Abstract
The genetic diversity of hepatitis B virus (HBV) strains has evolved through mutations such as point mutations, deletions or insertions, and recombination. We identified and characterized a novel type of mutation which is a complex of external insertion, deletion, and internal duplication in sequences from one of six patients with chronic hepatitis B virus genotype E (HBV/E). We provisionally named this mutation a “replacement mutation”; the core promoter upstream regulatory sequence/basic core promoter was replaced with a part of the S1 promoter covering the hepatocyte nuclear factor 1 (HNF1) binding site, followed by a tandem repeat of the HNF1 site. A longitudinal analysis of the HBV population over 6 years showed the clonal change from wild-type HBV/E to replacement-mutant type, resulting in a lower hepatitis B (HB) e antigen titer, a high HBV DNA level in serum, and progression of liver fibrosis. In an in vitro study using a replication model, the replacement-mutant HBV showed higher replication levels than the wild-type HBV/E replicon, probably mediated by altered transcription factor binding. Additionally, this HNF1 site replacement mutation was associated with excessive HB nucleocapsid protein expression in hepatocytes, in both in vivo and in vitro studies. This novel mutation may be specific to HBV genotype E, and its prevalence requires further investigation.
Viral genetic diversification occurs, in general, through mutation, recombination, and reassortment (44). Since reassortment does not occur in the hepatitis B virus (HBV) genome, HBV strains diversify through mutations and recombinations. The viral mutations are divided into point mutations, deletions, and insertions (8). Furthermore, the insertions in the HBV genome can be of two types. The first one is the insertion of a nucleotide sequence which does not exist close to the affected sequence; examples include the 36-bp insertion observed in HBV genotype G (4, 15, 34) and the insertion of the hepatocyte nuclear factor 1 (HNF1) binding site (10, 19, 20, 30). The second type is the insertion of a nucleotide sequence located close to the inserted portion, which is termed internal duplication or tandem repeat (10). The traditional concept is that these mutations occur independently.
HBV genotypes are determined by nucleotide differences of more than 8% (25). Each genotype has its distinct geographical distribution. The accumulating evidence suggests that there are correlations between different HBV genotypes and specific viral mutations. For example, HBV subgenotype Aa (HBV/Aa), which is distributed in Asia and Africa, has a subgenotype-specific mutation just prior to the precore open reading frame (ORF) start which was shown to reduce HBV e antigen (HBeAg) expression (2, 35). HBV genotypes B, C, and D are prone to develop precore stop codons at position 1896, based on the nucleotide base-pairing of the stem-loop structure (16). Genotype A has a genotype-specific insertion of six nucleotides in the core gene. HBV genotype E (HBV/E) is restricted to West Africa, and its virological and clinical characteristics are not well defined.
The HNF1 binding site is a necessary part of the S1 promoter for maximal transcriptional activity (32). Cases with an HNF1 site insertion in the basic core promoter (BCP) have been reported in patients with various clinical statuses (10, 19, 20, 30). An HNF1 site insertion in the BCP elevates core/pregenomic mRNA transcription activity and excessive HBcAg deposition in the nuclei and cytoplasm of infected hepatocytes (30). The well-known double mutation in the core promoter, G1762T/G1764A (24), is thought to create an HNF1 site in the BCP and enhanced pregenomic mRNA transcription in an in vitro study (21). These studies have shown that the presence of an HNF1 site in the BCP affects pregenomic RNA transcription.
In the present study we discovered and describe the characteristics of a novel type of HBV genome rearrangement, which is a combination of external insertion, deletion, and internal duplication of a single sequence in a patient with chronic hepatitis due to HBV/E. This novel mutation was provisionally given the name “replacement mutation,” and its uniqueness is that a part of the S1 promoter covering the HNF1 site is tandemly repeated in the core promoter upstream regulatory sequence (CURS)/BCP. Additionally, functional analyses of this mutation are conducted in vivo and in vitro.
MATERIALS AND METHODS
Patients.
Six patients with chronic hepatitis B, all infected with HBV genotype E, were included in this study (Table 1). The presence of HBV/E was determined by the HBV genotyping assay (Innogenetics) and further confirmed by sequencing and a phylogenetic analysis. All six patients were originally from West Africa but were all residing in the United Kingdom at the time of the study. The longitudinal data of one patient (patient 2) were investigated later.
TABLE 1.
Characteristics of six patients with HBV/E in the United Kingdom
| Patient | Age (yr) | Sex | Ethnicity and country of origin | HBeAg | ALT (IU/liter) | Liver histology |
|---|---|---|---|---|---|---|
| 1 | 44 | Male | African, Nigeria | Negative | 135 | Grade 6, stage 3 |
| 2 | 35 | Male | African, Nigeria | Positive | 429 | Grade 6, stage 2 |
| 3 | 34 | Female | African, Nigeria | Positive | 170 | Grade 4, stage 1 |
| 4 | 35 | Male | African, Ghana | Positive | 81 | Grade 4, stage 3 |
| 5 | 52 | Male | African, Nigeria | Negative | 111 | Grade 4, stage 3-4 |
| 6 | 45 | Male | African, Nigeria | Negative | 74 | Grade 5, stage 3 |
Serological testing.
Levels of HBeAg and the antibody to HBeAg (anti-HBe) were determined semiquantitatively using a commercially available chemiluminescent-enzyme immunoassay (Lumipulse ƒ; Fujirebio Inc., Tokyo, Japan).
RTD-PCR.
Serum HBV DNA was quantitatively detected by real-time detection PCR (RTD-PCR) based on TaqMan chemistry as reported previously (1), with some modification (9). The lower limit of detection for this system was as little as 5 DNA copies/assay, and the linear standard curve was obtained from 5 to 106 DNA copies/assay.
PCR amplification and sequencing of HBV.
The serum samples were stored at −80°C until use. Total DNA was extracted from 100 μl of serum using microspin columns (QIAamp blood kit; QIAGEN K.K., Tokyo, Japan). Purified DNA was resuspended in 80 μl of distilled water. PCR was carried out as described previously (36). The digest was run by electrophoresis on 3% or 5% (wt/vol) agarose, stained with ethidium bromide, and observed in UV light. The nucleotide sequences of the amplicons were determined directly by the dideoxy method, using the ABI Prism BigDye Terminator cycle sequencing ready reaction kit with a fluorescent model 3100 DNA sequencer (Applied Biosystems, Foster City, CA).
Molecular cloning for sequencing.
To detect the CURS/BCP sequence changes in patient 2, sequences including the CURS/BCP sequence were determined in serial samples. Six samples from October 1999 to 2002 could be sequenced directly from PCR products; however, the samples from 1997 and 1998 could not. The amplified products were cloned into TA cloning vector (TOPO TA cloning kit; Invitrogen, Carlsbad, CA) and sequenced.
Plasmid construction for replication model (replicon).
Using DNA extracted from patient 2, two overlapping fragments, fragment A and fragment B, covering the full HBV genome of approximately 1,700 bp, were amplified by nested PCR. The primers used for fragment A were E0010S (nucleotides [nt] 10 to 39, 5′-ATTCCACCAAGCTCTGCTAGATCCCAGAGT-3′) and E1813R (nt 1783 to 1813, 5′-GGTGCTGGTGCGCAGACCAATTTATGCCTA-3′) for the first PCR and B0046S-C (nt 10 to 39, 5′-ATTCCACCAAGCTCTGCTAGATCC CAGAGT-3′) and B1760R-C (nt 1755 to 1731, 5′-TAATCTCCTCCCCCA ACTCCTCCCA-3′) for the second PCR. The primers used for fragment B were E1601S (nt 1601 to 1630, 5′-ACGTCGCATGGAGACCACCGT-3′) and E0266R (nt 262 to 232, 5′-ATGGCGTCTCAGATCTGAGCACCACCT GAA-3′) for the first PCR and E1601S and B0207R-C (nt 207 to 178, 5′-CCCGCCTGTAATACGAGCAGGGGTCCTAGG-3′) for the second PCR. These fragments were then ligated into pGEM-T vector (Promega, Madison, WI) and cloned in DH5α cells. Ten clones each (pGEM-fragA-1 to -10 and pGEM-fragB-1 to -10) were obtained, and the nucleotide sequences were determined. Of these, pGEM-fragA-3 and pGEM-fragB-2, with consensus sequences and without core deletion or replacement mutation (not major clones), were used as templates to construct HBV replicons. To produce fragment C, pGEM-fragA-3 and pGEM-fragB-2 were mixed and amplified with primers E1039F-HindIII and E2168R. The PCR product was digested with HindIII and AvrII, and fragment C-B-HindIII-AvrII was produced. The PCR product was also cloned, and pGEM-fragA-C was produced. Finally, the fragments C-B-HindIII-AvrII and pGEM-fragA-C, cut with AvrII and SacI, were cloned into pUC19 without promoters (Invitrogen) cut with HindIII and SacI, and a pUC19-HBV/E wild-type replicon encoding a replication-competent 1.35-unit-length HBV genome was produced. In addition, the pUC19-HBV/E wild-type replicon was digested by RsrIII and XbaI, the fragment with the replacement mutation from patient 2 (strain UK2), cut with RsrIII and XbalI, was ligated, and a pUC19-HBV/E replacement replicon was produced.
Cell culture and DNA transfection.
HuH-7 cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. For the standard replication assay, 10-cm-diameter dishes were seeded with 1 × 106 HuH-7 cells per dish. Sixteen hours postseeding, cells were transfected with 5 μg of DNA construct using the Fugene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) and harvested 3 days later. Transfection efficiency was measured by cotransfection of 1 μg of reporter plasmid expressing secreted alkaline phosphatase and determination of secreted alkaline phosphatase enzymatic activity in the cell culture supernatant.
Isolation of core-associated HBV DNA from transfected cells.
HBV DNA was purified from intracellular core particles by a method described by Turelli et al. (41), with minor modifications. Briefly, cells were suspended in 1.5 ml of lysis buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, and 1% NP-40. Nuclei were pelleted by centrifugation at 4°C and 15,000 rpm for 5 min. The supernatant was adjusted to 6 mM Mg acetate and treated with 200 μg/ml of DNase I and 100 μg/ml of RNase A for 2 h at 37°C. The reaction was stopped by the addition of EDTA to a final concentration of 10 mM, and then the mixture was incubated for 10 min at 65°C. Proteins of the sample were digested with 200 μg/ml of proteinase K, 1% sodium dodecyl sulfate, and 100 mM NaCl for 2 h at 37°C. Nucleic acids were purified by phenol-chloroform (1:1) extraction and ethanol precipitation after the addition of 20 μg of glycogen.
Preparation of total RNA.
Transfected cells were lysed by ISOGEN (Nippon Gene, Japan). After the addition of 500 μl of chloroform and 15 min of incubation on ice, the lysates were centrifuged for 15 min at 15,000 rpm. The aqueous phase was precipitated with isopropanol. Total RNA was pelleted by centrifugation, washed with ethanol, and dissolved in water.
Southern and Northern blot hybridization.
Isolated core-associated HBV DNAs were separated on a 1.2% agarose gel. Twenty micrograms of total RNA was separated on a 1% agarose-formaldehyde gel. DNAs and RNAs were transferred to a positively charged nylon membrane (Roche Diagnostics, Germany) and hybridized with either an alkaline phosphatase-labeled full-length HBV fragment or a 1.3-kb GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA fragment generated with a Gene Images AlkPhos direct labeling system (Amersham Biosciences, United Kingdom). The detection was performed with CDP-Star (Amersham Biosciences, United Kingdom). The signals were analyzed by using a LAS-1000 image analyzer (Fuji Photo Film, Japan).
Hepatic expression of HBcAg.
A liver biopsy was performed as part of a routine diagnostic assessment, and the grade of inflammation and fibrosis stage were scored according to established criteria (14). The expression of HBcAg in hepatocytes was determined by immunoperoxidase staining using rabbit polyclonal anti-HBc as a primary antibody (22) and an EnVision detection kit (Dako Ltd., Ely, England). A semiquantitative assessment of the immunoreactivity was carried out by scoring the proportion of positive cells in four microscopic fields at a magnification of ×250 (23).
Immunofluorescence assay for HBV core protein.
At 3 days posttransfection, monolayer cultures on coverslips were washed with phosphate-buffered saline three times before fixation. The cells attached to the coverslips were fixed in ice-cold acetone-methanol (1:1) for 10 min. After blocking using antibody diluent (Dako Co., Carpinteria, CA), hepatitis B core antigen (HBcAg) was stained with a diluted mouse monoclonal antibody (Hyb-3120; Institutes of Immunology, Tokyo, Japan) which recognizes a capsid conformation-specific epitope (6). Goat anti-mouse immunoglobulin G-fluorescein isothiocyanate was used as a secondary antibody for the experiments. The nuclei of cells were counterstained with 10 μg of 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO). The results were visualized under an ECLIPSE E800M fluorescence microscope (Nikon, Tokyo, Japan) and a Zeiss LSM 510 confocal laser scanning microscope (Carl Zeiss, Germany).
Molecular evolutionary analysis.
The complete sequences of the HBV/E strains isolated from six patients (strains UK1 to UK6) were aligned along with the complete HBV genome strains of different genotypes by use of the CLUSTAL W software program (40), and the alignment was confirmed by visual inspection. A homology search for the UK2 unique partial sequence was carried out using NCBI BLAST 2.2.6 (3). The HBV genome database search was conducted with the Hepatitis Virus Database (http://s2as02.genes.nig.ac.jp/).
Nucleotide sequence accession number(s).
The sequences reported in this paper have been deposited in GenBank/DDBJ/EMBL databases (accession numbers AB219529 to AB219534).
RESULTS
HBV genome alignment and identification of a specific CURS/BCP sequence.
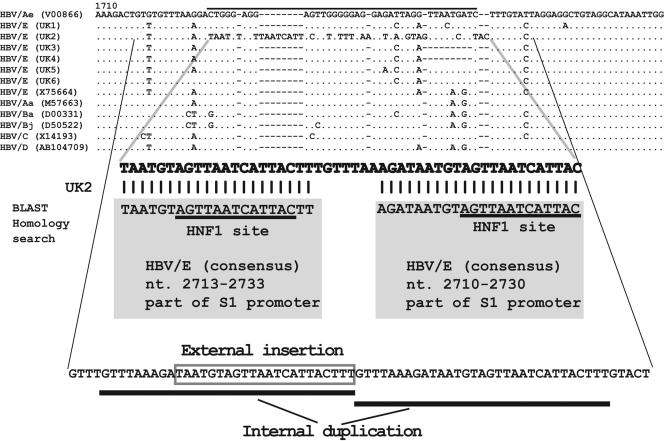
Six complete HBV/E genomes (for strains UK1 to UK6) were aligned with reference sequences of other genotypes (DNA data bank accession no. V00866, X75664, M57663, D00331, D50522, X14193, and AB104709). The alignment of the CURS/BCP region of the HBV genome is shown in Fig. 1. A new 50-nucleotide sequence was identified in UK2, starting from nt 1720.
FIG. 1.
Nucleotide alignment of the six HBV genotype E sequences of the core upstream regulatory region to the basic core promoter region, with references to other genotypes and subtypes. The sequence enlarged under the alignment shows the UK2-specific 50-nucleotide sequences which were analyzed by BLAST homology search. The 1st to 21st nucleotides and 29th to 50th nucleotides matched the conserved sequences of HBV genotype E, nt 2713 to 2733 and nt 2710 to 2730, shown in shaded rectangles. Furthermore, the consequences of mutations which occurred in the UK2 sequence are shown. The normal CURS/BCP sequences are deleted, the S1 promoter, including the HNF1 site, is externally inserted, and internal duplication occurs.
To clarify whether the region of 50 nucleotides is part of a particular structure or whether it represents a random accumulation of nucleotide substitutions, the 50-nucleotide sequence was examined by NCBI BLAST 2.2.6 (3). The result showed that the first 21 nucleotides match completely the well-conserved HBV/E nt 2713 to 2733, and the last 21 nucleotides completely matched well-conserved HBV/E nt 2710 to 2730. In HBV/E, nt 2706 to 2806 comprise the conserved S1 promoter region where the HNF1 binding site AGTTAA TCATAC is located. Therefore, the insertion of the UK2-specific 50 nucleotides in the HBV genome consists of an HNF1 site (S1 promoter) tandem repeat (Fig. 1).
Replacement mutation.
The mechanism of the aforementioned HNF1 site (S1 promoter) tandem repeat was sought next. A detailed inspection of the HBV DNA sequence showed that the mutation comprises three genomic variations. These include a deletion of the normal CURS/BCP sequence, insertion of part of the S1 promoter, and internal duplication of the sequence GTTTAAAGATAATGTAGTTAATCATTACTTT (Fig. 1). The internal duplication starts upstream from the 50-nucleotide BLAST-searched sequence. We therefore named this novel genetic rearrangement a “replacement mutation.”
Clinical characteristics of the HBV-infected patient with this HNF1 site replacement mutation.
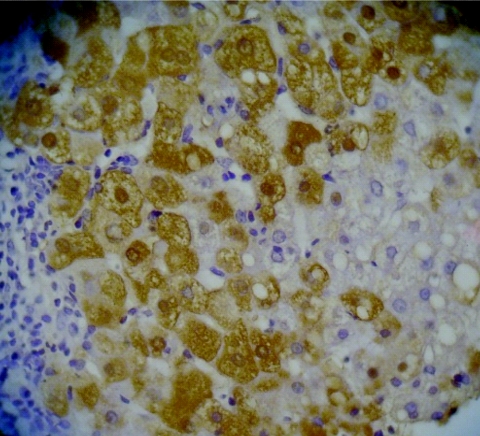
Three of six patients (patients 2, 3, and 4) were seropositive for HBeAg, and the other three (patients 1, 5, and 6) were anti-HBe positive. All three anti-HBe-positive patients had sequences with a double mutation, in nt 1762 and 1764, as well as the precore stop codon mutation (G1896A). Core deletions were observed in sequences from HBeAg-positive patients, and pre-S deletions were noted in anti-HBe-positive patients in this study. Strain UK2 had deletions from nt 2135 to 2308, UK3 from nt 2132 to 2229, and UK4 from nt 1989 to 2051 and nt 2118 to 2219. Among strains from anti-HBe-positive patients with pre-S deletions, UK1 had deletions from nt 44 to 55, UK5 from nt 3168 to 3170, and UK6 from nt 1 to 30, although these patients also had wild-type clones without any deletions, suggesting that these deletions are not always associated with HBeAg production. The patient with the replacement mutation had high HBV DNA levels (1.3 × 108 copies) in serum and active hepatitis (grade 6, stage 2, with an alanine aminotransferase [ALT] level of 429 IU/liter). Immunohistochemical analyses of liver specimens showed that this patient had hepatic HBcAg expression patterns in both nuclei and cytoplasm (Fig. 2) which were quite distinct from those of the other patients (Table 2).
FIG. 2.
Immunohistochemical detection of HBcAg in the liver of the patient 2. Strong staining is observed in both nuclei and cytoplasm.
TABLE 2.
HBV DNA levels and HBV genome mutations of the six patients
| Patient | HBeAgb | HBV DNA levels (copies/ml) | Mutation at nta:
|
Core deletionb | preS deletionb | % HBc stainingc
|
||
|---|---|---|---|---|---|---|---|---|
| 1762 and 1764 | 1896 | Nucleus | Cytoplasm | |||||
| 1 | − | 2.4 × 104 | Variant | Variant | − | + | NT | NT |
| 2 | + | 1.3 × 108 | Rep | No mutation | + | − | 75 | 75 |
| 3 | + | 4.2 × 108 | Deletion | No mutation | + | − | 5 | 40 |
| 4 | + | 2.2 × 108 | Deletion | No mutation | + | − | 1 | 10 |
| 5 | − | 5.4 × 104 | Variant | Variant | − | + | NT | NT |
| 6 | − | 5.2 × 104 | Variant | Variant | − | + | 20 | 0 |
Rep, replacement mutation.
+, presence; −, absence.
NT, not tested.
Longitudinal analysis of the replacement mutation.
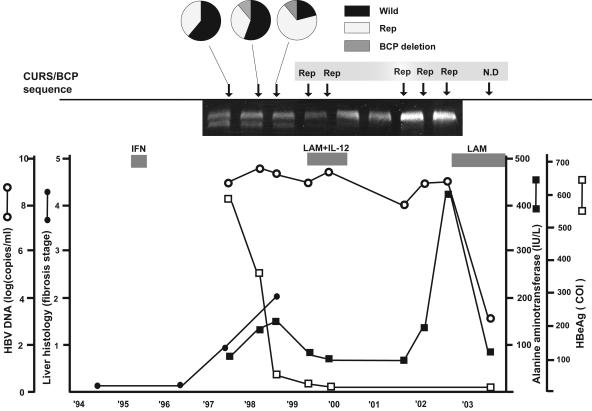
We investigated the evolution of the replacement mutation at nine time points from 1997 to 2003 with the corresponding clinical data. Patient 2 demonstrated the HNF1 site replacement mutation since October 1998, confirmed by direct sequencing of PCR products; however, the results for three earlier samples during 1997 and 1998 were not able to be determined by direct sequencing of PCR products, due to mixed viral populations. Sequencing analysis of 18 clones from the sample from 1997 showed 11 clones (61.1%) with wild-type sequences in CURS/BCP and 7 (38.9%) with the replacement mutation. Of nine clones in May 1998, 5 (55.6%) showed the wild type, 3 (33.3%) the replacement type, and 1 (11.1%) the BCP deletion type. Of an additional 19 clones from October 1998, 4 (21.1%) showed the wild type, 13 (68.4%) showed the replacement type, and 2 (10.5%) showed the BCP deletion type (Fig. 3). No other clinical events, except for initial interferon (IFN) therapy, were observed before 1997. In addition, the progression of the fibrosis stage occurs after the initial IFN therapy and correlates with a viral sequence change from a wild-type and replacement sequence mixed status to a replacement-dominant pattern. The titration of HBeAg decreased and the ratio of replacement increased during the time course.
FIG. 3.
Clinical course of patient 2 based on HBV DNA levels, fibrosis stage, HBeAg titer, and ALT levels. The three circular charts show how the proportions of wild type, replacement mutation (Rep) type, and BCP deletion type changed during the time course. The electropherogram (5%) shows that mixed types (wild type and Rep) are found during the first three points. ○, HBV DNA levels; •, fibrosis stage; □, HBeAg titer; ▪, ALT levels. The patient was treated with IFN monotherapy and then with the combination therapy of lamivudine (LAM) and interleukin-12 (IL-12). The patient has been treated with LAM since 2003. COI, cutoff index.
In vitro study using replication model (replicon).
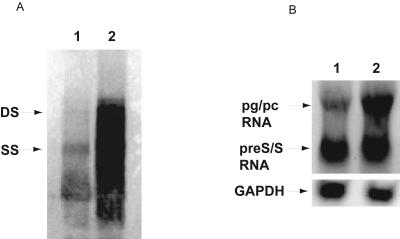
Southern blot analyses of total cellular DNA isolated from HuH-7 cells 72 h after transfection revealed that the pUC19-HBV/E replicon with the replacement mutation, which had the HBV/E construct with an HNF1 site tandem repeat in the CURS/BCP, replicated much more than the pUC19-HBV/E wild-type replicon (Fig. 4A). Northern blot analyses indicated that the replacement mutant with increased replication had higher pregenomic and pre-S/S RNA levels (Fig. 4B).
FIG. 4.
(A) Southern blot analysis of intracellular replication competence of replacement mutation with HNF1 site. Double-stranded (DS) DNA and single-stranded (SS) DNA are indicated by arrows. The pUC19-HBV/E replacement replicon (lane 2) replicates at a much higher rate than the pUC19-HBV/E wild-type replicon (lane 1). (B) Northern blot analysis of HBV transcripts. Lane 1, pUC19-HBV/E wild-type replicon; lane 2, pUC19-HBV/E replacement replicon. pg/pc RNA, pregenomic/precore RNA. A GAPDH probe was used to quantify RNA in each lane.
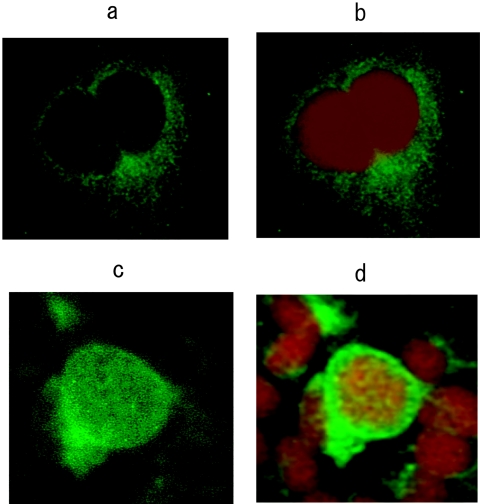
Immunofluorescence experiments were performed to investigate the intracellular localization of the viral protein products. After transfection of the pUC19-HBV/E wild-type replicon, homogenous cytoplasmic staining for HBcAg was evident, while no nuclear localization could be detected (Fig. 5A and B). In contrast, transfection of the pUC19-HBV/E replacement replicon indicated that HBcAg was localized in the nucleus and perinucleus, and only a faint cytoplasmic staining was found (Fig. 5C and D). These results were confirmed with a confocal laser scanning microscope.
FIG. 5.
Intracellular localization of HBcAg (magnification, ×400). Immunofluorescence staining of HBcAg in HuH-7 cells transfected with (a) pUC19-HBV/E wild-type replicon and (c) pUC19-HBV/E replacement replicon. (b and d) Nuclear staining for the same set of cells was performed by use of DNA staining. DAPI stain shows as red.
DISCUSSION
We report here an HNF1 site replacement mutation observed in a sequence from a chronic hepatitis patient with HBV/E. This report has dual impacts: one is that this mutation is a novel mode of viral mutation arising as a complex of known types of mutations, and the other is that an HNF1 site tandem repeat due to replacement mutation affects not only the RNA transcription and DNA replication efficiency of HBV but also accumulation of hepatitis B core protein in nuclei and cytoplasm of hepatocytes.
There are no previous reports that deletion, remote insertion, and internal duplication were observed in identical portions of the sequence. We provisionally named the novel mode of mutation “replacement mutation,” which is defined as a complex of insertion of remote sequence, deletion, and internal duplication in a single sequence portion. We examined 839 HBV strains with CURS/BCP nucleotide sequences which were deposited in the Hepatitis Virus Database (http://s2as02.genes.nig.ac.jp/); however, no similar mutations were found. In addition, we searched 530 complete genomes for this complex mutation in the HCV database; however, no similar mutations were found in any part of the complete genome sequences. This is the first report of replacement mutation as a viral mutation. Though it might be a rare mutation, researchers might have ignored the strange sequences as errors, especially if the strange sequences were seen in otherwise quite conserved parts of nucleotide sequences.
One of the impacts of this replacement mutation on viral mutation is that the virus will undergo large nucleotide changes in a relatively short period, and they are drastic changes compared with other mutations. Accumulation of point mutations, which occurs frequently, accounts for intragenotypic differences. One replacement mutation could cause a drastic change to the genomic sequence, which might explain the evolutionary events.
In this report, CURS/BCP was replaced with a part of the S1 promoter covering the HNF1 site. The S1 promoter originally is a large surface antigen promoter of approximately 100 bp, and this promoter includes the binding site for the liver-specific transcriptional factor HNF1 (28, 33). Without the HNF1 site, S1 promoter activity is reduced 10- to 20-fold (31, 32), indicating that the HNF1 site is accepted as the key part of the S1 promoter. There have been reports of insertion of an HNF1 site in BCP among HBV-infected patients with various clinical statuses (10, 19, 20, 30). One study has shown that HNF1 site insertion caused enhanced viral replication in a fulminant-hepatitis patient, and the accumulation of massive amounts of cytoplasmic and nuclear HBcAg was observed in infected hepatocytes (30). These data support our results that the HNF1 site replacement mutation causes accumulation of massive amounts of cytoplasmic and nuclear HBcAg in liver immunostaining and enhances viral replication on the basis of an in vitro replication model.
In our results, core deletions in sequences from three HBeAg-positive patients and pre-S deletions in sequences from three anti-HBe-positive patients were observed. Core deletions are reported to be related to the presence of HBeAg and seroconversion in the near future (43). In an in vitro experiment, the core deletion type can replicate more efficiently than the wild type when complemented with wild-type core protein, and the replication enhancement by core deletion is not through the enhancement of transcription (11). Although a minor wild-type clone might enhance the replication of a core deletion clone, HNF1 binding seems to be the major cause of replication enhancement. It is because only strain UK2 has a distinctively high percentage of both nuclear and cytoplasmic core protein expression, though all three HBeAg-positive patients demonstrated core deletions in the middle of core ORFs. Furthermore, the in vitro experiment shows that an HNF1 site tandem repeat enhances core/pregenomic RNA transcription (Fig. 4) when a part of the CURS/CP of the wild-type HBV/E replication clone was replaced by the HNF1 binding tandem repeat of UK2. Pre-S deletion is observed in cases of long-lasting HBV chronic infection and advanced liver diseases (27, 37). As the positions and lengths of pre-S deletions differ in each case, their effects on HBV replication have not been clearly elucidated. Additionally, the HNF1 tandem repeat causes X protein truncation, i.e., 27 amino acids of the C terminus are truncated. The role of X protein in HBV replication is controversial (5, 39). However, X protein truncation is reported to be related to hepatocellular carcinoma (13, 29). Further investigation into the relationship between the HNF1 tandem repeat and hepatocellular carcinoma is needed.
The analysis of serial samples revealed that the HBV population included both CURS/BCP wild-type and CURS/BCP replacement clones from 1997 to October 1998, and the proportion of the replacement type gradually rose and then became predominant. Insertions at the HNF1 site were observed in sequences from patients with immunosuppressive therapy (10, 19, 30); no therapy except IFN was included in this case. Although sequences for many patients undergoing IFN therapy have been reported (7, 12, 18, 42), the HNF1 replacement has not been reported, as mentioned in the database research results. However, most of the database-deposited HBV genome sequences for patients with IFN therapy are genotypes HBV/A, -B, -C, and -D. There have been no reports of HBV/E-related chronic hepatitis patients who were treated with IFN therapy. One possible explanation could be that HBV/E has specific sequences which allow the HNF1 site insertion and the BCP deletion from the wild type. As is known, HBV genotypes are determined by nucleotide differences of more than 8% (25). HBV genotypes reflect not only distinct geographical distributions but also genotype-specific mutation patterns. HBV/C has a higher G1762T/G1764A double mutation rate and a lower G1896A mutation rate than does HBV/B (26). A subgenotype of HBV/A, HBV/Aa (17, 35), which is distributed in Asia and Africa, has subgenotype-specific substitutions just prior to precore ORF start codons (Kozak sequences), and the mutation causes reduction of HBeAg (2, 38).
Though patient 2 has not had a very severe clinical course, probably due to the recent advancement of antiviral therapy, this HNF1 tandem repeat potentially could cause a severe form of hepatitis, as shown in the fulminant-hepatitis case with a single HNF1 site insertion (30). In addition, the HNF1 tandem repeat is correlated with not only HBeAg reduction but also viral replication and progression of liver fibrosis, as shown in our data. Further epidemiological and clinical studies will reveal the impact of the HNF1 tandem repeat on HBV infection.
Acknowledgments
This work was supported by a grant-in-aid from the Ministry of Health, Labor and Welfare of Japan (H16-kanen-3) and by the Uehara Memorial Foundation.
REFERENCES
- 1.Abe, A., K. Inoue, T. Tanaka, J. Kato, N. Kajiyama, R. Kawaguchi, S. Tanaka, M. Yoshiba, and M. Kohara. 1999. Quantitation of hepatitis B virus genomic DNA by real-time detection PCR. J. Clin. Microbiol. 37:2899-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, S. H., A. Kramvis, S. Kawai, H. C. Spangenberg, J. Li, G. Kimbi, M. Kew, J. Wands, and S. Tong. 2003. Sequence variation upstream of precore translation initiation codon reduces hepatitis B virus e antigen production. Gastroenterology 125:1370-1378. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat, R. A., P. P. Ulrich, and G. N. Vyas. 1990. Molecular characterization of a new variant of hepatitis B virus in a persistently infected homosexual man. Hepatology 11:271-276. [DOI] [PubMed] [Google Scholar]
- 5.Blum, H. E., Z.-S. Zhang, E. Galun, F. von Weizsäcker, B. Garner, T. J. Liang, and J. R. Wands. 1992. Hepatitis B virus X protein is not central to the viral life cycle in vitro. J. Virol. 66:1223-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway, J. F., N. R. Watts, D. M. Belnap, N. Cheng, S. J. Stahl, P. T. Wingfield, and A. C. Steven. 2003. Characterization of a conformational epitope on hepatitis B virus core antigen and quasiequivalent variations in antibody binding. J. Virol. 77:6466-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erhardt, A., U. Reineke, D. Blondin, W. H. Gerlich, O. Adams, T. Heintges, C. Niederau, and D. Haussinger. 2000. Mutations of the core promoter and response to interferon treatment in chronic replicative hepatitis B. Hepatology 31:716-725. [DOI] [PubMed] [Google Scholar]
- 8.Fields, B. N., D. M. Knipe, P. M. Howley, and D. E. Griffin. 2001. Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 9.Fujiwara, K., Y. Tanaka, E. Orito, T. Ohno, T. Kato, F. Sugauchi, S. Suzuki, Y. Hattori, M. Sakurai, I. Hasegawa, T. Ozasa, F. Kanie, H. Kano, R. Ueda, and M. Mizokami. 2004. Lack of association between occult hepatitis B virus DNA viral load and aminotransferase levels in patients with hepatitis C virus-related chronic liver disease. J. Gastroenterol. Hepatol. 19:1343-1347. [DOI] [PubMed] [Google Scholar]
- 10.Günther, S., N. Piwon, A. Iwanska, R. Schilling, H. Meisel, and H. Will. 1996. Type, prevalence, and significance of core promoter/enhancer II mutations in hepatitis B viruses from immunosuppressed patients with severe liver disease. J. Virol. 70:8318-8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunther, S., N. Piwon, A. Jung, A. Iwanska, H. Schmitz, and H. Will. 2000. Enhanced replication contributes to enrichment of hepatitis B virus with a deletion in the core gene. Virology 273:286-299. [DOI] [PubMed] [Google Scholar]
- 12.Hannoun, C., P. Horal, K. Krogsgaard, and M. Lindh. 2002. Mutations in the X region and core promoter are rare and have little impact on response to interferon therapy for chronic hepatitis B. J. Med. Virol. 66:171-178. [DOI] [PubMed] [Google Scholar]
- 13.Iavarone, M., J. B. Trabut, O. Delpuech, F. Carnot, M. Colombo, D. Kremsdorf, C. Brechot, and V. Thiers. 2003. Characterisation of hepatitis B virus X protein mutants in tumour and non-tumour liver cells using laser capture microdissection. J. Hepatol. 39:253-261. [DOI] [PubMed] [Google Scholar]
- 14.Ishak, K. G. 1994. Chronic hepatitis: morphology and nomenclature. Mod. Pathol. 7:690-713. [PubMed] [Google Scholar]
- 15.Kato, H., E. Orito, R. G. Gish, N. Bzowej, M. Newsom, F. Sugauchi, S. Suzuki, R. Ueda, Y. Miyakawa, and M. Mizokami. 2002. Hepatitis B e antigen in sera from individuals infected with hepatitis B virus of genotype G. Hepatology 35:922-929. [DOI] [PubMed] [Google Scholar]
- 16.Kramvis, A., and M. C. Kew. 1998. Structure and function of the encapsidation signal of hepadnaviridae. J. Viral Hepat. 5:357-367. [DOI] [PubMed] [Google Scholar]
- 17.Kramvis, A., L. Weitzmann, W. K. Owiredu, and M. C. Kew. 2002. Analysis of the complete genome of subgroup A′ hepatitis B virus isolates from South Africa. J. Gen. Virol. 83:835-839. [DOI] [PubMed] [Google Scholar]
- 18.Kuwahara, R., R. Kumashiro, S. Murashima, K. Ogata, K. Tanaka, A. Hisamochi, T. Hino, T. Ide, E. Tanaka, Y. Koga, and M. Sata. 2004. Genetic heterogeneity of the precore and the core promoter region of genotype C hepatitis B virus during lamivudine therapy. J. Med. Virol. 72:26-34. [DOI] [PubMed] [Google Scholar]
- 19.Laskus, T., J. Rakela, J. L. Steers, R. H. Wiesner, and D. H. Persing. 1994. Precore and contiguous regions of hepatitis B virus in liver transplantation for end-stage hepatitis B. Gastroenterology 107:1774-1780. [DOI] [PubMed] [Google Scholar]
- 20.Laskus, T., J. Rakela, M. J. Tong, M. J. Nowicki, J. W. Mosley, and D. H. Persing. 1994. Naturally occurring hepatitis B virus mutants with deletions in the core promoter region. J. Hepatol. 20:837-841. [DOI] [PubMed] [Google Scholar]
- 21.Li, J., V. E. Buckwold, M.-W. Hon, and J.-H. Ou. 1999. Mechanism of suppression of hepatitis B virus precore RNA transcription by a frequent double mutation. J. Virol. 73:1239-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naoumov, N. V., B. C. Portmann, R. S. Tedder, B. Ferns, A. L. Eddleston, G. J. Alexander, and R. Williams. 1990. Detection of hepatitis B virus antigens in liver tissue. A relation to viral replication and histology in chronic hepatitis B infection. Gastroenterology 99:1248-1253. [DOI] [PubMed] [Google Scholar]
- 23.Naoumov, N. V., K. A. Antonov, S. Miska, V. Bichko, R. Williams, and H. Will. 1997. Differentiation of core gene products of the hepatitis B virus in infected liver tissue using monoclonal antibodies. J. Med. Virol. 53:127-138. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto, H., F. Tsuda, Y. Akahane, Y. Sugai, M. Yoshiba, K. Moriyama, T. Tanaka, Y. Miyakawa, and M. Mayumi. 1994. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J. Virol. 68:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto, H., F. Tsuda, H. Sakugawa, R. I. Sastrosoewignjo, M. Imai, Y. Miyakawa, and M. Mayumi. 1988. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J. Gen. Virol. 69:2575-2583. [DOI] [PubMed] [Google Scholar]
- 26.Orito, E., M. Mizokami, H. Sakugawa, K. Michitaka, K. Ishikawa, T. Ichida, T. Okanoue, H. Yotsuyanagi, S. Iino, et al. 2001. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Hepatology 33:218-223. [DOI] [PubMed] [Google Scholar]
- 27.Pollicino, T., S. Campo, and G. Raimondo. 1995. PreS and core gene heterogeneity in hepatitis B virus (HBV) genomes isolated from patients with long-lasting HBV chronic infection. Virology 208:672-677. [DOI] [PubMed] [Google Scholar]
- 28.Pourcel, C., A. Louise, M. Gervais, N. Chenciner, M.-F. Dubois, and P. Tiollais. 1982. Transcription of the hepatitis B surface antigen gene in mouse cells transformed with cloned viral DNA. J. Virol. 42:100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poussin, K., H. Dienes, H. Sirma, S. Urban, M. Beaugrand, D. Franco, P. Schirmacher, C. Brechot, and P. Paterlini Brechot. 1999. Expression of mutated hepatitis B virus X genes in human hepatocellular carcinomas. Int. J. Cancer 80:497-505. [DOI] [PubMed] [Google Scholar]
- 30.Pult, I., T. Chouard, S. Wieland, R. Klemenz, M. Yaniv, and H. E. Blum. 1997. A hepatitis B virus mutant with a new hepatocyte nuclear factor 1 binding site emerging in transplant-transmitted fulminant hepatitis B. Hepatology 25:1507-1515. [DOI] [PubMed] [Google Scholar]
- 31.Raney, A. K., A. J. Easton, and A. McLachlan. 1994. Characterization of the minimal elements of the hepatitis B virus large surface antigen promoter. J. Gen. Virol. 75:2671-2679. [DOI] [PubMed] [Google Scholar]
- 32.Raney, A. K., D. R. Milich, A. J. Easton, and A. McLachlan. 1990. Differentiation-specific transcriptional regulation of the hepatitis B virus large surface antigen gene in human hepatoma cell lines. J. Virol. 64:2360-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui, A., S. Jameel, and J. Mapoles. 1987. Expression of the hepatitis B virus X gene in mammalian cells. Proc. Natl. Acad. Sci. USA 84:2513-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuyver, L., S. De Gendt, C. Van Geyt, F. Zoulim, M. Fried, R. F. Schinazi, and R. Rossau. 2000. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J. Gen. Virol. 81:67-74. [DOI] [PubMed] [Google Scholar]
- 35.Sugauchi, F., H. Kumada, S. A. Acharya, S. M. Shrestha, M. T. Gamutan, M. Khan, R. G. Gish, Y. Tanaka, T. Kato, E. Orito, R. Ueda, Y. Miyakawa, and M. Mizokami. 2004. Epidemiological and sequence differences between two subtypes (Ae and Aa) of hepatitis B virus genotype A. J. Gen. Virol. 85:811-820. [DOI] [PubMed] [Google Scholar]
- 36.Sugauchi, F., M. Mizokami, E. Orito, T. Ohno, H. Kato, S. Suzuki, Y. Kimura, R. Ueda, L. A. Butterworth, and W. G. Cooksley. 2001. A novel variant genotype C of hepatitis B virus identified in isolates from Australian Aborigines: complete genome sequence and phylogenetic relatedness. J. Gen. Virol. 82:883-892. [DOI] [PubMed] [Google Scholar]
- 37.Sugauchi, F., T. Ohno, E. Orito, H. Sakugawa, T. Ichida, M. Komatsu, T. Kuramitsu, R. Ueda, Y. Miyakawa, and M. Mizokami. 2003. Influence of hepatitis B virus genotypes on the development of preS deletions and advanced liver disease. J. Med. Virol. 70:537-544. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka, Y., I. Hasegawa, T. Kato, E. Orito, N. Hirashima, S. K. Acharya, R. G. Gish, A. Kramvis, M. C. Kew, N. Yoshihara, S. M. Shrestha, M. Khan, Y. Miyakawa, and M. Mizokami. 2004. A case-control study for differences among hepatitis B virus infections of genotypes A (subtypes Aa and Ae) and D. Hepatology 40:747-755. [DOI] [PubMed] [Google Scholar]
- 39.Tang, H., L. Delgermaa, F. Huang, N. Oishi, L. Liu, F. He, L. Zhao, and S. Murakami. 2005. The transcriptional transactivation function of HBx protein is important for its augmentation role in hepatitis B virus replication. J. Virol. 79:5548-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turelli, P., B. Mangeat, S. Jost, S. Vianin, and D. Trono. 2004. Inhibition of hepatitis B virus replication by APOBEC3G. Science 303:1829. [DOI] [PubMed] [Google Scholar]
- 42.Wai, C. T., C. J. Chu, M. Hussain, and A. S. Lok. 2002. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology 36:1425-1430. [DOI] [PubMed] [Google Scholar]
- 43.Wakita, T., S. Kakumu, M. Shibata, K. Yoshioka, Y. Ito, T. Shinagawa, T. Ishikawa, M. Takayanagi, and T. Morishima. 1991. Detection of pre-C and core region mutants of hepatitis B virus in chronic hepatitis B virus carriers. J. Clin. Investig. 88:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webby, R., E. Hoffmann, and R. Webster. 2004. Molecular constraints to interspecies transmission of viral pathogens. Nat. Med. 10:S77-S81. [DOI] [PMC free article] [PubMed] [Google Scholar]