Abstract
Most Epstein-Barr virus (EBV)-positive tumor cells contain one of the latent forms of viral infection. The role of lytic viral gene expression in EBV-associated malignancies is unknown. Here we show that EBV mutants that cannot undergo lytic viral replication are defective in promoting EBV-mediated lymphoproliferative disease (LPD). Early-passage lymphoblastoid cell lines (LCLs) derived from EBV mutants with a deletion of either viral immediate-early gene grew similarly to wild-type (WT) virus LCLs in vitro but were deficient in producing LPD when inoculated into SCID mice. Restoration of lytic EBV gene expression enhanced growth in SCID mice. Acyclovir, which prevents lytic viral replication but not expression of early lytic viral genes, did not inhibit the growth of WT LCLs in SCID mice. Early-passage LCLs derived from the lytic-defective viruses had substantially decreased expression of the cytokine interleukin-6 (IL-6), and restoration of lytic gene expression reversed this defect. Expression of cellular IL-10 and viral IL-10 was also diminished in lytic-defective LCLs. These results suggest that lytic EBV gene expression contributes to EBV-associated lymphoproliferative disease, potentially through induction of paracrine B-cell growth factors.
Epstein-Barr virus (EBV), a ubiquitous human herpes virus, can infect cells in either a latent or lytic state. Viral latency is characterized by the expression of a limited subset of viral genes that encode the viral proteins currently known to be required for EBV immortalization of primary B cells (45). Entry into the viral lytic cycle is triggered by expression of either of the two EBV immediate-early (IE) genes, BZLF1 and BRLF1 (reviewed in reference 50). BZLF1 and BRLF1 function as transcriptional activators and initiate an ordered cascade of viral lytic gene expression culminating in release of infectious virus and usually death of the host cell. In addition, BZLF1 and BRLF1 also activate expression of cellular genes that are thought to assist the virus in modulating the host immune response (4, 26, 28).
EBV is strongly associated with both B-cell and epithelial cell malignancies (44). The fact that all EBV-associated malignancies have a predominantly latent pattern of viral gene expression (44), coupled with studies demonstrating that several viral latency proteins are absolutely required for transformation of B cells in vitro (17, 22, 49, 57), has led to the view that only the latent phase of viral gene expression is important during the development of EBV-associated malignancies. Nevertheless, small numbers of lytically infected cells are frequently detected in biopsies of EBV-associated lymphoproliferative disease (LPD) in immunosuppressed individuals. One study reported that 92% of biopsies from EBV-positive LPD in immunosuppressed patients contained some tumor cells with lytic viral gene expression (36). However, as lytically infected cells are thought to die within a few days, such cells must be constantly regenerated within EBV-positive LPD.
There are several potential mechanisms by which EBV lytic gene expression could contribute to the growth of EBV-associated LPD in vivo. By increasing the horizontal transmission of the virus from cell to cell, lytic infection may increase the total number of latently infected cells. In addition, viral lytic genes, or cellular genes induced by viral lytic proteins, could potentially encode paracrine factors that promote tumor growth. For example, the Kaposi's sarcoma herpesvirus (KSHV) lytic protein ORF74 (where ORF is open reading frame) triggers paracrine secretion of the angiogenic factor vascular endothelial growth factor (2) and, thereby, may participate in the formation of Kaposi's sarcoma (35). Another KSHV lytic gene, viral interleukin-6 (vIL-6), encodes a homolog of IL-6 (37) that may function as a paracrine B-cell growth factor in KSHV-associated lymphomas (51).
EBV-associated LPD commonly occurs in immunosuppressed individuals following organ transplant (44) and displays a pattern of viral gene expression (latency III) that closely resembles that of peripheral blood B cells transformed by EBV in vitro (56). Lymphoblastoid cell lines (LCLs) obtained from EBV-transformed B cells grow vigorously when inoculated into SCID mice, providing an animal model for LPD (47). Interestingly, there is increasing evidence that EBV-immortalized LCLs are dependent on paracrine/autocrine secretion of growth factors for optimal growth (3, 58). For example, IL-6 enhances the growth of LCLs in mouse models of LPD (48, 55), and some freshly explanted cells from EBV-positive LPD are dependent on autocrine/paracrine secretion of IL-10 for growth and survival (30). Three different EBV gene products, including the latent LMP1 and EBV-encoded RNA gene products and the IE lytic protein, BZLF1, have been reported to induce expression of cellular IL-10 (cIL-10) (28) (24, 60). EBV also encodes a lytic viral homolog of IL-10, vIL-10 (16), which likewise enhances the growth of B cells (46). Deletion of vIL-10 from the EBV genome did not inhibit EBV transformation of primary B cells or growth of LCLs in SCID mice (54), although some studies have reported a contributory effect of vIL-10 on B-cell transformation (34, 53).
To determine whether lytic infection contributes to EBV-induced LPD in SCID mice, we have examined the phenotype of LCLs derived with wild-type (WT), BZLF1-deleted (Z-KO), or BRLF1-deleted (R-KO) viruses (9), using peripheral blood leukocytes from several different donors to generate LCLs. The Z-KO and R-KO viruses cannot express most viral lytic genes or replicate lytically unless the deleted gene product is supplied in trans (9). Somewhat surprisingly, we show that early-passage LCLs generated from the Z-KO and R-KO viruses (Z-KO and R-KO LCLs) are significantly impaired in comparison to the corresponding WT LCLs in growth in SCID mice. In contrast, acyclovir treatment (which prevents lytic viral replication but not IE and early lytic viral gene expression) did not inhibit the growth of WT LCLs in SCID mice, showing that release of infectious viral particles is not required for efficient tumor formation in SCID mice. The early-passage Z-KO and R-KO LCLs expressed substantially lower levels of IL-6 and somewhat lower levels of cIL-10 than did the WT LCLs, suggesting that the growth defect in SCID mice may be due at least partially to the loss of essential paracrine B-cell growth factors.
MATERIALS AND METHODS
EBV WT, Z-KO, and R-KO viruses and cell lines.
293 cells infected with the R-KO virus, Z-KO virus, and WT virus have been described previously (9). In the R-KO virus, nucleotides 103638 to 105083 (strain B95.8 coordinates, accession number V01555) within the BRLF1 gene were removed via insertion of a tetracycline resistance cassette. In the Z-KO virus, nucleotides 102389 to 103388 (B95.8 coordinates) within the BZLF1 gene were removed via insertion of a kanamycin resistance cassette. The R-KO, Z-KO, and WT viruses also encode enhanced green fluorescent protein and a hygromycin B resistance gene (both cloned into the B95.8 deletion of EBV where the second copy of oriLyt normally resides). LCLs containing the WT, Z-KO, and R-KO viruses were generated as described previously (14), using the supernatant of BZLF1- or BRLF1-transfected 293 cells infected with Z-KO or 293 R-KO virus, respectively, as a source of virus and peripheral blood leukocytes derived from several different anonymous donors (obtained from the American Red Cross). LCLs were maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS) and antibiotics. Z-KO and R-KO LCLs were verified to be BZLF1− and BRLF1− via PCR with BZLF1- and BRLF1-specific primers. LCLs used for in vitro and in vivo experiments were considered “early-passage” LCLs if they were in culture <4 months following the initial transformation event.
Selection of BZLF1-expressing Z-KO cell lines.
pREP9-Zp-Z (which contains the BZLF1 gene driven by its own promoter) was generated by cloning the 1.8-kb BamHI Z EBV fragment (strain B95.8 genome) into the BamHI site of a modified pREP9 vector (Invitrogen) in which the Rous sarcoma virus (RSV) promoter had been removed and a blasticidin resistance cassette inserted. pREP9-RSV-Z (which contains the BZLF1 gene driven by the RSV long terminal repeat promoter) was generated by cloning the KpnI/BglII fragment of the pSG5-Z vector (a gift from S. D. Hayward) into the KpnI/BamHI site of a modified pREP9 vector (pREP9-BSD) in which the G418 resistance cassette had been replaced with a blasticidin resistance cassette. Both pREP9 plasmids contain the EBV oriP element and hence can be stably maintained as episomes in EBNA-1 expressing LCLs. Stable Z-KO LCL lines containing each vector were generated using a nucleoporator (Amaxa) to transfect the cells as described previously (14), followed by drug selection with 7 μg/ml blasticidin (Invitrogen) or 300 μg/ml G418 (Gibco).
In vitro growth assays.
Early-passage LCLs were seeded at 2 × 105 cells/ml in RPMI medium supplemented with either 5% or 2% FBS and antibiotics. Aliquots were counted by trypan blue exclusion every 4 days, and cells were diluted back to 2 × 105 cells/ml with fresh medium at each time point in order to maintain log phase of growth.
In vivo LPD studies.
Female SCID mice (6 to 8 weeks old; National Institutes of Health) were maintained in a pathogen-free enclosure. A total of 5 × 106 early-passage LCLs in 150 μl of sterile 1× phosphate-buffered saline were injected subcutaneously into each flank. LPD size in three dimensions was measured every 2 to 3 days once lesions became palpable. For acylovir (ACV; American Pharmaceutical Partners, Inc.) studies, ACV was added to drinking water at a final concentration of 5 mg/ml starting at day 2 postinjection and was continued for the duration of the experiment. Based on previous studies, this dose of ACV (between 1,333 and 1,666 mg per kg of body weight per day) would result in mean plasma concentrations of between 6.4 and 10.9 μg/ml (11). ACV concentrations of 1.6 μg/ml in human plasma inhibit oropharyngeal lytic infection during infectious mononucleosis (33, 59). Mice were euthanized when lesions were greater than 1 cm3 or mice appeared ill. All experiments were performed in accordance with guidelines set forth by the Institutional Animal Care and Use Committee of the University of North Carolina, Chapel Hill. P values were calculated using an Excel t test.
Immunohistochemistry.
Upon harvesting, LPD lesions were fixed in 10% neutral-buffered formalin overnight, embedded in paraffin, and sectioned. Immunohistochemistry was performed using a BioGenex Super Sensitive non-biotin horseradish peroxidase detection system using a BMRF1 antibody (1:200 dilution; Research Diagnostics, Inc.) or BZLF1 antibody (1:20 dilution; Argene).
Reverse-transcription PCR (RT-PCR) analysis.
Total RNA was harvested from LCLs growing in vitro and reverse transcribed as previously described (26). Resulting cDNA was subjected to PCR using primers and conditions described in Table 1.
TABLE 1.
RT-PCR primers and conditions
| Genea | Primer sequence (5′-3′) | Ta (°C)b | Cycles | Reference |
|---|---|---|---|---|
| LMP2A | F: AGGAACGTGAATCTAATGAAGA | 55 | 38 | 61 |
| R: AAGTGACAACCGCAGTAAGCA | ||||
| EBER1 | F: AAAACATGCGGACCACCAGC | 55 | 25 | 61 |
| R: AGGACCTACGCTGCCCTAGA | ||||
| EBNA-LP | F: AGAGGAGGAGGTGGTAAGCGGTTC | 55 | 30 | 61 |
| R: CTGGCTAAGCCTGTGACTTAG | ||||
| BZLF1 | F: CACGGTAGTGCTGCAGCAGTTGC | 61 | 30 | |
| R: CCCAGAATCAACAGACTAACCAAGCCG | ||||
| BRLF1 | F: GCCATGAGGCCTAAAAAGGATGGC | 56 | 30 | |
| R: GCCCAGCACCTTGCTGTAGGTGTA | ||||
| VIL-10 | F: CGAAGGTTAGTGGTCACTCT | 52 | 35 | 34 |
| R: CACCTGGCTTTAATTGTCATG | ||||
| BHRF1 | F: GTCAAGGTTTCGTCTGTGTG | 55 | 40 | 13 |
| R: TTCTCTTGCTGCTAGCTCCA | ||||
| CIL-10 | F: CTGAGAACCAAGACCCAGACATCAAGG | 61 | 35 | 28 |
| R: CAATAAGGTTTCTCAAGGGGCTGGGTC | ||||
| IL-6 | F: CCACTCACCTCTTCAGAA | 49 | 35 | 40 |
| R: GCGCAGAATGAGATGAGT | ||||
| TGF-β | F: GCTGCACTTGCAGGAGCGCAC | 52 | 30 | 43 |
| R: AAATGGATCCACGAGCCCAA | ||||
| B2-microglobulin | F: TTCTGGCCTGGAGGGCATCC | 56 | 23 | 32 |
| R: ATCTTCAAACCTCCATGATG |
EBNA-LP, Epstein-Barr virus nuclear antigen leader protein; TGF-β, transforming growth factor β.
Ta, annealing temperature.
Immunoblotting.
Immunoblots were performed as previously described (14). Primary antibodies used were as follows: LMP1 (CS1-4; dilution, 1:100; Dako), α-EBNA1 (1 μg/ml; Abi;), α-EBNA2 (1:100; Dako), α-EBNA3A/α-EBNA3B/α-EBNA3C (2 μg/ml; Exalpha); α-BZLF1 (1:100; Argene), α-BRLF1 (1:100; Argene), α-BMRF1 (1:250; Vector Laboratories). Secondary antibodies were horseradish-peroxidase-conjugated secondary anti-mouse (1:10,000; Promega) or anti-sheep (1:5,000; Chemicon). Bound antibody was detected using the ECL system (Amersham). For detection of lytic proteins, LCLs were grown at high densities (>1 × 106 cells/ml) for 48 h, which maximizes detection of lytic proteins (unpublished observations). In addition, LCLs were treated with 5 μg/ml methotrexate (Immunex) for 48 h in order to induce lytic infection (10).
IL-6 and IL-10 ELISAs.
LCLs (WT-, Z-KO-, or R-KO-derived) from the same donor were plated at a density of 3 × 105 cells/ml in RPMI medium with 10% FBS. Enzyme-linked immunosorbent assays (ELISAs) for IL-6 or IL-10 were performed on the supernatants 2 days later using commercially available kits as specified by the manufacturer (R&D Systems).
Apoptosis and cell viability assays.
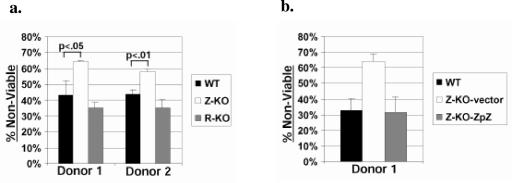
Early-passage LCLs were plated at a concentration of 2 × 105 cells/ml in 100 μl of RPMI medium (without phenol red) supplemented with 2% FBS and antibiotics in a 96-well plate. Anti-Fas antibody (clone CH11; Upstate) was added at 100 ng/ml. Cell viability was assessed 48 h later using trypan blue exclusion (see Fig. 7b) or the Cell Proliferation Kit II (Roche) (see Fig. 7a) according to the manufacturer's instructions. P values were calculated using an Excel t test.
FIG. 7.
Z-KO LCLs are more sensitive than WT LCLs to Fas-induced apoptosis in vitro. (a) Early-passage Z-KO, R-KO, or WT LCLs from two different donors were subjected to treatment with anti-Fas antibody for 48 h. Following treatment, cell viability was assessed as described in Materials and Methods. Data shown represent the mean of a representative experiment done in triplicate; error bars indicate standard deviation. (b) WT LCLs, Z-KO LCLs carrying a control vector (Z-KO-vector), and Z-KO LCLs carrying a BZLF1 expression vector (Z-KO-ZpZ) were subjected to anti-Fas treatment, followed by an assessment of viability. Data shown represent the mean of a representative experiment done in duplicate; error bars indicate standard deviation.
RESULTS
Early-passage Z-KO and R-KO LCLs are impaired for growth in SCID mice.
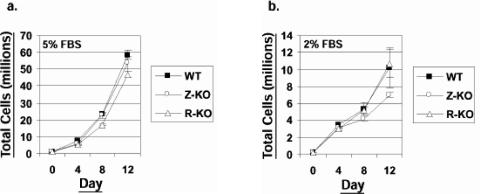
As previously described (9), LCLs obtained with the Z-KO, R-KO, and WT viruses grew at comparable rates in vitro in medium supplemented with 5% serum (Fig. 1a). Z-KO LCLs grew at a slightly slower rate than WT and R-KO LCLs in medium supplemented with 2% serum (Fig. 1b), but this difference was not statistically significant.
FIG. 1.
Z-KO and R-KO LCLs grow at a similar rate to WT LCLs in vitro. Early-passage WT, Z-KO, or R-KO LCLs were plated in medium containing either 5% (a) or 2% (b) FBS. Cells were counted every 4 days, and at each time point the cells were diluted back to 2 × 105 cells/ml to maintain log phase growth. The experiment was done in duplicate with mean total cells plotted on the y axis; error bars indicate standard deviation. Similar results were obtained in samples from two different donors; one representative donor is shown.
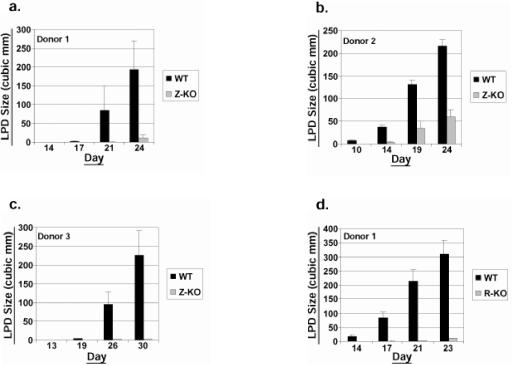
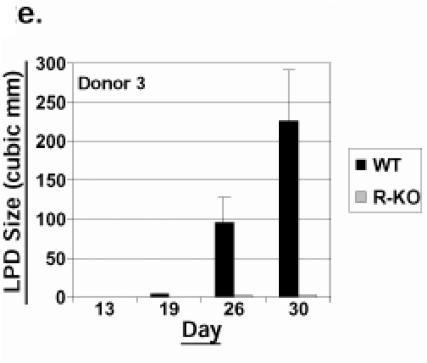
To examine whether lytic EBV infection contributes to the generation of LPD in SCID mice, equal numbers of early-passage (<4 months in culture following the initial transformation event) Z-KO and WT LCLs, both derived from the same donor, were injected subcutaneously into the flanks of SCID mice, and LPD development was monitored over time. Unexpectedly, the Z-KO LCLs from this donor (donor 1) formed LPD lesions at a significantly slower rate than WT LCLs (Fig. 2a). To determine if this defect was unique to this particular donor or Z-KO cell line, we generated WT and Z-KO LCLs from two additional donors. As shown in Fig. 2b and c, Z-KO LCLs derived from the two other donors also grew more slowly when implanted into SCID mice than the WT LCLs. Furthermore, early-passage R-KO LCLs derived from two of the three donors (donors 1 and 3) also exhibited an in vivo growth defect when compared to WT LCLs (Fig. 2d and e). In contrast, one of the three R-KO LCL lines (from donor 2) grew as well as the corresponding WT LCL (data not shown).
FIG.2.
Early-passage Z-KO and R-KO LCLs are impaired for growth in SCID mice. Early-passage WT, Z-KO, or R-KO LCLs were injected subcutaneously into the flanks of SCID mice, and LPD growth was monitored. y axis, mean LPD size in cubic millimeters; x axis, day postinjection. Error bars indicate standard error of the mean. (a) WT versus Z-KO LCL LPD growth (donor 1; eight injection sites per condition). (b) WT versus Z-KO LCL LPD growth (donor 2; eight injection sites per condition). (c) WT versus Z-KO LPD growth (donor 3; eight injection sites per WT condition and four injections sites per Z-KO condition). (d) WT versus R-KO LCL LPD growth (donor 1; eight injection sites per WT condition and four injection sites per R-KO condition). (e) WT versus R-KO LCL LPD growth (donor 3; eight injection sites per condition).
During the course of these experiments, we noted that extensive in vitro passaging of WT LCLs allowed them to grow more quickly (in comparison to the early-passage WT LCLs) when injected into SCID mice (data not shown). Interestingly, Z-KO and R-KO LCLs, which were clearly defective in comparison to the corresponding WT LCLs for growth in SCID mice when tested at early passage (within 4 months of immortalization), also acquired enhanced growth capacity in SCID mice with extended passaging in vitro and eventually became similar to the WT LCLs (data not shown). Thus, the growth defect of the Z-KO and R-KO LCLs was most apparent in newly derived lines.
These results suggested that one or more aspects of lytic EBV infection contribute to the growth of early-passage LCLs in SCID mice. To determine if the release of infectious viral particles is required for this effect, mice injected with WT LCLs were treated with or without the antiviral drug ACV, which inhibits the late phase of lytic replication without affecting the expression of IE or early lytic viral genes. ACV did not impair the growth of WT LCLs in SCID mice (data not shown). These results suggest that early lytic viral gene expression, but not the release of infectious viral particles, contributes to the enhanced growth of the early-passage WT LCLs in SCID mice.
BZLF1 expression rescues the in vivo growth defect of Z-KO LCLs in SCID mice.
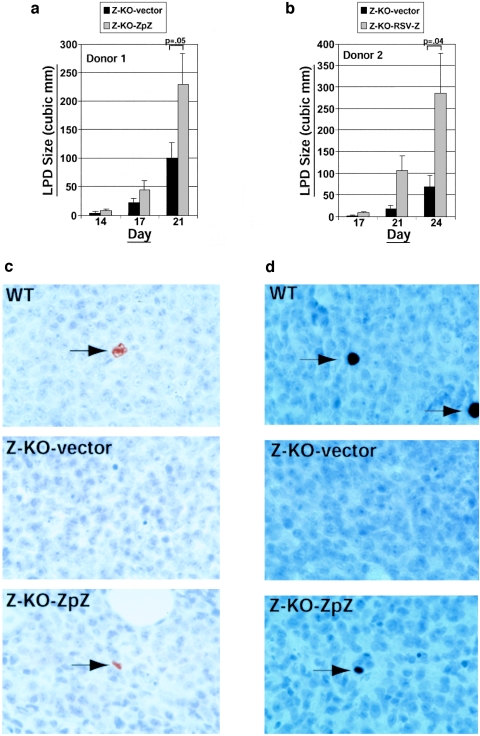
To ensure that the in vivo phenotype observed with the Z-KO LCLs was due to the lytic-defective state of the Z-KO LCLs, we determined whether restoration of BZLF1 expression in the Z-KO LCLs would reverse the phenotype in SCID mice. The BZLF1 gene was cloned under the control of its own promoter (donor 1) or the RSV promoter (donor 2) into the oriP-containing vector, pREP9, which can be stably maintained in EBV-positive cells as an episome. Z-KO LCLs stably expressing BZLF1 (or the vector control) were selected and amplified in vitro over a period of ∼2 months, and then tested for the ability to form LPD when injected into SCID mice. As shown in Fig. 3a and b, the growth-defective phenotype of Z-KO LCLs was somewhat attenuated by the number of in vitro passages required to select for stable cell lines. Nevertheless, Z-KO LCLs in which the BZLF1 gene was reexpressed under its own promoter or the RSV promoter, in the pREP9 vector clearly grew significantly faster than Z-KO LCLs carrying the vector control.
FIG. 3.
BZLF1 expression rescues the in vivo defect of Z-KO LCLs in SCID mice. Z-KO LCLs carrying a control vector (Z-KO-vector) or a BZLF1 expression vector driven by the endogenous BZLF1 promoter (Z-KO-ZpZ) (a) or a BZLF1 expression vector driven by the RSV promoter (Z-KO-RSV-Z) (b) were injected subcutaneously into the flanks of SCID mice, and LPD growth was monitored over time. y axis, mean LPD size in cubic millimeters; x axis, day postinjection. Error bars indicate standard error of the mean. Data shown in panel a represent the mean of six injection sites per condition for Z-KO-vector and Z-KO-ZpZ LCLs from donor 1. Data shown in panel b represent the mean of eight injection sites per condition for Z-KO-vector and Z-KO-RSV-Z LCLs from donor 2. (c) BZLF1 immunohistochemistry of LPD harvested from WT LCL-injected mice (WT), Z-KO-vector LCL-injected mice (Z-KO-vector), or Z-KO-ZpZ LCL-injected mice (Z-KO-ZpZ). Arrows indicate positively staining cells. (d) BMRF1 immunohistochemistry of LPD harvested from WT LCL-injected mice (WT), Z-KO-vector LCL-injected mice (Z-KO-vector), or Z-KO-ZpZ LCL-injected mice (Z-KO-ZpZ). Arrows indicate positively staining cells.
Immunohistochemical staining of the tumors was performed to compare the amount of lytic EBV gene expression in LPD lesions derived from WT LCLs, Z-KO LCLs carrying the control vector, or Z-KO LCLs carrying a BZLF1 expression vector. Expression of the lytic genes BZLF1 and BMRF1 was observed in approximately 1% of cells in the LPD lesions derived from WT LCL, whereas the LPD lesions derived from the Z-KO LCLs carrying the control vector, as expected, had no detectable lytic EBV gene expression (Fig. 3c and d). LPD lesions derived from the Z-KO LCLs carrying the BZLF1 expression vector (using the endogenous promoter) had a similar level of expression of the lytic genes BZLF1 and BMRF1 as the LPD lesions derived from WT LCLs (Fig. 3d). Other than the differences in LPD size and lytic EBV gene expression, no other obvious differences were observed between the WT versus Z-KO-derived LPD as assessed by hematoxylin and eosin staining (data not shown).
Viral gene expression in Z-KO, R-KO, and WT LCLs.
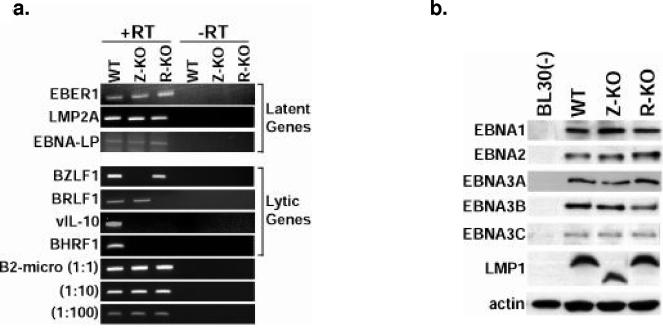
Latent viral gene expression was examined using both RT-PCR analysis and Western blotting (Fig. 4). Latent gene expression between WT, Z-KO, and R-KO LCLs was comparable among all latency genes, with the exception that the Z-KO LCLs expressed a truncated form of LMP1. Sequencing revealed that this truncated form of LMP1 was due to an in-frame deletion of residues 254 to 302 (data not shown). This region of LMP1, encoded by a variable number of a repeated sequence, was previously shown to be dispensable for EBV immortalization of B cells (6, 18) and does not overlap either of the essential signaling/transforming motifs of LMP1 (CTAR1 and CTAR2) (19, 20). As the R-KO LCLs, which express full-length LMP1, were also impaired for growth in SCID mice and reexpression of BZLF1 in the Z-KO LCLs rescued the in vivo phenotype, this LMP1 deletion is unlikely to contribute to the in vivo growth defect of the Z-KO and R-KO LCLs.
FIG.4.
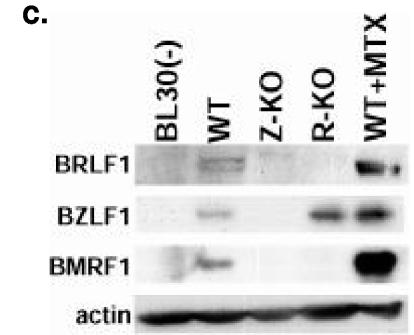
Viral gene expression in Z-KO, R-KO, and WT LCLs. (a) Analysis of viral gene expression in Z-KO, R-KO, and WT LCLs growing in vitro using RT-PCR analysis. Similar results were obtained in samples from two different donors; one representative donor is shown. To account for potential contamination by genomic DNA in the PCR, control reactions containing RNA not transcribed with reverse transcriptase were included (−RT). Serial dilutions of the cDNAs were also subjected to PCR analysis using primers for B2-microglobulin (B2-micro) as a loading control. (b) Analysis of latent viral gene expression using immunoblot analysis of total protein from LCLs growing in vitro. BL30(−) is an EBV-negative Burkitt lymphoma line. (c) Analysis of lytic viral gene expression of total protein from LCLs growing in vitro. WT LCLs treated with the lytic inducing agent methotrexate (WT+MTX) were included as a positive control for lytic protein expression.
The WT LCLs expressed both the BZLF1 and BRLF1 IE lytic genes (Fig. 4a and c). As expected, neither the Z-KO nor R-KO LCLs had detectable expression of their deleted genes (Fig. 4a). However, in contrast to the completely latent phenotype of these mutants in 293 cells (14), Z-KO LCLs expressed BRLF1 at the RNA level, and R-KO LCLs expressed BZLF1 at both the RNA and protein level (Fig. 4a). Both the Z-KO and R-KO LCLs had dramatically decreased expression of the early lytic gene, BHRF1 (Fig. 4a), which encodes a bcl-2 homolog (29), the early lytic gene, BMRF1 (Fig. 4c), and the late lytic gene, vIL-10 (Fig. 4a), in comparison to the WT LCLs.
Early-passage Z-KO and R-KO LCLs express lower levels of IL-6 and cIL-10 in vitro.
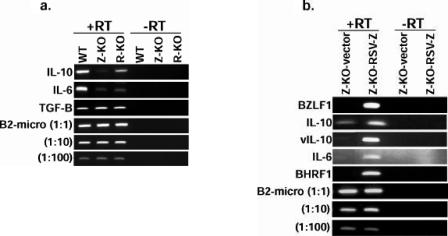
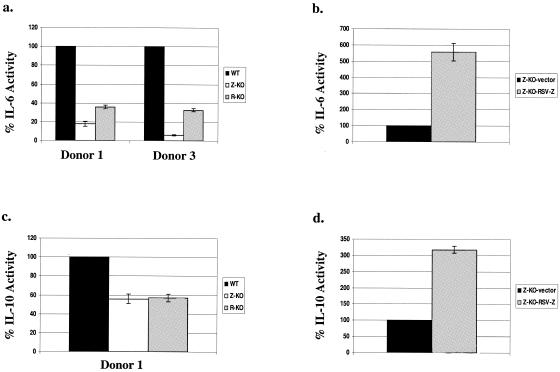
We next examined whether early-passage Z-KO and R-KO LCLs had reduced expression of the B-cell paracrine growth factors IL-6 and cIL-10 in comparison to WT LCLs by using semiquantitative RT-PCR techniques (Fig. 5a). Expression of IL-6 was markedly decreased in both the Z-KO and R-KO LCLs derived from two different donors in comparison to the corresponding WT LCLs. Z-KO and R-KO LCLs derived from two different donors also had decreased expression of cIL-10 in comparison to the corresponding WT LCLs (Fig. 5a). In contrast, although BZLF1 was previously shown to activate transforming growth factor β expression when expressed from a heterologous promoter in HeLa cells (4), transforming growth factor β expression was similar in WT, Z-KO, and R-KO LCLs. Importantly, reestablishment of BZLF1 expression in the Z-KO LCLs increased IL-6 and cIL-10 expression, as well as vIL-10 expression (Fig. 5b), confirming that the defects in cIL-10, IL-6, and vIL-10 mRNA expression were indeed secondary to the loss of lytic EBV gene expression.
FIG. 5.
Early-passage Z-KO and R-KO LCLs express lower levels of IL-6 and cIL-10 mRNA in vitro than the corresponding WT LCLs. (a) Total RNA from early-passage LCLs growing in vitro was subjected to RT-PCR analysis. Similar results were obtained in samples from two different donors; one representative donor is shown. −RT, no reverse transcriptase control. Serial dilutions of the cDNAs were also subjected to PCR analysis using primers for B2-microglobulin (B2-micro) as a loading control. (b) RT-PCR of RNA harvested from Z-KO LCLs carrying a control vector (Z-KO-vector) or a BZLF1-expression vector (Z-KO-RSV-Z).
To confirm that lytic-defective LCLs secrete less IL-6 and cIL-10 than the corresponding WT LCLs, ELISAs were performed to quantitate the amount of IL-6 and IL-10 in the supernatants of WT, Z-KO, and R-KO LCLs derived from the same donor. The supernatants from each of the three Z-KO LCLs contained considerably less IL-6 than the supernatants of the corresponding WT LCL (Fig. 6a and data not shown). Furthermore, restoration of BZLF1 expression in the Z-KO LCLs resulted in enhanced IL-6 secretion (Fig. 6b). Supernatants from two of the three R-KO LCLs also had reduced IL-6 in comparison to the WT LCLs (Fig. 6b and data not shown). Interestingly, the R-KO LCL derived from donor 2, which was the only R-KO LCL able to grow as efficiently as the WT LCL in SCID mice, expressed as much IL-6 as the corresponding WT LCL (data not shown).
FIG. 6.
Supernatants of early-passage Z-KO and R-KO LCLs have lower levels of IL-6 and cIL-10 in vitro than the corresponding WT LCLs. The amount of IL-6 and cIL-10 in the supernatants of early-passage LCLs growing in vitro was quantitated by ELISA as described in Materials and Methods. Similar results were obtained from the Z-KO LCLS in samples from three different donors; results from representative donors are shown. Similar results from the R-KO LCLs were obtained in two out of three donors as discussed in the text. (a) IL-6 levels in the supernatants of WT, Z-KO, and R-KO LCLS. Results are normalized such that the amount of IL-6 in each WT LCL supernatant is set at 100%. (b) IL-6 levels in the supernatants harvested from Z-KO LCLs carrying a control vector (Z-KO-vector) or a BZLF1-expression vector (Z-KO-RSV-Z). Results are normalized such that the amount of IL-6 in the supernatant of Z-KO LCLs carrying the control vector is set at 100%. (c) IL-10 levels in the supernatants of WT, Z-KO, and R-KO LCLS Results are normalized such that the amount of IL-10 in each WT LCL supernatant is set at 100%. (d) IL-10 levels in the supernatants harvested from Z-KO LCLs carrying a control vector (Z-KO-vector) or a BZLF1-expression vector (Z-KO-RSV-Z). Results are normalized such that the amount of IL-10 in the supernatant of Z-KO LCLs carrying the control vector is set at 100%.
The supernatants of the Z-KO LCLs and two of the three R-KO LCLs also had reduced amounts of cIL-10 in comparison to the WT LCLs (Fig. 6c and data not shown), although this effect was not as striking as the reduction of IL-6. As was the case for IL-6, the R-KO LCL from donor 2 was found to have a similar level of cIL-10 as the corresponding WT LCL (data not shown). Restoration of BZLF1 expression in the Z-KO LCLs increased the amount of cIL-10 in the supernatant (Fig. 6d). These results suggest that the in vivo growth defects of the early-passage Z-KO and R-KO LCLs may be due to diminished IL-6 and, to a lesser extent, cIL-10 secretion in comparison to the corresponding WT LCLs.
Z-KO LCLs are more sensitive than WT LCLs to Fas- induced apoptosis in vitro.
We also examined whether early-passage Z-KO or R-KO LCLs are more susceptible than WT-LCLs to Fas-mediated apoptosis, as both Z-KO and R-KO LCLs do not express the antiapoptotic lytic viral gene, BHRF1 (Fig. 4). Z-KO LCLs derived from two different donors exhibited a statistically significant increase in the percentage of cells killed following treatment with anti-Fas antibody in comparison to the corresponding WT LCLs (Fig. 7a), and this effect was reversed when BZLF1 was expressed in the Z-KO LCLs (Fig. 7b). However, no difference was noted between R-KO and WT LCLs, suggesting that the enhanced sensitivity of the Z-KO LCLs to apoptotic stimuli is not due to decreased BHRF1 but, instead, may reflect an antiapoptotic effect mediated directly by the BZLF1 protein. The level of Fas expression in WT versus lytic-defective LCLs was similar (data not shown).
DISCUSSION
In this paper we have examined the potential role of lytic viral gene expression in the development of EBV-induced LPD. We show that LCLs generated with lytic-defective viruses grow similarly to WT LCLs in vitro but at early passage are significantly impaired in their ability to form LPD in SCID mice. Furthermore, restoration of lytic gene expression in LCLs derived from lytic-defective viruses enhances their growth in SCID mice. As the lytic-defective LCLs also had decreased expression of several B-cell growth factors, in particular IL-6, which are known to be important for LCL growth in SCID mice (3, 46, 48, 55, 58), our results suggest that a small number of lytically infected cells contributes to the growth of the latently infected cells in LPD by inducing the release paracrine B-cell growth factors. In addition, as shown in the accompanying paper (15), lytic-defective LCLs also secrete less vascular endothelial growth factor than the WT LCLs and cannot support angiogenesis in vitro. Thus, lytically infected cells in EBV-positive tumors may contribute to tumor formation through the release of paracrine growth factors as well as angiogenesis factors.
Early-passage Z-KO and R-KO LCLs expressed lower levels of the B-cell growth factors IL-6, cIL-10, and vIL-10 than WT LCLs. Furthermore, this decrease in IL-6, cIL-10, and vIL-10 expression in Z-KO LCLs was reversed when BZLF1 was expressed in trans. Both cIL-10 and IL-6 have already been heavily implicated in the development of EBV-positive LPD, both in transplant patients (12, 23, 25) and in mouse models of EBV LPD (1, 3, 7, 31, 34, 39, 48). Most compellingly, when 12 patients with transplant-associated LPD were treated with an anti-IL-6 antibody, 8 of the patients had a remission or cure of the disease (12). In addition to the paracrine growth effects of cIL-6 and cIL-10, the virally encoded homologue of cIL-10, vIL-10, has also been shown to enhance viral transformation of B cells in vitro (34, 53) and to stimulate growth of B cells (46). Thus, the decrease in both cIL-10 and vIL-10 expression which occurs in the Z-KO and R-KO LCLs may have a greater impact on LPD growth in SCID mice than was previously observed using LCLs immortalized with a vIL-10-deleted EBV (12).
While early-passage LCLs derived from either the Z-KO or R-KO viruses were impaired for growth in SCID mice, the phenotype of the Z-KO LCLs was somewhat more severe. Although the R-KO LCLs formed tumors poorly in two different donors (Fig. 2), we identified one donor in which the Z-KO, but not R-KO, LCL was deficient in tumor growth (unpublished data). The efficiency at which EBV+ LPD develops in SCID mice is highly donor dependent (21, 38, 42). We speculate that BZLF1 is more important than BRLF1 for activation of cIL-10 and IL-6. Polymorphisms in the human IL-10 and IL-6 promoters affect their expression (5, 8), and LCLs derived from donors with higher constitutive levels of IL-6 and/or cIL-10 may be less dependent upon BZLF1-activated cytokine production. Of note, the one R-KO LCL (derived from donor 2) that grew efficiently in SCID mice at early passage was also the only R-KO LCL that expressed IL-6 and cIL-10 at a level similar to that of the corresponding WT LCL. Thus, the level of IL-6 and IL-10 expression in early-passage Z-KO and R-KO LCLs in comparison to the corresponding WT LCL was highly correlated with their ability to grow as efficiently as the WT LCL when injected into SCID mice.
Although we believe that the defective phenotype of early-passage lytic-defective LCLs is due to the loss of lytic viral protein expression, during the course of these studies we discovered that the Z-KO virus has a small in-frame deletion in the LMP1 gene. This small LMP1 deletion, which was observed in the Z-KO but not R-KO LCLs, is clearly not important for in vitro transformation of B cells but could conceivably contribute to the impaired growth of the Z-KO LCLs in SCID mice. However, as restoration of BZLF1 expression alone in trans was sufficient to enhance the growth of Z-KO LCLs, this suggests that the growth defect of Z-KO LCLs in SCID mice is due solely or primarily to the lack of lytic gene expression.
Another possible mechanism by which lytic EBV gene expression could contribute to LPD growth would be virally mediated resistance to apoptosis. Consistent with this, Z-KO LCLs were more sensitive to Fas-induced cytotoxicity than the WT LCLs, and this defect was reversed when BZLF1 was expressed in trans. This phenotype in the Z-KO LCLs was unlikely due to loss of BHRF1 expression, as the R-KO LCLs also had no BHRF1 expression and did not have enhanced apoptosis in response to Fas. BZLF1 itself may have an antiapoptotic effect in some circumstances, consistent with its ability to inhibit p53 function (32). If so, the BZLF1 expressed in R-KO LCLs may be sufficient to mediate this antiapoptotic effect.
While our results are most applicable to EBV-induced LPD, whether lytic infection contributes to other types of EBV-associated lymphomas (or epithelial cell tumors) remains unknown. Although EBV-positive Burkitt lymphomas also contain a portion of cells with lytic infection (62), lytically infected cells are rarely found in EBV-positive Hodgkin's disease (41). Our finding that lytic-defective LCLs maintained in culture for many passages eventually regained the ability to grow efficiently in SCID mice suggests that lytic EBV infection may be more important during the early stages of LPD and probably becomes unnecessary as tumor cells are selected for with additional growth-promoting alterations.
Interestingly, much of the anti-EBV cytotoxic T-cell response is directed against lytic viral antigens (52). Our results suggest that the decreased ability of immunosuppressed hosts to control the lytic form of EBV may promote the development of LPD not only by allowing enhanced horizontal transmission of the virus but also by increasing the number of lytically infected tumor cells. In addition, novel drugs that inhibit IE or early viral gene expression, rather than the later step of viral replication, could potentially be useful for decreasing EBV-induced LPD in high-risk patients. Agents that block fatty acid synthase activity were recently shown to inhibit early lytic EBV gene expression (27) and thus might be useful in this regard.
Acknowledgments
This work was supported by Public Health Service grants P01-CA19014, R01-CA66519, and R01-CA58853 (to S.C.K.) from the National Cancer Institute and P30 A150410 to the UNC Center for AIDS Research.
REFERENCES
- 1.Baiocchi, R. A., M. E. Ross, J. C. Tan, C. C. Chou, L. Sullivan, S. Haldar, M. Monne, M. V. Seiden, S. K. Narula, J. Sklar, et al. 1995. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood 85:1063-1074. [PubMed] [Google Scholar]
- 2.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 3.Beatty, P. R., S. M. Krams, and O. M. Martinez. 1997. Involvement of IL-10 in the autonomous growth of EBV-transformed B cell lines. J. Immunol. 158:4045-4051. [PubMed] [Google Scholar]
- 4.Cayrol, C., and E. K. Flemington. 1995. Identification of cellular target genes of the Epstein-Barr virus transactivator Zta: activation of transforming growth factor beta igh3 (TGF-beta igh3) and TGF-beta 1. J. Virol. 69: 4206-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordano, P., A. Lake, L. Shield, G. M. Taylor, F. E. Alexander, P. R. Taylor, J. White, and R. F. Jarrett. 2005. Effect of IL-6 promoter polymorphism on incidence and outcome in Hodgkin's lymphoma. Br. J. Haematol. 128: 493-495. [DOI] [PubMed] [Google Scholar]
- 6.Dirmeier, U., B. Neuhierl, E. Kilger, G. Reisbach, M. L. Sandberg, and W. Hammerschmidt. 2003. Latent membrane protein 1 is critical for efficient growth transformation of human B cells by Epstein-Barr virus. Cancer Res. 63:2982-2989. [PubMed] [Google Scholar]
- 7.Durandy, A., D. Emilie, M. Peuchmaur, M. Forveille, C. Clement, J. Wijdenes, and A. Fischer. 1994. Role of IL-6 in promoting growth of human EBV-induced B-cell tumors in severe combined immunodeficient mice. J. Immunol. 152:5361-5367. [PubMed] [Google Scholar]
- 8.Eskdale, J., G. Gallagher, C. L. Verweij, V. Keijsers, R. G. Westendorp, and T. W. Huizinga. 1998. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc. Natl. Acad. Sci. USA 95:9465-9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng, W. H., J. I. Cohen, S. Fischer, L. Li, M. Sneller, R. Goldbach-Mansky, N. Raab-Traub, H. J. Delecluse, and S. C. Kenney. 2004. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl. Cancer Inst. 96:1691-1702. [DOI] [PubMed] [Google Scholar]
- 11.Glasgow, L. A., J. T. Richards, and E. R. Kern. 1982. Effect of acyclovir treatment on acute and chronic murine cytomegalovirus infection. Am. J. Med. 73:132-137. [DOI] [PubMed] [Google Scholar]
- 12.Haddad, E., S. Paczesny, V. Leblond, J. M. Seigneurin, M. Stern, A. Achkar, M. Bauwens, V. Delwail, D. Debray, C. Duvoux, P. Hubert, B. Hurault de Ligny, J. Wijdenes, A. Durandy, and A. Fischer. 2001. Treatment of B-lymphoproliferative disorder with a monoclonal anti-interleukin-6 antibody in 12 patients: a multicenter phase 1-2 clinical trial. Blood 97:1590-1597. [DOI] [PubMed] [Google Scholar]
- 13.Hayes, D. P., A. A. Brink, M. B. Vervoort, J. M. Middeldorp, C. J. Meijer, and A. J. van den Brule. 1999. Expression of Epstein-Barr virus (EBV) transcripts encoding homologues to important human proteins in diverse EBV associated diseases. Mol. Pathol. 52:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong, G. K., H. J. Delecluse, H. Gruffat, T. E. Morrison, W. H. Feng, A. Sergeant, and S. C. Kenney. 2004. The BRRF1 early gene of Epstein-Barr virus encodes a transcription factor that enhances induction of lytic infection by BRLF1. J. Virol. 78:4983-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong, G. K., P. Kumar, L. Wang, B. Damania, M. L. Gulley, H.-J. Delecluse, P. J. Polverini, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 79:13984-13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu, D. H., R. de Waal Malefyt, D. F. Fiorentino, M. N. Dang, P. Vieira, J. de Vries, H. Spits, T. R. Mosmann, and K. W. Moore. 1990. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science 250:830-832. [DOI] [PubMed] [Google Scholar]
- 17.Humme, S., G. Reisbach, R. Feederle, H. J. Delecluse, K. Bousset, W. Hammerschmidt, and A. Schepers. 2003. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc. Natl. Acad. Sci. USA 100:10989-10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izumi, K. M., E. D. Cahir McFarland, E. A. Riley, D. Rizzo, Y. Chen, and E. Kieff. 1999. The residues between the two transformation effector sites of Epstein-Barr virus latent membrane protein 1 are not critical for B-lymphocyte growth transformation. J. Virol. 73:9908-9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumi, K. M., K. M. Kaye, and E. D. Kieff. 1997. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 94:1447-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johannessen, I., T. Haque, J. N′Jie-Jobe, and D. H. Crawford. 1998. Non-correlation of in vivo and in vitro parameters of Epstein-Barr virus persistence suggests heterogeneity of B cell infection. J. Gen. Virol. 79:1631-1636. [DOI] [PubMed] [Google Scholar]
- 22.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri, V. P., and M. A. Caligiuri. 1998. A review of the association between interleukin-10 and human B-cell malignancies. Cancer Immunol. Immunother. 46:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitagawa, N., M. Goto, K. Kurozumi, S. Maruo, M. Fukayama, T. Naoe, M. Yasukawa, K. Hino, T. Suzuki, S. Todo, and K. Takada. 2000. Epstein-Barr virus-encoded poly(A)− RNA supports Burkitt's lymphoma growth through interleukin-10 induction. EMBO J. 19:6742-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurzrock, R. 2001. Cytokine deregulation in cancer. Biomed. Pharmacother. 55:543-547. [DOI] [PubMed] [Google Scholar]
- 26.Li, Y., N. P. Mahajan, J. Webster-Cyriaque, P. Bhende, G. K. Hong, H. S. Earp, and S. Kenney. 2004. The C-mer gene is induced by Epstein-Barr virus immediate-early protein BRLF1. J. Virol. 78:11778-11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Y., J. Webster-Cyriaque, C. C. Tomlinson, M. Yohe, and S. Kenney. 2004. Fatty acid synthase expression is induced by the Epstein-Barr virus immediate-early protein BRLF1 and is required for lytic viral gene expression. J. Virol. 78:4197-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahot, S., A. Sergeant, E. Drouet, and H. Gruffat. 2003. A novel function for the Epstein-Barr virus transcription factor EB1/Zta: induction of transcription of the hIL-10 gene. J. Gen. Virol. 84:965-974. [DOI] [PubMed] [Google Scholar]
- 29.Marchini, A., B. Tomkinson, J. I. Cohen, and E. Kieff. 1991. BHRF1, the Epstein-Barr virus gene with homology to Bc12, is dispensable for B-lymphocyte transformation and virus replication. J. Virol. 65:5991-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masood, R., Y. Zhang, M. W. Bond, D. T. Scadden, T. Moudgil, R. E. Law, M. H. Kaplan, B. Jung, B. M. Espina, Y. Lunardi-Iskandar, et al. 1995. Interleukin-10 is an autocrine growth factor for acquired immunodeficiency syndrome-related B-cell lymphoma. Blood 85:3423-3430. [PubMed] [Google Scholar]
- 31.Mauray, S., M. T. Fuzzati-Armentero, P. Trouillet, M. Ruegg, G. Nicoloso, M. Hart, L. Aarden, M. Schapira, and M. A. Duchosal. 2000. Epstein-Barr virus-dependent lymphoproliferative disease: critical role of IL-6. Eur. J. Immunol. 30:2065-2073. [DOI] [PubMed] [Google Scholar]
- 32.Mauser, A., S. Saito, E. Appella, C. W. Anderson, W. T. Seaman, and S. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms. J. Virol. 76:12503-12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKendrick, M. W., J. I. McGill, J. E. White, and M. J. Wood. 1986. Oral acyclovir in acute herpes zoster. Br. Med. J. 293:1529-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazaki, I., R. K. Cheung, and H. M. Dosch. 1993. Viral interleukin 10 is critical for the induction of B cell growth transformation by Epstein-Barr virus. J. Exp. Med. 178:439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montaner, S., A. Sodhi, A. Molinolo, T. H. Bugge, E. T. Sawai, Y. He, Y. Li, P. E. Ray, and J. S. Gutkind. 2003. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3:23-36. [DOI] [PubMed] [Google Scholar]
- 36.Montone, K. T., R. L. Hodinka, K. E. Salhany, E. Lavi, A. Rostami, and J. E. Tomaszewski. 1996. Identification of Epstein-Barr virus lytic activity in post-transplantation lymphoproliferative disease. Mod. Pathol. 9:621-630. [PubMed] [Google Scholar]
- 37.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 38.Mosier, D. E., G. R. Picchio, S. M. Baird, R. Kobayashi, and T. J. Kipps. 1992. Epstein-Barr virus-induced human B-cell lymphomas in SCID mice reconstituted with human peripheral blood leukocytes. Cancer Res. 52(19 Suppl.):5552s-5553s. [PubMed] [Google Scholar]
- 39.Nepomuceno, R. R., C. E. Balatoni, Y. Natkunam, A. L. Snow, S. M. Krams, and O. M. Martinez. 2003. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein-Barr virus B-cell lymphomas. Cancer Res. 63:4472-4480. [PubMed] [Google Scholar]
- 40.Ogura, N., M. Tobe, H. Sakamaki, H. Kujiraoka, M. Akiba, Y. Abiko, and H. Nagura. 2002. Interleukin-1 beta induces interleukin-6 mRNA expression and protein production in synovial cells from human temporomandibular joint. J. Oral Pathol. Med. 31:353-360. [DOI] [PubMed] [Google Scholar]
- 41.Pallesen, G., K. Sandvej, S. J. Hamilton-Dutoit, M. Rowe, and L. S. Young. 1991. Activation of Epstein-Barr virus replication in Hodgkin and Reed-Sternberg cells. Blood 78:1162-1165. [PubMed] [Google Scholar]
- 42.Picchio, G. R., R. Kobayashi, M. Kirven, S. M. Baird, T. J. Kipps, and D. E. Mosier. 1992. Heterogeneity among Epstein-Barr virus-seropositive donors in the generation of immunoblastic B-cell lymphomas in SCID mice receiving human peripheral blood leukocyte grafts. Cancer Res. 52:2468-2477. [PubMed] [Google Scholar]
- 43.Raju, J., B. McCarthy, and R. P. Bird. 2002. Steady state levels of transforming growth factor-beta1 and -beta2 mRNA and protein expression are elevated in colonic tumors in vivo irrespective of dietary lipids intervention. Int. J. Cancer 100:635-641. [DOI] [PubMed] [Google Scholar]
- 44.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 45.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus and its replication, p. 2511-2553. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 46.Rousset, F., E. Garcia, T. Defrance, C. Peronne, N. Vezzio, D. H. Hsu, R. Kastelein, K. W. Moore, and J. Banchereau. 1992. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 89:1890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowe, M., L. S. Young, J. Crocker, H. Stokes, S. Henderson, and A. B. Rickinson. 1991. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J. Exp. Med. 173:147-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scala, G., I. Quinto, M. R. Ruocco, A. Arcucci, M. Mallardo, P. Caretto, G. Forni, and S. Venuta. 1990. Expression of an exogenous interleukin 6 gene in human Epstein-Barr virus B cells confers growth advantage and in vivo tumorigenicity. J. Exp. Med. 172:61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skare, J., J. Farley, J. L. Strominger, K. O. Fresen, M. S. Cho, and H. zur Hausen. 1985. Transformation by Epstein-Barr virus requires DNA sequences in the region of BamHI fragments Y and H. J. Virol. 55:286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Speck, S. H., T. Chatila, and E. Flemington. 1997. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol. 5:399-405. [DOI] [PubMed] [Google Scholar]
- 51.Staskus, K. A., R. Sun, G. Miller, P. Racz, A. Jaslowski, C. Metroka, H. Brett-Smith, and A. T. Haase. 1999. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J. Virol. 73:4181-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steven, N. M., N. E. Annels, A. Kumar, A. M. Leese, M. G. Kurilla, and A. B. Rickinson. 1997. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J. Exp. Med. 185:1605-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stuart, A. D., J. P. Stewart, J. R. Arrand, and M. Mackett. 1995. The Epstein-Barr virus encoded cytokine viral interleukin-10 enhances transformation of human B lymphocytes. Oncogene 11:1711-1719. [PubMed] [Google Scholar]
- 54.Swaminathan, S., R. Hesselton, J. Sullivan, and E. Kieff. 1993. Epstein-Barr virus recombinants with specifically mutated BCRF1 genes. J. Virol. 67:7406-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanner, J., and G. Tosato. 1991. Impairment of natural killer functions by interleukin 6 increases lymphoblastoid cell tumorigenicity in athymic mice. J. Clin. Investig. 88:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas, J. A., N. A. Hotchin, M. J. Allday, P. Amlot, M. Rose, M. Yacoub, and D. H. Crawford. 1990. Immunohistology of Epstein-Barr virus-associated antigens in B cell disorders from immunocompromised individuals. Transplantation 49:944-953. [DOI] [PubMed] [Google Scholar]
- 57.Tomkinson, B., E. Robertson, and E. Kieff. 1993. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J. Virol. 67:2014-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tosato, G., J. Tanner, K. D. Jones, M. Revel, and S. E. Pike. 1990. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J. Virol. 64:3033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tynell, E., E. Aurelius, A. Brandell, I. Julander, M. Wood, Q. Y. Yao, A. Rickinson, B. Akerlund, and J. Andersson. 1996. Acyclovir and prednisolone treatment of acute infectious mononucleosis: a multicenter, double-blind, placebo-controlled study. J. Infect. Dis. 174:324-331. [DOI] [PubMed] [Google Scholar]
- 60.Vockerodt, M., B. Haier, P. Buttgereit, H. Tesch, and D. Kube. 2001. The Epstein-Barr virus latent membrane protein 1 induces interleukin-10 in Burkitt's lymphoma cells but not in Hodgkin's cells involving the p38/SAPK2 pathway. Virology 280:183-198. [DOI] [PubMed] [Google Scholar]
- 61.Webster-Cyriaque, J., and N. Raab-Traub. 1998. Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia. Virology 248:53-65. [DOI] [PubMed] [Google Scholar]
- 62.Xue, S. A., L. G. Labrecque, Q. L. Lu, S. K. Ong, I. A. Lampert, P. Kazembe, E. Molyneux, R. L. Broadhead, E. Borgstein, and B. E. Griffin. 2002. Promiscuous expression of Epstein-Barr virus genes in Burkitt's lymphoma from the central African country Malawi. Int. J. Cancer 99:635-643. [DOI] [PubMed] [Google Scholar]