Abstract
Respiratory syncytial virus (RSV) is the leading viral pathogen responsible for bronchiolitis and pneumonia in infants and young children worldwide. We have previously shown in the mouse model that treatment with an anti-RSV neutralizing monoclonal antibody (MAb) against the F glycoprotein of RSV, palivizumab, decreased lung inflammation, airway obstruction, and postmethacholine airway hyperresponsiveness. MEDI-524, or Numax, is a new MAb derived from palivizumab with enhanced neutralizing activity against RSV. We compared the effects of these two MAbs on different markers of disease severity using the murine model of RSV infection. BALB/c mice were intranasally inoculated with RSV A2. Palivizumab or MEDI-524 was administered once at either 24 h before or 48 h after RSV inoculation. Regardless of the time of administration, all treated mice showed significantly decreased RSV loads in bronchoalveolar lavage samples measured by plaque assay. Only MEDI-524 given at −24 h significantly decreased lung RSV RNA loads on days 5 and 28 after RSV inoculation. Pulmonary histopathologic scores, airway obstruction, and postmethacholine airway hyperresponsiveness were significantly reduced in mice treated with MEDI-524 at 24 h before inoculation, compared with untreated controls and the other regimens evaluated. MEDI-524 was superior to palivizumab on several outcome variables of RSV disease assessed in the mouse model: viral replication, inflammatory and clinical markers of acute disease severity, and long-term pulmonary abnormalities.
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract disease in infants and young children worldwide. Although certain populations, such as children with chronic lung disease, congenital heart disease, prematurity, or immunodeficiency, are at increased risk for severe RSV disease, most infants hospitalized for RSV infection are previously healthy and have no known risk factors (3). Palivizumab (Pvz) (Synagis; MedImmune, Gaithersburg, MD) is a humanized neutralizing immunoglobulin G1 (IgG1) monoclonal antibody (MAb) directed against the F protein of RSV and is currently approved for prevention of severe RSV infection in high-risk children (13). Although this preventive strategy has dramatically reduced the number of hospitalizations due to RSV in the target populations (11, 13), there are still a small number of breakthrough hospitalizations. These hospitalizations do not appear to be related to the emergence of palivizumab-resistant mutants (10). Thus, the development of anti-RSV antibodies with more potent neutralizing activities, longer half-lives, or more favorable pharmacokinetic profiles and distribution characteristics has the potential to improve outcomes in patients with RSV disease. Potential benefits could be achieved not only for acute disease but also for the long-term consequences of RSV infection, such as recurrent wheezing and airway hyperresponsiveness (25, 27).
MEDI-524 (Numax) is a novel recombinant humanized IgG1 MAb derived from palivizumab, with more potent anti-RSV neutralizing activity. MEDI-524 was derived by in vitro affinity maturation of the murine complementary determining regions of the heavy and light chains of palivizumab. Like palivizumab, the specific activity of MEDI-524 is directed to a highly conserved neutralizing epitope of the F glycoprotein. Its binding affinity is approximately 70-fold greater than that of palivizumab, which is attributed to a 4-fold increase in the association rate and an approximately 17-fold decrease in the dissociation rate. In vitro studies indicate an 18-fold-increased neutralizing activity compared with that of palivizumab. In the cotton rat model, at equivalent serum concentrations, MEDI-524 is 50 to 100 times more potent than palivizumab. It decreases the RSV load in the lungs and, more importantly, in the upper respiratory tract (32). A phase III clinical study comparing MEDI-524 to palivizumab, to determine the safety and efficacy of MEDI-524 in reducing RSV hospitalization in high-risk children, is currently under way.
We have previously shown in the mouse model that RSV alone induced long-term airway disease, defined by persistent airway hyperresponsiveness and chronic inflammatory changes in the lungs for up to 154 days after infection (14). Treatment with palivizumab markedly decreased RSV replication and was associated with significant reduction of inflammatory and clinical markers of disease severity (17). The present study was designed to compare the effects of the two MAbs on different aspects of RSV respiratory infection during both the acute and chronic phases of the disease.
MATERIALS AND METHODS
Animals.
Seven-week-old female, pathogen-free BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in the animal care facility of our institution in separate filter top cages. Mice were housed in groups according to the experimental setup. Their virus-free status was confirmed by use of sentinel mice that were regularly tested for different pathogens as previously described (14, 17, 22). This study was approved by the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center at Dallas.
Virus.
Human RSV stock A2 (RSV A2) was grown and quantified on HEp-2 cells and was prepared and stored as previously described (14, 17). The HEp-2 cells were maintained in Eagle minimal essential medium (EMEM) supplemented with HEPES, glutamine, amphotericin, streptomycin, penicillin G, and 10% fetal bovine serum (10% EMEM). The virus titer was determined by a methylcellulose plaque assay, with a lower level of detection of 1.7 log10 PFU/ml. In addition, the RSV aliquot used for each individual experiment was tested in duplicate at the time of inoculation.
RSV inoculation.
Mice were anesthetized using inhaled methoxyflurane and intranasally inoculated on day 0 with 107.5 PFU/ml of RSV A2 in 100 μl of 10% EMEM (17). Uninfected control animals were sham inoculated with 100 μl of sterile 10% EMEM. Animals were allowed 30 seconds to aspirate the inoculum while held upright until fully recovered from the anesthesia.
Experimental design and sample collection.
Mice were anesthetized with an intraperitoneal injection of 75 mg/kg (body weight) of ketamine and 5 mg/kg of acepromazine before exsanguination by cardiac puncture. Four independent experiments were performed, and each included four to six mice per time point per treatment group. Mice were evaluated on days 1, 5, 28, and 70 after inoculation. Bronchoalveolar lavage (BAL) specimens were obtained as previously described to measure RSV loads (14, 17). Previous experiments in which blood was rinsed from the lungs at the time of sample collection showed that circulating palivizumab did not have a significant impact on decreasing the RSV loads measured in lungs and BAL samples (17). Whole-lung supernatants were also collected to determine RSV loads by plaque assay in parallel with real-time PCR (RLT-PCR) during both the acute (days 1 and 5) and chronic (days 28 and 70) phases of the disease (17). A histological evaluation was performed with a separate set of mice, using whole-lung specimens fixed with 10% buffered formalin solution.
To compare the effects of anti-RSV MAbs on viral replication and disease severity, mice were treated with a single dose (50 mg/kg; 1.25 mg per mouse) of one of the two different neutralizing MAbs against the RSV F protein. Palivizumab was reconstituted as previously described (17). MEDI-524 was provided by MedImmune, Inc. (Gaithersburg, MD); formulated at 102.9 mg/ml in 25 mM histidine-HCl buffer with a pH of 6.0, it was stored at 4°C until administration and was subsequently diluted in phosphate-buffered saline to achieve the desired concentration. Both antibodies were administered intraperitoneally in a total volume of 100 μl, either at 24 h before RSV intranasal inoculation as prophylaxis (Pvz −24 h versus MEDI-524 −24 h) or at 48 h after RSV inoculation as treatment (Pvz +48 h versus MEDI-524 +48 h). The doses of the anti-RSV MAbs used in the present experiments were selected on the basis of our previous studies, which had the purpose of comparing the effects of the MAbs on markers of disease severity rather than characterizing the pharmacokinetic properties of the MAbs in the mouse model (17). Control groups included RSV-infected untreated mice and medium-inoculated (uninfected) mice. In previous experiments with palivizumab, the administration of either phosphate-buffered saline or an IgG1 isotype-matched control antibody, MEDI-507, in RSV-infected mice as either prophylaxis (−24 h) or treatment (+48 h) did not affect any of the parameters evaluated, including RSV loads, BAL cytokine concentrations, lung histopathology, and pulmonary function tests (17).
Pulmonary function tests.
Unrestrained, whole-body plethysmography (Buxco, Troy, NY) was used to assess the degree of airway obstruction (AO) by measuring the baseline enhanced pause (Penh) daily during the first 2 weeks in all groups of mice. Airway hyperresponsiveness (AHR) after challenge with inhaled methacholine (50 mg/ml) was measured once a week from day 14 until day 70 after RSV inoculation. Penh is a value without dimension that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration (represented by the “pause”). Penh values were calculated according to the following formula: Penh = peak expiratory flow/peak inspiratory flow × pause. These values correlate with pulmonary airflow resistance or obstruction (7, 14, 17, 22). Measurements were obtained starting 5 min after the completion of each methacholine nebulization. Penh values measured during this period were averaged and expressed as delta Penh, which represents the difference between the maximum postmethacholine Penh values registered and the baseline Penh. Plethysmography was performed with the groups of mice to be sacrificed for sample collection at each time point and in an additional group of mice that was followed throughout each of the four independent experiments.
Histopathology.
Formalin-fixed lungs were paraffin embedded, sectioned, and stained with hematoxylin and eosin prior to light microscopy examination. The histopathologic scores (HPS) were determined by a pathologist unaware of the infection status of the animals. This HPS system assigns values from 0 to 21 and is based on grading of five different parameters: (i) peribronchiolar and bronchial infiltrates, (ii) bronchiolar and bronchial luminal exudates, (iii) perivascular infiltrates, (iv) monocytes, and (v) parenchymal pneumonia. The higher the score, the greater the inflammatory changes in the lung. This scoring system has been previously validated in the RSV mouse model (14, 17).
RSV quantitative culture and RLT-PCR.
Eighty-percent-confluent, 2-day-old HEp-2 cells were used to measure viral loads in BAL specimens and lung homogenate supernatants (14, 17). Lung homogenate supernatants were processed as described previously (17), and 100-μl samples were used immediately for determination of viral loads by a plaque assay. The remaining supernatant was frozen at −80°C for viral load measurements by RLT-PCR. One sample per mouse was evaluated as a single specimen. Briefly, RSV RNA was extracted using ion-exchange minicolumns (RNeasy mini kit; QIAGEN, Valencia, CA), and cDNA was prepared by reverse transcription using 2.5 μM random hexamers for 10 min at 22°C, 30 min at 42°C, and 5 min at 95°C. Real-time PCR was performed with a Perkin-Elmer/Applied Biosystems 7700 sequence detector (Foster City, CA) using 10 μl cDNA in a total volume of 50 μl master mix with the following run conditions: 1 cycle of 2 min at 50°C and 10 min at 95°C, followed by 50 cycles of 15 seconds at 95°C and 60 seconds at 60°C. Known concentrations of RSV A2 were used to derive a standard curve. Standards and negative controls were run together with each PCR assay. The lower limit of detection of the assay is 5 copies/ml. Quantitative RLT-PCR was used, targeting the conserved region of the RSV N gene. Forward (5′-AGA TCA ACT TCT GTC ATC CAG CAA) and reverse (5′-TTC TGC ACA TCA TAA TTA GGA GTA TCA AT) primers amplified an 85-bp region containing the 25-mer 6-carboxyfluorescein-labeled probe (5′-CAC CAT CCA ACG GAG CAC AGG AGA T), as described elsewhere (6, 31).
To further evaluate the significance of the RSV load measured by RLT-PCR, we conducted a set of experiments comparing mice inoculated with live RSV (106.8 PFU), UV-inactivated RSV (UV cross-linker for 2 h; Fisher Biotech), and control medium (EMEM). Results showed that on day 5, mice inoculated with live RSV had a median of 7.57 (range, 7.48 to 7.68) log10 copies/ml, UV-inactivated RSV had a median of 2.51 (range, 2.15 to 3.01) log10 copies/ml, and medium-inoculated controls had <100 copies/ml (P < 0.001). Likewise, on day 42, mice inoculated with live RSV had a median of 3.99 (range, 3.98 to 4.01) log10 copies/ml, while UV-inactivated RSV had <100 copies/ml and medium controls had <100 copies/ml. These experiments suggest that our ability to detect RSV RNA in the chronic phase of the disease is highly dependent on inoculation with live virus.
Statistical methods.
Differences between groups were tested using the Kruskal-Wallis one-way analysis of variance on ranks, since most of the data did not follow a normal distribution. When this test demonstrated a significant difference between groups (P < 0.05), two different methods of correcting for multiple comparisons were used (Dunn's and Tukey's tests). For all statistical analyses, Sigma Stat 2000 software (SPSS Science, Chicago, IL) was used.
RESULTS
Comparative effects of anti-RSV antibodies on RSV replication determined by quantitative culture and RLT-PCR. (i) Plaque assay tissue culture.
As shown in our previous studies in this mouse model, viral loads in RSV-infected untreated mice significantly increased from day 1 to day 5, both in BAL samples (median [25th to 75th percentile], 2.6 [2.26 to 2.72] PFU/ml log10 on day 1 versus 3.05 [2.62 to 3.6] PFU/ml log10 on day 5; P < 0.01) and in lung specimens (median [25th to 75th percentile], 2.56 [2.4 to 3.05] on day 1 versus 3.49 [3.18 to 3.59] on day 5; P < 0.01), providing evidence of active viral replication.
(a) BAL.
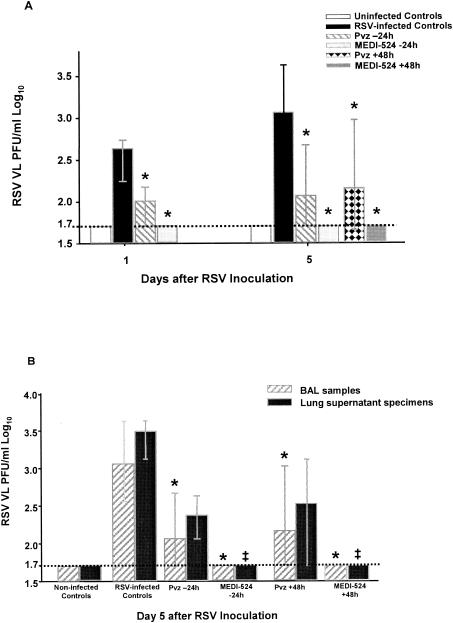
Administration of palivizumab and MEDI-524 resulted in significant reductions of RSV loads in BAL samples at all time points evaluated in all treatment groups. Treatment of mice before inoculation (Pvz −24 h and MEDI-524 −24 h) and after inoculation but before the onset of clinical disease (Pvz +48 h and MEDI-524 +48 h) was effective in significantly decreasing RSV loads in BAL samples compared with RSV-infected untreated controls (P < 0.001) (Fig. 1A). On days 1 and 5, the number of plaques determined by quantitative culture was reduced to below the limit of detection of the assay (1.7 PFU/ml log10) only in mice treated with MEDI-524 at 24 h before or 48 h after RSV inoculation. On days 28 and 70 postinoculation, no virus was detected by the plaque assay.
FIG. 1.
Comparative effects of anti-RSV antibodies on RSV replication determined by quantitative culture of BAL samples and lung supernatant homogenates. Mice were intranasally inoculated with RSV (107.5 PFU/ml) or 10% EMEM (uninfected controls) and treated with a single dose of either palivizumab or MEDI-524 at one of several different time points. On days 1 and 5 after RSV inoculation, mice were sacrificed, and BAL and lung samples from the same mice were harvested (n = 8 to 16). The RSV loads in BAL samples (A) and both BAL samples and lung specimens (B) were determined by plaque assays. (A) The treatment groups consisted of uninfected controls, RSV-infected untreated mice, and RSV-infected mice receiving either palivizumab or MEDI-524 at either −24 h (before) or +48 h (after) with respect to RSV infection. (B) Viral load was significantly higher (P < 0.05) on day 5 in lung specimens than in BAL samples for RSV-infected untreated mice and mice that received palivizumab at −24 h or +48 h of RSV infection. The values are represented as median RSV log10 PFU/ml (with error bars indicating 25th to 75th percentiles) in BAL fluid (*, P < 0.001) or lung supernatant (‡, P < 0.001) by way of Kruskal-Wallis analysis of variance on ranks for comparison with RSV-infected untreated controls. VL, viral load; dashed line, lower limit of detection of the plaque assay.
(b) Lungs.
Lung RSV loads were reduced by >1 log on days 1 and 5 after inoculation in treated mice, regardless of the time of MAb administration or the regimen used.
On day 1, both prophylactic regimens significantly decreased lung RSV loads compared with those of RSV-infected untreated controls (median [25 to 57th percentile], 2.56 [2.4 to 3.05] PFU/ml log10 in RSV-infected untreated controls versus <1.7 [<1.7] PFU/ml log10 in MEDI-524 −24 h and versus <1.7 [1.7 to 1.92] PFU/ml log10 in Pvz −24 h; P < 0.001). At this time point no significant differences were found in RSV loads measured in BAL and lung samples within each group of mice.
On day 5, which corresponds to peak viral replication in this model (14), RSV loads in lung specimens were significantly greater than in BAL samples in RSV-infected untreated mice (P < 0.01) and in mice treated with palivizumab (P < 0.05). Only MEDI-524 significantly reduced RSV loads in both BAL and lung specimens to below the limit of detection of the plaque assay (Fig. 1B).
(ii) RLT-PCR.
To further characterize the dynamics of RSV infection, we measured viral loads in lung supernatants by RLT-PCR in parallel with plaque assays in two independent experiments, each including four mice per time point per treatment group. Following a similar pattern as for the plaque assays, RSV RNA loads measured by RLT-PCR peaked around day 5 after inoculation (5, 6). On day 1, mice treated with either MEDI-524 or Pvz at −24 h had lower RSV RNA loads, but only in one experiment was the reduction statistically significant compared with that for the RSV-infected untreated mice. On day 5, MEDI-524 given 24 h before inoculation consistently decreased RSV RNA loads, compared with RSV-infected untreated controls (P < 0.001) (Table 1). Palivizumab treatment at −24 h also showed reduced RSV RNA, but the difference did not reach statistical significance (Table 1). In contrast to RSV loads measured by plaque assay, which became undetectable by day 7 after inoculation, RSV RNA loads measured by RLT-PCR remained consistently positive for 70 days in lung supernatants. At 4 weeks after inoculation, there was a trend towards lower RSV RNA loads in mice treated with palivizumab at −24 h or +48 h. MEDI-524 given at −24 h or +48 h significantly decreased the RSV RNA loads compared to those of RSV-infected untreated controls. Ten weeks after inoculation, RSV RNA was still detected in all RSV-infected mice, although at much lower concentrations. Mice treated with MEDI-524 at −24 h showed a clear trend toward lower RSV RNA loads, but the reduction did not achieve statistical significance (Table 1). All uninfected controls showed consistently negative values by RSV real-time PCR at all time points evaluated.
TABLE 1.
Comparative effect of anti-RSV neutralizing monoclonal antibodies on lung RSV-RNA loads, determined by real-time PCR
| Mouse group | Acute
|
Chronic
|
||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 VL | P | Day 5 VL | P | Day 28 VL | P | Day 70 VL | P | |
| Expt 1 | ||||||||
| RSV infected, untreated | 6.8 ± 0.18 | 6.5 ± 0.17 | 156 ± 10.2 | |||||
| Pvz-24 h | 6.23 ± 0.34 | 0.001 | 6.03 ± 0.44 | NSb | 226 ± 56.2 | NS | ||
| MEDI-524 −24 h | 5.72 ± 0.28 | 0.001 | 5.5 ± 0.29 | <0.001 | 44.7 ± 89.4 | NS | ||
| Pvz +48 h | 6.76 ± 0.39 | NS | 271 ± 60.4 | NS | ||||
| MEDI-524 +48 h | 6.49 ± 0.31 | NS | 396 ± 185 | NS | ||||
| Expt 2 | ||||||||
| RSV infected, untreated | 8.00 ± 0.77 | 8.6 ± 0.40 | 2,825 ± 1,038 | 54.3 ± 42.2 | ||||
| Pvz-24 h | 7.92 ± 0.26 | NS | 7.42 ± 0.03 | 0.005 | 1,429 ± 715 | NS | 28.1 ± 3.07 | NS |
| MEDI-524 −24 h | 7.26 ± 0.19 | NS | 7.22 ± 0.56 | 0.005 | 1,121 ± 684 | 0.03 | 5.17 ± 4.58 | NS |
| Pvz +48 h | 7.97 ± 0.33 | NS | 1,192 ± 764 | NS | 31.1 ± 29.4 | NS | ||
| MEDI-524 +48 h | 7.57 ± 0.17 | 0.005 | 787 ± 537 | 0.03 | 6.03 ± 6.96 | NS | ||
Data are presented as means ± standard deviations of log10 RNA copies/ml of lung supernatant on days 1 and 5, and as means ± standard deviations of RNA copies/ml on days 28 and 70 from two independent experiments using four mice per time point. Comparisons were made with RSV-infected, untreated controls by one-way analysis of variance.
NS, not significant.
Effects of anti-RSV antibodies on lung inflammation.
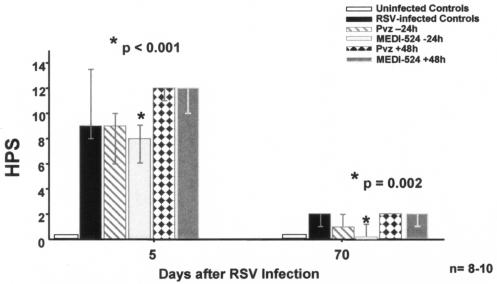
The RSV-infected untreated mice, compared to sham-inoculated controls, showed significantly greater HPS, with dense inflammatory infiltrates and severe pneumonia which peaked on day 5 after inoculation (Fig. 2). Although the acute inflammatory infiltrates gradually declined and changed over time, MEDI-524 given at −24 h significantly decreased lung inflammation during both the acute (day 5) and chronic phases of the disease. At week 10, the lung inflammatory infiltrates partially involved the larger vessels and airways and were composed mostly of lymphocytes, macrophages, and plasma cells. No eosinophils were observed. At this late time point (week 10), the lung HPS of mice that received MEDI-524 at −24 h were comparable to those of the uninfected controls. In contrast, neither palivizumab given at −24 h or +48 h nor MEDI-524 at +48 h significantly reduced lung inflammation (Fig. 3). Since whole-lung specimens were used to measure either RSV RNA loads or HPS, we could not determine correlations between RSV RNA loads and lung HPS.
FIG. 2.
Impact of treatment with anti-RSV neutralizing MAbs on lung HPS of mice infected with RSV. Mice were inoculated with RSV and treated as described in Materials and Methods. On days 5 and 70, lung specimens were obtained and subsequently fixed and stained for further evaluation by a pathologist unaware of the status of the animals. Bars represent the results of three independent experiments with 8 to 10 mice per time point in each treatment group. Treatment groups consisted of uninfected controls, RSV-infected untreated mice, and RSV-infected mice treated with either palivizumab or MEDI-524 at −24 h (before) or at +48 h (after) with respect to RSV infection. Values are shown as medians, with error bars indicating 25th to 75th percentiles. *, P of <0.001 (day 5) and P of 0.002 (day 70) by Kruskal-Wallis analysis of variance on ranks for comparison with RSV-infected untreated controls.
FIG. 3.
Effect of MEDI-524 on lung histopathology of RSV-infected mice. Lung histopathology results for days 5 and 70 postinfection of sham-inoculated controls, RSV-infected untreated controls, and RSV-infected mice treated with MEDI-524 at −24 h are shown. Hematoxylin and eosin stain. Magnification, ×40.
Effects of the anti-RSV antibodies on airway obstruction and airway hyperresponsiveness. (i) Airway obstruction.
In the acute phase of the disease, RSV-infected untreated mice, compared with uninfected controls, developed significant AO as defined by increased spontaneous Penh values, with two peaks on days 1 and 5. The AO peak on day 5 correlated with the worst HPS and clinical signs of disease in RSV-infected mice (P < 0.001; r = 0.4). The regimen of palivizumab at −24 h significantly reduced AO on day 5 but not on day 1, while mice that received MEDI-524 at −24 h developed significantly less AO on both days 1 and 5 (P < 0.001). Mice treated with either MAb at −24 h remained clinically asymptomatic throughout the disease course. On visual inspection, mice that received the MAbs before inoculation behaved like uninfected mice, with normal activities, shiny coats, and general well-being. The administration of either anti-RSV MAb 48 h after inoculation did not have any impact on AO (Table 2). After day 5, AO in all groups of mice gradually declined over time, and Penh returned to baseline values by day 14.
TABLE 2.
AO in RSV-infected mice treated with different anti-RSV monoclonal antibodies
| Mouse group | Airway obstructiona
|
||||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| Uninfected controls | 0.52 (0.48-0.58)* | 0.52 (0.47-0.63)* | 0.49 (0.45-0.59)* | 0.52 (0.47-0.57)* | 0.50 (0.47-0.57)* |
| RSV infected, untreated | 1.77 (1.62-2.43) | 0.76 (0.61-1.32) | 0.70 (0.64-0.83) | 0.67 (0.61-0.71) | 1.11 (1.01-2.03) |
| RSV Pvz −24 h | 1.49 (1.13-1.96) | 0.65 (0.60-0.80) | 0.45 (0.44-0.48)* | 0.53 (0.45-0.58)* | 0.59 (0.55-0.75)* |
| RSV MEDI-524 −24 h | 0.73 (0.61-1.02)* | 0.58 (0.54-0.64)* | 0.52 (0.49-0.66) | 0.54 (0.47-0.57)* | 0.55 (0.52-0.61)* |
| RSV Pvz +48 h | 1.77 (1.09-2.71) | 0.62 (0.53-0.89) | 0.54 (0.50-0.57)* | 0.57 (0.52-0.62) | 1.08 (0.91-1.30) |
| RSV MEDI-524 +48 h | 1.67 (1.07-2.85) | 0.57 (0.55-0.94) | 0.55 (0.51-0.61) | 0.57 (0.52-0.62) | 0.99 (0.83-1.65) |
Values represent basal Penh as a measurement of airway obstruction. Penh values are shown as medians and 25th to 75th percentiles. *, P of <0.001 for comparison between RSV-infected untreated mice and the other regimens (n = 12 to 16 per group).
(ii) AHR.
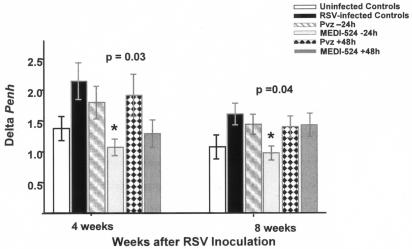
Aerosolized-methacholine challenge elicited increases in Penh in RSV-infected untreated mice, demonstrated by greater postmethacholine delta Penh values than those of uninfected controls (14, 17). Although mice treated with all different anti-RSV MAb regimens showed a trend toward less AHR compared with RSV-infected untreated mice, only MEDI-524 at −24 h significantly decreased AHR at 4 and 8 weeks postinoculation (Fig. 4).
FIG. 4.
Effects of anti-RSV neutralizing antibodies on AHR. To assess AHR, mice were challenged weekly with aerosolized methacholine, and delta Penh was recorded for up to 10 weeks postinfection. Bars represent means ± standard errors of the means for 12 to 16 mice per group. *, P of 0.03 and 0.04 by one-way analysis of variance for comparison with RSV-infected untreated controls at 4 and 8 weeks after infection, respectively.
DISCUSSION
Despite the fact that approximately one-third (25, 27) of patients who recovered from acute RSV bronchiolitis developed recurrent respiratory problems, specific therapeutic options for acute RSV infection are limited, and currently only the antiviral agent ribavirin is approved for this indication (2). The surface glycoproteins G and F are the major proteins involved in RSV infection. The F glycoprotein appears more important for induction of protective immunity and is associated with production of high serum neutralizing antibody response (8); therefore, this glycoprotein has been a major focus for therapeutic intervention in RSV disease (15, 21). Previous studies have shown that RSV loads measured shortly after hospitalization in nasal secretions of infants with RSV bronchiolitis were an independent predictor of disease severity (4). Passive administration of anti-RSV neutralizing antibody against the F protein is safe and effective for prevention of severe RSV disease in animal models and humans (11, 13, 17). However, the administration of the anti-RSV MAb as treatment for established RSV infection did not modify the clinical course of the disease, despite significant reductions of RSV loads measured in tracheal aspirates of children intubated for severe RSV disease (16). A double-blind, randomized, placebo control study assessing the safety and tolerance of intravenous palivizumab versus placebo in children admitted to the hospital with RSV lower respiratory tract infection (LRTI) showed a trend for better outcomes in the palivizumab arm (15 mg/kg) than in the placebo recipients. It should be noted that these children were treated within 3 days of the initiation of symptoms and were not intubated (23). Since none of the aforementioned studies were adequately powered to detect significant differences in clinical outcomes, the efficacy of palivizumab for treatment of RSV LRTI remains unclear.
The results of the present study in the mouse model demonstrate that passive administration of two different anti-RSV neutralizing MAbs against the F glycoprotein effectively reduced RSV replication when administered as either preexposure prophylaxis or early treatment. MEDI-524 had superior neutralizing activity against RSV compared with palivizumab, as measured by plaque assay. It reduced RSV loads to below the limit of detection of the assay in both BAL and lung specimens, regardless of the timing of administration and despite the greater viral loads detected in lung samples (A. Mejías, S. Chávez-Bueno, A. M. Ríos, M. Fonseca-Aten, E. Peromingo, P. Soni, K. Olsen, A. M. Gómez, D. Pfarr, H. Wu, P. A. Kiener, H. S. Jafri, and O. Ramilo, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. V-1687, 2004). In this model, RSV could not be cultured after day 7 postinfection, but genomic RSV RNA was detected by RLT-PCR in lung homogenates for up to 10 weeks after inoculation.
The evidence that the same or different strains of RSV can cause repeated infections throughout patients' lifetimes (29) suggests that RSV does not induce durable immunity. Recent studies of animal models have shown that RSV infection may result in “latency” or persistence in the lower respiratory tract (6, 24). The persistence of viral RNA, regardless of the presence of specific neutralizing antibodies, has been shown not only in animal models of RSV (6, 12, 24) but also with human metapneumovirus, a new member of the Metapneumovirus genus closely related to RSV (1). The significance of the RSV RNA detected in lung specimens during the chronic phase of the disease is yet to be defined. It is possible, however, that viral persistence could contribute to the delayed effects of severe RSV disease, such as chronic airway inflammation, by means of low-grade replication in the lower respiratory tract as suggested by Schwarze et al., who recovered low levels of infectious RSV from the lungs of mice depleted of T cells 150 days postinfection (24). Our results demonstrate that the administration of the more potent neutralizing MAb, MEDI-524, significantly decreased the number of RSV RNA copies during both the acute and the chronic phases of the disease. To our knowledge, this is the first demonstration that a MAb can reduce the RSV RNA load in the lungs and that this reduction was associated with improved markers of disease severity in RSV infection. Indeed, mice treated with MEDI-524 at −24 h, compared with those treated with palivizumab, showed significantly less lung inflammation not only on day 5, when mice showed the worst degree of pneumonia, but more importantly in the chronic phase. On day 70 after RSV infection, the lung pathology of mice treated with MEDI-524 at −24 h was very similar to that of the uninfected controls (HPS median [range], 0 [0 to 1] in MEDI-524-treated mice at −24 h versus 0 [0, 0] in uninfected controls) (Fig. 2). The administration of the anti-RSV MAbs at 48 h after inoculation also showed a trend toward decreased RSV RNA loads but did not modify the lung inflammation and disease course. Taken together, these results indicate that the timing of administration of the MAb has a significant impact on the markers of disease severity.
These findings are similar to previous experiences reported for children and animal models when palivizumab was administered after infection (17, 18, 20, 23) and suggest that although direct viral cytopathology plays an important role in initiating RSV-induced disease, it appears that much of the lung injury caused by this infection is the result of the host inflammatory response. Hence, when the MAbs were given after infection, they could not reverse the respiratory pathology that had already occurred. Despite the enhanced neutralizing activity of MEDI-524, viral RNA was still detectable in both the acute and chronic phases of the disease, although at significantly lower concentrations. These results suggest that either the anti-F neutralizing antibody was not completely effective or that in addition to the F glycoprotein, there are other mechanisms that contribute to RSV-induced disease. It appears that there are at least two types of human antibody responses to RSV infection: a response to a mature envelope on virions that is neutralizing, and a response to immature forms of envelope that is not. Overlap between the responses is limited. Parren et al. have suggested that viral evasion could be explained in part by the expression by the virus of a conformationally altered mature envelope protein that is less susceptible to the anti-F glycoprotein neutralizing activity (19). Recently, it has been suggested that RSV may resist the host antiviral response by modulating the type I interferon pathway by mechanisms associated with the expression of the nonstructural NS1/NS2 proteins (26). In addition, it is known that the RSV G glycoprotein exhibits extensive genetic diversity that may contribute to antigenic variation of neutralizing epitopes. The G glycoprotein has also been associated with altered chemokine expression in pulmonary leukocytes as well as altered pulmonary leukocyte trafficking in response to infection (28, 30).
Little is known about the long-term consequences of RSV prophylaxis in high-risk children. Preliminary results from a prospective case-cohort multicenter study suggested that preterm infants who received palivizumab during the first RSV season had a lower risk of subsequent recurrent wheezing at the 12-month follow-up compared with those who did not receive prophylaxis (E. A. Simoes, X. Carbonell-Estrany, J. Kimpen, C. H. L. Rieger, D. Morris, P. F. Pollack, and J. R. Groothuis, 14th European Respiratory Society Annual Congress, abstr. 1349, 2004).
Although children hospitalized for RSV LRTI after receiving palivizumab prophylaxis had lower nasopharyngeal RSV loads than similar infants not receiving palivizumab, they still carried the virus in the nasal mucosa (9). When assessing the pulmonary dynamics in our experiments, the administration of either palivizumab or MEDI-524 as preexposure prophylaxis markedly decreased the signs of RSV disease objectively measured with the noninvasive plethysmograph. Both “prophylactic” groups of mice remained asymptomatic throughout the course of disease, and the baseline Penh values were comparable to those of uninfected controls. Only MEDI-524 at −24 h was able to significantly decrease the peak of AO on day 1. Indeed, these mice developed significantly less long-term AHR at 4 and 8 weeks after the infection. There was a trend toward lower AHR in the mice that received MEDI-524 at +48 h or palivizumab at −24 h and +48 h although it was not significant. It is possible that the use of the multiple-test comparisons for the statistical analysis could have influenced these results. Thus, it could be argued that if the experiment included fewer treatment groups, possibly other MAb regimens would have demonstrated significant reductions of AHR as well. Taken together, these results emphasize the importance of early events in RSV infection in determining the long-term consequences of the disease. Despite the fact that the new neutralizing MAb could not totally prevent the establishment of the infection, it certainly modulated its consequences.
In summary, the present study demonstrated the superiority of MEDI-524 over palivizumab in preventing the manifestations of RSV disease. The greater reductions in RSV loads in the lower respiratory tract achieved during the acute and chronic phases of the disease resulted in further attenuation of the lung inflammation and long-term airway disease. Studies to determine whether the anti-RSV antibodies can decrease RSV RNA loads in the respiratory tracts of children and whether those reductions are associated with diminished acute and long-term morbidity induced by RSV are warranted.
Acknowledgments
This work was supported in part by grants by MedImmune, Inc. (to O.R. and H.J.) and the American Lung Association (to H.J.). A.M. was supported in part by the Pediatric Fellowship Award in Viral Respiratory Infectious Diseases from MedImmune, Inc., presented at the Pediatric Academic Societies Annual Meeting, and the RGK Foundation fellowship in Infectious Diseases at Children's Medical Center of Dallas.
We thank Aneta Wozniakowski for technical assistance with RLT-PCR and tissue culture assays and Dave Pfarr and H. Wu at MedImmune for preparation of the monoclonal antibodies.
REFERENCES
- 1.Alvarez, R., K. S. Harrod, W.-J. Shieh, S. Zaki, and R. A. Tripp. 2004. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J. Virol. 78:14003-14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2003. American Academy of Pediatrics. 2003. 2003 report of the Committee on Infectious Diseases, 26th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
- 3.Boyce, T. G., B. G. Mellen, E. F. Mitchel, Jr., P. F. Wright, and M. R. Griffin. 2000. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J. Pediatr. 137:865-870. [DOI] [PubMed] [Google Scholar]
- 4.Buckingham, S. C., A. J. Bush, and J. P. Devincenzo. 2000. Nasal quantity of respiratory syncytical virus correlates with disease severity in hospitalized infants. Pediatr. Infect. Dis. J. 19:113-117. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, E. M., S. L. Kunkel, R. M. Strieter, and N. W. Lukacs. 1998. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J. Immunol. 161:7047-7053. [PubMed] [Google Scholar]
- 6.Chavez-Bueno, S., A. Mejias, A. M. Gomez, K. D. Olsen, A. M. Rios, M. Fonseca-Aten, O. Ramilo, and H. S. Jafri. 2005. Respiratory syncytial virus-induced acute and chronic airway disease is independent of genetic background: an experimental murine model. Virol. J. 2:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chunn, J. L., H. W. Young, S. K. Banerjee, G. N. Colasurdo, and M. R. Blackburn. 2001. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J. Immunol. 167:4676-4685. [DOI] [PubMed] [Google Scholar]
- 8.Connors, M., P. L. Collins, C.-Y. Firestone, and B. R. Murphy. 1991. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 65:1634-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVincenzo, J. P., J. Aitken, and L. Harrison. 2003. Respiratory syncytial virus (RSV) loads in premature infants with and without prophylactic RSV fusion protein monoclonal antibody. J. Pediatr. 143:123-126. [DOI] [PubMed] [Google Scholar]
- 10.DeVincenzo, J. P., C. B. Hall, D. W. Kimberlin, P. J. Sanchez, W. J. Rodriguez, B. A. Jantausch, L. Corey, J. S. Kahn, J. A. Englund, J. A. Suzich, F. J. Palmer-Hill, L. Branco, S. Johnson, N. K. Patel, and F. M. Piazza. 2004. Surveillance of clinical isolates of respiratory syncytial virus for palivizumab (Synagis)-resistant mutants. J. Infect. Dis. 190:975-978. [DOI] [PubMed] [Google Scholar]
- 11.Feltes, T. F., A. K. Cabalka, H. C. Meissner, F. M. Piazza, D. A. Carlin, F. H. Top, Jr., E. M. Connor, and H. M. Sondheimer. 2003. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J. Pediatr. 143:532-540. [DOI] [PubMed] [Google Scholar]
- 12.Hegele, R. G., S. Hayashi, A. M. Bramley, and J. C. Hogg. 1994. Persistence of respiratory syncytial virus genome and protein after acute bronchiolitis in guinea pigs. Chest 105:1848-1854. [DOI] [PubMed] [Google Scholar]
- 13.The IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531-537. [PubMed] [Google Scholar]
- 14.Jafri, H. S., S. Chavez-Bueno, A. Mejias, A. M. Gomez, A. M. Rios, S. S. Nassi, M. Yusuf, P. Kapur, R. D. Hardy, J. Hatfield, B. B. Rogers, K. Krisher, and O. Ramilo. 2004. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J. Infect. Dis. 189:1856-1865. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, S., C. Oliver, G. A. Prince, V. G. Hemming, D. S. Pfarr, S. C. Wang, M. Dormitzer, J. O'Grady, S. Koenig, J. K. Tamura, R. Woods, G. Bansal, D. Couchenour, E. Tsao, W. C. Hall, and J. F. Young. 1997. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 176:1215-1224. [DOI] [PubMed] [Google Scholar]
- 16.Malley, R., J. DeVincenzo, O. Ramilo, P. H. Dennehy, H. C. Meissner, W. C. Gruber, P. J. Sanchez, H. Jafri, J. Balsley, D. Carlin, S. Buckingham, L. Vernacchio, and D. M. Ambrosino. 1998. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J. Infect. Dis. 178:1555-1561. [DOI] [PubMed] [Google Scholar]
- 17.Mejías, A., S. Chávez-Bueno, A. M. Ríos, J. Saavedra-Lozano, M. Fonseca Aten, J. Hatfield, P. Kapur, A. M. Gómez, H. S. Jafri, and O. Ramilo. 2004. Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model. Antimicrob. Agents Chemother. 48:1811-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottolini, M. G., S. J. Curtis, D. D. Porter, A. Mathews, J. Y. Richardson, V. G. Hemming, and G. A. Prince. 2002. Comparison of corticosteroids for treatment of respiratory syncytial virus bronchiolitis and pneumonia in cotton rats. Antimicrob. Agents Chemother. 46:2299-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parren, P. W., P. Poignard, H. J. Ditzel, R. A. Williamson, and D. R. Burton. 2000. Antibodies in human infectious disease. Immunol. Res. 21:265-278. [DOI] [PubMed] [Google Scholar]
- 20.Prince, G. A., A. Mathews, S. J. Curtis, and D. D. Porter. 2000. Treatment of respiratory syncytial virus bronchiolitis and pneumonia in a cotton rat model with systemically administered monoclonal antibody (Palivizumab) and glucocorticosteroid. J. Infect. Dis. 182:1326-1330. [Google Scholar]
- 21.Razinkov, V., C. Huntley, G. Ellestad, and G. Krishnamurthy. 2002. RSV entry inhibitors block F-protein mediated fusion with model membranes. Antivir. Res. 55:189-200. [DOI] [PubMed] [Google Scholar]
- 22.Ríos, A. M., A. Mejías, S. Chávez-Bueno, M. Fonseca-Aten, K. Katz, J. Hatfield, A. M. Gómez, H. S. Jafri, G. H. McCracken, Jr., O. Ramilo, and R. D. Hardy. 2004. Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection. Antimicrob. Agents Chemother. 48:2897-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saez-Llorens, X., M. T. Moreno, O. Ramilo, P. J. Sanchez, F. H. Top, Jr., and E. M. Connor. 2004. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 23:707-712. [DOI] [PubMed] [Google Scholar]
- 24.Schwarze, J., D. R. O'Donnell, A. Rohwedder, and P. J. Openshaw. 2004. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am. J. Respir. Crit. Care Med. 169:801-805. [DOI] [PubMed] [Google Scholar]
- 25.Sigurs, N., P. M. Gustafsson, R. Bjarnason, F. Lundberg, S. Schmidt, F. Sigurbergsson, and B. Kjellman. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 171:137-141. [DOI] [PubMed] [Google Scholar]
- 26.Spann, K. M., K.-C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and gamma interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein, R. T., D. Sherrill, W. J. Morgan, C. J. Holberg, M. Halonen, L. M. Taussig, A. L. Wright, and F. D. Martinez. 1999. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354:541-545. [DOI] [PubMed] [Google Scholar]
- 28.Sullender, W. M. 2000. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 13:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullender, W. M., M. A. Mufson, G. A. Prince, L. J. Anderson, and G. W. Wertz. 1998. Antigenic and genetic diversity among the attachment proteins of group A respiratory syncytial viruses that have caused repeat infections in children. J. Infect. Dis. 178:925-932. [DOI] [PubMed] [Google Scholar]
- 30.Tripp, R. A., L. Jones, and L. J. Anderson. 2000. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J. Virol. 74:6227-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Woensel, J. B., R. Lutter, M. H. Biezeveld, T. Dekker, M. Nijhuis, W. M. van Aalderen, and T. W. Kuijpers. 2003. Effect of dexamethasone on tracheal viral load and interleukin-8 tracheal concentration in children with respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 22:721-726. [DOI] [PubMed] [Google Scholar]
- 32.Wu, H., D. S. Pfarr, Y. Tang, L. L. An, N. K. Patel, J. D. Watkins, W. D. Huse, P. A. Kiener, and J. F. Young. 2005. Ultra-potent antibodies against respiratory syncytial virus: effects of binding kinetics and binding valence on viral neutralization. J. Mol. Biol. 350:126-144. [DOI] [PubMed] [Google Scholar]