Abstract
In a community of Tanzania where trachoma is endemic, we cultured conjunctival swabs from all residents who had active trachoma and were PCR positive for ocular Chlamydia trachomatis, both before (43 isolates) and 2 months after (9 isolates) mass antibiotic treatment. No clinically or programmatically significant increase in azithromycin or tetracycline resistance was observed.
Trachoma is caused by repeated conjunctival infection with Chlamydia trachomatis (3, 5). It is a major cause of blindness in poor communities (7). The World Health Organization (WHO) recommends annual mass antibiotic treatment (treatment of every resident of the community) wherever the prevalence of the clinical sign “trachomatous inflammation—follicular” (14) in 1- to 9-year-old children is 10% or greater (8). There are two alternative antibiotic regimens: 1% tetracycline eye ointment applied twice daily to both eyes for 6 weeks (18) or (where available) one oral dose of 20 mg azithromycin per kilogram body weight (or equivalent dose determined by height), to a maximum of 1 g (1).
The efficacy of single-dose azithromycin is the foundation upon which hopes for the global elimination of blinding trachoma by 2020 (17) have been built. There is concern, however, that mass distribution of azithromycin will select for macrolide-resistant strains of C. trachomatis or other pathogens (2).
After obtaining approval from the ethics committees of the London School of Hygiene & Tropical Medicine, London, United Kingdom, and the Kilimanjaro Christian Medical Centre, Tumaini University, Moshi, Tanzania, we invited all residents of Kahe Mpya subvillage, Rombo District, Tanzania (10), to participate. Written informed consent was obtained from each participant or their guardian. All consenting residents were examined using the WHO simplified system (14). In this system, “trachomatous inflammation—follicular” and/or “trachomatous inflammation—intense” constitutes the presence of “active trachoma.” A swab was taken from the right tarsal conjunctiva for PCR against C. trachomatis DNA (9, 10). All subjects with right eye active trachoma had a second right eye swab taken for C. trachomatis culture (10). Specimen handling has been described elsewhere (9, 10).
Every resident was offered azithromycin, or tetracycline eye ointment if azithromycin was contraindicated (9). Examination and swabbing were repeated 2 months later.
Swabs for PCR were tested by Amplicor (9, 10). Swabs in 2-SP medium (10) were cultured from subjects who proved PCR positive.
We used a previously described C. trachomatis isolation method (11) with minor adaptations. We treated confluent 24-h-old HeLa 229 cell monolayers with 30 μg/ml DEAE-dextran for 20 min. Each culture swab was vortexed with sterile glass beads in its 2-SP medium for 15 s; 100 μl was then inoculated onto each of three DEAE-treated HeLa cell monolayers in 12-well tissue culture plates and incubated at 35°C in 5% CO2 for 1 h. One milliliter of isolation medium (minimal essential medium, 10% fetal calf serum, 100 mM glutamine, 1 μg/ml cycloheximide) was added to each well, and cultures were centrifuged at 1,240 × g for 60 min at 25°C. An additional 1 ml of isolation medium was added to each well, and cultures were incubated at 35°C in 5% CO2. At 72 h, one of the three triplicate monolayers was stained with C. trachomatis-specific fluorescein-conjugated monoclonal antibody (Syva Co., Palo Alto, Calif.) to identify inclusions; the other two were blindly passaged onto fresh HeLa cells. Specimens were considered culture negative if no inclusions were detected after 10 blind passages.
To provide duplicate controls, we cultured and determined sensitivities for two samples (no. 0002497 and 0001452) twice. One sample of each pair was randomly selected as the “original” isolate for use in subsequent analyses; the other became the “duplicate.”
C. trachomatis strains IU823, IU824, and IU825 (serovar E isolates [4], provided courtesy of R. B. Jones) were our tetracycline-resistant reference strains. Type strains for serovar A (strain HAR-13) and serovar D (strain UW-3/CX) (both provided courtesy of C.-C. Kuo) were our susceptible reference strains.
For determination of antimicrobial sensitivities, an inoculum of 100 μl (average of 20,000 inclusion-forming units) was added to confluent DEAE-treated HeLa 229 cell monolayers in 24-well culture plates. One milliliter of isolation medium containing the appropriate concentration of antibiotic was added to each set of triplicate wells and to the control wells. The plates were centrifuged at 1,240 × g for 60 min at 25°C and then incubated at 35°C in 5% CO2 for 48 h. One set of cultures was fixed with methanol and stained with monoclonal antibody to C. trachomatis (Syva). To determine the minimum chlamydicidal concentration (MCC), a second set of cultures was prepared by scraping cells off into sucrose-phosphate-glutamic acid medium, sonicating the cell suspensions, inoculating fresh 18- to 24-h-old HeLa 229 monolayers, and incubating them without addition of antibiotics. The MCC was defined as the lowest concentration of antimicrobial that prevented inclusion formation following antimicrobial removal and continued culture for one passage (15).
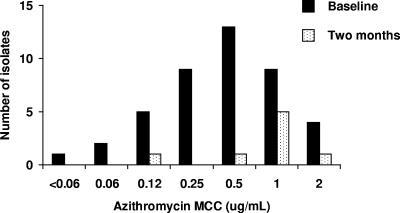
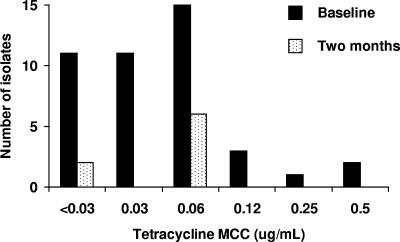
At baseline, as reported elsewhere (9, 10), 956 (98%) of the 978 residents of Kahe Mpya were examined. A total of 174 subjects (18%) had right eye active trachoma; 56 of these (32% of 174; 6% of all subjects) were Amplicor positive. A total of 46 (82%) of 56 subjects were culture positive (10). Mean azithromycin (Fig. 1) and tetracycline (Fig. 2) MCCs for the 43 isolates for which sensitivities could be determined were 0.6 μg/ml and 0.07 μg/ml, respectively.
FIG. 1.
MCCs of azithromycin against C. trachomatis isolates obtained at baseline and 2 months after mass antibiotic treatment.
FIG. 2.
MCCs of tetracycline against C. trachomatis isolates obtained at baseline and 2 months after mass antibiotic treatment.
For each pair of duplicate cultures, MCCs of both azithromycin and tetracycline were within one twofold dilution (Table 1). Only one of the tetracycline-resistant controls (IU824) appeared tetracycline resistant (MCC, 2 μg/ml); IU823 and IU825, which were recovered from the same patient as IU824 (4), had tetracycline MCCs of 0.25 μg/ml and 0.12 μg/ml, respectively (Table 1).
TABLE 1.
Antibiotic susceptibilities of duplicates and controls
| Isolate | Type of isolate | MCC (μg/ml) of:
|
|
|---|---|---|---|
| Azithromycin | Tetracycline | ||
| 0002497A | Original | 2 | 0.06 |
| 0002497B | Duplicate | 2 | 0.12 |
| 0001452A | Original | 0.5 | 0.06 |
| 0001452B | Duplicate | 0.5 | 0.06 |
| IU823 | Resistant controla | 1 | 0.25 |
| IU824 | Resistant controla | 1 | 2 |
| IU825 | Resistant controla | 0.06 | 0.12 |
| A | Susceptible control | 1 | 0.06 |
| D | Susceptible control | 1 | 0.12 |
Resistant to tetracycline.
After baseline swabbing, 916 people (94% of 978 subvillage residents) received azithromycin and 39 (4%) received tetracycline eye ointment (9). Two months later, 905 (94%) of the 959 people then resident in Kahe Mpya were examined; 49 (5%) had right eye active trachoma, and 9 of these were Amplicor positive. Eight (89%) of 9 were culture positive; antimicrobial sensitivities of these isolates are shown in Fig. 1 and 2. Mean azithromycin and tetracycline MCCs at 2 months were 1.0 μg/ml and 0.05 μg/ml.
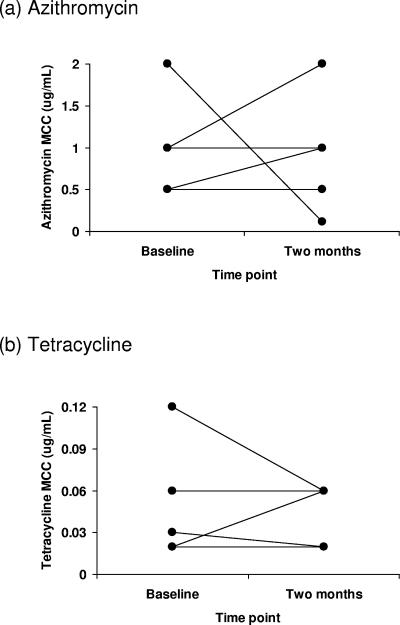
Five subjects had C. trachomatis isolates at both baseline and 2 months. All five were given azithromycin immediately after baseline swabbing. MCCs of azithromycin were higher in 2-month isolates than baseline isolates in two individuals, lower in one, and the same in two; MCCs of tetracycline were higher in 2-month isolates than baseline isolates in one individual, lower in two, and the same in two (Fig. 3).
FIG. 3.
MCCs of (a) azithromycin and (b) tetracycline against isolates taken from the five individuals from whom C. trachomatis was cultured at both baseline and 2 months after mass antibiotic treatment. Each line joins the two isolates taken from the same subject.
Evaluation of antimicrobial resistance in C. trachomatis is an evolving science and remains somewhat controversial (12, 15). Our protocol is based on the consensus of an expert group on antimicrobial susceptibility methods (6) and is routinely employed in the reference laboratory of the Public Health Agency of Canada. In accordance with recent recommendations (15), antibiotic solutions were prepared immediately prior to addition to infected cells and added immediately after HeLa cell inoculation; MCCs were determined after 48 h in antibiotic-containing medium followed by 48 h in antibiotic-free medium.
Both before and 2 months after mass antibiotic treatment, MCCs of azithromycin for all C. trachomatis strains isolated were ≤2.0 μg/ml. Tissue concentrations of azithromycin obtained after oral administration are much higher than this: in four patients aged 43 or more years given 1 g azithromycin 24 h before cataract surgery, the mean (±standard deviation) concentration in conjunctival tissue at operation was 24.5 (±9.7) μg/g (13). All C. trachomatis isolates obtained from Kahe Mpya subjects before mass treatment had MCCs of tetracycline of ≤0.5 μg/ml, while all isolates obtained at 2 months had MCCs of tetracycline of ≤0.06 μg/ml. We conclude that no clinically or programmatically significant changes in C. trachomatis azithromycin or tetracycline susceptibilities were induced by mass antibiotic treatment in this community.
Acknowledgments
We are grateful to the village leaders and residents of Kahe for their advice, assistance, and enthusiastic participation; to our project steering committee; to the management and maintenance staff of Bonite Bottlers, Moshi, for generously supplying the liquid CO2 from which we made dry ice; the International Trachoma Initiative, Dar es Salaam, for donation of azithromycin; and the Ministry of Health, United Republic of Tanzania, for support and encouragement.
The study was funded by grants from the Edna McConnell Clark Foundation (99100), Wellcome Trust/Burroughs Wellcome Fund (059134), the International Trachoma Initiative (01/032), and Pfizer Inc.
REFERENCES
- 1.Bailey, R. L., P. Arullendran, H. C. Whittle, and D. C. Mabey. 1993. Randomised controlled trial of single-dose azithromycin in treatment of trachoma. Lancet 342:453-456. [DOI] [PubMed] [Google Scholar]
- 2.Fry, A. M., H. C. Jha, T. M. Lietman, J. S. Chaudhary, R. C. Bhatta, J. Elliott, T. Hyde, A. Schuchat, B. Gaynor, and S. F. Dowell. 2002. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin. Infect. Dis. 35:395-402. [DOI] [PubMed] [Google Scholar]
- 3.Grayston, J. T., S. P. Wang, L. J. Yeh, and C. C. Kuo. 1985. Importance of reinfection in the pathogenesis of trachoma. Rev. Infect. Dis. 7:717-725. [DOI] [PubMed] [Google Scholar]
- 4.Jones, R. B., B. Van der Pol, D. H. Martin, and M. K. Shepard. 1990. Partial characterization of Chlamydia trachomatis isolates resistant to multiple antibiotics. J. Infect. Dis. 162:1309-1315. [DOI] [PubMed] [Google Scholar]
- 5.Mabey, D. C., A. W. Solomon, and A. Foster.2003. Trachoma. Lancet 362:223-229. [DOI] [PubMed] [Google Scholar]
- 6.Peeling, R. W., W. R. Bowie, J. R. Dillon, R. Johnson, R. B. Jones, B. Van der Pol, D. T. Low, D. H. Martin, J. Newhall, J. Orfila, R. Rice, J. Schachter, and J. Moncada. 1994. Standardisation of antimicrobial susceptibility testing for Chlamydia trachomatis, p. 346-349. In J. Orfila, G. I. Byrne, M. A. Chernesky, J. T. Grayston, R. B. Jones, G. L. Ridgway, P. Saikku, J. Schachter, W. E. Stamm, and R. S. Stephens (ed.), Chlamydial infections. Proceedings of the Eighth International Symposium on Human Chlamydial Infections. Societa Editrice Esculapio, Bologna, Italy.
- 7.Resnikoff, S., D. Pascolini, D. Etya'ale, I. Kocur, R. Pararajasegaram, G. P. Pokharel, and S. P. Mariotti. 2004. Global data on visual impairment in the year 2002. Bull. W. H. O. 82:844-851. [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon, A., and M. Burton. 2004. What's new in azithromycin? Commun. Eye Health 17:54-56. [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon, A. W., M. J. Holland, N. D. Alexander, P. A. Massae, A. Aguirre, A. Natividad-Sancho, S. Molina, S. Safari, J. F. Shao, P. Courtright, R. W. Peeling, S. K. West, R. L. Bailey, A. Foster, and D. C. Mabey. 2004. Mass treatment with single-dose azithromycin for trachoma. N. Engl. J. Med. 351:1962-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon, A. W., M. J. Holland, M. J. Burton, S. K. West, N. D. Alexander, A. Aguirre, P. A. Massae, H. Mkocha, B. Munoz, G. J. Johnson, R. W. Peeling, R. L. Bailey, A. Foster, and D. C. Mabey. 2003. Strategies for control of trachoma: observational study with quantitative PCR. Lancet 362: 198-204. [DOI] [PubMed] [Google Scholar]
- 11.Stephens, R. S., C.-C. Kuo, and M. R. Tam. 1982. Sensitivity of immunofluorescence with monoclonal antibodies for detection of Chlamydia trachomatis inclusions in cell culture. J. Clin. Microbiol. 16:4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suchland, R. J., W. M. Geisler, and W. E. Stamm. 2003. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob. Agents Chemother. 47:636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabbara, K. F., S. A. al-Kharashi, S. M. al-Mansouri, O. M. al-Omar, H. Cooper, A. M. el-Asrar, and G. Foulds. 1998. Ocular levels of azithromycin. Arch. Ophthalmol. 116:1625-1628. [DOI] [PubMed] [Google Scholar]
- 14.Thylefors, B., C. R. Dawson, B. R. Jones, S. K. West, and H. R. Taylor. 1987. A simple system for the assessment of trachoma and its complications. Bull. W. H. O. 65:477-483. [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, S. A., J. R. Papp, W. E. Stamm, R. W. Peeling, D. H. Martin, and K. K. Holmes. 2005. Evaluation of antimicrobial resistance and treatment failures for Chlamydia trachomatis: a meeting report. J. Infect. Dis. 191:917-923. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.World Health Organization. 1997. Planning for the global elimination of trachoma (GET): report of a W.H.O. consultation (W.H.O./PBL/97.60). World Health Organization, Geneva, Switzerland.
- 18.World Health Organization. 1993. Primary health care level management of trachoma (W.H.O./PBL/93.33). World Health Organization, Geneva, Switzerland.