Abstract
Pneumococci frequently colonize the upper respiratory tract, and these pneumococci are believed to act as a reservoir for infection of the lower respiratory tract and bacteremia. We investigated how the pneumococcal toxin pneumolysin affects the capacity of pneumococci to infect the upper and lower respiratory tract of the mouse. Wild-type Streptococcus pneumoniae serotype 2 and 3 strains, a serotype 2 pneumolysin-deficient mutant, and a serotype 2 mutant with the pneumolysin gene reinserted were used to study differences in colonization and disease. In addition, we also examined a pneumococcal chimeric mutant (capsule type switched from serotype 2 to serotype 3) to gain further insight into the role that capsule plays in nasopharyngeal infection. Absence of pneumolysin was found to be associated with significantly lower numbers of pneumococci in the nasopharynx, trachea, and lungs. Differences in pneumococcal capsule type were found to have significant effects on pneumococcal infection of the nasopharynx, trachea, and lungs. However, it was the combination of capsule type and genetic background that was important, and the influence of this combination varied with the site of infection. For example, in the nasopharynx the wild-type serotype 3 strain and the capsule-switched mutant behaved similarly, whereas in the lungs the mutant that was switched to serotype 3 survived less well than the wild-type serotype 3 strain. The combination of capsule type and genetic background also determined virulence. Thus, the wild-type serotype 3 strain was virulent, whereas the capsule-switched mutant was avirulent.
Streptococcus pneumoniae (the pneumococcus) is a major cause of morbidity and mortality. It causes a wide variety of diseases ranging from pneumonia, meningitis, otitis media, septicemia, and sinusitis to comparatively benign soft tissue infections. The pneumococcus can also colonize the upper respiratory tract. It has been estimated that the upper respiratory tracts of 40% or more of children carry pneumococci. Furthermore, the pneumococcus is asymptomatically carried by healthy people in the upper respiratory tract, and depending on the age of onset, the environment of the host, and the presence of underlying respiratory infections, the carriage rate can vary from 5 to 70% (4, 20, 29).
It is generally accepted that pneumococcal infection begins with the colonization of the nasopharynx, which allows progression of pneumococci into the lower parts of the respiratory tract, eventually leading to systemic disease (6, 23). Nasopharyngeal colonization also serves as an important reservoir of transmission to other susceptible individuals. The rapid increase in multiple-antibiotic-resistant strains and the high rate of pneumococcal carriage in children and the elderly, plus the problems that asymptomatic carriage presents for diagnosis, warrant further study of the virulence factors involved in nasopharyngeal colonization.
The pneumococcal polysaccharide capsule and the multifunctional pneumococcal toxin pneumolysin are known to be important virulence factors. The capsule is identified as a virulence factor by virtue of its antiphagocytic activity (28). The capsule clearly plays a role in disease, and acapsular mutants are avirulent; however, the role of the capsule in colonization is less clear. Furthermore, the combined effect on colonization of the capsule structure and the underlying strain differences are unknown.
Pneumolysin exhibits a wide variety of activities consistent with virulence. In vivo, a pneumolysin-deficient pneumococcus exhibits reduced virulence in the mouse, with slower growth in the lungs and delayed development of the cellular inflammatory response (8, 15). In addition to being a cytolytic toxin, at sublytic concentrations pneumolysin alters the functioning of immune cells (1, 18), inhibits ciliary beating on the human respiratory epithelium (10, 11), stimulates tumor necrosis factor alpha and interleukin-1β release from human monocytes (14), activates phospholipase A2 in pulmonary cells (24), and separates epithelial cell tight junctions (23). Pneumolysin is also known to inhibit neutrophil respiratory burst (21) and to activate the classical complement pathway in the absence of anti-pneumolysin antibody (19). It is unclear however, which, if any, of these attributes of pneumolysin are required for virulence.
MATERIALS AND METHODS
Bacteria.
S. pneumoniae serotype 2 strain D39 (NCTC 7466) and serotype 3 strain A66 were obtained from the National Collection of Type Cultures, London, United Kingdom. The pneumolysin-negative mutant PLN-A and the pneumolysin-positive strain Pn+ are insertion duplication mutants of D39 (7). Strain FP50 is a derivative of D39 in which the type 2 capsule locus of D39 was replaced by a kanamycin resistance gene and then the uncapsulated derivative was transformed with a PCR fragment containing the type 3 locus of A66. S. pneumoniae strain A66 (5, 22) was originally obtained by H. P. Bernheimer. Bacteria were identified as pneumococci prior to infection by Gram staining, by the catalase test, by α-hemolysis on blood agar plates, and by optochin sensitivity. The capsular polysaccharide serotypes were confirmed by the Quellung reaction.
For use in in vivo infection experiments, pneumococci were cultured and passaged in mice as described previously (8), and they were subsequently stored at −70°C. When required, suspensions were thawed at room temperature and bacteria were harvested by centrifugation before resuspension in sterile phosphate-buffered saline.
Infection of mice.
Female MF1 outbred mice that were 9 weeks old and weighed 30 to 35 g (Harlan Olac, Bichester, United Kingdom) were lightly anesthetized with 2.5% (vol/vol) fluo-thane (AstraZeneca, Macclesfield, United Kingdom) over oxygen (1.5 to 2 liters/min). As described previously (1, 8, 15), 50 μl of phosphate-buffered saline containing 1 × 106 CFU of S. pneumoniae was then administered into the nostrils of each mouse. The inoculum was confirmed by plating on blood agar plates following infection.
At preselected times following infection, preselected groups of mice were killed by cervical dislocation. The nasopharyneal tissue was exposed, and cartilage and associated soft tissue within the nasopharynx were removed into 10 ml of sterile distilled water and weighed. The trachea and lungs also were removed separately into 10 ml of sterile distilled water and weighed. All tissues then were homogenized in a Stomacher-Lab blender (Seward Medical, London, United Kingdom). Viable counts in homogenates were determined as described previously (8). The mice did not have detectable levels of anti-type 2, anti-type 3, or anti-pneumolysin antibodies.
Statistics.
Data were analyzed by Student's t test or one-way analysis of variance, and the Mann-Whitney U test was used for analysis of virulence studies. A P value of <0.05 was considered statistically significant.
RESULTS
Wild-type S. pneumoniae serotype 2 and 3 strains, a serotype 2 pneumolysin-deficient mutant, and a serotype 2 mutant with the pneumolysin gene reinserted were used to study the effect of pneumolysin and capsule on colonization and disease. A pneumococcal chimeric mutant (capsule type switched from serotype 2 to serotype 3) also was used to gain insight into the combined effect that capsule type and genetic background have in nasopharyngeal infection and virulence. A serotype 2 strain with the wild-type pneumolysin gene reinserted into the chromosome (Pn+) was used, and the behavior of this strain was identical to that of the wild-type serotype 2 strain in all assays; in no case were the results obtained with the Pn+ strain significantly different statistically (P > 0.05) from the results obtained with serotype 2 parent strain D39. All of the strains exhibited very similar growth rates under in vitro conditions.
Nasopharyngeal infection.
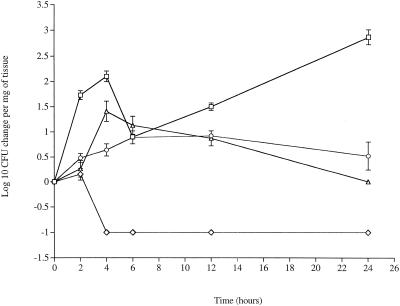
The serotype 2 strain, strain D39, showed significantly increased numbers (P < 0.05) over 24 h in the nasopharynx compared to the time zero levels (Fig. 1). Pneumolysin was essential for this increase and for viability in the nasopharynx. The number of PLN-A cells began to decline sharply after 2 h postinfection, and no pneumococci were recovered at 4 h postinfection or later (Fig. 1).
FIG. 1.
Changes in numbers of pneumococci in the nasopharynx tissue over 24 h. Symbols: □, wild-type serotype 2 strain D39 (zero log value, 0.6); ⋄, pneumolysin-deficient strain D39 (zero log value, 1); ○, wild-type serotype 3 strain A66 (zero log value, 2); ▵, FP50 mutant in which the capsule type was switched from serotype 2 to serotype 3 (zero log value, 1.55). The values are means ± standard errors of the means (n = 8 for each time point).
The serotype made a significant contribution to pneumococcal behavior in the nasopharynx. Thus, serotype 3 strain A66 showed no significant increase in numbers (P > 0.05) after 24 h. When the serotype 2 strain (D39) was switched to a serotype 3 capsule (FP50), it behaved like the wild-type serotype 3 strain (P > 0.05).
No pneumococci were recovered from blood during the first 12 h of infection. Pneumococci were present in blood at 12 and 24 h postinfection, as described previously (15).
Tracheal infection.
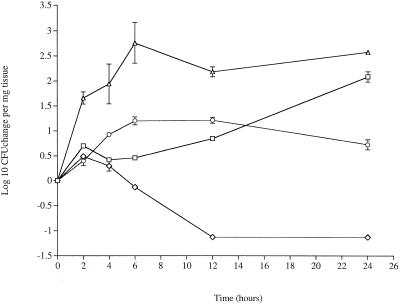
In the trachea, serotype 2 pneumococci showed a significant increase by 24 h postinfection (P < 0.05) compared to time zero levels (Fig. 2). As in the nasopharynx, the absence of pneumolysin had a dramatic impact, although the decline in the number of PLN-A cells was slower than the decline in the nasopharynx. No PLN-A was recovered after 12 h postinfection.
FIG. 2.
Changes in numbers of pneumococci in the trachea over a 24-h period. Symbols: □, wild-type serotype 2 strain D39 (zero log value, 1.41); ⋄, pneumolysin-deficient strain D39 (zero log value, 1.13); ○, wild-type serotype 3 strain A66 (zero log value, 1); ▵, FP50 mutant in which the capsule type was switched from serotype 2 to serotype 3 (zero log value, 1.4). The values are means ± standard errors of the means (n = 8 for each time point).
The serotype 3 levels were not significantly different from the serotype 2 levels over the 24-h experiment, although the serotype 2 levels had begun to increase by 24 h postinfection compared to the serotype 3 levels (although not significantly so). The behavior of FP50 showed, however, that the strain background can affect the influence of the capsule serotype in the trachea. The FP50 levels were significantly greater than the levels of both D39 during the first 12 h postinfection (P < 0.05) and serotype 3 strain A66 throughout the 24-h experiment (P < 0.05).
Lung infection.
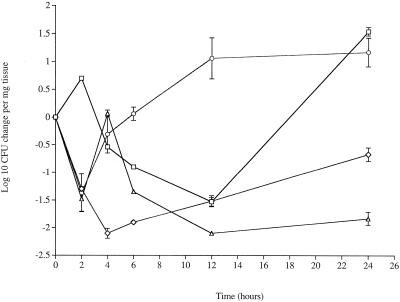
As we have reported previously (8), the numbers of both D39 and PLN-A cells decreased in the lungs during the first 12 h postinfection (Fig. 3); then the numbers of cells of both isolates increased, but by 24 h postinfection the numbers of D39 cells were significantly higher (P < 0.05) than the numbers of PLN-A cells.
FIG. 3.
Changes in numbers of pneumococci in the lungs over a 24-h period. Symbols: □, wild-type serotype 2 strain D39 (zero log value, 3.13); ⋄, pneumolysin-deficient strain D39 (zero log value, 2.3); ○, wild-type serotype 3 strain A66 (zero log value, 3.2); ▵, FP50 mutant in which the capsule type was switched from serotype 2 to serotype 3 (zero log value, 3.27). The values are means ± standard errors of the means (n = 8 for each time point).
Both capsular serotype and strain background affected behavior in the lungs. The numbers of D39 (serotype 2) cells were significantly greater than the numbers of A66 (serotype 3) cells at 2 h postinfection, but the serotype 3 levels increased significantly between 4 and 12 h compared to the serotype 2 levels (P < 0.05). However, the two strains reached equivalent levels by 24 h postinfection (Fig. 3). The FP50 levels, on the other hand, showed that capsule had a major impact early in infection, with the levels similar to wild-type serotype 3 levels during the first 4 h postinfection. However after 4 h the apparently advantageous effect of the serotype 3 capsule was found to be dependent on the strain background. Thus, the number of serotype 3 strain FP50 cells declined significantly (P < 0.05), in contrast to the number of wild-type serotype 3 strain A66 cells.
Virulence assay.
Virulence was assessed by determining the time necessary to attain a moribund state following intranasal infection. As reported before (8, 15), D39 rendered all mice moribund by around 45 h. It has also been reported before (1) that PLN-A is completely avirulent; all mice survive without symptoms of disease. The serotype 3 strain (A66) also was virulent, and all infected mice became moribund (mean time, 69 h). However, when the serotype 3 capsule replaced the serotype 2 capsule in D39, the resulting strain (FP50) was avirulent and all mice survived for the 7 days of the experiment without symptoms (P < 0.05 compared to D39 and A66).
DISCUSSION
Bacterial adherence is regarded as the first step in colonization of respiratory surfaces (13). Adherence to bronchoepithelial cells in vivo is followed by cytokine-induced cell activation and subsequent invasion into or between these cells (16; B. Smith, Q. Cheng, and M. Hostetter, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. P-233, 1998). Pneumococci adhere to nasopharyngeal buccal epithelial cells, to lung vascular endothelial and bronchoepithelial cells, and also to lung resting pneumocytes (2, 3, 9, 27, 28) via lectin-like interactions (25, 27) or protein-protein interactions (26, 30). It has been suggested that pneumococci bind to at least two different cell surface sugar moieties on noninflamed pulmonary epithelia and vascular endothelia (25), and the receptors involved are known to be different for nasopharyngeal cells and for lung cells (9, 27). Likewise, the platelet-activating factor receptor (pafR) has been suggested to serve as a ligand in the lung (28), whereas the polymeric immunoglobulin receptor (pigR) has this role in the nasopharynx (26). The pneumococcal adherence mechanisms of resting and activated respiratory cells also differ, however, with the platelet-activating factor receptor implicated only in activated tissue cells (12, 28). Up-regulation of pigR by proinflammatory cytokines also has been widely reported (17, 26).
It seems, therefore, that the pneumococcus has a wide range of molecules to utilize for colonization, but the identity of the host ligand used varies with the environment. The data in this paper suggest that pneumolysin is essential if the pneumococcus is to successfully exploit the host tissue cell ligands in the nasopharynx and trachea, as well as the lung. Thus, pneumolysin must be added to the collection of pneumococcal factors that have been suggested to regulate adherence, including the opacity locus, permeases, and cell wall structural proteins (25, 29). Although this toxin avidly binds to eukaryote membranes, it seems unlikely that it acts directly as an adhesin. It is also uncertain how the toxin may regulate pneumococcal adhesions. What appears to be more likely is that it functions via its effect on the host. Indeed, the in vitro activities of pneumolysin, such as separation of epithelial cell tight junctions and inhibition of the mucociliary beat (10, 11, 23), are in keeping with such a role. However, pneumolysin is also a potent inducer of inflammation, and perhaps a level of inflammation in the upper and lower respiratory tract is required for expression of the ligands for pneumococcal binding. The rapid elimination of pneumolysin-deficient pneumococci, especially from the nasopharynx, suggests that inflammation-induced ligands are the key ligands.
The importance of pneumolysin for colonization is consistent with the in vitro data of Rubins et al. (25). These authors found that pneumolysin-deficient pneumococci, including a deficient version of strain D39, attached significantly less well to respiratory epithelial cells. However, an strain effect was apparent from their data, since a pneumolysin-deficient serotype 3 strain was not significantly different from the wild type. A further indication that the contribution of the toxin can vary from strain to strain comes from a comparison of our in vivo data. Rubins and colleagues (25) concluded that pneumolysin is not a major determinant of colonization of the murine nasopharynx on the basis their work with a pneumolysin-deficient serotype 14 strain. Clearly, our data challenge this uncomplicated conclusion and suggest that the context of other pneumococcal factors determines the overall contribution of pneumolysin.
The conclusion that the overall context of the strain determines the importance of individual factors is supported by our investigation of the role of capsule in colonization. In addition to pneumolysin, differences in pneumococcal serotype also influenced upper and lower respiratory tract colonization, but the effect of serotype differed with the location of the pneumococcus and also with the strain. The influence of strain was most obvious when survival after intranasal infection was determined. The serotype 3 chimeric strain FP50 was significantly less virulent than the wild-type serotype 3 strain or the serotype 2 parent strain. This result was reflected by growth in the lungs, where FP50 fared significantly less well than either of the wild-type strains. In the trachea, the strain background also influenced the impact of capsule type. In contrast, in the nasopharyx the serotype 3 pneumococci fared less well than any serotype 2 strain.
These data support previous results (17) obtained by using intraperitoneal infection, which suggested that the strain background determined the virulence of a particular serotype. It was found that a highly virulent serotype 5 strain became avirulent when the capsule type was switched to serotype 3, whereas a serotype 6B strain became significantly more virulent when the capsule type was switched to serotype 3 (17). Interestingly, however, Kelly et al. found that alteration of the D39 capsular serotype from serotype 2 to serotype 3 had no affect on the virulence after intraperitoneal infection (17). In contrast, we found that alteration of strain D39 from serotype 2 to serotype 3 significantly reduced virulence after intranasal infection. Thus, it appears that not only should the combination of capsule and strain be considered but also the site of infection determines the importance of the interaction. Although the capsule is an important virulence factor, it appears to play no direct role in adhesion (17). Changes in virulence and colonization resulting from capsule switching probably are due to changes in exposure of pneumococcal surface molecules used to adhere to host cell receptors. Such pneumococcal surface changes also may result in different levels of binding of humoral factors.
Overall, the data in this paper combined with other published observations emphasize the multifactorial nature of pneumococcal virulence and the conclusion that the contribution of the factors to virulence, alone and in combination, varies with the site of infection. We now intend to investigate the various host inflammatory mediators stimulated by pneumolysin and involved during nasopharyngeal infection, along with their relative contributions to nasopharyngeal pathology.
Editor: E. I. Tuomanen
REFERENCES
- 1.Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behavior of pneumococci in pneumonia. Microb. Pathog. 24:167-174. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, B., C. Svanborg, and L. A. Hanson. 1982. Pneumococcal adhesion to human pharyngeal cells. Scand. J. Dis. Suppl. 33:96-97. [PubMed]
- 3.Andersson, B., J. Dahmen, T. Frejg, H. Leffler, G. Magnusson, and C. Svanborg. 1983. Identification of a active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J. Exp. Med. 158:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austrian, R. 1986. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 18(Suppl. A):35-45. [DOI] [PubMed] [Google Scholar]
- 5.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of tranformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthelson, R., A. Mobasseri, D. Zopf, and P. Simon. 1998. Adherence of Streptococcus pneumoniae to respiratory epithelial cells is inhibited by sialylated oligosaccharides. Infect. Immun. 66:1439-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57: 2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canvin, J. R., A. P. Marvin, M. Sivakumaran, J. C. Paton, G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 172:119-123. [DOI] [PubMed] [Google Scholar]
- 9.Cundell, D., and E. Tuomanen. 1994. Receptor specificity of adherence of Streptococcus pneumoniae to human type 2 pneumocytes and vascular endothelial cells in vitro. Microb. Pathog. 17:361-374. [DOI] [PubMed] [Google Scholar]
- 10.Feldman, C., T. J. Mitchell, P. W. Andrew, G. J. Boulnois, S. C. Reed, H. C. Todd, P. J. Cole, and R. Wilson. 1990. The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb. Pathog. 9:275-284. [DOI] [PubMed] [Google Scholar]
- 11.Feldman, C., R. Reed, A. Rutman, N. C. Munro, D. K. Jeffrey, A. Brain, V. Lund, T. J. Mitchell, P. W. Andrew, G. J. Boulnois, H. C. Todd, P. J. Cole, and R. Wilson. 1991. The effect of Streptococcus pneumoniae on intact respiratory epithelium. Eur. Respir. J. 5:576-583. [PubMed] [Google Scholar]
- 12.Gerard, C., I. Idanpann-Heikkila, and E. Tuomanen. 1995. Streptococcus pneumoniae anchors to activated eukaryotic cells by the receptor for platelet activating factor. Nature 377:435-438. [DOI] [PubMed] [Google Scholar]
- 13.Hakansson, A., I. Carlstedt, J. Davies, A.-K. Mossberg, H. Sabharwal, and C. Svanborg. 1996. Aspects on the interaction of Streptococcus pneumoniae and Haemophilus influenzae with human respiratory tract mucosa. Am. J. Respir. Crit. Care Med. 154:S187-S191. [DOI] [PubMed]
- 14.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadioglu, A., N. Gingles, K. Grattan, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadioglu, A., J. Sharpe, I. Lazou, C. Svanborg, C. Ockleford, T. J. Mitchell, and P. W. Andrew. 2001. Use of green fluorescent protein in visualisation of pneumococcal invasion of broncho epithelial cells in vivo. FEMS Microiol. Lett. 194:105-110. [DOI] [PubMed] [Google Scholar]
- 17.Kelly, T., J. P. Dillard, and J. E. Yother. 1994. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect. Immun. 62:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell, T. J., P. W. Andrew, G. J. Boulnois, C. J. Lee, R. A. Lock, and J. C. Paton. 1992. Molecular studies of pneumolysin as an aid to vaccine design. Zentralbl. Bakteriol. 23:429-438.
- 19.Mitchell, T. J., P. W. Andrew, F. K. Saunders, A. N. Smith, and G. J. Boulnois. 1991. Complement activation and antibody binding by pneumolysin via a region homologous to a human acute phase protein. Mol. Microbiol. 5:1883-1888. [DOI] [PubMed] [Google Scholar]
- 20.Obaro, S. K., M. A. Monteil, and D. C. Henderson. 1996. The pneumococcal problem. Br. Med. J. 312:1521-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzi, G., L. Masala, F. Iannelli, R. Manganelli, L. S. Havarstein, L. Piccoli, D. Simon, and D. A. Morrison. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rayner, C. F. J., A. D. Jackson, A. Rutman, A. Dewar, T. J. Mitchell, P. W. Andrew, P. J. Cole, and R. Wilson. 1995. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect. Immun. 63:442-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubins, J. B., P. W. Andrew, T. J. Mitchell, and D. E. Niewoehner. 1994. Pneumolysin activates phospholipase A2 in pulmonary artery endothelial cells. Infect. Immun. 62:3829-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubins, J. B., A. H. Paddock, D. Charboneau, A. M. Berry, J. C. Paton, and E. N. Janoff. 1998. Pneumolysin in pneumococcal adherence and colonization. Microb. Pathog. 25:337-342. [DOI] [PubMed] [Google Scholar]
- 26.Sollid, L. M., D. Kvale, P. Brandtzaeg, G. Markussen, and E. Thorsby. 1987. Interferon gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J. Immunol. 138:4303-4306. [PubMed] [Google Scholar]
- 27.Tuomanen, E. 1997. The biology of pneumococcal infection. Pediatr. Res. 42:253-258. [DOI] [PubMed] [Google Scholar]
- 28.Tuomanen, E. I., and R. H. Masure. 2000. Molecular and cellular biology of pneumococcal infection, p. 295-308. In A. Tomasz (ed.), Streptococcus pneumoniae molecular biology and mechanisms of disease. Mary Ann Liebert, New York, N.Y.
- 29.Wu, H.-Y., A. Virolainen, B. Mathews, J. King, M. W. Russell, and D. E. Briles. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonisation model in adult mice. Microb. Pathog. 23:127-137. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, J., I. Idanpaan-Heikkila, and E. Tuomanen. 1999. The pneumococcal lic locus: involvement in choline metabolism and virulence. Mol. Microbiol. 31:1477-1488. [DOI] [PubMed] [Google Scholar]