Abstract
In a randomized, double-blind, controlled trial, 546 patients with complicated skin and skin structure infections received tigecycline 100 mg/day (a 100-mg initial dose and then 50 mg intravenously twice daily) or the combination of vancomycin 2 g/day (1 g intravenously twice daily) and aztreonam 4 g/day (2 g intravenously twice daily) for up to 14 days. The primary end point was the clinical response in the clinical modified intent-to-treat (c-mITT) and clinically evaluable (CE) populations at the test-of-cure visit 12 to 92 days after the last dose. The microbiologic response at the test-of-cure visit was also assessed. Safety was assessed by physical examination, laboratory results, and adverse event reporting. Five hundred twenty patients were included in the c-mITT population (tigecycline group, n = 261; combination group, n = 259), and 436 were clinically evaluable (tigecycline group, n = 223; combination group, n = 213). The clinical responses in the tigecycline and the combination vancomycin and aztreonam groups were similar in the c-mITT population (84.3% versus 86.9%; difference, −2.6% [95% confidence interval, −9.0, 3.8]; P = 0.4755) and the CE population (89.7% versus 94.4%; difference, −4.7% [95% confidence interval, −10.2, 0.8]; P = 0.1015). Microbiologic eradication (documented or presumed) occurred in 84.8% of the patients receiving tigecycline and 93.2% of the patients receiving vancomycin and aztreonam (difference, −8.5 [95% confidence interval, −16.0, −1.0]; P = 0.0243). The numbers of patients reporting adverse events were similar in the two groups, with increased nausea and vomiting rates in the tigecycline group and an increased incidence of rash and increases in alanine aminotransferase and aspartate aminotransferase levels in the combination vancomycin and aztreonam group. Tigecycline was shown to be safe and effective for the treatment of complicated skin and skin structure infections.
The treatment of skin and skin structure infections (SSSIs) with antibiotics is challenging. The disease in patients with SSSIs has a diverse bacterial etiology, with polymicrobial cutaneous infections that often include gram-positive organisms, Pseudomonas aeruginosa, and enteric gram-negative bacilli and anaerobes (4, 8, 9, 11, 13, 33). In addition, a long-standing concern with SSSIs in the hospital is the emergence of methicillin-resistant Staphylococcus aureus (MRSA) (3). Inadequate coverage in the treatment of SSSIs may result in the development of antimicrobial resistance and clinical failure (9, 10, 35). The appearance of MRSA highlights the urgent need for new first-line therapies that are empirically effective against suspected pathogens, including resistant organisms (5, 38).
Tigecycline has been approved by the U.S. Food and Drug Administration as the first glycylcycline (36) that is indicated for the treatment of complicated SSSIs (cSSSIs) caused by Escherichia coli, Enterococcus faecalis (vancomycin-susceptible isolates only), Staphylococcus aureus (methicillin-susceptible and -resistant isolates), Streptococcus agalactiae, the Streptococcus anginosus group (which includes S. anginosus, S. intermedius, and S. constellatus), Streptococcus pyogenes, and Bacteroides fragilis (40). Tigecycline exhibits a spectrum of in vitro activity expanded beyond that of other broad-spectrum antibiotics because of its activity against pathogens that are susceptible and resistant to other antibiotics (1, 15, 29). Tigecycline inhibits protein synthesis and cell growth in bacteria, presumably by binding to the bacterial 30S ribosomal subunit and blocking aminoacyl-tRNA molecules from entering the A site of the ribosome (32). Tigecycline was designed to circumvent two common resistance mechanisms in bacteria: efflux and ribosomal protection (6). The activity of tigecycline is unaffected by the presence of extended-spectrum β-lactamases, penicillin binding protein mutations, or gyrase mutations (2, 16, 30). Tigecycline has shown potent in vitro activity against a broad spectrum of bacteria commonly found in cSSSI infections, which includes MRSA, methicillin-susceptible S. aureus (MSSA), Escherichia coli, Enterococcus faecalis, Enterococcus faecium, Bacteroides spp., Clostridium spp., Peptostreptococcus spp., and Fusobacterium spp. (12, 14, 22, 30, 33).
In a phase 2 study, tigecycline appeared to be efficacious and showed an acceptable safety profile in hospitalized patients with complicated SSSIs (31). We therefore compared the safety and efficacy of tigecycline with those of current antibacterial treatments for SSSIs. Vancomycin is a tricyclic glycopeptide antibiotic with activity against a wide variety of gram-positive organisms (21). However, it lacks activity against gram-negative organisms; therefore, aztreonam was added to the comparator treatment. Aztreonam is indicated for the treatment of serious SSSIs caused by susceptible gram-negative microorganisms, including Escherichia coli, Proteus mirabilis, Serratia marcescens, Enterobacter spp., P. aeruginosa, Klebsiella pneumoniae, and Citrobacter spp. (20). We conducted the present trial to determine the efficacy and safety of tigecycline monotherapy and the combination of vancomycin and aztreonam (V/A) and to compare the noninferiority of tigecycline to V/A in hospitalized patients with skin and skin structure infections.
(These results were presented in part at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, 30 October to 2 November 2004, Washington, D.C.)
MATERIALS AND METHODS
Patients.
A phase 3, randomized, double-blind study was conducted with patients with cSSSIs between November 2002 and December 2003 at 65 investigational sites worldwide (21 countries, including countries in Europe and Asia as well as Australia and South Africa). The protocol was reviewed and approved by the institutional review board or ethical review committee of each participating center. Before the study began written informed consent was obtained from each patient according to the guidelines of each institution. The trial was conducted in accordance with the Declaration of Helsinki and its amendments.
Eligible patients were hospitalized men and women aged 18 years or older with cSSSIs that either involved deep soft tissue, including extensive cellulitis at least 10 cm in width or length; required surgical intervention; or was associated with significant underlying disease (e.g., diabetes mellitus, peripheral vascular disease, peripheral neuropathy, and lower venous insufficiency). In addition to the infection, the patient had to have at least two of the following signs and symptoms: drainage or discharge, fever, erythema, swelling, localized warmth, pain, and/or white blood cell count >10,000/mm3. After the original sample for culture was obtained, the subjects could not receive more than two doses of a nonstudy antibacterial therapy. If a patient was considered a prior antibiotic treatment failure, a Gram stain showing a potential isolate or a sample for baseline culture of the infected site was obtained before the study drug was administered.
Patients were excluded if they had necrotizing fasciitis, gangrene, osteomyelitis, plasmapheresis, hemoperfusion, neutropenia, a severely impaired arterial blood supply, or any condition or medication that would impair the ability to eradicate infections. If patients had the presence of hepatic disease (aspartate aminotransferase [AST] or alanine aminotransferase [ALT] levels more than 10 times the upper limit of normal [ULN], a bilirubin level more than 3 times the ULN, or the presence of acute hepatic failure or acute decompensation of chronic hepatic failure), they were excluded from the study.
Patients were also excluded if they were hypersensitive to tigecycline, vancomycin, aztreonam, or tetracycline agents or had a known or suspected concomitant infection that required treatment with another antimicrobial agent. If a patient had an uncomplicated SSSI (e.g., simple abscesses, folliculitis, impetiginous lesions, furunculosis, or superficial cellulitis) or an SSSI that could be treated by surgery alone, he or she was excluded from the study.
Patients meeting the inclusion and exclusion criteria were included in the intent-to-treat (ITT) population, patients who received at least one dose of study drug were included in the modified ITT (mITT) population, and patients in the mITT population who had clinical evidence of a cSSSI by meeting the minimal disease criteria were included in the clinical modified (c-mITT) population.
Procedures.
We conducted the present trial to determine the noninferiority of tigecycline to V/A in hospitalized patients with skin and skin structure infections. The primary objective was to determine the safety and efficacy of tigecycline compared with those of the combination of V/A for the treatment of cSSSIs in hospitalized patients. The secondary objectives, which were exploratory in nature, were to obtain additional in vitro susceptibility data on tigecycline for a range of bacteria that cause cSSSIs and to compare its microbiological efficacy among treatment arms in patients with cSSSIs.
Patients were randomly assigned (1:1) to receive either tigecycline with placebo or the combination of V/A intravenously (i.v.) for up to 14 days. For patients assigned to the tigecycline group, the initial i.v. dose of 100 mg tigecycline was followed by 50 mg twice a day (approximately every 12 h) in 250 ml of normal saline infused over 60 min. Following each tigecycline infusion, patients received 100 ml normal saline placebo infused over 60 min. For patients assigned to the combination V/A group, 1 g vancomycin in 250 ml of normal saline was administered i.v. over 60 min, followed by 2 g aztreonam in 100 ml of saline over 60 min, twice a day (approximately every 12 h). Aztreonam could be discontinued after 48 h, according to the investigator's clinical judgment.
When baseline culture results became available, the investigator reviewed the results and assessed them for the presence of a gram-negative pathogen. The investigator could then decide that aztreonam or placebo coverage was no longer necessary; however, aztreonam could be continued at the investigator's discretion, even in the absence of a gram-negative pathogen. The unblinded dispenser discontinued aztreonam or placebo infusion when the dispenser was ordered to do so by the investigator.
Clinical and microbiologic assessments.
Samples of blood were obtained, and samples of the infection site for culture were obtained with superficial swab. Patients in the c-mITT population were considered to be clinically evaluable (CE) if they did not have P. aeruginosa as a sole baseline isolate, received no concomitant antibiotic after their first dose of study medication (tigecycline or V/A), and had an assessment of cure or failure at the test-of-cure visit. All other patients were considered unevaluable. Patients included in the microbiologically evaluable (ME) population were CE patients for whom one or more causative isolates were identified from the baseline culture and classification of the microbiologic response (eradication [documented or presumed], persistence, or superinfection [on-therapy emergence of a new isolate at the site of the infection with the emergence or worsening of signs and symptoms of infection; i.e., the patient was deemed a clinical failure]) at the test-of-cure visit could be determined. The test-of-cure assessment took place at least 12 days but no more than 92 days after the last dose of study.
The clinical response within the CE and c-mITT populations at the test-of-cure visit (12 to 92 days after last dose) was the primary efficacy end point. An investigator blinded to treatment assessed drainage and/or discharge, fever, erythema, swelling and/or induration, pain and/or tenderness to palpation, extent of infection (width and length), and localized warmth. Based on these assessments, the investigator evaluated the subject's clinical response to therapy (cure, failure, or indeterminate). At the test of cure, the patients were considered by the investigator to have a clinical cure if the patients had resolution of signs and symptoms such that no further antibiotic therapy was required. Patients were considered clinical failures if they had an inadequate response to therapy that required additional antibiotic therapy at any point during the study.
Microbiologic efficacy was evaluated at both the patient level (eradication [documented or presumed], persistence, superinfection, or indeterminate) and the isolate level (eradication [documented or presumed], persistence, or indeterminate). At both the patient and the isolate levels, the documented eradication rate (the primary baseline pathogen and all baseline isolates were not present in a repeat culture of a sample taken from the original site of infection) and the presumed eradication rate (a clinical response of cure precluded the availability of a specimen for culture) were determined. Skin cultures were the principal source of the baseline isolate; however, a blood isolate could be used if no baseline isolate was identified from the skin source. All specimens (blood cultures and aerobic and anaerobic cultures of specimens from the primary site of infection) were sent to local laboratories for primary identification of the isolates and were tested for susceptibility to tigecycline by Kirby-Bauer disk diffusion tests. The isolates recovered were subcultured and tested for susceptibility at a central laboratory by using both broth microdilution tests, to determine the MIC, and Kirby-Bauer disk diffusion tests by procedures published by the CLSI (formerly NCCLS) (26-28). MICs for tigecycline were determined by a reference broth microdilution method with fresh Mueller-Hinton medium. The provisional MIC breakpoints, based on previous preclinical investigations, were as follows: ≤2 μg/ml for susceptible; >2 to <8 μg/ml for intermediate; and ≥8 μg/ml for resistant. The MIC50 and the MIC90 represent the concentration of antibiotic that inhibited the growth of 50% and 90% of the isolates, respectively. Organisms isolated from baseline cultures were considered to be the primary baseline isolate based on the frequency with which those organisms are identified in the particular disease state (13, 17-19, 37).
Safety evaluation.
The safety population comprised all patients who received at least one dose of study medication, i.e., the mITT population. Safety assessments included a physical examination and 12-lead electrocardiograms at the baseline. Vital signs (temperature, heart rate, blood pressure) and clinical laboratory parameters (hematology, blood chemistry evaluations, and coagulation parameters) were assessed at the baseline, day 3, day 7, day 14 or the last day of therapy, and the test-of-cure visit. Adverse events (AEs) and treatment-emergent AEs (TEAEs), i.e., AEs that occurred or worsened during treatment, were recorded throughout the study period. Since renal failure is a frequent complication of bacteremia in hospitalized patients (35) and the vancomycin dosage could be adjusted according to the creatinine clearance levels for patients with compromised renal function, as suggested by the vancomycin label (21), serum creatinine levels were determined at the baseline, day 3, day 7, day 14, or the last day of therapy, and at the test-of-cure visit. We had no requirement for monitoring of vancomycin levels.
Statistical analysis.
In order to determine the noninferiority of tigecycline to the combination of V/A for clinical and microbiologic responses, a two-sided 95% confidence interval (CI) for the true difference in efficacy (tigecycline minus the combination of V/A) was used. The CI was corrected for continuity. Noninferiority was concluded if the lower limit of the two-sided 95% CI was greater than or equal to −15 (i.e., −15%). For all the subpopulation analyses (e.g., subgroup analyses and analyses for monomicrobial versus polymicrobial infections), an adjusted difference between treatment groups with its 95% CI was calculated from a generalized linear model with a binomial probability function and an identity link. For end points involving comparisons of tigecycline and the combination of V/A with small sample sizes, the method of Wilson (39), corrected for continuity, was used. The method used to compute the two-sided 95% CI for a single proportion was the “exact” method of Clopper and Pearson (7). Throughout this article, “significant” refers to a P value less than 0.05. All analyses other than that for the primary end point are considered secondary, sensitivity, or exploratory analyses. P values for these additional analyses have not been corrected for multiplicity and should be considered descriptive statistics only.
RESULTS
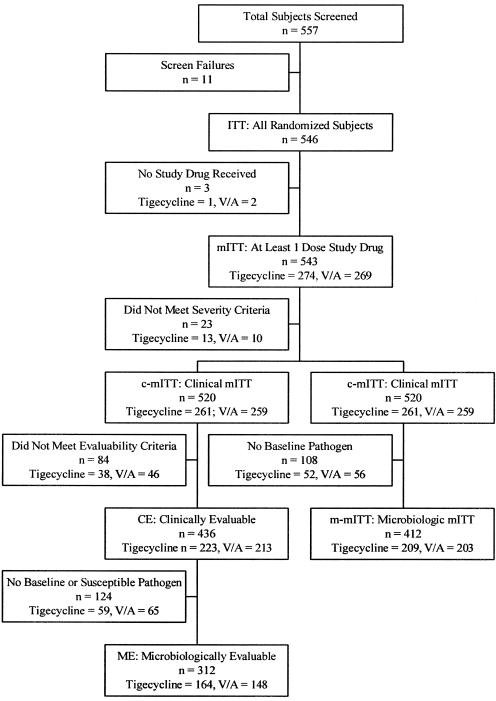
Of the 546 patients randomly assigned, 3 did not receive any drug, 274 received tigecycline (50.5%), and 269 (49.5%) received the combination of V/A (Fig. 1) and were included in the mITT population. Because 23 patients did not meet the criteria for the severity of infection, 520 patients (tigecycline group, n = 261; combination group, n = 259) were included in the c-mITT population. Eighty-four patients of the c-mITT population did not meet the evaluability criteria, and 436 patients (tigecycline group, n = 223; V/A combination group, n = 213) were included in the CE population. No baseline and/or susceptible isolates were found in 124 CE patients; therefore, 312 patients (tigecycline group, n = 164; V/A combination group, n = 148) were included in the ME population.
FIG. 1.
Population analyzed.
Both treatment groups had similar demographic characteristics, clinical diagnoses, causes of infection, and comorbid conditions (Table 1). The most common diagnosis in both treatment groups was deep soft tissue infection involving cellulitis. Approximately half of the original infections were spontaneous, about 30% were due to traumatic injury, and 12% resulted from surgeries. In the mITT population, fewer patients in the tigecycline group (n = 13; 4.7%) than in the V/A combination group (n = 27; 10%) received concomitant antibiotics, administered after the start of treatment with the study drug through the last dose of study drug (Fisher's exact test, P = 0.021).
TABLE 1.
Demographic and baseline characteristics of mITT population
| Characteristic | Tigecycline group (n = 274) | V/A group (n = 269) |
|---|---|---|
| Age (yr) (mean [SD]) | 48.8 (17.0) | 50.1 (17.8) |
| Sex (no. [%] of patients) | ||
| Men | 167 (60.9) | 163 (60.6) |
| Women | 107 (39.1) | 106 (39.4) |
| Ethnic origin (no. [%] of patients) | ||
| White | 227 (82.8) | 223 (82.9) |
| Black | 20 (7.3) | 20 (7.4) |
| Asian | 19 (6.9) | 22 (8.2) |
| Other | 8 (2.9) | 4 (1.5) |
| Wt (kg) (mean [SD]) | 82.5 (21.0) | 81.5 (20.5) |
| Creatinine clearance (ml/min) (mean [SD]) | 109.4 (42.4) | 104.3 (41.2) |
| Chief clinical diagnosis (no. [%] of patients) | ||
| Infected ulcers | 25 (9.1) | 19 (7.1) |
| Major abscesses | 73 (26.6) | 84 (31.2) |
| Burns | 9 (3.3) | 8 (3.0) |
| Deep soft tissue infection | 167 (60.9) | 157 (58.4) |
| Cellulitis | 160 (58.4) | 148 (55.0) |
| Complicated underlying disease | 26 (9.5) | 26 (9.7) |
| ≥10 cm (where anatomically applicable) | 144 (52.6) | 130 (48.3) |
| Requiring surgery or drainage | 71 (25.9) | 73 (27.1) |
| Wound infection | 7 (2.6) | 9 (3.3) |
| Other | 0 | 1 (0.4)a |
| Cause of infection (no. [%] of patients) | ||
| Trauma | 80 (29.2) | 81 (30.1) |
| Spontaneous | 144 (52.6) | 132 (49.1) |
| Bite (human, insect, animal) | 6 (2.2) | 15 (5.6) |
| Surgery | 33 (12.0) | 32 (11.9) |
| Injection | 10 (3.6) | 9 (3.3) |
| Other | 1 (0.4) | 0 |
| Comorbidity conditions (no. [%] of patients) | ||
| Diabetes mellitus | 41 (15.0) | 32 (11.9) |
| Peripheral vascular disease | 21 (7.7) | 19 (7.1) |
This patient had purulent drainage at peripheral i.v. catheter sites.
Approximately 80% of the mITT population in each treatment group completed therapy and were included in the CE population. In both groups, the reasons for exclusion from the CE population were broken treatment blind (12 of 274 patients [4.4%] and 17 of 269 patients [6.3%] in the tigecycline and the V/A groups, respectively), failure to meet the inclusion criteria and/or the minimal disease criteria (13 of 274 patients [4.7%] and 11 of 269 patients [4.1%] in the tigecycline and the V/A groups, respectively), no clinical evaluation at test of cure (10 of 274 patients [3.6%] and 8 of 269 patients [3.0%] in the tigecycline and the V/A groups, respectively), the presence of Pseudomonas at the baseline (1 of 274 patients [0.4%] and 3 of 269 patients [1.1%] in the tigecycline and the V/A groups, respectively), and the receipt of more than two prior doses of antibiotic after the sample for the baseline culture was obtained (1 of 274 patients [0.4%] and 2 of 269 patients [0.7%] in the tigecycline and the V/A groups, respectively).
The trial met the predefined statistical criteria for demonstration that the efficacy of the tigecycline montherapy was not inferior to that of combination V/A therapy (Table 2). For the CE population, the success rates were 89.7% and 94.4% (difference, −4.7 [95% CI differences, −10.2 to 0.8]) for the tigecycline and the V/A treatment groups, respectively. In the c-mITT population, the cure rates were 84.3% and 86.9% (difference, −2.6 [95% CI differences, −9.0, 3.8]) for the tigecycline and the V/A treatment groups, respectively. In the secondary analyses, the results for the ME population were generally consistent with those for the CE and the c-mITT populations. At the test-of-cure visit, tigecycline was noninferior to V/A and thus may be as effective as the combination of V/A in the ME and microbiologic modified ITT (m-mITT) patients with both monomicrobial and polymicrobial infections (Table 2).
TABLE 2.
Clinical success rates by study population at test-of-cure visit
| Population | Tigecycline
|
V/A
|
% (95% CI) for difference (tigecycline − V/A) | P value for test for noninferiority | Test for differences | ||
|---|---|---|---|---|---|---|---|
| No. of patients in population/total no. | % (95% CI) | No. of patients in population/total no. | % (95% CI) | ||||
| CE | 200/223 | 89.7 (84.9, 93.3) | 201/213 | 94.4 (90.4, 97.1) | −4.7 (−10.2, 0.8) | <0.001 | 0.1015 |
| c-mITT | 220/261 | 84.3 (79.3, 88.5) | 225/259 | 86.9 (82.1, 90.7) | −2.6 (−9.0, 3.8) | <0.001 | 0.4755 |
| ME | 148/164 | 90.2 (84.6, 94.3) | 143/148 | 96.6 (92.3, 98.9) | −6.4 (−12.4, −0.3) | 0.0019 | 0.0372 |
| Monomicrobial | 83/90 | 92.2 (84.6, 96.8) | 78/81 | 96.3 (89.6, 99.2) | −4.1 (−12.6, 4.6) | ||
| Polymicrobial | 65/74 | 87.8 (78.2, 94.3) | 65/67 | 97.0 (89.6, 99.6) | −9.2 (−19.6, 1.2) | −6.2 (−11.7, −0.7)a | |
| m-mITT | 180/204 | 88.2 (83.0, 92.3) | 177/196 | 90.3 (85.3, 94.1) | −2.1 (−8.6, 4.5) | <0.001 | 0.6114 |
| Monomicrobial | 104/114 | 91.2 (84.5, 95.7) | 99/111 | 89.2 (81.9, 94.3) | 2.0 (−6.6, 10.8) | ||
| Polymicrobial | 76/90 | 84.4 (75.3, 91.2) | 78/85 | 91.8 (83.8, 96.6) | −7.3 (−17.9, 3.4) | −1.7 (−7.9, 4.5)a | |
Adjusted difference.
The clinical cure rates were also equivalent in the two treatment groups when they were compared across the baseline diagnoses of infection (Table 3). Although there is little power to determine differences between the dose groups, tigecycline monotherapy was comparable to V/A for the subsets of patients with baseline diagnoses of diabetes, peripheral vascular disease, or bacteremia. A small number of CE patients presented with bacteremia at the baseline (15 patients in the tigecycline arm and 10 patients in the V/A arm). Although the calculations are based on asymptotic properties, which may not be verified in the case of a small sample size, no differences in cure rates were seen between the two treatment groups in the presence or absence of bacteremia. Similarly, no differences in the cure rates were seen between the two treatment groups in the few patients in the CE population with peripheral vascular disease (10 and 12 patients in the tigecycline and the V/A treatment groups, respectively). In the ME population, the microbial eradication rates (documented and presumed) were 84.8% (95% CI, 78.3, 89.9) and 93.2% (95% CI, 87.9, 96.7) for tigecycline and the combination of V/A, respectively, at the test-of-cure visit (difference, −8.5 [95% CI differences, −16.0, −1.0]) (Table 4).
TABLE 3.
Clinical success rates by exploratory subgroupsa at test-of-cure visit
| Clinical diagnosis | Tigecycline
|
V/A
|
Difference (tigecycline − V/A)
|
|||
|---|---|---|---|---|---|---|
| n/Nb | % (95% CI) | n/N | % (95% CI) | % | (95% CI) | |
| Soft tissue infection | 118/130 | 90.8 (84.4, 95.1) | 108/118 | 91.5 (85.0, 95.9) | −0.8 | (−8.6, 7.3) |
| Abscesses | 58/66 | 87.9 (77.5, 94.6) | 73/75 | 97.3 (90.7, 99.7) | −9.5 | (−20.6, 0.4) |
| Ulcers | 15/18 | 83.3 (58.6, 96.4) | 12/12 | 100.0 (73.5, 100.0) | −16.7 | (−42.3, 15.9) |
| Burns | 9/9 | 100.0 (66.4, 100.0) | 8/8 | 100.0 (63.1, 100.0) | 0.0 | (−37.1, 40.2) |
| Diabetes | 21/25 | 84.0 (63.9, 95.5) | 23/27 | 85.2 (66.3, 95.8) | −1.2 | (−24.4, 21.3) |
| Peripheral vascular disease | 7/10 | 70.0 (34.8, 93.3) | 10/12 | 83.3 (51.6, 97.9) | −13.3 | (−50.6, 25.8) |
| Baseline bacteremia | 14/15 | 93.3 (68.1, 99.8) | 10/10 | 100.0 (69.2, 100.0) | −6.7 | (−34.0, 28.4) |
The exploratory subgroups were the baseline diagnosis of the investigator, patients with diabetes or peripheral vascular disease, or baseline bacteremia status (CE population).
n, number of patients with clinical success; N, total number of patients.
TABLE 4.
Microbiologic response at the subject level (ME population) at test-of-cure visit
| Response | Tigecycline
|
V/A
|
Difference (tigecycline − V/A)
|
||||
|---|---|---|---|---|---|---|---|
| n/Na | % (95% CI) | n/N | % (95% CI) | % (95% CI) | Test for noninferiority | Test for difference | |
| Eradication | 139/164 | 84.8 (78.3, 89.9) | 138/148 | 93.2 (87.9, 96.7) | −8.5 (−16.0, −1.0) | 0.0460 | 0.0243 |
| Documented | 9/139 | 6.5 | 13/138 | 9.4 | |||
| Presumed | 130/139 | 93.5 | 125/138 | 90.6 | |||
| Monomicrobial | 80/90 | 88.9 (80.5, 94.5) | 75/81 | 92.6 (84.6, 97.2) | −3.7 (−13.5, 6.4) | ||
| Polymicrobial | 59/74 | 79.7 (68.8, 88.2) | 63/67 | 94.0 (85.4, 98.3) | −14.3 (−26.3, −1.9) | ||
| Persistence | 21/164 | 12.8 | 9/148 | 6.1 | |||
| Documented | 11/21 | 52.4 | 6/9 | 66.7 | |||
| Presumed | 10/21 | 47.6 | 3/9 | 33.3 | |||
| Monomicrobial | 9/90 | 10.0 | 5/81 | 6.2 | |||
| Polymicrobial | 12/74 | 16.2 | 4/67 | 6.0 | |||
| Superinfection | 4/164 | 2.4 | 1/148 | 0.7 | |||
| Monomicrobial | 1/90 | 1.1 | 1/81 | 1.2 | |||
| Polymicrobial | 3/74 | 4.1 | 0/67 | 0.0 | |||
n, number of patients with microbiologic response; N, total number of patients.
The microbiologic eradication rates (documented and presumed) at the test-of-cure visit for seven selected isolates of clinical interest, as they are the causes of cSSSIs, were comparable in the tigecycline and the V/A groups (Table 5). Among patients infected with MRSA and MSSA, the clinical and the microbiologic success rates were 83% and 87%, respectively, for tigecycline-treated patients and 50% and 95%, respectively, for the V/A-treated patients. MIC90 values for tigecycline were uniformly low for the most prevalent isolates, including the MRSA and MSSA isolates, compared with those for V/A (Table 6).
TABLE 5.
Microbiologic response at isolate level: selected baseline isolates at test-of-cure visit (ME population)
| Isolate | Tigecycline
|
V/A
|
||
|---|---|---|---|---|
| n/Na | % (95% CI) | n/N | % (95% CI) | |
| Staphylococcus aureus (MRSA) | 5/6 | 83.3 (35.9-99.6) | 3/6 | 50.0 (11.8-88.2) |
| Documented | 0/5 | 0.0 | 0/3 | 0.0 |
| Presumed | 5/5 | 100.0 | 3/3 | 100.0 |
| Staphylococcus aureus (MSSA) | 54/62 | 87.1 (76.1-94.3) | 55/58 | 94.8 (85.6-98.9) |
| Documented | 6/54 | 11.1 | 2/55 | 3.6 |
| Presumed | 48/54 | 88.9 | 53/55 | 96.4 |
| Streptococcus pyogenes | 21/22 | 95.5 (77.2-99.9) | 16/16 | 100.0 (79.4-100.0) |
| Documented | 3/21 | 14.3 | 2/16 | 12.5 |
| Presumed | 18/21 | 85.7 | 14/16 | 87.5 |
| Streptococcus agalactiae | 4/5 | 80.0 (28.4-99.5) | 3/3 | 100.0 (29.2-100.0) |
| Documented | 1/4 | 25.0 | 0/3 | 0.0 |
| Presumed | 3/4 | 75.0 | 3/3 | 100.0 |
| Enterococcus faecalisb | 3/4 | 75.0 (19.4-99.4) | 5/5 | 100.0 (47.8-100.0) |
| Documented | 1/3 | 33.3 | 2/5 | 40.0 |
| Presumed | 2/3 | 66.7 | 3/5 | 60.0 |
| Escherichia coli | 15/16 | 93.8 (69.8-99.8) | 13/14 | 92.9 (66.1-99.8) |
| Documented | 1/15 | 6.7 | 1/13 | 7.7 |
| Presumed | 14/15 | 93.3 | 12/13 | 92.3 |
| Bacteroides fragilis | 0/0 | NAc (NA) | 1/1 | 100.0 (2.5-100.0) |
| Documented | 0/0 | NA | 0/1 | 0.0 |
| Presumed | 0/0 | NA | 1/1 | 100.0 |
n, number of patients with microbiologic response; N, total number of patients.
In this study, all E. faecalis primary isolates were non-vancomycin-resistant Enterococcus isolates.
NA, not applicable.
TABLE 6.
MIC range, MIC50s, and MIC90s of selected primary baseline isolates (ME population)
| Isolate | Tigecycline
|
Vancomycin
|
Aztreonam
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | No. of isolates | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | No. of isolates | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | |
| Staphylococcus aureus (MRSA) | 12 | 0.12-0.25 | 0.12 | 0.25 | 12 | 0.50-4.00 | 1.00 | 2.00 | 12 | 128.0-128.0 | 128.0 | 128.0 |
| Staphylococcus aureus (MSSA) | 120 | 0.12-0.25 | 0.12 | 0.25 | 120 | 1.00-4.00 | 1.00 | 2.00 | 120 | 128.0-128.0 | 128.0 | 128.0 |
| Streptococcus pyogenes | 38 | 0.03-0.25 | 0.06 | 0.12 | 38 | 0.25-1.00 | 0.50 | 1.00 | 38 | 16.0-64.0 | 32.0 | 32.0 |
| Streptococcus agalactiae | 8 | 0.06-0.25 | NAb | NA | 8 | 0.50-1.00 | NA | NA | 8 | 128.0-128.0 | NA | NA |
| Enterococcus faecalisa | 9 | 0.12-0.25 | NA | NA | 9 | 1.00-2.00 | NA | NA | 9 | 128.0-128.0 | NA | NA |
| Escherichia coli | 30 | 0.12-0.50 | 0.25 | 0.50 | 30 | 128.0-128.0 | 128.0 | 128.0 | 30 | 0.12-0.50 | 0.12 | 0.25 |
| Bacteroides fragilis | 1 | 0.50-0.50 | NA | NA | 1 | 32.00-32.00 | NA | NA | ||||
In this study, all E. faecalis primary isolates were non-vancomycin-resistant Enterococcus isolates.
NA, MIC50 and MIC90 values are not valid if the number of isolates is less than 10.
Isolates with a fourfold or greater increase in the MIC from the baseline were evaluated for patients treated with tigecycline. Two patients in the study were identified to be infected with isolates deemed to be indeterminate or resistant to tigecycline, as defined by the provisional breakpoints, based on previous preclinical investigations.
Ribotyping analysis showed that the first patient acquired a second strain of K. pneumoniae (resistant) after the baseline culture grew a susceptible strain. In the second patient, K. pneumoniae grew only in a culture of a sample taken after the baseline culture, and ribotyping analysis was not performed. These results showed that neither of the strains developed documented resistance to tigecycline during therapy.
Data from all patients in the mITT population were used for the analysis of safety. Tigecycline had a safety profile consistent with that seen in phase 1 (23, 24, 34) and phase 2 (25, 31) trials. Table 7 shows the frequency and distribution of TEAEs that occurred in at least 3% of the patients. The majority of AEs were considered unrelated to a study medication and were mild to moderate in intensity. Nausea and vomiting were the most commonly reported TEAEs in the tigecycline group, occurring in 25% and 12% of patients, respectively, whereas they occurred in 5% and 2% of patients, respectively, in the V/A combination group. Although nausea and vomiting were usually related to tigecycline, approximately 96% of the incidents were mild to moderate in severity (grade 1 or 2). Significantly more tigecycline patients than patients in the V/A combination group (P < 0.001) received antiemetic therapy. The most common antiemetic treatment was metoclopramide.
TABLE 7.
Treatment emergent adverse events that occurred in ≥3% of patients
| Body system and adverse event | No. (%) of patients
|
|
|---|---|---|
| Tigecycline (n = 274) | V/A (n = 269) | |
| Any adverse event | 143 (52.2) | 118 (43.9) |
| Body as a whole | 46 (16.8) | 39 (14.5) |
| Headache | 13 (4.7) | 11 (4.1) |
| Cardiovascular system | 16 (5.8) | 26 (9.7) |
| Hypertension | 7 (2.6) | 14 (5.2) |
| Digestive systema | 89 (32.5) | 38 (14.1) |
| Diarrhea | 11 (4.0) | 4 (1.5) |
| Nauseaa | 69 (25.2) | 14 (5.2) |
| Vomitinga | 33 (12.0) | 6 (2.2) |
| Hemic and lymphatic system | 18 (6.6) | 14 (5.2) |
| Anemia | 4 (1.5) | 9 (3.3) |
| Metabolic and nutritional | 34 (12.4) | 30 (11.2) |
| AST level increaseda | 4 (1.5) | 14 (5.2) |
| ALT level increaseda | 5 (1.8) | 18 (6.7) |
| Skin and appendagesa | 20 (7.3) | 37 (13.8) |
| Pruritus | 11 (4.0) | 10 (3.7) |
| Rash | 3 (1.1) | 10 (3.7) |
Significant between-group difference (P < 0.05).
Serious adverse events were reported for 28 patients during the study: 15 patients in the tigecycline group (5.5%) and 13 patients in the V/A combination group (4.8%). The most frequent SAEs in the tigecycline-treated patients were cellulitis and infection (three patients each; 2.2% combined). The most frequent serious adverse event among V/A combination-treated patients was cellulitis (two patients; 0.7%).
Adverse events were the most frequent primary reason for discontinuation of the study drug (tigecycline, n = 6 [2.2%]; V/A combination, n = 13 [4.8%]). Three tigecycline patients discontinued treatment because of nausea. Seven V/A combination patients but no tigecycline patients (P < 0.007) discontinued treatment because of skin disorders: dermatitis allergic, furunculosis, pruritic rash, pruritis, and rash.
There was one death during the study. A 74-year-old man died from adenocarcinoma, but in the investigators' opinion, the death was not related to the study treatment. The patient received 7 days of treatment with tigecycline and died 1 day after the completion of therapy. Adenocarcinoma was present at the start of the study but was not discovered until surgery was performed.
No hematologic or serum chemistry abnormalities were associated with the use of tigecycline. In the V/A combination group, AST and ALT liver enzyme level increases were the most commonly reported TEAEs, occurring in 5% and 7% of patients, respectively, whereas they occurred in 2% and 2% of patients in the tigecycline group, respectively. Elevations in AST and ALT levels have been reported in <1% of aztreonam-treated patients (20) but are not described in the vancomycin prescription information (21). Significantly more patients in the V/A combination group (13.9%) had low lymphocyte levels compared with the number in the tigecycline group (4.4%) (P < 0.001). A higher percentage of patients in the tigecycline group (15.4%) had a high platelet count compared with the percentage of patients in the V/A combination group (8.9%) (P = 0.024). All but one patient (in the V/A combination group) with low platelet counts entered the study with preexisting thrombocytopenia.
DISCUSSION
The increasing rate of antibiotic resistance in patients presenting with complicated SSSIs (9, 10, 35) has spurred the need to develop new antimicrobial therapies. Tigecycline is the first glycylcycline that is indicated for the treatment of cSSSIs caused by a number of pathogens common in cSSSIs (40). Tigecycline's spectrum of in vitro activity is expanded beyond that of other broad-spectrum antibiotics because it is active against pathogens that are susceptible and resistant to other antibiotics (1, 15, 29).
In this phase 3 study, the safety and efficacy of tigecycline monotherapy for the treatment of cSSSIs were compared with the safety and efficacy of current standard therapy, which requires combination therapy, i.e., vancomycin and aztreonam, to provide adequate empirical coverage. Complicated SSSIs may be caused by gram-positive and/or gram-negative bacteria, including resistant strains (4, 8, 9, 11, 13, 33). The efficacy of tigecycline (a 50-mg infusion every 12 h after an initial dose of 100 mg) was noninferior to that of V/A (a 1-g/2-g infusion every 12 h), as seen in various predefined patient populations (CE, c-mITT, ME, m-mITT populations) at the test-of-cure visit. These results were consistent across different species of infecting pathogens and across different types of infections.
Tigecycline demonstrated efficacy against many isolates commonly linked to cSSSIs, including Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Streptococcus agalactiae, and Enterococcus faecalis. MIC90 values for tigecycline were uniformly low for the most prevalent isolates, including MRSA and MSSA isolates (MIC90 = 0.25 μg/ml for both groups). There was no evidence of the development of decreased susceptibility to tigecycline in this study.
The overall numbers of patients reporting adverse events were similar in the tigecycline and the V/A groups, with increased nausea and vomiting rates in the tigecycline group and increased incidence of rash and increases in ALT levels and AST levels in the combination V/A group.
The effective coverage of tigecycline for the treatment of cSSSIs seen in this study and in a previous phase 2 study (31) makes tigecycline a worthy candidate for the treatment of SSSIs requiring hospitalization. Additionally, this study compared tigecycline monotherapy with combined therapy with vancomycin and aztreonam, highlighting its expanded broad spectrum of activity for the treatment of cSSSIs. Based on these results and the increasing need for new antibiotics, tigecycline is a promising agent for the treatment of cSSSIs, especially in the setting when empirical coverage of gram-positive and gram-negative pathogens is warranted.
Acknowledgments
We thank the Tigecycline 305 cSSSI Study Group investigators for their valuable involvement in this study: Mickael Aoun, Eugeniusz Baran, Giedrius Barauskas, Andrei Bazarov, Miles Beaman, Johannes Breedt, José María Callejas Pérez, Patrick Carroll, Petr Cech, Chia-Ming Chang, Valeriy Chernyak, Mircea Dan Chiotan, Yin-Ching Chuang, Emil Lyubomirov Danchev, Georgi Petrov Deenitchin, Pierre Johannes Truter de Villiers, Jitka Dobesova, Marek Dobosz, Matthew Dryden, Carmen Fariñas Alvaréz, Georgi Ivanov Fichev, Ioan Florescu, Peter Fomin, Janis Gardovskis, Helen Giamarellou, David Gordon, Audrius Gradauskas, Georgi Borisov Gurbev, Shervanthi Homer-Vanniasinkam, Christian Joukhadar, Andrzej Kaszuba, Helmut Kerl, Natalia Klimusheva, Krzysztof Kołomecki, Peter Kujath, Uldis Kupcs, Lenoid Laberco, Frans Jacobus Maritz, John McBride, José Mensa Pueyo, Dušan Mištuna, Borislav Tzvetanov Ninov, Attila Olah, Remus Orasan, Andrejs Pavars, George Petrikkos, Waldemar Placek, T. Polyakova, Guntars Pupelis, Willem Jacobus Rabie, Arturas Razbadauskas, Brent Richards, Douglas Patrick Ross, Sorin Rugina, Luis Salmeron Febres, Oleg Samoylov, Slavko Schoenwald, Gerhard Tappeiner, Boris Teleshov, Jüri Teras, Milan Travnik, Eugene Tretiakov, Sergei Turkov, Tiit Vaasna, and Dah-Shyong Yu. We thank Wyeth Research employees Patricia Bradford for microbiological analysis and Donna Simcoe for professional medical writing services.
Wyeth Research, Collegeville, Pa., supported and funded this study.
J.B., J.T., J.G., F.J.M., T.V., and D.P.R. are investigators for this tigecycline study sponsored by Wyeth. M.G.-P., Dartois, E.J.E.-G., and E.L. are employees of Wyeth.
REFERENCES
- 1.Betriu, C., E. Culebras, I. Rodriguez-Avial, M. Gomez, B. A. Sanchez, and J. J. Picazo. 2004. In vitro activities of tigecycline against erythromycin-resistant Streptococcus pyogenes and Streptococcus agalactiae: mechanisms of macrolide and tetracycline resistance. Antimicrob. Agents Chemother. 48:323-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biedenbach, D. J., M. L. Beach, and R. N. Jones. 2001. In vitro antimicrobial activity of GAR-936 tested against antibiotic-resistant gram-positive blood stream infection isolates and strains producing extended-spectrum beta-lactamases. Diagn. Microbiol. Infect. Dis. 40:173-177. [DOI] [PubMed] [Google Scholar]
- 3.Boyce, J. M. 1992. Methicillin-resistant Staphylococcus aureus in hospitals and long-term care facilities: microbiology, epidemiology, and preventive measures. Infect. Control Hosp. Epidemiol. 13:725-737. [DOI] [PubMed] [Google Scholar]
- 4.Brook, I., and E. H. Frazier. 1990. Aerobic and anaerobic bacteriology of wounds and cutaneous abscesses. Arch. Surg. 125:1445-1451. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 6.Chopra, I. 2001. Glycylcyclines: third-generation tetracycline antibiotics. Curr. Opin. Pharmacol. 1:464-469. [DOI] [PubMed] [Google Scholar]
- 7.Clopper, C. J., and E. S. Pearson. 1934. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404-413. [Google Scholar]
- 8.Doern, G. V., R. N. Jones, M. A. Pfaller, K. C. Kugler, and M. L. Beach. 1999. Bacterial pathogens isolated from patients with skin and soft tissue infections: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). SENTRY Study Group (North America). Diagn. Microbiol. Infect. Dis. 34:65-72. [DOI] [PubMed] [Google Scholar]
- 9.Emori, T. G., and R. P. Gaynes. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falagas, M. E., L. Barefoot, J. Griffith, R. Ruthazar, and D. R. Snydman. 1996. Risk factors leading to clinical failure in the treatment of intra-abdominal or skin/soft tissue infections. Eur. J. Clin. Microbiol. Infect. Dis. 15:913-921. [DOI] [PubMed] [Google Scholar]
- 11.Fluit, A. C., F. J. Schmitz, J. Verhoef, and S. P. G. European. 2001. Frequency of isolation of pathogens from bloodstream, nosocomial pneumonia, skin and soft tissue, and urinary tract infections occurring in European patients. Eur. J. Clin. Microbiol. Infect. Dis. 20:188-191. [DOI] [PubMed] [Google Scholar]
- 12.Gales, A. C., and R. N. Jones. 2000. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936 tested against 1,203 recent clinical bacterial isolates. Diagn. Microbiol. Infect. Dis. 36:19-36. [DOI] [PubMed] [Google Scholar]
- 13.Ghoneim, A. T., J. McGoldrick, P. W. Blick, M. W. Flowers, A. K. Marsden, and D. H. Wilson. 1981. Aerobic and anaerobic bacteriology of subcutaneous abscesses. Br. J. Surg. 68:498-500. [DOI] [PubMed] [Google Scholar]
- 14.Hedberg, M. N. C. E. 1999. In vitro activity of anaerobic bacteria to GAR-936, a new glycylcycline. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 411, p. 26. American Society for Microbiology, Washington, D.C.
- 15.Henwood, C. J., T. Gatward, M. Warner, D. James, M. W. Stockdale, R. P. Spence, K. J. Towner, D. M. Livermore, and N. Woodford. 2002. Antibiotic resistance among clinical isolates of Acinetobacter in the UK, and in vitro evaluation of tigecycline (GAR-936). J. Antimicrob. Chemother. 49:479-487. [DOI] [PubMed] [Google Scholar]
- 16.Hoellman, D. B., G. A. Pankuch, M. R. Jacobs, and P. C. Appelbaum. 2000. Antipneumococcal activity of GAR 936, a new glycylcycline, compared to those of nine other agents against penicillin-susceptible and -resistant pneumococci. Antimicrob. Agents Chemother. 44:1085-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, M. E., F. J. Schmitz, A. C. Fluit, J. Acar, R. Gupta, J. Verhoef, et al. 1999. Frequency of occurrence and antimicrobial susceptibility of bacterial pathogens associated with skin and soft tissue infections during 1997 from an international surveillance programme. Eur. J. Clin. Microbiol. Infect. Dis. 18:403-408. [DOI] [PubMed] [Google Scholar]
- 18.Kontiainen, S., and E. Rinne. 1987. Bacteria isolated from skin and soft tissue lesions. Eur. J. Clin. Microbiol. 6:420-422. [DOI] [PubMed] [Google Scholar]
- 19.Mayhall, C. G. 2003. The epidemiology of burn wound infections: then and now. Clin. Infect. Dis. 37:543-550. [DOI] [PubMed] [Google Scholar]
- 20.Medical Economics. 2001. Azactam®. Aztreonam for injection. In Physicians' desk reference, 55th ed. Medical Economics, Montvale, N.J.
- 21.Medical Economics. 2001. Vancocin® HCl. Sterile vancomycin hydrochloride; intravenous. In Physicians' desk reference, 55th ed. Medical Economics, Montvale, N.J.
- 22.Milatovic, D., F.-J. Schmitz, J. Verhoef, and A. C. Fluit. 2003. Activities of the glycylcycline tigecycline (GAR-936) against 1,924 recent European clinical bacterial isolates. Antimicrob. Agents Chemother. 47:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muralidharan, G., J. Getsy, P. Mayer, I. Paty, M. Micalizzi, J. Speth, B. Webter, and P. Mojaverian. 1999. Pharmacokinetics (PK), safety and tolerability of GAR-936, a novel glycylcycline antibiotic, in healthy subjects. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 416, p. 303. American Society for Microbiology, Washington, D.C.
- 24.Muralidharan, G., P. Mojaverian, M. Micalizzi, J. Speth, S. Tse, R. Stroshane, J. Getsy, and P. Mayer. 2000. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 502, p. 17. American Society for Microbiology, Washington, D.C.
- 25.Murray, J., S. Wilson, S. Klein, A. Yellin, and E. Loh. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-739, p. 416. American Society for Microbiology, Washington, D.C.
- 26.National Committee for Clinical Laboratory Standards. 2001. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard, fifth ed. NCCLS document M11-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for microbes that grow aerobically; approved standard, sixth ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 28.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests; approved standard, eighth ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 29.Petersen, P. J., P. A. Bradford, W. J. Weiss, T. M. Murphy, P. E. Sum, and S. J. Projan. 2002. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrob. Agents Chemother. 46:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen, P. J., N. V. Jacobus, W. J. Weiss, P. E. Sum, and R. T. Testa. 1999. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postier, R. G., S. L. Green, S. R. Klein, E. J. Ellis-Grosse, E. Loh, and Tigecycline-200 Study Group. 2004. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin. Ther. 26:704-714. [DOI] [PubMed] [Google Scholar]
- 32.Projan, S. J. 2000. Preclinical pharmacology of GAR-936, a novel glycylcycline antibacterial agent. Pharmacotherapy 20:219S-223S. [DOI] [PubMed] [Google Scholar]
- 33.Rennie, R. P., R. N. Jones, and A. H. Mutnick. 2003. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn. Microbiol. Infect. Dis. 45:287-293. [DOI] [PubMed] [Google Scholar]
- 34.Sesoko, S., K. Umemura, and M. Nakashima. 2002. Pharmacokinetics (PK), safety, and tolerability of tigecycline (GAR-936) in healthy Japanese males. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother, abstr. 1761, p. 22. American Society for Microbiology, Washington, D.C.
- 35.Shmuely, H., S. Pitlik, M. Drucker, Z. Samra, H. Konisberger, and L. Leibovici. 2000. Prediction of mortality in patients with bacteremia: the importance of pre-existing renal insufficiency. Renal Failure 22:99-108. [DOI] [PubMed] [Google Scholar]
- 36.Sum, P. E., and P. Petersen. 1999. Synthesis and structure-activity relationship of novel glycylcycline derivatives leading to the discovery of GAR-936. Bioorg. Med. Chem. Lett. 9:1459-1462. [DOI] [PubMed] [Google Scholar]
- 37.Swartz, M. N. 2004. Clinical practice. Cellulitis. N. Engl. J. Med. 350:904-912. [DOI] [PubMed] [Google Scholar]
- 38.Tenover, F. C., J. W. Biddle, and M. V. Lancaster. 2001. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerg. Infect. Dis. 7:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson, E. B. 1927. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 22:209-212. [Google Scholar]
- 40. Wyeth Pharmaceuticals Inc. 2005. Tygacil™ package insert. Wyeth Pharmaceuticals Inc., Philadelphia, Pa. Available at http://www.fda.gov/cder/foi/label/2005/021821lbl.pdf. Accessed June 20, 2005.