Abstract
Cpl-1, a pneumococcal phage lytic enzyme, was tested in rats with experimental endocarditis due to Streptococcus pneumoniae WB4. High-dose regimen Cpl-1 eliminated pneumococci from blood within 30 min and decreased bacterial titers in vegetations (>4 log10 CFU/g) within 2 h. Rapid bacterial lysis induced by Cpl-1 treatment increased cytokine secretion noticeably.
Streptococcus pneumoniae, an important human pathogen, is becoming increasingly resistant to multiple classes of antimicrobial agents (6, 10). Alternative treatment strategies, therefore, need to be explored. Lysin Cpl-1, a pneumococcal phage lytic enzyme, is a muramidase that rapidly kills these bacteria (2, 7, 8). Hence, Cpl-1 is a potential therapeutic agent against multidrug-resistant pneumococci. In this study, the activity of Cpl-1 against a multidrug-resistant S. pneumoniae strain was assessed in vitro and in experimental endocarditis in rats.
The S. pneumoniae WB4 isolate, resistant to penicillin and erythromycin, was used (1). The MICs of Cpl-1 and vancomycin for this strain were 16 and 0.12 μg/ml, respectively. S. pneumoniae WB4 was grown either in brain heart infusion (BHI; Difco Laboratories, Detroit, MI) or on tryptic soy agar plates (Difco) supplemented with 5% sheep blood in 5% CO2 at 37°C.
Time-kill curves were determined with Cpl-1 at 100 μg/ml in either 50 mM phosphate buffer (pH 7.0) (buffer) or BHI at 37°C. Both the turbidity at an optical density at 600 nm and cell viability were monitored for 2 h. In time-kill experiments, Cpl-1 eradicated ≥6 log10 CFU/ml of S. pneumoniae WB4 within 15 min in both buffer and BHI medium.
Production and purification of Cpl-1 have been described elsewhere (9). The half-life of Cpl-1 in rat plasma was determined after administration of an intravenous (i.v.) bolus of 125 mg of Cpl-1/kg of body weight. Cpl-1 concentrations were measured over a 2-h period by spot densitometry on a Western blot and compared to standard Cpl-1 concentrations (8). In rats, the Cpl-1 half-life was approximately 20 min.
Sterile aortic vegetations were produced in rats and infusion pumps to deliver Cpl-1 were installed in rats as described previously (4, 5). Bacterial endocarditis was induced 24 h later by i.v. challenge of the animals with 107 CFU log-phase pneumococci. Animals were randomized for treatment, starting 16 h after infection, with Cpl-1 at a “low-continuous” dose (i.v. bolus of 10 mg/kg, followed by continuous infusion of 5 mg/kg/h for 6 h). Control rats received buffer. Rats receiving Cpl-1 therapy were sacrificed at 30 min or 2 or 6 h, those receiving buffer were killed at 0 (baseline), 2, and 6 h. Quantitative blood and vegetation cultures were performed, and bacterial densities were expressed as log10 CFU per milliliter or gram of tissue, respectively. In vivo data were analyzed by either Fisher's exact test or the nonparametric Mann-Whitney test. A P of <0.05 was considered to be statistically significant.
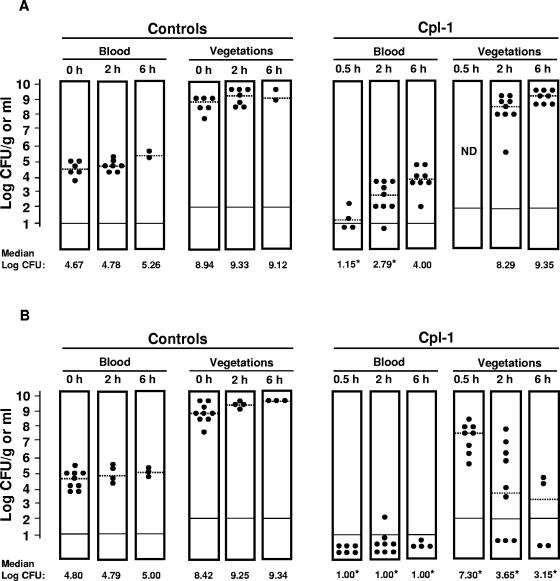
Figure 1A shows therapeutic results. At treatment onset, both the blood samples and vegetations of control rats were heavily infected. Colony counts progressively increased in both compartments with time. In infected rats, the “low-continuous” dose of Cpl-1 produced a decrease of >3 log10 CFU/ml in blood within 30 min. Bacterial titers in blood were still significantly lower than in controls for 2 h but reached values comparable to pretreatment counts after 6 h. Vegetation bacterial densities were not affected by the treatment. No resistant bacteria were detected when the persistent pneumococci were retested for Cpl-1 susceptibility in vitro.
FIG. 1.
Dose response to Cpl-1 in rats infected with the multidrug-resistant S. pneumoniae WB4. (A) “Low-continuous infusion” (bolus of 10 mg/kg, followed by a continuous infusion of 5 mg/kg/h for 6 h). (B) “High-continuous infusion” (bolus of 250 mg/kg, followed by continuous infusion of 250 mg/kg/h for 6 h). Each black circle corresponds to a single animal. Dashed lines represent the median bacterial densities. Solid black lines at the bottom of the columns indicate the bacterial detection limit. Values that were significantly different from the values for controls at treatment onset (P < 0.05) are indicated by asterisks.
These results suggested that Cpl-1 efficacy could be improved by the administration of higher doses. Thus, in the following set of experiments, rats received 25 times more Cpl-1, a “high-continuous” dose (i.v. bolus of 250 mg/kg, followed by continuous infusion of 250 mg/kg/h for 6 h). As shown in Fig. 1B, no pneumococci were detected in blood 30 min after therapy and a nearly complete bacterial eradication was documented over the next 6 h. Moreover, vegetation bacterial titers decreased significantly as early as 30 min after treatment.
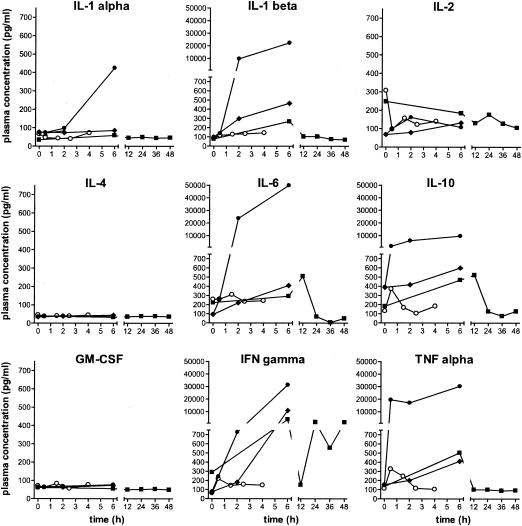
Since cytokine release had never been measured before after very rapid bacterial killing with Cpl-1, we performed multiplex cytokine enzyme-linked immunosorbent assays on rat plasma samples, which were separated from the blood samples used for bacterial counts and kept at −80°C until assayed. Cytokine activity was determined in duplicate samples with a Bio-Plex rat cytokine nineplex panel (Bio-Rad, Hercules, CA). Beads were analyzed with a Luminex 100 workstation (Luminex Corporation, Austin, TX) using Starstation software (Applied Cytometry Systems, Inc., Sacramento, CA). Untreated pneumococcal endocarditis induced the release of interleukin-1α (IL-1α), IL-1β, IL-6, IL-10, gamma interferon, and tumor necrosis factor, but not IL-2, IL-4, or granulocyte-macrophage colony-stimulating factor (Fig. 2). The rapid killing of S. pneumoniae and the presumably massive release of cell wall fragments with the high-dose regimen of Cpl-1 accentuated this cytokine secretion.
FIG. 2.
Cytokine measurements in plasma. Several interleukins, granulocyte-macrophage colony-stimulating factor (GM-CSF), gamma interferon (IFN gamma), and tumor necrosis factor alpha (TNF alpha) were measured. Open circles, uninfected rats that received a “high-continuous” infusion of Cpl-1; filled diamonds, infected rats that were not treated; filled circles, infected rats treated with “high-continuous” Cpl-1; filled squares, infected rats treated with vancomycin.
To put the bactericidal efficacy and the cytokine release with Cpl-1 into perspective, a control treatment with vancomycin was performed. Vancomycin was administered as previously described (3) for 48 h. Vancomycin-treated rats were sacrificed 6 h after the start of therapy and 8 h after the trough level of the last dose.
Vancomycin cleared pneumococci from blood in only one of five rats after 6 h of therapy (significantly different from values obtained with high-dose Cpl-1 [P < 0.05]). The blood had a median bacterial titer of 1.9 log10 CFU/ml (significantly different from values obtained with baseline titers at 0 h [P < 0.05] and from values obtained with high-dose Cpl-1 at 6 h [P = 0.06]). At 48 h, all eight rat blood cultures were negative. In the vegetations, titers decreased to 5.3 log10 CFU/g after 6 h (significantly different from values obtained with controls [P < 0.05] and from values obtained with high-dose Cpl-1 at 6 h [P = 0.06]), and seven of eight valve samples were culture negative at 48 h, with the remaining valve containing 3.1 log10 CFU/g. While vancomycin treatment was efficient, Cpl-1 seemed to act much faster, although the small number of animals made the difference short of significant. Vancomycin induced a less pronounced cytokine release than Cpl-1 at high doses (Fig. 2), likely due to the slower release of cell wall fragments following bacterial killing. Indeed, both the optical density at 600 nm and viable bacterial counts in vitro at 30 min were two to three times lower with either 5 or 40 μg/ml of vancomycin than with 100 μg/ml of Cpl-1.
Our findings support the use of Cpl-1 as an approach against S. pneumoniae infections not only in places where it can spread rapidly, e.g., in blood, but also in deep foci where bacteria might be protected by a physical hindrance. However, the doses of Cpl-1 necessary to achieve therapeutic concentrations in humans and its safety remain to be investigated.
Acknowledgments
We thank Marlyse Giddey and Jacques Vouillamoz for excellent technical assistance. We acknowledge Melissa Pope and Laurence Vachot for generous help with the Luminex experiments.
This work was supported by grants 3200-6571/2 and 3200B0-100667 of the Swiss National Fund for Scientific Research and a grant from the Defense Advanced Research Project Agency (DARPA) (to V.A.F.).
REFERENCES
- 1.Cottagnoud, P., M. Cottagnoud, F. Acosta, L. Flatz, F. Kuhn, A. Stucki, and J. Entenza. 2003. Meropenem prevents levofloxacin-induced resistance in penicillin-resistant pneumococci and acts synergistically with levofloxacin in experimental meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 22:656-662. [DOI] [PubMed] [Google Scholar]
- 2.Djurkovic, S., J. M. Loeffler, and V. A. Fischetti. 2005. Synergistic killing of Streptococcus pneumoniae with the bacteriophage lytic enzyme Cpl-1 and penicillin or gentamicin depends on the level of penicillin resistance. Antimicrob. Agents Chemother. 49:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Entenza, J. M., Y. A. Que, J. Vouillamoz, M. P. Glauser, and P. Moreillon. 2001. Efficacies of moxifloxacin, ciprofloxacin, and vancomycin against experimental endocarditis due to methicillin-resistant Staphylococcus aureus expressing various degrees of ciprofloxacin resistance. Antimicrob. Agents Chemother. 45:3076-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fluckiger, U., P. Moreillon, J. Blaser, M. Bickle, M. P. Glauser, and P. Francioli. 1994. Simulation of amoxicillin pharmacokinetics in humans for the prevention of streptococcal endocarditis in rats. Antimicrob. Agents Chemother. 38:2846-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heraief, E., M. P. Glauser, and L. R. Freedman. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs, M. R. 2004. Streptococcus pneumoniae: epidemiology and patterns of resistance. Am. J. Med. 117(Suppl. 3A):3S-15S. [DOI] [PubMed] [Google Scholar]
- 7.Jado, I., R. Lopez, E. Garcia, A. Fenoll, J. Casal, and P. Garcia on behalf of the Spanish Pneumococcal Infection Study Network. 2003. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Agents 52:967-973. [DOI] [PubMed] [Google Scholar]
- 8.Loeffler, J. M., S. Djurkovic, and V. A. Fischetti. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 71:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeffler, J. M., and V. A. Fischetti. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 47:375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrag, S. J., L. McGee, C. G. Whitney, B. Beall, A. S. Craig, M. E. Choate, J. H. Jorgensen, R. R. Facklam, K. P. Klugman, and the Active Bacterial Core Surveillance Team. 2004. Emergence of Streptococcus pneumoniae with very-high-level resistance to penicillin. Antimicrob. Agents Chemother. 48:3016-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]