Abstract
The first small-molecule CCR5 antagonist, TAK-779, could not be developed as an anti-human immunodeficiency virus type (anti-HIV-1) agent because of its poor oral bioavailability. TAK-652 is an orally bioavailable TAK-779 derivative with potent anti-HIV-1 activity. TAK-652 inhibited the binding of RANTES (regulated on activation, normal T-cell expressed and secreted), macrophage inflammatory protein 1α (MIP-1α), and MIP-1β to CCR5-expressing cells at nanomolar concentrations. TAK-652 could also suppress the binding of monocyte chemotactic protein 1 (MCP-1) to CCR2b-expressing cells. However, its inhibitory effect on ligand binding to other chemokine receptors was limited. TAK-652 was active against CCR5-using (R5) HIV-1 but totally inactive against CXCR4-using (X4) HIV-1. The compound was active against R5 HIV-1 clinical isolates containing reverse transcriptase and protease inhibitor-resistant mutations, with a mean 50% effective concentration (EC50) and EC90 of 0.061 and 0.25 nM, respectively. In addition, recombinant R5 viruses carrying different subtype (A to G) envelope proteins were equally susceptible to TAK-652. A single oral administration of TAK-652 up to 100 mg was safe and well tolerated in humans. The compound displayed favorable pharmacokinetics, and its plasma concentration was 7.2 ng/ml (9.1 nM) even 24 h after the administration of 25 mg. Thus, TAK-652 is a promising candidate as a novel entry inhibitor of HIV-1.
The introduction of highly active antiretroviral therapy (HAART) with reverse transcriptase and protease inhibitors has achieved dramatic improvements in the prognosis for patients suffering from human immunodeficiency virus type 1 (HIV-1) infection, leading to a remarkable decline in the death rate of AIDS (6, 16, 17). At present, one entry inhibitor, eight nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), three nonnucleoside reverse transcriptase inhibitors (NNRTIs), and eight protease inhibitors (PIs) are available in clinics (28). Although HAART can suppress the emergence of drug-resistant mutants through simultaneously attacking different targets, the emergence of multidrug-resistant mutants often results in the failure of therapy (8). Therefore, it still seems mandatory to discover anti-HIV-1 agents with a novel mode of action.
The chemokine receptors CCR5 and CXCR4 act as major coreceptors of HIV-1 in consort with the primary receptor CD4 (3, 14, 15). It has been reported that CCR5-using (R5) HIV-1 is isolated predominantly during the asymptomatic stage (4). R5 HIV-1 is also responsible for virus transmission between individuals. Furthermore, it has been reported that R5 HIV-1 seems to play a major role even in the advanced stage of the disease (11, 26). Therefore, an attempt to suppress R5 HIV-1 replication may be able to block viral transmission and delay disease progression. This hypothesis has been supported by the finding that individuals having homozygous CCR5-Δ32, a truncated and nonfunctional form of CCR5, display profound resistance to HIV-1 infection without obvious health problems (5, 12, 20). These lines of evidence gave us the idea that CCR5 antagonists may be effective as anti-HIV-1 agents without serious side effects.
In 1999, we reported the first small-molecule nonpeptidic CCR5 antagonist, TAK-779, to be a potent and selective inhibitor of HIV-1 replication (2). This compound blocks R5 HIV-1 replication by binding in a pocket between the transmembrane helices near the extracellular surface (7). However, TAK-779 is an anilide derivative with a quaternary ammonium moiety and could not be further developed because of its poor oral bioavailability. Replacement of the quaternary ammonium moiety of TAK-779 with a polar sulfoxide moiety, a ring expansion of (6,7)-fused nuclei to (6,8)-fused nuclei, and substitution of a 4-(2-butoxyethoxy) group for the methyl group led to an increase in bioavailability and potency. Finally, we have recently identified TAK-652, a novel and orally bioavailable TAK-779 derivative. In this paper, we describe the results of a preclinical evaluation of TAK-652 in vitro and its pharmacokinetic profiles in humans.
MATERIALS AND METHODS
Cells.
CCR1-, CCR2b-, CCR3-, CCR4-, CCR5-, and CCR7-expressing Chinese hamster ovary (CHO) cells and CCR5-expressing HeLa cells were maintained in Ham's F-12 medium supplemented with 10% fetal bovine serum (FBS) and 50 μg/ml gentamicin. COS-7 cells were obtained through the Health Science Research Resources Bank (Osaka, Japan) and maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS and antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin). U87 astroglioma cells expressing human CD4 and either CCR5 or CXCR4 (U87.CD4.CCR5 cells or U87.CD4.CXCR4 cells, respectively) were obtained from D. Littman (New York University School of Medicine, New York, NY) and maintained in DMEM supplemented with 10% FBS, 300 μg/ml Geneticin, 1 μg/ml puromycin, and antibiotics. The above medium without Geneticin and puromycin was used in viral replication assays. MOLT-4/CCR5 cells, i.e., the T-lymphoblastoid cell line MOLT-4 expressing human CCR5, were maintained in RPMI 1640 medium supplemented with 10% FBS, 1 mg/ml Geneticin, and antibiotics (1). MOLT-4/CCR5/Luc+ cells, which are MOLT-4/CCR5 cells carrying an integrated copy of the HIV-1 long terminal repeat-driven luciferase reporter gene, were maintained in RPMI 1640 medium supplemented with 10% FBS, 500 μg/ml Geneticin, and antibiotics. Peripheral blood mononuclear cells (PBMCs) obtained from healthy volunteers were isolated by Ficoll-Hypaque gradient density centrifugation and stimulated with 5 μg/ml phytohemagglutinin (PHA) in RPMI 1640 medium supplemented with 20% FBS, 100 U/ml recombinant human interleukin 2 (Takeda Pharmaceutical Company, Osaka, Japan), and antibiotics for 3 days. The above medium without PHA was used in viral replication assays.
Compounds.
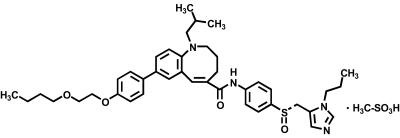
TAK-652, (S)-8-[4-(2-butoxyethoxy)phenyl]-1-isobutyl-N-(4-{[(1-propyl-1H-imidazol-5-yl)methyl]sulfinyl}phenyl)-1,2,3,4-tetrahydro-1-benzazocine-5-carboxamide monomethanesulfonate, and the CXCR4 antagonist AMD-3100 were synthesized by Takeda Pharmaceutical Company. The chemical structure of TAK-652 is shown in Fig. 1.
FIG. 1.
Chemical structure of TAK-652.
Viruses.
Seven R5 HIV-1 isolates (JR-FL, KK, CTV, HKW, HNK, HTN, and HHA), one CXCR4-using (X4) isolate (SW), and one CCR5- and CXCR4-using (R5X4) HIV-1 isolate (HE) were used for viral replication assays in U87 astroglioma cells and PBMCs. KK, CTV, HKW, HNK, HTN, HHA, and SW were clinical isolates from HIV-1-infected patients in Japan. For viral entry assays using recombinant HIV-1, a customized panel of 32 recombinant viruses was prepared from ViroLogic's specimen library. These viruses express genetically distinct HIV-1 envelope glycoproteins classified as subtypes A, B, C, D, E (now CRE01_AE), F, and G.
Chemokine binding assay.
The assay procedure for chemokine binding inhibition by test compounds has been described previously (2). In brief, CCR5-expressing CHO cells were incubated with various concentrations of TAK-652 in binding buffer (Ham's F-12 medium containing 20 mM HEPES and 0.5% bovine serum albumin, pH 7.2) containing either 200 pM 125I-regulated on activation, normal T-cell expressed and secreted (RANTES) (Amersham Pharmacia, Piscataway, NJ), 125I-macrophage inflammatory protein 1α (MIP-1α), or 125I-MIP-1β (Perkin-Elmer, Inc., Wellesley, MA). Binding reactions were performed at room temperature for 40 min. The binding reaction was terminated by washing out the cell-free ligand twice with cold phosphate-buffered saline (PBS). The cell-associated radioactivity was recorded with a scintillation counter (Top-count; Packard, Tokyo, Japan). Assays of the inhibitory effect of TAK-652 on the binding of 125I-RANTES to CCR1, 125I-monocyte chemotactic protein 1 (MCP-1) to CCR2b, 125I-eotaxin to CCR3, 125I-thymus and activation-regulated chemokine (TARC) to CCR4, and 125I-MIP-3β to CCR7 were carried out in a similar manner.
Envelope-mediated membrane fusion assay.
An assay of HIV-1 envelope-mediated membrane fusion was carried out according to a previously described method (22), with some modifications. COS-7 cells were seeded in a six-well plate at 5 × 105 cells/well. The culture supernatants were removed on the next day, and the cells were transfected with 0.6 μg of either pSG322-env, pHXB2-env, or pBluescript (Stratagene, La Jolla, CA), 0.2 μg of pSG5-rev, and 1.0 μg of pSG5-tat with Lipofectamine 2000 (Invitrogen, Gaithersburg, MD). pSG322-env and pHXB2-env encode the JR-FL (R5) and HXB2 (X4) envelope glycoproteins, respectively. After incubation for 6 h at 37°C, the supernatants were removed, and the cells were incubated with fresh culture medium for 2 days at 37°C. The transfected COS-7 cells and MOLT-4/CCR5/Luc+ cells were seeded in a 96-well plate at 1 × 104 cells (each) per well, and various concentrations of test compounds were added to the wells. The cell suspension was incubated at 37°C. A mixture of DMEM and RPMI 1640 medium supplemented with 10% FBS and antibiotics was used for membrane fusion. After an overnight incubation, Luc-Screen (Tropix, Foster City, CA) was added to each well, and the mixtures were incubated at room temperature for 10 min. The luciferase activity was measured with a luminometer (Wallac 1420 ARVO SX; Wallac Berthold Japan, Tokyo, Japan).
Antiviral assay with U87 astroglioma cells.
U87.CD4.CCR5 or U87.CD4.CXCR4 cells were seeded into a 48-well plate (3 × 104 cells/well) and incubated overnight at 37°C. The culture supernatants were removed, and the cells were inoculated with 1,000 50% cell culture infective doses of R5X4 HIV-1 (HE) per well in the presence of test compounds (100 nM) in a total volume of 400 μl. After incubation for 6 h, the cells were washed to remove unadsorbed viral particles and further incubated in the presence of the same concentration of test compounds for 3 days. On day 3 after infection, the culture supernatants were collected and tested for their p24 antigen levels with an enzyme-linked immunosorbent assay (ELISA) kit (ZeptoMetrix Corp., Buffalo, NY).
Antiviral assay with PBMCs.
PHA-stimulated PBMCs were inoculated with 500 50% cell culture infective doses of JR-FL or 11 to 55 ng of p24 from HIV-1 clinical isolates per 4 × 106 cells and incubated for 4 h. The cells were washed with culture medium to remove unadsorbed viral particles and then seeded into a 96-well plate (2 × 105 cells/well) with culture medium containing various concentrations of test compounds. On day 4 after infection, the cells were subcultured at 1:2 with culture medium containing the same concentrations of the test compounds. On day 7 after infection, the culture supernatants were collected and tested for their p24 antigen levels with an ELISA kit.
Viral entry assay using recombinant HIV-1.
An HIV-1 entry assay was developed by modifying the PhenoSense HIV assay, which is a novel phenotypic assay for drug susceptibility to HIV-1 (19). In brief, nucleic acid amplification (reverse transcriptase PCR) was carried out to obtain HIV-1 gp160 sequences derived from HIV-1-positive plasma samples. The amplified envelope sequences were incorporated into an expression vector (pCXAS) using conventional cloning methods. Envelope expression vectors (pHIVenv) were prepared as large pools of sequences that accurately represent the viral quasispecies in patients at the time of sample collection. Recombinant HIV-1 stocks containing viral envelope glycoproteins from patients were prepared by cotransfecting human embryonic kidney 293 cells with an HIV-1 genomic viral vector and an appropriate envelope expression vector. The genomic vector (pHIVlucΔU3) was replication defective and contained a luciferase expression cassette within a deleted region of the envelope gene. Recombinant virus particles were harvested 48 h after transfection and used for subsequent infection of U87.CD4.CCR5 or U87.CD4.CXCR4 cells. The infected cells were cultured in the presence of various concentrations of TAK-652 for 48 h. Viral entry followed by single-round replication was determined by measuring the luciferase activity of the cells.
Cytotoxicity evaluation.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Chemical Co. (St. Louis, MO) and used to determine the cytotoxicity of test compounds in mock-infected cells (18). MTT was added to each well and incubated at 37°C for 2 h, and then acidified isopropyl alcohol was added to dissolve formazan crystals. The optical density was determined with a microplate reader (model 550; Bio-Rad Laboratories, Hercules, CA).
Single-dose safety and pharmacokinetics in humans.
A double-blind phase I trial was conducted to evaluate the safety, tolerability, and pharmacokinetics of a single oral administration of TAK-652 in humans. Twenty-four healthy volunteers were enrolled in this study (two for a placebo and six for each dose), and three doses (25, 50, and 100 mg) of TAK-652 were administered orally as a solution to individuals in a fasted state. The TAK-652 solution was formulated in 0.5% (wt/vol) methylcellulose with 0.1% (wt/vol) Polysorbate 80 and 2 mM hydrochloric acid in distilled water. The placebo solution was 0.5% (wt/vol) methylcellulose with 0.1% (wt/vol) Polysorbate 80 and 2 mM hydrochloric acid in distilled water. Doses were selected based on allometric scaling of preclinical pharmacokinetic data and considerations of preclinical toxicology (no observed adverse effects). Screening was performed in the 3-week period prior to dosing, and poststudy assessments were carried out at 5 to 7 days postdosing. Safety and tolerability were evaluated by physical examinations (screening and poststudy), recording of vital signs (screening, predose, 1, 2, 4, 8, and 24 h postdose, and poststudy), electrocardiograms (ECG; screening, predose, 2, 6, and 24 h postdose, and poststudy), clinical laboratory evaluations (hematology, serum chemistry, and urinalysis; screening, predose, 24 h postdose, and poststudy), and recording of adverse events (predose, 3, 12, and 24 h postdose, and poststudy). Serial blood samples were collected to determine the plasma concentration of TAK-652. Blood samples were collected prior to drug administration (0 h) and then 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after administration. The samples were immediately processed, and the plasma concentration of TAK-652 was quantified by liquid chromatography/tandem mass spectrometry. The lower limit of TAK-652 quantification in plasma was 0.05 ng/ml. Pharmacokinetic parameters were estimated by noncompartmental procedures using WinNonlin, version 3.2, Enterprise (Pharsight Corporation, Mountain View, CA). The maximum plasma concentration (Cmax) and time to reach Cmax (Tmax) for each subject were calculated from the measured concentrations. The area under the plasma concentration-time curve from time zero to the last quantifiable concentration (AUC0-tz) for each subject was calculated from the measured concentrations by the trapezoidal rule.
Data analysis.
Fifty and ninety percent inhibitory concentrations were calculated using the SAS system procedure NLIN, which produces least-square estimates of the parameters of a nonlinear model (logistic model).
RESULTS
Inhibition of chemokine binding to receptor-expressing cells.
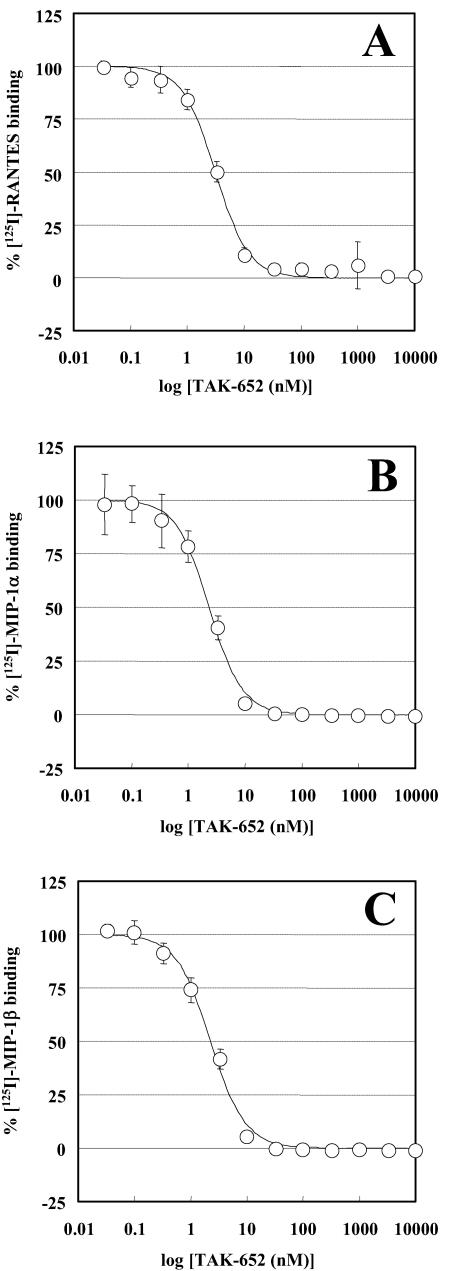
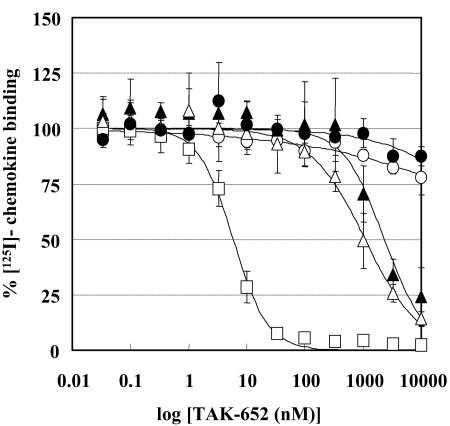
The inhibitory effect of TAK-652 on the binding of RANTES, MIP-1α, and MIP-1β to CCR5-expressing CHO cells was examined. The drug inhibited the binding of RANTES to CCR5 in a dose-dependent manner (Fig. 2A), and the 50% inhibitory concentration (IC50) for RANTES binding was 3.1 nM. The compound also blocked the binding of MIP-1α and MIP-1β to CCR5, with an IC50 of 2.3 nM (Fig. 2B and C). When the inhibitory effect of TAK-652 on the binding of other chemokines was investigated, TAK-652 did not affect the binding of RANTES and MIP-3β to CCR1- and CCR7-expressing CHO cells, respectively, at concentrations of up to 10,000 nM (Fig. 3). It modestly suppressed the binding of eotaxin and TARC to CCR3- and CCR4-expressing cells, with IC50s of 2,400 and 1,100 nM, respectively. TAK-652 inhibited the binding of MCP-1 to CCR2b, with an IC50 of 5.9 nM (Fig. 3), suggesting that the compound is a potent inhibitor of CCR5 and CCR2b. Furthermore, TAK-652 abrogated RANTES-induced Ca2+ mobilization in CCR5-expressing HeLa cells, but not in CCR1-expressing HeLa cells (data not shown), indicating that TAK-652 interacts with the chemokine receptor but not with its ligands.
FIG. 2.
Inhibitory effect of TAK-652 on binding of RANTES (A), MIP-1α (B), and MIP-1β (C) to CCR5. CCR5-expressing CHO cells were incubated with various concentrations of TAK-652 in binding buffer containing 125I-labeled RANTES, MIP-1α, or MIP-1β. Binding reactions were performed at room temperature and terminated by washing out the cell-free ligand with PBS. The cell-associated radioactivity was measured with a scintillation counter. Data represent means ± standard deviations for triplicate wells.
FIG. 3.
Inhibitory effect of TAK-652 on ligand binding to various chemokine receptors. CHO cells expressing CCR1 (open circles), CCR2b (open squares), CCR3 (filled triangles), CCR4 (open triangles), or CCR7 (filled circles) were incubated with various concentrations of TAK-652 in binding buffer containing 125I-labeled RANTES, MCP-1, eotaxin, TARC, or MIP-3β, respectively. Binding reactions were performed at room temperature and terminated by washing out the cell-free ligand with PBS. The cell-associated radioactivity was measured with a scintillation counter. Data represent means ± standard deviations for triplicate wells.
Inhibition of R5 envelope-mediated membrane fusion.
In the next experiment, TAK-652 was examined for its inhibitory effect on fusion between the HIV-1 envelope and the cell membrane, using envelope-expressing cells and CD4- and coreceptor-expressing cells. TAK-652 inhibited R5 HIV-1 (JR-FL) envelope-mediated membrane fusion, with an IC50 value of 0.10 nM, but did not affect X4 HIV-1 (HXB2) envelope-mediated membrane fusion, even at concentrations up to 1,000 nM (Table 1). In contrast, the CXCR4 antagonist AMD-3100 inhibited X4 HIV-1 envelope-mediated membrane fusion, with an IC50 value of 44 nM, yet had no effect on R5 HIV-1 envelope-mediated membrane fusion, even at a concentration of 1,000 nM (Table 1).
TABLE 1.
Inhibitory effect of TAK-652 and AMD3100 on HIV-1 envelope-mediated membrane fusion
| Compound | IC50 (nM)a
|
|
|---|---|---|
| JR-FL (R5) | HXB2 (X4) | |
| TAK-652 | 0.10 (0.070-0.14) | >1,000 |
| AMD-3100 | >1,000 | 44 (35-55) |
Assays were carried out in triplicate wells, and values in parentheses represent 95% confidence intervals.
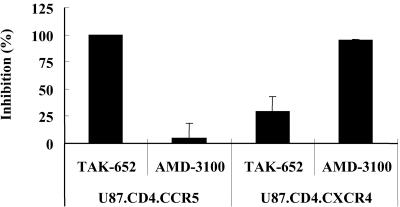
Inhibition of CCR5-mediated HIV-1 infection.
The anti-HIV-1 activity of TAK-652 was examined in two cell lines that are identical except for their coreceptor expression, namely, U87.CD4.CCR5 and U87.CD4.CXCR4 cells. TAK-652 completely inhibited the replication of R5X4 HIV-1 (HE) in U87.CD4.CCR5 cells at a concentration of 100 nM but was inactive against the same strain in U87.CD4.CXCR4 cells (Fig. 4). In contrast, AMD-3100 displayed an apparent inhibition of R5X4 HIV-1 replication only in U87.CD4.CXCR4 cells.
FIG. 4.
Antiviral activity of TAK-652 against R5X4 HIV-1 in U87.CD4.CCR5 and U87.CD4.CXCR4 cells. The cells were infected with R5X4 HIV-1 (HE) and incubated in the presence of test compounds (100 nM). After incubation for 6 h, the cells were washed to remove unadsorbed viral particles and further incubated in the presence of the same concentration of the test compounds for 3 days. On day 3 after virus infection, the culture supernatants were collected and tested for their p24 antigen levels by ELISA. The percent inhibition was calculated as follows: 100 × (1 − p24 antigen level in the presence of compound/p24 antigen level in the absence of compound). Data represent means ± standard deviations for triplicate wells.
Anti-HIV-1 activity against clinical isolates in PBMCs.
To estimate the efficacy of TAK-652 in HIV-1-infected patients, the anti-HIV-1 activity of TAK-652 was examined with six R5 and one X4 HIV-1 clinical isolate in PBMCs. The anti-HIV-1 activity was also tested with one R5X4 strain. TAK-652 inhibited the replication of all R5 isolates, with 50% effective concentrations (EC50s) and EC90s ranging from 0.024 to 0.089 nM and from 0.13 to 0.36 nM, respectively (Table 2). The mean EC50 and mean EC90 were 0.061 and 0.25 nM, respectively. On the other hand, the compound did not inhibit the replication of R5X4 HIV-1 (HE) and X4 HIV-1 (SW), even at a concentration of 10,000 nM (Table 2). TAK-652 did not affect the viability and proliferation of uninfected PBMCs at concentrations up to 10,000 nM (data not shown). Thus, TAK-652 was found to be a potent and selective inhibitor of R5 HIV-1 clinical isolates in PBMCs.
TABLE 2.
Anti-HIV-1 activity of TAK-652 for HIV-1 clinical isolates in PBMCsa
| Strain | Tropismb | No. of assays | EC50 (nM)
|
EC90 (nM)
|
||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| KK | R5 | 3 | 0.043 | 0.027 | 0.19 | 0.12 |
| CTV | R5 | 4 | 0.070 | 0.029 | 0.31 | 0.20 |
| HKW | R5 | 3 | 0.049 | 0.014 | 0.16 | 0.15 |
| HNK | R5 | 3 | 0.087 | 0.046 | 0.36 | 0.14 |
| HTN | R5 | 4 | 0.089 | 0.097 | 0.32 | 0.25 |
| HHA | R5 | 4 | 0.024 | 0.0084 | 0.13 | 0.054 |
| HE | R5X4 | 1 | >10,000 | >10,000 | ||
| SW | X4 | 2 | >10,000 | >10,000 | ||
PBMCs were obtained from different donors and used in each assay. The anti-HIV-1 activity was determined by measuring the p24 antigen levels in culture supernatants on day 7 after virus infection. Assays were carried out in triplicate wells and repeated the indicated number of times.
The tropism of clinical isolates was determined by their infectivity in U87.CD4.CCR5 and U87.CD4.CXCR4 cells.
Anti-HIV-1 activity in the presence of human serum.
To further estimate the efficacy of TAK-652 in vivo, the influence of human serum (HS) on its anti-HIV-1 activity was examined. The mean EC50 of TAK-652 in PBMCs obtained from four different donors was 0.085 nM in the presence of 20% FBS and 0.29 nM in the presence of 40% HS plus 10% FBS (Table 3). The ratios of EC50s for both conditions (EC50 in the presence of HS/EC50 in the absence of HS) for each individual ranged from 2.2 to 8.2, and the mean ratio among the four donors was 5.0 (Table 3).
TABLE 3.
Anti-HIV-1 activity of TAK-652 in PBMCs in the presence of high concentrations of human seruma
| Donorb | EC50 (nM) in presence of:
|
Ratioc | |
|---|---|---|---|
| 20% FBS | 40% HS + 10% FBS | ||
| 1 | 0.021 | 0.13 | 6.2 |
| 2 | 0.068 | 0.25 | 3.7 |
| 3 | 0.041 | 0.33 | 8.2 |
| 4 | 0.21 | 0.46 | 2.2 |
| Mean ± SD | 0.085 ± 0.087 | 0.29 ± 0.14 | 5.0 ± 2.7 |
Cells were infected with R5 HIV-1 (JR-FL) and incubated in the presence of various concentrations of TAK-652 and either 20% FBS alone or 40% HS plus 10% FBS.
PBMCs from four different healthy donors were used.
Ratio of EC50 in the presence of 20% FBS to EC50 in the presence of 40% HS plus 10% FBS.
Anti-HIV-1 activity in PBMCs from different donors.
It appears that the anti-HIV-1 activity of compounds is often affected by host cells obtained from different donors. Therefore, the activity of TAK-652 against two R5 strains (JR-FL and KK) was examined in PBMCs from eight different donors. In the absence of the compound, the p24 antigen levels in culture supernatants ranged from 1.5 to 270 ng/ml for the JR-FL strain and from 2.0 to 350 ng/ml for the KK strain on day 7 after virus infection (Table 4). These results indicate that the replication efficiency of R5 HIV-1 differed considerably from one donor to another. However, this difference in HIV-1 replication scarcely influenced the anti-HIV-1 activity of TAK-652. The drug inhibited R5 HIV-1 replication, with EC50s ranging from 0.021 to 0.21 nM for the JR-FL strain and from 0.033 to 0.091 nM for the KK strain (Table 4). In particular, the EC50s for the clinical isolate KK varied less than threefold among the PBMCs from the eight donors.
TABLE 4.
Anti-HIV-1 activity of TAK-652 in PBMCs from eight different donorsa
| Donor | Value in presence of JR-FL
|
Value in presence of KK
|
||
|---|---|---|---|---|
| EC50 (nM) | p24 (ng/ml) | EC50 (nM) | p24 (ng/ml) | |
| 1 | 0.10 | 3.9 | 0.068 | 7.6 |
| 2 | 0.021 | 28 | 0.047 | 30 |
| 3 | 0.037 | 6.3 | 0.075 | 3.8 |
| 4 | 0.068 | 65 | 0.043 | 44 |
| 5 | 0.041 | 16 | 0.055 | 21 |
| 6 | 0.21 | 270 | 0.091 | 350 |
| 7 | 0.10 | 11 | 0.039 | 11 |
| 8 | 0.033 | 1.5 | 0.033 | 2.0 |
| Median | 0.054 | 13 | 0.051 | 16 |
The anti-HIV-1 activity was determined by measuring the p24 antigen levels in culture supernatants on day 7 after virus infection. Assays were carried out in triplicate wells.
Activity against recombinant HIV-1 expressing different subtype envelope proteins.
TAK-652 was examined for its inhibitory effect on the replication of recombinant viruses containing 26 R5, 3 X4, and 3 R5X4 HIV-1 envelope glycoproteins in U87.CD4.CCR5 and U87.CD4.CXCR4 cells. TAK-652 blocked the infection of all R5 and R5X4 HIV-1 strains in U87.CD4.CCR5 cells, with EC50s ranging from 0.4 to 2.4 nM (Table 5). All subtypes evaluated in this study (A, B, C, D, E, F, and G) were found to be highly susceptible to TAK-652. The variation in their susceptibility to TAK-652 was approximately sixfold and was independent of the envelope subtype. The drug did not inhibit the infection of X4 or R5X4 HIV-1 in U87.CD4.CXCR4 cells, even at a concentration of 500 nM (Table 5). These results suggest that the anti-HIV-1 activity of TAK-652 is coreceptor dependent and subtype independent.
TABLE 5.
Anti-HIV-1 activity of TAK-652 for recombinant HIV-1 strains expressing different subtype envelope glycoproteins
| Tropism | Subtype and sample no. | EC50 (nM)a
|
|
|---|---|---|---|
| U87.CD4.CCR5 cells | U87.CD4.CXCR4 cells | ||
| R5 | A_R5_1 | 0.7 | NR |
| A_R5_2 | 1.2 | NR | |
| A_R5_3 | 0.9 | NR | |
| B_R5_1 | 1.0 | NR | |
| B_R5_2 | 1.0 | NR | |
| B_R5_3 | 0.9 | NR | |
| B_R5_4 | 1.1 | NR | |
| B_R5_5 | 2.4 | NR | |
| B_R5_6 | 0.4 | NR | |
| B_R5_7 | 0.8 | NR | |
| B_R5_8 | 0.9 | NR | |
| B_R5_9 | 1.1 | NR | |
| B_R5_10 | 2.4 | NR | |
| C_R5_1 | 1.0 | NR | |
| C_R5_2 | 0.8 | NR | |
| C_R5_3 | 1.0 | NR | |
| D_R5_1 | 0.5 | NR | |
| D_R5_2 | 0.7 | NR | |
| D_R5_3 | 1.0 | NR | |
| E_R5_1 | 0.7 | NR | |
| E_R5_2 | 1.6 | NR | |
| E_R5_3 | 0.8 | NR | |
| F_R5_1 | 1.0 | NR | |
| F_R5_2 | 1.1 | NR | |
| F_R5_3 | 0.7 | NR | |
| G_R5_RU570 | 1.1 | NR | |
| R5X4 | B_Dual_1 | 1.0 | >500 |
| B_Dual_2 | 0.9 | >500 | |
| B_Dual_3 | 0.5 | >500 | |
| X4 | B_X4_1 | NR | >500 |
| B_X4_2 | NR | >500 | |
| B_X4_3 | NR | >500 | |
Data represent means for two separate experiments. NR, not replicable.
Safety, tolerability, and pharmacokinetics in humans.
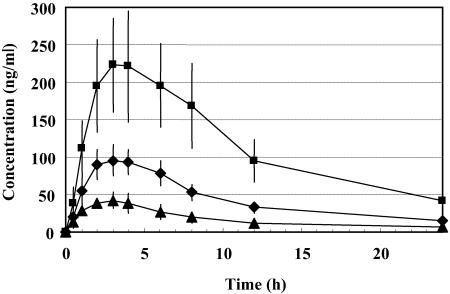
No withdrawal due to adverse events occurred among the 24 treated subjects. A total of six clinical adverse events were reported for four subjects. Among the six events, two dose-independent symptoms (headache and fatigue) were judged to be possibly related to the study drug. The other four mild events (headache, nasopharyngitis, hypoesthesia, and dizziness) did not seem to be attributable to the administration of TAK-652, yet this conclusion should be confirmed by further studies. No treatment- or dose-related trends in serum chemistry, hematology, and urinalysis data were observed during the study. There were no dose-related trends in supine systolic and diastolic blood pressure and pulse rate. No apparent treatment- or dose-related trends in vital signs or ECG were noted for any subjects during the course of this study. In particular, there was no evidence of a prolongation of the QTc interval at any dose of TAK-652. No clinically important findings were observed in ECG morphology for individuals receiving any dose. No clinically significant changes were noted poststudy. Thus, single oral doses (25, 50, and 100 mg in solution) of TAK-652 were safe and well tolerated in healthy male subjects. The mean plasma concentrations of TAK-652 for each dose from 30 min to 24 h postadministration are shown in Fig. 5. For all doses, the drug was at detectable levels in plasma at 30 min and after 24 h. The estimated pharmacokinetic parameters after single oral administration for healthy volunteers are presented in Table 6. Overall, TAK-652 had good oral absorption and a rather long half-life in plasma. Its plasma concentration at 24 h after a 25-mg administration was 7.2 ng/ml, which corresponds to 9.1 nM.
FIG. 5.
Plasma concentration-time profiles after single oral administration of TAK-652 to humans. Twenty-four healthy volunteers were enrolled in this study (two for the placebo and six for each dose). TAK-652 was administered orally in solution at a dose of 25 mg (filled triangles), 50 mg (filled diamonds), or 100 mg (filled squares). Blood samples were collected prior to drug administration (0 h) and 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after administration. Data represent means ± standard deviations for six subjects.
TABLE 6.
Pharmacokinetic parameters of TAK-652 after single oral administration to humansa
| Parameter | Value for indicated dose (mg)
|
||
|---|---|---|---|
| 25 | 50 | 100 | |
| AUC0-tz (ng · h/ml) | 416 (122) | 1,040 (213) | 2,760 (847) |
| Cmax (ng/ml) | 42.9 (9.4) | 97.6 (20.2) | 229 (69) |
| Tmax (h)b | 3.00 (2.00-3.00) | 4.00 (3.00-6.00) | 3.5 (2.00-4.00) |
| T1/2 (h) | 12.2 (2.3) | 8.77 (0.65) | 8.39 (1.32) |
| C12h (ng/ml) | 11.8 (3.8) | 32.5 (7.7) | 94.5 (28.5) |
| C24h (ng/ml) | 7.2 (3.0) | 14.4 (3.7) | 42.3 (15.0) |
Twenty-four healthy volunteers were enrolled in this study (two for the placebo and six for each dose), and TAK-652 was administered orally in solution. Data represent means (standard deviations) for six subjects.
Data are medians (minimum-maximum).
DISCUSSION
The present study has clearly demonstrated that TAK-652, an orally bioavailable TAK-779 derivative, is a potent inhibitor of HIV-1 replication in vitro. In addition, the compound was found to equally inhibit diverse strains of HIV-1. The R5 clinical isolates HKW, HNK, and HTN are highly resistant to several NRTIs, NNRTIs, and PIs used in clinics (S. Oka, International Medical Center of Japan, unpublished data). TAK-652 inhibited the replication of these multidrug-resistant strains at concentrations similar to those that suppressed the replication of the KK strain, an isolate from a treatment-naïve patient (Table 2). The activity of TAK-652 was not affected by different PBMC donors (Table 3). Furthermore, the assay results for recombinant viruses indicated that the activity spectrum of TAK-652 covered a variety of HIV-1 subtypes, including subtype G RU570 (Table 4), which was reported to be unsusceptible to SCH-C (21).
In terms of chemokine binding inhibition, TAK-652 also suppressed the binding of MCP-1 to CCR2b (Fig. 3). This property was also observed for TAK-779 (2). In contrast, TAK-220, another member of a class of CCR5 antagonists recently reported by our groups, was found to be highly specific to CCR5 (23). TAK-220 was inhibitory to the binding of RANTES and MIP-1α, but not that of MIP-1β, to CCR5, whereas TAK-652 equally blocked the binding of these three ligands to CCR5 (Fig. 2). These results indicate that due to the complete difference in chemical structure between TAK-652 and TAK-220 (anilide versus piperidine), their binding sites to CCR5 and mechanisms of HIV-1 inhibition may also differ. In fact, TAK-220 blocked the binding of the anti-CCR5 monoclonal antibodies (MAbs) 45531.111 and 2D7, which recognize different regions of the second extracellular loop (ECL2) of CCR5, but had no effect on the binding of the anti-CCR5 MAb 3A9, which is specific to the N terminus of CCR5 (10, 27). Interestingly, TAK-652 did not affect the binding of these MAbs to CCR5 (data not shown). Unlike RANTES, TAK-652 did not induce CCR5 internalization in CCR5-expressing cells (data not shown). Therefore, it is possible that TAK-652 could inhibit the interaction between HIV-1 gp120 and CCR5 through a conformational change of the gp120 binding site after binding to a domain of CCR5 other than ECL2 or the N terminus, presumably a site close to the TAK-779 binding site (7).
Another important issue that remains to be determined is the resistance to TAK-652. In general, HIV-1 strains that are resistant to an existing class of anti-HIV-1 agents often show cross-resistance to other compounds in the same class. Once such strains have emerged in patients, the choice of alternative agents becomes narrow for current HAART. Several pharmaceutical companies are now developing CCR5 antagonists, such as UK-427,857 (P. Door et al., 10th Conf. Retrovir. Opportunistic Infect., abstr. 12, 2003), SCH-D (D. Schurmann et al., 11th Conf. Retrovir. Opportunistic Infect., abstr. 140LB, 2004), AK602/ONO4128/GW873140 (13), and PRO140 (24). It was reported that an escape mutant resistant to AD101, a CCR5 antagonist structurally related to SCH-C, could be obtained through serial passages of an R5 primary isolate in PBMCs with increasing concentrations of the compound (25). The mutant was >20,000-fold less susceptible than the wild type to AD101 and was cross-resistant to SCH-C. However, no change in coreceptor usage (from CCR5 to CXCR4) was observed for the mutant. A subsequent analysis of the resistant virus revealed that amino acid changes in the V3 loop of gp120 were primarily responsible for the resistance to AD101 (9). More recently, the in vitro establishment and characterization of UK-427,857-resistant HIV-1 have also been presented (M. Westby et al., 13th Int. HIV Drug Resist. Workshop, abstr. 6, 2004). Thus, it is of particular importance to establish TAK-652-resistant mutants and to clarify whether they also show cross-resistance to other CCR5 antagonists. Long-term culture experiments with PBMCs infected with R5 HIV-1 in the presence of TAK-652 are in progress.
Pharmacological and toxicological tests of TAK-652 were conducted in animals, and the compound was found to be orally absorbable and quite safe (data not shown). However, there was significant variability among the oral absorption levels in animals. Therefore, an exploratory phase I trial was attempted to evaluate the safety, tolerability, and pharmacokinetics in humans. TAK-652 was found to show favorable oral absorption and pharmacokinetics in this study. It is noteworthy that TAK-652 had a long half-life in plasma. Plasma drug concentrations were 7.2 and 14.4 ng/ml 24 h after the single oral administration of 25 and 50 mg, respectively (Table 6). From the results in Table 2, we have calculated that the mean EC90 of TAK-652 for the inhibition of R5 HIV-1 clinical isolates is 0.25 nM, which corresponds to 0.2 ng/ml. The anti-HIV-1 activity of TAK-652 was not affected by the PBMC donor or the HIV-1 subtype (Tables 4 and 5). Since its anti-HIV-1 activity was diminished approximately fivefold in the presence of a high concentration of human serum (Table 3), the practical EC90 of TAK-652 in humans appears to be 1 ng/ml (target concentration). Thus, TAK-652 may be able to retain a plasma concentration sufficiently higher than the target concentration by once-daily administration at a reasonable dose. Further trials are ongoing to determine the safety and pharmacokinetics during consecutive administration of TAK-652.
In conclusion, TAK-652 is a novel small-molecule CCR5 antagonist and a potent and selective inhibitor of R5 HIV-1 replication. Pharmacokinetic and toxicity studies of TAK-652 indicate that the compound is safe and orally available in humans. Thus, TAK-652 has proved to be a promising therapeutic agent for HIV-1 infection, and an evaluation of its clinical efficacy in HIV-1-infected individuals will be initiated.
Acknowledgments
We thank K. Kuroshima, M. Inanami, and S. Shiki for their excellent technical assistance. pHXB2-env was obtained through the NIH AIDS Research and Reference Reagent Program, NIAID, Bethesda, MD (by contributors Kathleen Page and Dan Littman). The clinical isolates CTV and HHA were kindly provided by Shuzo Matsushita (Kumamoto University, Kumamoto, Japan). The clinical isolates HKW, HNK, and HTN were kindly provided by Shin-ichi Oka (International Medical Center of Japan, Tokyo, Japan).
This work was supported in part by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (grant 15390174 to M.B.).
REFERENCES
- 1.Baba, M., H. Miyake, M. Okamoto, Y. Iizawa, and K. Okonogi. 2000. Establishment of a CCR5-expressing T-lymphoblastoid cell line highly susceptible to R5 HIV type 1. AIDS Res. Hum. Retrovir. 16:935-941. [DOI] [PubMed] [Google Scholar]
- 2.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 4.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 6.Detels, R., A. Munoz, G. McFarlane, L. A. Kingsley, J. B. Margolick, J. Giorgi, L. K. Schrager, and J. P. Phair. 1998. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA 280:1497-1503. [DOI] [PubMed] [Google Scholar]
- 7.Dragic, T., A. Trkola, D. A. Thompson, E. G. Cormier, F. A. Kajumo, E. Maxwell, S. W. Lin, W. Ying, S. O. Smith, T. P. Sakmar, and J. P. Moore. 2000. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. USA 97:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch, M. S., F. Brun-Vezinet, B. Clotet, B. Conway, D. R. Kuritzkes, R. T. D'Aquila, L. M. Demeter, S. M. Hammer, V. A. Johnson, C. Loveday, J. W. Mellors, D. M. Jacobsen, and D. D. Richman. 2003. Antiretroviral drug resistance testing in adults infected with human immunodeficiency virus type 1: 2003 recommendations of an International AIDS Society-USA Panel. Clin. Infect. Dis. 37:113-128. [DOI] [PubMed] [Google Scholar]
- 9.Kuhmann, S. E., P. Pugach, K. J. Kunstman, J. Taylor, R. L. Stanfield, A. Snyder, J. M. Strizki, J. Riley, B. M. Baroudy, I. A. Wilson, B. T. Korber, S. M. Wolinsky, and J. P. Moore. 2004. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J. Virol. 78:2790-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274:9617-9626. [DOI] [PubMed] [Google Scholar]
- 11.Li, S., J. Juarez, M. Alali, D. Dwyer, R. Collman, A. Cunningham, and H. M. Naif. 1999. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J. Virol. 73:9741-9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 13.Maeda, K., H. Nakata, Y. Koh, T. Miyakawa, H. Ogata, Y. Takaoka, S. Shibayama, K. Sagawa, D. Fukushima, J. Moravek, Y. Koyanagi, and H. Mitsuya. 2004. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J. Virol. 78:8654-8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore, J. P., A. Trkola, and T. Dragic. 1997. Co-receptors for HIV-1 entry. Curr. Opin. Immunol. 9:551-562. [DOI] [PubMed] [Google Scholar]
- 15.Moore, J. P., S. G. Kitchen, P. Pugach, and J. A. Zack. 2004. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS. Res. Hum. Retrovir. 20:111-126. [DOI] [PubMed] [Google Scholar]
- 16.Moore, R. D., and R. E. Chaisson. 1999. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS 13:1933-1942. [DOI] [PubMed] [Google Scholar]
- 17.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 18.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-312. [DOI] [PubMed] [Google Scholar]
- 19.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 21.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takashima, K., H. Miyake, R. A. Furuta, J. Fujisawa, Y. Iizawa, N. Kanzaki, M. Shiraishi, K. Okonogi, and M. Baba. 2001. Inhibitory effects of small-molecule CCR5 antagonists on human immunodeficiency virus type 1 envelope-mediated membrane fusion and viral replication. Antimicrob. Agents Chemother. 45:3538-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takashima, K., H. Miyake, N. Kanzaki, Y. Tagawa, X. Wang, Y. Sugihara, Y. Iizawa, and M. Baba. 2005. Highly potent inhibition of human immunodeficiency virus type 1 replication by TAK-220, an orally bioavailable small molecule CCR5 antagonist. Antimicrob. Agents Chemother. 49:3474-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuttle, D. L., C. B. Anders, M. J. Aquino-De Jesus, P. P. Poole, S. L. Lamers, D. R. Briggs, S. M. Pomeroy, L. Alexander, K. W. Peden, W. A. Andiman, J. W. Sleasman, and M. M. Goodenow. 2002. Increased replication of non-syncytium-inducing HIV type 1 isolates in monocyte-derived macrophages is linked to advanced disease in infected children. AIDS Res. Hum. Retrovir. 18:353-362. [DOI] [PubMed] [Google Scholar]
- 27.Wu, L., G. LaRosa, N. Kassam, C. J. Gordon, H. Heath, N. Ruffing, H. Chen, J. Humblias, M. Samson, M. Parmentier, J. P. Moore, and C. R. Mackay. 1997. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 186:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeni, P. G., S. M. Hammer, M. S. Hirsch, M. S. Saag, M. Schechter, C. C. Carpenter, M. A. Fischl, J. M. Gatell, B. G. Gazzard, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2004. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 292:251-265. [DOI] [PubMed] [Google Scholar]