Abstract
Dengue fever and dengue hemorrhagic fever are caused by infection with any one of the four dengue viruses (DVs) and are significant public health burdens throughout the tropics. Higher viremia levels are associated with greater dengue disease severity. A therapeutic intervention to suppress viremia early in DV infection could potentially ameliorate severe disease. Recombinant alpha interferon 2a (rIFN-α-2a, Roferon-A) suppressed DV replication in human peripheral blood mononuclear cells in vitro. We therefore examined the effects of rIFN-α-2a and pegylated recombinant IFN-α-2a (PEG-rIFN-α-2a, PEGASYS) on DV serotype 2 (DV-2) viremia in rhesus monkeys. Flavivirus-naïve monkeys were inoculated with DV-2 and randomized to receive a single dose of rIFN-α-2a (10 million international units/m2) versus placebo or PEG-rIFN-α-2a (6 μg/kg) versus placebo 1 day after the onset of viremia. Serial daily viremia levels were measured, and convalescent-phase DV-2 neutralizing antibody titers were determined. Compared to placebo, a single injection of rIFN-α-2a temporarily suppressed DV-2 replication and delayed the time to peak viremia by a median of 3 days. However, measures of total viral burden were not different between the two groups. A single injection of PEG-rIFN-α-2a significantly lowered daily viremia levels and improved virus clearance, starting 48 h after administration. There were no significant differences in DV-2 neutralizing antibody titers between the treatment and placebo groups at 30 and 90 days postinfection. Based on their individual effects, future studies should investigate a combination of rIFN-α-2a and PEG-rIFN-α-2a for suppression of dengue virus viremia and as a potential therapeutic intervention.
Dengue is an emerging arboviral disease caused by infection with one of the dengue viruses (DVs), a group of four antigenically related mosquito-borne flaviviruses (18). A spectrum of disease is recognized following DV infection, ranging from asymptomatic or a self-limited mild febrile illness to classic dengue fever to the most severe form of illness, dengue hemorrhagic fever. Dengue hemorrhagic fever is characterized by the rapid development of plasma leakage and a hemorrhagic diathesis around the time of defervescence and viremia resolution. Morbidity and mortality are the result of hypotension and shock, at times accompanied by severe disseminated intravascular coagulation and bleeding (33). Over 1 billion people are at risk of infection and more than 100 countries have endemic DV transmission. There are approximately 50 to 100 million cases of dengue fever and 250,000 to 500,000 cases of dengue hemorrhagic fever annually in the world, and the incidence is rising (13, 35, 43). During peak seasons and epidemic years, hospitals and community health resources in the tropics are often overwhelmed caring for the high number of severe dengue cases, particularly among children (5). At the present time, no specific intervention exists for the treatment of severe dengue fever or dengue hemorrhagic fever. Close observation and supportive care is the current mainstay of treatment.
Dengue hemorrhagic fever is associated with higher viral burdens and viremia levels than dengue fever (25, 26, 39). Peak levels of viremia occur during the febrile phase of illness and well before the manifestations of dengue hemorrhagic fever occur. Effective suppression of dengue virus viremia early in illness could ameliorate the severity of DV infections, the morbidity and mortality of dengue hemorrhagic fever and the burden they place on limited hospital and other health resources in developing countries.
Alpha interferon (IFN-α) is the predominant type I IFN, a family of cytokines that were discovered by their ability to render cells resistant to viral infection (21). There are 14 subtypes of human IFN-α. Two recombinant forms, rIFN-α-2a and rIFN-α-2b, are available for clinical use. rIFN-α-2a (Roferon-A) has been used to treat chronic viral infections, such as hepatitis B and C, but it requires frequent dosing (15). The inconvenience of frequent repeated injections for the treatment of chronic disorders led to attempts to develop long acting IFNs which might be more convenient and efficacious. Pegylated rIFN-α-2a (PEG-rIFN-α-2a, PEGASYS) is rIFN-α-2a modified by the covalent conjugation of a 40-kDa branched methoxy-polyethylene glycol (PEG) molecule. Pegylation decreases systemic clearance of rIFN-α-2a and produced better clinical outcomes in hepatitis C than nonpegylated rIFN-α-2a (17, 45).
IFN-α can inhibit the replication of DVs and other related flaviviruses in vitro (6, 16, 40). We were interested in studying whether rIFN-α-2a or PEG-rIFN-α-2a could inhibit DV replication in vivo in a clinically relevant manner. There is no animal model of dengue hemorrhagic fever. Nonhuman primates develop a measurable viremia after inoculation with certain DV strains, but do not become ill (14). We studied the ability of rIFN-α-2a and PEG-rIFN-α-2a to suppress dengue virus viremia in rhesus monkeys when administered after the onset of viremia and found that these rIFN-α preparations can inhibit DV replication in vivo.
MATERIALS AND METHODS
Animals.
Healthy adult colony-born rhesus monkeys (Macaca mulatta) of Indian origin, free of neutralizing antibodies to DVs 1, 2, 3, and 4, and Japanese encephalitis virus were selected for this investigation. All the animals were 4 to 10 years old, weighed between 5 and 10 kg, and were caged individually in a double-screened modified outdoor housing facility. Tranquilization with ketamine (5 to 20 mg/kg, intramuscularly) was used for some animal manipulations associated with physical examination and phlebotomy.
Monkeys were also trained for pole and collar restraining for obtaining 1-ml blood samples from peripheral veins without anesthesia. The animal care and use was conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations relating to animals and experiments involving animals, and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, 1996 edition. The two animal protocols were reviewed and approved by the United States Army Medical Component, Armed Forces Research Institute of Medical Sciences, Institutional Animal Care and Use Committee, and by the Animal Use Review Division of the United States Army Medical Research and Material Command. The United States Army Medical Component, Armed Forces Research Institute of Medical Sciences, Animal Care and Use Program is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Viruses, cells, and IFN-α.
For the in vitro experiments, DV-2 strain 16681 was used to infect human peripheral blood mononuclear cells (PBMC) obtained from healthy volunteers. PBMC were isolated from heparinized whole blood by Ficoll-Hypaque density centrifugation and maintained in cell culture with RPMI medium plus 10% fetal calf serum. For infection of monkeys, DV-2 strain S16803 was used. The passage history of the DV-2 S16803 stock has been previously described (8). The DV-2 stocks were titered by limiting dilution plaque assay on LLC-MK2 cells (36). DV-2 S16803 plaque titers were determined within 4 weeks prior to the inoculation, and residual virus was repeat titered postinoculation. Vials of rIFN-α-2a (Roferon-A, Roche Laboratories) and PEG-rIFN-α-2a (PEGASYS, Roche Laboratories) were reconstituted according to the manufacturer's instructions. Animals in the placebo groups received normal saline solution.
Detection and quantification of DV-2.
A qualitative, serotype-specific reverse transcription (RT)-PCR assay (24) was used to identify the onset of viremia in plasma collected daily from the monkeys. DV-2 RNA copies/ml in cell culture supernatants and macaque plasma were quantified by a serotype-specific, fluorogenic RT-PCR assay, as previously described (19, 25). For quantification, all samples were tested in triplicate and in a blinded fashion. Interassay precision was determined by high-positive, low-positive, and negative controls. The lower limit of quantification was 3.80 log10 DV-2 RNA copies/ml.
Neutralizing antibody assay.
Plaque reduction neutralizing antibody titers against reference strains of DVs 1 to 4 and Japanese encephalitis virus were performed on LLC-MK2 cells by use of standard methods (36). The 50% plaque reduction neutralizing antibody titer (PRNT50) was calculated using a log probit regression method and reported as the reciprocal titer. A PRNT50 of <10 was considered undetectable.
Nonhuman primate study designs. (i) rIFN-α-2a study.
We randomized 24 monkeys into two groups, treatment (n = 12) and placebo (n = 12). All animals had blood drawn 42 days prior to study entry and on study day 0 in order to confirm that they had not been previously infected with DVs 1 to 4 or Japanese encephalitis virus. All animals were pole and collar trained to allow daily venipuncture without anesthesia. On study day 0, all animals were inoculated subcutaneously with 1 × 105 PFU DV-2 S16803. Plasma samples for DV-2 RT-PCR were collected serially every morning for 14 days and then again on study day 20. The onset of dengue virus viremia was monitored by the qualitative DV-2 RT-PCR assay on daily plasma samples; 24 h after viremia was first detected in an animal, those in the treatment group were given a single subcutaneous injection of rIFN-α-2a (10 million international units [MIU]/m2). The placebo group was injected subcutaneously with an equivalent volume of sterile normal saline solution.
(ii) PEG-rIFN-α-2a study.
Ten male monkeys were randomized into two groups, treatment (n = 5) and placebo (n = 5). The study design was the same as described above, except that the treatment group was injected subcutaneously with PEG-rIFN-α-2a (6 μg/kg) 24 h after viremia was first detected. Also, serial serum samples for DV-2 RT-PCR were collected daily for 20 days. Sera were also collected on study days 30 and 90 for plaque reduction neutralizing antibody titers.
Calculation of viral doubling time and elimination half-life.
Viremia increased in an exponential manner. The doubling time of DV-2 S16803 in individual animals was estimated by the following equation: doubling time = ln 2/[(log virusp-log virusf)/(study dayp − study dayf)], where log virusp is the log peak viremia level, log virusf is the log of the first viremia level measured above the lower limit of detection, study dayp is the day of peak viremia, and study dayf is the day of first viremia level measured above the lower limit of detection.
After reaching a peak level, viral clearance followed either one or two phases of single-order kinetics. The elimination half-life (t[1/2]) of DV-2 S16803 immediately following peak viremia in individual animals was estimated by the following equation: t[1/2] = −ln 2/[(log viruse-log virusp)/(study daye − study dayp)], where log viruse is the log of the viremia level at the end of the single-order kinetic elimination phase (first phase after peak if there were two phases), and study daye is the day of the viremia level at the end of the single-order kinetic elimination phase (first phase after peak if there were two phases).
Statistical analysis.
The SPSS software package (version 12.0) was used for statistical analyses. For normally distributed variables, comparisons between two groups were analyzed using Student's t test. For nonnormally distributed variables, comparisons between two groups were analyzed using the nonparametric Mann-Whitney U test. A P value of <0.05 was considered significant; 0.05 ≤ P ≤ 0.10 was considered a nonsignificant trend.
RESULTS
In vitro inhibition of DV-2 replication by rIFN-α-2a.
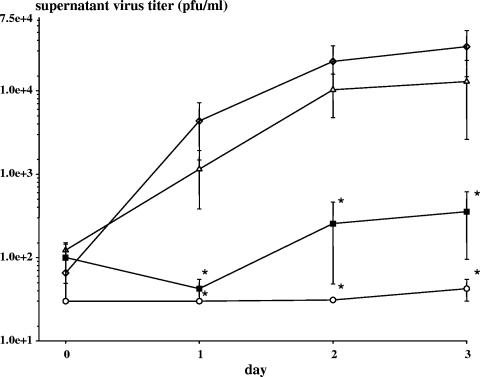
We examined the effect of exogenous rIFN-α-2a on DV-2 replication in healthy donor PBMC. Following virus adsorption and washing, rIFN-α-2a was added to DV-2 infected PBMC over a dose range from 5 to 500 U/ml. rIFN-α-2a inhibited DV-2 replication in PBMC in a dose-dependent fashion (Fig. 1). DV-2 supernatant titers decreased 100-fold with 50 U/ml rIFN-α-2a, a concentration that is achieved in the serum of adults receiving pharmacological doses of rIFN-α (1).
FIG. 1.
In vitro effect of rIFN-α-2a on DV-2 replication in PBMC. Healthy donor PBMC (n = 3) were adsorbed with DV-2 16681 for 2 h at a multiplicity of infection of 1, washed, and then cultured in medium with and without rIFN-α-2a. The virus titers in the cell culture supernatants were measured over the next 3 days. Symbols: ⋄, medium alone; ▵, rIFN-α-2a 5 U/ml; ▪, rIFN-α-2a 50 U/ml; ○, rIFN-α-2a 500 U/ml. Values are means ± standard error of the mean. *, P ≤ 0.05 compared to medium alone.
Effect of rIFN-α-2a on DV-2 viremia in nonhuman primates.
We next studied the in vivo effect of rIFN-α-2a in rhesus monkeys following DV-2 infection. 24 flavivirus-naïve monkeys were inoculated subcutaneously with 105 PFU of DV-2 strain S16803. As expected, none of the animals developed signs or symptoms of illness during the course of the study. Viremia began 2 to 5 days after DV-2 inoculation as measured by RT-PCR.
In order to approximate the potential use of rIFN-α-2a in a clinical setting, a single subcutaneous injection of 10 MIU/m2 rIFN-α-2a (n = 12) or placebo (n = 12) was given 1 day after the onset of detectable viremia. The duration of viremia in all animals ranged between 3 to 6 days. Time to peak viremia was delayed by a median of 3 days in the rIFN-α-2a group compared to placebo, and mean viremia levels were significantly lower in the rIFN-α-2a group compared to placebo over the third to fifth days of viremia (Fig. 2). However, mean peak viremia levels (peak log DV-2 RNA copies/ml, rIFN-α-2a, 5.73 ± 0.97, versus placebo, 5.40 ± 0.56, mean ± standard deviation) and the areas under the concentration-time curve (AUC) (rIFN-α-2a, 1.4 × 106 DV-2 RNA copies/ml/day, versus placebo, 5.7 × 105 DV-2 RNA copies/ml/day, median values) were not significantly different between the two groups. rIFN-α-2a appeared to have a short-lived suppressive effect on DV-2 viremia. Repeated, frequent administration of rIFN-α-2a is unlikely to be clinically feasible for treating dengue; therefore, we next studied the use of a longer-acting form of rIFN-α-2a, PEG-rIFN-α-2a.
FIG. 2.
Effect of rIFN-α-2a on DV-2 viremia in rhesus monkeys. We inoculated 24 flavivirus-naïve monkeys subcutaneously with DV-2 S16803; 24 h after onset of viremia was detected by qualitative RT-PCR, 12 animals were given a single subcutaneous injection of rIFN-α-2a (10 MIU/m2), and 12 animals were given placebo. Daily plasma viremia levels were measured by a quantitative, fluorogenic RT-PCR assay. The dotted line represents the lower limit of detection. Symbols: □, placebo group; •, treatment group, rIFN-α-2a 10 MIU/m2. ↑, time when rIFN-α-2a or placebo was given. Day of viremia is numbered consecutively from the first day viremia was detected by the qualitative RT-PCR assay (day 1). Values are means ± standard error of the mean. *, P < 0.05, rIFN-α-2a group versus placebo.
Effect of PEG-rIFN-α-2a on DV-2 viremia in nonhuman primates.
Using the same design as in the earlier rIFN-α-2a study, we examined the effect of a single injection of PEG-rIFN-α-2a on rhesus monkeys with DV-2 infection. 10 flavivirus-naïve monkeys were inoculated subcutaneously with 1 × 105 to 3 × 105 PFU of DV-2 strain S16803. In this study, all the animals had onset of viremia 1 day after virus inoculation, and the virus doubling time was significantly faster than in the previous monkey trial of rIFN-α-2a (DV-2 doubling time in placebo treated animals, 0.7 days versus 1.3 days [median values], PEG-rIFN-α-2a and rIFN-α-2a trials, respectively, P = 0.03). The duration of viremia in all animals ranged between 4 and 9 days; 48 h after intervention, monkeys who received PEG-rIFN-α-2a had mean daily viremia levels 0.6 to 1.4 logs lower than placebo over the ensuing 4 days (Table 1). Viremia levels decreased to undetectable levels subsequently in both groups.
TABLE 1.
Daily viremia levels in PEG-rIFN-α-2a-treated and placebo-treated monkeysa
| Groupb | Mean log10 DV-2 RNA copies/ml plasma ± SD on day of viremia:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2c | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | |
| PEG-rIFN-α | 4.65 ± 0.69 | 6.16 ± 0.45 | 5.81 ± 0.55 | 5.30 ± 0.40 | 4.36 ± 0.59 | 4.24 ± 0.51 | 4.11 ± 0.28 | 3.80 ± 0d | 3.88 ± 0.19 | 3.80 ± 0d |
| Placebo | 4.88 ± 0.36 | 6.17 ± 0.34 | 6.15 ± 0.53 | 5.93 ± 0.39 | 5.76 ± 0.45 | 5.06 ± 0.91 | 4.93 ± 0.95 | 4.15 ± 0.52 | 3.87 ± 0.17 | 3.80 ± 0d |
| Pe | >0.20 | >0.20 | >0.20 | 0.04 | 0.009 | 0.17 | 0.07 | >0.20 | >0.20 | >0.20 |
Day of viremia is numbered consecutively from the first day viremia was detected by the qualitative RT-PCR assay (day 1).
Five monkeys per group.
PEG-rIFN-α-2a (6 μg/kg) or placebo was given after blood was collected on viremia day 2.
Viremia level at or below the lower limit of detection.
P for comparison between PEG-rIFN-α-2a group and placebo.
Due to the rapid rise of DV-2 viremia in these animals, PEG-rIFN-α-2a or placebo was injected near peak levels of viremia. As such, there were no significant differences in mean peak viremia levels (peak log DV-2 RNA copies/ml: PEG-rIFN-α-2a, 6.16 ± 0.45, versus placebo, 6.39 ± 0.37, mean ± standard deviation, P = 0.7) or AUC ((DV-2 RNA copies/ml)/day: PEG-rIFN-α-2a, 2.6 × 106 versus placebo,5.9 × 106, median values, P = 0.2) between the two groups. In the placebo group, viremia levels in 3/5 monkeys continued to increase after intervention; whereas, viremia levels began to decrease immediately in 5/5 monkeys after PEG-rIFN-α-2a injection. After achieving peak viremia levels, the monkeys in the PEG-rIFN-α-2a group cleared DV-2 more rapidly than the placebo group (Fig. 3, nonsignificant trend).
FIG. 3.
Clearance of DV-2 in the plasma of monkeys after injection with PEG-rIFN-α-2a versus placebo. The elimination t[1/2] of circulating DV-2 immediately following peak viremia levels was calculated for each animal, as described in the Materials and Methods section. Symbols: ▵, placebo group (n = 5), ▾, treatment group, PEG-rIFN-α-2a 6 μg/kg (n = 5). Bars represent median values. *, P = 0.08, PEG-rIFN-α-2a group versus placebo.
Neutralizing antibody titers.
In the PEG-rIFN-α-2a study, plaque reduction neutralizing antibody titers to DV-2 were determined on sera collected 30 and 90 days after DV-2 inoculation. All animals in the PEG-rIFN-α-2a and placebo groups had detectable neutralizing antibodies against DV-2 on days 30 and 90. The PRNT50 values were not significantly different between the PEG-rIFN-α-2a and placebo groups at either time point (day 30 geometric mean PRNT50 1,282 versus 1,684, PEG-rIFN-α-2a versus placebo, respectively, P = 0.3; day 90 geometric mean PRNT50 1,161 versus 1,281, PEG-rIFN-α-2a versus placebo, respectively, P = 0.5).
DISCUSSION
Our data suggest that pharmacologic administration of rIFN-α preparations early in illness has the potential to be a useful therapeutic intervention for dengue. The antiviral effects of IFN-α have been well characterized, and IFN-α has an established track record of therapeutic use in certain malignancies and chronic viral infections (12, 32). rIFN-α was shown to inhibit DV replication in vitro when added before viral infection to human, nonhuman primate, and mosquito cell lines and primary human fibroblasts (6, 40). We have now demonstrated that rIFN-α can inhibit DV replication in primary human PBMC when added after virus adsorption and entry. Importantly, significant decreases in viral titers occurred with clinically achievable concentrations of rIFN-a.
In the nonhuman primate model of dengue virus viremia, our goal was to evaluate rIFN-α preparations in a manner that would best approximate its practical use in a clinical setting. Therefore, we gave the animals a single injection of the rIFN-α preparation at a dose that has been used safely in humans (15), and after viremia began. When given early after the onset of viremia, a single injection of rIFN-α-2a was able to inhibit DV-2 replication in vivo. Unfortunately, the antiviral effect was temporary and only delayed the subsequent rise and clearance of DV-2 in the monkeys. Pharmacokinetic studies of rIFN-α-2a in healthy human volunteers have shown that IFN-α-2a reaches maximal serum concentration in 7 to 12 h and then rapidly declines with an elimination half-life of 3 to 8 h (1, 15). Limited studies have found similar pharmacokinetics of rIFN-α in nonhuman primates (30, 41, 42). Pharmacodynamic studies of rIFN-α-2a in human volunteers have shown that serum activity of 2′,5′-oligoadenylate synthetase, a marker of IFN-α antiviral activity, reaches peak levels 24 h after treatment and then rapidly declines (15, 28). The rapid absorption but short half-life and antiviral effect of rIFN-α-2a were most likely responsible for the rightward shift of the viremia curve in rIFN-α-2a treated monkeys compared to placebo.
Although PEG-IFN-α-2a has a slower absorption rate than rIFN-α-2a (Tmax = 80 h), it has a longer elimination half-life (t[1/2] = 65 h). After a single dose of PEG-rIFN-α-2a in adult volunteers, serum 2′,5′-oligoadenylate synthetase activity reaches peak levels by 48 h and remains near peak levels for 1 week (31, 34). Supporting these pharmacodynamic data, in monkeys, we observed improved viral clearance and significantly lower daily viremia levels starting 48 h after PEG-rIFN-α-2a injection compared to placebo.
Two factors hampered the ability of PEG-rIFN-α-2a to produce a significant decrease in peak viremia or total viral burden. One was the slower absorption rate and longer time to reach peak antiviral activity for pegylated versus nonpegylated rIFN-α-2a. The second was the more rapid rise in viremia following DV-2 inoculation in the monkeys used in the PEG-rIFN-α-2a trial compared to the rIFN-α-2a trial. The reason for the faster virus doubling time and higher peak viremia levels in these animals is unclear. The ages, weights, and inoculation sites were comparable in both studies. No gender differences in viremia were observed (data not shown). All animals were flavivirus-naïve based on PRNT50 < 10 to DVs 1 to 4 and Japanese encephalitis virus from sera collected the day of virus inoculation.
Using postinoculation plaque titers, the animals in the PEG-rIFN-α-2a trial may have received up to 3 × 105 PFU of DV-2 instead of 105 PFU This potential difference in virus inoculum is unlikely to explain the faster doubling time and nearly 1 log higher peak viremia levels seen in these animals. By review of the animal medical records, 75% of the monkeys in the rIFN-α-2a trial were >2 years beyond any prior vaccinations or infections; the remaining 25% had received a malaria vaccine 1 year earlier. Whereas in the PEG-rIFN-α-2a trial, 70% of the monkeys received a malaria vaccine 9 months prior to DV-2 inoculation, and the remaining 30% received a challenge with Shigella bacteria 2 months prior to DV-2 inoculation. We do not know if the shorter interval between prior immune stimulation and DV-2 inoculation in the monkeys of the PEG-rIFN-α-2a trial contributed to higher DV-2 doubling times and peak levels. Activation of the immune system by immunizations to unrelated antigens have been shown to transiently increase human immunodeficiency virus type 1 viremia up to 1 month later (37).
Both preparations of IFN-α demonstrated some in vivo inhibition of DV-2 replication when given after viremia began. A combination of the rapid absorption and early antiviral effect of rIFN-α-2a, and the sustained antiviral activity of PEG-rIFN-α-2a, would have an excellent chance to significantly decrease viral burden in treated individuals. Prospective studies of DV infections in children have shown that viremia reaches peak levels within the first 72 h of febrile illness (25, 26, 39), and that higher peak viremia levels are associated with greater disease severity (25, 39). Presentation and recognition of dengue during this time period would allow early intervention, as reflected in the two nonhuman primate trials here. The magnitude of decrease in viremia produced by PEG-rIFN-α-2a was equivalent to the difference in mean viremia levels seen between dengue fever and dengue hemorrhagic fever (0.7 to 1.0 log viral RNA copies/ml) (9, 25, 26). A nearly 1-log suppression of dengue virus viremia by rIFN-α-2a plus PEG-rIFN-α-2a given within the first 72 h of illness might stem the subsequent immunological cascade that leads to hospitalized dengue fever or dengue hemorrhagic fever.
As potential therapeutic agents, rIFN-α-2a and PEG-rIFN-α-2a compare favorably to other antiviral compounds that have been proposed for dengue. They have an excellent record of clinical safety and tolerability in adults and children, especially with short-term therapy (15, 22, 31). Delivering a parenteral combination of rIFN-α-2a plus PEG-rIFN-α-2a at a single outpatient visit is simple, practical, and likely to be an affordable and cost-effective approach in many dengue endemic countries. Ribavirin can inhibit DV replication in vitro (20) and has also been considered as a potential therapeutic agent for dengue. However, a 10-day course of ribavirin beginning one day before virus inoculation had no effect on DV-1 viremia in rhesus monkeys (27).
While ribavirin monotherapy is unlikely to be successful in rapidly suppressing dengue virus viremia, it might have a role in combination therapy with rIFN-α, as has been seen with treatment of hepatitis C (11). Amantadine has been reported to inhibit DV replication in vitro (23). At the doses used to inhibit DV, amantadine inhibits replication of several other non-influenza A viruses due to its lysosomotropic effect (38). In vivo efficacy has only been seen against influenza A virus infections. Mycophenolic acid, an immunosuppressive agent used in solid organ transplantation, has also been reported to inhibit DV replication in human cell lines (7). Mycophenolic acid and other T-cell immunosuppressive agents can inhibit several viruses in vitro (3, 4, 29, 44), but this activity has never translated to in vivo efficacy with these agents (2, 10). Furthermore, the potential risks of using a T-cell immunosuppressive agent in a predominantly pediatric population with an acute systemic viral infection would be a significant concern.
Ours is only the second study in the published literature examining an antiviral strategy in the nonhuman primate model of dengue virus viremia and the first to demonstrate in vivo inhibition of DV replication. Future studies should test a single-dose combination of rIFN-α-2a and PEG-rIFN-α-2a in nonhuman primates with dengue virus viremia and, if promising, move to trials in the clinical setting.
Acknowledgments
This work was supported by the National Institutes of Health (NIH PO1 AI34533), the U.S. Army Medical Research and Materiel Command, and a Roche Laboratories Investigator Initiated Research Program grant.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the U.S. Department of Defense.
We also thank the Armed Forces Research Institute of Medical Sciences Virology staff, Sumitda Narupiti and Vipa Thirawuth, for the RT-PCR and fluorogenic RT-PCR assays; Naowayubol Nutkumhaeng and Somsak Imlarp for providing and titering virus stocks; Somkiat Changnak and Napaporn Latthiwongsakorn for serological assays; Choompun Manomuth, Tippawan Thipwong, Songdej Sangsri, Thongchai Khiankaew, and Prachakkra Panthusi for specimen processing and storage, Charles M. VanHoven and Wipa Chawachalasai for quality assurance; and Veterinary Medicine department staffs and Rawiwan Imerbsin, Srawauth Komchareon, Arvuth Kaewsupo, Phongsak Maneerat, Mana Saithasao, and Alongkorn Harnrujirakomjorn for handling and assessing animals.
REFERENCES
- 1.Anonymous. 2004. Physicians' desk reference. Medical Economics, Montvale, N.J.
- 2.Calabrese, L. H., M. M. Lederman, J. Spritzler, R. W. Coombs, L. Fox, B. Schock, B. Yen-Lieberman, R. Johnson, D. Mildvan, and N. Parekh. 2002. Placebo-controlled trial of cyclosporin-A in HIV-1 disease: implications for solid organ transplantation. J. Acquir. Immune Defic. Syndr. 29:356-362. [DOI] [PubMed] [Google Scholar]
- 3.Chapuis, A. G., G. Paolo Rizzardi, C. D'Agostino, A. Attinger, C. Knabenhans, S. Fleury, H. Acha-Orbea, and G. Pantaleo. 2000. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat. Med. 6:762-768. [DOI] [PubMed] [Google Scholar]
- 4.De Clercq, E., L. Naesens, L. De Bolle, D. Schols, Y. Zhang, and J. Neyts. 2001. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 11:381-395. [DOI] [PubMed] [Google Scholar]
- 5.DeRoeck, D., J. Deen, and J. D. Clemens. 2003. Policymakers' views on dengue fever/dengue haemorrhagic fever and the need for dengue vaccines in four southeast Asian countries. Vaccine 22:121-129. [DOI] [PubMed] [Google Scholar]
- 6.Diamond, M. S., T. G. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, M. S., M. Zachariah, and E. Harris. 2002. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology 304:211-221. [DOI] [PubMed] [Google Scholar]
- 8.Eckels, K. H., D. R. Dubois, R. Putnak, D. W. Vaughn, B. L. Innis, E. A. Henchal, and C. H. Hoke, Jr. 2003. Modification of dengue virus strains by passage in primary dog kidney cells: preparation of candidate vaccines and immunization of monkeys. Am. J. Trop. Med. Hyg. 69:12-16. [DOI] [PubMed] [Google Scholar]
- 9.Endy, T. P., A. Nisalak, S. Chunsuttiwat, D. W. Vaughn, S. Green, F. A. Ennis, A. L. Rothman, and D. H. Libraty. 2004. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J. Infect. Dis. 189:990-1000. [DOI] [PubMed] [Google Scholar]
- 10.Firpi, R. J., D. R. Nelson, and G. L. Davis. 2003. Lack of antiviral effect of a short course of mycophenolate mofetil in patients with chronic hepatitis C virus infection. Liver Transpl. 9:57-61. [DOI] [PubMed] [Google Scholar]
- 11.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 12.Gutterman, J. U. 1994. Cytokine therapeutics: lessons from interferon alpha. Proc. Natl. Acad. Sci. USA 91:1198-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman, M. G., and G. Kouri. 2004. Dengue diagnosis, advances and challenges. Int. J. Infect. Dis. 8:69-80. [DOI] [PubMed] [Google Scholar]
- 14.Halstead, S. B., H. Shotwell, and J. Casals. 1973. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J. Infect. Dis. 128:7-14. [DOI] [PubMed] [Google Scholar]
- 15.Haria, M., and P. Benfield. 1995. Interferon-α-2a: a review of its pharmacological properties and therapeutic use in the management of viral hepatitis. Drug Eval. 50:873-896. [DOI] [PubMed] [Google Scholar]
- 16.Harinasuta, C., C. Wasi, and S. Vithanomsat. 1984. The effect of interferon on Japanese encephalitis virus in vitro. Southeast Asian J. Trop. Med. Public Health 15:564-568. [PubMed] [Google Scholar]
- 17.Heathcote, E. J., M. L. Shiffman, W. G. Cooksley, G. M. Dusheiko, S. S. Lee, L. Balart, R. Reindollar, R. K. Reddy, T. L. Wright, A. Lin, J. Hoffman, and J. De Pamphilis. 2000. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N. Engl. J. Med. 343:1673-1680. [DOI] [PubMed] [Google Scholar]
- 18.Henchal, E. A., and J. R. Putnak. 1990. The dengue viruses. Clin. Microbiol. Rev. 3:376-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houng, H. S. H., R. C. M. Chen, D. W. Vaughn, and N. Kanesathasan. 2001. Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1,2,3 and 4, using conserved and serotype-specific 3′ non-coding sequences. J. Virol. Methods 95:19-32. [DOI] [PubMed] [Google Scholar]
- 20.Huggins, J. W. 1989. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev. Infect. Dis. 11(Suppl. 4):S750-S761. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs, A., and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:258-267. [PubMed] [Google Scholar]
- 22.Jonas, M. M. 1996. Interferon-alpha for viral hepatitis. J. Pediatr. Gastroenterol. Nutr. 23:93-106. [DOI] [PubMed] [Google Scholar]
- 23.Koff, W. C., J. L. Elm, Jr., and S. B. Halstead. 1980. Inhibition of dengue virus replication by amantadine hydrochloride. Antimicrob Agents Chemother. 18:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libraty, D. H., T. P. Endy, H. S. Houng, S. Green, S. Kalayanarooj, S. Suntayakorn, W. Chansiriwongs, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 185:1213-1221. [DOI] [PubMed] [Google Scholar]
- 26.Libraty, D. H., P. R. Young, D. Pickering, T. P. Endy, S. Kalayanarooj, S. Green, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186:1165-1168. [DOI] [PubMed] [Google Scholar]
- 27.Malinoski, F. J., S. E. Hasty, M. A. Ussery, and J. M. Dalrymple. 1990. Prophylactic ribavirin treatment of dengue type 1 infection in rhesus monkeys. Antiviral Res. 13:139-149. [DOI] [PubMed] [Google Scholar]
- 28.Merritt, J. A., L. A. Ball, K. M. Sielaff, D. M. Meltzer, and E. C. Borden. 1986. Modulation of 2′,5′-oligoadenylate synthetase in patients treated with alpha-interferon: effects of dose, schedule, and route of administration. J. Interferon Res. 6:189-198. [DOI] [PubMed] [Google Scholar]
- 29.Morrey, J. D., D. F. Smee, R. W. Sidwell, and C. Tseng. 2002. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antiviral Res. 55:107-116. [DOI] [PubMed] [Google Scholar]
- 30.Morrill, J. C., G. B. Jennings, T. M. Cosgriff, P. H. Gibbs, and C. J. Peters. 1989. Prevention of Rift Valley fever in rhesus monkeys with interferon-α. Rev. Infect. Dis. 11:S815-S825. [DOI] [PubMed] [Google Scholar]
- 31.Motzer, R. J., A. Rakhit, J. Thompson, H. Gurney, P. Selby, R. Figlin, S. Negrier, S. Ernst, M. Siebels, M. Ginsberg, K. Rittweger, and L. Hooftman. 2002. Phase II trial of branched peginterferon-alpha 2a (40 kDa) for patients with advanced renal cell carcinoma. Ann. Oncol. 13:1799-1805. [DOI] [PubMed] [Google Scholar]
- 32.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 33.Nimmannitya, S. 1997. Dengue hemorrhagic fever: diagnosis and management, p. 133-146. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 34.Reddy, K. R. 2004. Development and pharmacokinetics and pharmacodynamics of pegylated interferon alfa-2a (40 kD). Semin. Liver Dis. 24(Suppl. 2):33-38. [DOI] [PubMed] [Google Scholar]
- 35.Rigau-Perez, J. G., G. G. Clark, D. J. Gubler, P. Reiter, E. J. Sanders, and A. V. Vorndam. 1998. Dengue and dengue haemorrhagic fever. Lancet 352:971-977. [DOI] [PubMed] [Google Scholar]
- 36.Russell, P. K., A. Nisalak, P. Sukhavachana, and S. Vivona. 1967. A plaque reduction test for dengue virus neutralization antibodies. J. Immunol. 99:285-290. [PubMed] [Google Scholar]
- 37.Stanley, S. K., M. A. Ostrowski, J. S. Justement, K. Gantt, S. Hedayati, M. Mannix, K. Roche, D. J. Schwartzentruber, C. H. Fox, and A. S. Fauci. 1996. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N. Engl. J. Med. 334:1222-1230. [DOI] [PubMed] [Google Scholar]
- 38.Takeda, M., A. Pekosz, K. Shuck, L. H. Pinto, and R. A. Lamb. 2002. Influenza A virus M2 ion channel activity is essential for efficient replication in tissue culture. J. Virol. 76:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughn, D. W., S. Green, S. Kalayanarooj, B. Innis, S. Nimmannitya, S. Suntayakorn, T. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue virus viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 40.Vithanomsat, S., C. Wasi, C. Harinasuta, and P. Thongcharoen. 1984. The effect of interferon on flaviviruses in vitro: a preliminary study. Southeast Asian J. Trop. Med. Public Health 15:27-31. [PubMed] [Google Scholar]
- 41.Wills, R. J., and K. F. Soike. 1988. Pharmacokinetics of human recombinant interferon-alpha I after i.v. infusion and im injection in African green monkeys. J. Interferon Res. 8:427-432. [DOI] [PubMed] [Google Scholar]
- 42.Wills, R. J., H. E. Spiegel, and K. F. Soike. 1984. Pharmacokinetics of recombinant alpha A interferon following I.V. infusion and bolus, I. M., and P.O. administrations to African green monkeys. J. Interferon Res. 4:399-409. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 5. May 2005. W.H.O. DengueNet. http://www.who.int/GlobalAtlas/home.asp.
- 44.Wu, J., H. Y. Xie, G. P. Jiang, X. Xu, and S. S. Zheng. 2003. The effect of mycophenolate acid on hepatitis B virus replication in vitro. Hepatobiliary Pancreat. Dis. Int. 2:410-413. [PubMed] [Google Scholar]
- 45.Zeuzem, S., S. V. Feinman, J. Rasenack, E. J. Heathcote, M. Y. Lai, E. Gane, J. O'Grady, J. Reichen, M. Diago, A. Lin, J. Hoffman, and M. J. Brunda. 2000. Peginterferon alfa-2a in patients with chronic hepatitis C. N. Engl. J. Med. 343:1666-1672. [DOI] [PubMed] [Google Scholar]