Abstract
UV radiation from the sun is the primary germicide in the environment. The goal of this study was to estimate inactivation of viruses by solar exposure. We reviewed published reports on 254-nm UV inactivation and tabulated the sensitivities of a wide variety of viruses, including those with double-stranded DNA, single-stranded DNA, double-stranded RNA, or single-stranded RNA genomes. We calculated D37 values (fluence producing on average one lethal hit per virion and reducing viable virus to 37%) from all available data. We defined “size-normalized sensitivity” (SnS) by multiplying UV254 sensitivities (D37 values) by the genome size, and SnS values were relatively constant for viruses with similar genetic composition. In addition, SnS values were similar for complete virions and their defective particles, even when the corresponding D37 values were significantly different. We used SnS to estimate the UV254 sensitivities of viruses for which the genome composition and size were known but no UV inactivation data were available, including smallpox virus, Ebola, Marburg, Crimean-Congo, Junin, and other hemorrhagic viruses, and Venezuelan equine encephalitis and other encephalitis viruses. We compiled available data on virus inactivation as a function of wavelength and calculated a composite action spectrum that allowed extrapolation from the 254-nm data to solar UV. We combined our estimates of virus sensitivity with solar measurements at different geographical locations to predict virus inactivation. Our predictions agreed with the available experimental data. This work should be a useful step to understanding and eventually predicting the survival of viruses after their release in the environment.
It is often assumed that viruses pose a lower potential threat for use in biological warfare or bioterrorism than bacterial counterparts, because they are expected to persist for shorter times when released into the environment than bacteria. However, viral agents are hardier and reach further into the environment than previously expected. For example, an information leak in 2002 from the former Soviet Union reported an accidental infection in 1971 of naval personnel 11 miles offshore from a smallpox testing site in the Soviet city of Aralsk (73).
Sunlight or, more specifically, solar UV radiation (UV) acts as the principal natural virucide in the environment. UV radiation kills viruses by chemically modifying their genetic material, DNA and RNA. The most effective wavelength for inactivation, 260 nm (55), falls in the UVC range, so-named to differentiate it from near-UV found in ground-level sunlight, i.e., the UVB and UVA portions of the spectrum, 290 to 320 nm and 320 to 380 nm, respectively (51). Nucleic acids are damaged also by UVB and UVA but with lower efficiency than by UVC radiation (64).
Two issues must be considered to determine solar inactivation of biothreat viruses: estimating the UV sensitivity of viruses for which there is little or no experimental data and estimating the solar UV at specific geographic locations.
The overwhelming majority of published information on UV inactivation of viruses has been based upon exposure to UVC (UV254) radiation from a low-pressure mercury vapor (germicidal) lamp, with the primary emission at 254 nm. However, UV254 is not found in the sunlight that reaches the earth's surface; the ground-level virucidal solar UV wavelengths fall above 290 nm (16). Fortunately, the primary photochemical processes that damage the viral DNA or RNA occur at all the solar UV wavelengths, varying only in the efficiency of the different wavelengths (55). Since there are few published data that describe the survival of viruses, and none for threat viruses, following exposure to solar UV radiation, extrapolation from UV254 data will be required for most viruses. This extrapolation can be made using wavelength dependence (action spectrum) data.
The nucleic acid within the virus particle plays a crucial role in the absorption of UV radiation and in virus inactivation. In most viruses the other major constituents of the virus particles play relatively minor roles in inactivation by UV (55). For example, whole MS2, f2, Qβ, encephalomyocarditis virus (EMCV), and murine polyoma viruses and their respective free nucleic acids have essentially the same UV254 sensitivities (14, 30, 75, 80, 86). (Virus abbreviations are those indicated in the Virus Taxonomy report [76].) The number of bases in the DNA or RNA is important for determining sensitivity to UV inactivation, because the more target molecules, the more likely the genome will be damaged at a given level of UV exposure. Another important difference in sensitivity between viral nucleic acid types occurs because the most common lethal photoproducts of UV are pyrimidine dimers, particularly thymine dimers (12). Since DNA but not RNA contains thymine, DNA-containing viruses are generally more sensitive to damage by UV than RNA-containing viruses (45, 55). In addition, repair can reduce the lethal effect of UV, especially for viruses possessing double-stranded (ds) nucleic acids (34).
In the present study we attempted to develop a method to compare UV254 sensitivities among viruses of different sizes. These sensitivities could then be used to predict the sensitivities to UV254 of viruses of particular interest in biodefense, including smallpox, Ebola, Marburg, Congo Crimean, Junin and other hemorrhagic viruses and Venezuelan equine encephalitis and other encephalitis viruses.
The overall goal of this report was to assess the extent to which UV in sunlight might inactivate various viruses in the environment. Although other variables (discussed later) may affect the survival of viruses in the environment, inactivation by sunlight should provide a baseline for predicting the recovery time of contaminated areas after a virus-mediated biological attack.
MATERIALS AND METHODS
Compilation of virus UV254 sensitivities.
We collected the published UV254 inactivation data for viruses that infect vertebrates. The UV sensitivity of a virus is determined via a survival curve, with the log viral surviving fraction as a function of UV exposure (D is measured as fluence in J/m2). The simplest and most common survival curve for viruses follows single-hit kinetics, i.e., n/no = e−kD, where n/no is the virus surviving fraction and k is the slope of the survival curve when ln(n/no) is plotted versus D. The UV exposure that produces an average of one lethal hit per virion occurs when D = 1/k and is called the D37 (n/no = 0.37) (66). This term is used here to denote UV sensitivity. In some reports, D37 values were provided or information (e.g., a survival curve) was presented from which we could calculate D37.
Action spectra for virus inactivation.
The published action spectra for virus inactivation were compiled and analyzed. Since these data were to be combined subsequently with D37 values for UV254, all data for other wavelengths were normalized to those at 254 nm by calculating the relative sensitivity (ratio of D37 for the wavelength of interest to the D37 at 254 nm). Thus, for example, a relative sensitivity of 0.5 indicates that the wavelength of interest is only half as effective for virus inactivation as UV254 at the same J/m2 exposure level. Only action spectra that included 254 nm were usable. Since our interest is in UV wavelengths available from sunlight, only wavelengths above 280 nm were analyzed.
The effective solar spectrum.
The solar UV radiation that reaches ground level has two components, the direct beam from the sun, which depends primarily on the solar zenith angle (SZA), the angle from the vertical from an Earth location to the sun, and the scattered radiation from the sky in general (16, 23). The dependence on SZA is further twofold: (i) the direct beam of radiation is spread over a larger surface area when the SZA is larger (the sun is lower in the sky), and (ii) the beam must traverse a longer path through the stratospheric ozone layer. Because the ozone layer has a strong effect on the shorter wavelengths of UVB, this is the more important factor.
The SZA also changes during the day, being minimum at midday (solar noon) (16). The radiation level is at a maximum at that time and decreases roughly symmetrically before and after that maximum, i.e., the levels at 1 hour before and after the maximum are equal. Because the shorter wavelengths are more sharply attenuated at greater SZAs, on a clear day their contribution to the daily total effective irradiance is greatest at solar noon and decreases faster than that of the longer wavelengths as the time before or after noon increases. It should be noted that most solar irradiance data that might be useful for estimating virucidal activity at specific locations are only available at specific wavelengths (e.g., at the USDA/CSU website; see below).
Complementary operational information can be obtained by individuals with the proper clearances by referring to DOD document no. ECBC-TR-411 (classified), “Inactivation of viruses by solar UV radiation after release in U.S. cities” by J.-L. Sagripanti and D. Lytle, U.S. Army, Aberdeen Proving Ground, Md., November 2004. Scientists interested in pursuing related research should contact the corresponding author regarding collaboration and funding.
RESULTS
UV254 sensitivity of different viruses.
The UV254 fluences that provided one lethal hit per virus (D37) are presented in Tables 1 and 2. Median values are presented for each family for which there were published data. Median values were used instead of mean vales to reduce the effect of “outliers” in published data. The UV254 sensitivity of most viruses ranged from 1.1 J/m2 for viral hemorrhagic septicaemia virus (VHSV) in the Rhabdoviridae family (50) to 250 J/m2 for simian virus 40 (SV40) in the Polyomaviridae family (6). The Herpesviridae family was the only family that consistently displayed two-component survival curves. There were considerable variations in the D37 values for a number of viruses. We found the best agreement among the single-stranded DNA (ssDNA) viruses (Table 1), where variation of the D37 values was within 20% of the median. The variations among viruses in all other types were often 30% or more. Thus, with a few exceptions, the UV254 sensitivity of any given virus is known within a 25 to 30% error. The different D37 values among similar viruses hindered extrapolation to D37 values for unreported viruses and promoted the investigation of improved alternatives to express UV254 sensitivity.
TABLE 1.
Measured and predicted UV254 sensitivities for DNA-containing virus families whose hosts are vertebrates
| Familya | Measured UV254D37 (J/m2)b | Genome size rangec | Measured or predicted SnS (J/m2 · kb)d | Representative virus(es)e | Predicted UV254D37 range for entire family (J/m2)f | Reference(s) |
|---|---|---|---|---|---|---|
| dsDNA viruses | ||||||
| Adenoviridae | 130 (100-220) | 26-45 | 9,200 (7,300-16,000) | SAdV-7, HAdV-5, -40, -41 | 100-180 | 6, 9, 42 |
| Asfarviridae | 170-190 | 5,100 p | 13-15 | |||
| Herpesviridae | 19 (6.2-28) | 125-240 | 6,400 (1,600-9,600) | HHV-1, -2, -5, SuHV-1, MuHV-1, EHV-1 | 13-26 | 1, 20, 35, 37, 53, 62, 67, 79, 88 |
| 2nd 140g | 2nd 54,000g | 2nd 110-220g | ||||
| (38-490) | (12,000-120,000) | |||||
| Iridoviridae | 16 | 98-170 | 5,100 | FV-3 | 15-26 | 38 |
| Papillomaviridae | 6.8-8.4 | 5,100 p | 300-380 | |||
| Polyomaviridae | 250 | 4.7-5.3 | 2,600 | MPyV, SV40 | 250-280 | 6, 30, 55, 75 |
| Poxviridae | 11 | 130-300 | 4,100 | VACV | 6.8-16 | 6, 7, 34, 36, 55 |
| ssDNA viruses | ||||||
| Circoviridae | 1.8-2.3 | 46 p | 20-26 | |||
| Hepadnaviridae | 3.0-3.3 | 46 p | 14-15 | |||
| Parvoviridae | 9.2 (8.6-10.0) | 4-6 | 46 (43-50) | KRV, MMV, H-1PV | 7.7-12 | 33, 57, 72, 77 |
Virus family name or genus name, if not assigned to a family, is according to the most recent Virus Taxonomy report (76).
Median UV254 D37 values (and ranges) were derived from the indicated reference(s) (far right column).
Genome size range is according to the latest Virus Taxonomy report (76). For dsDNA viruses, the genome size is presented as the number of kilobase pairs; for ssDNA viruses the size is the number of kilobases.
SnS values for individual viruses within the family were calculated by multiplying the measured D37s by the genome sizes, as total number of bases, i.e., the total number of bases in dsDNA viruses is twice the number of base pairs. Median SnS values for individual viruses were then used to determine a median SnS value (and range) for the entire family. When no D37 data were available for a virus family, the median SnS value for that DNA type, e.g., dsDNA, was used for a predicted SnS value, shown in italics and indicated by the letter p.
D37 data were found for the listed viruses. Virus abbreviations are those indicated in the latest Virus Taxonomy report (76).
Predicted D37 range was calculated by dividing the appropriate measured or predicted SnS value by the genome size range of that virus family.
The Herpesviridae family presented survival curves with two components. The values for the second, UV-resistant components are denoted by 2nd.
TABLE 2.
Measured and predicted UV254 sensitivities for RNA-containing virus families whose hosts are vertebratesa
| Familyb | Measured D37 (J/m2) | Genome size range | Measured or predicted SnS (J/m2 · kb) | Representative virus(es) | Predicted D37 range for entire family (J/m2) | Reference(s) |
|---|---|---|---|---|---|---|
| dsRNA viruses | ||||||
| Birnaviridae | 120 (110-170) | 5.7-6.2 | 1,400 (1,300-1,900) | 1PNV | 110-120 | 32, 50, 63 |
| Reoviridae | 89 (46-123) | 18.6-26.4 | 3,800 (1,700-5,800) | MRV-1, -2, -3, RV-A, KEMV-10, -91 | 72-100 | 3, 18, 19, 39, 55, 68, 87 |
| ssRNA viruses | ||||||
| Arenaviridae* | 11 | 140 p | 13 | |||
| Bornaviridae* | 34 | 8.9 | 300 | BDV | 34 | 8 |
| Bunyaviridae* | 11-19 | 140 p | 7.4-13 | |||
| Deltavirus* | 1.7 | 140 p | 82 | |||
| Filoviridae* | 19 | 140 p | 7.4 | |||
| Orthomyxoviridae* | 7.5 (4.8-10) | 10-15 | 110 (70-140) | FLUAV, ISAV | 7.3-11 | 50, 54 |
| Paramyxoviridae* | 11 (10-12) | 15-16 | 170 (150-190) | NDV, MeV | 11 | 10, 29, 31 |
| Rhabdoviridae* | 4.3 (1.1-23) | 11-15 | 51 (12-260) | VSV, RABV, VHSV | 3.4-4.6 | 2, 4, 8, 50, 63, 79 |
| Arterioviridae** | 13-16 | 295 p | 18-23 | |||
| Astroviridae** | 6.8-7.9 | 295 p | 37-44 | |||
| Caliciviridae** | 7.4-8.3 | 295 p | 36-40 | |||
| Coronaviridae** | 3.1 | 20-31 | 78 | BEV | 2.5-3.9 | 79 |
| Flaviviridae** | 9.6-12 | 295 p | 25-31 | |||
| “HEV-like” viruses** | 7.2 | 295 p | 41 | |||
| Nodaviridae** | 140 | 4.5 | 630 | SBNN | 140 | 11 |
| Picornaviridae** | 48 (25-70) | 7-8.5 | 370 (190-540) | PV-1, -2, -3, E-1, -11, CV-A9, -B1, -B5, HHAV, EMCV | 44-53 | 3, 6, 18, 19, 42, 43, 47, 55, 78, 86 |
| Togaviridae** | 19 (7.3-23) | 9-12 | 220 (83-260) | SINV, VEEV, SFV | 18-24 | 69, 79, 86 |
| Retroviridae*** | 89 (88-120) | 7-11 | 740 (620-980) | MLV, FeLV, MoMSV, RSV | 67-110 | 5, 25, 31, 46, 49, 70, 83-85 |
See footnotes to Table 1 for explanations of columns. In addition, ssRNA viruses are listed according to sense of genetic information and/or mode of replication.
*, family has negative-sense ssRNA; **, family has positive-sense ssRNA; ***, family replicates via reverse transcriptase.
UV sensitivity normalized to target nucleic acid.
The sensitivity to inactivation depended on the type of nucleic acid (both the base composition and strandedness) and its size (length). A term designated “inactivation quantum yield” (ϕ), defined as the ratio of the inactivation cross section (σ) to the absorption cross section (s), represents the probability that an absorbed photon will lead to an inactivated virion (66). This value should be relatively constant for a given type of nucleic acid regardless of size but will differ among nucleic acid types (55). The product of inactivation quantum yield and absorption cross section gives the inactivation cross section, i.e., the UV sensitivity, and may be used to predict the UV sensitivity of untested viruses. Unfortunately, neither the inactivation quantum yield nor the absorption cross sections are likely to be known for most viruses in general and for threat viruses, in particular.
We searched for a quantity proportional to the inactivation quantum yield that could be estimated from similar viruses and extrapolated to untested viruses. Since the inactivation cross section is proportional to the inactivation constant, k, and the absorption cross section is proportional to the genome size, we proposed the following: ϕ = σ/s = αk/GS = α(D37 × GS)−1, where α is a proportionality constant.
Thus, the inactivation quantum yield is proportional to the reciprocal of the product of D37 and the genome size (GS). This product, which we hereby designate “size-normalized sensitivity,” or SnS, has the practical advantage of consisting of quantities already known for similar viruses. Note that a high value of SnS indicates a high D37, i.e., a low sensitivity to UV inactivation. As with the inactivation quantum yield, the SnS should be relatively constant for each type of viral nucleic acid and is expected to be independent of nucleic acid size, i.e., it represents a means to normalize the UV sensitivity with regard to viral target size. Further, dividing the SnS by the genome size of an untested virus yields an estimate of the D37 for that virus. We calculated SnS values for viruses of different sizes and have included the median values (and ranges) for the different virus families in Tables 1 and 2.
This approach is supported by nearly identical SnS values for the full vesicular stomatitis virus (VSV) and its defective particles that have measurable biological activities. Although the defective particles have only 11% and 29% of the full genome size, the SnS values were nearly the same as that for the full VSV (Table 3). The benefit of using SnS is also supported by data on Qβ bacteriophage and its Qβmidi particle, which have similar SnS values, in contrast to a 15-fold difference in their D37 values (Table 3).
TABLE 3.
UV254 inactivation data for two viruses and their defective particles, together with calculated SnS values
| Virusa | D37 (J/m2) | Genome size (kb) | SnS (J/m2 · kb) | Reference |
|---|---|---|---|---|
| VSV | 23 | 11.2 | 260 | 4 |
| VSV tsG31DI | 200 | 1.2 | 240 | 4 |
| VSV tsHuDI | 79 | 3.2 | 250 | 4 |
| Qβ | 51 | 4.2 | 210 | 14 |
| Qβ midi | 860 | 0.22 | 190 | 48 |
VSV, vesicular stomatitis virus, Rhabdoviridae family, or enterobacteria phage Qβ, Leviviridae family.
The SnS values for DNA viruses are displayed in Table 1. There were three ssDNA viruses representing one family, the Parvoviridae family, with a median SnS value of 46. There were 14 dsDNA viruses belonging to five families, all having SnS values above 1,000, with a median value of 5,100, much larger than for those of ssDNA viruses. Two basic factors contributed to the lower sensitivity of dsDNA viruses to UV254 inactivation: the innate genetic redundancy of the cDNA strands and the occurrence of repair of DNA damage (38). While the details of repair are important for virus survival, our purpose here is to consider conditions that are likely to occur after released environmental exposure of a normal human population. Therefore, all the data shown in Tables 1 and 2 were determined with cells having normal (wild-type) repair.
As expected from the differential chemical reactivity to UV between ribonucleotides and deoxyribonucleotides, the SnS values for ssRNA viruses are higher than those for ssDNA viruses (Tables 1 and 2). The ssRNA viruses can be grouped into three types regarding their replication patterns: the negative-sense ssRNA viruses were similar in size and UV254 sensitivity, with a median SnS value of 140 (range, 51 to 300); the positive-sense ssRNA viruses had a median SnS value of 295 (range, 78 to 630); the Retroviridae family, which incorporates reverse transcriptase in replication, had the highest median SnS value, 740. The SnS values of dsRNA viruses (Table 2) were above 1,000, similar to the values obtained for dsDNA viruses (Table 1).
The values of SnS indicate that viruses containing dsDNA or dsRNA are generally more resistant (with SnS values in the thousands of J/m2 · kb) than viruses whose genomes are ssDNA or ssRNA (SnS values ranging from 50 to several hundred J/m2 · kb). This finding is particularly exemplified by the SnS values of Mengo virus EMCV and its free ssRNA (SnS of 180 for free RNA and 220 for whole virus), being much lower than that for the dsRNA replicative form of EMCV, which had a higher SnS value of 2,100, similar to that of double-stranded viruses (86).
There were considerable variations found in the different published values for the D37s for many of the viruses, often covering a twofold range or greater. Therefore, as expected, the variations in SnS values from one virus family to another were also considerable. However, for the viruses of greater threat to humans, i.e., those with dsDNA (Table 1) and those with negative-sense or positive-sense ssRNA (Table 2) genomes, the variations in SnS values were significantly lower than those for the D37 values.
Predicting sensitivity to UV254 for untested viruses.
Tables 1 and 2 list all the virus families that infect vertebrates, including those for which there are no data for UV254 inactivation. We used the median SnS values of virus families for which there were data as the predicted SnS value for virus families having a similar genome type. (For example, see the predicted SnS value for Papillomaviridae in the dsDNA group [Table 1] or Filoviridae in the negative-sense ssRNA group [Table 2].) The SnS values calculated or predicted and presented in Tables 1 and 2 allowed prediction of D37 values for every member of each entire virus family, including members of families that had no available UV254 inactivation data. The predicted D37 range for each family was calculated as the SnS value for that family divided by the genome size range of the family members. The expected D37 for any specific virus can be more precisely calculated by dividing the SnS by the genome size of the particular virus.
The method of extrapolation using the principle of SnS and genome size appears to be consistent with basic biophysical principles but has variation from one family to another within a virus genomic type. The typical range of D37s from the median was about a factor of 2 of the median. Thus, extrapolation from known viruses to untested viruses has an uncertainty of about a factor of 2 in the SnS and, hence, in the UV254 exposure required to reduce the virus survival to any given level.
Predicted UV254 sensitivities of viruses of relevance in biodefense.
The UV254 sensitivity of one virus of relevance in biodefense, Venezuelan equine encephalitis virus (VEEV), has been reported once (69), and two families that contain viruses of relevance, Poxviridae and Togaviridae, have had other members reported. Most viruses of relevance in biodefense have no published UV inactivation data. With the known genome size and SnS calculated as described above, we estimated the UV254 sensitivity of several relevant viruses (Table 4). The family most sensitive to UV254 inactivation is Filoviridae; the least sensitive is Flaviviridae. For variola virus (smallpox) and equine encephalitic viruses (Western EEV [WEEV] and VEEV), the available data of closely related viruses reduce the uncertainties in those viruses to perhaps 30% in the predicted D37.
TABLE 4.
Predicted UV254 sensitivities for viruses of particular interest in biodefense
| Virus family | Virus(es) of interest | Genome sizea | Inactivation data for related virus | Predicted UV254D37 (J/m2) | UV254 for 1 log inactivation (J/m2) |
|---|---|---|---|---|---|
| Filoviridae | Marburg | 19.1 | None | 7.3 | 17 |
| Ebola | 18.9 | None | 7.4 | 17 | |
| Poxviridae | Variola | 185 | Vaccinia | 11.0 | 25 |
| Bunyaviridae | Hanta virus | 11.8 | None | 12.0 | 28 |
| Rift Valley fever | 12.0 | None | 12.0 | 28 | |
| Arenaviridae | Lassa | 11.0 | None | 13.0 | 30 |
| Junin, etc. | 11.0 | None | 13.0 | 30 | |
| Togaviridae | WEEV | 11.4 | VEEV | 19.0 | 44 |
| VEEV | 11.4 | VEEV | 23.0b | 53 | |
| Flaviviridae | West Nile | 11 | None | 24.0 | 55 |
Genome sizes are in kilobases except for Poxviridae genome size, which is in kilobase pairs.
Published value for VEEV (69).
Action spectrum for virus inactivation.
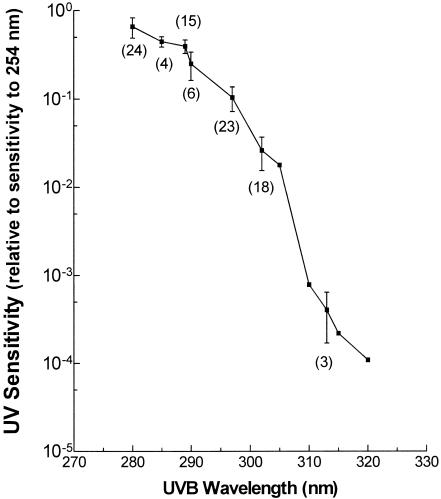
All available action spectrum data for virus inactivation were compiled, with bacteriophages providing the only data above 302 nm. The averaged values for the different types of nucleic acids demonstrated that there was little, if any, difference between action spectra calculated for DNA and for RNA viruses. Therefore, we pooled the data and constructed a curve describing the relative UV sensitivity (normalized to 254 nm) throughout the wavelength range required for this analysis (Fig. 1). There were more data for those wavelengths strongly emitted by mercury vapor lamps: 280, 289, 297, and 302 nm. There were much fewer published data at wavelengths above 302 nm. The inactivation action spectrum that we calculated parallels the nucleic acid absorption spectrum (65).
FIG. 1.
UVA/UVB action spectrum for virus inactivation normalized to 254 nm. The graph shows the relative sensitivity of viruses, calculated as the ratio of D37 at 254 nm to the D37 at the selected wavelength; hence, there are no units. Data represent the mean from n values for diverse viruses at a given wavelength. Values in parentheses indicate the number of data points at each wavelength. The bars represent the standard deviations from the means. Data were obtained from references 13, 15, 21, 22, 24, 27, 28, 40, 52-56, 58, 61, 64, 71, 74, 82, and 89.
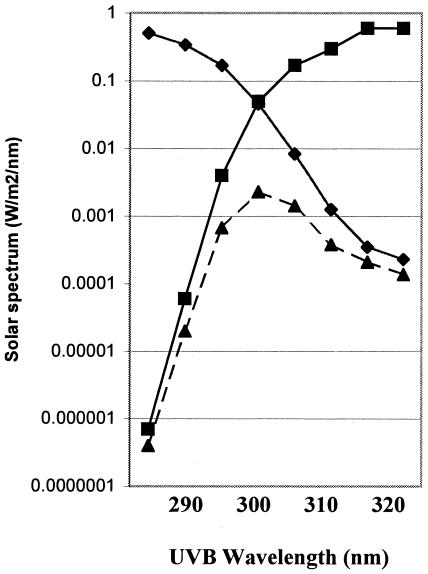
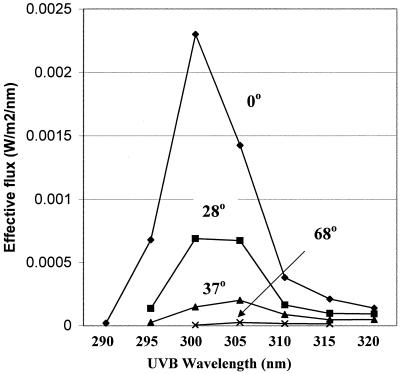
Figure 2 shows a solar spectrum at 0° SZA (sun straight overhead; flux in W/m2, =J/m2/s) together with the 254-nm-normalized action spectrum for virus inactivation and the corresponding calculated spectrum of the effective solar flux. The effective solar flux was calculated by multiplying the solar spectrum times the 254-nm-normalized action spectrum at each wavelength, with the result expressed in 254-nm equivalent flux (W/m2254). The effective solar flux spectra at different SZAs are presented on a linear scale in Fig. 3. At an SZA of 75°, the effective solar flux was much lower (500-fold at 300 nm) and the total effective solar flux was nearly 100-fold less than at a SZA of 0°.
FIG. 2.
Solar spectrum (squares) at SZA = 0° (sun directly overhead) (18), UV virus sensitivity normalized to 254 nm (diamonds) (see Fig. 1), and virus inactivation effective spectrum (triangles). The inactivation effective spectrum was calculated by multiplying the solar spectrum times the normalized UV sensitivity at selected wavelengths.
FIG. 3.
Virus inactivation effective spectra at different SZAs. The solar radiometry data are for 0° (from reference 16), 28° and 68° (reference 41), and 37° (reference 81).
Method for estimation of virus inactivation by solar exposure.
Estimation of virus inactivation by solar exposure begins by determining the total effective solar flux. The effective solar flux at each wavelength was obtained by measuring the fluxes (in W/m2/nm) (at the UVB and UVA wavelengths available from the USDA/CSU website [http://uvb.nrel.colostate.edu/] or other sources) and multiplying these amounts by the 254-nm-normalized action spectrum values appropriate for that wavelength. The total effective solar flux can be calculated by adding the contributions of the different wavelengths (in most instances only the UVB wavelengths will be needed).
Validation of approach by available data.
We were interested in determining whether our approach predicted effective solar fluxes consistent with available data for sunlight-inactivated virus. Although there are few published data to fully demonstrate the consistency of our approach, the available data are compared to our estimates in Table 5. Three laboratories have made direct comparisons for viruses between inactivation by UV254 and solar exposure. Murphy (44) compared noon-time solar inactivation of TMV-RNA in Davis, California, with inactivation by a germicidal lamp, 254 nm, and Furusawa et al. (13) and Ronto et al. (58-60) conducted similar experiments with bacteriophages T1 and T7, respectively. We used the reported D37s for UV254 inactivation (in J/m2) and for solar inactivation (in minutes) to calculate a 254-nm-equivalent solar flux (in J/m2254 per minute) for the experimental data. The direct comparisons between reported experimental data and our predictions are shown in Table 5.
TABLE 5.
Comparison of calculated and reported times for solar exposure to inactivate viruses to 37% survivala
| Target organism (reference) | Reported
|
Calculatedb
|
||
|---|---|---|---|---|
| Equivalent solar flux (J/m2254/min) (UV254 exposure [J/m2] for 37% survival) | Time of solar exposure for 37% survival (min) (location, SZA, date) | Equivalent solar flux (J/m2254/min) (location, SZA, date) | Time of solar exposure for 37% survival (min) | |
| TMV-RNA (44) | 0.42 (25) | 60 (Davis, Calif., 17.0°, 16 July) | 0.35 (Davis, Calif., 17.0°, 15 July) | 71 |
| T1 (13) | 0.30 (2.6) | 8.6 (Isehara, Japan, 12.2°, 14 June) | 0.31 (Griffin, Ga., 9.7°, 21 June) | 8.4 |
| T7 (58-60) | 0.60 (8.5) | 14.2 (Northern Hungary, 28.6°, 26 July) | 0.30 (Fort Peck, Mont., 29.6°, 28 July) | 28 |
For each virus, the same assay system was used to assess virus survival after solar exposure or after UV254 exposure. Solar exposure is at midday (approximately solar noon).
See text for method of calculating 254-nm-effective solar flux. Solar flux values from the USDA/CSU website (see text) for the stated location and date in 2003 were used for sites at similar latitudes to those of the reported viruses.
Our calculated values were determined using solar radiometric data from the USDA/CSU website (http://uvb.nrel.colostate.edu/) for the same location (Davis, Calif.) and calendar date or U.S. sites at similar elevations on dates that had similar SZAs. Two of the three calculated J/m2254/min values and calculated solar exposure times to produce 37% survival were very close to the reported experimental values: within 20% for TMV-RNA and 3% for T1. The other calculated value was approximately twice the experimental value, still within reasonable expectations given the different locations with unknown information on the exact times of exposure and the amounts of atmospheric ozone (26), air pollution, etc. The bottom-line conclusion is that our method of calculating the 254-nm-equivalent solar flux and solar exposure time needed to inactivate viruses gives results in reasonable agreement with the published data.
Predicted inactivation of a virus of relevance to biodefense at different locations.
To determine how the virucidal effect of solar UV compared from one location to another, data from the USDA/CSU website were used to calculate midday (solar noon-time) effective solar fluxes for selected locations at selected times of the year (Table 6). The data were chosen for days with clear skies, i.e., where the shape of the flux curve over the day displayed the typical symmetrical bell shape. The data in Table 6 demonstrate a general correlation between solar flux and SZA, with higher effective solar flux at lower SZA.
TABLE 6.
Estimated midday effective solar flux and predicted inactivation of filoviruses on clear days at different locations and dates of the year
| SZA (°N) | Location | Latitude (°N) | Date | Elevation (m) | Effective solar fluxa (J/m2254/min) | Time for 1 log decreaseb, Filoviridae (min) |
|---|---|---|---|---|---|---|
| 0.6 | Hilo, Hawaii | 19.5 | 28 July | 3,397 | 0.80 | 21 |
| 2.4 | Everglades, Fla. | 25.6 | 30 June | 0 | 0.50 | 34 |
| 8.2 | Hilo, Hawaii | 19.5 | 20 Apr. | 3,397 | 0.72 | 24 |
| 9.1 | Las Cruces, N.Mex. | 32.6 | 21 June | 1,317 | 0.55 | 31 |
| 9.7 | Griffin, Ga. | 33.2 | 21 June | 270 | 0.31 | 55 |
| 17.7 | Hilo, Hawaii | 19.5 | 16 Sept. | 3,397 | 0.83 | 20 |
| 17.6 | Las Cruces, N.Mex. | 32.6 | 1 May | 1,317 | 0.33 | 52 |
| 15.1 | Griffin, Ga. | 33.2 | 12 May | 270 | 0.28 | 61 |
| 17.0 | Davis, Calif. | 38.5 | 15 July | 18 | 0.35 | 49 |
| 24.3 | Hilo, Hawaii | 19.5 | 10 Mar. | 3,397 | 0.67 | 25 |
| 23.7 | Griffin, Ga. | 33.2 | 15 Apr. | 270 | 0.17 | 100 |
| 29.6 | Fort Peck, Mont. | 48.5 | 28 July | 634 | 0.30 | 57 |
| 41.8 | Hilo, Hawaii | 19.5 | 8 Jan. | 3,397 | 0.41 | 41 |
| 42.2 | Las Cruces, N.Mex. | 32.6 | 15 Oct. | 1,317 | 0.26 | 65 |
| 42.8 | Griffin, Ga. | 33.2 | 15 Oct. | 270 | 0.19 | 89 |
See text for method of calculating 254-nm-effective solar flux. Solar radiometry values are from the USDA/CSU website for the stated location and date in 2003.
UV254 fluence required for 1 log inactivation was estimated to be 17 J/m2 for members of the Filoviridae family (Table 4).
Although the SZA is important, there are other variables affecting the effective solar flux received at a particular location at a given time. Higher elevation results in higher effective solar flux, as can be seen by comparing data for Hilo, HA, Las Cruces, New Mexico, and Griffin, Georgia, locations of similar SZAs but different elevations. Interestingly, Davis, California, at an SZA near 17°, had as much effective solar flux as Las Cruces, New Mexico, also at an SZA near 17° but at an elevation over 1,300 m (4,000 ft) higher than Davis. Presumably, differences in atmospheric ozone and pollution account for these variations. These calculations also indicate that there were variations in sunlight exposure not completely accounted for by SZA and elevation. Thus, direct measurement of the solar spectrum at the specific location provides the most accurate determination of solar UV at any specific location and time. However, data in Table 5 suggest that errors can remain relatively low when using values obtained at other carefully selected locations when direct measurements of solar radiation for a particular site are not available.
Included in Table 6 are estimated times for virus inactivation by midday solar exposure for each location and date. We selected the most UV-sensitive (Filoviridae) among the families of viruses of potential interest in biodefense (Table 4). The estimates of midday virus inactivation in Table 6 range from 20 min to well over 1 hour for 1-log inactivation (10% survival). This indicates that some viruses could be inactivated by solar radiation rather quickly, while other, less-UV-sensitive virus types could persist for a long time.
DISCUSSION
In order to estimate the UV254 sensitivities of viruses of relevance in biodefense, we reviewed published information on the sensitivities of different virus families. We collected the D37s when available or calculated D37 from the presented data.
The variations among D37 values precluded defining the UV254 sensitivity among viruses with similar genetic composition, primarily because of substantial differences in genome size. When we standardized the virus sensitivity to virus genome size by defining the SnS, the variation among viruses with the same nucleic acid content decreased (Tables 1 and 2). The validity of this approach was supported by finding similar SnS values calculated for full virions and for their defective particles (Table 3). The similar SnS values among viruses of similar genetic composition indicate that the UV254 data available for a limited number of viruses could be extrapolated to other viruses, allowing predictions of UV254 sensitivities of viruses for which the nucleic acid types and sizes are known but no UV254 inactivation data exist. The sensitivities of several biothreat viruses were thus estimated (Table 4).
The action spectra of virus inactivation were found to be similar for all viruses regardless of genome type. Thus, one composite action spectrum was used to represent all viruses.
Examination of Fig. 3 indicates that the wavelengths for peak effectiveness of solar inactivation lie between 300 and 305 nm for SZAs up to 37°. These wavelengths contributed more than two-thirds of the total effective solar flux. A midday solar effective flux of 0.17 J/m2254/min (implying a daily total fluence of approximately 50 J/m2254) might be “marginally effective” for inactivating viruses relevant to biodefense, e.g., a full-day exposure would produce about a 3-log decrease in infectivity for the more-UV-sensitive viruses and much less for less-UV-sensitive viruses.
The method developed here for estimating the effective solar flux was relatively straightforward and depended on available and reliable solar radiometry. We predicted virus inactivation by combining viral UV254 sensitivity with effective solar flux at particular geographical sites and times of year. Due mainly to environmental factors which would decrease the available solar UVB (e.g., clouds, air pollution, dust, etc.), the accuracy of the predicted time required for virus inactivation by solar UV is expected to be within a factor of 2 of the actual value (Table 5). This methodology could also be extended to nonhuman viruses of agricultural impact, such as those that infect livestock and major crops.
Although the parameters reported here may suffice to estimate viral survival in many scenarios, experimental research directed to address various knowledge gaps identified in this study is required to increase the accuracy of our predicted viral persistence after a release. Determining the sensitivities of all threat viruses to UV254 and to radiation from UVA/UVB solar simulators under laboratory-controlled conditions that provide reliable radiometry should improve the level of confidence for the viral survival predicted in this work. Ultimately, the survival of a few selected viruses, or more likely of adequate nonpathogenic viral simulants, should be determined under actual solar exposure at representative locations and times of the year. Since virtually all the available UV254 inactivation data are for virus particles suspended in an aqueous solution, data need to be obtained from similar experiments conducted with viruses on surfaces and with aerosolized virus particles. Eventually, more sophisticated modeling will be required that takes account of such variables as shadows providing protection from the solar UV (17).
Lacking specific experimental data, our approach can be used to estimate survival of a wide variety of viruses after their release at any location and time of the year. Our approach and estimations of virus survival should be useful to develop more efficient countermeasures and to develop improved quarantine guidelines for cities and other areas contaminated after a viral release.
Acknowledgments
This work was supported by the U.S. Department of Defense Chemical and Biological Defense program administered by the Defense Threat Reduction Agency.
REFERENCES
- 1.Albrecht, T., S. C. Jeor, F. D. Funk, and F. Rapp. 1974. Multiplicity reactivation of human cytomegalovirus inactivated by ultra-violet light. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 26:445-454. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, R., M. Daya, and J. Reeve. 1987. An evaluation of the contribution of membrane lipids to protection against ultraviolet radiation. Biochim. Biophys. Acta 905:227-230. [DOI] [PubMed] [Google Scholar]
- 3.Battigelli, D. A., M. D. Sobsey, and D. C. Lobe. 1993. The inactivation of hepatitis A virus and other model viruses by UV irradiation. Water Sci. Technol. 27:339-342. [Google Scholar]
- 4.Bay, P. H., and M. E. Reichmann. 1979. UV inactivation of the biological activity of defective interfering particles generated by vesicular stomatitis virus. J. Virol. 32:876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bister, K., H. E. Varmus, E. Stavnezer, E. Hunter, and P. K. Vogt. 1977. Biological and biochemical studies on the inactivation of avian oncoviruses by ultraviolet irradiation. Virology 77:689-704. [DOI] [PubMed] [Google Scholar]
- 6.Bockstahler, L. E., and C. D. Lytle. 1977. Radiation enhanced reactivation of nuclear replicating mammalian viruses. Photochem. Photobiol. 25:477-482. [DOI] [PubMed] [Google Scholar]
- 7.Bossart, W., D. L. Nuss, and E. Paoletti. 1978. Effect of UV irradiation on the expression of vaccinia virus gene products synthesized in a cell free system coupling transcription and translation. J. Virol. 26:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danner, K., and A. Mayr. 1979. In vitro studies on Borna virus. II. Properties of the virus. Arch. Virol. 61:261-271. [DOI] [PubMed] [Google Scholar]
- 9.Day, R. S., III, and C. Ziolkowski. 1978. Studies on UV-induced viral reversion, Cockayne's syndrome, and MNNG damage using adenovirus 5, p. 535-539. In P. C. Hanawalt, E. C. Friedberg, and C. F. Fox (ed.), DNA repair mechanisms. Academic Press, San Diego, Calif.
- 10.Di Stefano, R., G. Burgio, P. Ammatuna, A. Sinatra, and A. Chiarini. 1976. Thermal and ultraviolet inactivation of plaque purified measles virus clones. G. Batteriol. Virol. Immunol. 69:3-11. [PubMed] [Google Scholar]
- 11.Frerichs, G. N., A. Tweedie, W. G. Starkey, and R. H. Richards. 2000. Temperature, pH and electrolyte sensitivity, and heat, UV and disinfectant inactivation of sea bass (Dicentrarchus labrax) neuropathy nodavirus. Aquaculture 185:13-24. [Google Scholar]
- 12.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis, p. 24-31. ASM Press, Washington, D.C.
- 13.Furusawa, Y., K. Suzuki, and M. Sasaki. 1990. Biological and physical dosimeter for monitoring solar UV-B light. J. Radiat. Res. 31:189-206. [DOI] [PubMed] [Google Scholar]
- 14.Furuse, K., and I. Watanabe. 1971. Effects of ultraviolet (UV) irradiation on RNA phages in H2O and D2O. Virology 46:171-172. [DOI] [PubMed] [Google Scholar]
- 15.Gates, F. L. 1934. Results of irradiating Staphalococcus aureus bacteriophage with monochromatic ultraviolet light. J. Exp. Med. 60:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson, J. H. 2003. UVB radiation: definition and characteristics. USDA/CSU website. [Online.] http://uvb.nrel.colostate.edu.
- 17.Grant, R. H. 1997. Biologically active radiation in the vicinity of a single tree. Photochem. Photobiol. 65:974-982. [Google Scholar]
- 18.Harris, G. D., V. D. Adams, D. L. Sorensen, and M. S. Curtis. 1987. Ultraviolet inactivation of selected bacteria and viruses with photoreactivation of the bacteria. Water Res. 21:687-692. [Google Scholar]
- 19.Hill, W. F., Jr., F. E. Hamblet, W. H. Benton, and E. W. Akin. 1970. Ultraviolet devitalization of eight selected enteric viruses in estuarine water. Appl. Microbiol. 19:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirai, K., F. Maeda, and Y. Watanabe. 1977. Expression of early virus functions in human cytomegalovirus infected HEL cells: effect of ultraviolet light-irradiation of the virus. J. Gen. Virol. 38:121-133. [DOI] [PubMed] [Google Scholar]
- 21.Hollaender, A., and B. M. Duggar. 1936. Irradiation of plant viruses and of microorganisms with monochromatic light. III. Resistance of the virus of typical tobacco mosaic and Escherichia coli to radiation from 3000 Å to 2250 Å. Proc. Natl. Acad. Sci. USA 22:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollaender, A., and J. Oliphant. 1944. The inactivating effect of monochromatic ultraviolet radiation on influenza virus. J. Bacteriol. 48:447-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ireland, W., and R. Sacher. 1996. The angular distribution of solar ultraviolet, visible and near-infrared radiation from cloudless skies. Photochem. Photobiol. 63:483-486. [Google Scholar]
- 24.Kassanis, B., and A. Kleczkowski. 1965. Inactivation of a strain of tobacco necrosis virus and of the RNA isolated from it, by ultraviolet radiation of different wave-lengths. Photochem. Photobiol. 4:209-214. [DOI] [PubMed] [Google Scholar]
- 25.Kelloff, G., S. A. Aaronson, and R. V. Gilden. 1970. Inactivation of murine sarcoma and leukemia viruses by ultra-violet irradiation. Virology 42:1133-1135. [DOI] [PubMed] [Google Scholar]
- 26.Kerr, J. B., and C. T. Elroy. 1993. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 262:1032-1034. [DOI] [PubMed] [Google Scholar]
- 27.Kleczkowski, A., and D. A. Govier. 1969. Action spectrum for inactivation of the infectivity of potato virus X by U.V. radiation. Photochem. Photobiol. 10:53-59. [DOI] [PubMed] [Google Scholar]
- 28.Kleczkowski, J., and A. Kleczkowski. 1965. Inactivation of a Rhizobium bacteriophage by ultraviolet radiation of different wave-lengths. Photochem. Photobiol. 4:201-207. [DOI] [PubMed] [Google Scholar]
- 29.Kohase, M., and J. Vilcek. 1979. Interferon induction with Newcastle disease virus in FS-4 cells: effect of priming with interferon and of virus inactivating treatments. Jpn. J. Med. Sci. Biol. 32:281-294. [DOI] [PubMed] [Google Scholar]
- 30.Latarjet, R., R. Cramer, and L. Montagnier. 1967. Inactivation, by UV-, X-, and gamma-radiations, of the infecting and transforming capacities of polyoma virus. Virology 33:104-111. [DOI] [PubMed] [Google Scholar]
- 31.Levinson, W., and R. Rubin. 1966. Radiation studies of avian tumor viruses and of Newcastle disease virus. Virology 28:533-542. [DOI] [PubMed] [Google Scholar]
- 32.Liltved, H., H. Hektoen, and H. Efraimsen. 1995. Inactivation of bacterial and viral fish pathogens by ozonation or UV irradiation in water of different salinity. Aquacult. Eng. 14:107-122. [Google Scholar]
- 33.Lytle, C. D. 1979. Radiation-enhanced virus reactivation in mammalian cells. Natl. Cancer Inst. Monogr. 50:145-149. [PubMed] [Google Scholar]
- 34.Lytle, C. D., S. A. Aaronson, and E. Harvey. 1972. Host cell reactivation in mammalian cells. II. Survival of herpes simplex virus and vaccinia in normal human and xeroderma pigmentosum cells. Int. J. Radiat. Biol. 22:159-165. [PubMed] [Google Scholar]
- 35.Lytle, C. D., and S. G. Benane. 1974. Host cell reactivation. IV. Cell culture conditions affecting virus survival. Int. J. Radiat. Biol. 26:133-141. [DOI] [PubMed] [Google Scholar]
- 36.Lytle, C. D., S. G. Benane, and J. E. Stafford. 1976. Host cell reactivation in mammalian cells. V. Photoreactivation studies with herpesvirus in marsupial and human cells. Photochem. Photobiol. 23:331-336. [DOI] [PubMed] [Google Scholar]
- 37.Lytle, C. D., R. E. Tarone. S.F. Barrett, J. D. Wirtschafter, J. M. Dupuy, and J. H. Robbins. 1983. Host cell reactivation by fibroblasts from patients with pigmentary degeneration of the retina. Photochem. Photobiol. 37:503-508. [DOI] [PubMed] [Google Scholar]
- 38.Martin, J. P., A. M. Aubertin, and A. Kirn. 1982. Expression of frog virus 3 early genes after ultraviolet irradiation. Virology 122:402-410. [DOI] [PubMed] [Google Scholar]
- 39.McClain, M. E., and R. S. Spendlove. 1966. Multiplicity reactivation of reovirus particles after exposure to ultraviolet light. J. Bacteriol. 92: 1422-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCleary, L. O., and M. P. Gordon. 1973. Ultraviolet irradiation of potato virus X, its RNA and a hybrid virus particle: photoreactivation, kinetic isotope effects, and quantum yield of inactivation. Photochem. Photobiol. 18:9-15. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie, R. L., M. Ilyas, J. E. Frederick, and V. Filyushkin. 1992. Ultra-violet radiation changes. Presented at UV-B Monitoring Workshop: a review of the science and status of measuring and monitoring programs. Washington, D.C.
- 42.Meng, Q. S., and C. P. Gerba. 1996. Comparative inactivation of enteric adenoviruses, polioviruses and coliphages by ultraviolet irradiation. Water Res. 30:2665-2668. [Google Scholar]
- 43.Miller, R. L., and P. G. W. Plagemann. 1974. Effect of ultraviolet light on mengovirus: formation of uracil dimmers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J. Virol. 13:729-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy, T. M. 1973. Inactivation of TMV-RNA by ultra-violet radiation in sunlight. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 23:519-526. [DOI] [PubMed] [Google Scholar]
- 45.Murphy, T. M., and M. P. Gordon. 1981. Photobiology of RNA viruses, p. 285-351. In H. Fraenkel-Conrat and R. R. Wagner (ed.), Comprehensive virology. Plenum Press, New York, N.Y.
- 46.Nomura, S., R. H. Bassin, W. Turner, D. K. Haapala, and P. J. Fischinger. 1972. Ultraviolet inactivation of Moloney leukaemia virus: relative target size required for virus replication and rescue of “defective” murine sarcoma virus. J. Gen. Virol. 14:213-217. [DOI] [PubMed] [Google Scholar]
- 47.Nuanualsuwan, S., T. Mariam, S. Himathongkham, and D. O. Cliver. 2002. Ultraviolet inactivation of feline calicivirus, human enteric viruses and coliphages. Photochem. Photobiol. 76:406-410. [DOI] [PubMed] [Google Scholar]
- 48.O'Hara, P. J., and M. P. Gordon. 1980. Ultraviolet inactivation of the midi variant of Qβ RNA: the sites of UV-induced replication inhibition. Photochem. Photobiol. 31:47-54. [DOI] [PubMed] [Google Scholar]
- 49.Owada, M., S. Ihara, K. Toyoshima, Y. Sugino, and Y. Kozai. 1976. Ultraviolet inactivation of avian sarcoma viruses: biological and biochemical analysis. Virology 69:710-718. [DOI] [PubMed] [Google Scholar]
- 50.Oye, A. K., and E. Rimstad. 2001. Inactivation of infectious salmon anaemia virus, viral haemorrhagic septicaemia virus and infectious pancreatic necrosis virus in water using UVC irradiation. Dis. Aquat. Organ. 48:1-5. [DOI] [PubMed] [Google Scholar]
- 51.Parrish, J. A., R. R. Anderson, F. Urbach, and D. Pitts. 1978. Biological effects of ultraviolet radiation with emphasis on human responses to longwave ultraviolet. Plenum Press, New York, N.Y.
- 52.Peak, M. J., and J. G. Peak. 1978. Action spectra for the ultraviolet and visible light inactivation of phage T7: effect of host-cell reactivation. Radiat. Res. 76:325-330. [PubMed] [Google Scholar]
- 53.Powell, W. F. 1959. Radiosensitivity as an index of herpes simplex virus development. Virology 9:1-19. [DOI] [PubMed] [Google Scholar]
- 54.Powell, W. F., and R. B. Setlow. 1956. The effect of monochromatic ultraviolet radiation on the interfering property of influenza virus. Virology 2: 337-343. [DOI] [PubMed] [Google Scholar]
- 55.Rauth, A. M. 1965. The physical state of viral nucleic acid and the sensitivity of viruses to ultraviolet light. Biophys. J. 5:257-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivers, T. M., and F. L. Gates. 1928. Ultraviolet light and vaccine virus. II. The effect of monochromatic ultraviolet light upon vaccine virus. J. Exp. Med. 47:45-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rommelaere, J., J. M. Vos, J. J. Cornelis, and D. C. Ward. 1981. UV-enhanced reactivation of minute virus of mice: stimulation of a late step in the viral life cycle. Photochem. Photobiol. 33:845-854. [DOI] [PubMed] [Google Scholar]
- 58.Ronto, G., S. Gaspar, and A. Berces. 1992. Phages T7 in biological UV dose measurement. J. Photochem. Photobiol. B 12:285-294. [DOI] [PubMed] [Google Scholar]
- 59.Ronto, G., S. Gaspar, A. B. P. Grof, A. Berces, and Z. Gugolya. 1994. Ultraviolet dosimetry in outdoor measurements based on bacteriophage T7 as a biosenser. Photochem. Photobiol. 59:209-214. [Google Scholar]
- 60.Ronto, G., P. Grof, and S. Gaspar. 1995. Biological UV dosimetry—a comprehensive problem. J. Photochem. Photobiol. B 31:51-56. [Google Scholar]
- 61.Rushizky, G. W., C. A. Knight, and A. D. McLaren. 1960. A comparison of the ultraviolet-light inactivation of infectious ribonucleic acid preparations from tobacco mosaic virus and those of the native and reconstituted virus. Virology 12:32-47. [DOI] [PubMed] [Google Scholar]
- 62.Ryan, D. K. G., and A. J. Rainbow. 1986. Comparative studies of host-cell reactivation, cellular capacity and enhanced reactivation of herpes simplex virus in normal, xeroderma pigmentosum and Cockayne syndrome fibroblasts. Mutat. Res. 166:99-111. [DOI] [PubMed] [Google Scholar]
- 63.Sako, H., and M. Sorimachi. 1985. Susceptibility of fish pathogenic viruses, bacteria and fungus to ultraviolet irradiation and the disinfectant effect of U.V.-ozone water sterilizer on the pathogens in water. Bull. Natl. Res. Inst. Aquaculture 8:51-58. [Google Scholar]
- 64.Setlow, R. 1960. The use of action spectra to determine the physical state of DNA in vivo. Biochim. Biophys. Acta 39:180-181. [DOI] [PubMed] [Google Scholar]
- 65.Setlow, R. 1974. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc. Natl. Acad. Sci. USA 71:3363- 3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Setlow, R. B., and E. C. Pollard. 1962. Molecular biophysics. Addison-Wesley Publishing Co., Reading, Mass.
- 67.Shanley, J. D. 1982. Ultraviolet irradiation of murine cytomegalovirus. J. Gen. Virol. 63:251-254. [DOI] [PubMed] [Google Scholar]
- 68.Shaw, J. E., and D. C. Cox. 1973. Early inhibition of cellular DNA synthesis by high multiplicities of infectious and UV-inactivated reovirus. J. Virol. 12:704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smirnov, Y., S. P. Kapitulez, and N. V. Kaverin. 1992. Effects of UV-irradiation upon Venezuelan equine encephalomyelitis virus. Virus Res. 22:151-158. [DOI] [PubMed] [Google Scholar]
- 70.Stull, H. B., and A. F. Gazdar. 1976. Stability of Rauscher leukemia virus under certain laboratory conditions. Proc. Soc. Exp. Biol. Med. 152:554-556. [DOI] [PubMed] [Google Scholar]
- 71.Sturm, E., F. L. Gates, and J. B. Murphy. 1932. Properties of the causative agent of a chicken tumor. II. The inactivation of the tumor-producing agent by monochromatic ultraviolet light. J. Exp. Med. 55:441-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su, Z. Z., J. J. Cornelis, and J. Rommelaere. 1981. Mutagenesis of intact parvovirus H-1 is expressed co-ordinately with enhanced reactivation of ultraviolet irradiated virus in human and rat cells treated with 2-nitronaphthofurans. Carcinogenesis 2:1039-1043. [DOI] [PubMed] [Google Scholar]
- 73.Tucker, J. B., and R. A. Zilinskas. 2003. The 1971 smallpox outbreak in the Soviet city of Aralsk: implications for Variola virus as a bioterrorist threat. Crit. Rev. Microbiol. 29:81-95. [DOI] [PubMed] [Google Scholar]
- 74.Tyrrell, R. M. 1978. Solar dosimetry with repair deficient bacterial spores: action spectra, photoproduct measurements and a comparison with other biological systems. Photochem. Photobiol. 27:571-579. [DOI] [PubMed] [Google Scholar]
- 75.van der Eb, A. J., and J. A. Cohen. 1967. The effect of UV-irradiation on the plaque-forming ability of single- and double-stranded polyoma virus DNA. Biochem. Biophys. Res. Commun. 28:284-288. [DOI] [PubMed] [Google Scholar]
- 76.van Regenmortel, M. H. V., C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner. 2000. Classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 77.Vos, J. M., J. J. Cornelis, S. Limbosch, F. Zampetti-Bosseler, and J. Rommelaere. 1981. UV-irradiation of related mouse hybrid cells: similar increase in capacity to replicate intact minute-virus-of-mice but differential enhancement of survival of UV-irradiated virus. Mutat. Res. 83:171-178. [DOI] [PubMed] [Google Scholar]
- 78.Wang, C.-H., S.-Y. Tschen, and B. Flehmig. 1995. Antigenicity of hepatitis A virus after ultra-violet inactivation. Vaccine 13:835-840. [DOI] [PubMed] [Google Scholar]
- 79.Weiss, M., and M. C. Horzinek. 1986. Resistance of Berne virus to physical and chemical treatment. Vet. Microbiol. 11:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Werbin, H., R. C. Valentine, and A. D. McLaren. 1967. Photobiology of RNA bacteriophages. I. Ultraviolet inactivation and photoreactivation studies. Photochem. Photobiol. 6:205-213. [Google Scholar]
- 81.Wester, U. 1992. Non-U.S. national UV research and monitoring programs: Sweden. Presented at UV-B Monitoring Workshop: a review of the science and status of measuring and monitoring programs. Washington, D.C.
- 82.Winkler, U., H. E. Johns, and E. Kellenberger. 1962. Comparative study of some properties of bacteriophage T4D irradiated with monochromatic ultraviolet light. Virology 18:343-358. [DOI] [PubMed] [Google Scholar]
- 83.Yoshikura, H. 1971. Ultraviolet inactivation of murine leukemia and sarcoma viruses. Int. J. Cancer 7:131-140. [DOI] [PubMed] [Google Scholar]
- 84.Yoshikura, H. 1973. Ultraviolet inactivation of murine leukemia virus and its assay in permissive and non-permissive cells. Int. J. Cancer 11:739-746. [DOI] [PubMed] [Google Scholar]
- 85.Yoshikura, H. 1973. Ultraviolet sensitivity of helper function of murine leukemia virus. Arch. Biochem. Biophys. 154:76-83. [DOI] [PubMed] [Google Scholar]
- 86.Zavadova, Z., L. Gresland, and M. Rosenbergova. 1968. Inactivation of single- and double-stranded ribonucleic acid of encephalomyocarditis virus by ultraviolet light. Acta Virol. 12:515-522. [PubMed] [Google Scholar]
- 87.Zavadova, Z., and H. Libikova. 1975. Comparison of the sensitivity to ultraviolet irradiation of reovirus 3 and some viruses of the Kemerovo group. Acta Virol. 19:88-90. [PubMed] [Google Scholar]
- 88.Zavadova, Z., and J. Zavada. 1968. Host-cell repair of ultraviolet-irradiated pseudorabies virus in chick embryo cells. Acta Virol. 12:507-514. [PubMed] [Google Scholar]
- 89.Zelle, M. R., and A. Hollaender. 1954. Monochromatic ultraviolet action spectra and quantum yields for inactivation of T1 and T2 Escherichia coli bacteriophages. J. Bacteriol. 68:210-215. [DOI] [PMC free article] [PubMed] [Google Scholar]