Abstract
Herpes simplex virus type 1 (HSV-1) is the leading cause of virus-induced encephalitis; however, the viral genes that regulate encephalitis have not been well characterized. In this study, we tested whether the LAT (latency-associated transcript) locus regulates the frequency of encephalitis in male or female mice. Male BALB/c mice are more susceptible to HSV-1-induced encephalitis than age-matched female BALB/c mice. Deletion of LAT coding sequences reduced the frequency of encephalitis. A recombinant virus containing the first 1.5 kb of the LAT coding sequence induces levels of encephalitis in male BALB/c mice similar to those induced by wild-type HSV-1.
Approximately 90% of the population is infected with herpes simplex virus type 1 (HSV-1) (29, 55). Following acute infection, latency is established in sensory neurons in trigeminal ganglia or sacral dorsal root ganglia (19, 50). Reactivation from latency periodically occurs, resulting in virus transmission and recurrent disease (19, 20).
The latency-associated transcript (LAT) is abundantly transcribed in latently infected neurons (8, 10, 11, 22, 27, 44, 48, 51, 52). An 8.3-kb LAT transcript is spliced, yielding the stable 2-kb LAT intron that localizes to the nucleus (2, 31, 49) and an unstable 6.3-kb transcript (11, 44, 57). Proper splicing of LAT is necessary for establishment and maintenance of latency (21). The HSV-1 McKrae strain is frequently shed in tears of latently infected rabbits because of spontaneous reactivation (39-43), and LAT gene expression is crucial for spontaneous reactivation. LAT inhibits apoptosis in cultured cells and trigeminal ganglia of infected rabbits or mice (1, 14, 17, 18, 21, 37). The ability of LAT to interfere with apoptosis correlates with spontaneous reactivation (17, 18).
HSV-1-induced encephalitis is a severe form of focal necrotizing encephalitis that affects approximately 2,000 individuals each year in the United States (12, 24, 54, 55). Without antiviral therapy, the mortality rate can be 70% (45, 46). Approximately 2/3 of HSV-1-induced encephalitis cases are due to reactivation from latency (56); consequently, high morbidity and long-term complications occur despite antiviral treatment (23, 26, 45).
In general, females induce a stronger immune response because they express higher levels of lymphocytes and cytokines (3, 5, 16). Susceptibilities to encephalomyocarditis virus (9), vesicular stomatitis virus (4), coxsackievirus B3 (15), and HSV-1 infections in mice (13) differ based on gender. Furthermore, a HSV-2 vaccine trial in humans succeeded in females but not in males (47). In female mice, a HSV resistance locus (sex modifier locus) has been identified (25). HSV-1 hematogenous infection is more pathogenic to females (6), and estrogen influences pseudorabies virus infections in the central nervous system (53), suggesting that gender-specific factors, male as well as female, influence host-pathogen interactions.
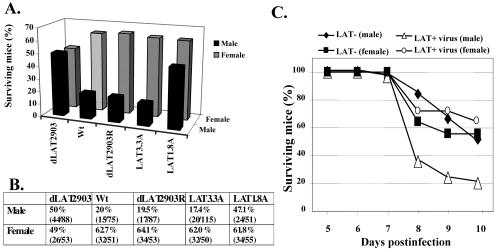
Based on the observations that gender can influence the pathogenic potential of certain viruses, we tested whether LAT influences the frequencies of encephalitis in male and female mice. Adult female or male BALB/c mice were infected with the wild-type (wt) McKrae strain or the LAT locus mutant, dLAT2903 (Fig. 1). As previously described for 129 Sv//Ev or RGKO male mice (13), male BALB/c mice infected with wt HSV-1 or dLAT2903R (rescued dLAT2903) (Fig. 1A) had frequencies of survival significantly lower than those of female BALB/c mice (Fig. 2A and B). A Tukey-Kramer multiple-comparison post-analysis of variance test revealed that survival rates for male and female mice infected with wt HSV-1 or dLAT2903R were significantly different (P = 0.004). Male mice infected with dLAT2903 had significantly higher (P = 0.003) levels of survival than those infected with wt HSV-1 or dLAT2903R. As previously reported (38), female mice infected with dLAT2903, wt HSV-1, or dLAT2903R exhibited similar survival rates.
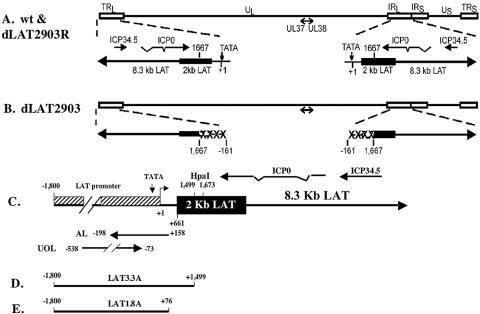
FIG. 1.
Schematic representation of wild-type and mutant viruses. (A) The prototypic HSV-1 genomic structure of the wt McKrae strain is shown at the top. The viral repeat regions are shown as open rectangles. TRL is the terminal long repeat. IRL is the internal (or inverted) long repeat. TRS is the terminal short repeat. IRS is the internal (or inverted) short repeat. The unique long (UL) and unique short (US) regions are each represented by a solid line. Below the genomic structure, the LAT region (one in each long repeat) is shown in expanded form. The primary 8.3-kb LAT is shown as a long arrow. The stable 2-kb LAT is shown as a solid rectangle. +1 and the small arrows indicate the starts of LAT transcription. The relative locations of mRNAs encoding ICP0 and ICP34.5 are shown for reference. (B) dLAT2903 is a LAT null mutant that contains deletions (XXXX) in both copies of LAT from LAT nucleotides −161 to + 1667. dLAT2903 is missing the core LAT promoter, a putative secondary LAT promoter just upstream of the 2-kb LAT, and the first 1,667 nucleotides of the primary LAT RNA. (C) Schematic of genes within the LAT locus. The primary LAT transcript is indicated by the large arrow. The solid rectangle represents the very stable 2-kb LAT intron. The LAT TATA box is indicated, and the striped box denotes the LAT promoter. The start of LAT transcription is indicated by the arrow at +1 (genomic nucleotide 118801). Several restriction enzyme sites and the relative locations of the ICP0, ICP34.5, UOL (upstream of LAT), and AL (antisense to LAT) transcripts are also shown for reference. The nucleotide positions of the 5′ and 3′ ends of UOL (30) and AL (36) are depicted and are relative to the start of LAT transcription. (D) LAT3.3A is a recombinant virus that was constructed using dLAT2903, and it contains a fragment spanning from −1800 to +1499. LAT sequences were inserted between UL37 and UL38. The construction of LAT3.3A was described previously (41). (E) LAT1.8A is a recombinant virus that was constructed using dLAT2903, and it contains a fragment spanning from −1800 to +76. LAT sequences were inserted between UL37 and UL38. The construction of LAT1.8A was described previously (38).
FIG. 2.
Survival of BALB/c mice after infection with HSV-1. Adult male and female BALB/c mice (54 to 82 days old) were obtained from Charles River Laboratory. Female and male mice were housed in separate rooms during the course of all experiments. Mice were ocularly infected with doses of 1 × 105 PFU/eye. Mice were bilaterally infected without scarification by placing the virus (2 μl of inoculum) into the conjunctival cul-de-sac, closing the eye, and rubbing the lid gently against the eye for 30 seconds. Prior to infection, mice were lightly anesthetized using isoflurane (Halocarbon Laboratories, River Edge, NJ). Mice were observed daily during the studies and were euthanized by CO2 inhalation if they exhibited severe neurological symptoms. (A) The percentages of surviving mice are presented. (B) The number of mice used for each virus and the percentages of mice that survived are shown. (C) Survival data were plotted based on the times that mice succumbed to fatal encephalitis after infection. There were no dramatic differences between wt McKrae, LAT3.3A, and dLAT2903R; consequently, these data were combined and designated as LAT+ virus data. Since there was no difference between dLAT2903 and LAT1.8A, these data were combined and designated as LAT− data. The mice that were euthanized because they were lethargic and unresponsive were scored as having undergone fatal encephalitis on the day they were euthanized.
The deletion within dLAT2903 prevents expression of at least four genetic elements (Fig. 1C): (i) the stable 2-kb LAT; (ii) the first 1.5 kb of LAT, which contains only part of the stable 2-kb LAT and is sufficient for high levels of spontaneous reactivation in the rabbit eye model (41) or for trigeminal ganglia explant-induced reactivation in mice (38); (iii) the AL transcript (36); and (iv) the UOL transcript (30). To localize regions of LAT necessary for enhancing encephalitis in male BALB/c mice, we tested LAT3.3A (Fig. 1D) and LAT1.8A (Fig. 1E). LAT3.3A expresses UOL, AL, and one copy of the first 1.5 kb of LAT but does not express the intact stable 2-kb LAT. LAT1.8A contains one copy of the minimal UOL coding sequences but not AL or LAT. Male, but not female, BALB/c mice infected with LAT3.3A had a frequency of encephalitis significantly higher than that for age-matched male mice infected with LAT1.8A (P = 0.003) (Fig. 2A and B). Furthermore, LAT3.3A, wt HSV-1, and dLAT2903R induced similar levels of encephalitis in male BALB/c mice, indicating that expression of the intact stable 2-kb LAT does not regulate gender-specific encephalitis. Finally, LAT1.8A induced levels of encephalitis in male BALB/c mice similar to those induced by dLAT2903.
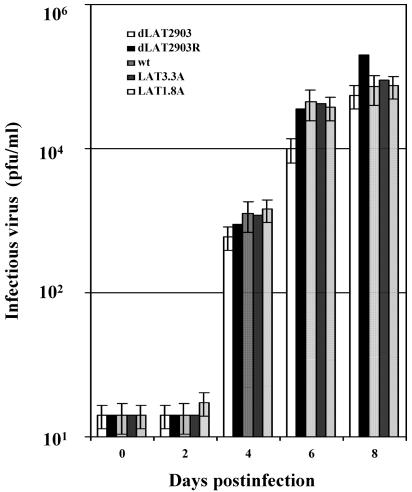
Following infection of male or female mice with the respective viruses, the mice that died typically succumbed to fatal encephalitis between 7 and 10 days after infection (Fig. 2C). Similar virus titers were also detected in brain homogenates at 2, 4, or 8 days after infection, regardless of the virus used to infect male (Fig. 3) or female BALB/c mice (data not shown). Consistent with previous studies (28, 35, 38), we also found similar levels of infectious virus in ocular swabs from male and female mice following infection with the virus strains. These studies suggested that the reduced frequency of encephalitis in male BALB/c mice infected with dLAT2903 was not due to reduced viral titers in the brain.
FIG. 3.
Analysis of virus titers in brains of male BALB/c mice following infection with HSV-1. Male BALB/c mice were infected with the designated virus as described in the legend for Fig. 2. Brains from individual mice were minced into small pieces and suspended in 2 ml of phosphate-buffered saline, and tissue was disrupted using a Polytron tissue homogenizer. Samples were then subjected to three cycles of freeze-thawing (−86°C to 37°C), and debris was removed by centrifugation (18,000 × g for 30 min at 4°C). One-tenth of the supernatant from a single brain was used for a measurement of the amount of infectious virus, which was performed as previously described (35, 38). The data represent values obtained from brains of eight mice for each virus and time point. The average of these results are shown.
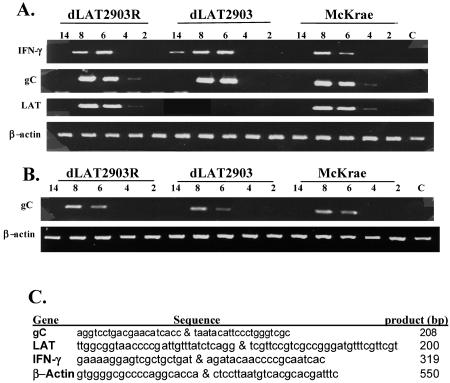
Since the LAT locus inhibits interferon (IFN) RNA expression in neuronal cell types (34), we tested whether IFN was differentially expressed in brains of mice during acute infection (2 to 8 days after infection) or after latency was established (14 days after infection). Using primers that specifically amplify mouse gamma IFN (IFN-γ) (Fig. 4C), male mice infected with dLAT2903 contained readily detectable levels of IFN-γ RNA at 6, 8, and 14 days after infection, but mock-infected mice did not (Fig. 4A). In contrast, male mice infected with the McKrae strain or dLAT2903R expressed IFN-γ RNA at 6 and 8 days, but not at 14 days, after infection. Viral glycoprotein (gC) was detected on days 6 and 8 regardless of the virus used for infection. At 4 days after infection, low levels of gC RNA were detected in male mice infected with the McKrae strain or with dLAT2903R but not in those infected with dLAT2903. gC RNA expression levels were similar in brains of male mice at 6 and 8 days after infection, regardless of which virus was used. As expected, LAT was not detected in mice infected with dLAT2903 (Fig. 4A). Female mice infected with dLAT2903, dLAT2903R, or wt HSV-1 expressed IFN-γ RNA 6 and 8 days after infection (Fig. 4B). These findings suggested that a correlation exists between survival of male mice infected with dLAT2903 and prolonged IFN-γ RNA expression in brains. Our results are also consistent with studies demonstrating that IFN-γ protects against HSV infection (7, 13).
FIG. 4.
Gene expression in male mice infected with HSV-1. As described in the legend for Fig. 2, adult male (A) and female (B) BALB/c mice were infected with the designated strains of HSV-1. Brains from individual mice were collected following euthanasia and snap-frozen on dry ice. One ml of TRIZOL reagent was added (Invitrogen, Carlsbad, CA), and RNA was prepared according to the manufacturer's instructions. For the designated times after infection (numbers of days shown at the tops of the gels), RNA was prepared from brains of three mice. RNA samples were subjected to DNase I digestion to remove trace amounts of contaminating DNA. Samples from mock-infected mice are designated C. Semiquantitative reverse transcriptase PCR was performed as described previously (34) using RNA from the brain of a single mouse. RNA concentrations were determined initially by measuring the optical density at 260 nm. Running a 1.2% formaldehyde agarose gel using 0.5 to 1 μg of RNA monitored the integrity of total RNA and confirmed the optical density reading. First-strand cDNA synthesis was performed with the SuperScript first-strand synthesis system for reverse transcriptase PCR (Invitrogen) using equal amounts of total RNA (1 to 2 μg) and oligo(dT)12-16 (for cellular genes) or random hexamers (for HSV-1 genes). PCRs were carried out in 20-μl reaction mixtures that contained 0.5 μM of the designated gene-specific primers shown in panel A, 1/10 of the cDNA reaction mixture, 2.5 to 5 mM MgCl2, 25 to 100 μM dNTP, and 1 U Taq DNA polymerase. LAT cDNA was amplified using the GC-rich PCR system (Roche). Without reverse transcriptase in the reaction mixture, there were no specific amplified products (data not shown). These results are representative of three separate experiments using RNA prepared from different mice. Upper gel in panel B, IFN-γ; lower gel in panel B, β-actin. (C) All primers were described previously (32, 34) and are presented in a 5′-to-3′ direction. For each gene, the first primer is the forward primer and the second is the reverse primer.
In summary, these studies indicated that the ability of HSV-1 to induce high levels of fatal encephalitis in male BALB/c mice was dependent on expression of the first 1.5 kb of LAT, expression of UOL, and/or expression of AL. We further suggest that the male mouse model may be useful for identifying sequences in the LAT locus that regulate the latency-reactivation cycle and neuropathogenesis. The first 1.5 kb of LAT promotes spontaneous reactivation (40, 41) and inhibits caspase 8-induced apoptosis efficiently (33). The functions of AL and UOL are not known. Additional studies are required to prove whether AL, UOL, or the 1.5-kb LAT sequence regulates neurovirulence in male BALB/c mice. Since expressions of the 1.5-kb LAT, of UOL, and of AL are regulated by the same promoter and their coding sequences overlap one another, preparing recombinant viruses that express just one of the respective gene products is a challenge.
Acknowledgments
This work was supported by Public Health Service grant 1P20RR15635 and by two USDA grants (2002-35204 and 2003-02213).
REFERENCES
- 1.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, M., and N. Fraser. 2001. Herpes simplex virus type 1 2-kilobase latency-associated transcript intron associates with ribosomal proteins and splicing factors. J. Virol. 75:12070-12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aida, T., N. Ishikawa, and K. Shinkai. 1998. Sex differences in immune responses to cephalothin in guinea pigs. J. Toxicol. Sci. 23:87-91. [DOI] [PubMed] [Google Scholar]
- 4.Barna, M., T. Komatsu, Z. Bi, and C. S. Reiss. 1996. Sex differences in susceptibility to viral infection of the central nervous system. J. Neuroimmunol. 67:31-39. [DOI] [PubMed] [Google Scholar]
- 5.Barrat, F., B. Lesourd, H. J. Boulouis, D. Thibault, N. S. Vincent, B. Gjata, A. Louise, T. Neway, and C. Pilet. 1997. Sex and parity modulate cytokine production during murine aging. Clin. Exp. Immunol. 109:562-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgos, J. S., C. Ramirez, I. Sastre, J. M. Alfaro, and F. Valdivieso. 2005. Herpes simplex virus type 1 infection via the bloodstream with apolipoprotein E dependence in the gonads is influenced by gender. J. Virol. 79:1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantin, E., B. Tanamachi, H. Openshaw, J. Mann, and K. Clarke. 1999. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J. Virol. 73:5196-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croen, K. D., J. M. Ostrove, L. J. Dragovic, J. E. Smialek, and S. E. Straus. 1987. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene “anti-sense” transcript by in situ hybridization. N. Engl. J. Med. 317:1427-1432. [DOI] [PubMed] [Google Scholar]
- 9.Curiel, R. E., M. H. Miller, R. Ishikawa, D. C. Thomas, and N. J. Bigley. 1993. Does the gender difference in interferon production seen in picornavirus-infected spleen cell cultures from ICR Swiss mice have any in vivo significance? J. Interferon Res. 13:387-395. [DOI] [PubMed] [Google Scholar]
- 10.Deatly, A. M., J. G. Spivack, E. Lavi, and N. W. Fraser. 1987. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc. Natl. Acad. Sci. USA 84:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deatly, A. M., J. G. Spivack, E. Lavi, D. R. D. O'Boyle, and N. W. Fraser. 1988. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J. Virol. 62:749-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gesser, R. M., and S. C. Koo. 1997. Latent herpes simplex virus type 1 gene expression in ganglia innervating the human gastrointestinal tract. J. Virol. 71:4103-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, X., P. Lundeberg, B. Tanamachi, H. Openshaw, J. Longmate, and E. Cantin. 2001. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J. Virol. 75:3048-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson, G., W. Peng, L. Jin, G.-C. Perng, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2002. Regulation of caspase 8- and caspase 9-induced apoptosis by the herpes simplex virus latency-associated transcript. J. Neurovirol. 8:103-111. [DOI] [PubMed] [Google Scholar]
- 15.Huber, S., and B. Pfaeffle. 1994. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J. Virol. 68:5126-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huppert, F. A., W. Solomou, S. O'Connor, K. Morgan, P. Sussams, and C. Brayne. 1998. Aging and lymphocyte subpopulations: whole-blood analysis of immune markers in a large population sample of healthy elderly individuals. Exp. Gerontol. 33:593-600. [DOI] [PubMed] [Google Scholar]
- 17.Inman, M., G. C. Perng, G. Henderson, H. Ghiasi, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2001. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 75:3636-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, L., W. Peng, G.-C. Perng, A. B. Nesburn, C. Jones, and S. L. Wechsler. 2003. Identification of herpes simplex virus type 1 latency associated transcript sequences that both inhibit apoptosis and enhance the spontaneous reactivation phenotype. J. Virol. 77:6556-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 51:81-133. [DOI] [PubMed] [Google Scholar]
- 20.Jones, C. 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin. Microbiol. Rev. 16:79-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang, W., R. Mukerjee, and N. F. Fraser. 2003. Establishment and maintenance of HSV latent infection is mediated through correct splicing of the LAT primary transcript. Virology 312:233-244. [DOI] [PubMed] [Google Scholar]
- 22.Krause, P. R., K. D. Croen, S. E. Straus, and J. M. Ostrove. 1988. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J. Virol. 62:4819-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahat, E., J. Barr, G. Barkai, G. Paret, N. Brand, and A. Barzilai. 1999. Long term neurological outcome of herpes encephalitis. Arch. Dis. Child. 80:69-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohr, J. M., J. A. Nelson, and M. B. Oldstone. 1990. Is herpes simplex virus associated with peptic ulcer disease? J. Virol. 64:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundberg, P., P. Welander, H. Openshaw, C. Nalibandian, C. Edwards, L. Moldawer, and E. Cantin. 2003. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J. Virol. 77:11661-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath, N., N. E. Anderson, M. C. Croxson, and K. F. Powell. 1997. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J. Neurol. Neurosurg. Psychiatry 63:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell, W. J., R. P. Lirette, and N. W. Fraser. 1990. Mapping of low abundance latency-associated RNA in the trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J. Gen. Virol. 71:125-132. [DOI] [PubMed] [Google Scholar]
- 28.Mott, K., N. Osorio, L. Jin, D. Brick, J. Naito, J. Cooper, G. Henderson, M. Inman, C. Jones, S. L. Wechsler, and G.-C Perng. 2003. The bovine herpesvirus 1 LR ORF2 is crucial for this gene's ability to restore the high reactivation phenotype to a herpes simplex virus-1 LAT null mutant. J. Gen. Virol. 84:2975-2985. [DOI] [PubMed] [Google Scholar]
- 29.Nahmias, A. J., and B. Roizman. 1973. Infection with herpes-simplex viruses 1 and 2. 3. N. Engl. J. Med. 289:781-789. [DOI] [PubMed] [Google Scholar]
- 30.Naito, J., R. Mukerjee, K. R. Mott, W. Kang, N. Osorio, N. W. Fraser, and G.-C. Perng. 2005. Identification of a protein encoded in the herpes simplex virus type 1 latency associated transcript promoter region. Virus Res. 108:101-110. [DOI] [PubMed] [Google Scholar]
- 31.Nicosia, M., J. M. Zabolotny, R. P. Lirette, and N. W. Fraser. 1994. The HSV-1 2-kb latency-associated transcript is found in the cytoplasm comigrating with ribosomal subunits during productive infection. Virology 204:717-728. [DOI] [PubMed] [Google Scholar]
- 32.Peng, W., G. Henderson, G.-C. Perng, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2003. The gene that encodes the herpes simplex virus type 1 latency-associated transcript influences the accumulation of transcripts (Bcl-xL and Bcl-xS) that encode apoptotic regulatory proteins. J. Virol. 77:10714-10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng, W., L. Jin, G. Henderson, G.-C. Perng, D. J. Brick, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2004. Mapping herpes simplex virus type 1 (HSV-1) LAT sequences that protect from apoptosis mediated by a plasmid expressing caspase-8. J. Neurovirol. 10:260-265. [DOI] [PubMed] [Google Scholar]
- 34.Peng, W., G. Henderson, M. Inman, L. BenMohamed, G.-C. Perng, S. L. Wechsler, and C. Jones. 2005. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal ganglia of acutely infected mice. J. Virol. 79:6162-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perng, G.-C., B. Maguen, L. Jin, K. R. Mott, N. Osorio, S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, M. Inman, G. Henderson, C. Jones, and S. L. Wechsler. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 76:1224-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perng, G.-C., B. Maguen, L. Jin, K. R. Mott, J. Kurylo, L. BenMohamed, A. Yukht, N. Osorio, A. B. Nesburn, G. Henderson, M. Inman, C. Jones, and S. L. Wechsler. 2002. A novel herpes simplex virus type 1 transcript (AL-RNA) antisense to the 5′ end of latency-associated transcript produces a protein in infected rabbits. J. Virol. 76:8003-8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perng, G.-C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hoffman, H. Ghiasi, A. B. Nesburn, and S. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript (LAT). Science 287:1500-1503. [DOI] [PubMed] [Google Scholar]
- 38.Perng, G.-C., D. Esmail, S. Slanina, A. Yukht, H. Ghiasi, N. Osorio, K. R. Mott, B. Maguen, L. Jin, A. B. Nesburn, and S. L. Wechsler. 2001. Three herpes simplex virus type 1 latency-associated transcript mutants with distinct and asymmetric effects on virulence in mice compared with rabbits. J. Virol. 75:9018-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perng, G. C., K. Chokephaibulkit, R. L. Thompson, N. M. Sawtell, S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J. Virol. 70:282-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perng, G. C., E. C. Dunkel, P. A. Geary, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perng, G. C., H. Ghiasi, S. M. Slanina, A. B. Nesburn, and S. L. Wechsler. 1996. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J. Virol. 70:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perng, G. C., S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J. Virol. 70:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perng, G. C., S. M. Slanina, A. Yukht, B. S. Drolet, W. Keleher, Jr., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1999. A herpes simplex virus type 1 latency-associated transcript mutant with increased virulence and reduced spontaneous reactivation. J. Virol. 73:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rock, D. L., A. B. Nesburn, H. Ghiasi, J. Ong, T. L. Lewis, J. R. Lokensgard, and S. L. Wechsler. 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 61:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skoldenberg, B. 1991. Herpes simplex encephalitis. Scand. J. Infect. Dis. Suppl. 80:40-46. [PubMed] [Google Scholar]
- 46.Skoldenberg, B., M. Forsgren, K. Alestig, T. Bergstrom, L. Burman, E. Dahlqvist, A. Forkman, A. Fryden, K. Lovgren, and K. Norlin. 1984. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet ii:707-711. [DOI] [PubMed] [Google Scholar]
- 47.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin, for the GlaxoSmithKline Herpes Vaccine Efficacy Study Group. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 48.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056-1059. [DOI] [PubMed] [Google Scholar]
- 49.Thomas, D. L., M. Lock, J. M. Zabolotny, B. R. Mohan, and N. W. Fraser. 2002. The 2-kilobase intron of the herpes simplex virus type 1 latency-associated transcript has a half-life of approximately 24 hours in SY5Y and COS-1 cells. J. Virol. 76:532-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner, E. K., G. Devi-Rao, L. T. Feldman, A. T. Dobson, Y. F. Zhang, W. M. Flanagan, and J. G. Stevens. 1988. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J. Virol. 62:1194-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner, E. K., W. M. Flanagan, G. Devi-Rao, Y. F. Zhang, J. M. Hill, K. P. Anderson, and J. G. Stevens. 1988. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J. Virol. 62:4577-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss, M. L., M. E. Dobbs, P. S. MohanKumar, S. I. Chowdhury, K. Sawrey, R. Guevara-Guzman, and J. Huang. 2001. The estrous cycle affects pseudorabies virus (PRV) infection of the CNS. Brain Res. 893:215-226. [DOI] [PubMed] [Google Scholar]
- 54.Whitley, R. 1991. Herpes simplex virus infections of the central nervous system. Encephalitis and neonatal herpes. Drugs 42:406-427. [DOI] [PubMed] [Google Scholar]
- 55.Whitley, R. 1997. Herpes simplex virus. Lippincott-Raven Publishers, Philadelphia, Pa.
- 56.Yamada, S., T. Kameyama, S. Nagaya, Y. Hashizume, and M. Yoshida. 2002. Relapsing herpes simplex encephalitis: pathological confirmation of viral reactivation. J. Neurol. Neurosurg. Psychiatry 74:262-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zwaagstra, J. C., H. Ghiasi, S. M. Slanina, A. B. Nesburn, S. C. Wheatley, K. Lillycrop, J. Wood, D. S. Latchman, K. Patel, and S. L. Wechsler. 1990. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J. Virol. 64:5019-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]