Abstract
We compared the efficacies of amphotericin B, fluconazole, flucytosine, and micafungin in a systemic murine infection by three isolates of Candida glabrata. Amphotericin B showed the best results, although none of the drugs dramatically reduced mortality or tissue burden in liver or spleen.
Candida glabrata is one of the most common Candida species other than C. albicans (3), and it causes a high mortality rate (12). Fluconazole (FLC) and amphotericin B (AMB) are the drugs recommended for treating C. glabrata infections (10). However, some strains have inherent resistance to FLC (2, 10, 11) and because the patients suffering from this infection are usually in a critical condition the use of AMB is limited. In some animal studies, it has been demonstrated that the efficacy of FLC is equivalent to that of AMB (4), but in others AMB was clearly shown to be more efficient than FLC at clearing fungal burden (1, 5). Therefore, we believe that if the in vivo activities of the available drugs are to be understood, numerous strains from different sources and with different in vitro antifungal susceptibilities should be tested. Up to now, only seven strains of C. glabrata have been tested with animal models (1, 4, 5), and in only one of these studies could the animals' survival rates be evaluated for only one drug (5). We have compared the efficacies of FLC, AMB, flucytosine (5FC), and micafungin (MFG) in an immunocompromised murine model of disseminated infection by C. glabrata.
Three clinical isolates, FMR 8489, FMR 8497, and FMR 8766, from urine, exudates, and blood, respectively, were used in the study. They were subcultured on Sabouraud dextrose agar plates and incubated at 35°C for 24 h. In vitro susceptibilities of the three strains to AMB, FLC, 5FC, and MFG were tested using a microdilution reference method (7). For 5FC and FLC, the MIC was defined as the lowest drug concentration that reduced control growth by 50%. For AMB and MFG, the MIC endpoint was defined as 100% inhibition.
Male OF1 mice were immunosuppressed by a single intraperitoneal injection of 200 mg of cyclophosphamide/kg of body weight, plus a single intravenous injection of 150 mg of 5-fluorouracil/kg on the day of infection (9). The procedure standards approved by the Animal Welfare Committee of the Rovira i Virgili University were used.
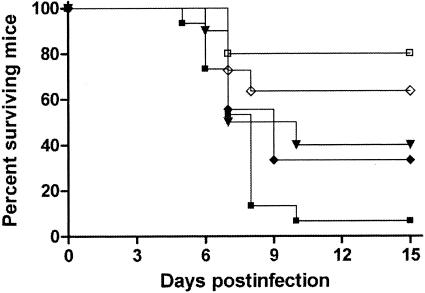
For the survival studies, a group of 10 mice was established for each strain and each treatment. Mice were challenged with 2 × 108 CFU in 0.2 ml into the lateral tail vein, except for strain FMR 8766, which required an inoculum of 6 × 108 CFU to attain a lethality similar to that of the other strains. Preliminary experiments testing several strains (Fig. 1) demonstrated that these concentrations of fungal elements were the optimal doses for producing an acute infection, with 60 to 100% of animals dying within 10 days. The different groups were treated as follows: AMB at 1.5 mg/kg of body weight/dose given intraperitoneally daily (8), FLC at 40 mg/kg given orally (5) twice daily, MFG at 5 mg/kg given subcutaneously (6) twice daily, and 5FC at 80 mg/kg orally three times a day (1). All treatments began 24 h after challenge, and the therapy lasted for 5 days. Mice were checked daily for 15 days.
FIG. 1.
Survival of mice after inoculation with C. glabrata. Mice were inoculated intravenously with either 2 × 107 (FMR 8489, □), 2 × 108 (FMR 8489, ▪; FMR 8497, ▾; FMR 8766, ⋄), or 6 × 108 (FMR 8766, ⧫) CFU/animal.
For tissue burden studies, five groups of 10 mice, one for each treatment and one for controls, were established for each of the two strains FMR 8489 and FMR 8497. For all treatments, therapy began 24 h after challenge and lasted 5 days. One day after the treatment finished, five of the surviving mice were sacrificed. Spleens and kidneys were aseptically removed, and the entire organs were homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated on Sabouraud dextrose agar, incubated at 35°C, and examined daily for 3 days. The numbers of CFU per gram of tissue were calculated.
Mean survival times were estimated by the Kaplan-Meier method and compared among groups by using the log rank test. CFU counts were analyzed by the Mann-Whitney U test. SPSS for Windows, version 11.0, was used.
MICs of AMB, FLC, 5FC, and MFG were similar for the three strains, and the differences among them were never higher than 1 dilution (Table 1).
TABLE 1.
In vitro antifungal activities of AMB, FLC, MFG, and 5FC against three strains of C. glabrata
| Strain | MIC-0a (μg/ml)
|
MIC-2a (μg/ml)
|
||
|---|---|---|---|---|
| AMB | MFG | FLC | 5FC | |
| FMR 8497 | 1 | 0.25 | 8 | <0.06 |
| FMR 8489 | 1 | 0.25 | 8 | <0.06 |
| FMR 8766 | 0.5 | 0.25 | 4 | 0.06 |
MIC-0 corresponds to a 100% inhibition of growth, and MIC-2 corresponds to a 50% inhibition of growth.
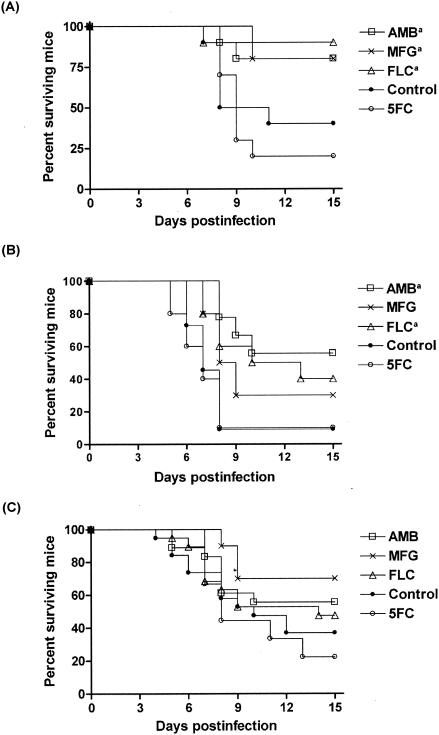
While all treatments, with the exception of 5FC, prolonged survival of mice infected with strain FMR 8497, only AMB and FLC significantly prolonged survival of mice infected with strain FMR 8489. In the case of the FMR 8766 strain, FLC, AMB, and MFG tended to prolong survival but none of them did so significantly (Fig. 2).
FIG. 2.
Cumulative mortality of mice infected with C. glabrata FMR 8497 (A), FMR 8489 (B), and FMR 8766 (C). a, P value of <0.05 versus control.
AMB reduced the fungal loads in kidneys and spleens for strain 8489 and in kidneys for strain 8497. Tissue burdens were also reduced for both strains by 5FC, but only in kidneys. The MFG and FLC regimens did not significantly reduce the counts for any organ or strain (Fig. 3).
FIG. 3.
Effects of the antifungal treatments on colony counts of the C. glabrata strains FMR 8497 (A) and FMR 8489 (B) in spleens and kidneys of mice. a, P value of <0.05 versus control. Horizontal lines indicate mean values.
The in vivo responses for all three strains tested were different even though the in vitro susceptibilities were similar for the four drugs. For one strain, practically all of the drugs tested significantly prolonged survival; for another strain, only two of the four drugs tended to prolong survival; and for the third, none of them prolonged it. However, AMB showed some advantages since it prolonged survival for two of three strains tested and was the only drug that was able to reduce fungal load in spleen for the two strains tested. The results, however, were not very impressive.
Our results do not agree with those of Atkinson et al. (1), who suggested that FLC could be useful for treating urinary infections, because FLC was not able to reduce fungal loads in the kidneys of mice infected with any of the two strains.
Although each of the four drugs tested showed some degree of effectiveness at prolonging survival and/or reducing fungal organ counts, none of them was able to sterilize organs or to reduce mortality dramatically. Because C. glabrata is a common cause of adult candidemia and has high mortality rates, finding effective treatments is an urgent need.
Acknowledgments
This work was supported by a grant from Fondo de Investigaciones Sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 020114).
REFERENCES
- 1.Atkinson, B. A., C. Bouthet, R. Bocanegra, A. Correa, M. F. Luther, and J. R. Graybill. 1995. Comparison of fluconazole, amphotericin B and flucytosine in treatment of a murine model of disseminated infection with Candida glabrata in immunocompromised mice. J. Antimicrob. Chemother. 35:631-640. [DOI] [PubMed] [Google Scholar]
- 2.Cuenca-Estrella, M., D. Rodríguez, B. Almirante, J. Morgan, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, M. Salvado, D. W. Warnock, A. Pahissa, and J. L. Rodriguez-Tudela on behalf of the Barcelona Candidemia Project Study Group. 2005. In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002-2003. J. Antimicrob. Chemother. 55:194-199. [DOI] [PubMed] [Google Scholar]
- 3.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher, M. A., S.-H. Shen, J. Haddad, and W. F. Tarry. 1989. Comparison of in vivo activity of fluconazole with that of amphotericin B against Candida tropicalis, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 33:1443-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju, J. Y., C. Polhamus, K. A. Marr, S. M. Holland, and J. E. Bennett. 2002. Efficacies of fluconazole, caspofungin, and amphotericin B in Candida glabrata-infected p47phox−/− knockout mice. Antimicrob. Agents Chemother. 46:1240-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luque, J. C., K. V. Clemons, and D. A. Stevens. 2003. Efficacy of micafungin alone or in combination against systemic murine aspergillosis. Antimicrob. Agents Chemother. 47:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility of yeasts. Approved standard M27-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Ortoneda, M., J. Capilla, F. J. Pastor, I. Pujol, and J. Guarro. 2002. Efficacy of liposomal amphotericin B in treatment of systemic murine fusariosis. Antimicrob. Agents Chemother. 46:2273-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortoneda, M., J. Capilla, I. Pujol, F. J. Pastor, E. Mayayo, J. Fernandez-Ballart, and J. Guarro. 2002. Liposomal amphotericin B and granulocyte colony-stimulating factor therapy in a murine model of invasive infection by Scedosporium prolificans. J. Antimicrob. Chemother. 49:525-529. [DOI] [PubMed] [Google Scholar]
- 10.Pappas, P. G., J. H. Rex., J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller, M. A., and D. J. Diekema for the International Fungal Surveillance Participant Group. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 12.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]