Abstract
The Mycobacterium tuberculosis mmpL7 gene, encoding a hypothetical resistance nodulation division transporter, confers a high resistance level to isoniazid when overexpressed in Mycobacterium smegmatis. The resistance level decreased in the presence of the efflux pump inhibitors reserpine and CCCP (carbonyl cyanide m-chlorophenylhydrazone). Energy-dependent efflux of isoniazid from M. smegmatis cells expressing the mmpL7 gene was observed.
The intrinsic resistance of Mycobacterium tuberculosis to most antibiotics is generally attributed to the low permeability of the mycobacterial cell wall, due to its specific lipid-rich composition and structure (2). However, since the intracellular concentration of a given drug depends on the balance between its influx and efflux, along with cell wall permeability, active efflux systems also provide resistance by extruding the drug molecules that enter the cell.
Bacterial drug efflux pumps have been classified into five families (11). The genome of M. tuberculosis contains genes encoding drug efflux transporters from all of these families (http://www.membranetransport.org), and, as described in a recent review, several mycobacterial drug efflux pumps have been identified and characterized experimentally (8).
Resistance nodulation division (RND) transporters have been found in all major kingdoms of living organisms, but they seem to be involved in drug efflux only in gram-negative bacteria. The AcrAB/TolC drug efflux pump of Escherichia coli provides a prototype for such export systems, with AcrB constituting the membrane pump itself, AcrA the membrane fusion protein, and TolC the outer membrane component (12).
Interestingly, the genome sequence of M. tuberculosis revealed the presence of 13 putative transmembrane proteins, predicted to be transport proteins of the RND superfamily (5). Since these proteins appear to be confined to mycobacteria, they have been designated MmpL (mycobacterial membrane proteins, large). The hydrophobic nature of the MmpL proteins and the close association of four of their genes with those involved in lipid metabolism suggest that they may be naturally involved in the transport of fatty acids (13). Indeed, MmpL8 is involved in sulfolipid-1 biosynthesis by transporting a precursor of this molecule (6, 9), while the MmpL7 protein catalyzes the export of phthiocerol dimycocerosate (PDIM) in M. tuberculosis (3). A mutant lacking the mmpL7 gene was severely attenuated for growth in the lungs (7).
Given the similarities with other RND transporters, it is possible that the MmpL proteins can also act in drug efflux.
In this paper, we demonstrate that the mmpL7 gene from M. tuberculosis confers a high level of resistance to isoniazid (INH) when overexpressed in Mycobacterium smegmatis. The resistance level significantly decreases in the presence of efflux inhibitors. We also observed energy-dependent efflux of INH from M. smegmatis cells expressing the mmpL7 gene.
Transformation of the M. tuberculosis cosmid library into M. smegmatis and selection for INH resistance.
A cosmid library of M. tuberculosis H37Rv constructed in the pYUB18 cosmid (kindly provided by S. Cole) was electroporated into M. smegmatis mc2155, and the transformants were selected on Middlebrook 7H11 agar supplemented with 10% (vol/vol) Middlebrook oleic acid-albumin-dextrose-catalase enrichment and 0.2% (vol/vol) glycerol containing different concentrations of INH. Three clones showed a high level of INH resistance (512 μg/ml, 16 times the MIC), while one clone had a moderate resistance (128 μg/ml, 4 times the MIC). Partial DNA sequence analyses revealed that all the cosmids responsible for a high INH resistance contained the inhA gene (1) and were discarded. The INH16 cosmid clone with moderate INH resistance was chosen for further characterization. The DNA fragment contained in this cosmid extends from nucleotides 3277340 to 3311634 of the M. tuberculosis genome and contains several genes, including mmpL7, on which we focused our attention. MmpL7, a protein of 920 amino acids with a predicted molecular mass of 95.1 kDa, contains 12 transmembrane domains (TMDs) and two large hydrophilic extracytoplasmic domains between TMD 1 and TMD 2 and between TMD 7 and TMD 8. All of these characteristics are described as typical of RND efflux pumps (14). Pairwise alignment of amino acid sequences showed that MmpL7 is 14% identical to and 46% similar to AcrB of E. coli (8).
mmpL7 gene overexpression is responsible for increased INH MIC of M. smegmatis.
One way to validate the hypothesis that a gene protects against the toxic effects of a drug is to determine whether constitutive overexpression of the gene results in increased resistance to this drug. Consequently, to understand whether the INH resistance of M. smegmatis was conferred by the mmpL7 gene, the gene was amplified from INH16 cosmid DNA by PCR by using the 5′-TGAAGCTTATGCCTAGTCCGG-3′ sense primer and the 5′-ATAAGCTTCGTGGCATGGGGTCT-3′ antisense primer. The PCR product was cloned into pGEM-T Easy vector and sequenced on both strands. The mmpL7 gene was then cloned into pSODIT-2 shuttle expression vector containing the determinant of hygromycin resistance (kindly provided by D. Young). pSODIT-2 and pSODIT/mmpL7 plasmids were introduced into M. smegmatis mc2155 cells plated onto Middlebrook 7H11 agar containing hygromycin and INH concentrations ranging from 32 μg/ml to 512 μg/ml. The isoniazid resistance level shown by M. smegmatis cells expressing the mmpL7 gene was very high, more than 16 times the wild-type MIC (>512 μg/ml versus 32 μg/ml). Susceptibilities to ethambutol, ciprofloxacin, ofloxacin, tetracycline, rifampin, ethidium bromide, doxorubicin, and tetraphenylphosphonium were not affected (data not shown). Consequently, the mmpL7 gene seems to be responsible for the INH resistance.
The MIC of ethionamide, a structural analog of INH and a useful second-line antituberculosis drug, was also determined. The overexpression of the MmpL7 protein caused a fourfold increase in the MIC of ethionamide for M. smegmatis cells expressing the mmpL7 gene compared to the MIC for M. smegmatis cells containing the vector pSODIT-2 alone (100 μg/ml versus 25 μg/ml).
The INH MIC was also determined in the presence of the efflux inhibitors reserpine (12 μg/ml), CCCP (carbonyl cyanide m-chlorophenylhydrazone; 15 μg/ml), and verapamil (40 μg/ml) in order to evaluate the effects of different, well-known efflux pump inhibitors on resistance levels. M. smegmatis cells containing the recombinant plasmid pSODIT/mmpL7 and the pSODIT-2 expression vector were grown on the same medium used for MIC testing containing different isoniazid concentrations and the inhibitors described above. As shown in Fig. 1, reserpine and CCCP reduced the isoniazid resistance eightfold (512 μg/ml versus 64 μg/ml) in M. smegmatis cells carrying the plasmid pSODIT/mmpL7, while verapamil had no effect on isoniazid resistance. The decrease in the INH MIC is presumably due to the inhibition of drug efflux mediating by the mmpL7 gene product. On the contrary, the INH MIC of M. smegmatis cells carrying the vector pSODIT-2 was not affected by any of the tested inhibitors (Fig. 1).
FIG. 1.
Effect of reserpine, CCCP, and verapamil on INH MICs of M. smegmatis transformants carrying pSODIT-2 or pSODIT/mmpL7. The results are the average of results for four replicates, and error bars indicate standard deviations.
Accumulation and efflux of isoniazid.
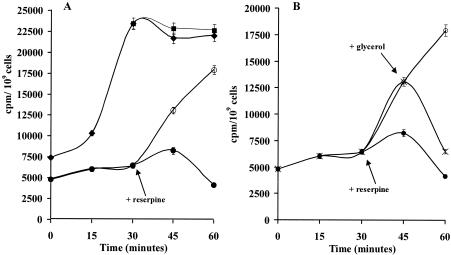
We hypothesized that the MmpL7 protein might confer INH resistance by directly transporting the drug out of the cell. To determine whether the resistance to isoniazid was due to an active drug efflux mechanism, the accumulation of isoniazid was monitored in M. smegmatis mc2155 cells carrying either pSODIT/mmpL7 and pSODIT-2, as described previously (4). The M. smegmatis recombinant strains were incubated with [14C]INH, and the INH intracellular concentration was measured at various time points. As shown in Fig. 2A, cells harboring the expression vector pSODIT-2 took up [14C]INH rapidly and achieved a steady-state level of accumulation within 30 min of incubation. This accumulation was approximately 65% lower in cells harboring the pSODIT/mmpL7 recombinant plasmid.
FIG. 2.
(A) Effect of the addition of reserpine on the accumulation of isoniazid by M. smegmatis cells carrying the pSODIT/mmpL7 plasmid (•, no reserpine addition; ○, addition of reserpine) or pSODIT-2 expression vector (♦, no reserpine addition; ▪, addition of reserpine). (B) Effect of the addition of reserpine and glycerol on the accumulation and efflux of isoniazid by M. smegmatis cells carrying the pSODIT/mmpL7 plasmid (•, no reserpine addition; ○, addition of reserpine; X, addition of glycerol). The arrows indicate the times of addition of reserpine and glycerol. The results are the averages of results for three replicates, and error bars indicate standard deviations.
A reduced level of accumulation of the drug may be caused either by a decreased level of drug permeation or by active drug extrusion through the cytoplasmic membrane. To determine the effect of membrane deenergization on the uptake of INH, reserpine was added to cells containing [14C]INH. Upon the addition of reserpine, the level of INH accumulation in the pSODIT/mmpL7-harboring strain increased to levels close to the values shown by the strain containing the expression vector pSODIT-2 (Fig. 2A). These results indicate that reserpine inhibits the efflux pump and that MmpL7 protein pumps out INH in an energy-dependent process. In contrast, under our conditions, reserpine had no significant effect on the level of isoniazid accumulation in the control strain carrying the vector pSODIT-2 (Fig. 2A).
To test if the available source of energy could lead to drug efflux, 0.2% (vol/vol) glycerol was added 15 min after the addition of reserpine. As shown in Fig. 2B, M. smegmatis cells expressing the mmpL7 gene, treated with reserpine and glycerol, rapidly eliminated INH, whereas the cells treated with reserpine only did not extrude significant amounts of the drug. On the contrary, the addition of glycerol had no effect on the level of INH accumulation in the case of M. smegmatis cells, carrying the pSODIT-2 vector, which were treated with reserpine (data not shown).
Together, these results strongly indicate that the MmpL7 protein actively pumps out isoniazid in M. smegmatis. To our knowledge, MmpL7 is the first example of an RND-like transporter responsible for isoniazid resistance in M. smegmatis.
In order to begin to understand the range of efflux substrates for the MmpL proteins and to assess any potential role in drug resistance in M. tuberculosis, Domenech et al. (10) constructed mutant strains with 11 out of 13 of the mmpL genes inactivated. Since the drug susceptibilities of these mutants to a broad spectrum of agents are unaltered, the authors suggest that, unlike their function in other organisms, these proteins do not play a significant role in the intrinsic drug resistance of M. tuberculosis. It is noteworthy that the mmpL7 knockout in M. tuberculosis seems to have no effect on INH MICs. This only appears to be in contrast with our results, since in M. smegmatis the environmental conditions could be completely different. A search of the available M. smegmatis genome database revealed that the MmpL7 protein is not present in this organism, and this finding is in agreement with the statement that the PDIM molecule was not found in M. smegmatis (3). Consequently, the M. tuberculosis MmpL7 protein can utilize INH as a substrate when it is expressed in M. smegmatis, while in M. tuberculosis INH could compete with the natural substrate (PDIM) of MmpL7 since its principal physiological role appears to be the export of complex lipids to the cell exterior. However, we cannot exclude the possitibility that an overexpression of the mmpL7 gene in M. tuberculosis could be responsible for the low-level INH resistance in those clinical isolates for which no mutation in the known gene targets has been identified. Analysis of mmpL7 gene expression in these strains could confirm its role in low-level INH resistance.
Acknowledgments
This research was supported by the European Union research project “Quality of life and management of living resources” (contract QLK2-CT-2000-01761), by the Fondo d'Ateneo per la Ricerca 2005, and by the Istituto Superiore Sanità, Ricerca Finalizzata, 2005.
We thank S. T. Cole for kindly providing the M. tuberculosis cosmid library and D. Young for pSODIT-2 vector.
M. R. Pasca and P. Guglierame contributed equally to the work.
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 2.Brennan, P. J. 2003. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 83:91-97. [DOI] [PubMed] [Google Scholar]
- 3.Camacho, L. R., P. Constant, C. Raynaud, M. A. Laneelle, J. A. Triccas, B. Gicquel, M. Daffe, and C. Guilhot. 2001. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 276:19845-19854. [DOI] [PubMed] [Google Scholar]
- 4.Choudhuri, B. S., S. Sen, and P. Chakrabarti. 1999. Isoniazid accumulation in Mycobacterium smegmatis is modulated by proton motive force-driven and ATP-dependent extrusion systems. Biochem. Biophys. Res. Commun. 256:682-684. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Converse, S. E., J. D. Mongous, M. D. Leavell, J. A. Leary, C. R. Bertozzi, and J. S. Cox. 2003. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl. Acad. Sci. USA 100:6121-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox, J. S., B. Chen, M. McNeil, and W. R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79-83. [DOI] [PubMed] [Google Scholar]
- 8.De Rossi, E., J. A. Aínsa, and G. Riccardi. Role of mycobacterial efflux transporters in drug resistance: an unresolved question. FEMS Microbiol. Rev., in press. [DOI] [PubMed]
- 9.Domenech, P., M. B. Reed, C. S. Dowd, C. Manca, G. Kaplan, and C. E. Barry, III. 2004. The role of MmpL8 in sulfatide biogenesis and virulence of Mycobacterium tuberculosis. J. Biol. Chem. 279:21257-21265. [DOI] [PubMed] [Google Scholar]
- 10.Domenech, P., M. B. Reed, and C. E. Barry III. 2005. Contribution of the Mycobacterium tuberculosis MmpL protein family to virulence and drug resistance. Infect. Immun. 73:3492-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, X. Z., and H. Nikaido. 2004. Efflux-mediated drug resistance in bacteria. Drugs 64:159-204. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido, H., and H. I. Zgurskaya. 2001. AcrAB and related multidrug efflux pumps of Escherichia coli. J. Mol. Microbiol. Biotechnol. 3:215-218. [PubMed] [Google Scholar]
- 13.Tekaia, F., S. V. Gordon, T. Garnier, R. Brosch, B. G. Barrell, and S. T. Cole. 1999. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 79:329-342. [DOI] [PubMed] [Google Scholar]
- 14.Tseng, T. T., K. S. Gratwick, J. Kollman, D. Park, D. H. Nies, A. Goffeau, and M. H. Saier, Jr. 1999. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1:107-125. [PubMed] [Google Scholar]