Abstract
Methylene blue (MB) represents a promising antimalarial drug candidate for combination therapies against drug-resistant parasite strains. To support and facilitate the application of MB in future field trials, we studied its antiparasitic effects in vitro. MB is active against all blood stages of both chloroquine (CQ)-sensitive and CQ-resistant P. falciparum strains with 50% inhibitory concentration (IC50) values in the lower nanomolar range. Ring stages showed the highest susceptibility. As demonstrated by high-performance liquid chromatography-tandem mass spectrometry on different cell culture compartments, MB is accumulated in malarial parasites. In drug combination assays, MB was found to be antagonistic with CQ and other quinoline antimalarials like piperaquine and amodiaquine; with mefloquine and quinine, MB showed additive effects. In contrast, we observed synergistic effects of MB with artemisinin, artesunate, and artemether for all tested parasite strains. Artemisinin/MB combination concentration ratios of 3:1 were found to be advantageous, demonstrating that the combination of artemisinin with a smaller amount of MB can be recommended for reaching maximal therapeutic effects. Our in vitro data indicate that combinations of MB with artemisinin and related endoperoxides might be a promising option for treating drug-resistant malaria and should be studied in future field trials. Resistance development under this drug combination is unlikely to occur.
Methylene blue (MB)—a drug clinically applied in methemoglobinopathies—has also been shown to have antimalarial effects and was identified as a specific inhibitor of Plasmodium falciparum glutathione reductase (GR). Thus, MB was recently reconsidered as a useful antimalarial drug (12, 22, 32). Advantages of MB include its intrinsic inhibition of heme polymerization within the food vacuole (2), its prevention of methemoglobinemia—a serious complication of malaria anemia (1)—and its relatively low price. Furthermore, MB shows high selectivity indices with respect to the viability of the human monocytic leukemia-derived cell line J-111 (2, 36), indicating that its cytotoxicity for mammalian cells is low. Considerable side effects of MB have been reported (16, 27), but they are likely to be restricted to persons with certain forms of inherited glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Due to increasing drug resistance, the development of chloroquine (CQ) sensitizers in combination with CQ is given high priority in antimalarial drug research (35). CQ-resistant parasites were shown to possess significantly increased concentrations of reduced glutathione (GSH) when compared with sensitive parasites. Thus, the reduction of glutathione disulfide by the flavoenzyme glutathione reductase and/or the de novo synthesis of GSH seems to be more efficient in resistant parasites (10, 15, 23). In Plasmodium, GSH is likely to be involved in buffering the reducing milieu, in antioxidant defense, redox signaling, DNA synthesis, and heme degradation, and in detoxification reactions catalyzed by glutathione S-transferase (3, 4, 14, 18). Furthermore, the combination of CQ derivatives with the selective P. falciparum GR inhibitor 6-[2-(3-methyl)-naphthoquinolyl]hexanoic acid as a double-drug conjugate inhibited the growth of CQ-resistant Plasmodium species both in vitro and in vivo (7). These and other observations suggest that the combination of CQ with MB—as a P. falciparum GR inhibitor and thus CQ sensitizer—might be useful. This approach has recently been tested in clinical trials in the Nouna District of Burkina Faso (6, 22). In this study, cases of MB toxicity or intolerance were not reported; however, a clear advantage over CQ monotherapy could not be observed either (24). Several reasons were proposed to be responsible for this clinical failure. They include (i) the possibility that the dosage of the highly water-soluble MB was not high enough (24), (ii) the possibility that MB and CQ do act antagonistically, and (iii) the possible formation of stable blue pigments in blood cells by MB leading to false-positive counts of persistent parasitemia. As summarized by Wainwright and Amaral (37), the latter cause can be largely excluded.
In the work reported here, we systematically studied the effects of MB on P. falciparum in cell culture, including growth inhibitory effects on different parasite strains, stage specificity, uptake, and staining effects. In addition, we tested the combination of MB with other clinically used antimalarials in a search for potential clinical drug combination therapies.
MATERIALS AND METHODS
Drugs and chemicals.
MB was obtained from Roth (Karlsruhe, Germany); RPMI 1640 medium was from GIBCO Invitrogen Life Technologies (Paisley, Scotland); chloroquine, amodiaquine, and pyrimethamine were from Sigma-Aldrich (Steinheim, Germany); mefloquine was from Roche (Mannheim, Germany); artemisinin and primaquine diphosphate were from Aldrich Chemical Co. (Milwaukee, Wis.); and quinine was from Acrös Organics (Geel, Belgium). MB for analytical purposes was purchased from Calbiochem/Merck (Darmstadt, Germany). Artemisinin derivatives (artemether and artesunate) as well as piperaquine tetraphosphate were kindly provided by the Swiss Tropical Institute (Basel, Switzerland) and J. Carl Craft, Medicines for Malaria Venture (Geneva, Switzerland), respectively.
Cultivation of Plasmodium falciparum.
CQ-sensitive (3D7-Netherlands and HB3-Honduras) and resistant (K1-South-East Asia and Dd2-Indochina) strains of P. falciparum were grown in continuous culture as described by Trager and Jensen (34) with slight modifications. Unless otherwise stated, parasites were maintained at 1 to 10% parasitemia and 3.3% hematocrit in an RPMI 1640 culture medium supplemented with A+ erythrocytes, 4% A+ human serum, 0.2% lipid-rich bovine serum albumin (Albumax), 9 mM (0.16%) glucose, 0.2 mM hypoxanthine, 2.1 mM l-glutamine, and 22 μg/ml gentamicin. All incubations were carried out at 37°C in 3% O2, 3% CO2, and 94% N2. Synchronization of parasites in culture to ring stages was carried out by treatment with 5% (wt/vol) sorbitol (20). For the conditions described above, the CQ sensitivities, expressed as 50% inhibitory concentration (IC50) values, were found to be 8.6 ± 0.4 nM for strain 3D7, 16.8 ± 0.5 nM for HB3, 90.2 ± 10.6 nM for Dd2, and 155 ± 11.4 nM for K1. The parasites were used for the experiments delineated below.
Stage specificity of MB action.
IC50 values for MB were determined on different synchronized blood-stage forms (rings, trophozoites, and schizonts) of P. falciparum strain K1. For this purpose, aliquots of a parasite culture synchronized to the ring stage (t = 0) were drawn every 3 to 6 h throughout the 48-h cycle and exposed to various MB concentrations. Considering the multiplication of the parasites towards the end of the cycle, the parasites to be treated at 39, 42, and 48 h were diluted with A+ erythrocytes by factors of 1:2, 1:4, and 1:7, respectively. Parasites were exposed to the drug for 6 h followed by a change of the medium and undisturbed growth for 42 h to complete a full cycle. Parasite growth and parasitemia were monitored by assessing Giemsa-stained blood smears under the microscope.
Effect of drug combinations with MB on P. falciparum.
Isotopic drug sensitivity assays by means of the semiautomated microdilution technique (9) were employed to investigate the effects of MB in combination with other clinically used antimalarials. The method which depends on the incorporation of radioactive [3H]hypoxanthine—which is taken up by the parasite as a precursor of purine deoxynucleotides for DNA synthesis—was performed according to the modifications of Fivelman et al. (13). In 96-well microtiter plates (Nunc), a twofold serial dilution of the starting concentration of each drug to be tested was carried out. Two drugs to be tested in combination were applied alone and in fixed concentration ratios of 1:1, 1:3, and 3:1 as described by Fivelman et al. (13). Parasites were incubated at a parasitemia of 0.125% (>70% ring forms) and 1.25% hematocrit in hypoxanthine-free medium. After 48 h, 0.5 μCi of [3H]hypoxanthine was added to each well and the plates were incubated for another 24 h. The cells of each well were harvested on a glass fiber filter (Perkin-Elmer, Rodgau-Jügesheim, Germany), washed, and dried. Their radioactivity in counts per minute was considered to be proportional to the respective growth of P. falciparum in the well. IC50 values (drug concentrations that produce 50% reduction in the uptake of [3H]hypoxanthine) and IC90 values were calculated; the fractional inhibitory concentrations (FIC) of the respective drugs were determined (13) based on the following definitions: FIC50 A = [IC50 (A + B)]/IC50 A; FIC50 B = [IC50 (B + A)]/IC50 B; and FIC50 = FIC50 A + FIC50 B (25). For these studies, P. falciparum strains with different CQ sensitivities (3D7, HB3, Dd2, and K1) were employed (33).
Studies on MB uptake.
Young P. falciparum trophozoites of the strain Dd2 were incubated for 12 h with 13 nM, 26 nM, or 39 nM MB. Nonparasitized red blood cells (NPRBC) were treated identically. After 12 h, cell cultures were centrifuged at 750 × g for 3 min. The supernatants representing the cell culture medium were immediately frozen. In parasitized red blood cells (PRBC), erythrocytic and parasitophorous membranes were lysed by treatment with 0.02% (wt/vol) saponin for 10 min at 37°C. After another centrifugation step, the supernatants were stored at −80°C. The parasites in the pellet were washed three times in saponin buffer and disrupted by three rounds of freezing and thawing in liquid nitrogen followed by sonication on ice. After centrifugation (31,000 × g, 30 min, 4°C), parasite lysate was obtained in the supernatant and parasite debris (consisting mainly of membranes) was obtained in the pellet. NPRBC were washed twice in phosphate-buffered saline before being disrupted by freezing and thawing. MB was determined in various fractions by high-performance liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) (28). In brief, in vitro samples (up to 100 μl of incubation medium, cell suspensions, or parasite lysates) were processed by adding 200 μl of precipitation reagent (acetonitrile), vortex mixing for 15 s, and centrifugation for 10 min at 2,500 × g. The clear supernatants were transferred into autosampler vials for LC/MS/MS analysis.
Statistics.
IC50 values were determined for each drug alone and for drugs in fixed concentration ratios by fitting a logistic dose-response equation to the concentration-response curves. IC50 and IC90 values were used to calculate FIC50 and FIC90. Mean IC50 and FIC values obtained for the various drugs and corresponding parasite strains were used for statistical analyses (13, 25). The unpaired Student's t test was used to compare differences between IC50 values of MB obtained for CQ-sensitive and CQ-resistant strains. Pearson correlation was used to assess the correlation between IC values of MB and CQ as well as between MB and the artemisinins. Least square means (LSMeans) was used to compare differences in FICs obtained in the combination of MB with the artemisinins with respect to the three different artemisinins and the different combination ratios. Statistical Analysis Software (SAS) was used for statistical computations. For all statistical tests, the significance level (P) was set at 0.05.
RESULTS
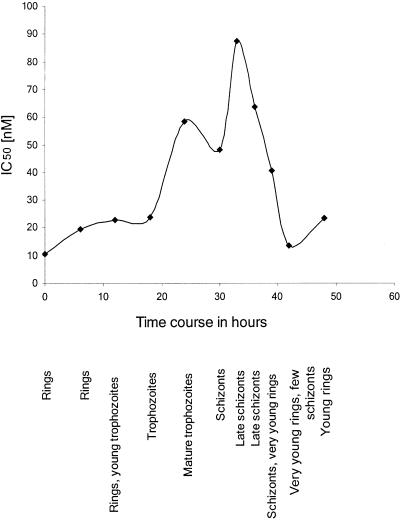
The stage-specific IC50 values of MB on P. falciparum blood-stage cultures are given in Fig. 1. The lowest IC50 values of about 11 nM were found for the very young ring stages of the parasites. Schizonts showed the lowest susceptibility to the drug, with IC50 values as high as 88 nM. The IC50 values determined in these experiments are higher than those in the drug combination assays. This is based on the fact that the stage-specificity data were obtained after exposing the parasites to MB for only 6 h, whereas the other data were obtained after 72 h of exposure. In addition, methodological differences might account for this phenomenon since stage specificity was assessed by counting parasitemia under the microscope rather than by determining the incorporation of [3H]hypoxanthine.
FIG. 1.
Stage specificity of methylene blue action on the CQ-resistant P. falciparum strain K1. IC50 values obtained for the various developmental stages of the 48-h life cycle are given. The data shown were derived from two parallel experiments which differed by less than 10%.
The data on the uptake of MB into various compartments of PRBC and NPRBC are given in Table 1. After incubation with MB (13, 26, and 39 nM) and preparation of the different lysates, MB was determined by LC/MS/MS. The total amount of MB added to the cell cultures could not be recovered quantitatively in the different fractions. This is most likely due to the various washing steps required and to the fact that MB binds strongly to the surfaces of reaction tubes (5).
TABLE 1.
Clearance of MB from the medium by P. falciparum-parasitized erythrocytes
| Initial MB concn (nM) in medium | MB concn (nM) after 12 h of incubation
|
||||
|---|---|---|---|---|---|
| Medium
|
Parasite
|
||||
| Without cells | Healthy erythrocytes | Parasitized erythrocytes | Lysate | Membrane pellet | |
| 13 | 6.1 | 4.0 | <2 | 8.2 | 79 |
| 26 | 14.7 | 6.0 | <2 | 16.3 | 152 |
| 39 | 16.6 | 19.2 | <2 | 21.7 | 265 |
The effects of several drug combinations on the growth of the P. falciparum strain K1 in vitro is given in Table 2. The combinations of MB with mefloquine or quinine were additive, whereas the combinations of MB with chloroquine (Fig. 2) and all other tested quinolines as well as with pyrimethamine were antagonistic. An antagonistic action of MB and CQ was also determined for the chloroquine-sensitive strain 3D7 (Fig. 2). MB, however, was found to act synergistically with artemisinins.
TABLE 2.
In vitro drug combination assays of methylene blue with clinically used antimalarials on P. falciparum strain K1a
IC values for the partner drugs of MB (columns 2 and 3). Mean values and standard deviations are given for values which had been obtained from four independent measurements; for values that were reproduced only once, no standard deviation is given. For MB itself, IC50 is 6.5 ± 1.8 nM and IC90 is 12.4 ± 2.3 under the chosen conditions. FIC50 values were determined according to the fixed-ratio method of Fivelman et al. (13). FIC50 of <1, synergistic drug action; FIC50 of 1, additive action; FIC50 of >1, antagonistic action. FIC50 A = [IC50 (A + B)]/IC50 A; FIC50 B = [IC50 (B + A)]/IC50 B; FIC50 = FIC50 A + FIC50 B (25).
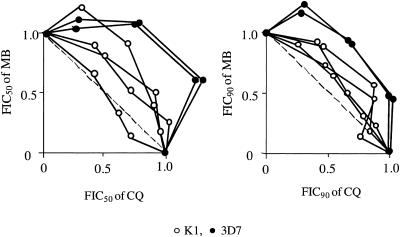
FIG. 2.
FIC50 (left) and FIC90 values (right) of MB and CQ determined at various dosage ratios (1:1, 1:3, and 3:1) and in independent experiments (indicated by the lines) for CQ-resistant (K1) and CQ-sensitive (3D7) strains of P. falciparum. The resulting antagonistic effect of the drug combination is indicated by the convex isobolograms.
When testing the three endoperoxides in combination with CQ on P. falciparum strains with different degrees of CQ resistance (Table 3), all three drug combinations acted synergistically on all strains tested as all FIC50 and FIC90 values were significantly lower than 1 (FIC50, P < 0.0001; FIC90, P < 0.0001). The degree of synergism was highest on the HB3 strain (mean FIC50 = 0.59, mean FIC90 = 0.59), followed by K1 (mean FIC50 = 0.76, P = 0.013 describing the significance of difference when compared with HB3; mean FIC90 = 0.78, P = 0.0003), 3D7 (mean FIC50 = 0.97, P < 0.0001; mean FIC90 = 0.86, P < 0.0001), and finally Dd2 (mean FIC50 = 0.99, P < 0.0001; mean FIC90 = 0.90, P = <0.0001). On the basis of the FIC values (Table 3) and the isobolograms shown in Fig. 3, synergistic effects were most pronounced in the artemisinin-MB combination. FIC values for the three different endoperoxides in combination with MB were reproducibly lowest for artemisinin (mean FIC50 = 0.64, mean FIC90 = 0.62) in comparison with artesunate (mean FIC50 = 1.00, P = 0.0001; mean FIC90 = 0.88, P = 0.0001) and artemether (mean FIC50 = 0.82, P = 0.0008). FIC50 values were reproducibly lower at combination ratios of 3:1 (mean FIC50 = 0.69) than at a combination ratio of 1:1 (mean FIC50 = 0.80, P = 0.049) or 1:3 (mean FIC50 = 0.98, P = 0.0001). No significant correlation was observed between the IC50 or IC90 values of MB and artemether (P = 0.209 and P = 0.358, respectively), artesunate (P = 0.572 and P = 0.659, respectively), and artemisinin (P = 0.164 and P = 0.128, respectively).
TABLE 3.
In vitro drug combination assays of endoperoxides with MB on P. falciparum strains with different CQ sensitivitiesa
| Drug A | P. falciparum strain tested | IC50 (nM)
|
FIC at ratio of:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug A | MB | 1:1
|
1:3
|
3:1
|
|||||
| FIC50 | FIC90 | FIC50 | FIC90 | FIC50 | FIC90 | ||||
| Artemether | K1 | 1.76 ± 0.07 | 7.94 ± 0.35 | 0.75 ± 0.21 | 0.80 ± 0.14 | 0.65 ± 0.21 | 0.90 ± 0.14 | 0.65 ± 0.07 | 0.70 ± 0.14 |
| Dd2 | 8.40 ± 1.63 | 5.24 ± 0.18 | 1.00 ± 0.14 | 0.90 ± 0.10 | 1.35 ± 0.35 | 1.20 ± 0.10 | 0.70 ± 0.14 | 0.70 ± 0.10 | |
| HB3 | 5.10 ± 0.83 | 4.98 ± 2.59 | 0.63 ± 0.12 | 0.63 ± 0.12 | 0.80 ± 0.17 | 0.80 ± 0.17 | 0.53 ± 0.12 | 0.57 ± 0.12 | |
| 3D7 | 5.87 ± 0.45 | 3.26 ± 0.57 | 0.95 ± 0.35 | 0.95 ± 0.21 | 1.25 ± 0.21 | 1.10 ± 0.14 | 0.70 ± 0.14 | 0.75 ± 0.21 | |
| Artesunate | K1 | 1.28 ± 0.16 | 5.45 ± 2.05 | 0.85 ± 0.07 | 0.70 ± 0.10 | 1.00 ± 0.10 | 0.95 ± 0.07 | 0.90 ± 0.14 | 0.80 ± 0.10 |
| Dd2 | 5.22 ± 0.40 | 6.00 ± 0.32 | 1.25 ± 0.07 | 1.05 ± 0.07 | 1.30 ± 0.14 | 1.15 ± 0.07 | 1.05 ± 0.07 | 0.90 ± 0.14 | |
| HB3 | 6.49 ± 0.81 | 6.10 ± 3.46 | 0.50 ± 0.14 | 0.55 ± 0.07 | 0.80 ± 0.14 | 0.90 ± 0.14 | 0.55 ± 0.07 | 0.50 ± 0.10 | |
| 3D7 | 4.35 ± 0.29 | 2.99 ± 0.34 | 1.30 ± 0.14 | 1.00 ± 0.10 | 1.55 ± 0.07 | 1.10 ± 0.10 | 1.00 ± 0.28 | 0.95 ± 0.07 | |
| Artemisinin | K1 | 3.98 ± 0.77 | 6.37 ± 1.10 | 0.67 ± 0.06 | 0.70 ± 0.10 | 0.73 ± 0.15 | 0.77 ± 0.21 | 0.73 ± 0.06 | 0.73 ± 0.06 |
| Dd2 | 20.36 ± 2.09 | 5.72 ± 0.06 | 0.65 ± 0.07 | 0.70 ± 0.10 | 0.95 ± 0.07 | 0.95 ± 0.07 | 0.50 ± 0.10 | 0.55 ± 0.07 | |
| HB3 | 13.67 ± 2.92 | 3.84 ± 0.24 | 0.47 ± 0.12 | 0.43 ± 0.06 | 0.60 ± 0.10 | 0.63 ± 0.15 | 0.43 ± 0.06 | 0.37 ± 0.06 | |
| 3D7 | 17.29 ± 1.53 | 3.22 ± 0.21 | 0.65 ± 0.21 | 0.55 ± 0.07 | 0.90 ± 0.14 | 0.80 ± 0.28 | 0.60 ± 0.28 | 0.55 ± 0.07 | |
Given are mean values and standard deviations. Data have been obtained from four independent experiments. FIC50 of <1, synergistic drug action; FIC50 of 1, additive action; FIC50 of >1, antagonistic action. The ratios 1:1, 1:3, and 3:1 refer to fixed dosage ratios for drug A to MB.
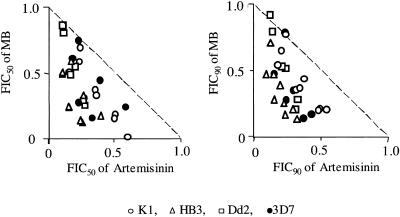
FIG. 3.
FIC50 (left) and FIC90 values (right) of MB and artemisinin at various fixed dosage ratios for different P. falciparum strains. All data points were plotted to represent the complete range of interactions seen at the concentration ratios evaluated instead of presenting individual representative isobolograms. The concave isobolograms for these data points indicate a synergistic effect of the combination. A characteristic curve can be drawn for the triangles representing the strain HB3.
DISCUSSION
MB is a promising antimalarial drug acting in vitro in the nanomolar range and in patients in the micromolar range. A mechanism of reverting chloroquine resistance by MB as a glutathione reductase inhibitor has been proposed (10, 15, 32), and clinical trials assessing the safety and effectiveness of MB are already under way (6, 24). In order to facilitate the design of future field studies and the interpretation of respective results, we have characterized the effects of the drug in cell cultures in detail.
As indicated by our studies on stage specificity of MB action, young ring stages of P. falciparum show the highest susceptibility to the drug and schizonts show the lowest. The differences in IC50 values are as high as a factor of 8. In addition, we studied the uptake of MB into various compartments of PRBC and NPRBC (Table 1). The half-life of MB in the blood after intravenous administration was estimated to be 5.25 h (26). Thus in cell culture it is likely to be much longer. The MB concentrations in the medium of PRBC were repeatedly below the detection limit of 2 nM, whereas the medium of NPRBC showed concentration-dependent MB levels. This indicates that MB is taken up particularly by PRBC and is thus available for antiparasitic activity. This hypothesis is further supported by the fact that in the parasite lysates a higher MB concentration was determined than in the medium. In the parasite membrane pellet, the accumulation was even more pronounced (factors of 9 to 12). Lysates of PRBC and NPRBC had MB concentrations below 4 nM. Thus, our data demonstrate selective uptake of MB by malarial parasites and indicate that concentrations required for efficient turncoat inhibition of glutathione reductase can be reached in Plasmodium.
Subsequently, we tested the effects of several drug combinations on P. falciparum strain K1 in vitro (Table 2). The combinations of MB with mefloquine or quinine were additive, whereas the combinations of MB with chloroquine (Fig. 2) were antagonistic. A significant positive correlation was detected between the IC50 values of CQ and MB (r = 0.7008, P = 0.0001) indicating an increased likelihood for cross-resistance. The antagonistic actions of chloroquine and methylene blue were also shown for the chloroquine-sensitive strain 3D7 (Fig. 2). The mode of action of CQ involves the inhibition of heme polymerization into nontoxic hemozoin (30). Also MB was shown to inhibit heme polymerization (2), in addition to its effects as a subversive inhibitor of P. falciparum GR (6). The effect of MB on glutathione reduction (15) is probably reflected in the observation that the antagonism between MB and CQ is less pronounced for the CQ-resistant strain K1 than for the sensitive strain 3D7.
Most interestingly, the combination of MB with artemisinin and its derivatives artemether and artesunate was found to act synergistically on the CQ-resistant strain K1 (Table 2). The combinations of MB with artemisinin or artemether were advantageous over the MB-artesunate combination. All four drugs exhibited IC50 values in the nanomolar range when given alone.
We therefore studied if the synergistic action of MB and artemisinin derivatives differs between P. falciparum strains with different degrees of CQ sensitivity. Based on data obtained by Su et al. (33) and our own experience, strains 3D7 and HB3 were chosen as CQ-sensitive strains while Dd2 was chosen in addition to K1 as a CQ-resistant strain. All three drug combinations acted synergistically on all strains tested. The best effects were observed on the HB3 strain, and the artemisinin-MB combination was found to be slightly advantageous over MB combined with artemether and artesunate, although the two latter compounds were more potent than artemisinin when studied as single drugs. Remarkably, FIC50 values were lowest at artemisinin/MB combination ratios of 3:1, indicating that only low MB concentrations might be required for maximal therapeutic effects. The lack of correlation between IC50 or IC90 values of MB and artemisinin indicates that cross-resistance between the endoperoxides and MB is unlikely to occur—an observation which is most promising for clinical application of the drug combination.
Artemisinin and its pharmaceutical derivatives are the most effective antimalarial drugs presently available and indeed have become the focus of antimalarial approaches worldwide (17, 21, 38). No definite parasite resistance to artemisinins has been reported yet, but multidrug-resistant parasites with elevated IC50 values of artemisinin have been recently produced in the laboratory (19). Furthermore, the short half-life of the artemisinins necessitates treatment for at least 7 days to achieve complete parasite clearance, and possible noncompliance of the patients with this regime could facilitate the development of drug resistance (8). Thus, continuous usage of the drug in monotherapy could lead to development of resistance in the near future, and consequently artemisinin combination therapies have been endorsed by the World Health Organization as the “policy standard” for the treatment of falciparum malaria (31).
The antimalarial activities of the artemisinins have been postulated to result from the inhibition of a P. falciparum Ca2+-ATPase (11, 29). As described in the introduction, MB has pleiotropic antimalarial activities (32) which do not seem to be related to those of artemisinin. Resistance development under this drug combination is therefore expected to be unlikely, suggesting that the MB-artemisinin combination is worth studying in field trials.
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft (Be 1540/4-4 and SFB 544.B2). Monique Akoachere is funded by a scholarship from the Deutscher Akademischer Austauschdienst (DAAD).
REFERENCES
- 1.Anstey, N. M., M. Y. Hassanali, J. Mlalasi, D. Manyenga, and E. D. Mwaikambo. 1996. Elevated levels of methaemoglobin in Tanzanian children with severe and uncomplicated malaria. Trans. R. Soc. Trop. Med. Hyg. 90:147-151. [DOI] [PubMed] [Google Scholar]
- 2.Atamna, H., M. Krugliak, G. Shalmiev, E. Deharo, G. Pescarmona, and H. Ginsburg. 1996. Mode of antimalarial effect of methylene blue and some of its analogues on Plasmodium falciparum in culture and their inhibition of P. vinkei petteri and P. yoelii nigeriensis in vivo. Biochem. Pharmacol. 51:693-700. [DOI] [PubMed] [Google Scholar]
- 3.Becker, K., S. Rahlfs, C. Nickel, and R. H. Schirmer. 2003. Glutathione—functions and metabolism in the malarial parasite Plasmodium falciparum. Biol. Chem. 384:551-566. [DOI] [PubMed] [Google Scholar]
- 4.Becker, K., L. Tilley, J. L. Vennerstrom, D. Roberts, S. Rogerson, and H. Ginsburg. 2004. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int. J. Parasitol. 34:163-189. [DOI] [PubMed] [Google Scholar]
- 5.Clark, W. M., B. Cohen, and H. D. Gibbs. 1925. Studies on oxidation-reduction. VIII. Methylene blue. Public Health Rep. 40:1131-1202. [Google Scholar]
- 6.Coulibaly, B., J. Eubel, S. Gromer, and R. H. Schirmer. 2005. Biochemistry based health care research. Methylene blue, malaria, methemoglobin, and malnutrition, p. 285-292. In H. Becher and B. Kouyaté (ed.), Health research in developing countries. Springer-Verlag, Heidelberg, Germany.
- 7.Davioud-Charvet, E., S. Delarue, C. Biot, B. Schwöbel, C. C. Boehme, A. Müssigbrodt, L. Maes, C. Sergheraert, P. Grellier, R. H. Schirmer, and K. Becker. 2001. A prodrug form of a Plasmodium falciparum glutathione reductase inhibitor conjugated with a 4-anilinoquinoline. J. Med. Chem. 44:4268-4276. [DOI] [PubMed] [Google Scholar]
- 8.Davis, T. M. E., H. A. Karunajeewa, and K. F. Ilett. 2005. Artemisinin-based combination therapies for uncomplicated malaria. Med. J. Aust. 182:181-185. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois, V. L., D. F. Platel, G. Pauly, and J. Tribouley-Duret. 1995. Plasmodium berghei: implication of intracellular glutathione and its related enzymes in chloroquine resistance in vivo. Exp. Parasitol. 81:117-124. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein-Ludwig, U., R. J. Webb, I. D. A. van Goethem, J. M. East, A. G. Lee, M. Kimura, P. M. O'Neill, P. G. Bray, S. A. Ward, and S. Krishna. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957-961. [DOI] [PubMed] [Google Scholar]
- 12.Färber, P. M., L. D. Arscott, C. H. Williams, K. Becker, and R. H. Schirmer. 1998. Recombinant Plasmodium falciparum glutathione reductase is inhibited by the antimalarial dye methylene blue. FEBS Lett. 422:311-314. [DOI] [PubMed] [Google Scholar]
- 13.Fivelman, Q. L., I. S. Adagu, and D. C. Warhurst. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsburg, H. (ed.). 2003. Redox metabolism in malaria: from genes, through biochemistry and pathology, to drugs. Redox Rep. 8:231-317. [DOI] [PubMed] [Google Scholar]
- 15.Ginsburg, H., and J. Golenser. 2003. Glutathione is involved in the antimalarial action of chloroquine and its modulation affects drug sensitivity of human and murine species of Plasmodium. Redox Rep. 8:276-279. [DOI] [PubMed] [Google Scholar]
- 16.Goluboff, N., and R. Wheaton. 1961. Methylene blue induced cyanosis and acute haemolytic anemia complicating the treatment of methemoglobinaemia. J. Pediatr. 58:86-89. [DOI] [PubMed] [Google Scholar]
- 17.Haynes, R. K., and S. Krishna. 2004. Artemisinins: activities and actions. Microbes Infect. 6:1339-1346. [DOI] [PubMed] [Google Scholar]
- 18.Krauth-Siegel, R. L., H. Bauer, and R. H. Schirmer. 2005. Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia. Angew. Chem. Int. Ed. Engl. 44:690-715. [DOI] [PubMed] [Google Scholar]
- 19.Krishna, S. 2005. New ideas about how artemisinins work, p. 2. First Annu. BIOMALPAR Conf. Biol. Pathol. Malaria Parasite. EMBL, Heidelberg, Germany.
- 20.Lambros, C., and J. P. Vanderberg. 1979. Synchronisation of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 21.Maitland, K., M. Makanga, and T. N. Williams. 2004. Falciparum malaria: current therapeutic challenges. Curr. Opin. Infect. Dis. 17:405-412. [DOI] [PubMed] [Google Scholar]
- 22.Mandi, G., S. Witte, P. Meissner, B. Coulibaly, U. Mansmann, J. Rengelshausen, W. Schiek, A. Jahn, M. Sanon, K. Wüst, I. Walter-Sack, G. Mikus, J. Burhenne, K. D. Riedel, R. H. Schirmer, B. Kouyaté, and O. Müller. 2005. Safety of the combination of chloroquine and methylene blue in healthy adult men with G6PD deficiency from rural Burkina Faso. Trop. Med. Int. Health 10:32-38. [DOI] [PubMed] [Google Scholar]
- 23.Meierjohann, S., R. D. Walter, and S. Müller. 2002. Regulation of the intracellular glutathione levels in erythrocytes infected with chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. Biochem. J. 368:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meissner, P., G. Mandi, S. Witte, B. Coulibaly, U. Mansmann, J. Rengelshausen, W. Schiek, A. Jahn, M. Sanon, T. Tapsoba, I. Walter-Sack, G. Mikus, J. Burhenne, K. D. Riedel, R. H. Schirmer, B. Kouyaté, and O. Müller. Safety of the combination of chloroquine and methylene blue in the treatment of uncomplicated falciparum malaria in young children of Burkina Faso. Malar. J., in press. [DOI] [PMC free article] [PubMed]
- 25.Ohrt, C., G. D. Willingmyre, P. Lee, C. Knirsch, and W. Milhous. 2002. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 46:2518-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter, C., D. Hongwan, A. Küpfer, and B. H. Lauterburg. 2000. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 56:247-250. [DOI] [PubMed] [Google Scholar]
- 27.Peters, W. 1970. Chemotherapy and drug resistance in malaria. Academic Press, London, United Kingdom.
- 28.Rengelshausen, J., J. Burhenne, M. Fröhlich, Y. Tayrouz, S. K. Singh, K.-D. Riedel, O. Müller, T. Hoppe-Tichy, W. E. Haefeli, G. Mikus, and I. Walter-Sack. 2004. Pharmacokinetic interaction of chloroquine and methylene blue combination against malaria. Eur. J. Clin. Pharmacol. 60:709-715. [DOI] [PubMed] [Google Scholar]
- 29.Ridley, R. G. 2003. To kill a parasite. Nature 424:887-889. [DOI] [PubMed] [Google Scholar]
- 30.Ridley, R. G., A. Dorn, S. Vippagunta, and J. Vennerstrom. 1997. Haematin (haem) polymerization and its inhibition by quinoline antimalarials. Ann. Trop. Med. Parasitol. 91:559-566. [DOI] [PubMed] [Google Scholar]
- 31.Roll Back Malaria. June. 2004, posting date. The RBM Partnership's global response: a programmatic strategy 2004-2008. [Online.] http//rbm.who.int/partnership/board/meetings/docs/strategy_rev.pdf.
- 32.Schirmer, R. H., B. Coulibaly, A. Stich, M. Scheiwein, H. Merkle, J. Eubel, K. Becker, H. Becher, O. Muller, T. Zich, W. Schiek, and B. Kouyate. 2003. Methylene blue as an antimalarial agent. Redox Rep. 8:272-276. [DOI] [PubMed] [Google Scholar]
- 33.Su, X., L. A. Kirkman, H. Fujioka, and T. E. Wellems. 1997. Complex polymorphisms in an ∼330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell 91:593-603. [DOI] [PubMed] [Google Scholar]
- 34.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 35.Valecha, N., S. Biswas, A. Srivastava, and U. Devi. 1992. Potentiation of chloroquine action against Plasmodium falciparum in vitro by verapamil and cyproheptadine. Indian J. Pharmacol. 24:158-162. [Google Scholar]
- 36.Vennerstrom, J. L., M. T. Makler, C. K. Angerhofer, and J. A. Williams. 1995. Antimalarial dyes revisited: xanthenes, azines, oxazines, and thiazines. Antimicrob. Agents Chemother. 39:2671-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wainwright, M., and L. Amaral. 2005. The phenothiazonium chromophore and the evolution of antimalarial drugs. Trop. Med. Int. Health 10:501-511. [DOI] [PubMed] [Google Scholar]
- 38.Yeung, S., W. Pongtavornpinyo, I. M. Hastings, A. J. Mills, and N. J. White. 2005. Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am. J. Trop. Med. Hyg. 71:179-186. [PubMed] [Google Scholar]