism.
Selectivity profile of maraviroc.
In light of the general role of chemokines in immune function, the activity of maraviroc was evaluated in a number of human in vitro immune function assays, including activity in a number of related chemokine receptor assays. No significant activity was observed in any of these assays at concentrations in excess of 1,000 times the IC50s for maraviroc (Table 4). Of greatest significance, maraviroc showed no evidence of activity against CCR2, which has the closest sequence identity to CCR5 and is known to be susceptible to previously described CCR5 antagonists (35, 54). Maraviroc also showed no significant activity against a range of pharmacologically relevant enzymes, ion channels, and receptors at concentrations up to 10 μM, as measured in binding competition and functional assays. It was also well tolerated in mouse and rat with no significant effects on the central, peripheral, renal, or respiratory system (C. Napier, P. Dorr, R. Gladue, R. Halliday, D. Leishman, I. Machin, R. Mitchell, A. Nedderman, M. Perros, S. Roffey, D. Walker, and R. Webster, Abstr. 11th Conf. Retrovir. Opportun. Infect., abstr. 546a, 2003). This included the cardiac potassium channel hERG (human ether-a-go-go-related gene), where maraviroc showed only very weak affinity (19% ± 3% inhibition at 10 μM; n = 4).
TABLE 4.
Evaluation of maraviroc using in vitro models of human immune system function
| Selectivity assaya | Activity of maraviroc (IC50)b |
|---|---|
| MCP-3-induced intracellular Ca2+ | >10 μM |
| release (CCR2) | |
| IL-2-stimulated T-cell proliferation | >10 μM |
| LPS-stimulated TNF-α release by | >4 μM |
| differentiated THP-1 cells | |
| Antigen-stimulated lymphocyte proliferationc | >10 μM |
| MIP-1α-induced chemotaxis of THP-1 cells | >25 μM |
| (CCR1)d | |
| MCP-1-induced chemotaxis of THP-1 cells (CCR2) | >25 μM |
| ITAC-induced chemotaxis by H9 cells (CCR3) | >25 μM |
| SLC-induced chemotaxis by H9 cells (CCR7) | >25 μM |
| IL-8-induced chemotaxis of neutrophils | >25 μM |
| (CXCR1/CXCR2) | |
| Gro-α-induced chemotaxis of neutrophils | >25 μM |
| (CXCR2) | |
| MIP-1α binding to CCR1e | >10 μM |
| Eotaxin binding to CCR3 | >10 μM |
| TARC binding to CCR4 | >10 μM |
| rhMIP-3β binding to CCR7 | >10 μM |
| I309 binding to CCR8 | >10 μM |
| Superoxide production in neutrophils | >10 μM |
| IL-4-stimulated IgE synthesis in lymphocytes | ≥10 μM (2 donors); 1 to 10 μM (1 donor) |
Each assay consisted of one to three experiments. TNF-α, tumor necrosis factor alpha; LPS, lipopolysaccharide; ITAC, interferon inducible T-cell-α chemoattractant; SLC, secondary lymphoid tissue chemokine; TARC, thymus and activation regulated chemokine; rhMIP-3β, recombinant human MIP-3β.
Maraviroc was tested for inhibitory activity up to the doses indicated according to protocols developed at Pfizer and based upon methods previously reported (see footnotes c to e).
See reference 30.
See reference 45.
Pharmacokinetic profile of maraviroc.
Clearance values were moderate to high in both rat and dog species following i.v. administration (74 and 21 ml/min/kg, respectively). The compound also had a moderate volume of distribution in both species (4.3 to 6.5 liters/kg). The half-life values of maraviroc were 0.9 h in the rat and 2.3 h in the dog. Following oral administration (2 mg/kg) to the dog, the Cmax (256 ng/ml) occurred 1.5 h. postdose, and the bioavailability was 40%. For the rat, investigation of the concentrations obtained in the portal vein following oral administration indicated that approximately 30% of the administered dose was absorbed from the intestinal tract. Allometric scaling to humans with these data indicated the potential to achieve efficacy under a low-dose oral regimen. This was supported by phase I clinical trial data which showed continuous systemic exposure above the geometric mean antiviral IC90 following oral dosing at 100 mg twice daily (b.i.d.) (see Table 5).
TABLE 5.
Predicted and measured human pharmacokinetic profile for maraviroca
| Parameterb | Predicted value for maraviroc from rat and dog | Measured value for maraviroc (30-mg i.v single dose or 100 mg b.i.d. oral) |
|---|---|---|
| Clearance (ml/min/kg) | 11 | 10.5 ± 1.3 |
| V (liters/kg) | 2 | 2.8 ± 0.9 |
| t1/2 (h) | 3 | 13.2 ± 2.8 |
| Absorption (%) | 30 | 55 |
| Bioavailability (%) | 20 | 23 (19.2-27.8) |
| Cmin (nM unbound) at 100 mg b.i.d. | 1 | 5.4 ± 1.4 |
The predicted pharmacokinetic values were made by allometric scaling to man from the plasma compound levels measured following single-dose intravenous and oral administration to both male Sprague-Dawley rats (1 mg/kg i.v. and 10 mg/kg p.o.; n = 2) and male beagle dogs (0.5 mg/kg i.v. and 2 mg/kg p.o.; n = 4). Values measured from clinical studies are the means ± SD, except for bioavailability, which was derived from a ratio following an oral (100 mg) and i.v. (30 mg) single-dose crossover study with volunteers (95% CI values shown; n = 12). The measured clearance, volume (steady state) and t1/2 values were derived following single i.v. dosing to volunteers (n = 8). Absorption value in humans was estimated from bioavailability and radiolabel mass balance studies. Cmin (minimum concentration of drug in serum) value was measured in HIV+ patients (n = 8) at steady state (day 10) following a dosing regimen of 100 mg b.i.d.
DISCUSSION
Maraviroc is a novel small-molecule inhibitor of CCR5 with potent anti-HIV-1 activity. The compound is the end product of a high-throughput screen and medicinal chemistry program, which optimized the pharmacological and pharmacokinetic properties of the chemical series. Maraviroc inhibits HIV-1 gp120 binding to CCR5, thus preventing gp160-CCR5-mediated cell-cell fusion. The compound blocks chemokine binding and CCR5-mediated signaling, as was demonstrated in Ca2+ mobilization and γ-S-GTP binding assays. The reduction of basal γ-S-GTP binding observed in the latter may indicate that maraviroc is acting as an inverse agonist to promote the formation of CCR5 in an inactive state. This would functionally mimic the Δ32 “null” phenotype in human, which is known to have no apparent consequences for the immune status or general health of the subjects. In contrast, the consequences of partial or altered CCR5-mediated signaling cannot be determined from any known CCR5 genetic variations. It would require long-term clinical trials to assert the safety of inhibitors that would reproduce the above phenotype.
One of the attractive properties of a new class of inhibitors is the expectation that they will be effective against HIV-1 strains regardless of the previous drug experience of the patient. Consistent with this, maraviroc is active against R5 strains from drug-naive subjects, as well as against viruses isolated from patients with experience in one or more of the preexisting classes. Furthermore, there was a tight distribution of responses to the 200 viruses tested, as indicated by the biological cutoff of 1.7-fold. This compares well with biological cutoffs for existing antiretroviral drugs tested in a similar pseudotyped-virus assay, using recombinant RT/protease derived from patient plasma (33). The distribution of the response to maraviroc is also tighter than that reported for the fusion inhibitor, enfuvirtide, which displayed a biological cutoff of 7.5 in the same assay when tested against baseline samples of patients enrolled in the “TORO” phase 3 clinical trials (M. Greenberg, Abstr. 2nd Eur. HIV Drug Resist. Workshop, abstr. 8, session 3, 2004). We have also extended our analysis to strains from diverse geographic origins. HIV-1 is comprised of three subgroups based upon phylogenetic clustering, of which the M (“Main”) group is responsible for the majority of global infections. This is further divided into nine recognized subtypes, or “clades,” clustering into independent branches based upon env amino acid sequences. While clade B is responsible for the majority of infections in the Western world, the epidemic is predominantly caused by viruses from other clades (A and C in particular; see UNAIDS 2004 report on global AIDS epidemic), and global spread of various clades is increasing (37). An earlier reported CCR5 antagonist, SCH-C, showed potent antiviral activity against most R5 strains but was poorly active against representatives from subtype G (43). Encouragingly, maraviroc had potent cross-clade activity against all CCR5-tropic HIV-1 primary isolates tested, with a less than 10-fold difference between the most- and least-susceptible clades and a less than 30-fold difference between the most- and least-susceptible individual isolates (among more than 40 isolates tested). In particular, the two subtype G strains which we tested are within threefold of the geometric mean IC90 for all primary isolates, with the RU570 isolate falling within sixfold. This broad-spectrum activity is a desirable attribute for an anti-infective agent and may indicate that different antagonists have distinct binding patterns for CCR5, presenting the coreceptor to HIV-1 in diverse states.
The considerable structural differentiation between maraviroc and previously reported CCR5 antagonists underpins their different pharmacological and pharmacokinetic behaviors. Maraviroc is a basic compound (tropane) which shows a pan-cognate chemokine blockade for both binding and signaling inhibition as reported here. This is in contrast to ONO4128/GW873140 (a zwitterionic spirodiketopiperazine [25]), which does not inhibit RANTES binding to CCR5 but does inhibit RANTES signaling via this receptor (26, 50).
Preclinical pharmacokinetic profiling indicates that unbound drug minimum concentrations in serum in excess of the antiviral IC90 are readily achievable through once- or twice-daily administration, a prediction that has been achieved in recent clinical trials (S. Abel, E. Van Der Ryst, G. J. Muirehead, M. Rosario, A. Edgington, and G. Weissgerber, Abstr. 11th Conf. Retrovir. Opportun. Infect., abstr. 547, 2003; A. L. Pozniac, G. Fatkenheuer, M. Johnson, I. M. Hoepelman, J. Rockstroh, F. Goebel, S. Abel, I. James, M. Rosario, C. Medhurst, J. Sullivan, M. Youle, and E. Van Der Ryst, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-443, 2003). Pharmacokinetic differences with respect to oral bioavailability and clearance have been observed between the CCR5 antagonists in clinical development (1; S. Abel, C. Russell, C. Ridgway, and G. Muirehead, Abstr. 6th Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 76, 2005; D. Schurmann, R. Rouzier, R. Nougerede, J. Reyes, G. Fatkenheuer, F. Raffi, C. Michelet, A. Tarral, C. Hoffmann, J. Kiunke, H. Sprenger, J. vanLier, A. Sansone, M. Jackson, and M. Laughlin, Abstr. 11th Conf. Retrovir. Opportun. Infect., abstr. 140LB, 2004; J. Demarest, K. Adkison, S. Sparks, A. Shachoy-Clark, K. Schell, S. Reddy, L. Fang, K. O'Mara, S. Shibayama, M. Berrey, S. Piscitelli, Abstr. 11th Conf. Retrovir. Opportun. Infect., abstr. 139, 2004).
In addition to its low nanomolar antiviral (IC90) potency and broad-spectrum antiviral activity, maraviroc has a prolonged CCR5 physical and functional occupancy (50; P. Dorr, M. Macartney, G. Rickett, C. Smith-Burchnell, S. Dobbs, J. Mori, P. Griffin, J. Lok, R. Irvine, M. Westby, C. Hitchcock, B. Stammen, D. Price, D. Armour, A. Wood, and M. Perros, Abstr. 10th Conf. Retrovir. Opportun. Infect., abstr. 12, 2003). Both intrinsic antiviral potency and prolonged receptor occupancy are believed to be important factors that contribute to the antiviral efficacy of CCR5 antagonists in clinical trials. However, the continuous supply of de novo CCR5-expressing HIV-1-susceptible cells in patients indicates a pharmacokinetic profile that ensures sustained exposure of available antagonists to achieve clinically relevant reductions in viral load (K. Adkison, Y. Lou, L. Fang, A. Shachoy-Clark, J. Demarest, M. Berry, S. and Piscitelli, Abstr. 6th Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 77, 2005). Achieving a complementary balance between primary antiviral CCR5 pharmacology and favorable pharmacokinetics to achieve sufficient exposure in vivo even without boosting with cytochrome p450 inhibitors is a key differentiating factor for maraviroc.
The pharmacokinetic and in vitro effects in the presence of other antiretrovirals and HIV-1 medications is an important consideration in light of the need for multiple drug therapy for maximal efficacy and reduced emergence of resistance. Combination studies in vitro demonstrate that maraviroc is not antagonistic to existing antiretroviral agents, and data in vivo reveal a drug-drug interaction profile commensurate with a convenient dosing regime, without affecting the pharmacokinetics of potential comedications (S. Abel, C. Russell, C. Ridgway, and G. Muirehead, Abstr. 6th Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 76, 2005).
One of the major questions surrounding this new class of inhibitors is how resistance will develop in humans. Since CCR5 antagonists are selective for R5 viruses, selection for CXCR4-using variants would lead to virus escape. Indeed, treatment of R5-infected hu-SCID mice with AOP-RANTES (which exerts its antiviral activity through retention of CCR5 in intracellular compartments) resulted in emergence of X4 strains (28). A possible explanation for this tropism switch is that since CCR5 is no longer expressed on the cell surface, the only viral variants that are positively selected are those with increased affinity for alternative coreceptors. In contrast to these data, in vitro studies aimed at generating HIV-1 resistance to CCR5 antagonists have not led to a rapid emergence of CXCR4-using variants (47; C. Stoddart, S. Xu, J. Wojcik, J. Riley, and J. Strizki, Abstr. 10th Conf. Retrovir. Opportun. Infect., abstr. 614, 2003; M. Westby, C. Smith-Burchnell, J. Mori, M. Lewis, R. Mansfield, J. Whitcomb, C. J. Petropoulos, and M. Perros, Abstr. XIII Int. HIV Drug Resist. Workshop, abstr. 6, 2004). This is consistent with the observation that maraviroc blocks binding of the virus to CCR5 without altering receptor levels on the cell surface, thereby selecting for variants with increased affinity for the inhibitor-bound receptor. Despite these encouraging preclinical findings, the emergence of X4 HIV-1 variants during CCR5 antagonist therapy will need close monitoring during clinical trials. Three examples of emergence of CXCR4-using variants have been described during short-term monotherapy studies of CCR5 antagonists (M. E. Lewis, E. van der Ryst, M. Youle, T. Jenkins, I. James, C. Medhurst, and M. Westby, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-584b, 2004; K. Kitrinos, C. LaBranche, M. Stanhope, H. Madsen, and J. Demarest, Abstr. XIV Int. HIV Drug Resist. Workshop, abstr. 61, 2005). Clonal analysis of virus before and after treatment has shown that in each case virus emerged from preexisting CXCR4-using virus reservoirs rather than a switch of R5 variants on treatment.
Finally, a key attribute of any novel antiretroviral treatment is convenience of dosing and lack of side effects when taken chronically. With excellent safety windows, in particular against the hERG channel, which is responsible for QT prolongation, and no measurable activity across a range of immune function assays, maraviroc fulfilled our desired preclinical profile in terms of safety of administration and expected lack of significant side effects and immunological consequences. On the basis of the data above, we believe that maraviroc has the potential to become a safe, well-tolerated, and easily administered effective HIV-1 inhibitor with broad-spectrum anti-HIV-1 activity. Further clinical development is ongoing.
Acknowledgments
We thank Rachel Halliday and Don Walker in the Pharmacokinetics and Drug Metabolism Department of Pfizer for their contribution to the pharmacokinetic studies. We also thank Samantha Abel of the Clinical R&D Department of Pfizer for her help with clinical pharmacokinetic data.
REFERENCES
- 1.Adkison, K. K., A. Shachoy-Clark, L. Fang, Y. Lou, K. O'Mara, M. M. Berrey, and S. C. Piscitelli. 2005. Pharmacokinetics and short-term safety of 873140, a novel CCR5 antagonist, in healthy adult subjects. Antimicrob. Agents Chemother. 49:2802-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber, C. G. 2004. CCR5 antagonists for the treatment of HIV. Curr. Opin. Investig. Drugs 5:851-861. [PubMed] [Google Scholar]
- 3.Beatty, C., M. Bradley, D. Brambilla, F. Breakenridge, J. W. Bremer, J. Dargavon, B. Ladd, D. Livnatt, C. Michel, C. Mundy, V. Price, T. Ramacciotti, P. Reichelderfer, B. Staes, C. Starkey, and M. Winters. 1997. DAIDS Virology Manual for HIV Laboratories, p. 73-76. Division of Acquired Immunodeficiency Syndrome, NIAID, NIH, Bethesda, Md.
- 4.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 5.Bradley, J., J. Gill, F. Bertelli, S. Letafat, R. Corbau, P. Hayter, P. Harrison, A. Tee, W. Keighley, M. Perros, G. Ciaramella, A. Sewing, and C. Williams. 2004. Development and automation of a 384-well cell fusion assay to identify inhibitors of CCR5/CD4-mediated HIV virus entry. J. Biomol. Screen 9:516-524. [DOI] [PubMed] [Google Scholar]
- 6.Bruhl, H., J. Cihak, M. Stangassinger, D. Schlondorff, and M. Mack. 2001. Depletion of CCR5-expressing cells with bispecific antibodies and chemokine toxins: a new strategy in the treatment of chronic inflammatory diseases and HIV. J. Immunology. 166:2420-2426. [DOI] [PubMed] [Google Scholar]
- 7.Chen, R. Y., J. M. Kilby, and M. S. Saag. 2002. Enfuvirtide. Expert Opin. Investig. Drugs 11:1837-1843. [DOI] [PubMed] [Google Scholar]
- 8.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 9.Cole, K. E., C. A. Strick, T. J. Paradis, K. T. Ogborne, M. Loetscher, R. P. Gladue, W. Lin, J. G. Boyd, B. Moser, D. E. Wood, B. G. Sahagan, and K. Neote. 1998. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 187:2009-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Combadiere, C., S. K. Ahuja, H. L. Tiffany, and P. M. Murphy. 1996. Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1(alpha), MIP-1(beta), and RANTES. J. Leukoc. Biol. 60:147-152. [DOI] [PubMed] [Google Scholar]
- 11.De Clercq, E. 1999. The emerging role of fusion inhibitors in HIV infection. Drugs R&D 2:321-331. [DOI] [PubMed] [Google Scholar]
- 12.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273:1856-1862. (Erratum, Science 274: 1069.) [DOI] [PubMed] [Google Scholar]
- 13.Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, Z. Zhang, W. A. O'Brien, L. Ratner, G. M. Shaw, and E. Hunter. 2001. Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor. J. Virology. 75:8605-8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobbs, S., M. Perros, and G. A. Rickett. July. 2001. An assay method for determining whether an agent is capable of modulating the interaction of CCR5 with gp120. Patent EP1118858.
- 15.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 16.Guo, Q., H. T. Ho, I. Dicker, L. Fan, N. Zhou, J. Friborg, T. Wang, B. V. McAuliffe, H. G. Wang, R. E. Rose, H. Fang, H. T. Scarnati, D. R. Langley, N. A. Meanwell, R. Abraham, R. J. Colonno, and P. F. Lin. 2003. Biochemical and genetic characterizations of a novel human immunodeficiency virus type 1 inhibitor that blocks gp120-CD4 interactions. J. Virol. 77:10528-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ickovics, J., and C. Meade. 2002. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care 14:309-318. [DOI] [PubMed] [Google Scholar]
- 18.Jansson, C. C., K. Pohjanoksa, J. Lang, S. Wurster, J. M. Savola, and M. Scheinin. 1999. alpha(2)-adrenoceptor agonists stimulate high-affinity GTPase activity in a receptor subtype-selective manner. Eur. J. Pharmacol. 374:137-146. [DOI] [PubMed] [Google Scholar]
- 19.Kazmierski, W. M., L. Boone, W. Lawrence, C. Watson, and T. Kenakin. 2002. CCR5 chemokine receptors: gatekeepers of HIV-1 infection. Curr. Drug Targets Infect. Disord. 2:265-278. [DOI] [PubMed] [Google Scholar]
- 20.Kutilek, V. D., D. A. Sheeter, J. H. Elder, and B. E. Torbett. 2003. Is resistance futile? Curr. Drug Targets Infect. Disord. 3:295-309. [DOI] [PubMed] [Google Scholar]
- 21.Lin, P. F., W. Blair, T. Wang, T. Spicer, Q. Guo, N. Zhou, Y. F. Gong, H. G. Wang, R. Rose, G. Yamanaka, B. Robinson, C. B. Li, R. Fridell, C. Deminie, G. Demers, Z. Yang, L. Zadjura, N. Meanwell, and R. Colonno. 2003. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc. Natl. Acad. Sci. USA 100:11013-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little, S. J., S. Holte, J. P. Routy, E. S. Daar, M. Markowitz, A. C. Collier, R. A. Koup, J. W. Mellors, E. Connick, B. Conway, M. Kilby, L. Wang, J. M. Whitcomb, N. S. Hellmann, and D. D. Richman. 2002. Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med. 347:385-394. [DOI] [PubMed] [Google Scholar]
- 23.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 24.Mack, M., B. Luckow, P. J. Nelson, J. Cihak, G. Simmons, P. R. Clapham, N. Signoret, M. Marsh, M. Stangassinger, F. Borlat, T. N. Wells, D. Schlondorff, and A. E. Proudfoot. 1998. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 187:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda, K., H. Nakata, Y. Koh, T. Miyakawa, H. Ogata, Y. Takaoka, S. Shibayama, K. Sagawa, D. Fukushima, J. Moravek, Y. Koyanagi, and H. Mitsuya. 2004. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J. Virol. 78:8654-8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda, K., K. Yoshimura, S. Shibayama, H. Habashita, H. Tada, K. Sagawa, T. Miyakawa, M. Aoki, D. Fukushima, and H. Mitsuya. 2001. Novel low molecular weight spirodiketopiperazine derivatives potently inhibit R5 HIV-1 infection through their antagonistic effects on CCR5. J. Biol. Chem. 276:35194-35200. [DOI] [PubMed] [Google Scholar]
- 27.Meanwell, N. A., and J. F. Kadow. 2003. Inhibitors of the entry of HIV into host cells. Curr. Opin. Drug Discov. Dev. 6:451-461. [PubMed] [Google Scholar]
- 28.Mosier, D. E., G. R. Picchio, R. J. Gulizia, R. Sabbe, P. Poignard, L. Picard, R. E. Offord, D. A. Thompson, and J. Wilken. 1999. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J. Virol. 73:3544-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller, A., N. G. Mahmoud, M. C. Goedecke, J. A. McKeating, and P. G. Strange. 2002. Pharmacological characterization of the chemokine receptor, CCR5. Br. J. Pharmacol. 135:1033-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak, E. J., A. W. Liu, G. T. Nepom, and W. W. Kwok. 1999. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J. Clin. Investig. 104:R63-R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien, S. J., and J. P. Moore. 2000. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol. Rev. 177:99-111. [DOI] [PubMed] [Google Scholar]
- 32.Owen, S. M., D. Rudolph, D. Schols, N. Fujii, N. Yamamoto, and R. B. Lal. 2002. Susceptibility of diverse primary HIV isolates with varying co-receptor specificity's to CXCR4 antagonistic compounds. J. Med. Virol. 68:147-155. [DOI] [PubMed] [Google Scholar]
- 33.Parkin, N. T., N. S. Hellmann, J. M. Whitcomb, L. Kiss, C. Chappey, and C. J. Petropoulos. 2004. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasi, K. J., C. A. Sabin, P. V. Jenkins, H. L. Devereux, C. Ononye, and C. A. Lee. 2000. The effects of the 32-bp CCR-5 deletion on HIV transmission and HIV disease progression in individuals with haemophilia. Br. J. Haematol. 111:136-142. [DOI] [PubMed] [Google Scholar]
- 35.Paterlini, M. G. 2002. Structure modeling of the chemokine receptor CCR5: implications for ligand binding and selectivity. Biophys. J. 83:3012-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peltonen, J. M., M. Pihlavisto, and M. Scheinin. 1998. Subtype-specific stimulation of [S-35]Gtp-gamma-S binding by recombinant alpha(2)-adrenoceptors. Eur. J. Pharmacol. 355:275-279. [DOI] [PubMed] [Google Scholar]
- 37.Perrin, L., L. Kaiser, and S. Yerly. 2003. Travel and the spread of HIV-1 genetic variants. Lancet Infect. Dis. 3:22-27. [DOI] [PubMed] [Google Scholar]
- 38.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antivir. Res. 14:181-205. [DOI] [PubMed] [Google Scholar]
- 39.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. (First published 18 March 2003; 10.1073/pnas.0630530100.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 41.Scozzafava, A., A. Mastrolorenzo, and C. T. Supuran. 2002. Non-peptidic chemokine receptors antagonists as emerging anti-HIV agents. J. Enzyme Inhibition Med. Chem. 17:69-76. [DOI] [PubMed] [Google Scholar]
- 42.Seibert, C., and T. P. Sakmar. 2004. Small-molecule antagonists of CCR5 and CXCR4: a promising new class of anti-HIV-1 drugs. Curr. Pharm. Des. 10:2041-2062. [DOI] [PubMed] [Google Scholar]
- 43.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tagat, J. R., S. W. McCombie, D. Nazareno, M. A. Labroli, Y. Xiao, R. W. Steensma, J. M. Strizki, B. M. Baroudy, K. Cox, J. Lachowicz, G. Varty, and R. Watkins. 2004. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]-4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]-4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagonist. J. Med. Chem. 47:2405-2408. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka, H., T. Minoshima, and N. Endo. 1995. The effect of a synthetic 7-thiaprostaglandin E1 derivative, TEI-6122, on monocyte chemoattractant protein-1 induced chemotaxis in THP-1 cells. Br. J. Pharmacol. 116:2298-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor, J. M., Y. Wang, L. Ahdieh, J. S. Chmiel, R. Detels, J. V. Giorgi, R. Kaslow, L. Kingsley, and J. Margolick. 2000. Causal pathways for CCR5 genotype and HIV progression. J. Acquir. Immune Defic. Syndr. 23:160-171. [DOI] [PubMed] [Google Scholar]
- 47.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsou, C. L., R. P. Gladue, L. A. Carroll, T. Paradis, J. G. Boyd, R. T. Nelson, K. Neote, and I. F. Charo. 1998. Identification of C-C chemokine receptor 1 (CCR1) as the monocyte hemofiltrate C-C chemokine (HCC)-1 receptor. J. Exp. Med. 188:603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volberg, W. A., B. J. Koci, W. Su, J. Lin, and J. Zhou. 2002. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J. Pharmacol. Exp. Ther. 302:320-327. [DOI] [PubMed] [Google Scholar]
- 50.Watson, C., S. Jenkinson, W. Kazmierski, and T. Kenakin. 2005. The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitor. Mol. Pharmacol. 67:1268-1282. (First published 11 January 2005; 10.1124/mol.104.008565.) [DOI] [PubMed] [Google Scholar]
- 51.Winters, M. A., J. M. Schapiro, J. Lawrence, and T. C. Merigan. 1998. Human immunodeficiency virus type 1 protease genotypes and in vitro protease inhibitor susceptibilities of isolates from individuals who were switched to other protease inhibitors after long-term saquinavir treatment. J. Virol. 72:5303-5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yerly, S., L. Kaiser, E. Race, J. P. Bru, F. Clavel, and L. Perrin. 1999. Transmission of antiretroviral-drug-resistant HIV-1 variants. Lancet 354:729-733. [DOI] [PubMed] [Google Scholar]
- 53.Yerly, S., S. Vora, P. Rizzardi, J. P. Chave, P. L. Vernazza, M. Flepp, A. Telenti, M. Battegay, A. L. Veuthey, J. P. Bru, M. Rickenbach, B. Hirschel, and L. Perrin. 2001. Acute HIV infection: impact on the spread of HIV and transmission of drug resistance. AIDS 15:2287-2292. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, Y., B. Lou, R. B. Lal, A. Gettie, P. A. Marx, and J. P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou, Y., T. Kurihara, R. P. Ryseck, Y. Yang, C. Ryan, J. Loy, G. Warr, and R. Bravo. 1998. Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J. Immunol. 160:4018-4025. [PubMed] [Google Scholar]