Abstract
We compared the efficacies of 17 agents against rapidly growing and starved Mycobacterium tuberculosis H37Rv. Few compounds have significant activity at attainable concentrations. However, two phenothiazine compounds at similar concentrations were bactericidal for starved and growing cells. These drugs appear to target a process important in both replicating and nonreplicating bacteria.
The cure for Mycobacterium tuberculosis infections remains problematic, requiring extended periods of treatment with multiple drugs. Because compliance is necessary throughout the treatment period, directly observed therapy is recommended, thus necessitating the costs and complexity of a large infrastructure. Inadequate treatment results not only in failure for the individual patient, but also in the selection of drug-resistant strains that can then infect others. The currently available antituberculous drugs are quite effective in vitro, resulting in rapid bacterial killing. This, however, is not the case in vivo. Clinically, patients who do not receive prolonged therapy, extending long after the resolution of symptoms, often relapse. In animal models, bacterial killing is much slower than in vitro, despite the presence of the host's immune response (4). Several groups have shown that mycobacteria can become tolerant to antibiotics under various in vitro conditions (8, 15). In particular, slow bacterial growth is associated with decreased sensitivity to antibiotics (5, 6, 12).
One simple system for inducing antibiotic tolerance in vitro is starvation. Prolonged deprivation of nutrients results in a marked slowing of bacterial growth and concomitant phenotypic antibiotic resistance (2, 7, 11, 14). As bacteria can easily grow upon being returned to nutrient-rich media, this model allows easy quantification of antibiotic effectiveness.
We have measured the bactericidal concentrations of a number of compounds with antituberculous activities, including currently employed first- and second-line drugs in both log-phase and starved cells. For starvation experiments, M. tuberculosis H37Rv cells were grown in Middlebrook 7H9 medium supplemented with 0.2% (vol/vol) glycerol, 10% (vol/vol) Middlebrook oleic acid-albumin-dextrose-catalase (OADC) enrichment, and 0.025% (vol/vol) Tween 80 at 37°C with constant rolling at 2 rpm until they reached an optical density at 600 nm of ∼0.6. The cells were then washed twice and resuspended in phosphate-buffered saline (PBS) at the same cell density. Cells (50 ml of culture) were incubated at 37°C for an additional 6 weeks in 1-liter roller bottles. Log-phase bacteria were grown in 7H9 OADC-supplemented medium until they reached an optical density at 600 nm of ∼0.6. Compounds, dissolved in appropriate solvents, were added to either 1 ml PBS containing ∼1 × 107 starved M. tuberculosis H37Rv cells or 1 ml 7H9 containing ∼1 × 105 log-phase M. tuberculosis H37Rv cells at various concentrations. Cultures were incubated in 15-ml conical tubes at 37°C with constant shaking for 7 days and then washed twice in PBS before dilutions were plated on Middlebrook 7H11 plates supplemented with 0.2% (vol/vol) glycerol, 10% (vol/vol) Middlebrook OADC enrichment, and 0.025% (vol/vol) Tween 80, containing no antibiotics. Bacterial counts were determined after incubation for 4 weeks at 37°C. All values were determined in triplicate.
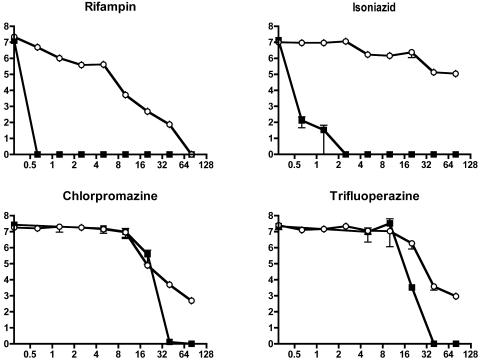
Of the 17 agents tested, only 4 were able to kill 99% of the starting bacterial population at minimum bactericidal concentrations (MBC99s) of up to 160 mg/liter (Table 1). As previously observed (2), rifampin retained bactericidal activity, although it is considerably less active against nongrowing than against log-phase cells (Fig. 1). Isoniazid had poor activity against starved cells. Even at the highest concentration tested, incubation with isoniazid barely killed 99% of bacteria. Most other compounds had little or no activity in this assay. However, the two tested phenothiazines, chlorpromazine and trifluoperazine, were able to kill starved bacteria at an MBC99 similar to that obtained against rapidly growing cells. There were differences, however, in the degree of bacterial death. In log-phase cells, treatment with either of these drugs resulted in complete killing at high concentrations. However, starved cultures could not be sterilized even with the highest concentrations of drugs.
TABLE 1.
MBC99s of 17 drugs for log-phase and 6-week-starved M. tuberculosis H37Rv
| Drug | MBC (mg/liter)
|
|
|---|---|---|
| Log-phase cells | 6-Wk-starved cells | |
| Rifampin | <0.625 | 10 |
| Trifluoperazine | ca. 10-20 | 40 |
| Chlorpromazine | ca. 10-20 | 40 |
| Isoniazid | <0.625 | 80 |
| Apramycin | <1.25 | >160 |
| Ethionamide | <0.625 | >160 |
| Capreomycin sulfate | 0.625 | >160 |
| Amikacin sulfate | <0.625 | >160 |
| Thiacetazone | <0.625 | >160 |
| Ethambutol | 0.625 | >160 |
| Streptomycin sulfate | <0.625 | >160 |
| p-Aminosalicylic acid | <0.625 | >160 |
| Ofloxacin | <0.625 | >160 |
| Tetracycline | ca. 10-20 | >160 |
| Cycloserine | ca. 10-20 | >160 |
| Erythromycin | 40 | >160 |
| Dapsone | >40 | >160 |
FIG. 1.
Drug susceptibility of log-phase (▪) and 6-week-starved (○) M. tuberculosis H37Rv. Cultures were treated in triplicate with rifampin, isoniazid, chlorpromazine, or trifluoperazine at different concentrations for 7 days. Control samples of both starved and log-phase M. tuberculosis H37Rv were incubated without drugs for 7 days. Then cultures were washed twice with PBS and their viability assessed by plating followed by CFU counting. Values represent the means ± standard errors of the means of triplicate determinations (except with the starved-cell rifampin test, which was performed in duplicate). Units on the y axis are bacterial counts (log10 CFU/ml), and units on x axis are concentrations in mg/ml.
Heifets et al. (9) recently showed that when M. tuberculosis was cultured under anaerobic conditions, only capreomycin had any significant bactericidal effect, reducing mycobacterial survival 100-fold when used at a concentration of 8 μg/ml. Our data demonstrate that in starved cells capreomycin has little effect, even at much higher concentrations. This suggests that the nongrowing states induced by starvation and anaerobiosis differ in fundamental ways.
Several mechanisms may be involved in the killing of nongrowing cells. The lack of efficacy of isoniazid (which targets cell wall biosynthesis) in starved cells suggests that cell wall synthesis is not important during starvation, a phenomenon that has been commonly observed in other bacteria (10). Drugs could target processes that are critical for survival even when bacteria are not replicating, such as transcription, allowing drugs like rifampin to retain some activity. Recent studies suggest that phenothiazines target respiration (3, 16), suggesting that this might be an essential process during starvation. Nongrowing cells might have alterations in their cell walls that result in changed permeability to antibiotics. Finally, a drug might associate with the bacteria during incubation and not be removed by washing, thus manifesting its antibacterial effects during outgrowth. Any or all of these mechanisms might be functional in vivo, where a persistent antibacterial effect might lead to more-rapid clearance of infection during treatment.
Phenothiazine compounds have previously been shown to have antituberculous activity (1). These drugs have only moderate activity in pure bacterial cultures but are concentrated in macrophages (13) and, thus, may be more effective in vivo. Because of this, they have been proposed as alternative treatments as part of a multidrug regimen to treat antibiotic-resistant tuberculosis. Our data suggest that this class of drugs might accelerate the clearance of bacteria. Perhaps more importantly, these drugs identify respiration as a possible target for new antibiotics that might shorten the required course of tuberculosis therapy.
Acknowledgments
We thank members of the Rubin lab for their helpful comments.
This work was supported by Public Health Service grant no. AI51929 from the National Institute of Allergy and Infectious Diseases to E.J.R.
REFERENCES
- 1.Amaral, L., J. E. Kristiansen, L. S. Abebe, and W. Millett. 1996. Inhibition of the respiration of multi-drug resistant clinical isolates of Mycobacterium tuberculosis by thioridazine: potential use for initial therapy of freshly diagnosed tuberculosis. J. Antimicrob. Chemother. 38:1049-1053. [DOI] [PubMed] [Google Scholar]
- 2.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 3.Boshoff, H. I., T. G. Myers, B. R. Copp, M. R. McNeil, M. A. Wilson, and C. E. Barry III. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J. Biol. Chem. 279:40174-40184. [DOI] [PubMed] [Google Scholar]
- 4.Burman, W. J. 1997. The value of in vitro drug activity and pharmacokinetics in predicting the effectiveness of antimycobacterial therapy: a critical review. Am. J. Med. Sci. 313:355-363. [DOI] [PubMed] [Google Scholar]
- 5.Chuard, C., P. E. Vaudaux, R. A. Proctor, and D. P. Lew. 1997. Decreased susceptibility to antibiotic killing of a stable small colony variant of Staphylococcus aureus in fluid phase and on fibronectin-coated surfaces. J. Antimicrob. Chemother. 39:603-608. [DOI] [PubMed] [Google Scholar]
- 6.Eng, R. H. K., F. T. Padberg, S. M. Smith, E. N. Tan, and C. E. Cherubin. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob. Agents Chemother. 35:1824-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giard, J. C., A. Hartke, S. Flahaut, A. Benachour, P. Boutibonnes, and Y. Auffray. 1996. Starvation-induced multiresistance in Enterococcus faecalis JH2-2. Curr. Microbiol. 32:264-271. [DOI] [PubMed] [Google Scholar]
- 8.Gomez, J. E., and J. D. McKinney. 2004. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinburgh) 84:29-44. [DOI] [PubMed] [Google Scholar]
- 9.Heifets, L., J. Simon, and V. Pham. 2005. Capreomycin is active against non-replicating M. tuberculosis. Ann. Clin. Microbiol. Antimicrob. 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbert, K. C., and S. J. Foster. 2001. Starvation survival in Listeria monocytogenes: characterization of the response and the role of known and novel components. Microbiology 147:2275-2284. [DOI] [PubMed] [Google Scholar]
- 11.McLeod, G. I., and M. P. Spector. 1996. Starvation- and stationary-phase-induced resistance to the antimicrobial peptide polymyxin B in Salmonella typhimurium is RpoS (σS) independent and occurs through both phoP-dependent and -independent pathways. J. Bacteriol. 178:3683-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millar, M. R., and J. Pike. 1992. Bactericidal activity of antimicrobial agents against slowly growing Helicobacter pylori. Antimicrob. Agents Chemother. 36:185-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ordway, D., M. Viveiros, C. Leandro, R. Bettencourt, J. Almeida, M. Martins, J. E. Kristiansen, J. Molnar, and L. Amaral. 2003. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 47:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuomanen, E., and A. Tomasz. 1990. Mechanism of phenotypic tolerance of nongrowing pneumococci to beta-lactam antibiotics. Scand. J. Infect. Dis. Suppl. 74:102-112. [PubMed] [Google Scholar]
- 15.Wallis, R. S., S. Patil, S.-H. Cheon, K. Edmonds, M. Phillips, M. D. Perkins, M. Joloba, A. Namale, J. L. Johnson, L. Teixeira, R. Dietze, S. Siddiqi, R. D. Mugerwa, K. Eisenach, and J. J. Ellner. 1999. Drug tolerance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 43:2600-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein, E. A., T. Yano, L. S. Li, D. Avarbock, A. Avarbock, D. Helm, A. A. McColm, K. Duncan, J. T. Lonsdale, and H. Rubin. 2005. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc. Natl. Acad. Sci. USA 102:4548-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]