Abstract
“Helicobacter heilmannii” (proposed name) type 1 colonizes the human stomach. It has been shown to be identical to “Candidatus Helicobacter suis,” a Helicobacter species colonizing the stomachs of >60% of slaughter pigs. This bacterium has not been isolated in vitro until now. Antibiotic susceptibility testing of “Candidatus Helicobacter suis” has not been carried out so far. For the present study, a mouse model was adopted to evaluate the antibiotic susceptibility of this organism. Mice infected with “Candidatus Helicobacter suis” were treated with amoxicillin and omeprazole, a therapy which is used to treat H. heilmannii infections in humans. Two different isolates of “Candidatus Helicobacter suis” were tested. The excretion of bacterial DNA was assessed during treatment, using PCR on fecal samples. At the end of the experiment, 8 days after the cessation of treatment, the presence of infection was evaluated using a urease test and a PCR test on stomach samples. A marked decrease in the excretion of bacterial DNA was observed a few days after the onset of treatment, and the level remained low until the end of the experiment. A difference in susceptibility between the two “Candidatus Helicobacter suis” isolates was pointed out. The in vivo mouse model infected with “Candidatus Helicobacter suis” will be useful for further screening of potential therapeutic regimens.
Helicobacter pylori infections in humans are a major cause of gastric and duodenal ulceration (14, 30) as well as gastric cancer (1). Triple therapy involving a proton pump inhibitor, clarithromycin, and amoxicillin is recommended as the first-line treatment (13). However, H. pylori strains may differ in their antibiotic susceptibilities. Results from in vitro susceptibility testing often correlate poorly with in vivo susceptibility (8, 11). In this respect, the in vivo H. pylori (strain SS1) mouse model is very useful for testing different treatment regimens (31).
H. pylori is not the only Helicobacter species capable of colonizing the human gastric mucosa. “Helicobacter heilmannii” (proposed name) has been found in approximately 0.96% of gastric biopsies in humans (7). This organism is strongly associated with gastritis but is also associated with peptic ulceration, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma (5, 17). Recent evidence indicates that “H. heilmannii” is not a single species but represents different bacterial species with similar spiral morphologies, most of which are probably of zoonotic origin (15, 23, 25, 27, 28). Classification into “H. heilmannii” type 1 and “H. heilmannii” type 2 was established on the basis of 16S rRNA gene sequences (24). More than 50% of the “H. heilmannii” infections in humans are due to “H. heilmannii” type 1 (27). “H. heilmannii” type 1 has been shown to be identical to “Candidatus Helicobacter suis” (19), a hitherto nonculturable spiral bacterium that colonizes the stomachs of >60% of slaughter pigs (2, 6, 16, 20). The actual role of “Candidatus Helicobacter suis” in gastric disease in pigs is still a matter of debate, but it has been suggested that this bacterium is associated with gastric ulceration of the pars oesophagea (21) and with chronic pyloric gastritis (20). Since in vitro cultivation of “Candidatus Helicobacter suis” still has not been achieved, mouse inoculation was used to isolate this bacterium from infected pig stomach mucosa (4).
Antimicrobial treatment of “H. heilmannii” infections in humans is based on clinical experience (9, 18, 26, 29). Mostly, treatment schemes successful in eradicating H. pylori are also used to treat “H. heilmannii.” To our knowledge, antibiotic susceptibility testing of “H. heilmannii” type 1 has not been carried out so far. The aim of the present study was to adopt an in vivo mouse model infected with “Candidatus Helicobacter suis” for evaluating the antibiotic susceptibility of this organism.
MATERIALS AND METHODS
“Candidatus Helicobacter suis” isolates.
Since in vitro isolation of “Candidatus Helicobacter suis” is not possible with current methods, in vivo isolation was performed by mouse inoculation.
Two different isolates were used in the antibiotic treatment study. For each isolate, pig stomachs were obtained from a slaughterhouse and transported to the lab. This was done on different days for the two isolates, by which two isolates of “Candidatus Helicobacter suis” from two different pig farms were obtained.
The stomachs were opened, and the remaining food was rinsed off with autoclaved tap water (37°C). A small mucosal fragment from the antrum (1 cm from the torus pyloricus) was taken to screen for the presence of “Candidatus Helicobacter suis.” One-half of this fragment was used for a rapid urease test (CUT; Temmler Pharma, Marburg, Germany) (37°C for 1 h). The other half of the fragment was frozen (−20°C) and used for the specific detection of “Candidatus Helicobacter suis” by PCR as described below. For one stomach that yielded a positive urease test, the upper cell layers and mucus were scraped off the antrum. Scrapings were homogenized in lyophilization medium (LYM) consisting of 2 volumes of horse serum, 1 volume of brain heart infusion broth (Oxoid, England), and 10% glucose. The homogenate was then centrifuged (5,000 × g, 5 min; Beckman Allegra 6R centrifuge) to remove large particles. The supernatant was diluted 1/10 in LYM and intragastrically inoculated into three 6-week-old BALB/c mice (0.3 ml/mouse) (Harlan, Horst, The Netherlands). The isolates used in this study received three (isolate 1) and four (isolate 2) mouse passages. Each mouse passage was performed 2 weeks after inoculation by homogenizing whole urease-positive mouse stomachs in LYM (5 ml LYM/stomach). The mouse stomach homogenate was then used as an inoculum (0.3 ml/mouse) without a previous centrifugation step. For each mouse passage, except for the last one, three new BALB/c mice were inoculated. The last mouse passage was performed with 15 BALB/c mice, from which urease-positive stomachs were pooled and homogenized. The homogenate was frozen at −70°C.
Experimental protocol.
Eighty-five specific-pathogen-free, 6-week-old BALB/c mice were obtained from an authorized breeder (Harlan). Animals were housed individually in autoclaved filter-top cages, fed a commercial diet of autoclaved pellets (Teklad; Harlan), and given autoclaved water ad libitum. Forty-five mice were used for the isolation of “Candidatus Helicobacter suis” as described above. Forty mice were used in the antibiotic treatment study. Of these 40 animals, 5 were left uninoculated. The remaining animals (35) were divided into the following two groups: a first group of 15 mice for isolate 1 and a second group of 20 mice for isolate 2. The animals in group 1 and group 2 were inoculated with isolate 1 and isolate 2 of “Candidatus Helicobacter suis,” respectively. For this purpose, the frozen stock of mouse stomach homogenate was placed at 37°C for 15 min and diluted 1/10 in LYM. Each mouse was administered 0.3 ml of the inoculum intragastrically, using a ball-tipped gavage needle. Five and 10 of the infected animals were left untreated for isolate 1 and isolate 2, respectively.
Two weeks after infection, 10 mice each from group 1 and group 2 were treated with omeprazole (Astra Zeneca, Brussels, Belgium) and amoxicillin (SmithKline Beecham, Genval, Belgium). The dosages of omeprazole and amoxicillin were chosen according to the results of a study by Morgner et al. (18). The weight of each mouse was approximated to be 20 g, and treatment amounts were calculated with the formula of Riviere (22). Amoxicillin and omeprazole were diluted in sterile distilled water and 0.2 M sodium bicarbonate (pH 8), respectively, for appropriate dosing. All treatments were administered intragastrically three times daily for 14 days.
The effect of treatment was evaluated with two parameters. The first parameter was the presence of bacterial DNA in fecal samples from 1 day after the onset of treatment until 1 week after treatment, which was investigated by PCR on fecal material (PCR-SK). Starting from 1 day after the onset of treatment, 200 mg of feces was collected daily from each individual animal for DNA extraction followed by PCR-SK (see below).
Eight days after the cessation of treatment, the animals were euthanized, and results from immunohistochemistry, urease tests, and PCR on stomach samples (PCR-SL) were used as a second parameter to evaluate the clearance of infection. Therefore, three gastric tissue samples were taken. The first gastric tissue sample was fixed in buffered formalin (24 h) and embedded in paraffin for immunohistochemical staining. The second sample was used for urease testing by the CUTest (Temmler Pharma, Marburg, Germany) and incubated at 37°C. The urease test was regarded as positive when the solution turned red within 1 h. The third gastric tissue sample was used for DNA extraction followed by PCR-SL (see below).
PCR-SL.
DNAs from stomach samples were extracted using DNeasy tissue kits (QIAGEN, Hilden, Germany). PCR was performed as described previously (3). PCR products were run in 1.5% agarose gels containing 50 ng/ml ethidium bromide. After 1 h at 160 V, the products were visualized with a UV transilluminator.
PCR-SK.
At the time of the study, a PCR assay for the specific detection of “Candidatus Helicobacter suis” in stomach samples was already described (3). This PCR amplifies a 433-bp fragment of the 16S rRNA gene of “Candidatus Helicobacter suis” but could not detect the bacterial DNA in the feces of infected mice. In feces, fragmentation of DNA has been shown to occur (12), and therefore other primers (HS 586 [5′-GGGAGGACAAGTCAGGTGTGAA-3′] and HS 641 [5′-TCTCCCACACTCCAGAAGGATAG-3′]) (Table 1) were selected from variable regions of the 16S rRNA gene of “Candidatus Helicobacter suis” (GenBank accession no. AF1027028). These primers were used to amplify a 79-bp fragment from mouse fecal samples. DNAs from 200-mg fecal samples were extracted using a QIAamp DNA stool mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. The DNA yield was determined by measuring the DNA concentration in the DNA extract by its absorbance at 260 nm.
TABLE 1.
Specific detection of “Candidatus Helicobacter suis” infection in fecal samples from mice by PCR amplification of a 79-bp fragment of the 16S rRNA gene of “Candidatus Helicobacter suis”
| Primer or straina | Species or sequence (5′-3′) |
|---|---|
| HS 586 | GGGAGGACAAGTCAGGTGTGAA |
| HS641 | TCTCCCACACTCCAGAAGGATAG |
| CCUG 38995 | H. billis |
| CCUG 29260 | H. pametensis |
| CCUG 32350 | H. nemestrinae |
| NCTC 11961 | H. pylori |
| LMG 6444 | Campylobacter jejuni |
| LMG 16318 | H. pullorum |
| LMG 18044 | H. mustelae |
| LMG 16316 | H. hepaticus |
| LMG 18086 | H. canis |
| LMG 11759 | H. fenelliae |
| LMG 14378 | H. nemestrinae |
| LMG 12678 | H. pametensis |
| LMG 12684 | H. acinonychis |
| R 1051 | H. bizzozeronii |
| R 1053 | H. salomonis |
| R 3647 | H. felis |
| LMG 7543 | H. cinaedi |
Bacterial strains used for the evaluation of the specificity of the primers.
PCR mixtures (50 μl) contained 50 pmol of each primer (Invitrogen Life Technologies, Merelbeke, Belgium), a 200 μM concentration of each deoxynucleoside triphosphate (Amersham Pharmacia Biotech, Puurs, Belgium), 0.03 U/μl Taq Platinum, 1.5 mM MgCl2, and 1× PCR buffer (Invitrogen Life Technologies). Two microliters of template DNA was added to the vials. The PCR conditions were as follows: initial denaturation at 95°C for 3 min followed by 40 cycles of 30 s at 94°C, 30 s at 62°C, and 30 s at 72°C. A final extension was performed for 5 min at 72°C. PCR products were run in 1.5% agarose gels containing 50 ng/ml ethidium bromide. After 1 h at 160 V, the products were visualized with a UV transilluminator.
The specificity of the primers was tested on DNA extracts from 17 different Helicobacter species (Table 1). No amplification products were seen when Helicobacter species other than “Candidatus Helicobacter suis” were used as templates.
To test the sensitivity of the PCR-SK assay, 200-mg fecal mouse samples were spiked with 1010, 109, 108, 107, 106, 105, 104, 103, 102, and 10 copies of the 16S rRNA gene sequence from “Candidatus Helicobacter suis,” present as plasmid DNA. The 16S rRNA gene sequence (1.4 kbp) of “Candidatus Helicobacter suis” was then amplified by PCR as described by De Groote et al. (2). This was followed by cloning into the pCR2.1-TOPO vector by using a TOPO TA cloning kit (Invitrogen, Life Technologies) according to the manufacturer's instructions for chemical transformation. DNA sequencing further confirmed that the expected 16S rRNA gene sequence had been cloned into the pCR2.1-TOPO vector. Plasmid DNA was extracted with a S.N.A.P. Miniprep kit (Invitrogen, Life Technologies). The circular plasmid was eluted in sterile water and linearized with EcoRV (Invitrogen, Life Technologies). The absorbance of the DNA solution was measured three times at 260 nm on a UV/Vis spectrophotometer, and the mean value was taken as the actual absorbance. The copy number of the rRNA gene sequence was calculated as follows: copy number of 16S rRNA gene/μl = (concentration of linearized plasmid [g/μl]/molecular weight of pCR2.1-TOPO [g/mol]) × 6.023 × 1023 (copies/mol). Serial dilutions of the purified linear plasmid were made, and 10 μl of each dilution (ranging from 109 copies/μl to 1 copy/μl) was used to spike a 200-mg fecal sample. DNAs from spiked fecal samples were extracted using a QIAamp DNA stool mini kit (QIAGEN,Hilden, Germany) according to the manufacturer's instructions. Afterwards, PCR-SK was performed as described above.
To compare the sensitivity of PCR-SK with that of PCR-SL, serial dilutions of the linear plasmid containing the 16S rRNA gene sequence of “Candidatus Helicobacter suis,” starting from 1010 copies/μl, were used as template DNAs in both PCR tests. Thereafter, PCR-SK and PCR-SL were performed as described above.
The nucleotide sequences of the amplified PCR-SK products were determined to confirm the predicted sequence by indirect sequencing. The amplicons derived from three randomly selected positive fecal samples of isolate 1 and from three randomly selected positive fecal samples of isolate 2 were cloned into the pCR2.1-TOPO vector (TOPO TA cloning kit; Invitrogen, Life Technologies) according to the manufacturer's instructions for chemical transformation. Plasmids were purified using a S.N.A.P. Miniprep kit (Invitrogen, Life Technologies). Sequences were determined by using the M13 primers complementary to the pCR2.1-TOPO vector, a Big Dye Terminator cycle sequencing kit, and an ABI Prism 3100 DNA sequencer (Applied Biosystems, Tokyo, Japan). Sequences were entered into the Kodon software package (Applied Maths, Sint-Martens Latem, Belgium). A sequence similarity test was then done using the BLAST analysis tool (http://BLAST.genome.jp/).
Immunohistochemical staining for detection of Helicobacter bacteria.
Immunohistochemical staining was performed on formalin-fixed and paraffin-embedded gastric tissue samples as described by De Groote et al. (2). Colonization was assessed on a three-point scale, as follows: negative, no bacteria present; weak, less than one-third of crypts colonized; and strong, two-thirds or more of crypts colonized. The antrum-body transitional zone was defined according to the method of Veldhuyzen van Zanten et al. (31). Colonization in the antrum-body transitional zone was evaluated according to the method of Veldhuyzen van Zanten et al. (31), with the following minor modifications: the center point of the antrum-body transitional zone was identified and then bacteria were counted within a six-gland radius on both sides.
Statistical analysis.
The differences in excretion of “Candidatus Helicobacter suis” DNA into the feces between treated and untreated groups were assessed by using Fisher's exact test with the statistics package SPSS. P values of <0.05 were regarded as statistically significant.
RESULTS
During the study, three animals, an untreated control animal and two treated animals, died from causes unrelated to the infection or treatment.
Detection of “Candidatus Helicobacter suis” DNA in feces by PCR-SK.
The mean DNA yield from the DNA stool mini kit was 135 μg DNA per gram of mouse feces. All fecal samples from uninoculated animals were negative by PCR-SK.
The excretion of “Candidatus Helicobacter suis” DNA into the feces of mice is shown in Fig. 1 and 2 for the first and second isolate, respectively. For both untreated control groups, “Candidatus Helicobacter suis” DNA was excreted throughout the observation period by the vast majority of animals, ranging from 70 to 100% of the inoculated mice. Indirect sequencing of randomly selected PCR-SK products amplified from fecal samples of positive control animals confirmed that the amplicon was identical to the 16S rRNA gene sequence of “Candidatus Helicobacter suis.”
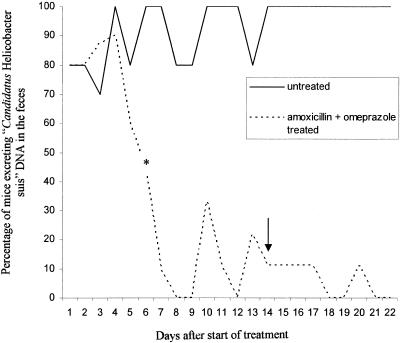
FIG. 1.
Percentages of mice from group 1 excreting “Candidatus Helicobacter suis” DNA from 1 day after the start of treatment until the end of the experiment. A significant difference was found starting from day 6 (*) of treatment and for all following days. The arrow indicates the last day of treatment.
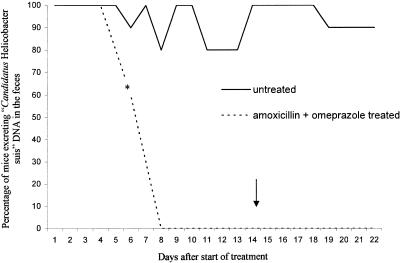
FIG. 2.
Percentages of mice from group 2 excreting “Candidatus Helicobacter suis” DNA from 1 day after the start of treatment until the end of the experiment. A significant difference (P < 0.05) was found starting from day 6 (*) of treatment and for all following days. The arrow indicates the last day of treatment.
For the group experimentally infected with isolate 1 and treated with omeprazole and amoxicillin, the treatment resulted in an overall decrease in excretion of “Candidatus Helicobacter suis” DNA compared to that for the untreated control group for isolate 1. This difference was statistically significant from day 6 after the start of the treatment until the end of the experiment. On the last day of sampling (day 8 after the end of treatment), all treated animals had negative fecal samples by PCR-SL.
For the group experimentally infected with isolate 2 and treated with omeprazole and amoxicillin, treatment also resulted in a decrease in “Candidatus Helicobacter suis” DNA excretion compared to that for untreated control animals for isolate 2. This difference in excretion was statistically significant from day 6 after the start of treatment until the end of the experiment. In fact, from day 10 after the onset of treatment until the end of the experiment, DNA of the target organism could not be detected by PCR-SK in the feces of any of the treated animals.
PCR-SK performed on fecal samples spiked with different amounts of the 16S rRNA gene sequence (ranging from 1010 to 101 copies) showed that PCR-SK on fecal samples could detect at least 105 copies of the 16S rRNA gene sequence of “Candidatus Helicobacter suis.”
The relative sensitivity of PCR-SK to that of PCR-SL was tested with purified plasmid DNA as the PCR template. Both PCR tests had the same sensitivity and could detect at least 200 copies of the 16S rRNA gene sequence.
Detection of Helicobacter bacteria in stomach samples by immunohistochemistry, urease tests, and PCR.
The results of immunohistochemistry, urease tests, and PCR-SL performed on stomach samples are summarized in Table 2. Gastric samples taken for immunohistochemistry consisted of the antrum (antral gland type) and the body (fundic gland type), except for six samples which contained only the antrum, seven samples which contained only the fundus, and one sample which contained only the antrum and the antrum-body transitional zone. Antrum and body stomach regions were scored separately, as was the antrum-body transitional zone (Table 2).
TABLE 2.
Results of immunohistochemistry, urease testing, and PCR-SL for mouse stomach samplesa
| Mouse sample | Immunohistochemistry result
|
Urease test result (gastric tissue) | PCR-SL test result (gastric tissue) | ||
|---|---|---|---|---|---|
| Antrum | A-B | Body | |||
| Negative control 1 | − | − | − | − | − |
| Negative control 2 | − | − | − | − | − |
| Negative control 3 | − | − | − | − | − |
| Negative control 4 | − | − | ND | − | − |
| Negative control 5 | − | − | − | − | − |
| Samples for isolate 1 | |||||
| Positive control 1 | ++ | ++ | ++ | + | + |
| Positive control 2 | ND | ND | ++ | + | + |
| Positive control 3 | ND | ND | ++ | + | + |
| Positive control 5 | ++ | ++ | ++ | + | + |
| OA 1 | − | − | − | − | − |
| OA 2 | − | − | − | − | − |
| OA 3 | ND | ND | − | − | − |
| OA 4 | − | − | − | − | − |
| OA 6 | − | − | − | − | − |
| OA 7 | ND | ND | − | − | − |
| OA 9 | ND | ND | − | − | − |
| OA 10 | − | − | − | − | − |
| Samples for isolate 2 | |||||
| Positive control 1 | ++ | ++ | ++ | + | + |
| Positive control 2 | ++ | ++ | ++ | + | + |
| Positive control 3 | ++ | ND | ND | + | + |
| Positive control 4 | ND | ND | ++ | + | + |
| Positive control 5 | ++ | ++ | ++ | + | + |
| Positive control 6 | ++ | ++ | ++ | + | + |
| Positive control 7 | ++ | ND | ND | + | + |
| Positive control 8 | ++ | ND | ND | + | + |
| Positive control 9 | ++ | ND | ND | + | + |
| Positive control 10 | ++ | ++ | ++ | + | + |
| OA 1 | − | − | + | + | + |
| OA 2 | − | − | − | − | − |
| OA 3 | ND | − | − | − | − |
| OA 4 | + | ND | ND | + | + |
| OA 5 | + | ND | + | + | + |
| OA 6 | − | − | − | − | + |
| OA 7 | ND | ND | + | + | + |
| OA 8 | − | ND | ND | − | + |
| OA 9 | − | − | − | + | + |
| OA 10 | − | − | − | + | + |
−, negative result; + positive result; ++, strong positive result; A-B, antrum-body transition; ND, not determined, i.e., specific stomach region was not present; OA, omeprazole and amoxicillin treatment. Positive control animal 4, OA animal 5, and OA animal 8 from the isolate 1 group died during the experiment.
Helicobacter bacteria could not be detected in uninoculated control animals. Untreated control animals infected with isolate 1 were strongly positive in the antrum, the antrum-body transitional zone, and the fundus (two-thirds of crypts were colonized with Helicobacter bacteria), as determined by immunohistochemistry. They were positive by the urease test and also by PCR-SL. All mice challenged with isolate 1 followed by antibiotic treatment were negative at euthanasia with all methods used (immunohistochemistry, urease test, and PCR-SL). The results of immunohistochemical staining showed that the antrum, the antrum-body transitional zone, and the body (fundic mucosa) were negative.
Untreated control animals infected with isolate 2 were positive for bacteria, as determined by the three methods used. In these animals, two-thirds of the glandular crypts in the antrum, the antrum-body transitional zone, and the fundus were colonized with Helicobacter bacteria. An immunohistochemical evaluation of mice challenged with isolate 2 followed by antibiotic treatment showed that in 4/10 animals one-third of the glandular crypts were colonized with Helicobacter bacteria in the antrum and/or body. The remaining six were negative by immunohistochemistry. All animals that were positive by immunohistochemistry were also positive by the urease test; additionally, two other animals were shown to be positive by the urease test. Positive PCR-SL results correlated well with the positive results of the other tests, with two exceptions (OA6 and OA8) (Table 2), which were negative by the urease test and immunohistochemistry.
DISCUSSION
To our knowledge, this is the first study in which the antibiotic susceptibility of “H. heilmannii” type 1 or “Candidatus Helicobacter suis” was tested by means of an in vivo mouse model. In this mouse model, “Candidatus Helicobacter suis” was shown to colonize both antral and fundic mucosa, a colonization pattern similar to that observed in pigs naturally infected with “Candidatus Helicobacter suis” (21).
For H. pylori, the use of an in vivo mouse model is considered more reliable for antibiotic susceptibility testing than in vitro methods (31). With “Candidatus Helicobacter suis,” in vivo testing is the sole option since the bacterium cannot be cultured in vitro. Because of this, no phenotypic or genotypic data were available for the “Candidatus Helicobacter suis” isolates used in this study.
The colonization of both isolates of “Candidatus Helicobacter suis” was suppressed by treatment with amoxicillin and omeprazole. In order to define the efficacy of antibiotic treatment, two parameters were examined. The first parameter involved the excretion of bacterial DNA in the feces. Results from immunohistochemical staining, urease tests, and PCR-SL on gastric tissue samples at the end of the experiment comprised the second parameter used to assess the efficiency of the installed antimicrobial regimen. Treatment with amoxicillin and omeprazole resulted in a marked decrease in bacterial DNA excretion. This may lead to the assumption that the degree of bacterial colonization in the stomach of the treated animals has lessened. It is not clear, however, whether there is a direct link between stomach colonization and fecal excretion. Previously, other investigators have used PCR on fecal samples as an early indicator of the likely success of treatment for Helicobacter hepaticus and Helicobacter bilis infections of the cecum and colon in mice (10). These authors considered negative fecal samples to be an indicator of eradication of the infection.
For isolate 1, all treated animals tested negative at the end of the experiment, as determined by immunohistochemistry, urease tests, and PCR-SL. In contrast, the stomachs of the majority of the treated animals infected with isolate 2 proved positive by PCR-SL, although fecal samples were negative. Some of these animals had negative urease tests, and most of them were negative as determined by immunohistochemistry, which can be explained by the fact that immunohistochemistry and urease tests are not as sensitive as PCR for the detection of “Candidatus Helicobacter suis” (3). Taking all of the results into account, we can say that a decrease in, and even absence of, bacterial DNA in the feces is not sufficient for stating that animals have cleared the infection in the stomach. This may be partially explained by the fact that only 105 copies of the 16S rRNA gene can be detected by PCR-SK. Screening of gastric tissue samples for the presence of Helicobacter bacteria needs to be done to evaluate antimicrobial efficacies.
Treatment resulted in a decrease in bacterial colonization for isolate 1 and isolate 2, but true eradication cannot be claimed because only 1 week was left between the end of treatment and screening for the presence of Helicobacter bacteria. For humans, it is agreed that eradication can only be claimed when the patient is negative for Helicobacter for 4 weeks after the end of treatment.
In contrast with the observations made by Veldhuyzen van Zanten et al. (31) for H. pylori, treatment of “Candidatus Helicobacter suis” with amoxicillin and omeprazole was effective in the antrum but also in the antrum-body transitional zone. The difference in observations may be due to the higher total daily dosage of amoxicillin used in our study or to a difference in susceptibility between H. pylori and “Candidatus Helicobacter suis.” The observations made in our study that treatment with amoxicillin and omeprazole has an effect on colonization in both antral and fundic mucosa is in agreement with the study of Morgner et al. (18), who tested treatment with amoxicillin and omeprazole in humans infected with “H. heilmannii.”
In the present study, a difference in susceptibility between different isolates of “Candidatus Helicobacter suis” was pointed out. The dosages of the drugs given to the animals in the present study were calculated by extrapolation of the dosages given to people infected with “H. heilmannii” (18), using the formula of Riviere (22). It cannot be excluded that a higher dosage would have resulted in the clearance of “Candidatus Helicobacter suis” infection for the group infected with isolate 2, on the condition that this dosage is not toxic in the mouse model.
This is the first time that antimicrobial agents have been tested for their effect against “Candidatus Helicobacter suis.” In view of the potential benefit of the elimination of “Candidatus Helicobacter suis” on the management of gastric inflammation and ulceration in pigs and of certain gastric pathologies in humans, this model will be useful for further screening of potential therapeutic regimens.
Acknowledgments
This work was supported by the Federal Government Public Health Service, Food Chain Security and Environment grant S-6137, and the Pfizer Company.
We acknowledge Nathalie Van Rysselberghe and Karolien Hermy for their technical assistance.
REFERENCES
- 1.Asghar, R. J., and J. Parsonnet. 2001. Helicobacter pylori and risk for gastric adenocarcinoma. Semin. Gastrointest. Dis. 12:203-208. [PubMed] [Google Scholar]
- 2.De Groote, D., L.-J. van Doorn, R. Ducatelle, A. Verschuuren, F. Haesebrouck, W. G. V. Quint, K. Jalava, and P. Vandamme. 1999. “Candidatus Helicobacter suis,” a gastric helicobacter from pigs, and its phylogenetic relatedness to other gastrospirilla. Int. J. Syst. Bacteriol. 49:1769-1777. [DOI] [PubMed] [Google Scholar]
- 3.De Groote, D., R. Ducatelle, L. J. van Doorn, K. Timant, A. Verschuuren, and F. Haesebrouck. 2000. Detection of “Candidatus Helicobacter suis” in gastric samples of pigs by PCR: comparison with other invasive diagnostic techniques. J. Clin. Microbiol. 38:1131-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dick, E., A. Lee, G. Watson, and J. O'Rourke. 1989. Use of the mouse for the isolation and investigation of stomach-associated, spiral shaped bacteria from man and other animals. J. Med. Microbiol. 29:55-62. [DOI] [PubMed] [Google Scholar]
- 5.Goddard, A. F., R. P. H. Logan, J. C. Atherton, D. Jenkins, and R. C. Spiller. 1997. Healing of duodenal ulcer after eradication of Helicobacter heilmannii. Lancet 349:1815-1816. [DOI] [PubMed] [Google Scholar]
- 6.Grasso, G. M., G. Ripabelli, M. L. Sammarco, A. Ruberto, and G. Iannitto. 1996. Prevalence of Helicobacter-like organisms in porcine gastric mucosa: a study of swine slaughtered in Italy. Comp. Immunol. Microbiol. Infect. Dis. 19:213-217. [DOI] [PubMed] [Google Scholar]
- 7.Heilmann, K. L., and F. Borchard. 1991. Gastritis due to spiral shaped bacteria other then H. pylori: clinical, histological, and ultrastructural findings. Gut 32:137-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschl, A. M., and M. L. Rotter. 1996. Amoxicillin for the treatment of Helicobacter pylori infection. J. Gastroenterol. 31(Suppl. 9):44-47. [PubMed] [Google Scholar]
- 9.Kaklikkaya, N., O. Ozgur, F. Aydin, and U. Cobanoglu. 2002. Helicobacter heilmannii as causative agent of chronic active gastritis. Scand. J. Infect. Dis. 34:768-770. [DOI] [PubMed] [Google Scholar]
- 10.Kerton, A., and P. Warden. 2004. Review of successful treatment for Helicobacter spp. in laboratory mice. Eur. Soc. Lab. Anim. Vet. (ESLAV) 7:17-22. [DOI] [PubMed] [Google Scholar]
- 11.Kuipers, E. J., J. G. Kusters, and W. A. Deboer. 1997. Current therapies of Helicobacter pylori infection. Baillierre's Clin. Infect. Dis. 4:395-412. [Google Scholar]
- 12.Lantz, P. G., W. Abu al-Soud, R. Knutsson, B. Hahn-Hagerdal, and P. Radstrom. 2000. Biotechnical use of polymerase chain reaction for microbiological analysis of biological samples. Biotechnol. Annu. Rev. 5:87-130. [DOI] [PubMed] [Google Scholar]
- 13.Malfertheiner, P., F. Megraud, C. O'Morain, A. P. Hungin, R. Jones, A. Axon, D. Y. Graham, G. Tytgat, and European Helicobacter Pylori Study Group (EHPSG). 2002. Current concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report 2—2000. Aliment. Pharmacol. Ther. 16:167-180. [DOI] [PubMed] [Google Scholar]
- 14.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1:1311-1315. [DOI] [PubMed] [Google Scholar]
- 15.Meining, A., G. Kroher, and M. Stolte. 1998. Animal reservoirs in the transmission of Helicobacter heilmannnii. Results of a questionnaire-based study. Scand. J. Gastroenterol. 33:795-798. [DOI] [PubMed] [Google Scholar]
- 16.Mendes, E. N., D. M. M. Queiroz, G. A. Rocha, A. M. M. F. Nogueira, A. C. T. Carvalho, A. P. Lage, and A. J. A. Barbosa. 1991. Histopathological study of porcine gastric mucosa with and without a spiral bacterium (“Gastrospirillum suis”). J. Med. Microbiol. 35:345-348. [DOI] [PubMed] [Google Scholar]
- 17.Morgner, A., E. Bayerdörffer, A. Meining, M. Stolte, and G. Kroher. 1995. Helicobacter heilmannii and gastric cancer. Lancet 346:511-512. [DOI] [PubMed] [Google Scholar]
- 18.Morgner, A., N. Lehn, L. P. Andersen, C. Thiede, M. Bennedsen, K. Trebesius, B. Neubauer, A. Neubauer, M. Stolte, and E. Bayerdörffer. 2000. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology 118:821-828. [DOI] [PubMed] [Google Scholar]
- 19.O'Rourke, J. L., J. V. Solnick, B. A. Neilan, K. Seidel, R. Hayter, L. M. Hansen, and A. Lee. 2004. Description of “Candidatus Helicobacter heilmannii” based on DNA sequence analysis of 16S rRNA and urease genes. Int. J. Syst. Evol. Microbiol. 54:2203-2211. [DOI] [PubMed] [Google Scholar]
- 20.Park, J. H., B. J. Lee, Y. S. Lee, and J. H. Park. 2000. Association of tightly spiraled bacterial infection and gastritis in pigs. J. Vet. Med. Sci. 62:725-729. [DOI] [PubMed] [Google Scholar]
- 21.Queiroz, D. M. M., G. A. Rocha, E. N. Mendes, S. B. Mourra, A. M. R. Oliviera, and D. Miranda. 1996. Association between Helicobacter and gastric ulcer disease of the pars oesophagea in swine. Gastroenterology 111:19-27. [DOI] [PubMed] [Google Scholar]
- 22.Riviere, J. E. 1999. Interspecies extrapolations, p. 296-307. In J. E. Riviere (ed.), Comparative pharmacokinetics principles, techniques, and applications. University Press, Ames, Iowa.
- 23.Solnick, J. V. 2003. Clinical significance of Helicobacter species other than Helicobacter pylori. Clin. Infect. Dis. 36:349-354. [DOI] [PubMed] [Google Scholar]
- 24.Solnick, J. V., J. O'Rourke, A. Lee, B. J. Paster, F. E. Dewhirst, and L. S. Tompkins. 1993. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J. Infect. Dis. 168:379-385. [DOI] [PubMed] [Google Scholar]
- 25.Svec, A., P. Kordas, Z. Pavlis, and J. Novotny. 2000. High prevalence of Helicobacter heilmannii-associated gastritis in a small predominantly rural area: further evidence in support of a zoonosis? Scand. J. Gastroenterol. 9:925-928. [DOI] [PubMed] [Google Scholar]
- 26.Sykora, J., V. Hejda, J. Varvarovska, F. Stozicky, F. Gottrand, and K. Siala. 2003. Helicobacter heilmannii related gastric ulcer in childhood. J. Pediatr. Gastroenterol. Nutr. 36:410-413. [DOI] [PubMed] [Google Scholar]
- 27.Trebesius, K., K. Alder, M. Vieth, M. Stolte, and R. Haas. 2001. Specific detection and prevalence of Helicobacter heilmannii-like organisms in the human gastric mucosa by fluorescent in situ hybridization and partial 16S ribosomal DNA sequencing. J. Clin. Microbiol. 39:1510-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Bulck, K., A. Decostere, M. Baele, A. Driessen, J. C. Debongnie, A. Burette, M. Stolte, R. Ducatelle, and F. Haesebrouck. 2005. Identification of non-Helicobacter pylori spiral organisms in gastric samples from humans, dogs, and cats. J. Clin. Microbiol. 43:2256-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Loon, S., A. Bart, J. E. den Hertog, P. G. J. Nikkels, R. H. J. Houwen, J. E. A. R. De Schryver, and J. H. Oudshoorn. 2003. Helicobacter heilmannii gastritis caused by cat to child transmission. J. Pediatr. Gastroenterol. Nutr. 36:407-409. [DOI] [PubMed] [Google Scholar]
- 30.Veldhuyzen van Zanten, S. J. O., and P. M. Sherman. 1994. Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: a systematic overview. Can. Med. Assoc. J. 150:177-185. [PMC free article] [PubMed] [Google Scholar]
- 31.Veldhuyzen van Zanten, S. J. O., T. Kolesnikow, V. Leung, J. L. O'Rourke, and A. Lee. 2003. Gastric transitional zones, areas where Helicobacter treatment fails: results of a treatment trial using the Sydney strain mouse model. Antimicrob. Agents Chemother. 47:2249-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]