Abstract
Coxsackievirus B4 (CVB4)-induced production of alpha interferon (IFN-α) by peripheral blood mononuclear cells (PBMC) is enhanced in vitro by nonneutralizing anti-CVB4 antibodies from healthy subjects and, to a higher extent, from patients with insulin-dependent diabetes mellitus. In this study, we focused on identification of the viral target of these antibodies in CVB systems. High levels of IFN-α were obtained in supernatants of PBMC incubated with CVB4E2 or CVB3 and plasma from healthy subjects and, to a higher extent, from patients. The VP4 capsid proteins dissociated by heating at 56°C from CVB4E2 (VP4CVB4) and CVB3 (VP4CVB3) but not H antigen preincubated with plasma from healthy subjects or patients inhibited the plasma-dependent enhancement of CVB4E2- and CVB3-induced IFN-α synthesis. There was no cross-reaction between VP4CVB4 and VP4CVB3 in the inhibiting effect. IFN-α levels in culture supernatants showed dose-dependent correlation with anti-VP4 antibodies eluted from plasma specimens using VP4-coated plates. There were higher index values for anti-VP4 antibodies detected by enzyme-linked immunosorbent assay (ELISA) and higher proportions of positive detection in 40 patients than in 40 healthy subjects (80% versus 15% for anti-VP4CVB4). There was no relationship between the levels of anti-CVB neutralizing antibodies and the detection of anti-VP4 antibodies by ELISA. The CVB plasma-induced IFN-α levels obtained in PBMC cultures in the anti-VP4 antibody-positive groups were significantly higher than those obtained in the anti-VP4 antibody-negative groups regardless of the titers of anti-CVB neutralizing antibodies. These results show that VP4 is the target of antibodies involved in the plasma-dependent enhancement of CVB4E2- and CVB3-induced IFN-α synthesis by PBMC.
The six coxsackievirus B (CVB) serotypes (CVB1 to CVB6), together with echovirus serotypes, EV-69, and swine vesicular disease virus, belong to the Human enterovirus B species of the Enterovirus genus within the Picornaviridae family (37). CVB are small naked viruses (30 nm). They contain a single plus-strand of RNA protected by an icosahedral capsid which is a combination of 60 protomers of four polypeptides each: VP1 to VP4. VP1, VP2, and VP3 are exposed at the virion surface, whereas VP4 is an internal protein linked to the genome (29, 41, 42). Of the four proteins, VP1 exhibits the highest sequence variability and VP4 exhibits the lowest (21, 31, 36). The epitopes which bind neutralizing antibodies are mainly present on VP1. Nevertheless, minor epitopes are present on VP2 and VP3 (5, 26, 28, 32).
CVB are responsible for a broad spectrum of diseases, such as aseptic meningitis, myocarditis, encephalitis, acute hemorrhagic conjunctivitis, nonspecific febrile illnesses, upper respiratory tract infections, and other acute or chronic illnesses (27). There are arguments in favor of the involvement of CVB in insulin-dependent diabetes mellitus (IDDM) (20, 33, 35, 39).
Our team and others have reported the detection of enterovirus RNA with strong homology to CVB, especially CVB3 and CVB4, in the peripheral blood of IDDM patients at the onset of clinical manifestations of the disease (1, 9, 12, 25, 30). Overall, the average proportion of enteroviral RNA-positive patients in various studies was 33% compared to 4% of control subjects (19). CVB4 E2 was isolated from the pancreas of a child with ketoacidosis (45). This isolate is particularly important because it is able to induce insulitis, β-cell destruction, and overt diabetes when injected into mice in contrast to CVB4JVB, a nondiabetogenic prototype CVB4 strain (46, 47). Recently, Yin et al. detected enteroviral RNA by reverse transcription-PCR in peripheral blood mononuclear cells (PBMC) from patients with IDDM, and they showed that the viral nucleic acid sequences had homologies with CVB4E2 (43).
It has been demonstrated that human β cells in pancreatic islets could harbor a persistent CVB infection (CVB4JVB, CVB4E2, CVB3), which resulted in the expression of alpha interferon (IFN-α) by these cells, and that CVB-induced IFN-α played a role in the initiation and/or maintenance of chronic CVB infection in human islets (8). These results support the hypothesis that the expression of IFN-α by β cells in the pancreases of patients with IDDM reported by Foulis et al. may be due to CVB (15). Interestingly, Ylipaasto et al. reported recently that the enterovirus genome can be detected by in situ hybridization in the pancreases of patients with IDDM (44). IFN-α may be an initiator of autoimmunity against β cells through the activation of autoimmune (islet-reactive) CD4+ Th1 cells (7, 38, 40). Thus, IFN-α can partake in promoting the expression of IDDM.
It has been reported recently that, in 50% of cases, increased levels of IFN-α in plasma were associated with the presence of enterovirus sequences, particularly CVB3 and CVB4, in circulating blood of adults and children with IDDM (9). IFN-α mRNA was detected in blood cells from patients with IFN-α in their plasma, suggesting that IFN-α was produced during the course of CVB4 and CVB3 infection. Like other enteroviruses, CVB are weak IFN-α inducers, compared to strong IFN-α inducers like Sendai virus and herpes simplex virus type 1 (14). However, CVB4JVB-induced synthesis of IFN-α by PBMC in vitro can be enhanced through interactions between CVB4, specific antibodies segregated from neutralizing antibodies isolated from the plasma of healthy subjects, FcγRII, FcγRIII, and a receptor for CVB called CAR (coxsackievirus and adenovirus receptor) (10). These results suggest that antibodies can play a role in the IFN-α response to CVB4. Furthermore, it has also been demonstrated that CVB4JVB can infect monocytes by an antibody-dependent mechanism involving the virus, antiviral antibodies, and receptors (CAR, FcγRII, and FcγRIII) that result in IFN-α production (16).
It has been shown that CVB4JVB, through interactions with circulating and/or cell-bound immunoglobulin G (IgG), can strongly induce the production of IFN-α by PBMC in IDDM patients. Furthermore, the IFN-α-inducing activity of plasma from IDDM patients preincubated with CVB4JVB, prior to the addition to PBMC isolated from healthy subjects, was high in comparison to that of plasma from healthy subjects (17). The IFN-α-inducing activity of CVB4JVB was strongly enhanced by IgGs from type 1 diabetic patients in comparison to IgGs from healthy subjects. IDDM patients may therefore have a higher prevalence of anti-CVB4 antibodies that enhance CVB4-induced IFN-α synthesis than the appropriate controls. It was observed that there was no correlation between the titers of anti-CVB4 antibodies detected in patients by neutralization assay or by H-antigen-based enzyme-linked immunosorbent assay (ELISA) and the plasma-dependent enhancement of CVB4-induced IFN-α production (10, 17).
Thus, CVB4-induced production of IFN-α by PBMC is enhanced in vitro by nonneutralizing anti-CVB4 antibodies found in the circulating blood of healthy subjects and patients with IDDM. These antibodies can play a role in the interaction between CVB and PBMC. The aim of this study was to identify the viral target of antibodies contained in the plasma of healthy subjects and patients with IDDM and involved in the enhancement of CVB4E2- and CVB3-induced IFN-α synthesis.
MATERIALS AND METHODS
Blood donors.
Blood samples were obtained from 40 patients with newly diagnosed type 1 diabetes: 30 adults (18 males and 12 females; median age, 35 years; range, 18 to 50 years) and 10 children (5 males and 5 females; median age, 10 years; range, 2 to 16 years). All patients included in this study had metabolic decompensation (diabetic ketoacidosis or diabetic ketosis) and were treated with insulin. Informed consent was obtained from the adults and the children's parents, and the committee on research ethics approved the study protocol.
Blood samples were obtained from 40 healthy subjects: 30 adults (16 males and 14 females; median age, 25 years; range, 19 to 40 years), who were enlisted in the “Centre de Transfusion Sanguine” (Lille, France) and 10 children (5 males and 5 females; median age, 12 years; range, 7 to 15 years), who were inpatients or outpatients. Healthy donors had no suspected immunological, infectious, or metabolic disease.
Venous blood from patients and healthy donors was collected in sterile tubes (Becton Dickinson, Meylan, France) containing 20 IU heparin/ml (Heparin Choay, Sanofi, France). Plasma was separated, aliquoted, and stored frozen at −80°C.
Purification of viruses.
CVB3 (American Type Culture Collection, Manassas, Va.) and a CVB4 E2 diabetogenic strain (provided by Ji-Won Yoon, Julia McFarlane Diabetes Research Center, Calgary, Alberta, Canada) were propagated in Hep-2 cells (Biowhittaker, Verviers, Belgium) in Eagle's minimum essential medium (Gibco BRL, Eragny, France) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco BRL) and 1% l-glutamine (Eurobio, France). After incubation for 24 h at 37°C in a 5% CO2 atmosphere, the cell suspension was frozen and thawed three times and centrifuged at 2,000 × g for 10 min. Virus from the resulting supernatant was pelleted by centrifugation at 500,000 × g for 3 h at 4°C in a Beckman TLA-100.4 rotor. The pellet was suspended in 3 ml of 0.01 M Tris-HCl, pH 7.2, containing 0.5% (vol/vol) Nonidet P-40, incubated at 4°C for 20 h, homogenized, and centrifuged at 4,000 × g to remove the insoluble debris. Only 0.5 ml of the clarified virus suspension was layered over 0.5 ml sucrose (30%, wt/vol) and 3 ml CsCl (40%, wt/vol). After centrifugation at 348,000 × g at 4°C in a Beckman TLA-100.4 rotor for 4 h, gradient fractions were recovered and virus titers in gradient fractions were determined by the 50% tissue culture infectious dose assay on confluent culture of Hep-2 cells. Fractions containing peak infectivity titers were pooled, desalted, and equilibrated with 0.01 M Tris-HCl, pH 7.2, by centrifugation at 4,000 × g on a Macrosep membrane (Pall Filtron Corporation, Saint Germain en Laye, France) at molecular weight cutoffs (MWCO) of 300,000 molecular weight (300K). Aliquots were stored frozen at −80°C. Mock preparations were obtained according to the same procedure, except that the Hep-2 cells were sham infected by the virus solvent alone.
Dissociation of CVB.
VP4 protein and H antigen were dissociated from CVB3 and CVB4E2. Purified and concentrated CVB (∼1 mg) were incubated at 56°C for 5 min in 0.5 ml sodium buffer (0.1 M NaCl, 0.005 M NaCl, pH 7.0). This treatment resulted in the release of RNA and VP4, thus giving natural empty capsids, also called H antigen. VP4 was separated from the mixture by centrifugation at 4,000 × g on a Macrosep membrane at MWCO of 100K. H antigen and RNA were retained by the membrane, and VP4 passed through the membrane. RNA was degraded by adding 0.05 mg of bovine pancreatic RNase A (Roche Molecular Biochemicals, Mannheim, Germany) and incubating the mixture at 37°C for 10 min. H antigen and VP4 were desalted and equilibrated with phosphate-buffered saline (PBS), pH 7.2, by centrifugation at 4,000 × g on a Macrosep membrane at MWCO of 100K and 3K, respectively. Concentration of H antigen and VP4 was carried out using a Savant Speed Vac Concentrator SVC100H (Global Medical Instrumentation, Minnesota). Mock dissociation was also made with the supernatant of noninfected Hep-2 cells purified as described above. The concentration of protein was calculated from the absorbance at 280 nm by assuming an extinction coefficient of 1 mg/ml.
Isotopic labeling of CVB.
Hep-2 cells were infected by CVB3 and CVB4E2 at a multiplicity of infection (MOI) of 0.1 for 3 h at 37°C. The inoculum was removed, and serum-free, methionine-free growth medium (ICN Pharmaceuticals France, Orsay, France) was added to the monolayers. Mock and infected Hep-2 cells were incubated at 37°C for 1 h. Thereafter, 25 μCi of [35S]methionine (Amersham Biosciences Europe GmbH, Orsay, France) was added per ml of medium. At 24 h postinfection, virus was harvested and purified as described above. Fractions with peak radioactivity were pooled, divided into aliquots, and stored at −80°C.
Gel electrophoresis of radiolabeled CVB proteins.
Purified CVB, VP4, and H-antigen fractions were subjected to 16% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions (23). Bands were visualized by autoradiography on an X-ray film (KODAK X-OMAT AR, Amersham Biosciences Europe GmbH) for 15 days.
ELISA for anti-VP4 antibody detection.
To detect anti-VP4 antibodies in donors' plasma, 96-well microtiter plates (SEDAC Therapeutics, China) were coated overnight at room temperature (RT) with VP4 dissociated from CVB3 or CVB4E2 at 5 μg/ml in PBS, pH 7.4. In the same way, microtiter plates were coated with proteins obtained from a mock dissociation procedure. Wells were then washed three times with washing solution (PBS, pH 7.4, 0.05% Tween 20), saturated for 1 h at 37°C with saturation buffer (PBS, pH 7.4, 2.5% nonfat milk, 0.5% Tween 20), and washed again four times. Afterwards, 0.1 ml of specimens diluted in saturation buffer at optimal dilution (1/50, as shown by preliminary experiments) was added to the microwells. After 2 h of incubation at RT, wells were washed four times and 0.1 ml of horseradish peroxidase-labeled goat anti-human IgA, IgG, or IgM (Bio-Rad Life Science Group, Marnes la Coquette, France) diluted to 1/10,000 was added for 1 h at RT. After four washing cycles, 0.1 ml of the substrate solution (0.4 mg/ml o-phenylenediamine, 0.012% H2O2 in 0.05 M phosphate citrate buffer, pH 5.0) (Sigma-Aldrich Chimie, Saint Quentin Fallavier, France) was added for 30 min at RT. The reaction was stopped by the addition of 25 μl of 2 N sulfuric acid (Sigma-Aldrich Chimie). Absorbance readings were measured at 490 nm in a Dynex MRX microplate reader (Thermo Life Science, Cergy Pontoise, France). The immunoassay cutoff value was determined by adding up the specimen absorbance on mock plates to the blank one on VP4 plates. Test specimens with an index value (specimen/cutoff value ratio) higher than 1.0 were deemed positive for anti-VP4 antibodies.
Elution of anti-VP4 antibodies.
ELISA plates coated with dissociated VP4 or mock proteins were used to elute the corresponding antibodies as described previously (4). A triplicate ELISA procedure was conducted as described above up to the step of incubation with diluted plasma. After washing, the bound antibodies were eluted with 0.1 ml of 0.2 M glycine, pH 2.5, by incubating the plates overnight at 4°C. Afterwards, the supernatants corresponding to the same specimen were pooled, desalted, and equilibrated with PBS, pH 7.2, by centrifugation at 4,000 × g on a Macrosep membrane at MWCO of 50K. Immunoglobulin concentrations were estimated by using an assumed extinction coefficient of 1.43 for 1 mg/ml solution at 280 nm.
PBMC isolation and IFN-α induction.
Venous blood from healthy donors was collected in sterile 7.5-ml tubes (Becton Dickinson, Meylan, France) containing 20 IU heparin/ml (Heparin Choay, Sanofi, France). PBMC from heparinized blood were separated over a Ficoll-Paque solution (Diatrizoate Ficoll, Eurobio) as described previously (3). The mononuclear cells were collected, washed three times with RPMI 1640, adjusted to a concentration of 2 × 106 cells/ml in RPMI medium supplemented with 10% FCS and 1% l-glutamine, and then distributed as 0.1-ml aliquots into 96-well tissue culture plates. Virus-antibody complexes were made by incubation of either plasma at optimal dilution (1/10 or 1/100) or eluted anti-VP4 antibody fractions at various concentrations with CVB3 or CVB4E2 for 1 h at 37°C. PBMC were infected with these mixtures (MOI, 1). After 48 h of incubation, supernatants of culture were harvested, cleared, and stored until assayed for the presence of IFN-α. Cultures not treated with viruses served as controls.
Immunoassay for IFN-α.
The concentration of IFN-α was determined by a specific and sensitive dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) based on the direct sandwich technique using a mixture of two murine monoclonal antibodies (MAbs) to human IFN-α LT27:273 and LT27:293, which bind more than 90% of natural IFN-α subtypes coated onto microtiter plate wells (LKB WALLAC, Turku, Finland), and a europium-labeled murine anti-human IFN-α, as previously described (6). Samples (100 μl) diluted with an equal volume of dilution buffer containing irrelevant mouse monoclonal IgG1:B1:2 at 50 μg/ml were then added to the plate. After 2 h, plates were washed three times and 0.2 ml of the europium-conjugated antibody at 1/800 dilution was added per well in dilution buffer. After 1 h, the plates were washed six times and 0.2 ml of enhancement solution (LKB WALLAC, Turku, Finland) was added per well to promote the dissociation of Eu3+ cations from the labeled antibody into solution, where they formed fluorescent chelates with components of the enhancement solution. After 20 min, the fluorescence in the microtitration strip wells was measured in a time-resolved fluorometer (1230 Arcus Fluorometer, LKB WALLAC, Finland). The National Institutes of Health leukocyte reference interferon G-23-902-530 was used as a standard. Intra- and interassay variations were lower than 10%.
Plaque neutralization assay.
To assess the presence of anti-CVB3 and anti-CVB4 neutralizing antibodies in plasma, twofold serial dilutions of each sample in minimum essential medium supplemented with 2% FCS and 1% l-glutamine were incubated with 100 50% tissue culture infectious doses of CVB3 or CVB4E2 in 96-well microtiter plates for 2 h at 37°C. Hep-2 cells in suspension were then added at approximately 104 cells per well, and the plates were reincubated for 3 days at 37°C in a humidified incubator with 5% CO2. Results were expressed as the inverse final dilution (titer) of sample that totally inhibited the viral cytopathic effect.
Statistical analysis.
Data are summarized as means ± standard deviations. The significance of differences in IFN-α levels was determined by the Wilcoxon test or the Mann-Whitney U test. The χ2 test or χ2 test with Yates correction (χ2c) was used to compare the distribution of categorical variables between samples. Correlations were evaluated by the Spearman test.
RESULTS
CVB4E2- and CVB3-derived VP4 capsid proteins inhibit the plasma-dependent enhancement of CVB4E2- and CVB3-induced production of IFN-α, respectively.
CVB4E2 and CVB3 were preincubated with plasma from healthy subjects and patients with IDDM to induce IFN-α production by PBMC isolated from healthy donors, as described in Materials and Methods. The IFN-α levels in culture supernatants were assayed using DELFIA. The mean levels of IFN-α obtained with plasma from IDDM adults and IDDM children were significantly higher than those in healthy adults and children (Table 1). In presence of plasma from patients or healthy subjects alone, the level of IFN-α in PBMC culture supernatant was 0 IU/ml, and in presence of virus alone, the level of IFN-α was lower than 2 IU/ml (data not shown).
TABLE 1.
Levels of CVB/plasma-induced IFN-α in supernatants of PBMC cultures
| Subject group (n) | IFN-α production (IU/m)a induced by:
|
|
|---|---|---|
| CVB3/plasma | CVB4/plasma | |
| Healthy subjects | ||
| Adults (30) | 238 ± 123b | 100 ± 44 |
| Children (10) | 162 ± 69 | 94 ± 45 |
| IDDM patients | ||
| Adults (30) | 402 ± 178b | 260 ± 178b |
| Children (10) | 328 ± 152c | 262 ± 143c |
IFN-α levels were determined by dissociation-enhanced lanthanide fluoroimmunoassay and are presented as means ± standard deviations.
P < 0.001 versus healthy adults (Student's t test).
P < 0.001 versus healthy children (Student's t test).
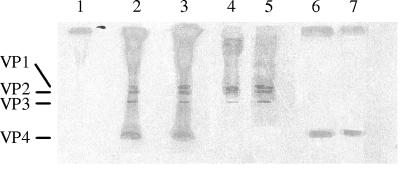
To identify the viral protein involved in the plasma-dependent enhancement of IFN-α production, VP4 and H antigen were dissociated from CVB4E2 and CVB3. On the basis of electrophoretic analysis under reducing and denaturing conditions, typical patterns of capsid polypeptides of 35S-labeled CVB were obtained. The estimated molecular masses for VP1, VP2, VP3, and VP4 were 37, 34, 27, and 7 kDa, respectively. Dissociation of 35S-labeled CVB4E2 and CVB3 at 56°C was accompanied by the release of protein which resolved at the expected molecular weight of VP4 protein. The remaining fraction corresponded to the VP1 to VP3 polypeptides (Fig. 1).
FIG. 1.
Autoradiogram of CVB capsid polypeptides on 16% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions. Virus polypeptides VP1, VP2, VP3, and VP4 are indicated by lines on the left. Lane 1, mock virus obtained from supernatant of noninfected Hep-2 cells; lanes 2 and 3, 35S-labeled CVB3 and 35S-labeled CVB4E2 from CVB3- and CVB4E2-infected Hep-2 cells, respectively; lanes 4 and 5, H antigen from 35S-labeled CVB3 and 35S-labeled CVB4, respectively, dissociated at 56°C as described in Materials and Methods; lanes 6 and 7, VP4 from 35S-labeled CVB3 and 35S-labeled CVB4E2, respectively, obtained after dissociation.
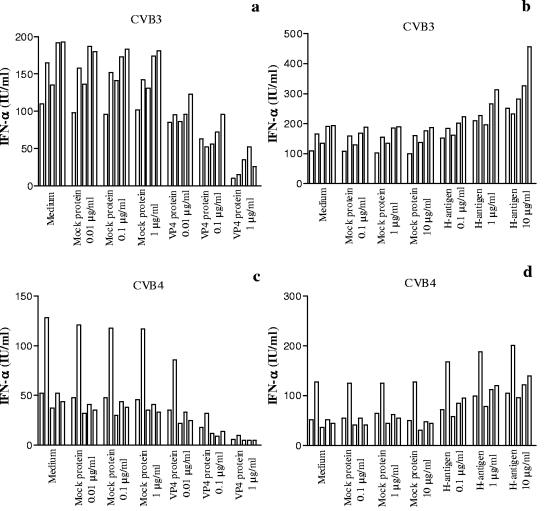
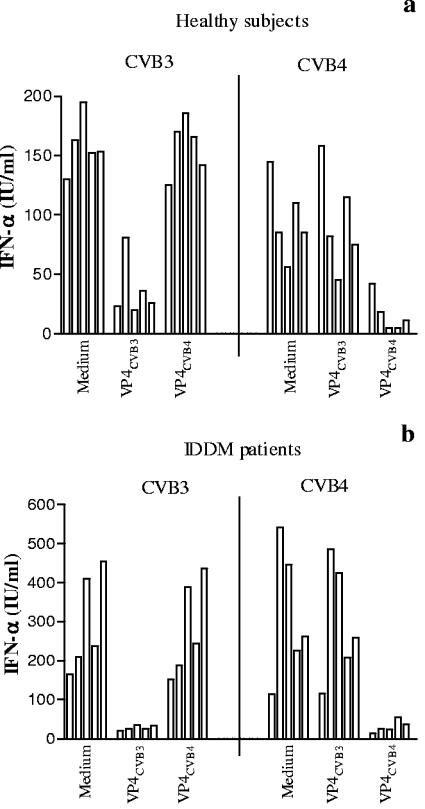
VP4 and H antigen were preincubated for 1 h at 37°C at various concentrations with plasma obtained from 5 healthy subjects and brought to optimal dilution (1/10 or 1/100). CVB4E2 or CVB3 was then added for 1 h before incubation with PBMC. As shown in Fig. 2a, a dose-dependent decrease in CVB3-plasma-induced IFN-α production was obtained in presence of VP4CVB3 protein, whereas in the presence of H antigen, a dose-dependent increase in IFN-α production was obtained (Fig. 2a). The incubation of PBMC with H antigen alone did not induce IFN-α production (data not shown). Similar patterns of results were obtained with VP4CVB4 in the CVB4E2-plasma system (Fig. 2b). On the contrary, the mock proteins isolated from noninfected Hep-2 cells had no effect on IFN-α production. These results showed that VP4 protein inhibits the CVB-plasma-induced IFN-α production.
FIG. 2.
Role of VP4 in the plasma-dependent enhancement of CVB-induced IFN-α production by PBMC. Plasma samples obtained from 5 healthy subjects were preincubated for 1 h at 37°C in the absence or presence of the VP4 fraction (a and c) or H antigen (b and d) dissociated from CVB3 or CVB4 (CVB4E2) at various concentrations before the addition of CVB3 or CVB4 (CVB4E2) (MOI, 1) for one additional hour at 37°C. PBMC were then infected with these mixtures. Culture supernatant samples were harvested 48 h postinfection. IFN-α levels in culture supernatants were determined by DELFIA. For each plasma sample, the experiments were performed in duplicate and the culture supernatants obtained for each plasma sample were pooled before the IFN-α assay was performed. Each individual result is presented. Mock protein means protein from mock-infected cells.
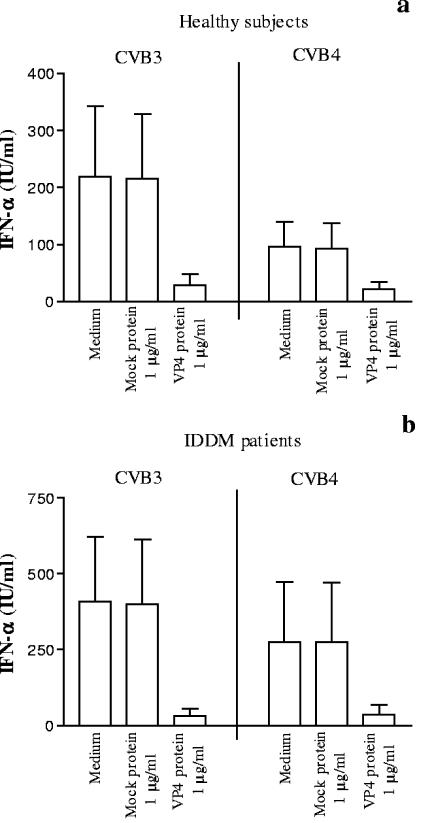
To confirm the effect of VP4 protein on CVB-induced IFN-α production, plasma which mediated a high level of CVB-induced IFN-α production in vitro was sorted from 20 healthy subjects and 20 IDDM patients and preincubated with VP4 protein (1 μg/ml) before the virus was added, and the IFN-α induction test was performed as described above. As shown in Fig. 3, CVB3-induced IFN-α production was significantly decreased in the presence of VP4CVB3 protein. In the same way, CVB4E2-induced IFN-α production was significantly decreased in the presence of VP4CVB4 protein.
FIG. 3.
Inhibition of the plasma-dependent enhancement of CVB-induced IFN-α production by PBMC by preincubating plasma with VP4 (1 μg/ml). Plasma samples were obtained from 20 healthy subjects (a) and 20 IDDM patients (b). The procedure is described in the legend to Fig. 2. Means and standard deviations are presented. In the presence of VP4CVB3 protein, the results were 219 ± 123 IU/ml versus 29 ± 20 IU/ml (P < 0.001, n = 20) for healthy subjects and 409 ± 212 IU/ml versus 32 ± 25 IU/ml (P < 0.001, n = 20) for IDDM patients. In the presence of VP4CVB4 protein, the results were 96 ± 44 IU/ml versus 21 ± 13 IU/ml (P < 0.001, n = 20) for healthy subjects and 276 ± 197 IU/ml versus 38 ± 32 IU/ml (P < 0.001, n = 20) for IDDM patients.
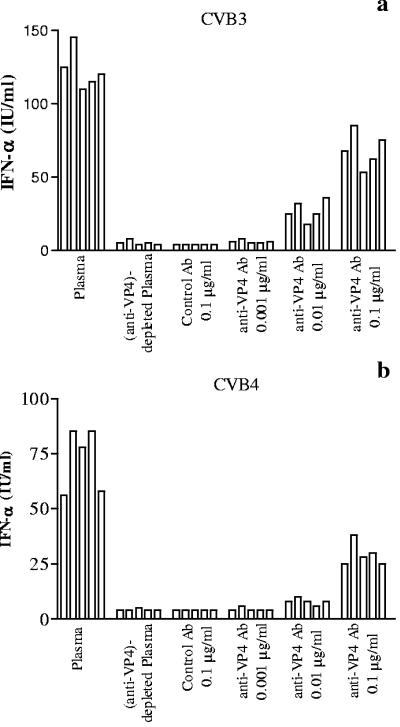
The mechanism of plasma-dependent enhancement of CVB3- and CVB4E2-induced production of IFN-α has been further investigated. Anti-VP4 antibodies were eluted from plasma specimens using VP4-coated plates as described in Materials and Methods. Eluted antibodies were then preincubated with CVB3 or CVB4E2 prior to the addition of PBMC. IFN-α levels in culture supernatants showed dose-dependent correlation with eluted antibody concentrations, whereas no IFN-α production was detected in the presence of anti-VP4-depleted plasma or control antibodies eluted from mock infection plates (Fig. 4).
FIG. 4.
Role of anti-VP4 antibodies in the plasma-dependent enhancement of CVB-induced IFN-α production by PBMC. CVB3 (a) or CVB4 (CVB4E2) (b) was preincubated for 1 h at 37°C with either plasma at optimal dilution obtained from 5 healthy subjects, eluted anti-VP4 antibody fractions at various concentrations obtained from the plasma specimen using VP4-coated plates, anti-VP4-depleted plasma, or control antibodies eluted from mock-infected plates as described in Materials and Methods.
The levels of IFN-α produced upon CVB3 challenge differed from those obtained upon CVB4 challenge (Table 1). These results suggest that there was no cross-reaction between antibodies directed against VP4 obtained from CVB3 and antibodies directed against VP4 obtained from CVB4. This hypothesis has been explored, and the results are shown in Fig. 5. Unlike VP4CVB3, when VP4CVB4 was preincubated with plasma from healthy subjects or IDDM patients before the addition of CVB3, levels of CVB3-induced IFN-α in PBMC cultures were not significantly altered (Fig. 5a). Similarly, VP4CVB3 protein dissociated from CVB3 did not affect the IFN-α levels induced by CVB4E2 (Fig. 5b). These data are in favor of the absence of cross-reaction between VP4 obtained from CVB3 and VP4 obtained from CVB4.
FIG. 5.
Absence of cross-reaction between VP4 fractions dissociated from CVB3 or CVB4 (CVB4E2) in the plasma-dependent enhancement of CVB-induced IFN-α production by PBMC. Plasma samples obtained from 5 healthy subjects (a) or 5 IDDM patients (b) were preincubated for 1 h at 37°C in the absence or presence of the VP4 fraction (1 μg/ml) dissociated from CVB3 or CVB4 (CVB4E2) before the addition of CVB3 or CVB4 (CVB4E2) (MOI, 1) for one additional hour at 37°C.
Detection of antibodies directed toward CVB4E2- and CVB3-derived VP4 in plasma from healthy subjects and patients with IDDM.
ELISA plates were further developed to screen anti-VP4 antibodies in plasma from healthy subjects and IDDM patients. The proportion of individuals positive for anti-VP4CVB3 antibodies was higher in IDDM patients (62.5%; 6/10 children and 19/30 adults) than in healthy subjects (35%; 3/10 children and 11/30 adults). The proportion of individuals positive for anti-VP4CVB4 antibodies was higher in IDDM patients (80%; 7/10 children and 25/30 adults) than in healthy subjects (15%; 1/10 children and 5/30 adults). The mean index values obtained in the group of IDDM patients were significantly higher than those obtained in the group of healthy subjects (Table 2). There was no relationship between the detection of anti-VP4CVB3 antibodies and anti-VP4CVB4 antibodies in both healthy and IDDM subjects (χ2c test, P = 0.82 [healthy subjects] and P = 0.38 [IDDM patients]; n = 40).
TABLE 2.
Detection by ELISA of anti-VP4 antibodies in plasma from healthy subjects and patients with IDDM
| Subject group (n) | Anti-VP4CVB3
|
Anti-VP4CVB4
|
||
|---|---|---|---|---|
| No. (%) with positive detectiona | Index valueb | No. (%) with positive detectiona | Index valueb | |
| Healthy subjects 40 | 14 (35) | 0.9 ± 0.32 | 6 (15) | 0.72 ± 0.34 |
| IDDM patients 40 | 25 (62.5)c | 1.88 ± 1.09d | 32 (80)e | 1.96 ± 0.85d |
Number of individuals with positive antibody detection by ELISA.
Index values of anti-VP4 antibodies detected by ELISA. Microtiter plates were coated with VP4 dissociated from either CVB3 or CVB4 (CVB4E2). In the same way, microtiter plates were coated with proteins obtained from a mock dissociation procedure. The immunoassay cutoff value was determined by adding the specimen absorbance on mock infection plates to the blank absorbance on VP4 plates. The index value is obtained by calculating the specimen/cutoff value ratio. Data are presented as means ± standard deviations.
P < 0.02 versus healthy subjects (χ2 test).
P < 0.001 versus healthy subjects (Student's t test).
P < 0.001 versus healthy subjects (χ2 test).
The relationship between the detection of anti-VP4 antibodies and anti-CVB neutralizing antibodies (anti-CVB NA) has been investigated. In plasma from 40 healthy subjects, the titers of anti-CVB3 and anti-CVB4 NA were low and ranged between 2 and 32, whereas in plasma from 40 IDDM patients, anti-CVB3 NA ranged between 2 and 4,096 and anti-CVB4 NA titers ranged between 4 and 512. There was a significant difference in the geometric mean of the anti-CVB NA titers between healthy subjects and IDDM patients: anti-CVB3 NA, 4.3 ± 7.7 versus 60.8 ± 715.8, P < 0.001; anti-CVB4 NA, 3.3 ± 6.0 versus 52.0 ± 162.7, P < 0.001 (data not shown).
In both healthy subjects and IDDM patients, there was no correlation between titers of anti-CVB3 NA obtained by the seroneutralization test and the index values of anti-VP4CVB3 antibodies obtained by ELISA: healthy subjects, r = 0.28, P = 0.08; IDDM patients, r = 0.08, P = 0.59. As regards anti-CVB4 antibodies, a weak but significant correlation was observed between the two methods for IDDM patients (r = 0.35, P = 0.03) but not for healthy subjects (r = −0.09, P = 0.55) (data not shown). When the highest values of anti-CVB4 NA antibody titers (512) were eliminated from the group of IDDM patients, the correlation was no longer significant (r = 0.18, P = 0.28, n = 36).
Relationship between detection of antibodies directed toward CVB4E2- and CVB3-derived VP4 and the plasma-dependent enhancement of CVB-induced production of IFN-α in control subjects and patients with IDDM.
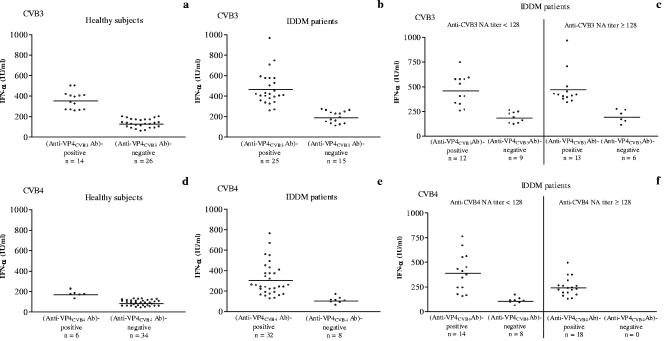
The levels of CVB/plasma-induced IFN-α in vitro were compared with the detection of anti-VP4 antibodies. Healthy subjects and IDDM patients were segregated into an anti-VP4 antibody (Ab)-positive group and an anti-VP4 Ab-negative group. As shown in Fig. 6, the CVB3/plasma-induced IFN-α levels obtained from healthy subjects and IDDM patients in the anti-VP4CVB3 Ab-positive groups were significantly higher than those obtained from healthy subjects and IDDM patients in the anti-VP4CVB3 Ab-negative groups (Fig. 6a and b). Similar patterns of results were obtained when IFN-α concentrations contained in the supernatants of CVB4/plasma-stimulated PBMC cultures were studied in individuals with, respectively, positive or negative detection of anti-VP4 antibodies on VP4CVB4-coated plates (Fig. 6d and e). The mean levels of IFN-α were higher for anti-VP4 Ab-positive IDDM patients than for anti-VP4 Ab-positive healthy subjects in the CVB3 (P < 0.01) and CVB4 (P < 0.02) systems. A similar pattern of results was obtained when anti-VP4 Ab-negative IDDM patients and healthy subjects were compared in the CVB3 system (P < 0.01), whereas the difference was not significant between anti-VP4 Ab-negative patients and controls in the CVB4 system (P = 0.09).
FIG. 6.
Individual representation of IFN-α levels in cultures of PBMC obtained from healthy subjects and IDDM patients selected according to the detection of anti-VP4 antibodies. The patients were further segregated according to the titers of neutralizing antibodies (e and f). PBMC were infected with CVB3 (a, b, and c) or CVB4 (CVB4E2) (d, e, and f) preincubated for 1 h at 37°C with plasma obtained from healthy subjects and IDDM patients. The detection of anti-VP4 antibodies was performed by ELISA on plates coated with VP4 dissociated from CVB3 or CVB4 (CVB4E2). Plasma specimens with an index value of anti-VP4 antibodies higher than 1.0 were deemed positive for anti-VP4 antibodies. The horizontal bars represent the means. For anti-VP4CVB3 Ab-positive groups versus anti-VP4CVB3 Ab-negative groups, the results for healthy subjects were 353 ± 87 IU/ml versus 127 ± 42 IU/ml (P < 0.001) and those for IDDM patients were 464 ± 161 IU/ml versus 187 ± 57 IU/ml (P < 0.001) (a and b). For anti-VP4CVB4 Ab-positive groups versus anti-VP4CVB4 Ab-negative groups, the results for healthy subjects were 171 ± 30 IU/ml versus 83 ± 28 IU/ml (P = 0.001) and those for IDDM patients were 305 ± 159 IU/ml versus 104 ± 31 IU/ml (P < 0.001) (d and e). For IDDM patients with low anti-CVB3 NA titers, the results for anti-VP4CVB3-positive antibodies versus anti-VP4CVB3-negative antibodies were 459 ± 154 IU/ml versus 183 ± 55 IU/ml (P < 0.001) (c). For IDDM patients with low anti-CVB4 NA titers, the results for anti-VP4CVB4-positive antibodies versus anti-VP4CVB4-negative antibodies were 388 ± 192 IU/ml versus 104 ± 31 IU/ml (P < 0.001) (f). For IDDM patients with high anti-CVB3 NA titers, the results for anti-VP4CVB3-positive antibodies versus anti-VP4CVB3-negative antibodies were 469 ± 173 IU/ml versus 193 ± 66 IU/ml (P < 0.001) (c). For IDDM patients with anti-VP4CVB4-positive antibodies and low anti-CVB4 NA titers versus high anti-CVB4 NA titers, the results were 388 ± 192 IU/ml versus 241 ± 92 IU/ml (P = 0.01) (f).
Following that, the levels of CVB/plasma-induced IFN-α were then compared with the titers of anti-CVB NA and the detection of anti-VP4 antibodies in IDDM patients. The patients were segregated into two groups: the first had NA titers of <128 (low) and the second had NA titers of ≥128 (high). There was no significant difference in IFN-α levels between low- and high-anti-CVB NA titer groups of IDDM patients (CVB3, 341 ± 184 IU/ml [n = 21] versus 382 ± 196 IU/ml [n = 19], P = 0.81; CVB4, 285 ± 206 IU/ml [n = 22] versus 241 ± 92 IU/ml [n = 18], P = 0.85) (data not shown). Each group was further segregated into anti-VP4 Ab-positive and anti-VP4 Ab-negative subgroups. Of the 21 IDDM patients with low anti-CVB3 NA titers, 12 (57%) were positive for anti-VP4CVB3 antibodies and had higher levels of CVB3-induced IFN-α than those obtained from 9 patients negative for anti-VP4CVB3 antibodies (Fig. 6c). Similarly, of the 22 patients with low anti-CVB4 NA titers, 14 (64%) were positive for anti-VP4CVB4 antibodies and had higher levels of CVB4-induced IFN-α than those obtained from 8 patients negative for anti-VP4CVB4 antibodies (Fig. 6f). On the other hand, 13 (68%) of 19 patients with high anti-CVB3 NA titers were positive for anti-VP4CVB3 antibodies and had higher levels of CVB3-induced IFN-α than 6 patients negative for anti-VP4CVB3 antibodies (Fig. 6c). As regards CVB4, 18/18 (100%) patients with high anti-CVB4 NA titers were positive for anti-VP4CVB4 antibodies (Fig. 6f). In this group of patients, the mean level of IFN-α was significantly lower than the one in patients with low anti-CVB4 NA titers and positive for anti-VP4CVB4 antibodies (P = 0.01).
DISCUSSION
We previously reported that CVB4JVB could strongly induce the synthesis of IFN-α in the presence of plasma from healthy subjects (10, 16) and IDDM patients (17). This enhancing effect resulted from the interaction of plasma IgG devoid of neutralizing activity with CVB4. In previous studies, we described that antibodies bind CVB4 and that complexes of virus and antibodies, through interaction with CAR (virus receptor) and FcγR (antibodies receptor), enhance CVB4-induced IFN-α production, whereas the virus alone is a poor IFN-α inducer (0 to 2 IU/ml IFN-α detected in PBMC culture supernatant) and that antibodies alone do not induce IFN-α (10). In the present study, it has been shown that plasma increases the CVB4E2- and CVB3-induced production of IFN-α by PBMC as well. The viral protein recognized by enhancing antibodies and involved in CVB/plasma-induced IFN-α production in supernatants of PBMC cultures has been identified. The significant decrease or increase in IFN-α production obtained when preincubating VP4 protein or H antigen, respectively, with plasma, suggests that these fractions interfere with the CVB binding of antibodies involved in the induction of IFN-α production. Therefore, VP4 appeared to bind antibodies involved in the enhancement of CVB-induced IFN-α production, and anti-VP4 antibodies remained free in the plasma and were not adsorbed by H antigen. Authors have reported that neutralizing epitopes are associated with H antigen obtained from poliovirus (34). These antibodies were raised against peptide sequences that are exposed normally at the virion surface. On the contrary, VP4 protein lies buried in close association with the RNA core (24) and appears to be inaccessible by antibodies. However, previous studies showed that the poliovirus particle is a dynamic entity capable of undergoing conformational alterations at physiological temperatures and that VP4, when exposed even partially during the normal “breathing motion” of the virus, can be bound by anti-VP4 antibodies (24). This conformational flexibility may provide an explanation for our observation of interaction between VP4 and plasma antibodies.
Anti-VP4 antibodies are directly involved in the enhancement of CVB-induced IFN-α as evidenced by the fact that (i) a dose-dependent decrease in IFN-α was seen when VP4 protein was preincubated with plasma before the virus was added, (ii) eluted antibodies bound on VP4-coated plates enhanced the CVB-induced IFN-α production in a dose-dependent manner, and (iii) plasma depleted on VP4-coated plates did not enhance the CVB-induced IFN-α production. Interestingly, VP4 released from CVB3 and preincubated with plasma did not affect the IFN-α levels induced by CVB4; conversely, VP4 released from CVB4 did not affect the IFN-α levels induced by CVB3. Furthermore, there was no relationship between the detection of anti-VP4CVB3 antibodies and anti-VP4CVB4 antibodies in both healthy and IDDM subjects. These results suggest that VP4CVB3 is serotypically distinct from VP4CVB4 and that plasma antibodies involved in the enhancement of CVB3-induced IFN-α synthesis are different from those involved in the enhancement of CVB4-induced IFN-α synthesis. Recent studies have demonstrated that VP4 nucleotide sequences are highly conserved among strains of one serotype, and attempts have been made to classify the human enteroviral serotypes by performing phylogenetic analysis on VP4 nucleotide sequences. The VP4 nucleotide sequences were sufficiently divergent to segregate the isolates from heterologous serotypes (11, 21, 22, 37), which can explain the absence of cross-reaction between anti-CVB3 and anti-CVB4 antibodies enhancing the synthesis of IFN-α.
This is the first report of an immunosorbent assay aimed at detecting anti-CVB antibodies based on the use of VP4 dissociated from CVB. There was no correlation between index values for anti-VP4 antibodies and neutralizing antibody titers. Indeed, high index values for anti-VP4 antibodies were obtained for controls and patients with low neutralizing antibody titers, which suggests that there are anti-CVB antibodies in plasma from healthy controls and patients which are not detected by methods usually available, such as neutralization assay. We cannot exclude that patients had neutralizing antibodies which remained undetected in our assay nor that they have neutralizing antibodies directed toward a CVB (a variant of CVB4, for example) with non-cross-reacting neutralizing epitopes (compared with the strain used in our assay) but with cross-reacting epitopes in VP4. The immunosorbent assay described in the present study allowed the detection of anti-VP4 antibodies with higher index values and in larger proportions of individuals in the IDDM patient group than in the healthy control group (80% versus 15% for anti-VP4CVB4 antibodies; 62.5% versus 32.5% for anti-VP4CVB3 antibodies). Our results are in favor of recent, repeated, or prolonged exposure to CVB for patients compared with controls and give further weight to the argument that CVB infections may be associated with IDDM.
The detection of anti-VP4 antibodies was negative in certain individuals (controls and patients), whereas an IFN-α-inducing activity of their plasma combined with CVB was obtained. The IFN-α-inducing activity of these plasma was absorbed with the VP4 fraction (data not shown), which suggests that they contained anti-VP4 antibodies but that the concentration was below the limit of the assay and that further studies are needed to increase the sensitivity of detection of these antibodies by ELISA. One explanation for low IFN-α levels and negative detection of anti-VP4 antibodies in certain patients can be the involvement of viral proteins other than VP4, since Li et al. reported that at physiological temperature, the VP1 NH2 term is exposed because of the “breathing motion” of the virus (24). The low IFN-α levels and negative detection of anti-VP4 antibodies in patients and controls, which reflect the low reactivity of their plasma in CVB3 or CVB4 systems, do not exclude the presence of anti-VP4 antibodies directed toward enteroviruses other than CVB3 or CVB4 in the plasma from these individuals. In so far as CVB2 and echoviruses can be detected in the blood of patients with IDDM (9, 20), other enterovirus serotypes are candidate viruses to be included in the plasma-dependent enhancement of virus-induced production of IFN-α in our system and for the detection of corresponding anti-VP4 antibodies.
The plasma-dependent enhancement of CVB3- or CVB4-induced production of IFN-α produced by PBMC was higher in patients with IDDM with positive detection of anti-VP4 antibodies. These data reveal a mechanism by which circulating CVB4 and CVB3 induce the production of IFN-α as reported previously for IDDM patients (9). As far as CVB3 infection is concerned, levels of IFN-α in anti-VP4CVB3 Ab-positive patients with low anti-CVB3 NA titers were not significantly higher than those in anti-VP4CVB3 Ab-positive patients with high anti-CVB3 NA titers. On the other hand, levels of IFN-α in anti-VP4CVB3 Ab-negative patients were significantly lower than those in anti-VP4CVB3 Ab-positive patients. These results suggest that plasma from IDDM patients with detectable anti-VP4CVB3 antibodies enhances IFN-α synthesis, even though it displays a high titer of anti-CVB3 NA. Interestingly, in the group of anti-VP4CVB4 Ab-positive patients, levels of CVB4-induced IFN-α obtained from patients with low anti-CVB4 NA titers (<128) were significantly higher than those obtained from patients with high anti-CVB4 NA titers (≥128), whereas the mean index value of anti-VP4CVB4 obtained from patients with low anti-CVB4 NA titers was not significantly different from the one obtained from patients with high anti-CVB4 NA titers (P = 0.39) (data not shown). These results suggest that, in IDDM patients with detectable anti-VP4CVB4 antibodies, an enhanced synthesis of IFN-α can occur in patients with low titers of anti-CVB4 NA. Insofar as we have reported in a previous study that antibody-dependent enhancement of IFN-α synthesis in PBMC resulted from an increase in CVB4 infectivity (16), our results suggest that plasma from individuals containing detectable anti-VP4CVB4 antibodies with low anti-CVB4 NA titers can enhance CVB4 infection. Altogether, these data indicate that there may be a balance between anti-CVB neutralizing antibodies and anti-VP4 enhancing antibodies, which can play a role in the pathogenesis of CVB infection. Further investigations are needed to explore this hypothesis.
Since humans are frequently exposed to sequential CVB challenges (18), since anti-VP4 antibodies combined with CVB increase the production of IFN-α, and since IFN-α can contribute to the development of autoimmune diseases and especially IDDM (2, 7, 13, 44), IFN-α can therefore be repeatedly produced, possibly at high levels, and may play a pathophysiological role in triggering or accelerating β-cell autoimmunity in genetically susceptible individuals (high-risk or prediabetic individuals), which results in clinical IDDM.
Further investigations are needed to determine whether anti-VP4 antibodies play a role in the pathogenesis of CVB infection, whether there is an association between anti-VP4 antibodies and the susceptibility to CVB infection resulting in IDDM, and whether the positive detection of anti-VP4 by ELISA as described here may have a predictive value of CVB-associated disease like IDDM. An alternative hypothesis is that the presence of high titers of anti-VP4 antibodies in IDDM patients could be a consequence of the diabetic condition.
Our results show that VP4CVB3 is serotypically distinct from VP4CVB4 and that plasma antibodies involved in the enhancement of CVB3-induced IFN-α synthesis are different from those involved in the enhancement of CVB4-induced IFN-α synthesis. Future studies will determine whether anti-VP4 antibodies are specific at the serotype or variant level and whether detection of anti-VP4 antibodies can be suitable for clinical diagnosis and epidemiological studies.
In conclusion, our results show that capsid protein VP4 is the target of antibodies contained in the plasma of healthy subjects and, to a higher extent, in the plasma of patients with IDDM. Capsid protein VP4 and specific anti-VP4 antibodies interact to play a role in the plasma-dependent enhancement of CVB4E2- and CVB3-induced synthesis of IFN-α by PBMC.
Acknowledgments
We thank Delphine Caloone for technical assistance and Mouna Lazrek for helpful discussions.
This work was supported by “CHRU Lille AOCHU98/1911,” Ministère de l'Education Nationale de la Recherche et de la Technologie and Université Lille 2, France (UPRES EA3610), ALFEDIAM, and a European Contract of 6th FP (Euro-thymaide).
REFERENCES
- 1.Andreoletti, L., D. Hober, C. Hober-Vandenberghe, S. Belaich, M. C. Vantyghem, J. Lefebvre, and P. Wattre. 1997. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J. Med. Virol. 52:121-127. [DOI] [PubMed] [Google Scholar]
- 2.Bosi, E., R. Minelli, E. Bazzigaluppi, and M. Salvi. 2001. Fulminant autoimmune type 1 diabetes during interferon-alpha therapy: a case of Th1-mediated disease? Diabet. Med. 18:329-332. [DOI] [PubMed] [Google Scholar]
- 3.Boyum, A. 1976. Isolation of lymphocytes, granulocytes and macrophages. Scand. J. Immunol. Suppl. 5:9-15. [PubMed] [Google Scholar]
- 4.Brahimi, K., J. L. Perignon, M. Bossus, H. Gras, A. Tartar, and P. Druilhe. 1993. Fast immunopurification of small amounts of specific antibodies on peptides bound to ELISA plates. J. Immunol. Methods 162:69-75. [DOI] [PubMed] [Google Scholar]
- 5.Caggana, M., P. Chan, and A. Ramsingh. 1993. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J. Virol. 67:4797-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cederblad, B., S. Blomberg, H. Vallin, A. Perers, G. V. Alm, and L. Ronnblom. 1998. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-alpha-producing cells. J. Autoimmun. 11:465-470. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti, D., B. Hultgren, and T. A. Stewart. 1996. IFN-alpha induces autoimmune T cells through the induction of intracellular adhesion molecule-1 and B7.2. J. Immunol. 157:522-528. [PubMed] [Google Scholar]
- 8.Chehadeh, W., J. Kerr-Conte, F. Pattou, G. Alm, J. Lefebvre, P. Wattre, and D. Hober. 2000. Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells. J. Virol. 74:10153-10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chehadeh, W., J. Weill, M. C. Vantyghem, G. Alm, J. Lefebvre, P. Wattre, and D. Hober. 2000. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J. Infect. Dis. 181:1929-1939. [DOI] [PubMed] [Google Scholar]
- 10.Chehadeh, W., A. Bouzidi, G. Alm, P. Wattre, and D. Hober. 2001. Human antibodies isolated from plasma by affinity chromatography increase the coxsackievirus B4-induced synthesis of interferon-alpha by human peripheral blood mononuclear cells in vitro. J. Gen. Virol. 82:1899-1907. [DOI] [PubMed] [Google Scholar]
- 11.Chu, P. Y., K. H. Lin, K. P. Hwang, L. C. Chou, C. F. Wang, S. R. Shih, J. R. Wang, Y. Shimada, and H. Ishiko. 2001. Molecular epidemiology of enterovirus 71 in Taiwan. Arch. Virol. 146:589-600. [DOI] [PubMed] [Google Scholar]
- 12.Clements, G. B., D. N. Galbraith, and K. W. Taylor. 1995. Coxsackie B virus infection and onset of childhood diabetes. Lancet 346:221-223. [DOI] [PubMed] [Google Scholar]
- 13.Fabris, P., C. Betterle, N. A. Greggio, R. Zanchetta, E. Bosi, M. R. Biasin, and F. de Lalla. 1998. Insulin-dependent diabetes mellitus during alpha-interferon therapy for chronic viral hepatitis. J. Hepatol. 28:514-517. [DOI] [PubMed] [Google Scholar]
- 14.Feldman, S. B., M. Ferraro, H. M. Zheng, N. Patel, S. Gould-Fogerite, and P. Fitzgerald-Bocarsly. 1994. Viral induction of low frequency interferon-a producing cells. Virology 204:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Foulis, A. K., M. A. Farquharson, and A. Meager. 1987. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet ii:1423-1427. [DOI] [PubMed] [Google Scholar]
- 16.Hober, D., W. Chehadeh, A. Bouzidi, and P. Wattre. 2001. Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-alpha synthesis. J. Infect. Dis. 184:1098-1108. [DOI] [PubMed] [Google Scholar]
- 17.Hober, D., W. Chehadeh, J. Weill, C. Hober, M. C. Vantyghem, P. Gronnier, and P. Wattré. 2002. Circulating and cell-bound antibodies increase the coxsackievirus B4-induced production of IFN-α by peripheral blood mononuclear cells of patients with type 1 diabetes. J. Gen. Virol. 83:2169-2176. [DOI] [PubMed] [Google Scholar]
- 18.Hyoty, H., M. Hiltunen, M. Knip, M. Laakkonen, P. Vahasalo, J. Karjalainen, P. Koskela, M. Roivainen, P. Leinikki, T. Hovi, et al. 1995. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes 44:652-657. [DOI] [PubMed] [Google Scholar]
- 19.Hyoty, H. 2002. Enterovirus infections and type 1 diabetes. Ann. Med. 34:138-147. [PubMed] [Google Scholar]
- 20.Hyoty, H., and K. W. Taylor. 2002. The role of viruses in human diabetes. Diabetologia 45:1353-1361. [DOI] [PubMed] [Google Scholar]
- 21.Ishiko, H., Y. Shimada, M. Yonaha, O. Hashimoto, A. Hayashi, K. Sakae, and N. Takeda. 2002. Molecular diagnosis of human enteroviruses by phylogeny-based classification by use of the VP4 sequence. J. Infect. Dis. 185:744-754. [DOI] [PubMed] [Google Scholar]
- 22.Kubo, H., N. Iritani, and Y. Seto. 2002. Molecular classification of enteroviruses not identified by neutralization tests. Emerg. Infect. Dis. 8:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Li, Q., A. G. Yafal, Y. M. Lee, J. Hogle, and M. Chow. 1994. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J. Virol. 68:3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonnrot, M., K. Salminen, M. Knip, K. Savola, P. Kulmala, P. Leinikki, T. Hyypia, H. K. Akerblom, and H. Hyoty. 2000. Enterovirus RNA in serum is a risk factor for beta-cell autoimmunity and clinical type 1 diabetes: a prospective study. Childhood Diabetes in Finland (DiMe) Study Group. J. Med. Virol. 61:214-220. [PubMed] [Google Scholar]
- 26.Mateu, M. G. 1995. Antibody recognition of picornaviruses and escape from neutralization: a structural view. Virus Res. 38:1-24. [DOI] [PubMed] [Google Scholar]
- 27.Melnick, J. L. 1996. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 655-712. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Channock, J. L. Melnick, T. P. Monath, et al. (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 28.Minor, P. D. 1990. Antigenic structure of picornaviruses. Curr. Top. Microbiol. Immunol. 161:121-154. [DOI] [PubMed] [Google Scholar]
- 29.Muckelbauer, J. K., M. Kremer, I. Minor, G. Diana, F. J. Dutko, J. Groarke, D. C. Pevear, and M. G. Rossmann. 1995. The structure of coxsackievirus B3 at 3.5 A resolution. Structure 3:653-667. [DOI] [PubMed] [Google Scholar]
- 30.Nairn, C., D. N. Galbraith, K. W. Taylor, and G. B. Clements. 1999. Enterovirus variants in the serum of children at the onset of type 1 diabetes mellitus. Diabet. Med. 16:509-513. [DOI] [PubMed] [Google Scholar]
- 31.Oberste, M. S., K. Maher, D. R. Kilpatrick, M. R. Flemister, B. A. Brown, and M. A. Pallansch. 1999. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 37:1288-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimann, B. Y., R. Zell, and R. Kandolf. 1991. Mapping of a neutralizing antigenic site of coxsackievirus B4 by construction of an antigen chimera. J. Virol. 65:3475-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rewers, M., and M. Atkinson. 1995. The possible role of enteroviruses in diabetes mellitus, p. 353-384. In H. A. Rotbart (ed.), Human enterovirus infections. Library of Congress, Washington, D.C.
- 34.Rombaut, B., R. Vrijsen, and A. Boeye. 1985. Stabilization by host cell components and Mg2+ of the neutralization epitopes of poliovirus. J. Gen. Virol. 66(Pt 2):303-307. [DOI] [PubMed] [Google Scholar]
- 35.See, D. M., and J. G. Tilles. 1995. Pathogenesis of virus-induced diabetes in mice. J. Infect. Dis. 171:1131-1138. [DOI] [PubMed] [Google Scholar]
- 36.Singh, S., V. T. Chow, K. P. Chan, A. E. Ling, and C. L. Poh. 2000. RT-PCR, nucleotide, amino acid and phylogenetic analyses of enterovirus type 71 strains from Asia. J. Virol. Methods 88:193-204. [DOI] [PubMed] [Google Scholar]
- 37.Stanway, G., T. Hovi, N. J. Knowles, and T. Hyypiä. 2002. Molecular and biological basis of picornavirus taxonomy, p. 17-21. In B. L. Semley and E. Wimmer (ed.), Molecular basis of picornavirus. ASM Press, Washington, D.C.
- 38.Stewart, T. A., B. Hultgren, X. Huang, S. Pitts-Meek, J. Hully, and N. J. MacLachlan. 1993. Induction of type I diabetes by interferon-alpha in transgenic mice. Science 260:1942-1946. [DOI] [PubMed] [Google Scholar]
- 39.Varela-Calvino, R., and M. Peakman. 2003. Enteroviruses and type 1 diabetes. Diabetes Metab. Res. Rev. 19:431-441. [DOI] [PubMed] [Google Scholar]
- 40.Wesche, B., E. Jaeckel, C. Trautwein, H. Wedemeyer, A. Falorni, H. Frank, M. A. von Zur, M. P. Manns, and G. Brabant. 2001. Induction of autoantibodies to the adrenal cortex and pancreatic islet cells by interferon alpha therapy for chronic hepatitis C. Gut 48:378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wetz, K., and K. O. Habermehl. 1979. Topographical studies on poliovirus capsid proteins by chemical modification and cross-linking with bifunctional reagents. J. Gen. Virol. 44:525-534. [DOI] [PubMed] [Google Scholar]
- 42.Wetz, K., and K. O. Habermehl. 1982. Specific cross-linking of capsid proteins to virus RNA by ultraviolet irradiation of poliovirus. J. Gen. Virol. 59:397-401. [DOI] [PubMed] [Google Scholar]
- 43.Yin, H., A. K. Berg, T. Tuvemo, and G. Frisk. 2002. Enterovirus RNA is found in peripheral blood mononuclear cells in a majority of type 1 diabetic children at onset. Diabetes 51:1964-1971. [DOI] [PubMed] [Google Scholar]
- 44.Ylipaasto, P., K. Klingel, A. M. Lindberg, T. Otonkoski, R. Kandolf, T. Hovi, and M. Roivainen. 2004. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47:225-239. [DOI] [PubMed] [Google Scholar]
- 45.Yoon, J. W., M. Austin, T. Onodera, and A. L. Notkins. 1979. Isolation of a virus from the pancreas of child with diabetic ketoacidosis. N. Engl. J. Med. 300:1173-1179. [DOI] [PubMed] [Google Scholar]
- 46.Yoon, J. W., T. Onodera, and A. L. Notkins. 1978. Virus-induced diabetes mellitus. XV. Beta cell damage and insulin-dependent hyperglycemia in mice infected with coxsackie virus B4. J. Exp. Med. 148:1068-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon, J. W. 1990. The role of viruses and environmental factors in the induction of diabetes. Curr. Top. Microbiol. Immunol. 164:95-123. [DOI] [PubMed] [Google Scholar]