Abstract
Leishmania amazonensis, a causative agent of cutaneous leishmaniasis, is susceptible in vitro to light-mediated cytolysis in the presence of or after pretreatment with the photosensitizer aluminum phthalocyanine chloride. Cytolysis of both promastigotes and axenic amastigotes required less photosensitizer (e.g., one μg · ml−1) and a lower light dose (e.g., 1.5 J · cm−2) than did the mammalian cells examined for comparison. Exposure of Leishmania cells to the photosensitizer alone had little effect on their viability, as judged from their motility, growth, and/or retention of green fluorescent proteins genetically engineered for episomal expression. Fluorimetric assays for cell-associated and released green fluorescence proteins proved to be even more sensitive for the evaluation of cell viability than microscopy for the evaluation of motility and/or integrity. Axenic amastigotes pretreated with the photosensitizer infected macrophages of the J774 line but were lysed intracellularly when the infected cells were exposed to light. Addition of the photosensitizer to the already infected cells produced no effect on their intracellular parasites. However, light irradiation lysed these macrophages and also those infected with parasites preincubated with the photosensitizer at a concentration of 5 μg · ml−1 or higher. Photosensitized Leishmania cells are highly susceptible to cytolysis, apparently due to the generation of reactive oxidative species on light illumination, suggestive of inefficiency of their antioxidant mechanisms. Efficient delivery of photosensitizers to intracellular Leishmania is expected to increase their therapeutic potentials against leishmaniasis.
Photodynamic therapy takes advantages of the properties associated with certain dyes, which are normally nontoxic, except when they are excited by light to generate reactive oxidative species (ROS). There are many potential applications of this in medicine, among which its antitumor and antimicrobial activities have received the greatest attention (5, 13, 20).
Recently, photodynamic therapy has been used to treat cutaneous leishmaniasis (16, 18), a skin disease caused by trypanosomatid protozoa in the genus Leishmania. It is transmitted by blood-sucking females of the sand fly vector, in which Leishmania parasites exist as extracellular motile promastigotes in the alimentary tract. Upon entry into a mammalian host, the parasites reside in the phagolysosomes of mononuclear phagocytes or macrophages, wherein the parasites replicate as nonmotile amastigotes. The transition of cutaneous leishmaniasis from clinical disease to spontaneous cure follows a chronic course. The disease phase is thought to result from immunopathology caused by Leishmania “pathoantigens,” and the curative phase that follows is due to protective immunity elicited by Leishmania“natural vaccines” (10). The development of drug resistance in chemotherapy of leishmaniasis is well documented, leading to the consideration of using physical means as alternative therapeutic methods to eliminate cutaneous Leishmania, i.e., cryotherapy (2), thermotherapy (27), and now photodynamic therapy. The potential effectiveness of photodynamic therapy against Leishmania is indicated by its capacity to kill both free-living protozoa (17) and parasitic protozoa (21), including other trypanosomatids (15). Of direct relevance is our observation of the photolysis of transgenic Leishmania, which were genetically engineered to develop cellular uroporphyria, on exposure to delta-aminolevulinate and light (29).
We report here on the photosensitivity of L. amazonensis in vitro after exposure to aluminum phthalocyanine chloride (AlPhCl). This photosensitizer was chosen since its congeners have an excellent safety margin for human clinical trials (26). Although AlPhCl has been reported to appear in the cytosolic and mitochondrial compartments of mammalian cells, it is also endocytic and lysosomal, due to its hydrophobic interactions with the cell membrane and serum proteins (24), in mononuclear phagocytes in vivo (8), the very site of intracellular Leishmania residence. In this study, both stages of Leishmania amazonensis were found to be significantly more sensitive than mammalian cells to this photosensitizer for light-induced cytolysis, suggestive of its potential selectivity against Leishmania. The differential sensitivity to AlPhCl between host cells and parasites was, however, no longer evident in J774 cells infected with Leishmania. The utility of AlPhCl for photodynamic therapy thus may be improved by considering specific targeting against intracellular amastigotes.
MATERIALS AND METHODS
Cells and their cultivation.
Leishmania amazonensis (RAT/BA/74/LV78) clone 12-1 and macrophages of the J774A1 line were grown as described previously (9). Briefly, J774 cells were cultured routinely in RPMI 1640 (Sigma), pH 7.4, plus 10% heat-inactivated fetal bovine serum (HIFBS) at 35°C. Promastigotes were cultured at 25°C in medium 199 (Sigma) with 10% HIFBS, pH 7.4. Amastigotes of the same species were cultured continuously at 33°C in Grace's insect cell culture medium (Invitrogen) with 20% HIFBS, pH 5.4 (23).
Leishmania transfectants expressing GFPs.
Leishmania amazonensis of the same clone was transfected with p6.5-gfp to express green fluorescent protein (GFP), as before (7), except that a monomeric gfp of 720 bp (courtesy of Dunne Fong) was used for the present study. Briefly, gfp was PCR amplified for cloning into pGEM-T, and inserts were removed with BamHI for cloning into a p6.5-a Leishmania-specific vector, which has a constitutive expression site and nagt as a selectable marker for tunicamycin resistance (7, 29). The transfectants obtained were grown stably under the same culture conditions as described for the promastigotes and axenic amastigotes in the presence of tunicamycin at 10 μg · ml−1 and 5 μg · ml−1, respectively.
In vitro infection and evaluation.
Infection of J774 cells with Leishmania and quantitative evaluation of intracellular parasites followed established procedures (9). We routinely used a parasite-to-host cell ratio of 5:1 to 10:1, i.e., 2 × 107 to 4 × 107 Leishmania parasites and 4 × 106 macrophages in 4 ml RPMI 1640 plus 20% HIFBS per 25-cm2 flask. Before use for infection, transfectants were either grown for one cycle in tunicamycin-free medium or washed once with serum-free medium. To facilitate light illumination of infected cells, we used 6- and 12-well culture plates with 2 ml and 1 ml of the infected culture per well, respectively. The infected cultures were maintained at 35°C, with daily medium renewal when necessary.
Infection of macrophages was quantitatively assessed microscopically by a well-established method described previously (9). Both infected and noninfected cultures contained loosely adherent macrophages together with floating cells in each well. The adherent cells were dislodged from the substratum by repeated aspiration with the preexisting medium in each well by using a disposable plastic Pasteur pipette until completion, as indicated by the absence of adherent cells or the presence of very few of them visible by microscopic examination. The macrophage cell suspension obtained from each well thus included both dislodged and originally floating cells. The number of infected macrophages and the intracellular amastigotes were evaluated by counting ∼100 macrophages under a phase-contrast microscope with a ×100 oil immersion lens. Infection of macrophages was presented as the total number of intracellular amastigotes per culture calculated from the following formula: the total number of macrophages × the percentage of infected cells × the average number of Leishmania parasites per cell.
Treatment of cells with aluminum phthalocyanine chloride.
AlPhCl (Sigma) was dissolved in dimethyl formamide (Sigma) at 1 mg · ml−1, filter sterilized, and kept in the dark. Appropriate amounts of this stock solution were added to cell suspensions immediately before experimentation. Before treatment, both Leishmania stages were grown to late log phase, centrifuged, and resuspended to 107 to 108 cells · ml−1 mostly in culture media but also in Hank's balanced salt solution (Invitrogen) buffered with HEPES to pH 7.4 with 0.01% bovine serum albumin. Cells resuspended to 107 · ml−1 were exposed to AlPhCl in graded concentrations up to 40 μg · ml−1 in the dark for various time periods at room temperature. Preincubation of the cells was also carried out under this or other culture conditions in complete media (see the figure legends for details). Infected and noninfected macrophages of the J774 line were treated at 106 cells · ml−1 under similar conditions.
Exposure of AlPhCl-sensitized cells to light.
AlPhCl-treated and control cell suspensions were incubated in culture plates, i.e., 107 Leishmania parasites · 0.2 ml−1 · well−1 in 96-well culture plates and 106 J774 cells · ml−1 at 1 and 2 ml · well−1 in 12-well and 6-well culture plates, respectively. They were exposed to light illumination at the red-range wavelengths of >650 nm via a red filter (part no. 650021; Smith-Victor Co., Barlett, IL) in two different ways. (i) The cells were exposed to light via a fiber optic illuminator (Reichert Jung, Cambridge Instrument Inc.) or a 300-W quartz lamp (General Electric Co.) at an irradiance of 1.67 to 2.5 mW · cm−2, which was measured by using an L1-250A light meter (LI-COR). The strengths of illumination were expressed in fluence as J · cm−2 by inclusion of the time factors into the equation as follows: irradiance (mW · cm−2) × time (min) × 60 × 10−3. The light source was placed 5 to 12 cm from the bottom of the well for maintaining an ambient temperature of ∼25°C for the duration of the exposure. The cells in each well were exposed for up to 30 min, and adjacent wells were covered with a black board. (ii) The cells were exposed to light as described above, except that the light intensity was increased by using a 75 end-emitting optical fiber cable 5 cm from the bottom of the well in conjunction with a 250-W metal halide lamp (Eclipse II; Supervision). Leishmania-infected J774 cells in the presence or absence of AlPhCl and those infected with Leishmania pretreated with AlPhCl were placed in 12-well culture plates at 1 ml per well and exposed to light under the conditions described above.
Analyses of cell viability: Leishmania GFP release and macrophage dye exclusion. (i) Fluorescent microscopy.
Control and experimental gfp transfectants were examined for their integrity first under a phase-contrast microscope and then an epifluorescence microscope for detection of GFP by using a filter set for fluorescein isothiocyanate (exciter band path at 485 nm, dichroic fluorescence transmitter at 510 nm, and emitter long path at 520 nm; Chroma) in a Zeiss microscope with a superpressure mercury lamp (50 W; HBO; Osram). Fluorescence images were taken with a Nikon Coolpix 4500 digital camera by using a fixed shutter speed and exposure.
(ii) Fluorimetry.
Photolysis of AlPhCl-treated transfectants was assessed fluorometrically by measuring the relative GFP fluorescence, which remained in the cells versus that released into the incubation medium. Treated and control samples in aliquots of 200 μl (107 cells) were centrifuged at 3,500 × g for 5 min at 4°C. The cell pellets and supernatants were each resuspended or adjusted to a final volume of 2 ml with phosphate-buffered saline for reading of the fluorescence intensity in a Perkin-Elmer fluorometer (model LS50B). Readings were taken at excitation and emission wavelengths of 488 nm and 507 nm, respectively, by using slit widths of 9 nm and 10 nm, respectively. The fluorescence intensity of GFP up to 400 was linear in relation to the number of gfp transfectants at cell densities of up to 108 · ml−1. The viabilities of the gfp transfectants after exposure to AlPhCl with or without light exposure was further assessed by inoculation of these samples in aliquots of 50 μl (2.5 × 106 cells) each into 1 ml of culture medium for cell growth. The increase in GFP fluorescence was measured fluorometrically daily for 4 days.
(iii) Trypan blue dye exclusion.
The suspension of macrophages from each well was mixed with 0.4% trypan blue solution at a ratio of 3:1 (vol/vol). The preparation was immediately assessed microscopically in a hemacytometer for enumeration of stained and unstained cells, taken as dead and living cells, respectively.
All experiments were repeated two to three times with wild-type cells and twice with the gfp transfectants. The results obtained were comparable among repeated experiments, irrespective of the Leishmania cell types used. The data presented represent the means ± standard errors of the values in duplicate or triplicate for each of the individual samples from representative experiments.
RESULTS
Aluminum phthalocyanine chloride is essentially nontoxic to both stages of Leishmania amazonensis but sensitizes them for light-mediated cytolysis.
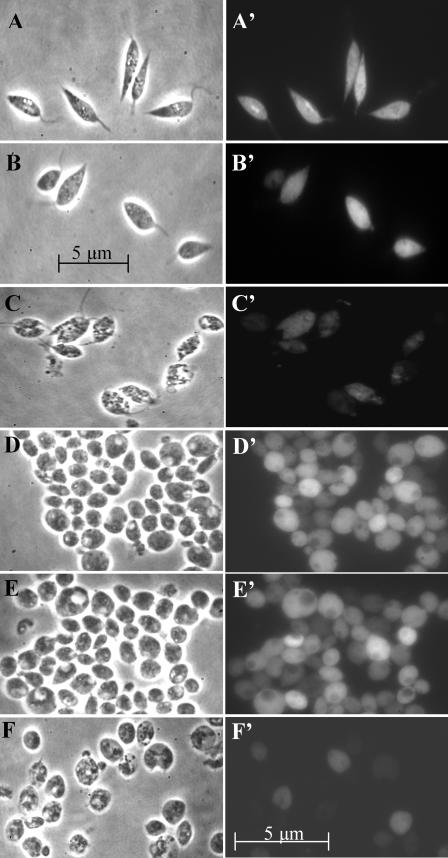
The lack of toxicity of AlPhCl to both stages of L. amazonensis was clearly indicated by observations of the wild-type cells (data not shown) and gfp transfectants under appropriate conditions of treatment by phase-contrast microscopy (Fig. 1A to F) and fluorescent microscopy (Fig. 1A′ to F′). On exposure to light alone (Fig. 1A and A′) or to AlPhCl alone up to 5 μg · ml−1 (Fig. 1B and B′), the promastigotes remained viable, as clearly indicated by their motility and structural integrity (Fig. 1A and B) and the homogeneous GFP fluorescence (Fig. 1A′ and B′). When the cells were exposed to the dye at 1 μg · ml−1 at a fluence of ∼3.6 J · cm−2, the cells were killed rapidly, as indicated by cessation of their flagellar motility, their appearance as ghost cells (Fig. 1C), and weak or patchy GFP fluorescence (Fig. 1C′). Under similar experimental conditions, axenic amastigotes maintained their structural integrity (Fig. 1D and E) and cellular fluorescence (D′ and E′) but became deformed and shriveled (Fig. 1F) and lost cellular fluorescence (Fig. 1F′) when AlPhCl treatment was followed by light exposure. The microscopic assessments of cytolysis for axenic amastigotes are substantiated by fluorimetric analysis of their cell and supernatant fractions for GFP fluorescence. Nearly all fluorescence was associated with cell fractions for the samples presented in Fig. 1D and E and with the supernatants for those shown in Fig. 1F (data not shown) (see the legends for Fig. 2C and 3B for information of relevance). The rapid and drastic morphological changes observed make it possible to quantitatively evaluate the photosensitivity of Leishmania to light-induced cytolysis based on this criterion. The loss of GFP fluorescence from photolysed cells provides additional and more sensitive criteria to verify their death, since some lysed cells remained structurally visible but were dimly fluorescent or totally nonfluorescent (compare Fig. 1C and C′ and Fig. 1F and F′). The GFP fluorescence assay proved superior to microscopy for assessment of the viability of axenic amastigotes (see below).
FIG.1.
Photosensitivity of Leishmania amazonensis promastigotes and axenic amastigotes in the presence of AlPhCl. Promastigotes and axenic amastigotes of Leishmania amazonensis expressing GFP were incubated at 50 × 106 · ml−1 with or without 1 to 5 μg · ml−1 AlPhCl at 25°C in the dark for 16 h in medium 199 plus 10% HIFBS, pH 7.4, for promastigotes and at 33°C in Grace's medium plus 20% HIFBS, pH 5.3, for axenic amastigotes. The cells were subsequently exposed to light at a fluence of 3.6 J · cm−2 (2.5 mW · cm−2 red light at >650 nm). After light exposure, cell suspensions in ∼5-μl aliquots was each placed on a glass slide and covered with a 25-mm2 coverslip for observation under a phase-contrast (A to F) or an epifluorescence (A′ to F′) microscope. (A, A′, D, and D′) Untreated controls; (B, B′, E, and E′) cells treated with 5 μg · ml−1 AlPhCl without light; (C, C′, F, and F′) cells treated with 1 μg · ml−1 of AlPhCl plus light. Note that both promastigotes (A to C) and amastigotes (D and E) remain intact and viable after light exposure alone (A and D) or after incubation with AlPhCl alone (B and E). Light plus photosensitizer induces the lysis of both promastigotes and amastigotes (C and F), resulting in the loss of cellular GFP fluorescence (C′ and F′).
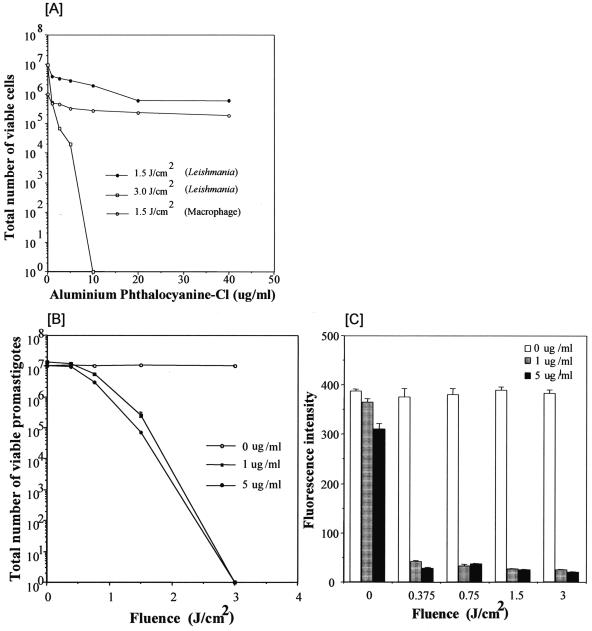
FIG. 2.
Photosensitivity of Leishmania amazonensis promastigotes and J774 macrophages in presence of AlPhCl. (A) Promastigotes grown to the stationary phase were resuspended to 107 · ml−1 in Hank's balanced salt solution plus 0.01% bovine serum albumin with increasing concentrations of AlPhCl, as indicated. J774 cells were similarly treated for comparison. Solid circles and open squares, AlPhCl-treated promastigotes apparently viable after exposure to 1.5 and 3 J · cm−2 of red light (at 1.67 mW · cm−2), respectively; open circles, viable and intact J774 cells, as determined by trypan blue exclusion. (B) Leishmania amazonensis promastigotes grown to the stationary phase were resuspended to 50 × 106 · ml−1 in medium 199 plus 10% HIFBS and treated with AlPhCl at 0, 1, and 5 μg · ml−1 for 16 h. Aliquots of 200 μl (107 cells) were withdrawn for exposure to red light fiber optic illumination at various fluences up to 3 J · cm−2 (2.5 mW · cm−2) (300-W quartz lamp; General Electric), as indicated. Immediately after exposure, cells were examined under a phase-contrast microscope by using a ×100 oil immersion lens to determine the ratio of the number of viable to the number of ghost cells. Fifty percent cell lysis was noted for 1 μg · ml−1 (solid square) and 5 μg · ml−1 (solid circle) at fluences of 0.8 J · cm−2 and 0.5 J · cm−2, respectively. Open circle, promastigotes remain viable after exposure to light illumination alone without preincubation with the dye. (C) Leishmania amazonensis promastigotes expressing GFP were grown to the stationary phase as described in Materials and Methods and resuspended to 50 × 106 · ml−1 in medium 199 plus 10% HIFBS. Cells were treated with AlPhCl at 0, 1, and 5 μg · ml−1 for 16 h and exposed to red light as described in the legend to panel B. Immediately after exposure, 2.5 × 106 cells in aliquots of 50 μl each were withdrawn and inoculated into 1 ml medium 199 plus 10% HIFBS and incubated at 25°C for 4 days to allow the survivors to grow. Their viability is estimated from GFP fluorescence levels fluorimetrically at 507 nm following excitation at 488 nm. See Materials and Methods for details.
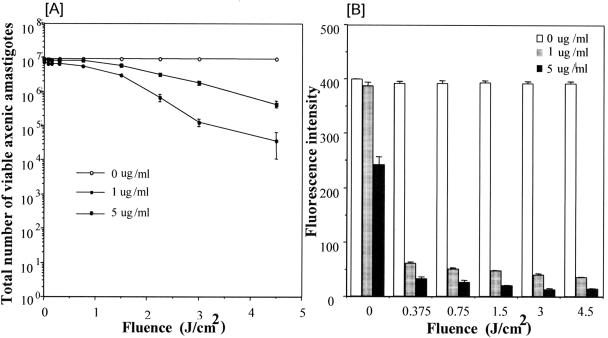
FIG. 3.
Photosensitivity of Leishmania amazonensis axenic amastigotes pretreated with AlPhCl. (A) Axenic amastigotes were treated under the same conditions as described for promastigotes in the legend to Fig. 2B. Cells were resuspended in Grace's medium plus 20% HIFBS at 50 × 106 · ml−1 in the presence of AlPhCl at 0, 1 and 5 μg · ml−1. After incubation for 16 h at 33°C, aliquots of 200 μl (107 cells) were each exposed to different doses of red light as indicated and viability was determined, as described in legend to Fig. 2B. Fifty percent cell lysis was noted for 1 μg · ml−1 (solid squares) and 5 μg · ml−1 (solid circles) of AlPhCl at fluences of 1.5 J · cm−2 and 1 J · cm−2, respectively. There was no cytolysis of axenic amastigotes exposed to light alone (open circles). (B) Leishmania amazonensis axenic amastigotes expressing GFP were grown to the stationary phase in Grace's medium plus 20% HIFBS under a selective pressure of 5 μg · ml−1 of tunicamycin. Stationary-phase cells were resuspended to 50 × 106 · ml−1 in the same medium without drug selection and treated with AlPhCl at 0, 1, and 5 μg · ml−1 for 16 h at 33°C. Treated cells were exposed to light, as described for panel A. Immediately after exposure, 2.5 × 106 cells in aliquots of 50 μl were each inoculated in 1 ml Grace's medium plus 20% HIFBS containing 5 μg · ml−1 tunicamycin and incubated at 33°C for 4 days to allow the growth of the survivors. The GFP fluorescence of cells grown from the survivors was assessed fluorimetrically, as described in the legend to Fig. 2C.
AlPhCl was initially evaluated quantitatively at graded concentrations up to 40 μg · ml−1 for its activity against motile promastigotes at a low cell density of 107 · ml−1 and at fluences of 1.5 and 3 J · cm−2, i.e., exposure of cells at an irradiance of 1.7 mW · cm−2 (Fig. 2A). Cells were exposed to light immediately after the addition of the dye in appropriate amounts. More than 100 cells from each sample were examined posttreatment by phase-contrast microscopy to count the ratio of the number of viable cells to the number of dead or ghost cells. The conditions used were found to be either suboptimal (Fig. 2A, solid circles) or outside the linear range of the dose-fluence effect curve (Fig. 2A, open squares). However, without light exposure, there was no apparent change in the integrity and motility of promastigotes even in the presence of AlPhCl up to 40 μg · ml−1 for the duration of the experiment (data not shown). Significantly, there was very limited lysis of the J774 cells immediately after exposure to AlPhCl up to 40 μg · ml−1 followed by illumination, as determined by trypan blue dye exclusion (Fig. 2A, open circles). The promastigotes thus appeared to be minimally 10- to 20-fold more sensitive than the J774 cells to photosensitization by AlPhCl when sensitivity was assessed immediately after treatment. After further incubation for 16 h posttreatment, although all cells used showed increased cytolysis with an increasing intensity of the treatments, the margin of differential viability increased to >800-fold in favor of the mammalian cells versus the Leishmania cells under the conditions of, for example, 1 μg AlPhCl · ml−1 and a fluence of 1.5 J · cm−2 (data not shown).
Additional experiments were thus carried out by varying the fluence by using promastigotes pretreated for 16 h with lower AlPhCl concentrations at no more than 5 μg · ml−1. Cells were preincubated with the dye overnight in attempt to “load” them to saturation, thereby eliminating this potential variable, as is the case in the first experiment when the cells were exposed to the dye and light illumination almost simultaneously. Under these experimental conditions, 50% cytolysis of either wild-type promastigotes (data not shown) or gfp transfectants was noted at a fluence of ∼1 J · cm−2, regardless of whether the dye was left in the cell suspensions or removed from the cells after preincubation (Fig. 2B). The difference in the dye concentrations between 1 μg · ml−1 (Fig. 2B, solid squares) and 5 μg · ml−1 (solid circles) was very marginal under these experimental conditions. In either case, no cytolysis was evident without light illumination (Fig. 2B). The viability of the gfp transfectants for all posttreatment samples presented in Fig. 2B was further assessed by their ability to grow. Equal aliquots of all samples at each time point were inoculated into culture medium in separate wells. After incubation for 4 days, cultures reached the turbidity of the stationary phase with a cell density of 40 × 106 to 50 × 106 cells · ml−1 among the controls, which were exposed to either light alone or AlPhCl alone, regardless of the fluence or the dye concentration used; in contrast, no motile promastigotes were microscopically visible among those which were exposed to the dye plus light illumination in various combinations except for the one with the mildest treatments of 1 μg · ml−1 of the dye and a fluence of 0.37 J · cm−2 (data not shown). Upon further incubation, few viable promastigotes in the latter eventually grew to full turbidity, while no growth was evident for the remainder of the promastigotes treated with dye-light combinations at greater strengths. These observations were substantiated quantitatively by fluorimetry of cells harvested from these samples for GFP fluorescence (Fig. 2C). All samples exposed to light alone at all fluences (Fig. 2C, blank bars at fluences of 0 to 3 J · cm−2) and samples exposed to dye alone (Fig. 2C, gray and dark bars at a fluence of 0 J · cm−2) gave the highest fluorescence intensities of ∼400 and >300, respectively, consistent with an increase in cellular GFP levels due to the growth of these cells. The fluorescence intensity was marginally detectable at levels of <50 among all samples exposed to various dye plus light combinations, indicative of the presence of few or no viable cells after these treatments (Fig. 2C, gray and dark bars at fluences of 0.37 to 3 J · cm−2). Clearly, evaluation of promastigote viability microscopically (Fig. 2B) is not as sensitive as evaluation by fluorimetry for GFP (Fig. 2C). This indicates that some promastigotes, which remain intact and motile immediately or shortly after treatments, subsequently lose their viability, even though growth inhibition under the conditions used cannot be totally ruled out.
When evaluated under the same experimental conditions, axenic amastigotes were found to be as susceptible or, at best, slightly less susceptible than promastigotes to photosentitization with AlPhCl for cytolysis. The GFP fluorescence assay for viability after growth was found to be more accurate than morphometric analysis of intact versus ghost cells by microscopy for axenic amastigotes. By the latter method of assessment, all axenic amastigotes remained intact when they were exposed to dye alone (Fig. 3A; 0 fluence) or light alone (Fig. 3, open circles, at all fluences), as found for promastigotes (Fig. 2A, open circles); however, complete lysis of axenic amastigotes was not visualized even at a fluence higher than that used for 100% cytolysis of promastigotes; and, in addition, 50% cytolysis of axenic amastigotes appeared to require fluences of ∼1 and ∼2 J · cm−2 at 5 and 1 μg · ml−1 of the dye, respectively (Fig. 3A, squares and solid circles, respectively). These values, which are higher than those noted for promastigotes assessed under the same experimental conditions, proved to be overestimated. This is indicated by GFP fluorescence assays for viability with a separate set of samples simultaneously prepared with gfp-transfected amastigotes, as already described for promastigote transfectants (Fig. 2C). Based on the results of this viability assay, axenic amastigotes appeared to be essentially as sensitive as promastigotes to all combinations of dye plus light exposure conditions used; notable was a slight but expected difference between the two dye concentrations (Fig. 3B, gray and dark bars at all fluences from 0.375 to 4.5 J · cm−2). The overestimation by microscopy apparently results from the properties of axenic amastigotes as small nonmotile cells, which are thus less amenable to morphological analysis than the larger and motile promastigotes. The fluorescence intensity of the sample exposed to the dye alone at 5 μg · ml−1 was lower than those for the samples not exposed to the dye at all or exposed to a lower concentration of the dye alone at 1 μg · ml−1 (Fig. 3B, 0 fluence, solid bar versus blank and gray bars, respectively). We attribute this to periodic brief exposure of the samples to light, by necessity, for observation. That AlPhCl alone is intrinsically nontoxic to Leishmania is supported by the observations from two separate experiments. When they were handled under the normal laboratory lighting conditions, both promastigotes and axenic amastigotes were found to grow, albeit at a slightly lower cell density than untreated cells, in the presence of up to 10 μg · ml−1 of AlPhCl. They subsequently sustained repeated subcultures and grew just as well as the untreated wild-type cells (data not shown). Thus, the photosensitizer alone is essentially nontoxic to Leishmania at the concentrations tested unless treated cells are further exposed to light at a high intensity.
The microscopic and fluorimetric data presented clearly indicate that both Leishmania promastigotes and amastigotes are susceptible to photosensitization, dependent on the concentrations of the photosensitizer and the fluence used.
Photolysis of intracellular parasites as well as J774 macrophages after infection with axenic amastigotes pretreated with aluminum phthalocyanine chloride.
Since AlPhCl alone at up to 5 μg · ml−1 had essentially no effect on the viability of Leishmania (Fig. 2 and 3), untreated axenic amastigotes and those pretreated with this dye at different concentrations (1 to 5 μg · ml−1) were used to infect J774 cells (Fig. 4 and 5). Preincubation of wild-type axenic amastigotes (data not shown) and gfp transfectant axenic amastigotes with the dye had no noticeable effect on their infectivity, as they were taken up by macrophages similarly to the untreated controls. In these infected cultures, untreated amastigotes with light exposure alone (Fig. 4A) and amastigotes pretreated with the dye but without light exposure (Fig. 4B) remained intact and were uniformly fluorescent (Fig. 4A′ and B′) in clear parasitophorous vacuoles (Fig. 4A and B). In contrast, those pretreated with 1 and 5 μg · ml−1 of AlPhCl underwent intracellular lysis after light exposure, as clearly shown by the loss of their structural integrity (Fig. 4C and D) and, in addition, by the release of GFP from these lysed amastigotes into the parasitophorous vacuoles, rendering the entire vacuoles fluorescent (Fig. 4C′ and D′, arrows).
FIG.4.
Preincubation of Leishmania amazonensis amastigotes with AlPhCl sensitizes them for light-induced intracellular degradation in macrophages of the J774 line. Axenic amastigotes expressing GFP were incubated in Grace's medium plus 20% HIFBS at 50 × 106 cells · ml−1 with or without AlPhCl (1 μg and 5 μg · ml−1) at 33°C for 16 h. Amastigotes (5 × 107) sedimented at 3,500 × g for 5 min were mixed with 5 × 106 J774 cells in RPMI 1640 plus 20% HIFBS for infection at 35°C. After infection for 5 h, all cultures were irradiated at a fluence of 4.5 J · cm−2. After light exposure, cells were observed by phase-contrast (A to D) and epifluorescence (A′ to D′) microscopy as described in the text. (A and A′) J774 cells infected with untreated axenic amastigotes and exposed to light illumination; (B and B′) cells infected with axenic amastigotes preincubated with 5 μg AlPhCl · ml−1 alone without light exposure; (C, C′, D, and D′) cells infected with axenic amastigotes preincubated with 1 μg (C and C′) and 5 μg (D and D′) AlPhCl · ml−1, followed by light exposure. Note that J774 cells phagocytized axenic amastigotes, regardless of whether they received pretreatment with AlPhCl. J774 cells contained intact amastigotes which were exposed to light alone without dye pretreatment (A and A′) or pretreated with the dye alone without light exposure (B and B′). Those preincubated with the dye were degraded intracellularly upon exposure to light (C and D). Intracellular degradation of amastigotes results in the release of GFP into parasitophorous vacuoles (C′ and D′, arrows).
FIG. 5.
Leishmania amazonensis axenic amastigotes preincubated with AlPhCl infect J774 cells but sensitize both for photolysis. Axenic amastigotes were resuspended to 50 × 106 · ml−1 for preincubation at 33°C with different concentrations of AlPhCl (0, 1, and 5 μg · ml−1) in Grace's medium containing 20% HIFBS for 16 h. J774 cells were mixed with AlPhCl-preincubated axenic amastigotes at a host cell-to-parasite ratio of 1:10 in RPMI 1640 containing 20% HIFBS. The mixture was plated at 106 J774 cells · ml−1 · well−1 in 12-well culture plates for infection at 35°C. After incubation for 5 h (A and B) and 24 h (C and D), samples in duplicate were irradiated individually with red light (>650 nm) at the indicated fluence, i.e., 2.5 mW · cm−2. Sixteen hours after irradiation, samples were counted in the presence of trypan blue to determine the number of viable J774 cells in a hemacytometer (A and C) and under a phase-contrast microscope to estimate the number of intact intracellular amastigotes (B and D). See Materials and Methods for experimental details of assessing the viability of J774 cells, inclusive of both already detached cells and those dislodged by medium aspiration. Note that only trypan blue-unstained and thus viable J774 cells are presented in panels A and C.
The fate of dye-pretreated axenic amastigotes versus untreated controls and that of infected macrophages were further analyzed quantitatively in greater detail. The viability of J774 cells was not assessed until 16 h after irradiation for more accurate evaluation to ensure the exclusion of those cells which were not immediately killed. The viability of Leishmania amastigotes in the infected macrophages was assessed microscopically for their integrity and GFP fluorescence intensity. All sets of cultures were exposed to light at increasing fluences up to 4.5 J · cm−2 (for 0 to 30 min) under otherwise identical conditions 5 h (Fig. 5A and B) and 24 h (Fig. 5C and D) after infection. In both cases, the total number of macrophages (Fig. 5A and C) and the total number of macrophage-associated amastigotes (Fig. 5B and D) per culture were estimated 16 h after light exposure. The total number of macrophages infected with untreated axenic amastigotes and the total number of untreated axenic amastigotes in these cultures (Fig. 5A to D, open circles) remained unchanged during the entire period of experimentation, as expected. Cultures infected with axenic amastigtoes preincubated with the dye showed a decrease in the total number of both macrophages and parasites. The level of decrease was dependent on the concentrations of the dye used to pretreat the inoculating amastigotes, the duration of infection, and the fluence (Fig. 5A to D, solid squares versus solid circles). Infected cultures with amastigotes pretreated with 1 μg · ml−1 of the dye showed a limited decrease in both macrophages (Fig. 5A and C, solid squares) and amastigotes (Fig. 5B and D, solid squares), although the latter decreased more proportionally with increasing fluence. Infected cultures with amastigotes pretreated with 5 μg · ml−1 of the dye showed decreases in the total number of both macrophages (Fig. 5A and C, solid circles) and parasites (Fig. 5B and D, solid circles) per culture not only with the fluence but also with the duration of the infection (Fig. 5A and B versus Fig. 5C and D, solid circles). After 5 and 24 h of infection without exposure to light, the total number of amastigotes per culture decreased by severalfold more than that for the untreated controls or those treated with 1 μg · ml−1 of AlPhCl (Fig. 5B and D, solid circle at time zero of light exposure versus solid square and open circles at the same zero time point). This suggests that pretreatment of amastigotes with 5 μg · ml−1 of the dye is indeed slightly inhibitory, which may result from inadvertent exposure to light and which may be further exacerbated after prolonged infection for 24 h with macrophages at 35°C. Cultures infected for 24 h with amastigotes pretreated with 5 μg · ml−1 of the dye did not contain either viable macrophages (Fig. 5C, solid circle, 4.5 J · cm−2) or viable amastigotes (Fig. 5D, solid circle, 4.5 J · cm−2) after exposure to the highest fluence. The results obtained indicate that pretreatment of axenic amastigotes not only sensitized them for intracellular degradation but also sensitized their host cells to meet a similar fate upon light exposure.
In three separate experiments, J774 cells already infected with amastigotes were treated with 1, 5, or 10 μg · ml−1 of the dye. The treatment alone produced no appreciable effect on these cultures in comparison to the appearance of the untreated controls. Upon exposure to light, infected macrophages were lysed in a dose-dependent manner in the presence of AlPhCl (data not shown), as expected.
DISCUSSION
Evidence is presented in the present study that AlPhCl sensitizes cultured Leishmania amazonensis for photolysis in vitro, indicative of its intrinsic sensitivity to photodynamic therapy. This observation supports the clinical utility of such therapeutic procedures for the treatment of cutaneous leishmaniasis (16, 18). Especially relevant is the demonstration that cytolysis of this cutaneous Leishmania species is rapid and extensive only with a combination of the photosensitizer and light but not with the individual treatments alone (Fig. 1 to 3). The cellular event observed is similar to the light-hastened suicidal photolysis of transgenic Leishmania in response to delta-aminolevulinate-induced uroporphyria (29). Both observations are consistent with the generation of ROS as the cytolytic mechanism via excitation of photosensitizers (3, 28), i.e., uroporphyrin from within in the previous case (29) and AlPhCl introduced externally in this study. Also notable in the present study is the fact that leishmanolysis occurs at low concentrations of AlPhCl in combination with low light intensity relative to the conditions needed to lyse J774 macrophages, as demonstrated here (Fig. 2 and 5). Further study is required to determine whether the cytolysis of Leishmania in both cases results from apoptosis or necrosis, or both, as reported for other eukaryotic cells (1, 12, 24). Of interest is the fact that AlPhCl mediates the photosensitivity of axenic amastigotes, which is clinically relevant, as it is equivalent to the intracellular stage of Leishmania spp. found in experimental and clinical leishmaniasis of mammalian hosts (11). This stage of Leishmania appears to be similarly sensitive as promastigotes or the insect stage to AlPhCl sensitization for photolysis (Fig. 1 to 3). This observation is somewhat unexpected, since amastigotes are often thought to withstand better than promastigotes the oxidative stress associated with the antimicrobial activities of the professional phagocytes, in which the former stage of Leishmania take residence in the mammalian hosts. This preliminary observation raises the question of whether Leishmania may indeed differ stage specifically in the handling of the oxygen free radicals, which are expected to form secondarily on exposure of AlPhCl to light, by their own antioxidative pathways, e.g., peroxidoxin, superoxide dismutase, and trypanothion reductase (4, 6, 22, 25). If not, whether they might somehow exploit the antioxidant mechanisms of their host cells for their survival is an even more intriguing issue for investigation.
Although cultured Leishmania is intrinsically sensitive to “photodynamic therapy” under the simple conditions presented, how it works in the presence of their host cells or macrophages in vivo and, by inference, in the clinical setting is apparently more complicated. Selective killing of intracellular amastigotes does not seem to occur by treating infected macrophages with AlPhCl or infecting these cells with amastigotes pretreated with AlPhCl followed by light illumination. Although intact macrophages with lysed or lysing intracellular amastigotes were noted (Fig. 4C and D), we were unable to find the “ideal” experimental conditions which specifically lysed intracellular amastigotes but which left their host cells substantially intact or viable. This is different from the observations of blood parasites, i.e., Trypanosoma cruzi (15), Plasmodium (19, 30), and Babesia (19), whose susceptibilities to photodynamic treatment have been exploited for their elimination from blood bank samples. It was also noted previously (29) that photosensitized Leishmania caused the cytolysis of infected host cells.
The therapeutic effects of photodynamic therapy observed for cutaneous leishmaniasis (16, 18) thus do not seem to result from the specific accumulation of the photosensitizers in the parasitophorous vacuoles, which then sensitizes the amastigotes therein for selective photolysis. We evaluated this in the present study with infected macrophages in vitro by using AlPhCl because its uptake by phagocytes is anticipated when it is associated with serum proteins. The therapeutic effectiveness of photosensitizers based on this principle may be improved by enhancing such uptake by conjugating them to ligands for high-capacity endocytosis via, for example, scavenger receptors of macrophages (13, 14, 20). Stimulation of the local immune response by irradiation coupled with the use of parasite antigens released from photodynamically lysed amastigotes as natural vaccines (10) may explain the therapeutic benefits of the procedures used for the treatment of cutaneous leishmaniasis (16, 18). The principle of photodynamic therapy offers new ways to improve prophylaxis and therapy of infectious and other diseases (29). A vast array of known photosensitizers with different physical and chemical properties is available, and these offer considerable modalities for cellular activity and photodynamic effects in conjunction with light illumination at different wavelengths and intensities for the effective treatment of diseases.
Acknowledgments
We thank Michael Hamblin for suggestions.
Financial support was obtained from NIH (grant AI-20486).
REFERENCES
- 1.Agarwal, M. L., M. E. Clay, E. J. Harvey, H. H. Evans, A. R. Antunez, and N. L. Oleinick. 1991. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 51:5993-5996. [PubMed] [Google Scholar]
- 2.Asilian, A., A. Sadeghinia, G. Faghihi, and A. Momeni. 2004. Comparative study of the efficacy of combined cryotherapy and intralesional meglumine antimoniate (Glucantime) vs. cryotherapy and intralesional meglumine antimoniate (Glucantime) alone for the treatment of cutaneous leishmaniasis. Int. J. Dermatol. 43:281-283. [DOI] [PubMed] [Google Scholar]
- 3.Bachowski, G. J., E. Ben-Hur, and A. W. Girotti. 1991. Phthalocyanine-sensitized lipid peroxidation in cell membranes: use of cholesterol and azide as probes of primary photochemictry. J. Photochem. Photobiol. 9:307-321. [DOI] [PubMed] [Google Scholar]
- 4.Barr, S. D., and L. Gedamu. 2001. Cloning and characterization of three differentially expressed peroxidoxin genes from Leishmania chagasi. Evidence for an enzymatic detoxification of hydroxyl radicals. J. Biol. Chem. 276:34279-34287. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Hur, E., and I. Rosenthal. 1985. The phthalocyanines: a new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 47:145-147. [DOI] [PubMed] [Google Scholar]
- 6.Castro, H., C. Sousa, M. Santos, A. Cordeiro-da-Silva, L. Flohe, and A. M. Tomas. 2002. Complementary antioxidant defense by cytoplasmic and mitochondrial peroxiredoxins in Leishmania infantum. Free Radic. Biol. Med. 33:1552-1562. [DOI] [PubMed] [Google Scholar]
- 7.Chan, M. M., J. C. Bulinski, K.-P. Chang, and D. Fong. 2003. A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitol. Res. 89:266-271. [DOI] [PubMed] [Google Scholar]
- 8.Chan, W. S., A. J. MacRobert, D. Phillips, and I. R. Hart. 1989. Use of charge coupled device camera for imaging of intracellular phthalocyanines. Photochem. Photobiol. 50:617-624. [DOI] [PubMed] [Google Scholar]
- 9.Chang, K.-P. 1980. Human cutaneous Leishmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science 209:1240-1242. [DOI] [PubMed] [Google Scholar]
- 10.Chang, K.-P., S. G. Reed, B. S. McGwire, and L. Soong. 2003. Leishmania model for microbial virulence: the relevance of parasite multiplication and pathoantigenicity. Acta Trop. 85:375-390. [DOI] [PubMed] [Google Scholar]
- 11.Debrabant, A., M. B. Joshi, P. F. Pimenta, and D. M. Dwyer. 2004. Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int. J. Parasitol. 34:205-217. [DOI] [PubMed] [Google Scholar]
- 12.de Castro Pazos, M., C. Pacheco-Soares, N. Soares da Silva, R. A. DaMatta, and M. T. Pacheco. 2003. Ultrastructural effects of two phthalocyanines in CHO-K1 and HeLa cells after laser irradiation. Biocell 27:301-309. [PubMed] [Google Scholar]
- 13.Demidova, T. N., and M. R. Hamblin. 2004. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 17:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries, H. E., A. C. Moor, T. M. Dubbelman, T. J. van Berkel, and J. Kuiper. 1999. Oxidized low-density lipoprotein as a delivery system for photosensitizers: implications for photodynamic therapy of atherosclerosis. J. Pharmacol. Exp. Ther. 289:528-534. [PubMed] [Google Scholar]
- 15.Docampo, R., S. N. Moreno, R. P. Muniz, F. S. Cruz, and R. P. Mason. 1983. Light-enhanced free radical formation and trypanocidal action of gentian violet (crystal violet). Science 220:1292-1295. [DOI] [PubMed] [Google Scholar]
- 16.Enk, C. D., C. Fritsch, F. Jonas, A. Nasereddin, A. Ingber, C. L. Jaffe, and T. Ruzicka. 2003. Treatment of cutaneous leishmaniasis with photodynamic therapy. Arch. Dermatol. 139:432-434. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, B. B., A. Krieger-Liszkay, and R. L. Eggen. 2004. Photosensitizers neutral red (type I) and rose Bengal (type II) cause light-dependent toxicity in Chlamydomonas reinhardtii and induce the Gpxh gene via increased singlet oxygen formation. Environ. Sci. Technol. 38:6307-6313. [DOI] [PubMed] [Google Scholar]
- 18.Gardlo, K., Z. Horska, C. D. Enk, L. Rauch, M. Megahed, T. Ruzicka, and C. Fritsch. 2003. Treatment of cutaneous leishmaniasis by photodynamic therapy. J. Am. Acad. Dermatol. 48:893-896. [DOI] [PubMed] [Google Scholar]
- 19.Grellier, P., R. Santus, E. Muoray, V. Agmon, J. C. Maziere, D. Rigomier, A. Dagan, S. Gatt, and J. Schrevel. 1997. Photosensitized inactivation of Plasmodium falciparum- and Babesia divergens-infected erythrocytes in whole blood by lipophilic pheophorbide derivatives. Vox Sang. 72:211-220. [DOI] [PubMed] [Google Scholar]
- 20.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassab, K., D. Dei, G. Ronucci, G. Jori, and O. Coppellotti. 2003. Phthalocyanine-photosensitized inactivation of pathogenic protozoan, Acanthamoeba palestinensis. Photochem. Photobiol. Sci. 2:668-672. [DOI] [PubMed] [Google Scholar]
- 22.Krauth-Siegel, R. L., H. Bauer, and R. H. Schirmer. 2005. Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia. Angew. Chem. Int. Ed. 44:690-715. [DOI] [PubMed] [Google Scholar]
- 23.Liu, X., and K.-P. Chang. 1994. Identification by extrachromosomal amplification and overexpression of a zeta-crystallin/NADPH-oxidoreductase homologue constitutively expressed in Leishmania spp. Mol. Biochem. Parasitol. 66:201-210. [DOI] [PubMed] [Google Scholar]
- 24.Luo, Y., and D. Kessel. 1997. Initiation of apoptosis versus necrosis by photodynamic therapy with chloroaluminum phthalocyanine. Photochem. Photobiol. 66:479-483. [DOI] [PubMed] [Google Scholar]
- 25.Plewes, K. A., S. D. Barr, and L. Gedamu. 2003. Iron superoxide dismutases targeted to the glycosomes of Leishmania chagasi are important for survival. Infect. Immun. 71:5910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pushpan, S. K., S. Venkatraman, V. G. Anand, J. Sankar, D. Parmeswaran, S. Ganesan, and T. K. Chandrashekar. 2002. Porphyrins in photodynamic therapy—a search for ideal photosensitizers. Curr. Med. Chem. Anti-Canc. Agents 2:187-207. [DOI] [PubMed] [Google Scholar]
- 27.Reithinger, R., M. Mohsen, M. Wahid, M. Bismullah, R. J. Quinnell, C. R. Davies, J. Kolaczinski, and J. R. David. 2005. Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a randomized, controlled trial. Clin. Infect. Dis. 40:1148-1155. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal, I., and E. Ben-Hur. 1992. Ascorbate-assisted, phthalocyanine-sensitized photohaemolysis of human erythrocytes. Int. J. Radiat. Biol. 62:481-486. [DOI] [PubMed] [Google Scholar]
- 29.Sah, J. F., H. Ito, B. K. Kolli, D. A. Peterson, S. Sassa, and K.-P. Chang. 2002. Genetic rescue of Leishmania deficiency in porphyrin biosynthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. J. Biol. Chem. 277:14902-14909. [DOI] [PubMed] [Google Scholar]
- 30.Zhao, X. J., S. Lustigman, Y. S. Li, M. E. Kenney, and E. Ben-Hur. 1997. Structure-activity and mechanism studies on silicon phthalocyanines with Plasmodium falciparum in the dark and under red light. Photochem. Photobiol. 66:282-287. [DOI] [PubMed] [Google Scholar]