Abstract
BK virus (BKV) is a common human polyomavirus infecting >80% of the population worldwide. Infection with BKV is asymptomatic, but reactivation in renal transplant recipients can lead to polyomavirus-associated nephropathy. In this report, we show that enzymatic removal of α(2,3)-linked sialic acid from cells inhibited BKV infection. Reconstitution of asialo cells with α(2,3)-specific sialyltransferase restored susceptibility to infection. Inhibition of N-linked glycosylation with tunicamycin reduced infection, but inhibition of O-linked glycosylation did not. An O-linked-specific α(2,3)-sialyltransferase was unable to restore infection in asialo cells. Taken together, these data indicate that an N-linked glycoprotein containing α(2,3)-linked sialic acid is a critical component of the cellular receptor for BKV.
BK virus (BKV) is 1 of 14 members of the polyomavirus family, a group of nonenveloped, small double-stranded DNA-containing viruses (14). BKV infects approximately 80% of the world's population early in life with an average age at seroconversion of approximately 5 years (9, 18, 38). Initial exposure to human polyomaviruses is common and asymptomatic. Once primary infection with BKV resolves, the virus persists in epithelial cells of the kidney and urogenital tract (6, 12, 43). Lifelong persistence of BKV within the kidney is subclinical in healthy individuals; however, in the context of immunosuppression, BKV can cause disease. An elevation of BKV load can induce hemorrhagic cystitis, ureteric stenosis, and retinitis, mostly in immunocompromised individuals (1, 9, 20, 24, 28, 31, 33). More recently, BKV has emerged as a significant and increasingly frequent complication leading to the loss of the transplanted organ in kidney allograft recipients (22, 32, 34, 37, 42). Polyomavirus-associated nephropathy afflicts 5 to 6% of kidney transplant recipients (9, 29, 32). In approximately 50% of polyomavirus-associated nephropathy cases, extensive fibrosis, calcification, inflammation, and cell death lead to progressive organ dysfunction, resulting ultimately in loss of the transplanted organ (9, 29, 32).
The cellular receptors for BKV have not been described. Early receptor studies elucidated a role for sialic acids and polysialylated gangliosides in BKV infectious entry into Vero cells and in virus binding to red blood cells (35, 40, 41). The linkage of sialic acid and the potential role of glycoproteins in infection were not elucidated in these early studies.
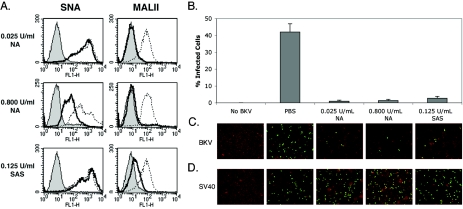
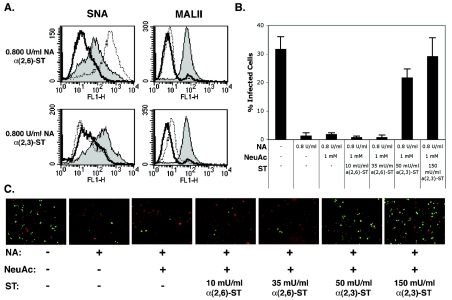
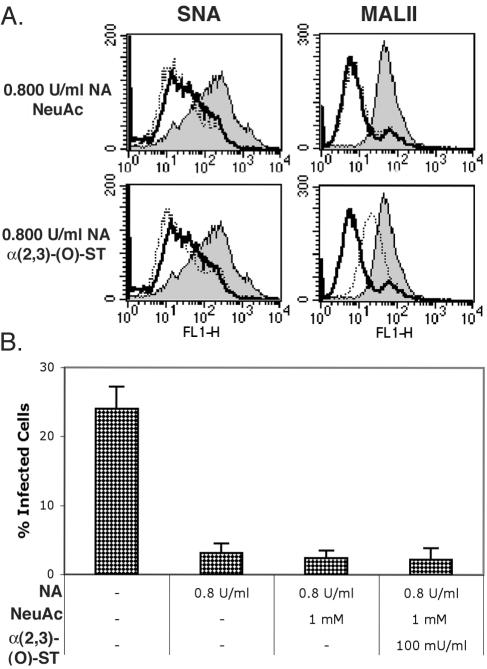
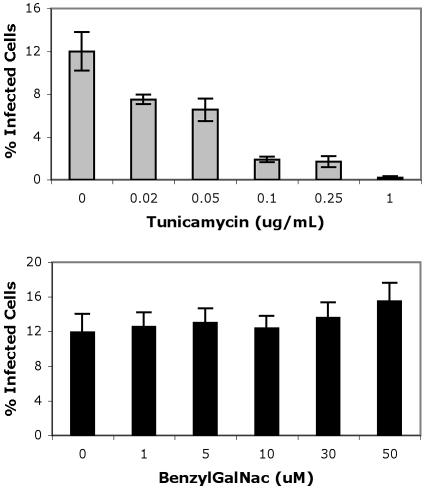
In this study, we show that an N-linked glycoprotein containing terminal α(2,3)-linked sialic acid is a critical determinant of BKV infection of Vero cells. Our first approach to defining a role for specific linkages of sialic acid in infection involved the enzymatic removal of terminal α(2,3)- and α(2,6)-linked sialic acids from Vero cells. Sialidase S (SAS) from Streptococcus pneumoniae specifically removes α(2,3)-linked sialic acid from glycoproteins and complex carbohydrates (27). Neuraminidase (NA) from Vibrio cholerae at high concentrations removes both α(2,3)- and α(2,6)-linked sialic acid from glycoproteins, gangliosides, and complex carbohydrates and at low concentration removes only the α(2,3) linkage (13, 19). The specificity of these sialidases was tested by measuring the binding of a biotinylated α(2,3)-linked sialic acid-specific lectin (MALII) and a biotinylated α(2,6)-specific lectin (SNA) to cells before and after treatment with each enzyme (Fig. 1A) (17, 39). The binding of biotinylated lectins was detected using Alexa Fluor 488-conjugated streptavidin. To begin to assess which linkage of sialic acid was important for BKV infection, Vero cells were treated with NA or SAS as indicated and challenged with virus. Infection was scored at 48 h postinfection by indirect immunofluorescence analysis of BKV T-antigen (Ag)-expressing cells. Both concentrations of NA inhibited infection, suggesting a role for α(2,3)-linked sialic acid in infection (Fig. 1B and C). The α(2,3)-specific SAS also inhibited infection, indicating that this linkage of sialic acid is critical for BKV infection (Fig. 1B and C). These experiments could not rule out a role for the α(2,6)-linked sialic acid, nor could they distinguish whether the sialic acid was attached to proteins or gangliosides. The effect of these enzymes on BKV infection, however, was specific, as no inhibition of infection was observed when enzyme-treated cells were challenged with simian virus 40 (Fig. 1D). To further examine the role of specific linkages of sialic acids in infection and to determine whether glycoproteins or gangliosides were involved, Vero cells were completely stripped of sialic acid with 0.800-U/ml NA and then incubated with α(2,3)-(N)-sialyltransferase [α(2,3)-ST] in the presence of 1 mM of a sialic acid donor, cytidine-5′-monophospho-N-acetylneuraminic acid sodium salt (NeuAc) for 1.5 h at 37°C. In the cell, this activated donor sugar is synthesized from neuraminic acid within the nucleus and transferred to carbohydrate chains on glycoproteins by sialyltransferases in the Golgi (8, 16, 30). The α(2,3) and α(2,6) sialyltransferases used in this study are highly specific and transfer sialic acid, or NeuAc, to a terminal galactose attached to N-acetylglucosamine (Gal- GlcNAc) on N-linked proteins (44). After sialyltransferase treatment, cells were washed and either stained with biotinylated lectins to determine if sialic acids were reconstituted on the cell surface or challenged with BKV. α(2,3)-ST was able to restore MALII binding to cells stripped of sialic acid (Fig. 2A), and this correlated with a restoration of infectivity (Fig. 2B and C). As this enzyme is specific for α(2,3) sialyation on N-linked glycoproteins, this experiment suggests that α(2,3)-linked sialic acid on an N-linked glycoprotein is sufficient to mediate infection (44). To control for endogenous sialyltransferase activity during the incubation, NA-treated cells received the sialic acid donor molecule, NeuAc, without any exogenous sialyltransferase. Histograms show that NeuAc alone was not capable of reconstituting any sialic acids at the cell membrane (Fig. 3A). Additionally, infection was not restored, indicating that in our system NeuAc is inert without a sialyltransferase enzyme (Fig. 2B and C and Fig. 3B). This experiment was repeated using SAS to remove only the α(2,3)-linked sialic acid from cells with similar results (data not shown). We next asked whether infection could be restored in asialo cells incubated with the neuraminic acid donor in the presence of a α(2,6)-sialyltransferase [α(2,6)-ST]. Like α(2,3)-ST, this enzyme specifically adds sialic acid to N-linked glycoproteins (44). The α(2,6)-ST restored SNA binding to asialo cells (Fig. 2A) but these cells remained refractory to infection with BKV, ruling out a role for the α(2,6)-linked sialic acids in BKV infection (Fig. 2B and C). Antennary oligosaccharide complexes consisting of a terminal α(2,3)-linked sialic acid with a branched α(2,6)-linked sialic acid side chain are not found in N-linked carbohydrates (2, 4, 25). Therefore, the possibility of BKV recognizing the branched form of this receptor is unlikely. To further support the role of N-linked glycoproteins as receptors for BKV, we made use of metabolic inhibitors of N- and O-linked glycosylation. Inhibitors of the two primary glycosylation pathways were used as previously described to further characterize the sialic acid-containing receptor (5, 15, 23, 36). Tunicamycin is a fungal toxin and antibiotic that prevents the assembly of UDP-N-acetylglucosamine onto the lipid carrier, dolichol phosphate, which is one of the first stages required in asparagine-initiated glycosylation (26). Benzyl N-acetyl-α-D-galactosaminide (benzylGalNAc) acts as a competitive inhibitor of N-acetyl-d-galactosaminyltransferase to serine or threonine, which is the beginning transfer step in O-linked glycosylation (3, 7, 11). Cells were preincubated and infected with BKV in the presence of these inhibitors. Tunicamycin induced a dose-dependent decrease in infection, while benzylGalNAc did not inhibit infection (Fig. 4). This experiment, together with the reconstitution experiments above using N-linked sialyltransferases, suggests that N-linked glycoproteins play an important role in BKV infection. It is possible that tunicamycin disables the function of endogenous sialyltransferases, thereby nonspecifically inhibiting infection by reducing sialic acid content. To further confirm the requirement for N-linked glycoproteins, we asked whether an α(2,3)-(O)-sialyltransferase [α(2,3)-(O)-ST] could rescue infection in asialo cells. Enzymatic treatment with α(2,3)-(O)-ST added sialic acids to galactose attached to N-acetyl galactosamine (Gal-GalNAc) of O-linked carbohydrates, gycolipids, and gangliosides (10, 21). Treatment of asialo cells with a neuraminic acid donor in the presence of the α(2,3)-(O)-ST restored MALIII binding but failed to restore infectivity (Fig. 3A and B).
FIG. 1.
Selective cleavage of α(2,3)- and α(2,6)-linked sialic acids from Vero cells treated with NA and SAS inhibits BKV infection. (A) The removal of α(2,3)- and α(2,6)-linked sialic acids was detected by flow cytometry using a biotinylated α(2,3)-specific lectin, MALII, and a biotinylated α(2,6)-specific lectin SNA. Lectin binding to untreated cells is indicated by dotted lines, and lectin binding to treated cells is indicated by boldface lines. The shaded histogram is the binding of streptavidin conjugated to Alexa Fluor 488 in the absence of added lectins. NA at 0.025 U/ml selectively cleaves α(2,3)-linked sialic acid, as indicated by inhibition of MALII binding and no inhibition of SNA binding (top). NA at 0.800 U/ml cleaves both α(2,3)- and α(2,6)-linked sialic acid (middle). The recombinant enzyme, SAS, selectively cleaves α(2,3)-linked sialic acids (bottom). (B) After Vero cells were treated with phosphate-buffered saline, 0.800-U/ml NA, 0.025-U/ml NA, or 0.125-U/ml SAS as indicated, the cells were infected with BKV. After 48 h of incubation, the percentage of infected cells was determined by cellular expression of the viral protein T-Ag. Error bars represent the standard deviation. All enzyme treatments significantly reduced infection (P < 0.001). The experiment was repeated 10 times with identical results. (C) Representative images of the data shown in panel B (magnification, ×100). (D) NA and SAS have no effect on simian virus 40 (SV40) infection of Vero cells. Green, cells positive for T-Ag expression.
FIG. 2.
α(2,3)-(N)-sialyltransferase but not α(2,6)-sialyltransferase restores BKV infection to asialo Vero cells. (A) Vero cells were untreated (shaded) or treated with 0.800-U/ml NA to remove almost all the sialic acids from cell surfaces. NA-treated cells were then incubated with 150-mU/ml α(2,3)-ST or 35-mU/ml α(2,6)-ST in the presence of NeuAc for 1.5 h at 37°C (dotted line) or incubated with medium alone (boldface line). Cells were then labeled with either SNA or MALII to determine if sialic acids had been restored. (B) Enzyme-treated cells described above were challenged with BKV, and infection was scored at after 48 h. Error bars represent standard deviations. This experiment was repeated three times. Treatment with 35-mU/ml α(2,6)-ST does not change infection levels (P > 0.1), while α(2,3)-ST significantly increases BKV infection (P < 0.001). (C) Immunofluorescent images of the data shown in panel B (magnification, ×100). Green, cells positive for T-Ag expression.
FIG. 3.
α(2,3)-(O)-Sialyltransferase can restore MALII binding but not BKV infection to asialo Vero cells. (A) Vero cells were either untreated (shaded) or treated with 0.800-U/ml NA. In histograms at the bottom, NA-treated cells were incubated with 100-mU/ml α(2,3)-(O)-ST and 1.0 mM NeuAc for 1.5 h at 37°C (dotted line) or incubated with medium alone (boldface line). In histograms at the top, NA treatment was followed by incubation with NeuAc without sialyltransferase (dotted line) or medium without NeuAc (boldface line). These cells were then labeled with either SNA or MALII. (B) Instead of lectin staining, cells shown in panel A were challenged with BKV, and infection was scored after 48 h. This experiment was repeated three times. NA inhibited infection, but α(2,3)-(O)-ST did not significantly restore infection (P > 0.18). Error bars represent standard deviations.
FIG. 4.
Tunicamycin but not benzylGalNAc inhibits BKV infection of Vero cells. Cells were incubated in medium containing tunicamycin for 4 h or benzylGalNAc for 12 h. The cells were then incubated with BKV in the presence of the inhibitors for 1 h at 4°C. The medium was removed and replaced by fresh medium without inhibitors. After 48 h, the percentage of BKV-infected cells was scored by indirect immunofluorescence analysis of T-Ag expression cells. This experiment was repeated three times. Tunicamycin inhibits infection at all concentrations (P < 0.001). BenzylGalNAc does not inhibit infection (P > 0.1) and at 25 and 50 μM slightly increases infection (P < 0.034). Error bars represent standard deviations.
Taken together, these data indicate that the functional receptor for BKV involves an N-linked glycoprotein with α(2,3)-linked sialic acid. The presence of α(2,3)-linked sialic acid on an N-linked glycoprotein is sufficient to mediate infection of these cells; however, these experiments do not rule out a role for gangliosides in contributing to BKV infection.
Acknowledgments
We thank all the members of the Atwood laboratory for critical discussions during the course of this work. We also thank Bethany O’Hara for technical assistance and Amanda Robinson, Amy Bozek, and Lorie St. Pierre for administrative assistance.
Aisling S. Dugan was supported by a Frederic Poole Gorham fellowship in biology and by G.A.A.N.N. training grant P200A030100 from the Department of Education. Work in our laboratory was supported by grant R01 CA71878 from the National Cancer Institute and by grant R01 NS43097 from the National Institute of Neurologic Disorders and Stroke.
REFERENCES
- 1.Barouch, D. H., W. C. Faquin, Y. Chen, I. J. Koralnik, G. K. Robbins, and B. T. Davis. 2002. BK virus-associated hemorrhagic cystitis in a human immunodeficiency virus-infected patient. Clin. Infect. Dis. 35:326-329. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, P. H., C. Cui, R. Liu, T. Stehle, S. C. Harrison, J. A. DeCaprio, and T. L. Benjamin. 1999. Discrimination between sialic acid-containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J. Virol. 73:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockhausen, I., G. Moller, A. Pollex-Kruger, V. Rutz, H. Paulsen, and K. L. Matta. 1992. Control of O-glycan synthesis: specificity and inhibition of O-glycan core 1 UDP-galactose:N-acetylgalactosamine-alpha-R beta 3-galactosyltransferase from rat liver. Biochem. Cell Biol. 70:99-108. [DOI] [PubMed] [Google Scholar]
- 4.Brockhausen, I., J. Schutzbach, and W. Kuhns. 1998. Glycoproteins and their relationship to human disease. Acta Anat. (Basel) 161:36-78. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M. H., and T. Benjamin. 1997. Roles of N-glycans with α2,6 as well as α2,3 linked sialic acid in infection by polyoma virus. Virology 233:440-442. [DOI] [PubMed] [Google Scholar]
- 6.Chesters, P. M., J. Heritage, and D. J. McCance. 1983. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 147:676-684. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero, C. A., S. Zarate, G. Corkidi, S. Lopez, and C. F. Arias. 2000. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 74:9362-9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harduin-Lepers, A., M. A. Recchi, and P. Delannoy. 1995. 1994, the year of sialyltransferases. Glycobiology 5:741-758. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch, H. H., and J. Steiger. 2003. Polyomavirus BK. Lancet Infect. Dis. 3:611-623. [DOI] [PubMed] [Google Scholar]
- 10.Horton, H. R., L. A. Moran, R. S. Ochs, J. D. Rawn, and K. G. Scrimgeour. 2002. Principles of biochemistry, 3rd ed. Prentice-Hall International, Upper Saddle River, NJ.
- 11.Huet, G., I. Kim, C. de Bolos, J. M. Lo-Guidice, O. Moreau, B. Hemon, C. Richet, P. Delannoy, F. X. Real, and P. Degand. 1995. Characterization of mucins and proteoglycans synthesized by a mucin-secreting HT-29 cell subpopulation. J. Cell Sci. 108:1275-1285. [DOI] [PubMed] [Google Scholar]
- 12.Imperiale, M. J. 2000. The human polyomaviruses, BKV and JCV: molecular pathogenesis of acute disease and potential role in cancer. Virology 267:1-7. [DOI] [PubMed] [Google Scholar]
- 13.Iwamori, M., Y. Ohta, Y. Uchida, and Y. Tsukada. 1997. Arthrobacter ureafaciens sialidase isoenzymes, L, M1 and M2, cleave fucosyl GM1. Glycoconj. J. 14:67-73. [DOI] [PubMed] [Google Scholar]
- 14.Johne, R., D. Enderlein, H. Nieper, and H. Muller. 2005. Novel polyomavirus detected in the feces of a chimpanzee by nested broad-spectrum PCR. J. Virol. 79:3883-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaludov, N., K. E. Brown, R. W. Walters, J. Zabner, and J. A. Chiorini. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75:6884-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kean, E. L. 1991. Sialic acid activation. Glycobiology 1:441-447. [DOI] [PubMed] [Google Scholar]
- 17.Knibbs, R. N., I. J. Goldstein, R. M. Ratcliffe, and N. Shibuya. 1991. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J. Biol. Chem. 266:83-88. [PubMed] [Google Scholar]
- 18.Knowles, W. A. 2001. The epidemiology of BK virus and the occurrence of antigenic and genomic subtypes, p. 527-560. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses: molecular and clinical perspectives. Wiley, New York, N.Y.
- 19.Komagome, R., H. Sawa, T. Suzuki, Y. Suzuki, S. Tanaka, W. J. Atwood, and K. Nagashima. 2002. Oligosaccharides as receptors for JC virus. J. Virol. 76:12992-13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak, E. J., R. A. Vilchez, P. Randhawa, R. Shapiro, J. S. Butel, and S. Kusne. 2002. Pathogenesis and management of polyomavirus infection in transplant recipients. Clin. Infect. Dis. 35:1081-1087. [DOI] [PubMed] [Google Scholar]
- 21.Lee, Y. C., N. Kojima, E. Wada, N. Kurosawa, T. Nakaoka, T. Hamamoto, and S. Tsuji. 1994. Cloning and expression of cDNA for a new type of Galβ1,3GalNAc α2,3-sialyltransferase. J. Biol. Chem. 269:10028-10033. [PubMed] [Google Scholar]
- 22.Lin, P. L., A. N. Vats, and M. Green. 2001. BK virus infection in renal transplant recipients. Pediatr. Transplant. 5:398-405. [PubMed] [Google Scholar]
- 23.Liu, C. K., G. Wei, and W. J. Atwood. 1998. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal α(2-6)-linked sialic acids. J. Virol. 72:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markowitz, R. B., H. C. Thompson, J. F. Mueller, J. A. Cohen, and W. S. Dynan. 1993. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J. Infect. Dis. 167:13-20. [DOI] [PubMed] [Google Scholar]
- 25.Marth, J. D. 1996. Complexity in O-linked oligosaccharide biosynthesis engendered by multiple polypeptide N-acetylgalactosaminyltransferases. Glycobiology 6:701-705. [DOI] [PubMed] [Google Scholar]
- 26.McDowell, W., and R. T. Schwarz. 1988. Dissecting glycoprotein biosynthesis by the use of specific inhibitors. Biochimie 70:1535-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, H. J., J. J. Klock, and C. Starr. January1997. Polynucleotide encoding alpha 2-3 neuraminidase. U.S. patent 5,591,839.
- 28.Mylonakis, E., N. Goes, R. H. Rubin, A. B. Cosimi, R. B. Colvin, and J. A. Fishman. 2001. BK virus in solid organ transplant recipients: an emerging syndrome. Transplantation 72:1587-1592. [DOI] [PubMed] [Google Scholar]
- 29.Nickeleit, V., T. Klimkait, I. F. Binet, P. Dalquen, V. Del Zenero, G. Thiel, M. J. Mihatsch, and H. H. Hirsch. 2000. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N. Engl. J. Med. 342:1309-1315. [DOI] [PubMed] [Google Scholar]
- 30.Oetke, C., S. Hinderlich, R. Brossmer, W. Reutter, M. Pawlita, and O. T. Keppler. 2001. Evidence for efficient uptake and incorporation of sialic acid by eukaryotic cells. Eur. J. Biochem. 2638:4553-4561. [DOI] [PubMed] [Google Scholar]
- 31.Pahari, A., and L. Rees. 2003. BK virus-associated renal problems—clinical implications. Pediatr. Nephrol. 18:743-748. [DOI] [PubMed] [Google Scholar]
- 32.Ponticelli, C. 2004. Renal transplantation 2004: where do we stand today? Nephrol. Dial. Transplant. 19:2937-2947. [DOI] [PubMed] [Google Scholar]
- 33.Priftakis, P., G. Bogdanovic, P. Kokhaei, H. Mellstedt, and T. Dalianis. 2003. BK virus (BKV) quantification in urine samples of bone marrow transplanted patients is helpful for diagnosis of hemorrhagic cystitis, although wide individual variations exist. J. Clin. Virol. 26:71-77. [DOI] [PubMed] [Google Scholar]
- 34.Randhawa, P. S., S. Finkelstein, V. Scantlebury, R. Shapiro, C. Vivas, M. Jordan, M. M. Picken, and A. J. Demetris. 1999. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 67:103-109. [DOI] [PubMed] [Google Scholar]
- 35.Seganti, L., P. Mastromarino, F. Superti, L. Sinibaldi, and N. Orsi. 1981. Receptors for BK virus on human erythrocytes. Acta Virol. 25:177-181. [PubMed] [Google Scholar]
- 36.Shah, A. H., and H. L. Lipton. 2002. Low-neurovirulence Theiler's viruses use sialic acid moieties on N-linked oligosaccharide structures for attachment. Virology 304:443-450. [DOI] [PubMed] [Google Scholar]
- 37.Shah, K. V. 2000. Human polyomavirus BKV and renal disease. Nephrol. Dial. Transplant. 15:754-755. [DOI] [PubMed] [Google Scholar]
- 38.Shah, K. V., R. W. Daniel, and R. M. Warszawski. 1973. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J. Infect. Dis. 128:784-787. [DOI] [PubMed] [Google Scholar]
- 39.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Nsimba-Lubaki, B. Peeters, and W. J. Peumans. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α 2-6)Gal/GalNAc sequence. J. Biol. Chem. 262:1596-1601. [PubMed] [Google Scholar]
- 40.Sinibaldi, L., P. Goldoni, V. Pietropaolo, C. Longhi, and N. Orsi. 1990. Involvement of gangliosides in the interaction between BK virus and Vero cells. Arch. Virol. 113:291-296. [DOI] [PubMed] [Google Scholar]
- 41.Sinibaldi, L., D. Viti, P. Goldoni, G. Cavallo, C. Caroni, and N. Orsi. 1987. Inhibition of BK virus haemagglutination by gangliosides. J. Gen. Virol. 68:879-883. [DOI] [PubMed] [Google Scholar]
- 42.Smith, R. D., J. H. Galla, K. Skahan, P. Anderson, C. C. Linnemann, Jr., G. S. Ault, C. F. Ryschkewitsch, and G. L. Stoner. 1998. Tubulointerstitial nephritis due to a mutant polyomavirus BK virus strain, BKV(Cin), causing end-stage renal disease. J. Clin. Microbiol. 36:1660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trofe, J., P. Roy-Chaudhury, J. Gordon, G. Wadih, D. Maru, M. A. Cardi, P. Succop, R. R. Alloway, K. Khalili, and E. S. Woodle. 2005. Outcomes of patients with rejection post-polyomavirus nephropathy. Transplant. Proc. 37:942-944. [DOI] [PubMed] [Google Scholar]
- 44.Williams, M. A., H. Kitagawa, A. K. Datta, J. C. Paulson, and J. C. Jamieson. 1995. Large-scale expression of recombinant sialyltransferases and comparison of their kinetic properties with native enzymes. Glycoconj. J. 12:755-761. [DOI] [PubMed] [Google Scholar]