Abstract
Pleconaril, a specific inhibitor of human picornaviruses, showed therapeutic efficacy against community-acquired colds caused by rhinoviruses in two placebo-controlled trials. Virological assessments were conducted during these trails, including virus culture and drug susceptibility testing. Nasal mucus samples collected from the enrolled patients were tested for the presence of picornavirus by reverse transcriptase PCR and culture. In total, 827 baseline nasal mucus samples were positive by virus culture (420 in the placebo group and 407 in the pleconaril group). Pleconaril treatment was associated with a more rapid loss of culturable virus. By study day 3, the number of samples positive by culture fell to 282 for the placebo-treated subjects and 202 for the pleconaril-treated subjects (P < 0.0001); and by day 6, the number of samples in the two groups positive by culture fell to 196 and 165, respectively (P = 0.07). The clinical benefit correlated strongly with the pleconaril susceptibility of the baseline virus isolate. Pleconaril-treated subjects infected with the more highly susceptible viruses (50% effective concentration ≤ 0.38 μg/ml) experienced a median 1.9- to 3.9-day reduction in symptom duration compared with that for the placebo-treated subjects. By contrast, subjects whose baseline virus isolate susceptibility was >0.38 μg/ml did not benefit from pleconaril treatment. These results indicate that the magnitude of symptomatic improvement in pleconaril-treated subjects with community-acquired colds is related to the drug susceptibility of the infecting virus, clearly linking the antiviral effects of the drug to clinical efficacy. Postbaseline virus isolates with reduced susceptibility or full resistance to pleconaril were recovered from 10.7% and 2.7% of drug-treated subjects, respectively. These patients shed low levels of virus and had no unusual clinical outcomes. Nevertheless, studies on the biologic properties and transmissibility of these variant viruses are warranted.
Human rhinoviruses (HRVs) are the predominant cause of viral respiratory tract infections (VRIs), particularly common colds (4, 8, 12, 17, 25, 30), as well as acute otitis media and sinusitis (5, 9, 32), and are associated with the induction of exacerbations of asthma in both children and adults (20, 21, 23, 28, 33). Among elderly individuals, rhinovirus infections are a significant cause of lower respiratory tract illnesses and in this population may present an overall health care burden similar to that for influenza (10, 26, 27). Enteroviruses also cause significant respiratory disease, including pharyngitis, croup, bronchitis, bronchiolitis, infectious asthma, pneumonia, pleurodynia, and the common cold (6, 37). The overall health care and societal burdens of picornavirus infections are enormous, but no specific antiviral therapy exists to prevent or treat picornavirus diseases.
Pleconaril is a novel, orally administered small-molecule inhibitor of human picornavirus replication that integrates into a hydrophobic pocket within the major coat protein of human picornaviruses (viral protein 1 [VP1]) and affects functions associated with this protein, including attachment of some viruses to their cellular receptor and the release of viral RNA to the cell cytoplasm (uncoating) (31, 39). Pleconaril has also been shown to associate with virus particles during virus assembly, blocking the infectivity of progeny virions (40). This VP1 hydrophobic pocket into which pleconaril integrates appears to be unique, in that the antiviral properties of pleconaril are specific to human picornaviruses. Virus variants with reduced susceptibilities to pleconaril have been shown to preexist at very low levels in susceptible virus populations but can be isolated in cell culture under drug selection pressure (11). Amino acid changes conferring reduced drug susceptibility are typically localized to amino acids in or near the drug-binding pocket (11, 16, 36).
During the fall season of 2000, two phase III, double-blind, placebo-controlled studies of community-acquired VRIs in otherwise healthy adults were conducted with pleconaril and showed that pleconaril treatment reduced the duration and the severity of common cold symptoms in picornavirus-infected individuals when it was administered within 24 h of symptom onset (15). This represented the first time that an antiviral drug was shown to influence the course of naturally occurring common colds when it was administered therapeutically. As would be expected for a specific antiviral drug such as pleconaril, treatment benefit was observed exclusively in subjects infected with a picornavirus. In the pivotal trials, about 65% of prospectively enrolled subjects were confirmed by reverse transcriptase PCR (RT-PCR) testing to have VRI of a picornavirus etiology.
Included in the design of these trials was an extensive virologic assessment of samples obtained from enrolled patients with respect to detection of picornaviruses and determination of in vitro susceptibilities of recovered isolates. The current studies were conducted to (i) determine the baseline virus isolate susceptibilities to pleconaril in cell culture, (ii) examine the relationship between in vitro susceptibility and the antiviral effects of pleconaril in vivo, (iii) examine the relationship between in vitro antiviral susceptibility and clinical efficacy, (iv) monitor the subjects for the emergence of variant viruses with reduced susceptibilities to pleconaril, and (v) assess the possible virologic and clinical consequences of resistance emergence for treated subjects. The results of these evaluations show that the symptomatic efficacy of pleconaril was linked to its in vivo antiviral effects and to the drug susceptibility of the infecting virus. The emergence of resistance to pleconaril occurred infrequently and was associated with a low virus titer and favorable clinical outcomes.
MATERIALS AND METHODS
Clinical study and outcome parameters.
The two phase III trials (studies 843-043 and 843-044) were randomized, double blind, and placebo controlled in design and enrolled 2,096 participants at 196 centers in North America during the autumn of 2000 (15). Patients were treated with either placebo or drug for 5 days. The clinical end points used to assess the response to pleconaril have been described previously (15). The primary efficacy end point of the phase III trials was the time from the initiation of therapy to the time of the alleviation of illness, defined as the number of days until complete resolution of rhinorrhea and five other cold symptoms (nasal congestion, sore throat, cough, malaise, and myalgia) self-assessed as absent or mild for 48 h without use of cold symptom relief medication (15).
Specimen collection.
Immediately prior to the first dose of study medication on study day 1, all enrolled subjects were instructed to expel nasal mucus onto plastic wrap (baseline or pretreatment specimen). Similarly, postbaseline nasal mucus specimens were obtained from patients on study days 3 (range, days 2 to 4) and 6 (range, days 5 to 9). If a subject was unable to produce an adequate nasal mucus specimen at follow-up, 0.5 ml of water was inhaled into each nostril and allowed to dwell for 15 to 30 s, and the subject then expelled the nasal contents into plastic wrap. All of the nasal mucus material was transferred with the aid of a plastic spoon and swab to a Starswab Multitrans Collection and Transport System tube (Starplex Scientific, Etobicoke, Ontario, Canada) containing 2 ml of transport medium and was eventually stored frozen at −80°C in aliquots.
RT-PCR testing.
The presence of picornavirus RNA in patient nasal mucus samples was detected by a picornavirus-specific, real-time, quantitative RT-PCR assay (TaqMan; Applied Biosystems), as described previously (15). This assay detects about 90% of prototype HRV serotypes (data not shown). If all three samples from a patient were negative or indeterminate by the TaqMan assay, then the baseline sample was retested by a modified enzyme-linked oligosorbent RT-PCR assay that detects all prototype HRV and enterovirus serotypes (3, 15). Negative results by the two independent RT-PCR assays were considered confirmatory of a true picornavirus-negative sample.
Virus culture.
If a sample was positive by either RT-PCR assay, then an aliquot was submitted for virus culture. Virus culture of baseline RT-PCR-positive samples from study 843-043 were sent to the laboratory of Frederick Hayden at the University of Virginia, Charlottesville; and those from study 843-044 were sent to the laboratory of Marilyn Menegus, University of Rochester Medical School, Rochester, NY. Virus culture at the two laboratories was performed with identical cell lines and virus culturing procedures. Briefly, samples were thawed in a 37°C water bath, vortexed, and spun at 800 × g for 10 min in a refrigerated centrifuge. Maintenance medium (Eagle minimal essential medium [BioWhittaker] with 5% Fetalclone serum [HyClone Labs, Logan, UT], antibiotics, and glutamine) was removed from subconfluent roller tube cultures of HeLa-I cells, a cell line that over expresses ICAM-1, the receptor for the majority of HRV serotypes (2). Each tube was inoculated with 200 μl of patient specimen. After 1 h of adsorption at 33°C, the inoculum was decanted and the cell monolayers were washed twice with 1 ml of Dulbecco's modified phosphate-buffered saline (BioWhittaker) to eliminate drug carryover from nasal mucus specimens. The monolayers were then overlaid with 1 ml of virus culture medium (McCoy's 5A medium containing 2% fetal bovine serum, 30 mM MgCl2, and antibiotics), and the tubes were incubated at 33 to 34°C in a revolving drum apparatus set at one revolution every 5 min. All tubes were monitored microscopically for the appearance of picornavirus cytopathic effect (CPE) three times per week. At the end of 7 days, all negative cultures were blind passaged onto fresh HeLa-I cell monolayers. The tubes were frozen at −80°C for a minimum of 2 h and then thawed in a 37°C water bath. Two hundred fifty microliters of the resulting cell lysate was inoculated onto a fresh roller tube of HeLa-I cells as described above. This tube was incubated for an additional 15 to 16 days. Picornavirus-positive samples were stored frozen at −80°C in aliquots. Confirmation of the presence of rhinovirus or enterovirus was done by pH stability testing of select samples.
In vitro pleconaril susceptibility testing.
The antiviral activity of pleconaril against clinical isolates was determined in a cell culture assay that measured virus-induced CPE (24). Five 0.5 log10 virus dilutions were tested in a matrix against seven concentrations of pleconaril in a 96-well microplate format with HeLa-I cells. The drug concentrations ranged from 0.004 μg/ml to 3.8 μg/ml, the highest pleconaril concentration testable in this assay. The purpose of running multiple dilutions of the test samples was to ensure that an appropriate level of virus-induced CPE was produced in the 5-day assay to allow measurement of antiviral effects (protection of cells from CPE) and to control for possible multiplicity-of-infection effects of the inoculum. At the end of the 5-day incubation period the cells were fixed with glutaraldehyde, stained with crystal violet, rinsed, and dried. Residual staining was measured spectrophotometrically at a wavelength of 570 nm on a 96-well Molecular Devices VersaMax tunable plate reader. The highest dilution of virus isolate that yielded at least a mean 80% destruction of the cell monolayers was used to calculate the 50% effective concentration (EC50) of pleconaril. Data from the seven-point EC50 curve were analyzed in a four-parameter curve-fitting program by using SoftMax Pro software, provided with the VersaMax plate reader. At least two independent tests were run with each virus outgrowth sample. An HRV 1B reference standard was included in every assay run. The mean EC50 for pleconaril against HRV 1B this virus was approximately 0.2 μM. For a given test run to be valid, the EC50 for pleconaril against HRV 1B had to fall within ±0.5 log10 of this EC50 value. All test runs met this criterion.
Data analysis.
Contingency tables were analyzed by a two-tailed Fisher's exact test by using a 95% confidence interval. The two-tailed Mann-Whitney nonparametric test was used to compare differences in medians between groups. P values of ≤0.05 between analysis groups were considered significant.
RESULTS
Virus recovery.
As shown in Table 1, 827 of 1,363 baseline RT-PCR-positive samples (61%) were positive by virus culture (420 in the placebo group and 407 in the pleconaril group). The vast majority of these outgrowths were likely rhinoviruses. Seventy-five randomly selected RT-PCR- and culture-positive samples were tested for sensitivity to inactivation at low pH, which is diagnostic of rhinoviruses (18, 19). The infectivities of 74 isolates were inactivated by exposure to low pH. The one virus resistant to a low pH was presumed to be an enterovirus.
TABLE 1.
Patients with positive virus culture at baseline and study day, by study and pooleda
| Patient group | No. (%) of patients
|
|||||
|---|---|---|---|---|---|---|
| Study 843-043
|
Study 843-044
|
Studies, pooled
|
||||
| Placebo | Pleconaril | Placebo | Pleconaril | Placebo | Pleconaril | |
| Patients with positive baseline virus culture | 196 | 201 | 224 | 206 | 420 | 407 |
| Patients with positive culture results by individual study days: | ||||||
| 2 | 23 (85) | 14 (52) | 26 (84) | 13 (72) | 49 (84) | 27 (60) |
| 3 | 94 (74) | 74 (55) | 103 (73) | 77 (54) | 197 (74) | 151 (55) |
| 4 | 15 (54) | 12 (50) | 21 (53) | 12 (35) | 36 (53) | 24 (41) |
| Total | 132 (73) | 100 (54) | 150 (71) | 102 (53) | 282 (72) | 202 (53) |
| P valueb (days 2 to 4) | 0.0003 | 0.0002 | <0.0001 | |||
| Patients with positive culture results by individual study days: | ||||||
| 5 | 8 (62) | 7 (70) | 7 (64) | 8 (47) | 15 (63) | 15 (56) |
| 6 | 55 (53) | 55 (54) | 66 (53) | 41 (37) | 121 (53) | 96 (45) |
| 7 | 27 (54) | 23 (48) | 22 (38) | 17 (37) | 49 (45) | 40 (43) |
| 8 | 3 (30) | 9 (41) | 7 (58) | 5 (42) | 10 (45) | 14 (41) |
| 9 | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) |
| Total | 94 (52) | 94 (51) | 102 (49) | 71 (37) | 196 (51) | 165 (44) |
| P value (days 5 to 9) | 0.83 | 0.016 | 0.070 | |||
The denominator for the baseline represents all RT-PCR-positive specimens; and for day 3 and day 6, the denominator represents all available postbaseline samples from baseline-positive patients in the respective treatment groups. The reasons for the reduced sample number from the baseline to day 3 and to day 6 included the facts that samples were not taken, not tested, or lost in transit; samples were collected outside the time windows; or samples had an inadequate quantity for testing.
P values were calculated from a Fisher's exact test. Data sets that are significantly different from one another are shown in boldface and italic and are for comparisons of the placebo and pleconaril groups.
Pleconaril administration was associated with a more rapid loss of culturable virus. By day 3 (range, days 2 to 4), the number of samples positive by virus culture fell to 72% among the placebo-treated subjects and 53% among the pleconaril-treated subjects (P < 0.0001). By day 6 (range, days 5 to 9), 51% of the placebo-treated subjects remained virus culture positive, whereas 44% of the pleconaril-treated group remained virus culture positive (P = 0.07). These results indicate that while the virus culture positivity rates dropped in both the placebo and the pleconaril treatment groups by study days 3 and 6, the rate of decline was accelerated in the pleconaril-treated subjects.
Pleconaril secretion into nasal mucus could potentially affect the ability to culture virus from these samples. While the procedures used for virus culture were designed to minimize drug carryover from nasal secretions, a randomly selected subset of nasal samples (n = 128) from pleconaril-treated subjects was tested for drug levels by a validated gas chromatography method with a minimum quantifiable limit (MQL) of 1 ng/ml. Pleconaril levels in nasal mucus specimens were exceedingly low (median level, 1.8 ng/ml; range of levels, <1 ng/ml to 79.4 ng/ml) (data not shown). The level of pleconaril was below the MQL in 51 of the 128 samples. Only 14 of the 128 samples had pleconaril levels above 10 ng/ml. Approximately 4% of virus isolates from the present studies had EC50 values of ≤1.8 ng/ml. Based on these data, drug carryover is unlikely to have significantly affected virus culture positivity rates.
Baseline virus isolate susceptibility to pleconaril.
All virus isolates were tested for susceptibility to pleconaril in the CPE protection assay. Valid EC50 values were generated for 744 of the 827 pretreatment (baseline) isolates. Valid EC50 values could not be generated for some samples due to insufficient virus growth in the 5-day CPE assay (43 from the pleconaril group and 40 from the placebo group). Of these 744 baseline samples, 649 (87%) were susceptible to pleconaril inhibition at or below the highest concentration testable in cell culture (EC50 ≤ 3.8 μg/ml) (Table 2). The consistency of the two clinical trials is reflected by the fact that the proportion of baseline isolates susceptible to pleconaril in both studies was 87% (Table 2).
TABLE 2.
Inhibition by pleconaril of virus isolates from baseline virus culture-positive samples for patients with a drug susceptibility result at baseline, by study and pooleda
| No. of samples with virus inhibited by pleconaril/total no. of samples (%)
| |||||
|---|---|---|---|---|---|
| 843-043
|
843-044
|
Pooled
|
|||
| Placebo | Pleconaril | Placebo | Pleconaril | Placebo | Pleconaril |
| 154/173 (89) | 147/173 (85) | 181/207 (87) | 167/191 (87) | 335/380 (88) | 314/364 (86) |
The denominator includes all baseline virus culture positive samples for which valid EC50 values were obtained. The reasons for the reduced sample number from virus culture-positive sample to samples reported to be inhibited by pleconaril in cell culture include the following: the samples were not tested, the samples grew too poorly in the drug testing assay, or the samples failed to provide reproducible EC50 values.
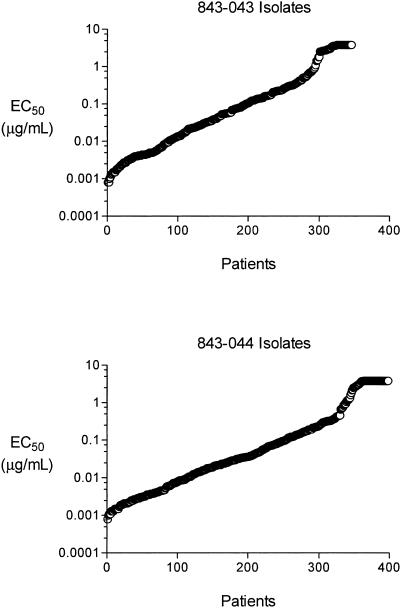
The distribution of pleconaril susceptibility for the pretreatment isolates is shown in Fig. 1. The EC50 values of the baseline isolates ranged over 3,000-fold, from less than 1 ng/ml to greater than 3.8 μg/ml. The replication of 50% of these isolates in cell culture was inhibited by a pleconaril concentration of ≤0.05 μg/ml, and the replication of 80% of these isolates was inhibited at a drug concentration of ≤0.42 μg/ml (Table 3). These values mirror the overall distribution of pleconaril susceptibility of the 101 HRV prototype strains (24), as well as those derived from the testing of 46 HRV clinical isolates (22). The results suggest that a large number of HRV serotypes were circulating in North America in the fall season of 2000.
FIG. 1.
Distribution of baseline susceptibility to pleconaril of virus isolates from protocols 843-043 and 843-044. Each EC50 value represents the average of at least two independent determinations.
TABLE 3.
Summary of virus susceptibility to pleconaril
| Study | No. of clinical isolates or strains | Concn (μg/ml) inhibiting:
|
EC50 range (μg/ml) | |
|---|---|---|---|---|
| 50% of isolates or strains | 80% of isolates or strains | |||
| 843-043a | 346 | 0.06 | 0.50 | 0.0008 to >3.8 |
| 843-044a | 398 | 0.04 | 0.35 | 0.0008 to >3.8 |
| Pooled | 744 | 0.05 | 0.42 | 0.0008 to >3.8 |
| Kaiser et al. (22) | 46 | 0.04 | 0.54 | <0.01 to >1.0 |
| ViroPharma (24) | 101b | 0.08 | 0.30 | 0.008 to >4.8 |
Baseline virus isolates.
Prototype HRV strains.
Relationship of in vitro susceptibility to postbaseline viral recovery.
As a measure of the antiviral effect of pleconaril in vivo, the rate of postbaseline sample culture positivity as a function of baseline virus susceptibility and treatment group was examined. With the range of susceptibilities identified for baseline isolates, we hypothesized that the largest in vivo antiviral effect would be observed in subjects infected with isolates with the lowest EC50 values. To test this hypothesis, patients were divided into quintiles of approximately equal sizes based on the baseline isolate EC50 value, and the percentage of postbaseline samples that were culture positive was determined. The percentage of culture-positive postbaseline samples remained relatively constant in the placebo-treated subjects, ranging from 58% to 68% across the five susceptibility quintiles (Fig. 2). By contrast, only 22.5% of the samples from pleconaril-treated subjects who had the most highly susceptible viruses at the baseline (EC50 range, 0.0008 to 0.005 μg/ml) were culture positive on days 3 and/or 6 (P < 0.0001 by Fisher's exact test), and only 37% of samples from pleconaril-treated subjects infected with baseline isolates with susceptibilities ranging from >0.005 to 0.025 μg/ml were culture positive postbaseline (P < 0.0001) (Fig. 2). Among the pleconaril-treated subjects who had baseline viruses with susceptibilities in the middle quintile (EC50 range, > 0.025 to 0.096 μg/ml), 48% of samples were culture positive on day 3 and/or 6, whereas 64% of those from placebo-treated subjects were culture positive on day 3 and/or 6 (P = 0.01). There was no significant difference in the rate of postbaseline isolate recovery in the fourth quintile (EC50 range, >0.096 to 0.38 μg/ml), and a slightly higher rate of postbaseline isolate recovery in pleconaril-treated subjects (75%) versus placebo-treated subjects (64%) in the highest quintile (EC50 range, >0.38 to >3.8 μg/ml; P = 0.04). The results indicate that pleconaril's antiviral effect in vivo was observed in subjects infected with the more highly susceptible baseline virus isolates.
FIG. 2.
Postbaseline culture positivity rate by treatment group and baseline drug susceptibility. Culture results are for both sampling days for each subject who was culture positive at enrollment. *, P < 0.05 by Fisher's exact test.
Clinical efficacy as a function of baseline virus susceptibility.
The relationship between clinical outcomes and the in vitro antiviral susceptibilities of the baseline virus isolates was analyzed in a similar manner. As shown in Table 4, pleconaril-treated subjects with baseline isolates in the first four susceptibility quintiles experienced a 1.9- to 3.9-day reduction in the median number of days to symptom resolution (overall median number of days to symptom resolution for the first four susceptibility quintiles, 8.1 for the placebo-treated subjects and 5.8 for the pleconaril-treated subjects [P < 0.0001 by Mann-Whitney two-tailed test]). Pleconaril-treated subjects with virus isolates in the least-susceptible quintile experienced no benefit from drug treatment, and in fact, their median time to disease resolution was longer than that for the placebo-treated subjects (see Discussion). The results indicate that beneficial clinical outcomes were observed in subjects with baseline virus isolates that had susceptibility values of ≤0.38 μg/ml.
TABLE 4.
Median time to alleviation of illness (primary end point) and relationship to baseline virus susceptibility to pleconaril
| Quintile | EC50 range (μg/ml) | Placebo
|
Pleconaril
|
P valuea | ||
|---|---|---|---|---|---|---|
| No. of samples | Median no. of days (95% CIb) | No. of samples | Median no. of days (95% CI) | |||
| 1 | 0.0008 to 0.005 | 68 | 6.7 (5.6-7.8) | 80 | 4.8 (3.8-5.8) | <0.05 |
| 2 | >0.005 to 0.025 | 78 | 8.8 (7.5-10.1) | 72 | 4.9 (3.9-5.9) | <0.05 |
| 3 | >0.025 to 0.096 | 78 | 9.0 (7.8-10.2) | 69 | 6.4 (5.3-7.5) | <0.05 |
| 4 | >0.096 to 0.38 | 81 | 7.9 (6.8-9.0) | 67 | 5.9 (4.5-7.3) | 0.14 |
| 5 | >0.38 to ≥3.8 | 75 | 5.7 (4.6-6.8) | 76 | 9.4 (8.3-10.5) | <0.05 |
Mann-Whitney two-tailed Student's t test.
CI, confidence interval.
Isolation of postbaseline viruses with reduced susceptibility to pleconaril.
The emergence in pleconaril-treated patients of virus variants with reduced drug susceptibility was investigated. Reduced drug susceptibility was defined as any postbaseline isolate (day 3 or 6) exhibiting a ≥10-fold increase in the EC50 value relative to that for its corresponding baseline isolate. The 10-fold cutoff value was set to be outside the range of variation among assay replicates (data not shown).
Virus variants with reductions in pleconaril susceptibility were recovered from 2 of 294 (0.7%) placebo-treated subjects and 28 of 263 (10.7%) pleconaril-treated subjects postbaseline (P < 0.0001 by Fisher's exact test). One of the two postbaseline isolates from placebo-treated subjects was resistant to inhibition by pleconaril in cell culture (EC50 > 3.8 μg/ml), while the other remained susceptible to the drug (EC50 = 2.5 μg/ml). Of the pleconaril-treated subjects with postbaseline virus variants with reduced susceptibility, 21 harbored viruses that remained susceptible to the drug, with a median EC50 value of 0.27 μg/ml. Fully resistant variants were recovered from 7 (2.7%) pleconaril-treated subjects.
To understand the potential importance of the emergence of variants with reduced drug susceptibility, we compared the virologic and clinical course of these pleconaril-treated subjects with those of pleconaril recipients in which there was no change in drug susceptibility between baseline and postbaseline virus isolates. Virus RNA levels in subjects from both groups were assessed by a quantitative RT-PCR (TaqMan) assay (14). As shown in Fig. 3, the median percentage of baseline virus RNA levels was exceedingly low in pleconaril-treated subjects from whom variant viruses with reduced susceptibilities were isolated compared to the baseline virus RNA levels.
FIG. 3.
Virus RNA levels in patients with treatment-emergent virus isolates with reduced susceptibility to pleconaril. Data were generated by a validated, quantitative RT-PCR (TaqMan) assay. The relative change in RNA level from the baseline was determined for each subject, and the data are expressed as the median percentage of the baseline virus RNA level remaining for the respective cohorts on study days 3 and 6.
Further, the clinical course of illness resolution in these subjects appeared to be less protracted than that of the overall pleconaril-treated population (Table 5). The data suggest that the emergence of viruses with reduced drug susceptibility does not adversely affect the clinical outcome in pleconaril-treated, immunocompetent adults with colds caused by rhinoviruses.
TABLE 5.
Clinical outcomes for patients with treatment-emergent virus isolates with reduced drug susceptibility
| Parameter | All placeboa | All pleconarilb | Reduced drug susceptibility |
|---|---|---|---|
| No. of patients | 380 | 364 | 28 |
| Duration of illness (median no. of days [95% CIc]) | 7.4 (6.9-7.9) | 6.3 (5.8-6.8) | 4.4 (2.9-6.0) |
Represents all placebo-treated subjects with baseline virus culture-positive samples for which valid EC50 values were obtained.
Represents all pleconaril-treated subjects with baseline virus culture-positive samples for which valid EC50 values were obtained.
CI, confidence interval.
DISCUSSION
We report here that the antiviral activity and therapeutic efficacy of pleconaril treatment of colds caused by rhinoviruses are significantly related to the drug susceptibility of the infecting virus. In addition, we show that the emergence of virus variants with reduced susceptibility to pleconaril after the initiation of treatment occurs at low frequency and is associated with very low virus titers in patients and no adverse clinical consequences.
Most patients were infected with susceptible rhinovirus strains and experienced clinical benefit. Over 87% of all baseline virus isolates were inhibited by pleconaril in cell culture. This result is consistent with the broad-spectrum antipicornavirus activity exhibited by pleconaril in other studies. In adults and children with colds between 1988 and 1998, Kaiser and coworkers reported that pleconaril inhibited 89% of 46 rhinovirus clinical isolates (22). In our own laboratory testing, pleconaril inhibited 92% of the 101 prototypic rhinovirus serotypes (24).
In the present clinical studies, while both viral RNA levels and virus culture positivity decreased in all patients postenrollment, the rate of decrease was significantly greater in pleconaril-treated subjects (15). For patients determined to be picornavirus infected by RT-PCR, virus-positive cultures were obtained from approximately two-thirds of patients at the baseline and from lower proportions on days 3 and 6. The differences in the proportion of pleconaril-treated patients with culturable virus compared to the proportion of placebo-treated patients with culturable virus were significantly reduced by day 2, demonstrating a very early antiviral effect in the pleconaril-treated patients.
We performed a series of analyses to explore the relationship between baseline virus susceptibility to pleconaril and the observed virologic and clinical responses to pleconaril treatment. Not surprisingly, susceptibility to pleconaril inhibition correlated with both antiviral effects and clinical benefit. Pleconaril-treated subjects infected with viruses poorly susceptible to inhibition by the drug in cell culture experienced neither antiviral nor treatment benefit. The in vitro susceptibility threshold for clinical benefit appeared with viruses having EC50 values to pleconaril of ≤0.38 μg/ml (Table 4). Subjects harboring viruses with EC50 values above this level received no apparent benefit from pleconaril treatment. Indeed, these subjects appeared to recover more slowly than the corresponding placebo recipients in both the rate of postbaseline virus recovery and clinical outcome. The significance and reproducibility of this paradoxical observation will require further clinical testing.
In cell culture testing, 80% of the baseline virus isolates from the two trials had EC50 values to pleconaril of <0.42 μg/ml (Table 3). These data suggest that most (∼80%) picornavirus-infected subjects enrolled in these studies had a virus with baseline susceptibility that would place it within this efficacy response window. Since about 65% of prospectively enrolled subjects were confirmed to be picornavirus infected by RT-PCR, roughly half of all patients enrolled in these trials fell within the response window. Of these subjects, the median times to achievement of the primary efficacy end point were 8.1 days for the placebo-treated subjects and 5.8 days for the pleconaril-treated subjects (P < 0.0001). Thus, pleconaril-treated patients harboring viruses with susceptibility in the efficacy response window recovered from their colds an average of 2.3 days more quickly than the corresponding placebo-treated cohort.
In addition to the assessment of antiviral effect, the present study sought to determine if picornaviruses with reduced drug susceptibility could be detected in patients during pleconaril treatment. Comparison of the drug susceptibilities of the baseline and the postbaseline (days 3 and 6) virus isolates showed that the majority of the virus isolates had no significant change in their EC50 values following treatment. However, postbaseline viruses with reduced drug susceptibility were isolated from pleconaril-treated patients at a higher frequency than from placebo-treated subjects. Postbaseline viruses with ≥10-fold reduced drug susceptibility relative to that at the baseline were detected in 10.7% of evaluable pleconaril-treated patients and 0.7% of placebo-treated subjects (P < 0.0001 by Fisher's exact test). It is likely that the two placebo-treated patients whose postbaseline isolate exhibited reduced susceptibility to pleconaril experienced a second infection with a nonsusceptible HRV serotype. Among the pleconaril-treated patients who originally harbored susceptible viruses at the baseline, only 2.7% had postbaseline viruses that were no longer inhibited by pleconaril in cell culture. These data indicate that the emergence of pleconaril resistance in patients with VRIs is relatively infrequent. For example, this is a higher frequency of resistance emergence than that found with oseltamivir treatment of acute influenza in adults (0.4%) (34) but a much lower frequency than that found with amantadine or rimantadine treatment of influenza A virus infection (approximately 30%) (14).
The clinical course in subjects with postbaseline viruses showing reduced susceptibility or a lack of pleconaril susceptibility had a median duration of illness that appeared to be shorter than that for all picornavirus-infected patients (Table 5). This observation may in part reflect the fact that all postbaseline viruses with reduced drug susceptibility were isolated from subjects whose baseline isolate fell within the first three susceptibility quintiles (EC50 values, 0.0008 to 0.096 μg/ml). Our data suggest that the emergence of viruses with reduced drug susceptibility does not affect the clinical outcome in pleconaril-treated, immunocompetent adults with colds caused by rhinoviruses. Moreover, based on the exceedingly low virus levels in the nasal secretions of these patients, such isolates may be unlikely to be transmitted to others. However, further clinical testing, such as household transmission studies, would need to be undertaken to confirm this point.
The transmissibility and virulence of treatment-emergent resistant picornavirus variants are unknown. However, there are several observations that bear on these points. First, from studies of coxsackievirus B3 (CVB3), virus variants selected from susceptible virus populations that were not inhibited by pleconaril in cell culture were found to be considerably attenuated in animals (11). An assessment of the virulence of three independently isolated pleconaril-resistant CVB3 viruses in a mouse lethal infection model showed that all drug-resistant variants had a reduced ability to cause death relative to that of the drug-susceptible parental virus. Moreover, the time to the onset of death was delayed in animals infected with the resistant variants. The reduced virulence of the resistant viruses in all cases was correlated with a 2- to 3-log10 reduction in the ability of these viruses to replicate in the tissues of infected mice. The molecular basis of drug resistance in 10 independently isolated resistant CVB3 isolates was attributed to an amino acid change in the VP1 protein in the drug-binding pocket.
Second, in clinical studies of pleconaril in enteroviral meningitis patients, postbaseline pleconaril-resistant viruses were isolated from two patients on treatment day 4 (unpublished data). However, no virus could be isolated from these same two patients on day 8, and both patients recovered fully by day 7. This observation suggests that the pleconaril-resistant viruses either replicated poorly or were cleared efficiently by the host immune response. The resistant variants isolated on day 4 were both shown to be less fit in cell culture than their respective drug-susceptible baseline viruses. Both resistant variants had an identical single amino acid change in the drug-binding pocket of VP1.
Finally, in work conducted by others, HRV 2 variants resistant to a capsid inhibitor were used in experimental infections of humans and were shown to be less virulent than the wild-type drug-susceptible parental virus (38). Wild-type HRV 2 and cell culture-derived variants that were either resistant to or dependent on the capsid inhibitor Ro 09-0410 were inoculated into the noses of human volunteers. Ro 09-0410 is a chalcone capsid inhibitor that acts by a mechanism of action similar to that for pleconaril, binding to the same pocket in the VP1 protein. Ro 09-0410-resistant viruses are cross-resistant to pleconaril-class compounds (1, 7, 29). In the study, the drug-resistant virus produced illness in one-half as many volunteers as the wild-type virus and infection in about one-third as many volunteers as the wild-type virus. The frequency of serological evidence of infection was significantly lower in the group challenged with the drug-resistant virus, and volunteers given the drug-resistant virus shed significantly less virus after virus challenge.
It appears that the changes required to confer pleconaril resistance also generally reduce the fitness of picornaviruses. This is not a surprising finding since the natural ligand of the drug-binding pocket is a fatty acid molecule (“pocket factor”) that has been shown to be important in imparting stability to the virus capsid (13, 35). The binding sites of pocket factor and antiviral capsid-binding compounds overlap considerably. Many of the amino acid changes associated with reduced susceptibility to pleconaril would be predicted to affect pocket factor binding as well, thus imparting reduced stability to the variant virus (24).
In summary, approximately 87% of picornaviruses isolated from patients with common colds were susceptible to inhibition by pleconaril in cell culture. The virologic and clinical responses to pleconaril treatment are related to the drug susceptibility of the isolate recovered prior to the initiation of treatment. The findings also raise the possibility that alternative treatment regimens, such as intranasal dosing, might exert greater antiviral and therapeutic effects. Assessments of the clinical course in patients with postbaseline viruses that had reduced drug susceptibility or that were no longer inhibited by pleconaril in cell culture indicate that there were no significant adverse consequences in their recovery from illness. The clinical implications of the emergence of viruses having reduced susceptibility to pleconaril require further study.
Acknowledgments
The studies described herein were sponsored and funded by ViroPharma, Incorporated.
REFERENCES
- 1.Ahmad, A. L., A. B. Dowsett, and D. A. Tyrrell. 1987. Studies of rhinovirus resistant to an antiviral chalcone. Antivir. Res. 8:27-39. [DOI] [PubMed] [Google Scholar]
- 2.Arruda, E., C. E. Crump, B. S. Rollins, A. Ohlin, and F. G. Hayden. 1996. Comparative susceptibilities of human embryonic fibroblasts and HeLa cells for isolation of human rhinoviruses. J. Clin. Microbiol. 34:1277-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruda, E., and F. G. Hayden. 1993. Detection of human rhinovirus RNA in nasal washings by PCR. Mol. Cell. Probes 7:373-379. [DOI] [PubMed] [Google Scholar]
- 4.Arruda, E., A. Pitkaranta, T. J. Witek, Jr., C. A. Doyle, and F. G. Hayden. 1997. Frequency and natural history of rhinovirus infections in adults during autumn. J. Clin. Microbiol. 35:2864-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman, C. A., W. J. Doyle, D. Skoner, P. Fireman, and J. M. Gwaltney. 1994. Otologic manifestations of experimental rhinovirus infection. Laryngoscope 104:1295-1299. [DOI] [PubMed] [Google Scholar]
- 6.Chonmaitree, T., and L. Mann. 1995. Respiratory infections, p. 255-270. In H. A. Rotbart, (ed.), Human enterovirus infections. ASM Press, Washington, D.C.
- 7.Dearden, C., W. al Nakib, K. Andries, R. Woestenborghs, and D. A. Tyrrell. 1989. Drug resistant rhinoviruses from the nose of experimentally treated volunteers. Arch. Virol. 109:71-81. [DOI] [PubMed] [Google Scholar]
- 8.Dingle, J. H., G. F. Badger, and W. S. Jordan. 1964. Ilness in the home: study of 25,000 illnesses in a group of Cleveland families. The Press of Western Reserve University, Cleveland, Ohio.
- 9.Elkhatieb, A., G. Hipskind, D. Woerner, and F. G. Hayden. 1993. Middle ear abnormalities during natural rhinovirus colds in adults. J. Infect. Dis. 168:618-621. [DOI] [PubMed] [Google Scholar]
- 10.Falsey, A. R., R. M. McCann, W. J. Hall, M. M. Criddle, M. A. Formica, D. Wycoff, and J. E. Kolassa. 1997. The “common cold” in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J. Am. Geriatr. Soc. 45:706-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groarke, J. M., and D. C. Pevear. 1999. Attenuated virulence of pleconaril-resistant coxsackievirus B3 variants. J. Infect. Dis. 179:1538-1541. [DOI] [PubMed] [Google Scholar]
- 12.Gwaltney, J. M., Jr., J. O. Hendley, G. Simon, and W. S. Jordan, Jr. 1966. Rhinovirus infections in an industrial population. I. The occurrence of illness. N. Engl. J. Med. 275:1261-1268. [DOI] [PubMed] [Google Scholar]
- 13.Hadfield, A. T., W. Lee, R. Zhao, M. A. Oliveira, I. Minor, R. R. Rueckert, and M. G. Rossmann. 1997. The refined structure of human rhinovirus 16 at 2.15 Å resolution: implications for the viral life cycle. Structure 5:427-441. [DOI] [PubMed] [Google Scholar]
- 14.Hayden, F. G., and A. J. Hay. 1992. Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr. Top. Microbiol. Immunol. 176:119-130. [DOI] [PubMed] [Google Scholar]
- 15.Hayden, F. G., D. T. Herrington, T. L. Coats, K. Kim, E. C. Cooper, S. A. Villano, S. Liu, S. Hudson, D. C. Pevear, M. Collett, and M. McKinlay. 2003. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 36:1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinz, B. A., R. R. Rueckert, D. A. Shepard, F. J. Dutko, M. A. McKinlay, M. Fancher, M. G. Rossmann, J. Badger, and T. J. Smith. 1989. Genetic and molecular analyses of spontaneous mutants of human rhinovirus 14 that are resistant to an antiviral compound. J. Virol. 63:2476-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendley, J. O. 1999. Clinical virology of rhinoviruses. Adv. Virus Res. 54:453-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes, J. H., S. Chema, N. Lin, R. M. Conant, and V. V. Hamparian. 1974. Acid lability of rhinoviruses: loss of C and D antigenicity after treatment at pH 3.0. J. Immunol. 112:919-925. [PubMed] [Google Scholar]
- 19.Hughes, J. H., D. C. Thomas, V. V. Hamparian, and H. G. Cramblett. 1973. Acid liability of rhinovirus type 14: effect of pH, time, and temperature. Proc. Soc. Exp. Biol. Med. 144:555-560. [DOI] [PubMed] [Google Scholar]
- 20.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, M. J. Campbell, L. K. Josephs, A. Cunningham, B. S. Robinson, S. H. Myint, M. E. Ward, D. A. Tyrrell, and S. T. Holgate. 1996. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am. J. Respir. Crit. Care Med. 154:654-660. [DOI] [PubMed] [Google Scholar]
- 21.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, F. Lampe, L. Josephs, P. Symington, S. O'Toole, S. H. Myint, and D. A. Tyrrell, and. 1995. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ 310:1225-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser, L., C. E. Crump, and F. G. Hayden. 2000. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinoviruses. Antivir. Res. 47:215-220. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. O., and R. L. Hodinka. 1998. Serious respiratory illness associated with rhinovirus infection in a pediatric population. Clin. Diagn. Virol. 10:57-65. [DOI] [PubMed] [Google Scholar]
- 24.Ledford, R. M., N. R. Patel, T. M. Demenczuk, A. Watanyar, T. Herbertz, M. S. Collett, and D. C. Pevear. 2004. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 78:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makela, M. J., T. Puhakka, O. Ruuskanen, M. Leinonen, P. Saikku, M. Kimpimaki, S. Blomqvist, T. Hyypia, and P. Arstila. 1998. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 36:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson, K. G., J. Kent, V. Hammersley, and E. Cancio. 1996. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ 313:1119-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson, K. G., J. Kent, V. Hammersley, and E. Cancio. 1997. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ 315:1060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson, K. G., J. Kent, and D. C. Ireland. 1993. Respiratory viruses and exacerbations of asthma in adults. BMJ 307:982-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ninomiya, Y., N. Shimma, and H. Ishitsuka. 1990. Comparative studies on the antirhinovirus activity and the mode of action of the rhinovirus capsid binding agents, chalcone amides. Antivir. Res. 13:61-74. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulos, N. G., P. J. Bates, P. G. Bardin, A. Papi, S. H. Leir, D. J. Fraenkel, J. Meyer, P. M. Lackie, G. Sanderson, S. T. Holgate, and S. L. Johnston. 2000. Rhinoviruses infect the lower airways. J. Infect. Dis. 181:1875-1884. [DOI] [PubMed] [Google Scholar]
- 31.Pevear, D. C., M. J. Fancher, P. J. Felock, M. G. Rossmann, M. S. Miller, G. Diana, A. M. Treasurywala, M. A. McKinlay, and F. J. Dutko. 1989. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J. Virol. 63:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitkaranta, A., E. Arruda, H. Malmberg, and F. G. Hayden. 1997. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J. Clin. Microbiol. 35:1791-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakes, G. P., E. Arruda, J. M. Ingram, G. E. Hoover, J. C. Zambrano, F. G. Hayden, T. A. Platts-Mills, and P. W. Heymann. 1999. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 159:785-790. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, N. A. 2001. Treatment of influenza with neuraminidase inhibitors: virological implications. Philos. Trans. R. Soc. London B Biol. Sci. 356:1895-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossmann, M. G. 1994. Viral cell recognition and entry. Protein Sci. 3:1712-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepard, D. A., B. A. Heinz, and R. R. Rueckert. 1993. WIN 52035-2 inhibits both attachment and eclipse of human rhinovirus 14. J. Virol. 67:2245-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strikas, R. A., L. J. Anderson, and R. A. Parker. 1986. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970-1983. J. Infect. Dis. 153:346-351. [DOI] [PubMed] [Google Scholar]
- 38.Yasin, S. R., W. al Nakib, and D. A. Tyrrell. 1990. Pathogenicity for humans of human rhinovirus type 2 mutants resistant to or dependent on chalcone Ro 09-0410. Antimicrob. Agents Chemother. 34:963-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeichhardt, H., M. J. Otto, M. A. McKinlay, P. Willingmann, and K. O. Habermehl. 1987. Inhibition of poliovirus uncoating by disoxaril (WIN 51711). Virology 160:281-285. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, Y., A. A. Simpson, R. M. Ledford, C. M. Bator, S. Chakravarty, G. A. Skochko, T. M. Demenczuk, A. Watanyar, D. C. Pevear, and M. G. Rossmann. 2004. Structural and virological studies of the stages of virus replication that are affected by antirhinovirus compounds. J. Virol. 78:11061-11069. [DOI] [PMC free article] [PubMed] [Google Scholar]