Abstract
Human prion diseases, such as Creutzfeldt-Jakob disease (CJD), are neurodegenerative and fatal. Sporadic CJD (sCJD) can be transmitted between humans through medical procedures involving highly infected organs, such as the central nervous system. However, in variant CJD (vCJD), which is due to human contamination with the bovine spongiform encephalopathy (BSE) agent, lymphoreticular tissue also harbors the transmissible spongiform encephalopathy-associated prion protein (PrPTSE), which poses a particularly acute risk for iatrogenic transmission. Two blood transfusion-related cases are already documented. In addition, the recent observation of PrPTSE in spleen and muscle in sCJD raised the possibility that peripheral PrPTSE is not limited to vCJD cases. We aimed to clarify the peripheral pathogenesis of human TSEs by using a nonhuman primate model which mimics human diseases. A highly sensitive enzyme-linked immunosorbent assay was adapted to the detection of extraneural PrPTSE. We show that affected organs can be divided into two groups. The first is peripheral organs accumulating large amounts of PrPTSE, which represent a high risk of iatrogenic transmission. This category comprises only lymphoreticular organs in the vCJD/BSE model. The second is organs with small amounts of PrPTSE associated with nervous structures. These are the muscles, adrenal glands, and enteric nervous system in the sporadic, iatrogenic, and variant CJD models. In contrast to the first set of organs, this low level of tissue contamination is not strain restricted and seems to be linked to secondary centrifugal spread of the agent through nerves. It might represent a risk for iatrogenic transmission, formerly underestimated despite previous reports of low rates of transmission from peripheral organs of humans to nonhuman primates (5, 10). This study provides an additional experimental basis for the classification of human organs into different risk categories and a rational re-evaluation of current risk management measures.
Prion diseases are fatal disorders of both humans and animals and can be acquired, spontaneous, or genetic in origin (28). All forms of Creutzfeldt-Jakob disease (CJD) are infectious (5, 12), and transmission of sporadic CJD (sCJD) has occurred through cross-contamination from neurosurgical devices or through therapeutic usage of human central nervous system (CNS) tissues, causing over 267 iatrogenic CJD (iCJD) cases worldwide (6). With the appearance of variant CJD (vCJD), a new risk of iatrogenic transmission emerged, since the misfolded form of the prion protein which accumulates in transmissible spongiform encephalopathies (PrPTSE) was found in peripheral organs, including lymphoid tissues and the rectum (32). Therefore, leukoreduction of blood units was implemented in 1999, and precautions have been recommended for gastrointestinal endoscopies (4). However, interhuman transmission of vCJD has probably already happened twice through blood transfusion from persons with preclinical vCJD (25, 27). This demonstrates a risk of interhuman disease transmission not only through high-titer tissue like the CNS but also through peripheral compartments. In addition, a recent study reported the presence of PrPTSE in peripheral organs of some sCJD patients (13). PrPTSE was found in spleen and muscle tissue. This report casts doubts on the consensus that vCJD is the only form of CJD with peripheral PrPTSE and raises questions about the determinants of tissue distribution in the various forms of CJD in humans. Therefore, a precise study on the tissue distribution of PrPTSE in CJDs of different origins under controlled conditions (especially with regard to the host genetics and the route of infection) is urgently needed to clarify the pathogenesis of these TSEs affecting humans. We infected nonhuman primates (Macaca fascicularis) with the agents of bovine spongiform encephalopathy (BSE), sCJD, iCJD (linked to growth hormone administration), and vCJD. Previous studies have shown that this animal model, which is closely related to humans, faithfully reproduces the main characteristics of vCJD, i.e., the clinical presentation, the typical neuropathology, and the possibility of oral transmission (23, 24). sCJD and iCJD can be readily distinguished from vCJD and from each other in these animals on the basis of the clinical signs and the neuropathology (unpublished data). Hence, we used this model to analyze systematically the involvement of peripheral organs of the different forms of CJD. First, we developed a highly sensitive PrPTSE purification procedure specifically adapted to each peripheral organ, coupled with an enzyme-linked immunosorbent assay (ELISA) detection method. This allowed us to quantify extraneural PrPTSE, which was expressed as a percentage of the amount found in brain. These results should be taken into consideration for human risk assessment studies supporting public health policies, as they are key in devising proportionate measures to avoid further iatrogenic transmission of human CJD.
MATERIALS AND METHODS
Primate model.
Cynomolgus macaques (Macaca fascicularis) were kept and handled in accordance with national guidelines. They were injected intracerebrally with 400 μl and by the intratonsillar route with 80 μl of a 10% brain homogenate obtained from one French and three British vCJD patients (each patient paired to a separate macaque), from one French sCJD patient (M/M homozygous at codon 129 of the PrP gene, harboring a type 2 PrPTSE in the binary classification), or from one French iCJD patient (M/M homozygous at codon 129 of the PrP gene, harboring a type 1 PrPTSE) following treatment with cadaveric human growth hormone. One primate was inoculated by the intratonsillar route with 80 μl of a 10% British vCJD brain homogenate alone. One animal was infected with a single dose of 5 g of cattle BSE brain by the oral route. Animals were euthanized for humane reasons when they became recumbent. Organs were dissected free of connective tissue and either snap-frozen in liquid nitrogen and stored at −80°C for biochemical analyses or fixed in Carnoy's fluid for histological examination. Cynomolgus macaques are all Met/Met homozygous at codon 129 of the PrP gene.
Purification of PrPTSE.
Organs were cut into 100-mg pieces and transferred into a Bio-Rad Ribolyzer tube containing 900 μl of dissociation buffer containing 25 mM HEPES (pH 7.2), 0.3 M sucrose, 53.6 μg Liberase Blendzyme 2 (Roche), and, for certain tissues, trypsin inhibitor (Sigma) from soybean (spleen, tonsils, lymph nodes, and kidney, 0.5% [wt/vol]; adrenal glands, 0.25% [wt/vol]; intestine, 0.1% [wt/vol]; pancreas, 3% [wt/vol]). A four-times-higher Liberase concentration was used for pancreas. Conditions were chosen in such a way that negative samples and mock samples spiked with normal brain homogenate gave the same background signal. Samples were incubated for 30 to 60 min at 37°C with a ribolyzing step performed every 10 min, until completely homogenized. The homogenate was then transferred into a new tube, and protease activity was stopped by adding complete mini-protease inhibitor (Roche). Samples were adjusted to a final concentration of 2% (wt/vol) Sarkosyl-phosphate-buffered saline (PBS), 0.3% (wt/vol) sodium phosphotungstic acid, and 12.6 mM MgCl2 at pH 7.4 and incubated for 1 h at 37°C with constant agitation. Then samples were centrifuged at 20,000 × g for 2 h in a microcentrifuge, and the pellet was washed with 250 μl of PBS containing 0.1% (wt/vol) Sarkosyl and 0.1 M EDTA. Following another centrifugation step at 20,000 × g for 15 min, the supernatant was discarded and the pellet resuspended in 50 μl of C1 buffer (TeSeE purification kit; Bio-Rad).
ELISA.
Following denaturation at 100°C for 5 min, the soluble PrPTSE was diluted fivefold in an appropriate buffer (R6, TeSeE detection kit; Bio-Rad) and analyzed using immunometric ELISA (TeSeE sheep detection kit; Bio-Rad). ELISA was done following the manufacturer's instructions using the antibodies Bar 224 and SAF 34 for PrP detection.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (17) with slight modifications. Briefly, peripheral tissues including adrenal glands and muscle fragments were fixed by immersion in Carnoy's fluid and transferred to butanol until paraffin embedding. Tissue sections (4 to 7 μm thick) were hydrated, treated (or not) with 2 μg/ml proteinase K (1/10,000, 5 min at room temperature; Eurobio) and immunolabeled for PrP with different monoclonal antibodies (provided by J. Grassi, CEA, Saclay, France). The best sensitivity was obtained with Bar 224 (1 mg/liter) and with Bar 233 (1 mg/liter), both raised against ovine recombinant PrP; similar results were also obtained in some cases with Saf 60 (10 mg/liter), an antibody raised against purified hamster 263K PrPTSE. The immunolabeled sites were then detected with the peroxidase-coupled polymer anti-mouse immunoglobulin Envision (Dako) followed by the chromogen NovaRed (Vector) and counterstained with Mayer's hemalum. Tyrosine hydroxylase-immunoreactive structures were labeled with the rabbit polyclonal antibody (1/1,000; Institut J. Boy) and neuronal structures with the rabbit polyclonal antibody anti-PGP 9.5 (ubiquitin C-terminal hydroxylase, 1/1,000; Dako) followed in both cases by the anti-rabbit immunoglobulin Envision system. The immunolabeled slides were examined under an Axiophot microscope (Zeiss) fitted to a charge-coupled device camera (DP 50; Olympus).
RESULTS
Highly sensitive ELISA for peripheral organs.
We developed an easy and straightforward purification method to detect PrPTSE in the following peripheral tissues: spleen, tonsils, intestine (Peyer's patches), muscles, adrenal glands, kidney, pancreas, lung, and liver (Fig. 1). Since the quantification of PrPTSE in different tissues requires that data be highly reproducible, we optimized PrPTSE purification by replacing traditional collagenase (collagenase A) (30) with an enzyme mix (Liberase Blendzyme 2; Roche), which avoided lot-to-lot variability. The protein digestion step was done at the same time using a neutral protease, which is included in the Liberase blend, to shorten incubation time and improve tissue dissociation.
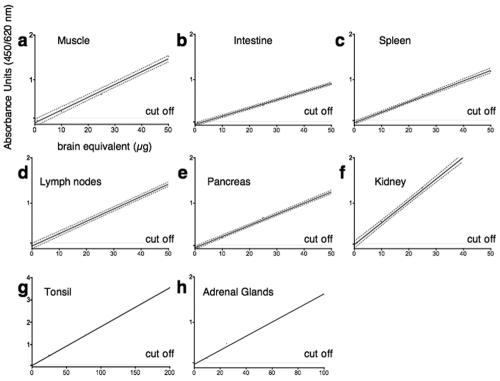
FIG. 1.
Sensitivity of PrPTSE detection in different peripheral organs. Tissue samples were spiked with different amounts of infectious brain homogenate to determine the detection level. Linear regression analysis was performed to generate a best-fit line. All samples were run at least in triplicate except for tonsil and adrenal gland samples, because of limited availability of those samples. The 95% confidence interval of the regression line was calculated for panels a to f using Prism statistics (GraphPad). r2 was >0.98 and linear regression was highly significant for all panels (P < 0.006). The cutoff value was calculated by multiplying the negative-control value by 2. Mock samples spiked with the corresponding amount of normal brain had values in the same range as negative samples (data not shown). In all peripheral tissues, we could detect PrPTSE at concentrations 104 times lower than in brain. Labels on the ordinate and abscissa in panel a apply to all panels.
Through the simple addition of different amounts of trypsin inhibitor (Sigma), the proteolytic activity obtained with the enzyme blend was easily adapted to the different compositions of peripheral organs, which avoided loss of detection sensitivity due to overconcentrated enzymes. In addition, trypsin inhibitor was the only efficient modifier that allowed complete inactivation of endogenous pancreatic proteases, a step necessary to avoid PrPTSE degradation during screening of pancreatic tissues. After organ homogenization using these modifications, PrPTSE was purified using phosphotungstic acid precipitation (32) and subsequently quantified by ELISA (Bio-Rad). With this method, we were able to detect PrPTSE in different organs even at concentrations 104 times lower than in brain (Fig. 1).
Primates succumb to the disease faster after vCJD infection than after sporadic or iatrogenic infection by similar routes.
Primates infected with sporadic or iatrogenic CJD had a much longer survival time than those infected with the same volume of 10% vCJD brain homogenates by the same route (both 56 months versus 25, 30, 32, and 37 months, respectively, by combined intratonsillar and intracerebral injection). Intratonsillar infection alone by vCJD led to a somewhat prolonged survival time of 44 months. Inoculation with the agent of cattle BSE by the oral route gave a survival time of 63 months.
Strain-dependent accumulation of PrPTSE in lymphoreticular organs of vCJD- and sCJD-infected primates (Table 1).
TABLE 1.
Semiquantitative assessment of PrPTSE in peripheral organs of inoculated primates
| Agent | Presence ina:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Lymphoid system
|
Peripheral nervous system
|
|||||||
| Spleen | Tonsils | LN | Intestine (PP) | Nerves | Muscle | Adrenal gland | Intestine (ENS) | |
| BSE | +++ | +++ | +++ | ++ | ++ | + | ++ | + |
| vCJD | +++ | +++ | +++ | ++ | ++ | + | + | + |
| sCJD | + | − | − | − | ++ | + | ++ | + |
| iCJD | ND | + | − | − | ++ | + | + | + |
+, <0.5 absorbance unit; ++, 0.5 to 2.0 absorbance units; +++, >2.0 absorbance units; −, no detectable PrPTSE. LN, mesenteric lymph nodes; PP, Peyer's patches; ENS, enteric nervous system.
Since a recent report showed that PrPTSE can be found in spleens of sCJD patients and not only in vCJD patients (13), we analyzed the lymphotropism of different CJD strains. Large amounts of PrPTSE were present in spleens and tonsils of primates after infection with the vCJD/BSE agent (Fig. 2). In spleens of vCJD- and BSE-infected primates, PrPTSE reached levels up to 4% of those in brain tissue (data not shown). Tonsils have already been reported to accumulate up to 10% of brain PrPTSE in primates inoculated intravenously or orally with the macaque-adapted BSE agent (17). In Peyer's patches the maximum amount of PrPTSE was 0.1% of that found in brain after vCJD/BSE infection (Fig. 2).
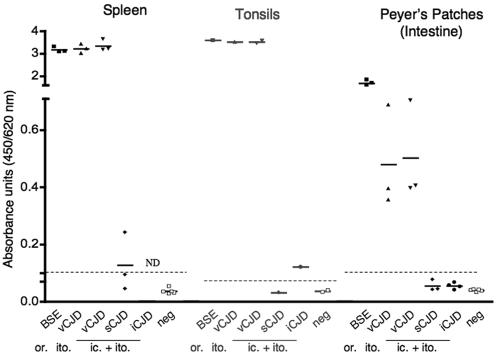
FIG. 2.
Detection of PrPTSE in lymphoreticular organs. Spleens, tonsils, and distal ileum (rich in Peyer's patches) from autopsies of BSE-, vCJD-, sCJD-, and iCJD-infected primates were homogenized, and amounts of PrPTSE were analyzed by ELISA as described in Materials and Methods. The column scatter graph shows each value in a group, with a line at the mean. Each group represents one primate, except for the vCJD (ic. + ito.) model, in which two to four animals were investigated. Samples were run at least three times for each animal unless specimens were limited (i.e., tonsils). The cutoff is indicated by a dotted line. The route of infection is indicated by “or.” (oral), “ito.” (intratonsillar), or “ic. + ito” (combined intracerebral and intratonsillar). ND, not determined.
In the primate infected with sCJD, only small amounts of PrPTSE (20,000 times less than in brain) were found in the spleen but not in the tonsils. However, the tonsils of the iCJD-infected primate were positive, with similar small amounts (Fig. 2). Peyer's patches were negative in both sCJD- and iCJD-infected animals. For these organs, the cutoff for negative samples was 20,000 times less PrPTSE than in brain. These results confirm that lymphoreticular organs are weakly positive in sCJD and iCJD, but their involvement is readily distinguishable from that observed in vCJD.
PrPTSE accumulation in muscle tissue of vCJD-, iCJD-, and sCJD-infected primates (Table 1).
Muscles accumulate detectable PrPTSE at the end of the incubation phase in rodents (3, 30, 31) and sheep (1). In humans, muscles were found to be positive in sCJD patients (13) but, surprisingly, samples of iCJD and vCJD have been reported to be negative in other studies (16). Therefore, we analyzed muscle specimens from each diseased primate. Figure 3a shows that PrPTSE is readily detectable in skeletal muscles (the biceps femoris) of sporadic as well as iatrogenic and variant CJD infected primates. Amounts of PrPTSE were about 10−4 those found in brain, with uninfected primates showing no detectable PrPTSE (i.e., 30,000 times less than that in brain) (Fig. 3a).
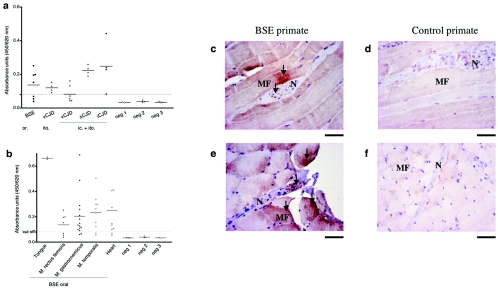
FIG. 3.
Detection and localization of PrPTSE in muscles of macaques infected with the BSE, vCJD, sCJD, or iCJD agent. (a) Analysis of biceps or quadriceps muscles of primates infected with the BSE, vCJD, sCJD, or iCJD agent. (b) Different muscle groups of a primate infected with BSE by the oral route. (a and b) Samples were analyzed by ELISA (Bio-Rad) as described in Materials and Methods. Each value in a group is shown as a dot, with a line at the mean. Samples with an optical density lower than the cutoff value (—-) were considered negative. Values situated just above the cutoff, like muscle samples of the vCJD (ic. + ito.)-infected primates, were interpreted as borderline positive. Neg 1-3 represents noninfected primate samples from three primates. (c to f) PrP immunostaining in diaphragmatic muscles of control and BSE-inoculated (orally) primates with (c and d) Bar 233 (after PK digestion) and (e and f) Bar 224 antibody. Arrows indicate PrP staining of nerve (N) and muscle fibers (MF). Bar = 50 μm.
To determine how the amount of PrPTSE is distributed in different types of muscles, we analyzed a branchiomeric muscle (the temporalis), somatic muscles (gastrocnemius and rectus femoris), the tongue muscle, and the heart muscle. This was performed in the primate infected with BSE by the oral route, which would best reflect vCJD pathogenesis. We found PrPTSE in all analyzed muscle groups at about the same level of 10,000 to 20,000 times less PrPTSE than in brain tissue, independent of their functional group (Fig. 3b). Only the tongue muscle gave average signals two to three times higher than other muscles (1/5,000) (Fig. 3b).
Intriguingly, we observed great variations in the amount of PrPTSE when samples were taken from different zones of the same muscle (Fig. 3b). For example, in the gastrocnemius, the amount of PrPTSE varied up to 10-fold depending on the sampling region, with 13% of the samples being below the cutoff. These variations were not observed with spiked muscle or pooled homogenates taken as controls for the reproducibility of the measurements (data not shown) and therefore represent true regional differences in PrPTSE content within a muscle. To investigate muscular PrPTSE localization, we performed immunohistochemistry staining. We observed that PrPTSE deposition occurred in those muscle fibers which were adjacent to PrPTSE-positive small nerve terminals (Fig. 3c and e). As nerve endings are located in specific muscle regions, this most probably explains why more or less PrPTSE can be found in one muscle sample, depending on the abundance of the innervation.
Centrifugal spread of prions to different peripheral organs (Table 1).
Since peripheral nerve and muscle involvement was not restricted to the vCJD model but was also observed in sCJD and iCJD, we set out to determine if this holds true for other highly innervated peripheral organs. We detected PrPTSE in adrenal glands in all transmitted TSEs (Fig. 4a). Again, there was a clear association of PrPTSE with nervous structures. PrPTSE staining was found in the nerves of the capsule surrounding the adrenal cortex (Fig. 4b) and in tyrosine hydroxylase-positive nerve fibers of the reticular zone of the adrenal cortex (Fig. 4c). Interestingly, TH-positive cell bodies of the adrenal medulla were negative (Fig. 4c).
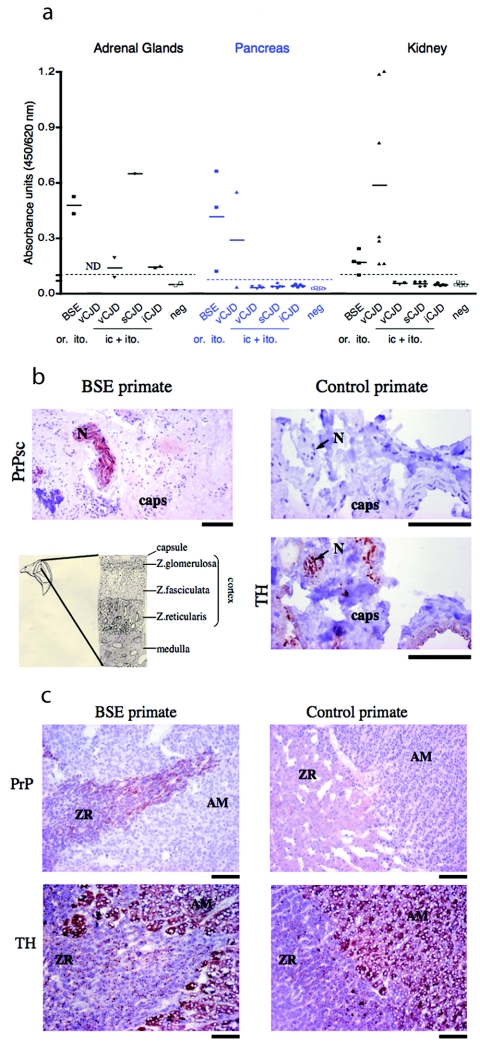
FIG. 4.
Detection and localization of PrPTSE in peripheral organs of macaques infected with the BSE, vCJD, sCJD, or iCJD agent. (a) Adrenal gland, pancreas, and kidney necropsy samples of each primate were analyzed for PrPTSE. All values are shown as dots with a line at the mean. Dotted lines correspond to cutoff values. (b and c) Hematoxylin-and-eosin-stained microscopic images of adrenal glands from BSE-inoculated and control primates. (b) PrPTSE labeling (with Bar 224) after PK digestion in a nerve fiber (N) of the adrenal capsule (caps) of a BSE-inoculated (orally) primate and control primate. Nerve labeling of the control section was done with a neuron-specific tyrosine hydroxylase (TH) antibody. (c) Sections illustrate the zona reticularis (ZR) of the adrenal cortex and the adrenal medulla (AM). PrPTSE was stained (with SAF 60) after PK digestion and cells of the adrenal medulla and nerve fibers of the zona reticularis were labeled with an anti-tyrosine hydroxylase (TH) antibody. (b and c) Bar = 100 μm.
Other organs, such as lung and liver, were always negative (detection limit of 10−4 times the level of PrPTSE in brain). However, kidney and pancreas were positive in BSE and vCJD primates, but only after infection by the peripheral route (oral or intratonsillar) (Fig. 4a). PrPTSE levels were similarly low in adrenal glands (2,500 to 10,000 times less than in brain), pancreas (6,000 and 8,500 times less than in brain), and kidney (8,000 and 30,000 times less than in brain).
DISCUSSION
If we are to devise rational measures to avoid iatrogenic contamination by human TSE agents, it is of paramount importance that we understand the pathogenesis of these diseases, but such understanding is still poor. This is mainly due to the scarcity of human autopsy samples available for PrPTSE analysis. In addition, the interpretation of results from a few patient tissues in drawing comparisons between the different human forms of the disease is extremely difficult, if not impossible, because of the different routes of infection involved (oral for vCJD, intramuscular for GH iCJD, unknown for sCJD) and the existence of differential susceptibility linked to PrP codon 129 in humans (harboring either V or M). We therefore studied the different forms of human prion diseases in the Old World primate cynomolgus macaque, which is one of the animal species most closely related to humans. All cynomolgus macaques are M/M at codon 129 of the PrP gene. The macaque model displays the same human “strain-specific” electrophoretic pattern of PrPTSE in sCJD and vCJD (23). In this way we could explore the “strain”-specific PrPTSE distribution in controlled inoculation conditions. PrPTSE is a useful marker for the presence of the infectious agent in the case, as here, that mouse bioassays do not fulfill the requirements for sensitivity due to a high species barrier effect.
A first observation of importance was that the incubation period in vCJD-infected primates was about half that of animals infected with sCJD or iCJD by the same route (combined intracerebral and intratonsillar). This is consistent with the observation that the sCJD agent repeatedly exhibits low transmission rates or lengthy incubation periods upon inoculation into mice, whereas the vCJD/BSE agent is readily transmitted to mice (8, 9, 24). This may be due to differential kinetics of replication of the agent strains. However, given our observation of PrPTSE levels in lymphoid organs, a more straightforward explanation would be the strong lymphotropism specific to the vCJD agent. Indeed, only in the animals infected with the latter could PrPTSE be detected in lymphoid organs at levels up to 10% of those found in the brain. Lymphotropism would allow the infectious agent to undergo an efficient primary replication step in lymphoreticular system (LRS) tissues, leading to a higher infectious load ready to be taken up by nerve endings on the one hand and shed in the bloodstream on the other hand, both accelerating the neuroinvasion process.
However, we also found very low levels of PrPTSE (<10,000 of that in brain) in the LRSs of sCJD- and iCJD-infected macaques, indicating that these tissues are, to some extent, permissive to infection by these TSE agent strains as well. These findings raise the question of the mechanism of lymphotropism in prion diseases. Does a lack of apparent lymphotropism result from a lower affinity of the prion strain for cellular components of lymphoid tissues? Alternatively, it could result from a prion strain triggering a more efficient defense reaction by the host, thus undermining the infection.
On the other hand, PrPTSE accumulation in lymphoreticular organs of nonhuman primates infected with the sCJD/iCJD agent might be due to a centrifugal spread of the agent, which, for example, was held responsible for the appearance of PrPTSE in retinas and olfactory epithelium of sCJD patients (15, 33). In accordance with this hypothesis, one macaque inoculated under the same conditions with a second passage of sCJD in nonhuman primates developed the disease much faster (26 months) and did not accumulate PrPTSE in the spleen and tonsils (17), suggesting that the shorter incubation might not allow enough time for extensive prion spread via nerves into these organs.
Overall, our study suggesting that a low PrPTSE load in lymphoid organs in sCJD might be related to longer incubation periods should be an incentive for investigation of these mechanisms, since it suggests a target for prophylactic intervention.
The high lymphotropism and presumable blood shedding of the BSE agent in primates also raises the concern that vCJD may be transmitted by blood transfusion or by the administration of blood derivatives. There is now evidence that the vCJD agent does indeed pose a greater risk in this respect than the sCJD agent. Studies in sheep and rodents have demonstrated that BSE can be transmitted by transfusion, and two cases of vCJD have been reported in which the patients had received blood cells from donors incubating vCJD (7, 20, 21, 25, 27). On the other hand, transmission of sporadic or iatrogenic CJD through blood transfusion has never been described.
One previous study showed that PrPTSE could be found in the spleen of certain sCJD patients (13), which is in accordance with this study, where we found very low levels of PrPTSE not only in the spleen but also in the tonsils in sCJD and iCJD, respectively. This suggests that all lymphoid tissues may be weakly positive in sCJD and iCJD patients. This is of importance, since these tissues are used for screening of subclinical vCJD carriers (18, 19). Great care should be taken to avoid false-positive vCJD case reporting when weakly positive samples or atypical LRS staining could indicate other forms of CJD. In any case, a positive result with a peripheral biopsy specimen should be analyzed, taking into account not only the total amount and type of PrPTSE found but also the patient's history, clinical stage, and PrP codon 129 genotype.
Our study also highlights the fact that other organs outside the LRS can accumulate PrPTSE independently of the strain's lymphotropism. This was particularly striking in highly innervated tissues, such as the muscle and adrenal glands, where PrPTSE deposits were associated with nerve endings and fibers, respectively. This suggests that centrifugal spread through the nerves might be responsible for PrPTSE accumulation in these organs. The presence of PrPTSE in these tissues was common to all CJD agents. Muscle biopsy would therefore in principle be able to detect CJD. A classification might even be possible through PrPTSE typing, and studies are under way to assess this point further. However, the difficulty of detecting PrPTSE in every patient, and negative findings in some studies, raises questions about the practicability of this method. Our study shows that PrPTSE detection does not vary between different functional or developmental muscle groups. Only the tongue showed elevated PrPTSE levels compared to other muscles. Variation arises much more in the uneven distribution of PrPTSE within a single muscle, as PrPTSE was found to accumulate next to nerve endings, following the regional distribution of muscle innervation. Hence, for diagnostic purposes, several samples from different regions would have to be analyzed using a highly sensitive method to avoid false-negative results.
High PrPTSE levels in the tongue in the hamster scrapie model have already been reported (2, 26). This is, at least in part, explained by the colocalization of PrPTSE with nerve endings, since this organ is highly innervated directly via four cranial nerves.
In addition to highly innervated organs, we screened for PrPTSE in peripheral organs, which have been suggested to harbor infectivity in human TSEs. Low rates of transmission from human kuru or CJD patients to nonhuman primates were obtained with spleen, lymph nodes, lung, liver, and kidney tissues (5). In addition, tissue pools of spleen, liver, and kidney from kuru-infected chimpanzees transmitted the disease to chimpanzees (10). Hence, we tested lung, liver, and kidney for PrPTSE. We were also interested in pancreatic tissue, since the pancreas was found to be positive for PrPTSE and infectivity in chronic wasting disease and scrapie (14, 29). We found PrPTSE in kidney and pancreas but only in nonhuman primates infected with vCJD (intratonsillar) and BSE (oral) (Fig. 4a). The significance of these results needs to be investigated further, but it seems that in our model only the vCJD/BSE agent is able to accumulate in these organs. Since primates infected with vCJD by the combined intracerebral and intratonsillar routes did not have detectable levels of PrPTSE in kidney and pancreas, we suppose that a prolonged incubation time is necessary for prions to reach these organs through centrifugal spread via the nerves. Further studies are necessary to elucidate the involvement of these organs.
Lung and liver tissues, on the other hand, harbored no detectable levels of PrPTSE, indicating that if PrPTSE was present, it was at very low levels—less than 1:10,000 of that found in brain.
Because we could quantify the PrPSc loads of different organs under defined experimental conditions, we propose a tentative tissue classification which should not be considered definitive until more primates have been examined (Table 1). Of note, our findings are reinforced by previous data showing very low rates of transmission of human TSEs to nonhuman primates using peripheral tissues, which led D. C. Gajdusek and coworkers to conclude that “all tissues of patients with Creutzfeldt-Jakob Disease must be considered potentially hazardous” (11). Affected peripheral organs, except lymphoid organs after vCJD/BSE infection, contained about 4 logs less PrPSc than brain tissue. Therefore, the possible risk arising from either of these two categories of tissues should be considered very small when compared with that posed by the LRS after BSE/vCJD infection: (i) muscle, adrenal glands, and non-LRS parts of the intestine from primates infected with any TSE and (ii) lymphoreticular tissues from iCJD- and sCJD-infected primates. These findings might help develop careful risk assessment plans to ensure the safety of medical procedures without compromising the benefits of sophisticated clinical practice.
Conclusions.
In terms of the pathogenesis of human TSE infections, our study highlights the following. (i) CJD strains associated with sCJD, vCJD, and iCJD can lead to detectable amounts of PrPTSE in organs that are affected by spreading through the nerves (i.e., muscles, intestine [enteric nervous system], and adrenal glands). These organs might not be targets for prion replication per se and represent a low risk of iatrogenic transmission. (ii) The vCJD/BSE strain exhibits a wider tropism in primates than sCJD and iCJD strains, leading to PrPTSE detection in a broader range of tissues, some of which (kidney and pancreas) harbor small amounts of PrPTSE and represent a low transmission risk. On the other hand, PrPTSE accumulates primarily and extensively in lymphoid organs (spleen, Peyer's patches, tonsils), which are high-risk organs. Therefore, the vCJD/BSE strain can be designated a lymphotropic strain for primates. This lymphotropism seems to correlate with the higher virulence in primates of the BSE strain compared to other human TSE strains. Further exploration of cellular and molecular mechanisms of lymphotropism are necessary, because they clearly affect the degree of peripheral PrPTSE accumulation and the rate of disease progression and might therefore be crucial for preclinical diagnosis and therapeutic approaches.
Acknowledgments
We thank R. Rioux, S. Jacquin, and A. Fort for expert animal care and J. Grassi (SPI, CEA) for providing high-quality antibodies. C.H. thanks P. Kaiser at the University of Tübingen for supporting his work.
This work was financed by the French Ministry of Research (GIS PRION) and EU grant QLK1-2002-01096. We have no conflicting financial interest.
REFERENCES
- 1.Andreoletti, O., S. Simon, C. Lacroux, N. Morel, G. Tabouret, A. Chabert, S. Lugan, F. Corbiere, P. Ferre, G. Foucras, H. Laude, F. Eychenne, J. Grassi, and F. Schelcher. 2004. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat. Med. 10:591-593. [DOI] [PubMed] [Google Scholar]
- 2.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2003. Rapid prion neuroinvasion following tongue infection. J. Virol. 77:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosque, P. J., C. Ryou, G. Telling, D. Peretz, G. Legname, S. J. DeArmond, and S. B. Prusiner. 2002. Prions in skeletal muscle. Proc. Natl. Acad. Sci. USA 99:3812-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramble, M. G., and J. W. Ironside. 2002. Creutzfeldt-Jakob disease: implications for gastroenterology. Gut 50:888-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, P., C. J. Gibbs, Jr., P. Rodgers-Johnson, D. M. Asher, M. P. Sulima, A. Bacote, L. G. Goldfarb, and D. C. Gajdusek. 1994. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann. Neurol. 35:513-529. [DOI] [PubMed] [Google Scholar]
- 6.Brown, P., M. Preece, J. P. Brandel, T. Sato, L. McShane, I. Zerr, A. Fletcher, R. G. Will, M. Pocchiari, N. R. Cashman, J. H. d'Aignaux, L. Cervenakova, J. Fradkin, L. B. Schonberger, and S. J. Collins. 2000. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology 55:1075-1081. [DOI] [PubMed] [Google Scholar]
- 7.Brown, P., R. G. Rohwer, B. C. Dunstan, C. MacAuley, D. C. Gajdusek, and W. N. Drohan. 1998. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion 38:810-816. [DOI] [PubMed] [Google Scholar]
- 8.Bruce, M. 1996. Presented at the IIIrd International Symposium on Transmissible Subacute Spongiform Encephalopathies: Prion Diseases, Val-de-Grâce, Paris, France.
- 9.Bruce, M. E., R. G. Will, J. W. Ironside, I. McConnell, D. Drummond, A. Suttie, L. McCardle, A. Chree, J. Hope, C. Birkett, S. Cousens, H. Fraser, and C. J. Bostock. 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 10.Gajdusek, D. C., and C. J. Gibbs, Jr. 1971. Transmission of two subacute spongiform encephalopathies of man (kuru and Creutzfeldt-Jakob disease) to New World monkeys. Nature 230:588-591. [DOI] [PubMed] [Google Scholar]
- 11.Gajdusek, D. C., C. J. Gibbs, Jr., D. M. Asher, P. Brown, A. Diwan, P. Hoffman, G. Nemo, R. Rohwer, and L. White. 1977. Precautions in medical care of, and in handling materials from, patients with transmissible virus dementia (Creutzfeldt-Jakob disease). N. Engl. J. Med. 297:1253-1258. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs, C. J., Jr., D. C. Gajdusek, D. M. Asher, M. P. Alpers, E. Beck, P. M. Daniel, and W. B. Matthews. 1968. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science 161:388-389. [DOI] [PubMed] [Google Scholar]
- 13.Glatzel, M., E. Abela, M. Maissen, and A. Aguzzi. 2003. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N. Engl. J. Med. 349:1812-1820. [DOI] [PubMed] [Google Scholar]
- 14.Hadlow, W. J., R. C. Kennedy, and R. E. Race. 1982. Natural infection of Suffolk sheep with scrapie virus. J Infect. Dis. 146:657-664. [DOI] [PubMed] [Google Scholar]
- 15.Head, M. W., V. Northcott, K. Rennison, D. Ritchie, L. McCardle, T. J. Bunn, N. F. McLennan, J. W. Ironside, A. B. Tullo, and R. E. Bonshek. 2003. Prion protein accumulation in eyes of patients with sporadic and variant Creutzfeldt-Jakob disease. Investig. Ophthalmol. Vis. Sci. 44:342-346. [DOI] [PubMed] [Google Scholar]
- 16.Head, M. W., D. Ritchie, N. Smith, V. McLoughlin, W. Nailon, S. Samad, S. Masson, M. Bishop, L. McCardle, and J. W. Ironside. 2004. Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt-Jakob disease: an immunohistochemical, quantitative, and biochemical study. Am. J. Pathol. 164:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog, C., N. Sales, N. Etchegaray, A. Charbonnier, S. Freire, D. Dormont, J. P. Deslys, and C. I. Lasmezas. 2004. Tissue distribution of bovine spongiform encephalopathy agent in primates after intravenous or oral infection. Lancet 363:422-428. [DOI] [PubMed] [Google Scholar]
- 18.Hill, A. F., R. J. Butterworth, S. Joiner, G. Jackson, M. N. Rossor, D. J. Thomas, A. Frosh, N. Tolley, J. E. Bell, M. Spencer, A. King, S. Al-Sarraj, J. W. Ironside, P. L. Lantos, and J. Collinge. 1999. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 353:183-189. [DOI] [PubMed] [Google Scholar]
- 19.Hilton, D. A., A. C. Ghani, L. Conyers, P. Edwards, L. McCardle, D. Ritchie, M. Penney, D. Hegazy, and J. W. Ironside. 2004. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J. Pathol. 203:733-739. [DOI] [PubMed] [Google Scholar]
- 20.Houston, F., J. D. Foster, A. Chong, N. Hunter, and C. J. Bostock. 2000. Transmission of BSE by blood transfusion in sheep. Lancet 356:999-1000. [DOI] [PubMed] [Google Scholar]
- 21.Hunter, N., J. Foster, A. Chong, S. McCutcheon, D. Parnham, S. Eaton, C. MacKenzie, and F. Houston. 2002. Transmission of prion diseases by blood transfusion. J Gen. Virol. 83:2897-2905. [DOI] [PubMed] [Google Scholar]
- 22.Lasmézas, C. I., J. Y. Cesbron, J. P. Deslys, R. Demaimay, K. T. Adjou, R. Rioux, C. Lemaire, C. Locht, and D. Dormont. 1996. Immune system-dependent and -independent replication of the scrapie agent. J. Virol. 70: 1292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lasmézas, C. I., E. Comoy, S. Hawkins, C. Herzog, F. Mouthon, T. Konold, F. Auvre, E. Correia, N. Lescoutra-Etchegaray, N. Sales, G. Wells, P. Brown, and J. P. Deslys. 2005. Risk of oral infection with bovine spongiform encephalopathy agent in primates. Lancet 365:781-783. [DOI] [PubMed] [Google Scholar]
- 24.Lasmézas, C. I., J. G. Fournier, V. Nouvel, H. Boe, D. Marce, F. Lamoury, N. Kopp, J. J. Hauw, J. Ironside, M. Bruce, D. Dormont, and J. P. Deslys. 2001. Adaptation of the bovine spongiform encephalopathy agent to primates and comparison with Creutzfeldt-Jakob disease: implications for human health. Proc. Natl. Acad. Sci. USA 98:4142-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llewelyn, C. A., P. E. Hewitt, R. S. Knight, K. Amar, S. Cousens, J. Mackenzie, and R. G. Will. 2004. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363:417-421. [DOI] [PubMed] [Google Scholar]
- 26.Mulcahy, E. R., J. C. Bartz, A. E. Kincaid, and R. A. Bessen. 2004. Prion infection of skeletal muscle cells and papillae in the tongue. J. Virol. 78:6792-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peden, A. H., M. W. Head, D. L. Ritchie, J. E. Bell, and J. W. Ironside. 2004. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364:527-529. [DOI] [PubMed] [Google Scholar]
- 28.Prusiner, S. B. 2001. Shattuck lecture—neurodegenerative diseases and prions. N. Engl. J. Med. 344:1516-1526. [DOI] [PubMed] [Google Scholar]
- 29.Sigurdson, C. J., T. R. Spraker, M. W. Miller, B. Oesch, and E. A. Hoover. 2001. PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen. Virol. 82:2327-2334. [DOI] [PubMed] [Google Scholar]
- 30.Thomzig, A., C. Kratzel, G. Lenz, D. Kruger, and M. Beekes. 2003. Widespread PrPSc accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 4:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomzig, A., W. Schulz-Schaeffer, C. Kratzel, J. Mai, and M. Beekes. 2004. Preclinical deposition of pathological prion protein PrPSc in muscles of hamsters orally exposed to scrapie. J. Clin. Investig. 113:1465-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadsworth, J. D., S. Joiner, A. F. Hill, T. A. Campbell, M. Desbruslais, P. J. Luthert, and J. Collinge. 2001. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 358:171-180. [DOI] [PubMed] [Google Scholar]
- 33.Zanusso, G., S. Ferrari, F. Cardone, P. Zampieri, M. Gelati, M. Fiorini, A. Farinazzo, M. Gardiman, T. Cavallaro, M. Bentivoglio, P. G. Righetti, M. Pocchiari, N. Rizzuto, and S. Monaco. 2003. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jakob disease. N. Engl. J. Med. 348:711-719. [DOI] [PubMed] [Google Scholar]