Abstract
Pyrrolidine dithiocarbamate (PDTC) is an antiviral compound that was shown to inhibit the replication of human rhinoviruses (HRVs), poliovirus, and influenza virus. To elucidate the mechanism of PDTC, the effects on the individual steps of the infection cycle of HRV were investigated. PDTC did not interfere with receptor binding or internalization by receptor mediated endocytosis of HRV2 particles into HeLa cells. But we demonstrate that the processing of the viral polyprotein was prevented by PDTC treatment in HeLa cells infected with HRV2. Furthermore, PDTC inhibited the replication of the viral RNA, even when added four hours post infection. As PDTC is described as a metal ion binding agent, we investigated the effect of other metal chelators on the multiplication of HRV2. We show that EDTA, ο-phenanthroline, and bathocuproine disulfonic acid do not exhibit any antiviral properties. Surprisingly, these substances, coadministered with PDTC, abolished the antiviral effect of PDTC, suggesting that metal ions play a pivotal role in the inhibition of virus multiplication. These results suggest that PDTC inhibits the activity of the viral proteases in a metal ion dependent way.
Human rhinoviruses (HRVs) are the most frequent cause of the common cold and are implicated in more than 50% of upper respiratory tract infections (45). Although not life threatening, infection with HRV can prepare the ground for more serious diseases, such as acute exacerbation of asthma (20, 30) or otitis media (5). As there are more than 100 HRV serotypes, vaccine development is unfeasible. Apart from symptomatic medication no causative treatment for HRV infections is currently available. Therefore, analysis of functions of new antiviral substances is of great interest and might also shed light on novel aspects of virus-cell interactions.
HRVs belong to the genus Picornaviridae. These viruses have a single-stranded positive-sense RNA genome of approximately 7,400 nucleotides. The viral RNA encodes four capsid proteins (VP1 through VP4) and seven nonstructural proteins that are involved in viral RNA replication and polyprotein processing. The HRV serotypes can be classified into A and B groups based on sequence alignments (36). Alternatively, HRVs are divided into two groups according to their receptor specificity (1, 49). Members of the major group HRVs bind to the intercellular adhesion molecule 1 (ICAM-1) (15, 44), whereas serotypes of the minor group use various members of the low-density lipoprotein receptor family (18).
Upon receptor binding, the viral particle is internalized by receptor-mediated endocytosis. After acidification of the late endosome and subsequent uncoating, the RNA of minor group HRVs is released into the cytoplasm (32). Then the viral RNA is translated into a single large polyprotein from an internal ribosome entry site (IRES), which is located in the 5′ untranslated region of the HRV genome. The polyprotein is processed into the mature viral proteins through a sequence of cleavages performed by two virus-encoded proteinases. The 2A proteinase (2Apro) cleaves between the C terminus of VP1 and its own N terminus to separate the capsid protein region from the nonstructural protein precursor (reviewed in reference 17). Further processing is carried out by the 3C proteinase (3Cpro) or its precursor 3CDpro. During infection the viral proteinases also attack several cellular targets such as transcription and translation factors or cytoskeletal proteins. The cleavage of eukaryotic initiation factors 4GI (eIF4GI) and 4GII by 2Apro leads to a shutoff of cap-dependent host cell protein synthesis (10, 14).
Viral replication via a minus-strand intermediate and subsequent synthesis of new positive-strand RNA is mediated by the virus-encoded RNA-dependent RNA polymerase 3Dpol.
Finally, RNA and capsid proteins are assembled into mature infectious viral particles, which are then released by cell destruction. During late stages of virus infection morphological changes of cells can be observed, known as cytopathic effects.
Over the last two decades several steps in the life cycle of HRV have been targets for antiviral therapy (39), such as viral attachment, uncoating, polyprotein processing, and RNA polymerization. So far, only limited success has been achieved by the use of recombinant soluble receptor fragments to block virus binding (26, 47). Promising clinical trials have been performed with the capsid binding substance pleconaril (34) and the 3Cpro inhibitor ruprintrivir (AG7088) (31).
Recently, we described pyrrolidine dithiocarbamate (PDTC) as a potent inhibitor of HRV multiplication. PDTC is effective against all tested HRV serotypes in several cell lines analyzed without being toxic (11). In HeLa cells, the IC 50 against HRV 14 is 60 μM, toxic effects can be observed at concentrations higher than 1 mM. Surprisingly, the antiviral activity of PDTC is not restricted to members of the Picornaviridae such as HRV or poliovirus, since PDTC was also shown to inhibit multiplication of influenza virus, a member of the Orthomyxoviridae (48; A. Grassauer, personal communication). Therefore, the antiviral activity of this compound seems to involve more general processes unidentified so far.
PDTC is a low-molecular-weight thiol compound that can exert numerous effects in biological systems. It is a widely used inhibitor of the activation of nuclear factor kappa B (NF-κB) (37, 38), although there are conflicting reports how this inhibition is accomplished. On the one hand it has been attributed to the antioxidative property of PDTC (2), on the other hand to the inhibition of the ubiquitin ligase activity, which is a prerequisite for NF-κB activation (16). Furthermore, modulation of the cellular availability of heavy metal ions is proposed as another mechanism of the inhibition of NF-κB activation by PDTC (2, 38). PDTC binds various metal ions, leading to the formation of lipophilic dithiocarbamate-metal complexes, which transport metal ions such as zinc and copper from the extracellular medium into the cell (9, 21, 43).
To gain insight into the mechanism by which PDTC blocks the replication of HRV, we examined the effects on the various steps of the viral life cycle in detail. Receptor binding and internalization of HRV2 are not affected by PDTC. However, we demonstrate that PDTC blocks processing of the viral polyprotein. Addition of PDTC, even at 4 h postinfection, results in a total inhibition of replication of both positive- and negative-strand RNA.
As PDTC is a metal binding substance and several viral processes were described to be influenced by various metals, we investigated if the metal ion binding function of PDTC is the mechanistic basis of the antiviral function. Interestingly, the antiviral action of PDTC is abolished by the addition of metal chelating agents, such as EDTA, ο-phenanthroline, or bathocuproine disulfonic acid (BCS) and can be restored by the concomitant addition of zinc and copper ions. These results suggest that metal ions play an important role for the antiviral property of PDTC.
MATERIALS AND METHODS
Cells, media, and reagents.
HeLa cells (strain Ohio; European Collection of Cell Cultures, Salisbury, United Kingdom) were cultured in RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen), 2 mM l-glutamine (Dipro, Austria), 100 U/ml penicillin (Dipro), and 100 μg/ml streptomycin (Dipro).
Chemicals were obtained from Sigma. PDTC was purchased from Alexis Biochemicals.
Virus preparation.
HRV2 was obtained from the American Type Culture Collection and routinely grown in suspension cultures of HeLa cells (strain Ohio; Flow Laboratories, McLean; Virginia) as described previously (40). Virus titers in 50% tissue culture infectious doses (TCID50)/ml were determined according to Reed and Muench (33).
Infection of cells.
HeLa cells seeded into 6-well tissue culture dishes were infected at 70% confluence with HRV2 in RPMI medium supplemented with 2% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (infection medium). PDTC was added simultaneously with HRV. Routinely, 125 μM PDTC was used, which is nontoxic to HeLa cells (11). Cells were incubated at 37°C and 5% CO2 in a humidified incubator. The input virus was removed 2 h postinfection, and cells were washed once with an acidic HEPES buffer (10 mM HEPES, 140 mM KCl; pH 5.3) and twice with phosphate-buffered saline (PBS). For further incubation, infection medium and PDTC were added when indicated.
Immunofluorescence.
Immunofluorescence was essentially performed as described elsewhere (3). Briefly, cells were grown on sterile coverslips in six-well tissue culture dishes. Fixation was done with 4% paraformaldehyde-PBS at room temperature (RT) for 10 min. Cells were permeabilized by incubation in 0.5% Trition X-100 in PBS for 5 min. Blocking was done at 4°C using 1% goat serum-PBS. As primary antibody the mouse monoclonal antibody 8F5 (1:200 in PBS-1% bovine serum albumin) against the virus capsid protein VP2 (1:200) was employed (29, 40). As secondary antibody, Alexa488-labeled goat anti-mouse immunoglobulin G conjugates were used at a dilution of 1:800 (Molecular Probes). Finally, slides were mounted with DAKO fluorescent mounting medium and examined with a Leica TCS-NT confocal microscope using fluorescein isothiocyanate settings.
Measurement of IRES-dependent translation.
HeLa cells were infected with HRV2 using the multiplicity of infection indicated in the figure legends. The culture medium was removed 6 h postinfection, the cells were pretreated with 125 μM PDTC or 125 μM cycloheximide for 15 min and washed twice with PBS. The cells were labeled in 1 ml per well of methionine- and cysteine-free RPMI medium supplemented with 300 μCi of [35S]methionine/cysteine (radiochemical purity by high-pressure liquid chromatography: 70% [35S]methionine, 25% [35S]cysteine; Hartmann Analytic, Braunschweig, Germany) for 1 h. The labeling was performed in the presence of PDTC or cycloheximide. Subsequently, proteins were precipitated by trichloroacetic acid, and the incorporated radioactivity was measured in a scintillation counter (Packard).
Pulse-labeling and immunoprecipitation.
HeLa cells were infected with HRV2. The infection medium was replaced by 1 ml per well of methionine- and cysteine-free RPMI medium supplemented with 600 μCi of [35S]methionine/cysteine (radiochemical purity by high-pressure liquid chromatography: 70% [35S]methionine, 25% [35S]cysteine; Hartmann Analytic, Braunschweig, Germany) at 6 h or 6.5 h postinfection During a labeling period of 1 h 125 μM PDTC, or 200 μM zVAD.fmk (benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone) were added. Protein extracts were prepared by addition of reducing sample buffer (20% glycerol, 20% 2-mercaptoethanol, 8% sodium dodecyl sulfate [SDS], 0.04% bromophenol blue) and examined by SDS-polyacrylamide gel electrophoresis (PAGE). Alternatively, the cells were incubated in infection medium supplemented with PDTC.
For immunoprecipitation the cells were washed twice with PBS, collected by scraping in 1 ml of ice-cold PBS and centrifugation at 300 × g for 4 min. Cell pellets were frozen in liquid N2 and stored at −20°C. They were resuspended by vortexing in 500 μl of lysis buffer (1% [wt/vol] Triton X-100, 50 mM Tris-Cl, 300 mM NaCl, 5 mM EDTA, 0.02% Na-azide; pH 7.4) supplemented with 10 mM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/ml leupeptin. The samples were incubated on ice for 30 min and centrifuged at high speed in an Eppendorf centrifuge at 4°C for 15 min. Meanwhile, 1 μg of the monoclonal antibody 8F5 specific for VP2 was coupled to 30 μl 50% nProtein A-Sepharose bead slurry (Amersham Bioscience) in 0.5 ml ice-cold PBS and 0.01% Triton X-100. The beads were washed three times with lysis buffer; 500 μl cell lysate and the antibody-bound beads were combined and tumbled at 4°C for 2 h. The protein-bead conjugates were washed twice with ice-cold PBS. Proteins were resuspended in sample buffer, separated by SDS-PAGE and visualized by exposure of the dried gel to BioMax MR x-ray film (Kodak).
Northern blotting.
Total RNA was extracted from infected HeLa cells using RNeasy mini kit (QIAGEN). 10 μg of each sample were fractionated on a 1% formaldehyde denaturing gel at 60 V, and the RNA quality was checked by ethidium bromide staining to visualize the 18S and 28S rRNAs. RNA was transferred to a Hybond N nylon membrane (Amersham) by wet blotting using 10x SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer overnight and UV-cross-linked (120 kJoule) in a Stratalinker (Stratagene). For synthesis of strand-specific RNA probes, primer extension was performed in the presence of [γ-32P]dCTP (purity >98%; Hartmann Analytic, Braunschweig, Germany) using the cDNA of HRV2 as a template (41).
The primers for the positive and negative strands were 5′-TGGCCCTTGGAATATAGCTG-3′ (located in the 2C coding region) and 5′-CAGCTAGAGGGCTTGAATGG-3′ (located in the 3C coding region), respectively. The primer extension reaction was set up as follows: 0.2 mM dATP, 0.2 mM dTTP, 0.2 mM dGTP, 2 μM dCTP, 54 μCi of [γ-32P]dCTP, 25 pmol primer, 2.5 U Taq polymerase (Finnzymes, Espoo, Finnland), 5 ng of HRV2 template in reaction buffer in a total volume of 50 μl. Membranes were incubated in prehybridization solution consisting of 25 ml 20x SSPE buffer (3 M NaCl, 0.25 M NaH2PO4, 0.02 M EDTA; pH 7.4), 50 ml 100% formamide, 10 ml blocking reagent (Roche), 1 ml 10% SDS, and H2O to 100 ml at 42°C for 2 h. Labeled RNA probes were denatured at 95°C for 5 min and added to the prehybridizing solution. Membranes were incubated at 42°C overnight. After stringency washes (2x SSC buffer, 0.1% SDS), membranes were exposed to BioMax MR X-ray film (Kodak).
RESULTS
PDTC does not alter the infectivity of HRV particles.
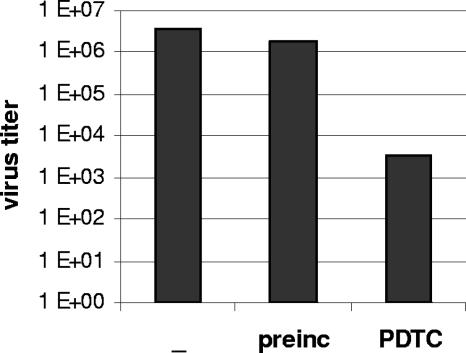
Over the past two decades, antirhinoviral agents inhibiting viral attachment and/or uncoating were developed, e.g., WIN compounds and pleconaril (4). To find out whether PDTC interacts with the viral capsid, HRV2 particles were incubated with PDTC at room temperature for 1 h. Afterwards, HeLa cells were incubated with PDTC-treated or untreated virus at 4°C for 1 h. Under these conditions, receptor binding but no further internalization can take place. Unbound virus was removed by extensive washing and infection was continued by cultivating cells in infection medium without PDTC at 37°C. The virus titer of the supernatant was determined by titration using TCID50 assays 24 h postinfection.
Infection of HeLa cells with PDTC-treated virus resulted in a titer of 1.8 × 106. A similar titer was obtained in control infections with untreated virus (Fig. 1). In contrast, continuous presence of PDTC during infection led to a significant reduction of the virus titer of three orders of magnitude (Fig. 1) (11). Thus, PDTC inhibits the multiplication of HRV2 when present during the infection, but it does not directly interact with or inactivate HRV2 particles.
FIG. 1.
PDTC does not inactivate or interact with the virus capsid. HRV2 virus was preincubated with 125 μM PDTC for 1 h. HeLa cells were infected with pretreated or untreated HRV2 (multiplicity of infection, 20) for 1 h at 4°C. Unbound virus was washed away and cells were incubated in infection medium supplemented with 125 μM PDTC where indicated at 37°C. Virus titers of the supernatants were determined by TCID50 assay after 24 h.
Receptor binding and internalization of HRV2 is not affected by PDTC.
HRV enters the cell by receptor-mediated endocytosis. Minor group serotypes like HRV2 bind to members of the low-density lipoprotein receptor family (18). To investigate the effect of PDTC on receptor binding and internalization of HRV2, the localization of the virus particles during infection was monitored by immunofluorescence staining of the virus capsid protein VP2.
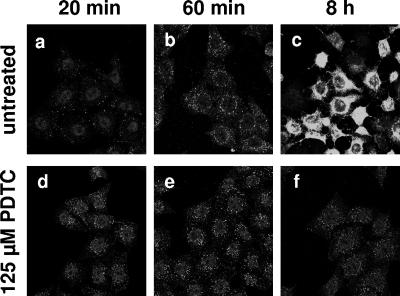
An infection period of 20 min resulted in a uniform distribution of the HRV2 particles in the cytoplasm of the HeLa cells (Fig. 2a); 60 min postinfection the virus accumulated in the perinuclear region, which is the characteristic localization of late endosomes. HeLa cells infected with HRV undergo morphological changes, like cell rounding and detachment from the substrate, described as cytopathic effect. As a complete life cycle of HRV2 in HeLa cells lasts approximately 10 h, the cytopathic effect became visible 8 h postinfection, and the cytoplasm of the infected cells were filled with progeny viral particles (Fig. 2c).
FIG. 2.
PDTC does not interfere with internalization of HRV2. HeLa cells grown on coverslips were infected with HRV2 (multiplicity of infection, 50) in the absence or presence of 125 μM PDTC. Cells were fixed using 4% paraformaldehyde 20 min, 60 min, and 8 h postinfection The virus capsid protein VP2 was localized by the monoclonal antibody 8F5 and visualized by immunofluorescence.
In the presence of PDTC the amount and distribution of HRV2 particles was similar to that of nontreated cells at 20 and 60 min postinfection (Fig. 2). Thus, the initial steps of virus infection such as receptor binding and internalization are not affected by PDTC.
However, treatment with PDTC completely inhibited the multiplication of viral particles at 8 h postinfection, and later on a loss of intracellular viral particles was observed (Fig. 2 and data not shown). In addition, PDTC prevented the formation of a visible cytopathic effect.
PDTC has no major effect on IRES-dependent translation.
During rhinoviral infection the cellular cap-dependent translation is inhibited by the host cell shut-off. This is achieved by cleavage of the cellular translation factors eIF4GI and eIF4GII by the viral protease 2Apro (10, 14). However, translation of the HRV RNA genome is unaffected, as it is initiated at an internal ribosomal entry site (IRES) via a cap-independent mechanism. In the presence of PDTC no significant synthesis of viral proteins can be detected (see Fig. 2A in reference 11). Among other reasons, this can be due to decreased viral translation or impaired polyprotein processing.
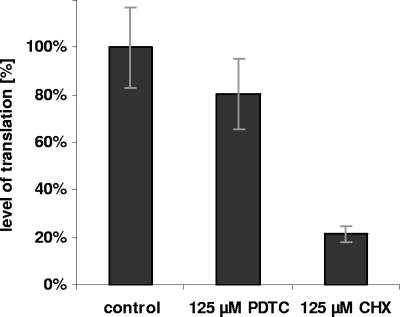
To investigate the effect of PDTC on IRES-dependent translation the level of protein synthesis was determined after onset of host cell shut-off in cells infected with HRV2 by incorporation of [35S]methionine/cysteine for 1 h followed by trichloroacetic acid precipitation of the proteins and scintillation counting. During the labeling period cells were treated with PDTC or cycloheximide, a strong inhibitor of both cap- and IRES-dependent translation. PDTC slightly reduced the level of IRES-dependent translation (about 80% compared to untreated cells), whereas cycloheximide significantly decreased the level of translation to about 20% (Fig. 3). This weak effect of PDTC on IRES-dependent translation is unlikely to be responsible for the absence of newly synthesized capsid proteins.
FIG. 3.
PDTC has no major effect on IRES dependent translation. At 6 h p.i. the level of IRES-dependent protein synthesis in HeLa cells, infected with HRV2 (multiplicity of infection, 100), was determined by metabolic labeling for 1 h; 125 μM PDTC or 125 μM cycloheximide (CHX) was added 15 min before the labeling and present during the period of the pulse. The amount of incorporated [35S]methionine/cysteine was determined by protein precipitation and measurement by scintillation counting. Error bars indicate the standard deviation from experiments carried out in triplicate.
PDTC interferes with polyprotein processing of HRV2.
Upon infection the HRV genome is translated into a large polyprotein precursor, which is subsequently cleaved by a sequence of proteolytic cleavages. To investigate the effect of PDTC on the processing of the viral polyprotein we analyzed the pattern of virus protein precursors in the presence and absence of PDTC by pulse-labeling. As a positive control we employed the methylated form of the caspase inhibitor zVAD.fmk, which was recently described to inhibit HRV 2Apro and possibly 3Cpro (8).
HeLa cells were infected with HRV2. After host cell shutoff newly synthesized proteins were labeled while administering PDTC or zVAD.fmk. Subsequently, the protein pattern was analyzed by SDS-PAGE and autoradiography.
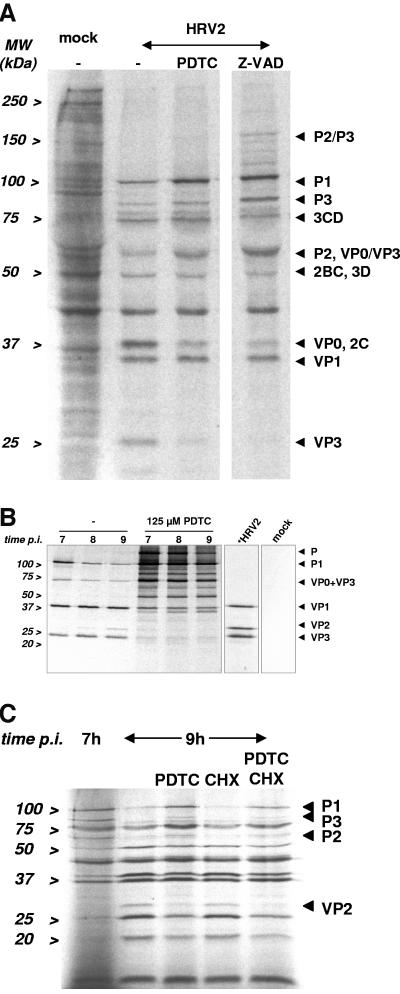
Infection with HRV2 resulted in complete inhibition of host cell protein synthesis at 6.5 h postinfection, but the viral proteins were clearly expressed (Fig. 4A). Addition of PDTC led to an accumulation of the uncleaved protein precursors P1, P2, and P3. A reduction of processed proteins, as VP0, 2C, and VP3 was observed. Incubation with zVAD.fmk caused a similar pattern as obtained in the presence of PDTC. This strongly suggests that PDTC interferes with polyprotein processing by interfering with protease activity.
FIG. 4.
PDTC interferes with the polyprotein processing. (A) Viral proteins in HeLa cells, infected with HRV2 (multiplicity of infection 100), were labeled by incorporation of [35S]methionine/cysteine for 1 h starting 6 h 30 min p.i. in the presence of 125 μM PDTC or 200 μM zVAD.fmk. Cell lysates were prepared and analyzed by SDS-PAGE and autoradiography. (B) HeLa cells, infected with HRV2 (multiplicity of infection, 50), were preincubated with PDTC for 30 min and subsequently incubated in medium supplemented with [35S]methionine/cysteine and 125 μM PDTC to label viral proteins for 60 min starting 6 h p.i. Then the cells were transferred to infection medium containing 125 μM PDTC. Cell lysates were prepared every hour. VP2 or VP2 comprising precursor proteins were isolated by immunoprecipitation employing the specific antibody 8F5 and analyzed by SDS-PAGE and autoradiography. Radiolabeled HRV2 was separated in parallel to identify viral capsid proteins (*HRV2). Mock designates immunoprecipitation from uninfected but labeled cells. (C) Viral proteins were labeled in virus-infected HeLa cells, infected with HRV2 (multiplicity of infection 50), for 15 min starting 6 h 45 min p.i. Subsequently the cells were treated with 125 μM PDTC or 125 μM cycloheximide for 2 h. Cell lysates were prepared and proteins were analyzed by SDS-PAGE and autoradiography.
The effects on polyprotein processing were investigated in more detail by analyzing the effects on P1 processing. To this end, a pulse-chase labeling experiment followed by immunoprecipitation with a VP2 specific antibody was performed. Newly synthesized proteins of cells infected with HRV2 were labeled for 60 min starting at 6 h postinfection after 30 min of incubation with 125 μM PDTC. Then the cells were incubated in infection medium supplemented with PDTC and cell lysates were prepared from 7 to 9 h postinfection. VP2 and VP2-containing intermediate precursor proteins were immunoprecipitated with the monoclonal antibody 8F5 (40), and analyzed by SDS-PAGE and autoradiography. To clearly identify VP2, bands were compared to radiolabeled HRV 2 virus separated simultaneously (lane *HRV2) (28).
In untreated cells the processing of the P1 precursor protein was clearly visible as a reduction of the amount of the corresponding polyprotein band. At the same time a smaller band comprising VP2 appeared (Fig. 4B). In the presence of PDTC the processing of the P1 protein was blocked and no cleavage products could be detected.
Furthermore, we were interested whether the inhibitory effect of PDTC on the processing of the polyprotein does depend on de novo protein synthesis. To this end, proteins of infected cells were labeled for 15 min starting at 6 h 45 min postinfection. Afterwards, the cells were treated with PDTC and/or cycloheximide for 2 h. Again, virus proteins were analyzed by SDS-PAGE and autoradiography.
In untreated cells the large precursor molecules were processed into the various viral proteins (Fig. 4C). Cycloheximide did not interfere with the viral polyprotein processing. However, P1 protein processing was impaired in the presence of PDTC, resulting in P1 accumulation. When PDTC and cycloheximide were coadministered, the pattern of the virus proteins was similar to that of PDTC-treated cells alone. This indicates that the inhibitory effect of PDTC on polyprotein processing does not depend on de novo protein synthesis.
PDTC decreases positive- and negative-strand RNA synthesis of HRV2.
To determine whether PDTC affects the synthesis of the positive or negative strand RNA of HRV, the amount of both RNA species was quantified by Northern blot analysis with strand-specific probes.
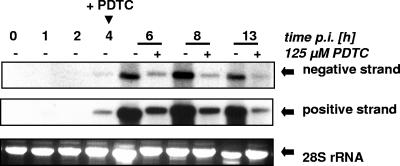
In untreated cells an increase in both positive- and negative-strand RNA levels was detected starting 4 h postinfection, which resulted in a peak RNA level at 8 h postinfection (Fig. 5). At 13 h postinfection the level of viral RNA decreased again due to the cytopathic effect. In contrast, addition of PDTC at the onset of viral RNA synthesis 4 h postinfection gave rise to an immediate and strong inhibition of both positive and negative strand RNA replication (Fig. 5). Viral RNA synthesis is also reduced to the same extent when PDTC is administered at the time of infection (data not shown).
FIG. 5.
PDTC decreases the replication of positive and negative strand RNA of HRV2. HeLa cells were infected with HRV2 (multiplicity of infection, 20). At the start of viral RNA replication at 4 h p.i. PDTC was added where indicated (+). Total RNA was isolated 1, 2, 4, 6, 8, and 13 h p.i. The level of positive- and negative-strand HRV2 RNA was quantitated by Northern blot analysis using strand-specific probes generated by primer extension in the presence of [γ-32P]dCTP and visualized by autoradiography. Analysis of the 28S rRNA ensured an equal loading.
Thus, PDTC strongly inhibits viral RNA synthesis of both positive and negative strands. A repression of replication of virus RNA by PDTC was also observed for the major group virus HRV14 (data not shown).
Antiviral effect of PDTC is abolished by metal ion chelating agents.
Previously, Erl et al. showed that PDTC can increase the intracellular concentration of various metal ions (9). As the activity of several viral enzymes was described to be influenced by metal ions, we employed metal-chelating substances. In this context it has to be noted that several biological effects of PDTC, such as inhibition of the activation of NF-κB or induction of apoptosis in endothelial cells, are abolished in the presence of metal ion chelators (21, 22).
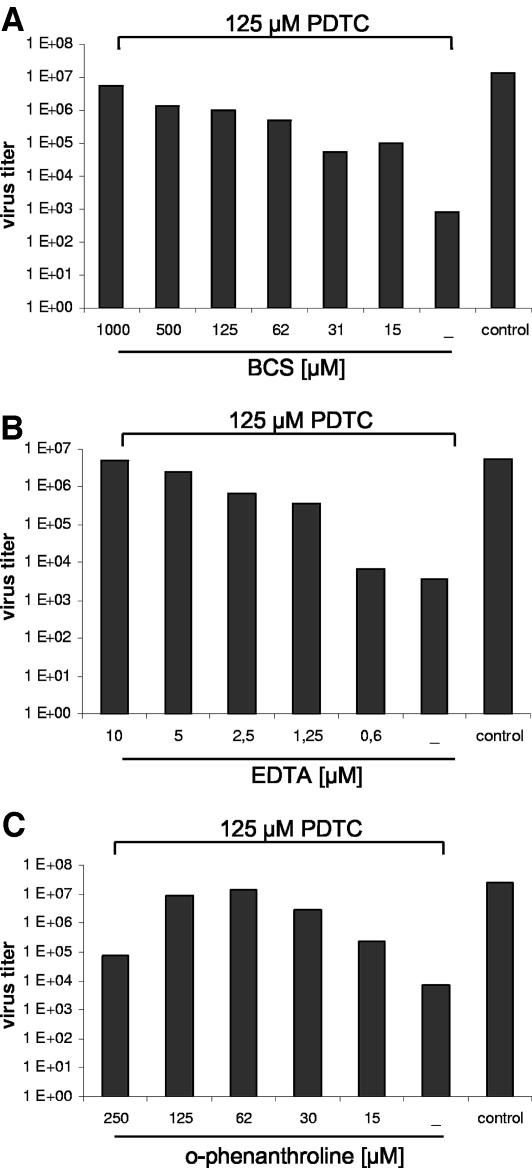
To investigate whether the antiviral effect of PDTC was dependent on its ability to bind metal ions, other metal ion chelators such as ο-phenanthroline, BCS, or free-base EDTA were added during virus infection. The amount of progeny virus in the supernatant was determined by TCID50 assays 24 h postinfection. The multiplication of HRV2 was not affected by these metal chelators at the concentrations used, which is in agreement with published data, where 1,000-fold higher concentrations of EDTA were shown to interfere with receptor binding (data not shown) (25). However, coadministration of metal chelators and PDTC abolished the antiviral effect of PDTC. As shown in Fig. 6 the virus titer of HRV2 was reduced by PDTC alone about three orders of magnitude compared to controls. When BCS was added simultaneously the antiviral effect was abolished in a dose-dependent manner, leading to normal virus replication as in untreated cells at 1,000 μM BCS (Fig. 6A). Similar effects were observed after coadministration of EDTA or ο-phenanthroline (Fig. 6B and C). EDTA was much more potent in terms of inhibiting the effect of PDTC, as 10 μM EDTA was already sufficient compared to 62 μM ο-phenanthroline. Interestingly, both the cell-permeable agent ο-phenanthroline and the non-cell-permeable substances EDTA and BCS were effective.
FIG. 6.
Antiviral effect of PDTC is abolished by metal chelators. HeLa cells, infected with HRV2 (multiplicity of infection 20), were treated with the metal chelators BCS (A), EDTA (B), or ο-phenanthroline (C). 125 μM PDTC were added simultaneously. The virus titers in the supernatant of the infected cells were determined by TCID50 assay 24 h p.i.
Copper and zinc ions are involved in the antiviral effect of PDTC.
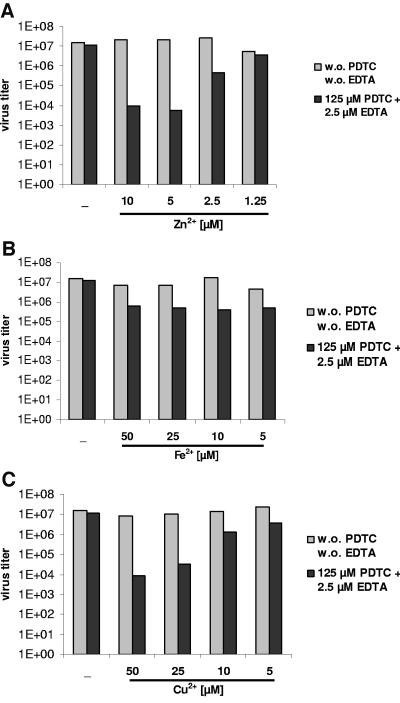
Several divalent metal ions, including copper and zinc, have been shown to contribute to some of the biological effects of PDTC, such as the inhibition of NF-κB and induction of cell apoptosis (6, 9, 21, 22). We analyzed whether zinc, iron, or copper ions could restore the antiviral effect of PDTC when it is suppressed by EDTA (as shown in Fig. 6). Addition of at least 2.5 μM Zn2+ ions or 25 μM Cu2+ ions reestablished the antiviral activity of PDTC in the presence of EDTA (Fig. 7A and C). Iron ions failed to show an effect (Fig. 7B). None of these metal ions alone showed any antiviral activity in the concentrations used (Fig. 7) and no cytotoxicity was observed (data not shown). These results suggest that Zn2+ or Cu2+ ions are an important factor for the antiviral effect of PDTC.
FIG. 7.
Zinc and copper ions are involved in the antiviral effect of PDTC. HeLa cells, infected with HRV2 (multiplicity of infection, 20), were treated with 125 μM PDTC and 2.5 μM EDTA (dark bars). Simultaneously, Zn2+ (A), Fe2+ (B), or Cu2+ (C) ions were added. The virus titers in the supernatant of the infected cells were determined by TCID50 assay at 24 h p.i. Light bars represent cells treated with the respective ions but without PDTC and without EDTA.
DISCUSSION
Currently, there is no cure available for the common cold apart from symptomatic treatment. PDTC was shown to be a potent inhibitor of the multiplication of HRV and poliovirus, both members of the Picornaviridae (11). Furthermore, PDTC turned out to prevent the multiplication of influenza virus, belonging to the Orthomyxoviridae (48). This indicates that the antiviral effect of PDTC is not restricted to one distinct virus family, and thus its mode of action might rely on more general mechanisms. The precise molecular basis of the inhibitory function has not yet been determined.
To elucidate the action of PDTC on HRV multiplication in more detail, we investigated the effect of PDTC on single steps of the infection cycle of HRV2. We demonstrated that PDTC did not affect the early steps of the viral infection cycle, such as receptor binding, virus entry, and internalization. Thus, PDTC does not interact with the capsid of HRV2, as preexposure of the virus to PDTC did not alter the infectivity of HRV particles (Fig. 1). Moreover, PDTC does not interfere with virus entry, since binding to the cellular receptor and receptor-mediated endocytosis were unaffected by PDTC (Fig. 2). Although PDTC slightly decreased IRES-dependent translation, this effect is too weak to be exclusively responsible for the antiviral property of PDTC (Fig. 3).
However, our data indicate that PDTC impedes the processing of the HRV polyprotein (Fig. 4). As PDTC treatment abolished the appearance of processed VP2 an inhibition of the viral proteases 2Apro and/or 3Cpro is proposed (Fig. 4B). As a consequence of the impaired polyprotein processing, the repressed replication of both positive- and negative-strand RNA in the presence of PDTC might be due to alterations in the processing and production of the nonstructural proteins (Fig. 5).
In account of their important roles in the processing of the viral polyprotein, blocking of the viral proteases 2Apro and 3Cpro is regarded as a promising approach to antiviral therapy (27, 35, 45). Recently, we demonstrated that inhibition of the viral protease 2Apro with the methylated form of the caspase inhibitor zVAD.fmk resulted in a significant decrease of multiplication of HRV (8). The most promising HRV protease inhibitor currently available is the 3Cpro inhibitor ruprintrivir, which shows potent activity for a broad spectrum of rhinovirus serotypes (31).
Previously, we have described that PDTC does not inhibit the activity of the HRV2 2Apro in vitro, as was shown by using purified protease and cleavage of synthetic peptide substrates (11). Furthermore, PDTC failed to inhibit 2Apro-mediated cleavage of eIF4GI and cytokeratin 8 in cell extracts. No inhibition of eIF4GI cleavage was found in rabbit reticulocyte lysates during translation of mRNAs encoding HRV2 2Apro (data not shown). Nevertheless, in a cellular context PDTC might change the cytoplasmic environment, thereby interfering with 2Apro and/or 3Cpro function.
Dithiocarbamates can chelate various metal ions, leading to the formation of lipophilic dithiocarbamate-metal complexes (43). Several studies have reported that dithiocarbamates can promote cell entry of metals, e.g., diethyldithiocarbamate and PDTC increased the intracellular copper levels in thymocytes (9). Kim et al. demonstrated that PDTC gave rise to uptake of zinc ions (22). These data suggest that PDTC might alter the tightly regulated balance of ions in cells, resulting in alterations of enzyme functions. In some reports it was described that various biological effects of PDTC can be prevented by the addition of metal chelators (21, 22). In this manuscript we demonstrate that the antiviral effect of PDTC was neutralized by addition of free-base EDTA, ο-phenanthroline, or BCS. Thus, it seems that the availability of ions is crucial for the antiviral action of PDTC (Fig. 6).
Among metal ions, zinc has been known to interfere with HRV replication for many years. As a mechanism, the inhibition of the posttranslational cleavage of the precursor protein was suggested (23, 24). Cordingley et al. described that 3Cpro is inhibited by zinc ions in an in vitro protease assay (7). In contrast, there is no effect of zinc ions on the 2Apro self-processing or cleavage of eIF4GI determined by in vitro translation of HRV2 mRNA in rabbit reticulocyte lysate (13).
Comparison of the antiviral activities of various zinc salts showed that the therapeutic index is very low (12). There are conflicting results on the effectiveness of zinc gluconate or zinc acetate in the treatment of the common cold in clinical trials (42, 46).
However, it cannot be excluded that PDTC has an impact on viral enzymes other than proteases. Zinc ions are described to inhibit the activity of the 3Dpol of HRV16 in vitro (19). Thus, by increasing the intracellular zinc ion concentration PDTC might directly inhibit polymerase function and replication.
In our report, we propose a model for the antiviral effect of PDTC on HRV. We demonstrated that PDTC interferes with polyprotein processing. Moreover, the level of virus RNA was decreased in PDTC-treated cells. However, our results exclude an action of PDTC on the early steps of virus infection, such as receptor binding or internalization. Since the antiviral effect of PDTC is abolished in the presence of several metal ion-chelating compounds, the antiviral activity of PDTC seems to depend on the availability of metal ions. Specifically, zinc or copper ions seem to play a crucial role, as they restore the antiviral property of PDTC in the presence of EDTA.
However, to date it is unclear if PDTC works as a carrier for the ion or if binding of an ion is necessary to allow PDTC to enter the cell. Elucidation of the antiviral mechanism of PDTC will be the subject of further research and will provide more information about the environmental requirements for the rhinovirus life cycle.
Acknowledgments
This paper is dedicated to the memory of our head and mentor Ernst Kuechler, who was substantially involved in the research presented in this article. Sadly, Ernst Kuechler passed away in March 2005.
We acknowledge support by grant P16642-B11 from the Austrian Science Foundation to E.K. and J. S. F.V.K is supported by grants from the Netherlands Organization for Scientific Research (NWO-VIDI-917.46.305) and the M. W. Beijerink Virology Fund (from the Royal Netherlands Academy of Sciences).
We thank Barbara Klein for technical help.
REFERENCES
- 1.Abraham, G., and R. J. Colonno. 1984. Many rhinovirus serotypes share the same cellular receptor. J. Virol. 51:340-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessho, R., K. Matsubara, M. Kubota, K. Kuwakado, H. Hirota, Y. Wakazono, Y. W. Lin, A. Okuda, M. Kawai, R. Nishikomori, and et al. 1994. Pyrrolidine dithiocarbamate, a potent inhibitor of nuclear factor kappa B (NF-kappa B) activation, prevents apoptosis in human promyelocytic leukemia HL-60 cells and thymocytes. Biochem. Pharmacol. 48:1883-1889. [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino, J. S. (ed.). 1998. Current protocols in cell biology. John Wiley & Sons Inc., New York, NY.
- 4.Carrasco, L. 1994. Picornavirus inhibitors. Pharmacol. Ther. 64:215-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chonmaitree, T., V. M. Howie, and A. L. Truant. 1986. Presence of respiratory viruses in middle ear fluids and nasal wash specimens from children with acute otitis media. Pediatrics 77:698-702. [PubMed] [Google Scholar]
- 6.Chung, K. C., J. H. Park, C. H. Kim, H. W. Lee, N. Sato, Y. Uchiyama, and Y. S. Ahn. 2000. Novel biphasic effect of pyrrolidine dithiocarbamate on neuronal cell viability is mediated by the differential regulation of intracellular zinc and copper ion levels, NF-kappaB, and MAP kinases. J. Neurosci Res. 59:117-125. [PubMed] [Google Scholar]
- 7.Cordingley, M. G., R. B. Register, P. L. Callahan, V. M. Garsky, and R. J. Colonno. 1989. Cleavage of small peptides in vitro by human rhinovirus 14 3C protease expressed in Escherichia coli. J. Virol. 63:5037-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deszcz, L., J. Seipelt, E. Vassilieva, A. Roetzer, and E. Kuechler. 2004. Antiviral activity of caspase inhibitors: effect on picornaviral 2A proteinase. FEBS Lett. 560:51-55. [DOI] [PubMed] [Google Scholar]
- 9.Erl, W., C. Weber, and G. K. Hansson. 2000. Pyrrolidine dithiocarbamate-induced apoptosis depends on cell type, density, and the presence of Cu2+ and Zn2+. Am. J. Physiol. Cell Physiol. 278:C1116-25. [DOI] [PubMed] [Google Scholar]
- 10.Etchison, D., and S. Fout. 1985. Hum. rhinovirus 14 infection of HeLa cells results in the proteolytic cleavage of the p220 cap-binding complex subunit and inactivates globin mRNA translation in vitro. J. Virol. 54:634-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudernak, E., J. Seipelt, A. Triendl, A. Grassauer, and E. Kuechler. 2002. Antiviral effects of pyrrolidine dithiocarbamate on human rhinoviruses. J. Virol. 76:6004-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geist, F. C., J. A. Bateman, and F. G. Hayden. 1987. In vitro activity of zinc salts against human rhinoviruses. Antimicrob Agents Chemother. 31:622-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser, W., A. Triendl, and T. Skern. 2003. The processing of eIF4GI by human rhinovirus type 2 2Apro: relationship to self-cleavage and role of zinc. J. Virol. 77:5021-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gradi, A., H. Imataka, Y. V. Svitkin, E. Rom, B. Raught, S. Morino, and N. Sonenberg. 1998. A novel functional human eukaryotic translation initiation factor 4G. Mol. Cell. Biol. 18:334-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. McClelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa, M., H. Miyashita, I. Sakamoto, M. Kitagawa, H. Tanaka, H. Yasuda, M. Karin, and K. Kikugawa. 2003. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 22:3356-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellen, C. U., H. G. Krausslich, and E. Wimmer. 1989. Proteolytic processing of polyproteins in the replication of RNA viruses. Biochemistry 28:9881-9890. [DOI] [PubMed] [Google Scholar]
- 18.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blass. 1994. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung, M., C. S. Gibbs, and M. Tsiang. 2002. Biochemical characterization of rhinovirus RNA-dependent RNA polymerase. Antiviral Res. 56:99-114. [DOI] [PubMed] [Google Scholar]
- 20.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, F. Lampe, L. Josephs, P. Symington, S. O'Toole, S. H. Myint, D. A. Tyrrell, et al. 1995. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Br. Med. J. 310:1225-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, C. H., J. H. Kim, C. Y. Hsu, and Y. S. Ahn. 1999. Zinc is required in pyrrolidine dithiocarbamate inhibition of NF-kappaB activation. FEBS Lett. 449:28-32. [DOI] [PubMed] [Google Scholar]
- 22.Kim, C. H., J. H. Kim, J. Xu, C. Y. Hsu, and Y. S. Ahn. 1999. Pyrrolidine dithiocarbamate induces bovine cerebral endothelial cell death by increasing the intracellular zinc level. J. Neurochem. 72:1586-1592. [DOI] [PubMed] [Google Scholar]
- 23.Korant, B. D., and B. E. Butterworth. 1976. Inhibition by zinc of rhinovirus protein cleavage: interaction of zinc with capsid polypeptides. J. Virol. 18:298-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korant, B. D., J. C. Kauer, and B. E. Butterworth. 1974. Zinc ions inhibit replication of rhinoviruses. Nature 248:588-590. [DOI] [PubMed] [Google Scholar]
- 25.Lonberg-Holm, K., and N. M. Whiteley. 1976. Physical and metabolic requirements for early interaction of poliovirus and human rhinovirus with HeLa cells. J. Virol. 19:857-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marlovits, T. C., T. Zechmeister, M. Gruenberger, B. Ronacher, H. Schwihla, and D. Blaas. 1998. Recombinant soluble low density lipoprotein receptor fragment inhibits minor group rhinovirus infection in vitro. FASEB J. 12:695-703. [DOI] [PubMed] [Google Scholar]
- 27.McKinlay, M. A. 2001. Recent advances in the treatment of rhinovirus infections. Curr. Opin. Pharmacol. 1:477-481. [DOI] [PubMed] [Google Scholar]
- 28.Mischak, H., C. Neubauer, B. Berger, E. Kuechler, and D. Blaas. 1988. Detection of the human rhinovirus minor group receptor on renaturing Western blots. J. Gen. Virol. 69:2653-2656. [DOI] [PubMed] [Google Scholar]
- 29.Neubauer, C., L. Frasel, E. Kuechler, and D. Blaas. 1987. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology 158:255-258. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson, K. G., J. Kent, and D. C. Ireland. 1993. Respiratory viruses and exacerbations of asthma in adults. Br. Med. J. 307:982-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patick, A. K., S. L. Binford, M. A. Brothers, R. L. Jackson, C. E. Ford, M. D. Diem, F. Maldonado, P. S. Dragovich, R. Zhou, T. J. Prins, S. A. Fuhrman, J. W. Meador, L. S. Zalman, D. A. Matthews, and S. T. Worland. 1999. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother. 43:2444-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed, L. J. a. M., H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 34.Romero, J. R. 2001. Pleconaril: a novel antipicornaviral drug. Expert Opin. Investig. Drugs 10:369-379. [DOI] [PubMed] [Google Scholar]
- 35.Rotbart, H. A. 2002. Treatment of picornavirus infections. Antiviral Res. 53:83-98. [DOI] [PubMed] [Google Scholar]
- 36.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83:333-340. [DOI] [PubMed] [Google Scholar]
- 37.Schreck, R., K. Albermann, and P. A. Baeuerle. 1992. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic. Res. Commun. 17:221-237. [DOI] [PubMed] [Google Scholar]
- 38.Schreck, R., B. Meier, D. N. Mannel, W. Droge, and P. A. Baeuerle. 1992. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 175:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shih, S. R., S. J. Chen, G. H. Hakimelahi, H. J. Liu, C. T. Tseng, and K. S. Shia. 2004. Selective human enterovirus and rhinovirus inhibitors: An overview of capsid-binding and protease-inhibiting molecules. Med. Res. Rev. 24:449-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skern, T., C. Neubauer, L. Frasel, P. Grundler, W. Sommergruber, M. Zorn, E. Kuechler, and D. Blaas. 1987. A neutralizing epitope on human rhinovirus type 2 includes amino acid residues between 153 and 164 of virus capsid protein VP2. J. Gen. Virol. 68:315-323. [DOI] [PubMed] [Google Scholar]
- 41.Skern, T., W. Sommergruber, D. Blaas, P. Gruendler, F. Fraundorfer, C. Pieler, I. Fogy, and E. Kuechler. 1985. Hum. rhinovirus 2: complete nucleotide sequence and proteolytic processing signals in the capsid protein region. Nucleic Acids Res. 13:2111-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson, K. H., and C. Orvig. 2003. Boon and bane of metal ions in medicine. Science 300:936-939. [DOI] [PubMed] [Google Scholar]
- 43.Thorn, G. D., and R. A. Ludwig. 1962. The dithiocarbamates and related compounds. Elsevier Publishing, Amsterdam, The Netherlands.
- 44.Tomassini, J. E., D. Graham, C. M. DeWitt, D. W. Lineberger, J. A. Rodkey, and R. J. Colonno. 1989. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 86:4907-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner, R. B. 2001. The treatment of rhinovirus infections: progress and potential. Antiviral Res. 49:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner, R. B., and W. E. Cetnarowski. 2000. Effect of treatment with zinc gluconate or zinc acetate on experimental and natural colds. Clin. Infect. Dis. 31:1202-1208. [DOI] [PubMed] [Google Scholar]
- 47.Turner, R. B., M. T. Wecker, G. Pohl, T. J. Witek, E. McNally, R. St George, B. Winther, and F. G. Hayden. 1999. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA 281:1797-1804. [DOI] [PubMed] [Google Scholar]
- 48.Uchide, N., K. Ohyama, T. Bessho, B. Yuan, and T. Yamakawa. 2002. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 56:207-217. [DOI] [PubMed] [Google Scholar]
- 49.Uncapher, C. R., C. M. DeWitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]