Abstract
β-Defensins are small (3 to 5 kDa in size) secreted antimicrobial and antiviral proteins that are components of innate immunity. β-Defensins are secreted by epithelial cells, and they are expressed at high levels in several mucosae, including the mouth, where the concentration of these proteins can reach 100 μg/ml. Because of these properties, we wondered whether they could be part of the defenses that lower oral transmission of human immunodeficiency virus (HIV) compared to other mucosal sites. Our data show that select β-defensins, especially human β-defensin 2 (hBD2) and hBD3, inhibit R5 and X4 HIV infection in a dose-dependent manner at doses that are compatible with or below those measured in the oral cavity. We observed that β-defensin treatment inhibited accumulation of early products of reverse transcription, as detected by PCR. We could not, however, detect any reproducible inhibition of env-mediated fusion, and we did not observe any modulation of HIV coreceptors following treatment with hBD1 and hBD2, in both resting and phytohemagglutinin-activated cells. Our data instead suggest that, besides a direct inactivation of HIV virions, hBD2 inhibits HIV replication in the intracellular environment. Therefore, we speculate that β-defensins mediate a novel antiretroviral mechanism that contributes to prevention of oral HIV transmission in the oral cavity. Immunohistochemical data on hBD2 expression in oral mucosal tissue shows that hBD2 is constitutively expressed, forming a barrier layer across the epithelium in healthy subjects, while in HIV-positive subjects levels of hBD2 expression are dramatically diminished. This may predispose HIV-positive subjects to increased incidence of oral complications associated with HIV infection.
Oral transmission of human immunodeficiency virus type 1 (HIV-1) is poorly understood, partly because data on this type of transmission are scarce and inherently problematic to analyze and interpret. Despite recent suggestions that the risk of becoming infected through the oral route may be higher than previously estimated, oral transmission of HIV is 8 to 10 times less likely to occur than vaginal or rectal transmission in humans (41, 50, 61). This is in contrast with studies performed in infant macaques, which are readily infected orally with simian immunodeficiency virus (3, 42). Therefore, it is possible that the oral mucosa in infants has a critical defect in immunity that renders them more susceptible to viral infection. A better understanding of the protection mechanism in the oral environment will likely provide information useful for preventing mother-to-child transmission of HIV-1.
Although the scarcity of data renders it difficult to envision a model for protection in the oral mucosa, there are a number of possible explanations for the relatively low rate of oral transmission of HIV (41, 50, 61). One possibility is that inhibitory factors are responsible for this phenomenon. Interestingly, saliva has been shown to inhibit HIV-1 replication, and several salivary proteins, including anti-HIV antibodies, mucins, thrombospondin, lysozyme, lactoferrin, lactoperoxidase, cystatins, complement, statherin, and secretory leukocyte protease inhibitor, have been characterized as HIV inhibitors (41, 50, 61). Other inhibitory proteins might also reside in the mucosa. β-Defensins, a subfamily of homologous antimicrobial peptides constituting an important component of innate immunity found predominantly in vertebrates, are among the proteins expressed at the highest levels in the oral mucosa (17, 20, 29, 33, 62).
Human defensins are cationic and Cys-rich proteins with molecular weights ranging from 3 to 5 kDa. Based on sequence homology and the connectivity of six conserved cysteine residues, human defensins are classified into α and β families. Human α-defensins were first discovered as natural peptide antibiotics (HNP1 to HNP4) stored in the azurophilic granules of neutrophils and released to combat ingested foreign microbes during phagocytosis (16, 48, 56). Other α-defensins, secreted in response to bacterial stimulation, have also been found in intestinal Paneth cells (2, 26, 27). In contrast, human β-defensins (hBDs) are found predominantly in various epithelial cells and tissues (4, 46, 64). hBD1, originally isolated from human blood filtrate (4), is constitutively expressed in the urogenital and airway tracts, suggesting a role in protecting mucosae from microbial infection (64). hBD2, first isolated from lesions of inflamed skin, is transcriptionally up-regulated by inflammatory stimuli such as cytokines and microorganisms (21). hBD3 was isolated originally from inflamed human skin, is inducibly expressed in various epithelia (18, 22, 25, 47), and possesses a broad spectrum of potent bactericidal activities in a salt-insensitive manner against both gram-positive and gram-negative bacteria (18, 22, 25, 46). Genomics studies have identified several new members of the β-defensin family (28, 40, 53). Recently, a circular form of antimicrobial peptides, called θ-defensins, has been characterized in macaques, while in humans all the genes encoding these peptides contain stop codons and are therefore likely to constitute expressed pseudogenes (12). Despite their diversity, defensins kill a broad range of microorganisms including bacteria, fungi, and certain enveloped viruses, presumably through membrane disruption, and constitute an important component in innate immunity. Thus, they could also affect HIV-1 infection by interfering with its envelope.
All classes of defensins reportedly can suppress HIV replication (9, 30, 32, 39, 58, 63). Nakashima et al. described α-defensins as antiviral in 1993 (32), and recent work had suggested that they might play a role in controlling HIV in some subjects (63). θ-Defensins, present only in nonhuman primates, might be part of the cross-species barrier to HIV. Recently, the anti-HIV activity of β-defensins has also been reported (39). However, for two reasons, it is difficult to extrapolate the antiviral activity of α- and θ-defensins to resistance against oral HIV transmission. First, θ-defensins are not expressed in humans (12). Second, α-defensins are not prominently expressed in the oral mucosa (13, 15). In contrast, β-defensins are highly expressed in the oral epithelium (13, 15, 43), with measured local concentration as high as 100 μg/ml in a 100-μm-thick layer (49). Therefore, β-defensins are candidates as components of innate resistance to oral HIV infection.
In this report, we show that hBD2 and hBD3 inhibit HIV-1Bal, an R5 isolate, and IIIB, an X4 isolate; hBD2 exerted its antiviral activity without affecting cellular proliferation. Further, our data indicate that inhibition of HIV occurs at an early stage. Surprisingly, our data ruled out a mechanism involving inhibition of membrane fusion, including downregulation of HIV receptors. Instead, our data are consistent with a dual mechanism of inhibition. One component of the HIV-suppressive activity of hBD2 is due to a direct inactivation of virions, while a second component is observed postentry. Therefore, hBD2 inhibits HIV replication through a mechanism, possibly related to that described for α-defensins (9, 9a), which is not merely based on membrane disruption. These results indicate that the study of the antiviral activity of hBD2 and hBD3 could be useful in providing new tools for HIV prevention (for example, as topical microbicides), in therapy (either as such or as a basis for developing new drugs), and as correlates of immunity in the oral cavity.
MATERIALS AND METHODS
Cells.
The TZM cell line was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. This indicator cell line is a HeLa cell line derivative that expresses high levels of CD4 and CCR5 along with endogenously expressed CXCR4. TZM cells contain HIV long terminal repeat (LTR)-driven β-galactosidase and luciferase reporter cassettes that are activated by HIV tat expression. TZM cells were routinely subcultured every 3 to 4 days by trypsinization and were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1× penicillin-streptomycin (complete medium). The infectious titer of all virus stocks was determined on TZM cells by direct counting of blue foci. HeLa and 293T cells were obtained from the American Type Culture Collection (Manassas, VA).
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood on a Ficoll gradient and activated in complete medium, 10 ng/ml interleukin-2 (IL-2), and 5 μg/ml phytohemagglutinin (PHA) for 48 h. Activated PBMC were maintained in complete RPMI medium containing 10 ng/ml IL-2 at a density of <2 × 106 cells/ml.
Cell metabolism assays.
PBMC and TZM cells treated with β-defensins were tested using the MTS [3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt]assay (Promega, Madison, WI), which measures conversion of MTS tetrazolium into formazan by cellular dehydrogenase enzymes in metabolically active cells. A total of 1 × 105 cells/well were cultured in 96-wells plates for 3 days in the presence of β-defensins or with medium alone as a control in triple replicates and then added to an MTS/phenazine methosulfate mixture and incubated for 1 to 4 h as indicated by the manufacturer's protocol prior to a spectrophotometric absorbance reading at 490 nm. Triplicate reading were averaged and optical density ratios of treated to control cells were calculated as percentages.
Total chemical synthesis of human β-defensins.
hBDs 1 to 3 were chemically synthesized by solid phase peptide synthesis using a custom-modified procedure tailored from the published in situ neutralization protocol developed for Boc chemistry (45). The syntheses, purification, folding, and characterizations were published previously (57). The beta connectivity of three disulfide bonds (Cys1-Cys5, Cys2-Cys4, and Cys3-Cys6) in highly pure synthetic hBDs 1 to 3 was independently verified by mass mapping of peptide fragments generated by enzymatic digestion and Edman degradation (57). Protein concentrations were determined by absorbance measurements at 280 nm using molar extinction coefficients calculated according to a published algorithm (36).
PBMC infectivity assay.
PHA-activated human PBMC (1 × 105 PBMC/well) were treated for 1 h with synthesized human β-defensins 1 to 3 (0.8 to 100 μg/ml), 3′-azido-3′-deoxythymidine, T20 (11.25 μg/ml), or RANTES (2 μg/ml) and then infected for 2 h with 500 50% tissue culture infective doses (TCID50) of HIVBaL, an R5 isolate, or HIVIIIB, an X4 isolate. After 2 h, cells were washed three times with phosphate-buffered saline (PBS), and complete medium was added with the appropriate treatment. Infection was monitored by p24 enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Perkin Elmer, Foster City, CA).
TZM cell line infectivity assay.
TZM cells (2 × 104 per well) were added to 96-well microtiter plate wells (Falcon, Lincoln Park, NJ) in 100 μl of complete medium and allowed to adhere 15 to 18 h at 37°C. An equivalent amount of each virus stock (multiplicity of infection [MOI] of 0.01) was added to the cell monolayers in the presence of 40 μg/ml DEAE-dextran in DMEM in a final volume of 100 μl. Viral infection was allowed to proceed for 2 h at 37°C, following which 100 μl of complete DMEM was added. Luciferase activity was measured after 15 to 18 h at 37°C with 5% CO2 in a humidified incubator using a Promega luciferase assay system kit (Madison, WI). Briefly, the supernatants were removed, and the cells were lysed with a Steady Glo luciferase assay system. The light intensity of each well was measured on a Reporter luminometer. Mock-infected cells were used to determine background luminescence. All infectivity assays were performed at least in duplicate.
Activation of the β-galactosidase gene was detected by fixing the cells in 0.25% glutaraldehyde-0.8% formaldehyde in PBS for 5 min at room temperature; cells were washed three times in PBS and subsequently stained with a solution containing 400 μg/ml of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), 4 mM MgCl2, 4 mM potassium ferrocyanide, and 4 mM potassium ferricyanide in PBS overnight at 37°C. The staining solution was then removed, and the cells were overlaid in PBS to allow for microscopic analysis.
Cell-cell fusion assay.
HIV-1 IIIB or BaL Env was transiently expressed on the surface of HeLa cells using the recombinant vaccinia constructs vPE16 and vCB-43, respectively. Target cells (either SupT1 or HeLa/CD4/R5) were labeled with the cytoplasmic dye CMTMR (5- and 6-{[(4-chloromethyl)benzoyl]amino}tetramethylrhodamine) at a concentration of 20 μM, and HIV-1 Env-expressing HeLa cells were labeled with calcein AM at a concentration of 10 μM for 1 h at 37°C. The dyes were purchased from Molecular Probes (Eugene, OR). Calcein-labeled Env cells were cocultured with CMTMR-labeled effector cells for 2 h at 37°C, and dye distribution was monitored microscopically as described previously (24). The inhibitor T20 was synthesized by Macromolecular Resources (Fort Collins, CO).
PBMC fusion assays.
PBMC fusion assays were performed as previously described (8, 34). To measure fusion driven by an X4 envelope, 5 × 104 HeLa cells were plated in each well of a 24-well plate and allowed to attach overnight. Cells were transfected with 0.2 μg/well of a T7 promoter-driven luciferase plasmid (BD Biosciences Clontech, Palo Alto, CA) using Fugene 6 (Roche Diagnostics), and incubated for 3 h at 37°C. The cells were then infected with vP1198 (IIIB envelope-expressing vaccinia obtained from the National Institutes of Health AIDS Research and Reference Reagent Program) or vCB-43 (HIVBaL envelope-expressing vaccinia) at an MOI of 1 PFU/cell for 2 h, washed two times with PBS, resuspended in appropriate medium, and incubated at 32°C overnight. Activated PBMC were simultaneously infected with vTF7-3 (T7 polymerase-expressing vaccinia virus) at an MOI of 10 PFU/cell for 2 h; cells were then washed and resuspended in complete RPMI medium with IL-2 and incubated as per HeLa cells. PBMC were aliquoted into individual tubes at 4 × 105 cells/tube and pretreated for 1 h with defensin or control compound (T20). Medium was removed from HeLa cells and replaced with 2 × 105 pretreated PBMC in RPMI medium supplemented with IL-2 and appropriate treatment. Cells were allowed to fuse at 37°C for 1 h; they were washed and lysed with 1× Reporter lysis buffer (Promega), and fusion was determined by luminescence, which was determined by mixing 10 μl of lysate with 100 μl of luciferase reagent (Promega) and placing the mixture in a Turner Luminometer.
Real-time PCR.
PHA-activated PBMC in complete RPMI medium supplemented with 10 ng/ml IL-2 were aliquoted at 2.2 × 106 cells/tube into 15-ml conical tubes at 1 × 106 cells/ml. Cells were pretreated with T20 (11.2 μg/ml) or RANTES (2 μg/ml) or with hBD2 (20 to 0.8 μg/ml) for 1 h at 37°C. HIV, strains IIIB and BaL, was added at an MOI of 0.005, and infection was carried out at 37°C for 2 h. Cells were washed two times and resuspended at 1 × 106 cells/ml in complete RPMI medium, 10 ng/ml IL-2, and appropriate treatment. Cells (1 × 106) were collected at 2, 4, and 20 h after infection and washed with PBS, and cell pellets were stored at −80°C.
Cell pellets were thawed and lysed in 100 μl of PCR buffer containing 0.45% Tween 20, 0.45% NP-40, and 100 μg/ml Proteinase K (Roche, Indianapolis, IN) at 56° for 2 h, followed by boiling for 10 min. Quantitative TaqMan PCRs were performed using a Sybr Green PCR System (Applied Biosystems, Foster City, CA): 1× Sybr Green PCR buffer, 2.5 mM MgCl2, a 0.625 mM concentration of each deoxynucleoside triphosphate, 0.125 U of AmpErase, 0.125 U of Taq, 7.5 pM forward primer, 7.5 pM reverse primer, and 5 μl of lysate in a final volume of 25 ml/reaction. Primers LTR/RU5F, LTR/RU5R, α-tubulinF, and α-tubulinR have been previously described (14, 44). PCR conditions were 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 30 s, 55°C (for HIV primers) or 57°C (for α-tubulin primers) for 30 s, and 72°C for 1 min, followed by a final extension at 72°C for 10 min. Copy number per sample was corrected for α-tubulin copy number as determined by PCR.
Flow cytometry.
Human PBMC were treated with hBD1, hBD2, or positive controls at the concentrations and times indicated in Results, after which the PBMC were processed for flow cytometric analysis. Briefly, the cells were harvested, washed in wash buffer, and then stained with monoclonal antibodies (MAbs). The following MAbs were used: fluorescein isothiocyanate (FITC)-CD4, phycoerythrin (PE)-CCR5, PE-CXCR4, peridinin chlorophyll protein (PerCP)-CD3, allophycocyanin (APC)-CCR5, and APC-CXCR4 (all from BD Pharmingen, San Jose, CA). After incubation with the MAbs for 30 min at 4°C in dark, the cells were washed and fixed in 1% paraformaldehyde solution for flow cytometric analysis using a FACSCalibur flow cytometer (BD Biosciences, CA). Live cells were gated according to forward and side scatter profiles. Data analysis was performed using FlowJo software (Tree Tree Star Inc., San Carlos, CA).
Immunohistochemical analyses.
Human oral mucosa was isolated as a routine procedure in the course of wisdom tooth extraction, from noninflamed regions, of HIV-negative and HIV-positive donors at a maxillofacial clinic. All samples were collected and processed at the S. Giovanni Battista Hospital, University of Torino, Turin, Italy, in accordance with Institutional Review Board guidelines. Tissues were processed by standard formalin fixation and paraffin-embedding methods. Archival samples from five HIV-positive and five HIV-negative subjects were randomly selected and processed for immunohistochemical analyses to determine baseline levels and distribution patterns of human defensin populations within the tissue. All tissue donors were male, between the ages of 29 to 41 years. HIV-positive subjects were under antiretroviral therapy and had viremia ranging from 250 to 55,000 copies/ml (except one case with undetectable viremia) and CD4 counts of 42 to 459 cells/mm3. Paraffin-embedded samples were cut to 4-μm thickness, and sections were stained with hematoxylin and eosin for gross morphological analysis. Tissue sections were stained by immunohistochemical analyses for the presence of human α-defensins 1 to 3 using a 1:1,000 dilution of mouse MAb anti-human defensin, clone DEF-3 (Serotec, Raleigh, NC). Human β-defensin-2 was detected using a 1:4,000 dilution of polyclonal rabbit anti-hBD-2 antiserum (Peptide Institute, Louisville, KY), as previously described (1). Human β-defensins 1 to 3 were detected following the described protocol below, using the respective affinity purified antibodies. All immunohistochemical procedures were performed using a VectaStain ABC Elite Kit (Vector Laboratories, Inc., Burlingame, CA) per the manufacturer's instructions. Briefly, tissue sections were baked in a vacuum oven at 55°C for 1 h and then dehydrated in xylene and rehydrated in a graded alcohol series. Endogenous peroxidase activity was destroyed by immersion in 0.3% hydrogen peroxide-methanol solution for 10 min. Antigen retrieval was achieved with a 10-min Pronase E digestion at 37°C. All tissues were blocked with 10% horse serum (Vector Laboratories) for 1 h at room temperature. The primary antibody was diluted in BD Antibody Diluent (Becton Dickinson, Franklin Lakes, NJ) per the manufacturer's recommendations. Tissues were incubated with primary antibody for 1 h at room temperature and shifted to 4°C overnight. All subsequent steps were performed at room temperature. After two 5-min washes with PBS, tissue sections were incubated with the respective biotinylated secondary antibodies (Serotec and Vector Laboratories) for 30 min, rinsed in PBS, and incubated with streptavidin-peroxidase conjugate for 30 min, according to the VectaStain Kit instructions. Color visualization of the complex was achieved by incubating tissue sections with diaminobenzidine tetrahydrochloride containing Ni2+ for 5 min. All tissue sections were briefly rinsed in water and counterstained with hematoxylin. Tissues incubated with control anti-immunoglobulin G1 (IgG1) isotype monoclonal antibody (for staining of α-defensins 1 to 3) or with control rabbit sera (for β-defensin 2 staining) or with secondary antibodies alone were included as a negative control with each staining procedure.
RESULTS
β-defensins inhibit HIV-1 infection.
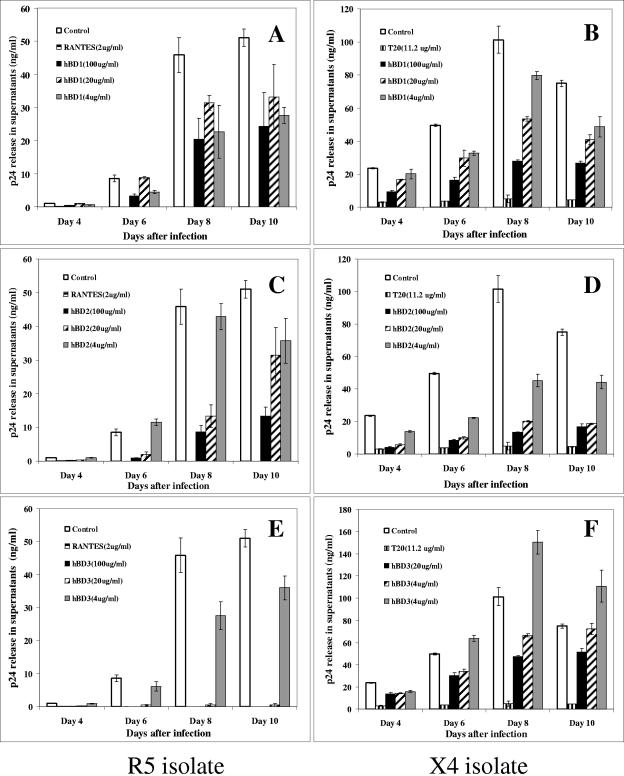
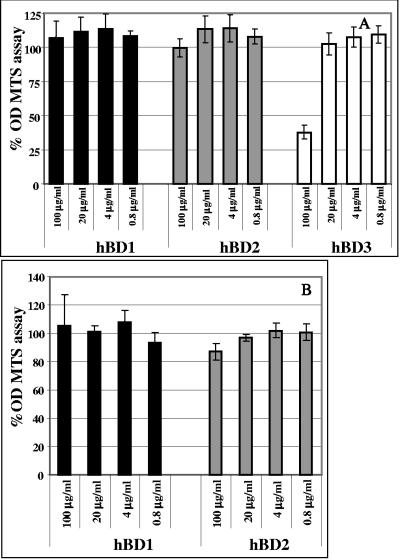
We tested hBD1, hBD2, and hBD3 in infectivity assays. In these assays, 1 × 105 cells were treated with defensins at concentrations ranging from 4 to 100 μg/ml. The chemokine RANTES at 2 μg/ml for R5 virus or the peptide T20 (11.25 μg/ml) for X4 virus was used as a positive control for inhibition. Subsequently, cells were washed with PBS and infected with 500 TCID50 of an R5 (BaL) or an X4 (IIIB) isolate for 2 h at 37°C. Cells were washed again three times with PBS and cultured at 37°C in 5% CO2 for up to 10 days. Defensins and RANTES or T20 were supplemented to the culture every 3 days. Release of HIV-1 p24 was quantified in supernatants every 2 days starting at day 4 after infection. Proliferation was monitored by [3H]thymidine incorporation. Figure 1 shows release of p24 by infected cells in the tissue culture supernatants in a representative experiment. All three defensins can inhibit HIV-1 replication to some degree. However, inhibition by hBD1 does not seem to be acting in a dose-dependent manner with the R5 virus (Fig. 1A), unlike hBD2 (Fig. 1B) and hBD3 (Fig. 1C). Further, while inhibition by hBD3 appears to be far more potent than that observed with hBD2, we observed a decrease in cell proliferation, as measured by the MTS assay, when cells where treated with hBD3 at the higher doses (Fig. 2). Therefore, it is possible that some or all of the antiviral effect of hBD3 is due to an influence on cellular proliferation. In contrast, hBD2 displays anti-HIV activity in the absence of negative effects on cell metabolism (Fig. 2). Because of the more desirable profile of HIV-1 suppression by hBD2 as opposed to hBD1 and the absence of toxicity compared to hBD3, we reasoned that hBD2 constitutes the best candidate for further studies aimed at understanding the step(s) of the infection process that is influenced by treatment with defensin. Nonetheless, in most of the subsequent experiments, we compared the activity of hBD2 to hBD1, as a control for specificity.
FIG. 1.
β-Defensins inhibit HIV replication. hBD1, hBD2, and hBD3 were used at concentrations ranging from 100 to 4 μg/ml to treat PBMC for 1 h prior to and after infection with HIV-1 BaL (R5) (A, C, and E) or IIIB (X4) (B, D, and F). RANTES, a CCR5 ligand (A, C, and E), or T20, a fusion inhibitor (B, D, and F) was used as a positive control. Cells were then infected for 2 h with 500 TCID50 of HIV-1, and infection was monitored by assaying supernatants for HIV p24 production by ELISA at the times indicated. Shown here are averages of three experiments. Bars indicate the ranges of the mean values.
FIG. 2.
Effect of β-defensins on cell metabolism. PBCM and TZM cells treated with β-defensins at concentrations ranging from 100 to 0.8 μg/ml were tested using the MTS assay, which measures conversion of MTS tetrazolium into formazan by cellular dehydrogenase enzymes in metabolically active cells. A total of 1 × 105 cells/well were cultured in 96-well plates for 3 days in presence of β-defensins or with media alone as a control in triple replicates, and then added to the MTS/ phenazine methosulfate mixture and incubated 1 to 4 h as indicated by the manufacturer's protocol prior to spectrophotometric absorbance reading at 490 nm. Triplicate readings were averaged, and optical density ratios of treated/control cells were calculated as percentages. Shown here are averages of three independent experiments. Bars indicate standard errors of the means.
hBD2, but not hBD1, suppresses single-round HIV infection.
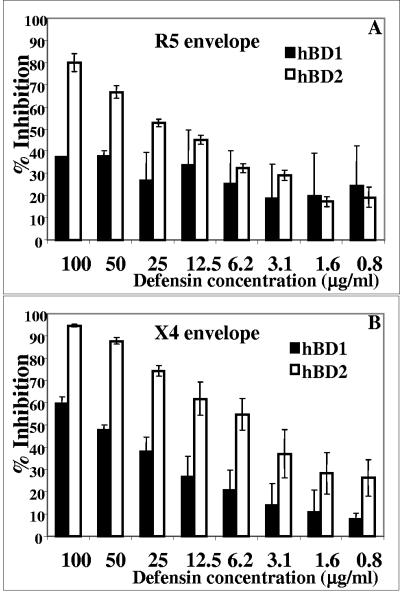
To further probe the inhibitory mechanism of hBD2, we employed a virus-cell infection system that utilizes an indicator cell line, TZM, that has been engineered to express CD4 and CCR5. As CXCR4 is endogenously expressed on this cell line, it is susceptible to infection by diverse HIV isolates. Following viral fusion the LTR-driven reporter gene products luciferase and β-galactosidase are expressed, allowing for quantitative measurement of viral infectivity as soon as 16 h after infection. Such an assay system allows us to determine virus-cell fusion inhibition as well as inhibition of early HIV life cycle events. When defensins are employed in this assay system, a robust dose-dependent inhibition of both X4 (NL4-3) and R5 (BaL) isolates by hBD2 (Fig. 3A and B) is clearly seen in accordance with PBMC infectivity results (Fig. 1), while hBD1 displays a significantly lower potency (Fig. 3A and B).
FIG. 3.
hBD2, but not hBD1, inhibits single-round HIV infection. The TZM cell line was incubated in the presence of 100 to 0.78 μg/ml of β-defensins for 1 h at 37°C, followed by infection with HIVBaL (A) or HIVNL4-3 (B) as detailed in Materials and Methods. After 16 h, the cells were lysed and luminescence was determined. All assays were performed in duplicate. Values represent percent inhibition compared to untreated controls; shown here are average data obtained from three independent experiments; error bars indicate standard errors of the means.
hBD2 inhibits viral replication at an early stage.
In order to gain some insight on the mechanism of inhibition of HIV replication, we investigated the presence of early reverse transcription products in PBMCs infected with HIV at 2, 4, and 16 h after infection. We used primers specific for “Strong stop” RNA (LTR/RU5), or a control, constitutively expressed RNA for α-tubulin. Our results show that hBD2 treatment inhibits the formation of these early products. Inhibition of product formation is already evident 2 h after infection with HIVBaL, when concentrations of 100, 20, and 4 μg/ml of hBD2 resulted in a 12%, 45%, and 16% average decrease, respectively, of LTR/RU5 copy number. However, at 4 h after infection, the inhibition using the same concentrations of defensins had increased significantly, to averages of 79%, 46%, and 27% of LTR/U5 products compared to the control untreated cells (Fig. 4A). In infections performed with the IIIB isolate, the decline in LTR/RU5 products was in the same range of potency (Fig. 4B) as with BaL. Control treatments with entry inhibitors RANTES (for R5 HIV) and T20 (for X4) resulted in inhibition of formation of early reverse transcription products at all time points. Overall, these pointed to a mechanism of inhibition of HIV by hBD2 occurring at an early stage in the infection process, between entry-fusion and reverse transcription.
FIG. 4.
hBD2 inhibits HIV-1 replication at an early stage. Activated PBMC were treated with 100, 20, and 4 μg/ml of hBD2. Control treatments consisted of RANTES (2 μg/ml, for the R5 isolate BaL) (A) or T20 (11.25 μg/ml, for the X4 isolate IIIB) (B). Treated cells were then infected with 500 TCID50 of HIV-1, washed, treated again, and collected after 2, 4, and 20 h. DNA was isolated from 1 × 106 cells and analyzed by quantitative TaqMan PCRs using the Sybr Green PCR System as described in Materials and Methods. Primers were used that amplified LTR/RU5 (“first jump” products of reverse transcription) or α-tubulin. Copy number values for each amplified product were standardized based on the number of amplified copies of α-tubulin, and percent inhibition was calculated compared to untreated controls. Shown here are average results of three independent experiments. Error bars indicate standard errors of the means.
β-Defensins 1 and 2 do not inhibit cell-cell fusion.
Defensins have previously defined membrane-disrupting properties (16, 17, 20, 29, 33, 62), and α- and θ-defensins can inhibit HIV by binding to the glycan moieties of CD4 and gp120 (12, 54, 55). Therefore, a reasonable hypothetical mode of HIV inhibition is due to perturbation of viral and/or cell membranes, culminating in the inhibition of membrane fusion. To verify whether β-defensins inhibit HIV gp160-mediated membrane fusion, we tested hBD1 and hBD2 in a dye redistribution fusion assay as previously described (24). HeLa cells, transiently overexpressing HIV-1 gp160 (IIIB or BaL) were labeled with the intracellular dye calcein AM and cocultured with labeled target cells expressing CD4 and the appropriate chemokine receptors. In these tests, neither hBD1 nor hBD2 inhibited cell-cell fusion, while the potent fusion inhibitor T20 inhibited the process as expected (data not shown). We also performed fusion tests using PBMC, reasoning that, while the fusion test system we used is valid, both viral and receptor proteins are overexpressed in a nonphysiological cell type and, thus, may lack some of the mechanisms of receptor down-modulation that are present in PBMC. We performed experiments using a fusion system where PBMC expressing T7 polymerase constituted the target cells and were incubated with HeLa cells expressing HIV gp160 and a T7 promoter-luciferase construct for enhanced signal detection. In these assays also, we did not observe a reproducible dose-dependent inhibition of cell-cell fusion mediated by either X4 or R5 HIV gp120 (data not shown). Thus, we ruled out a mechanism for HIV inhibition by β-defensins based on membrane fusion.
hBD1 and hBD2 do not modulate expression of HIV receptors on the cell membrane.
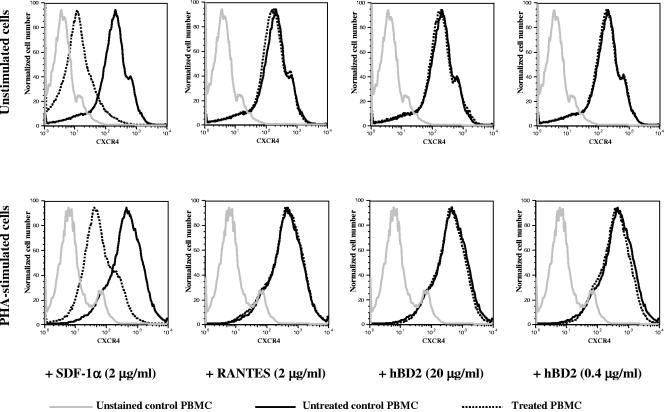
Since the role of HIV receptors and their colocalization has been intensely studied and one publication has shown that β-defensins alter HIV coreceptor expression (39), we evaluated whether β-defensins affect HIV receptor systems on the cell membrane. To this end, we treated uninfected PBMC with both hBD1 and hBD2 and investigated expression of CD4, CXCR4, and CCR5 on PBMC by flow cytometry. PBMC were treated with hBD1 and hBD2 for 2, 5, and 20 h and were then labeled with FITC-CD4, PerCP-CD3, PE-CCR5, or APC-CXCR4. To our surprise, we were not able to detect any modulation of CCR5 or CXCR4 expression on the cell surface by either hBD1 or hBD2, using both fresh unstimulated and PHA-activated cells. Figure 5 shows the results at 2 h after treatment with hBD2; similar results were obtained with 1 and 5 h of treatment, and with hBD1(not shown). These experiments were repeated at least three times (under both unstimulated and stimulated conditions) using cells from different donors. In contrast, treatment with the cognate chemokine SDF-1 (for CXCR4) (Fig. 5) and RANTES (for CCR5; not shown) induced a prompt reduction in receptor expression. In the case of CCR5, however, basal expression of CCR5 was close to undetectable in some donors, especially in unstimulated cells. We performed experiments also using APC-CCR5 and PE-CXCR4 with a similar outcome (not shown). Restricting the analysis on CD4+ cells also did not reveal modulation of HIV receptor expression (not shown). Our results show that treatment with hBD1 and hBD2 does not significantly modulate HIV receptors (Fig. 5).
FIG. 5.
hBD1 and hBD2 do not modulate expression of CXCR4 on the cell membrane. Uninfected PBMC were treated with hBD2, and expression of CXCR4 was evaluated by flow cytometry. PBMC were treated with hBD1 and hBD2 for 2, 5 and 20 h and then labeled with FITC-CD4, PerCP-CD3, and PE-CXCR4; cells were then washed and fixed in 1% paraformaldehyde solution for flow cytometric analysis using a FACSCalibur flow cytometer. Live cells were gated according to forward and side scatter profiles. Data analysis was performed using FlowJo software. Shown here are representative results, after 2 h of treatment, of three independent experiments.
hBD treatment does not induce release of HIV-inhibitory chemokines.
Since a study has shown that α-defensins can trigger the release of CCR5 ligands (which inhibit HIV replication by binding to their cognate receptor) in monocytes (19) and our own data show that the release of CCR5 from antigen-activated PBMC occurs with fast kinetics, compatible with inhibition of HIV infection in vitro (51), we investigated whether treatment with hBD1 and hBD2 could trigger release of HIV inhibitory chemokines (6, 11, 23, 35, 37) in PBMC. Supernatants derived from PBMC incubated for 3 days with doses of hBD1 and hBD2 ranging between 0.4 and 20 μg/ml were tested for the presence of chemokines RANTES, MIP-1α, MIP-1β, SDF-1, macrophage-derived chemokine, and I-309 by commercial ELISA kits. While we observed that hBD1 induces some increase in RANTES production (not shown), other chemokines were not detected at elevated levels at any time points. Further, treatment with hBD-2 did not induce an increase in production of any of the HIV-inhibitory chemokines under any experimental condition tested (not shown). It is unlikely that the lack of detection of release of CCR5 ligands compared to studies of α-defensins is due to differences in the detection system, since the ELISA-based assays that were used in both studies had similar thresholds of detection. Furthermore, concentrations of CCR5 ligands below the threshold of detection of the ELISA assay that we used (31 pg/ml) would be below the concentrations needed to efficiently inhibit HIV replication (6, 11, 23, 35, 37). Therefore, we ruled out the possibility that β-defensins, particularly hBD2, inhibit HIV-1 indirectly by inducing the release of chemokines.
hBD2 can directly target HIV-1.
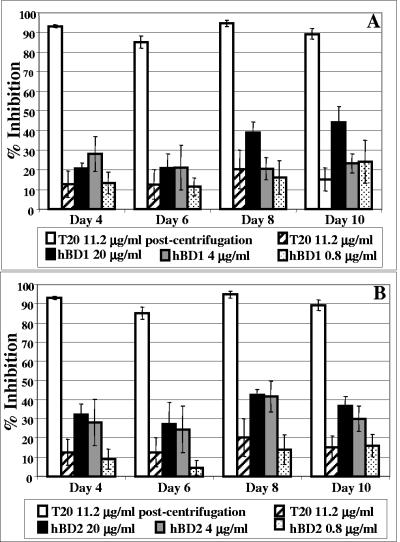
In order to gain some insight into the mechanism of action of hBD2, we tested whether this defensin is targeting the HIV virion. To this end, we treated both the X4 isolate IIIB and R5 isolate BaL with hBD2 at concentrations ranging from 20 to 0.8 μg/ml for 1 h at 37°C. As a control, virus was mock treated with PBS or with 11.2 μg/ml of the fusion inhibitor T20, which targets a fusion intermediate but has no direct virus-inactivating properties. Pretreated virus was then centrifuged at 100,000 × g for 1 h and used to infect target cells using the same viral dose indicated above. Infection was monitored by HIV p24 ELISA. In Fig. 6, we show that virus treated with either hBD1 (panel A) or hBD2 (panel B) display a relative decrease in infectivity. However, this activity did not inhibit viral infectivity more than about 30 to 40% on average at a dose of 4 μg/ml, and a higher dose of 20 μg/ml did not seem to further increase inhibitory activity. This suggests that this inhibition might occur due to a threshold mechanism rather than according to a dose-response curve. Further, this activity did not seem to be accountable per se for all of the decrease in infectivity that we observed when we tested β-defensins in a typical infectivity assay. Also, contrary to our observations in infectivity assays (Fig. 1 and 3), there was no significant difference in potency between hBD1 and hBD2. The control peptide, T20, did not affect virus infectivity when it was used prior to infection to pretreat the virus; when added at the time of infection, however, it reduced HIV replication more than 90%, as measured by p24 ELISA (Fig. 6). Similar results were observed with the R5 isolate BaL (not shown). Thus, a nonspecific component of the mechanism of HIV inhibition by β-defensins could be related to direct interference with HIV-1, partially lowering its infectivity.
FIG. 6.
hBD1 and hBD2 inhibit virus infectivity. The R5 isolate BaL (A) and the X4 isolate IIIB (B) were treated with hBD1 and hBD2 at concentrations ranging from 20 to 0.8 μg/ml for 1 h at 37°C. As a control, virus was mock treated with PBS or with 11.2 μg/ml of the fusion inhibitor T20, which is active only in the course of attachment/fusion events but has no direct virus-inactivating properties. Pretreated virus was then centrifuged at 100,000 × g for 1 h, washed, and used to infect target cells. T20 (11.2 μg/ml) was also used at the time of infection (postcentrifugation) as a positive control. Infection was monitored by HIV p24 ELISA for 10 days. Shown here are average results of four independent experiments. Bars indicate standard errors of the means.
hBD2, but not hBD1, displays long-acting HIV-suppressive activity.
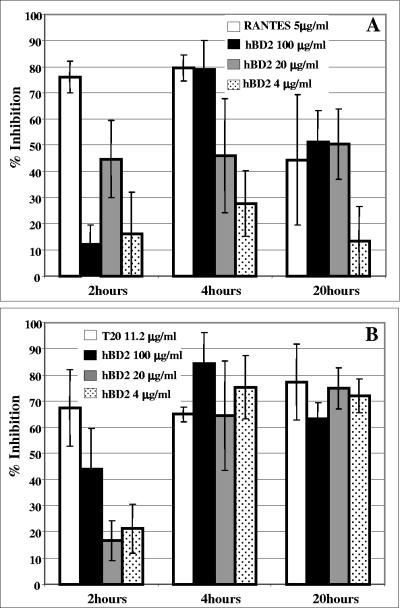
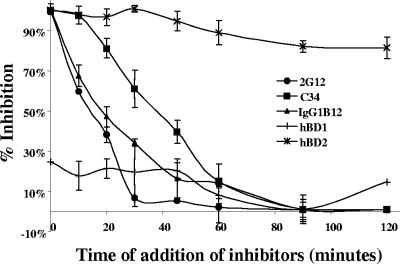
In order to gain more insight into the inhibitory activity of hBD2, we tested the kinetics of hBD2 inhibition and compared them with the kinetics of known HIV fusion inhibitors. Our PCR data showed a kinetic of inhibition occurring at an early stage of HIV replication; still, we observed no HIV entry inhibition, so that we reasoned that hBD2 is likely to inhibit at an early stage but postentry. Thus, we set up a time course experiment using R5 HIV (BaL envelope). Two inhibitors of HIV fusion that target disparate steps in the viral fusion cascade were utilized: IgG1B12, an anti gp120 antibody that blocks gp120-CD4 binding, and C34, which inhibits transition of the gp41 prehairpin intermediate into a stable six-helix bundle. Figure 7 shows that fusion is completely inhibited when virus and cells are incubated with all three inhibitors at the time of infection. When the inhibitors are added at later time points, the inhibitory effect is reduced (Fig. 7). As IgG1B12 and C34 target different intermediates in the fusion cascade, the kinetics of inhibition are clearly different. Figure 7 shows that, as expected, following 60 min of virus-cell coculture, IgG1B12 (used at 10 μg/ml) has lost 56% of its inhibitory ability while C34 (10 μg/ml) can still inhibit 70% of virus entry. Following 2 h of coculture, IgG1B12 no longer inhibits viral fusion as the viral envelope has already engaged CD4, while 21% of these attached viruses are still susceptible to C34, indicating that they have not yet undergone some of the terminal conformational changes that directly precede membrane fusion (31). However, hBD2 at 50 μg/ml retained >80% of its inhibitory activity even 2 h after infection. This is consistent with a mechanism of inhibition of viral replication beyond inhibition of entry/fusion and early steps of the viral life cycle. In contrast, hBD1 treatment at the same concentration showed marginal inhibitory activity (Fig. 7).
FIG. 7.
hBD2, but not hBD1, inhibits HIV replication postinfection. A time course experiment using HIVBaL was performed as indicated in Materials and Methods. hBD1 and hBD2 (50 μg/ml) were added either at the time of infection or 30 to 120 min after infection. Infected cells were harvested after 16 h, and infection was quantified by luciferase assays. The inhibitors of HIV fusion IgG1B12 (10 μg/ml), 2G12 (10 μg/ml), and C34 (10 μg/ml) were used as controls. Shown here are mean results from four experiments. Error bars indicate standard deviations.
hBD2 is expressed at higher levels than α-defensins 1 to 3 in adult oral mucosa, and its expression is decreased in HIV-l-infected subjects.
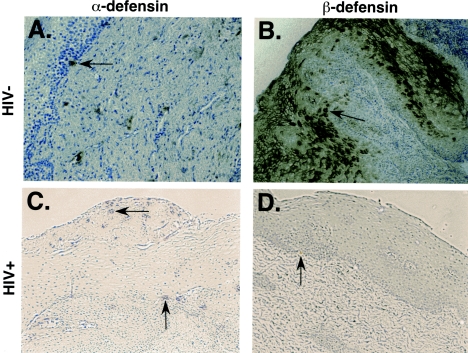
Immunohistochemical analyses were performed on adult keratinized and nonkeratinized oral mucosal specimens, for the detection of human α-defensins 1 to 3 and hBD2. Paraffin-embedded tissue samples were incubated with a mouse monoclonal antibody, Def-3, that recognizes α-defensins 1 to 3. Tissue specimens from 5 different donors demonstrated isolated positive-stained cells dispersed below the epithelium (Fig. 8A). Occasionally, positively stained cells were observed within the epithelium layer. These staining results are consistent with the finding that granulocytes are the predominant source of α-defensins within mucosal tissue (13, 15, 43). Tissue specimens were also incubated with an hBD2 antisera to detect the presence of hBD2. The result of these experiments demonstrated robust levels of hBD2, located across the epithelium of the oral mucosal tissue (Fig. 8B). The expression of hBD2 was confined to the upper epithelial area in all samples analyzed, thus forming a layer of positively stained cells (Fig. 8B). These results are consistent with the idea that epithelial cells in mucosal tissues are the source of hBD2 expression. Tissues incubated with each respective secondary antibody alone, anti-IgG1 isotype monoclonal antibody (for α-defensins 1 to 3), or with control rabbit sera served as a negative staining control.
FIG. 8.
The study of the expression of α-defensins 1 to 3 and of hBD2 in HIV seronegative and seropositive subjects reveals decreased hBD2 expression in HIV infection. Paraffin-embedded sections of nonkeratinized human oral mucosa were stained as described in Materials and Methods for α-defensins 1 to 3 (A and C) and for hBD2 (B and D). Shown here are representative results from one each (out of a total of five each) HIV-negative (upper panels) and HIV-positive (lower panels) subjects.
Samples of oral mucosal tissue obtained from HIV-positive individuals revealed levels and patterns of expression of α- defensins comparable to those observed in HIV-seronegative subjects (Fig. 8C). However, the expression of hBD2 in HIV-positive subjects was different from that observed in HIV-negative subjects both quantitatively and in its pattern. In HIV-positive subjects, we did not observe the presence of a continuous barrier layer of hBD2 across the top layers of epithelium. Instead, hBD2 appeared to be expressed in sparse foci below the basal epithelium in some of the sample analyzed, while in other samples we could not observe any staining (Fig. 8D).
CONCLUSIONS
Antimicrobial β-defensins, expressed at high levels in mucosae, are an important component of innate immunity in epithelia. Our results show that hBD-2 and 3 possess dose-dependent HIV-suppressive activity, while hBD1 is significantly less active. The antiviral activity was reproducibly observed with three isolates of HIV, two of which utilize CXCR4 (IIIB and NL4.3), while the third is CCR5 tropic (BaL), thus suggesting a broad-spectrum antiviral activity (Fig. 1 and 2). Interestingly, we noticed that, at least in the case of the isolates tested, hBD2 was more efficient in inhibiting replication of the X4 isolates (Fig. 1 and 2). These results are in accordance with the result of a recently published study (39). We observed some striking differences between our results and those of Quiñones-Mateu et al. (39). First, all of our data were gathered under high-salt, serum-rich conditions. Under high-salt conditions, the previous study detected no inhibition of R5 isolates, and even under low-salt conditions the inhibition of R5 isolates was marginal (39). In contrast, in our study inhibition of R5 isolates was very pronounced (Fig. 1), and one would expect to see even higher inhibition in serum-free conditions, as serum can sequester defensins (38). In addition, the inhibitory effect of hBD3 was accompanied by some level of cellular toxicity, especially at higher doses (100 μg/ml), so that we could not discriminate how this toxicity would affect viral replication, and we chose to compare hBD1 to hBD2 for further studies. It is conceivable that the different source of peptides (baculovirus-or Escherichia coli-expressed as opposed to synthetic) or the target cells used (the cell line GHOST, expressing HIV coreceptor, as opposed to PBMC) could account for the discrepancies that we observed. Our attempt to dissect the mechanism of action of these peptides yielded some unexpected results.
Defensins are known to disrupt membrane structure (16, 17, 20, 29, 33, 62), and θ-defensins tightly bind to the sugar moieties on gp120 and CD4 (12, 54, 55), so that we envisioned a mechanism of inhibition directed to both virus and cells. Our main hypothesis when we started our studies was that these effects would concur in inhibiting membrane fusion events mediated by HIV envelope. Consistently with a blockade of HIV at an early stage, we observed that concentrations comparable to those used in the infectivity assays could inhibit the accumulation of early products (4 h after infection) of reverse transcription, using both X4 and R5 isolates of HIV as detected by a quantitative real-time PCR assay (Fig. 4). To our surprise, however, we could not detect any reproducible inhibition of env-mediated fusion, despite the use of different experimental approaches to study the process and of both cell lines and primary cells. We evaluated the expression of coreceptors CD4, CXCR4, and CCR5 on the cell membranes by flow cytometry and did not observe any modulation following treatment with hBD1 and hBD2 in a range of concentrations between 20 and 0.8 μg/ml in both resting and PHA-activated cells (Fig. 5). This finding is at odds with the report by Quiñones-Mateu et al., who described a downregulation of CXCR4 upon hBD2 treatment (39). The only significant difference in their studies is the use of recombinant defensins, as opposed to our use of synthetic defensins. It is possible that the two molecules of different origin might have a different activity, and a comparative study will elucidate this discrepancy. Of note, lipopolysaccharide has been shown to downregulate CXCR4, although this is a potential contaminant of proteins expressed in bacteria and should therefore not be present in baculovirus-expressed proteins (52). We also studied release of HIV inhibitory chemokines, which have been described to mediate the anti-HIV activity of α-defensins in macrophages (19), and found that only hBD1 treatment results in some increase in RANTES release in PBMC, while none of the other chemokines showed detectable increases as measured by ELISA.
We could detect some decrease in HIV infectivity, but this decrease was not specific for hBD2, as also hBD1 treatment lowered HIV infectivity (Fig. 6). Therefore, while we think that direct inactivation of HIV might contribute to overall suppression, this is probably not the only mechanism responsible for it. Confirmation for this possibility comes from time course experiments where we treated cells from 10 to 120 min after infection; control infections were left untreated, while control infections for inhibition by defensins were treated prior to and at the time of infection, as in our pilot experiments. In addition, we treated cells with agents known to block fusion intermediates. As expected, the efficacy of these agents in blocking HIV infection decreased dramatically after 60 to 90 min after infection had been started (Fig. 7). In contrast, the efficacy of hBD2 remained ∼80% even when treatment was started 120 min after infection (Fig. 7). The significantly lower efficacy of treatment with the same concentration of hBD1 (∼20%) at all time points indicated that the inhibition is not a specific phenomenon. The finding that hBD2 inhibits HIV replication postinfection is of interest. Importantly, this result was obtained in a 16-h infection so that the observed suppression was not due to inhibition of spread. β-Defensins are known to signal through several receptors. For example, human α- and β-defensins selectively chemoattract different subsets of T lymphocytes and immature dendritic cells, thus playing important roles as immune modulators in adaptive immunity as well (10, 59, 60). For hBDs, chemotaxis of immature dendritic cells and memory T cells results from their direct binding and activation of the chemokine receptor CCR6, whose only known chemokine-ligand is MIP-3α (59). In addition, β-defensins induce intracellular signaling by interacting with chemokine and Toll-like receptors (5, 59), so that we cannot rule out that additional, late events in HIV replication might also be affected by β-defensins. These results are consistent with a model of inhibition by hBD2 due to either a receptor-triggered event or a change in membrane dynamics that, without affecting fusion events, results in inhibition of some signaling event. For example, disrupting membrane rafts can block signaling events. This model is compatible with observations made by Chang et al. (9, 9a), who studied the HIV-suppressive activity of α-defensins 1 to 3, and it is conceivable that a common or parallel mechanism of HIV inhibition exists between α-defensins and hBD2.
The range of concentration of hBD2 tested, between 100 and 0.8 μg/ml (i.e., in the 0.2 to 25 millimolar range), might seem high in comparison to the antiviral activity of other known soluble inhibitors, such as CCR5 ligands, which are active in the 10 to 100 μM range (11). However, it must be noted that this is the same concentration range for antibacterial activity. Further, expression of β-defensins at levels comparable to the ones used in our study has been described in the oral mucosa (49). Since transmission of HIV seems to occur less readily through the oral mucosa, we hypothesize that β-defensins might constitute a component of the innate immunity to this virus. In addition, it is possible that the immunodeficiency triggered by HIV infection decreases expression of this defensive mechanism, thus increasing occurrence of oral complications often observed in the course of HIV disease. In this respect, using immunohistochemical analyses, we observed that hBD2 expression, which in healthy individuals not infected with HIV is expressed at high levels in the epithelium of the oral mucosa and forms a thick barrier, is dramatically decreased in HIV-positive subjects, potentially leaving them more likely to contract opportunistic infections or other oral complications (Fig. 8).
The elucidation of the HIV-inhibitory activity of β-defensins and of their pattern of expression in the oral mucosa of HIV-negative and HIV-positive subjects has several important consequences. First, it is now imperative to investigate the expression of these proteins in mucosae from oral, rectal, and vaginal tissue, since it is possible that differential levels of expression of β-defensins might contribute to explain the documented lower rate of infection through oral mucosa. Further, it is conceivable that the oral transmission observed in infant macaques could be due to a less efficient expression of β-defensins in the oral mucosa in infants compared to adults, so that an investigation of the relative levels of expression in these two populations is warranted. Interestingly, a recent study has found a correlation between a polymorphism in the hBD1 gene and risk of HIV-1 infection in a population of 97 children (7). Finally, the finding that molecules that are constitutively expressed in the mucosa can inhibit HIV without being associated with inflammation has important consequences for both preventive and therapeutic approaches. In prevention, β-defensins or derivatives or small molecules modeled on them could be included in topical microbicide preparations; the same molecules could be also be evaluated in the growing arsenal of weapons to be used to treat HIV infection.
Acknowledgments
We thank Abigail Bowlsbey for technical help.
This study was supported by National Institutes of Health grant 1R21DE15508-01 (A.G.-D.), by grant AI056264 (W.L.), and by the Intramural Research Program of the NIH National Cancer Institute, Center for Cancer Research (R.B.).
Footnotes
A.G.-D. and P.G.-D. dedicate this study to the loving memory of Maresa Molaschi and Mario Garzino.
REFERENCES
- 1.Abiko, Y., A. Suraweera, M. Nishimura, T. Arakawa, T. Takuma, I. Mizoguchi, and T. Kaku. 2001. Differential expression of human beta-defensin 2 in keratinized and non-keratinized oral epithelial lesions; immunohistochemistry and in situ hybridization. Virchows Arch. 438:248-253. [DOI] [PubMed] [Google Scholar]
- 2.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nature Immunol. 1:99-100. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., A. M. Trichel, L. An, V. Liska, L. N. Martin, M. Murphey-Corb, and R. M. Ruprecht. 1996. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science 272:1486-1489. [DOI] [PubMed] [Google Scholar]
- 4.Bensch, K. W., M. Raida., H. J. Magert, P. Schulz-Knappe, and W. G. Forssmann. 1995. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 368:331-335. [DOI] [PubMed] [Google Scholar]
- 5.Biragyn, A., P. A. Ruffini, C. A. Leifer, E. Klyushnenkova, A. Shakhov, O. Chertov, A. K. Shirakawa, J. M. Farber, D. M. Segal, J. J. Oppenheim, and L. W. Kwak. 2002. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298:1025-1029. [DOI] [PubMed] [Google Scholar]
- 6.Bleul C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. [DOI] [PubMed] [Google Scholar]
- 7.Braida, L., M. Boniotto, A. Pontillo, P. A. Tovo, A. Amoroso, and S. Crovella. 2004. A single-nucleotide polymorphism in the human beta-defensin 1 gene is associated with HIV-1 infection in Italian children. AIDS 18:1598-1600. [DOI] [PubMed] [Google Scholar]
- 8.Broder, C. C., and E. A. Berger. 1995. Fusogenic selectivity of the envelope glycoprotein is a major determinant of human immunodeficiency virus type 1 tropism for CD4+ T-cell lines vs. primary macrophages. Proc. Natl. Acad. Sci. USA 92:9004-9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, T. L., F. Francois, A. Mosoian, and M. E. Klotman. 2003. CAF-mediated human immunodeficiency virus (HIV) type 1 transcriptional inhibition is distinct from alpha-defensin-1 HIV inhibition. J. Virol. 77:6777-6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Chang, T. L., J. Vargas, Jr., A. DelPortillo, and M. E. Klotman. 2005. Dual role of α-defensin-1 in anti-HIV-1 innate immunity. J. Clin. Investig. 115:765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chertov, O., D. F. Michiel, L. Xu, J. M. Wang, K. Tani, W. J. Murphy, D. L. Longo, D. D. Taub, and J. J. Oppenheim. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271:2935-2940. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 12.Cole, A. M., T. Hong, L. M. Boo, T. Nguyen, C. Zhao, G. Bristol, J. A. Zack, A. J. Waring, O. O. Yang, and R. I. Lehrer. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dale, B. A., J. R. Kimball, S. Krisanaprakornkit, F. Roberts, M. Robinovitch, R. O'Neal, E. V. Valore, T. Ganz, G. M. Anderson, and A. Weinberg. 2001. Localized antimicrobial peptide expression in human gingiva. J. Periodontal Res. 36:285-294. [DOI] [PubMed] [Google Scholar]
- 14.Désiré, N., A. Dehee, V. Schneider, C. Jacomet, C. Goujon, P.-M. Girard, W. Rozenbaum, and J. C. Nicolas. 2001. Quantitation of human immunodeficiency virus type 1 proviral load by a TaqMan real-time PCR assay. J. Clin. Microbiol. 39:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunsche, A., Y. Acil, R. Siebert, J. Harder, J. M. Schroder, and, S. Jepsen. 2001. Expression profile of human defensins and antimicrobial proteins in oral tissues. J. Oral Pathol. Med. 30:154-158. [DOI] [PubMed] [Google Scholar]
- 16.Ganz, T., M. E. Selsted, D. Szklarek, S. S. Harwig, K. Daher, D. F. Bainton, and R. I. Lehrer. 1985. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 76:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz, T., and R. I. Lehrer. 1998. Antimicrobial peptides of vertebrates. Curr. Opin. Immunol. 10:41-44. [DOI] [PubMed] [Google Scholar]
- 18.Garcia, J. R., F. Jaumann, S. Schulz, A. Krause, J. Rodriguez-Jimenez, U. Forssmann, K. Adermann, E. Kluver, C. Vogelmeier, D. Becker, R. Hedrich, W. G. Forssmann, and R. Bals. 2001. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity: its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 306:257-264. [DOI] [PubMed] [Google Scholar]
- 19.Guo, C. J., N. Tan, L. Song, S. D. Douglas, and W. Z. Ho. 2004. Alpha-defensins inhibit HIV infection of macrophages through upregulation of CC-chemokines. AIDS 18:1217-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock, R. E. W., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88. [DOI] [PubMed] [Google Scholar]
- 21.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 22.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 23.Horuk, R., J. Hesselgesser, Y. Zhou, D. Faulds, M. Halks-Miller, S. Harvey, D. Taub, M. Samson, M. Parmentier, J. Rucker, B. J. Doranz, and R. W. Doms. 1998. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J. Biol. Chem. 273:386-391. [DOI] [PubMed] [Google Scholar]
- 24.Hug, P., H. M. Lin, T. Korte, X. Xiao, D. S. Dimitrov, J. M. Wang, A. Puri, and R. Blumenthal. 2000. Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J. Virol. 74:6377-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia, H. P., B. C. Schutte, A. Schudy, R. Linzmeier, J. M. Guthmiller, G. K. Johnson, B. F. Tack, J. P. Mitros, A. Rosenthal, T. Ganz, and P. B. McCray, Jr. 2001. Discovery of new human beta-defensins using a genomics-based approach. Gene 263:211-218. [DOI] [PubMed] [Google Scholar]
- 26.Jones, D. E., and C. L. Bevins. 1992. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 267:23216-23225. [PubMed] [Google Scholar]
- 27.Jones, D. E., and C. L. Bevins. 1993. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 315:187-192. [DOI] [PubMed] [Google Scholar]
- 28.Kao, C. Y., Y. Chen, Y. H. Zhao, and R. Wu. 2003. ORFeome-based search of airway epithelial cell-specific novel human beta-defensin genes. Am. J. Respir. Cell Mol. Biol. 29:71-80. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer, R. I., and T. Ganz. 2002. Defensins of vertebrate animals. Curr. Opin. Immunol. 14:96-102. [DOI] [PubMed] [Google Scholar]
- 30.Mackewicz, C. E., J. Yuan, P. Tran, L. Diaz, E. Mack, M. E. Selsted, and J. A. Levy. 2003. α-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS 17:F23-32. [DOI] [PubMed] [Google Scholar]
- 31.Markosyan, R. M., X. Ma, M. Lu, F. S. Cohen, and G. B. Melikyan. 2002. The mechanism of inhibition of HIV-1 env-mediated cell-cell fusion by recombinant cores of gp41 ectodomain. Virology 302:174-184. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima, H., N. Yamamoto, M. Masuda, and N. Fujii. 1993. Defensins inhibit HIV replication in vitro. AIDS 7:1129. [DOI] [PubMed] [Google Scholar]
- 33.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 34.Nussbaum, O., C. C. Broder, and E. A. Berger. 1994. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J. Virol. 68:5411-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberlin, E., A. Amara, F. Bachelerie, C. Bessia, J. L. Virelizier, F. Arenzana-Seisdedos, O. Schwartz, J. M. Heard, I. Clark-Lewis, D. F. Legler, M. Loetscher, M. Baggiolini, and B. Moser. 1996. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382:833-835. [DOI] [PubMed] [Google Scholar]
- 36.Pace, C. N., F. Vajdos, L. Fee, G. Grimsley, and T. Gray. 1995. Protein Sci. 4:2411-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal, R., A. Garzino-Demo, P. D. Markham, J. Burns, M. Brown, R. C. Gallo, and A. L. DeVico. 1997. Inhibition of HIV-1 infection by the beta-chemokine MDC. Science 278:695-698. [DOI] [PubMed] [Google Scholar]
- 38.Panyutich, A., and T. Ganz. 1991. Activated alpha 2-macroglobulin is a principal defensin-binding protein. Am. J. Respir. Cell Mol. Biol. 5:101-106. [DOI] [PubMed] [Google Scholar]
- 39.Quiñones-Mateu, M. E., M. M. Lederman, Z. Feng, B. Chakraborty, J. Weber, H. R. Rangel, M. L. Marotta, M. Mirza, B. Jiang, P. Kiser, K. Medvik, S. F. Sieg, and A. Weinberg. 2003. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 17:F39-48. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Jimenez, F. J., A. Krause, S. Schulz, W. G. Forssmann, J. R. Conejo-Garcia, R. Schreeb, and D. Motzkus. 2003. Distribution of new human beta-defensin genes clustered on chromosome 20 in functionally different segments of epididymis. Genomics 81:175-183. [DOI] [PubMed] [Google Scholar]
- 41.Rothenberg, R. B., M. Scarlett, C. del Rio, D. Reznik, and C. O'Daniels. 1998. Oral transmission of HIV. AIDS 12:2095-2105. [DOI] [PubMed] [Google Scholar]
- 42.Ruprecht, R. M., T. W. Baba, V. Liska, S. Ayehunie, J. Andersen, D. C. Montefiori, A. Trichel, M. Murphey-Corb, L. Martin, T. A. Rizvi, B. J. Bernacky, S. J. Buchl, and M. Keeling. 1998. Oral SIV, SHIV, and HIV type 1 infection. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S97-S103. [PubMed] [Google Scholar]
- 43.Sawaki, K., N. Mizukawa, T. Yamaai, J. Fukunaga, and T. Sugahara. 2002. Immunohistochemical study on expression of alpha-defensin and beta-defensin-2 in human buccal epithelia with candidiasis. Oral Dis. 8:37-41. [DOI] [PubMed] [Google Scholar]
- 44.Schmidtmayerova, H., M. Alfano, G. Nuovo, and M. Bukrinsky. 1998. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4 and CXCR4 mediated pathway: replication is restricted at a post-entry level. J. Virol. 72:4633-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnolzer, M., P. Alewood, A. Jones, D. Alewood, and S. B. Kent. 1992. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int. J. Pept. Protein Res. 40: 180-193. [DOI] [PubMed] [Google Scholar]
- 46.Schutte, B. C. and P. B. McCray, Jr. 2002. β-defensins in lung host defense. Annu. Rev. Physiol. 64:709-748. [DOI] [PubMed] [Google Scholar]
- 47.Schutte, B. C., J. P. Mitros, J. A. Bartlett, J. D. Walters, H. P. Jia, M. J. Welsh, T. L. Casavant, and P. B. McCray, Jr. 2002. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. USA 99:2129-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selsted, M. E., S. S. Harwig, T. Ganz, J. W. Schilling, and R. I. Lehrer. 1985. Primary structures of three human neutrophil defensins. J. Clin. Investig. 76:1436-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi, J., G. Zhang, H. Wu, C. Ross, F. Blecha, and T. Ganz. 1999. Porcine epithelial β-defensin 1 is expressed in the dorsal tongue at antimicrobial concentrations. Infect. Immun. 67:3121-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shugars, D. C., and S. M. Wahl. 1998. The role of the oral environment in HIV-1 transmission. J. Am. Dent. Assoc. 129:851-858. [DOI] [PubMed] [Google Scholar]
- 51.Sun, L., S. F. Abdelwahab, G. K. Lewis, and A. Garzino-Demo. 2004. Recall antigen activation induces prompt release of CCR5 ligands from PBMC: implication in memory responses and immunization. Int. Immunol. 16: 1623-1631. [DOI] [PubMed] [Google Scholar]
- 52.Verani, A., F. Sironi, A. G. Siccardi, P. Lusso, and D. Vercelli. 2002. Inhibition of CXCR4-tropic HIV-1 infection by lipopolysaccharide: evidence of different mechanisms in macrophages and T lymphocytes. J. Immunol. 168:6388-6395. [DOI] [PubMed] [Google Scholar]
- 53.von Horsten, H. H., P. Derr, and C. Kirchhoff. 2002. Novel antimicrobial peptide of human epididymal duct origin. Biol. Reprod. 67:804-813. [DOI] [PubMed] [Google Scholar]
- 54.Wang, W., A. M. Cole, T. Hong, A. J. Waring, and R. I. Lehrer. 2003. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J. Immunol. 170:4708-4716. [DOI] [PubMed] [Google Scholar]
- 55.Wang, W., S. M. Owen, D. L. Rudolph, A. M. Cole, T. Hong, A. J. Waring, R. B. Lal, and R. I. Lehrer. 2004. Activity of alpha- and theta-defensins against primary isolates of HIV-1. J. Immunol. 173:515-520. [DOI] [PubMed] [Google Scholar]
- 56.Wilde, C. G., J. E. Griffith, M. N. Marra, J. L. Snable, and R. W. Scott. 1989. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J. Biol. Chem. 264:11200-11203. [PubMed] [Google Scholar]
- 57.Wu, Z., D. M. Hoover, D. Yang, C. Boulegue, F. Santamaria, J. J. Oppenheim, J. Lubkowski, and W. Lu. 2003. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc. Natl. Acad. Sci. USA 100:8880-8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, Z., F. Cocchi, D. Gentles, B. Ericksen, J. Lubkowski, A. Devico, R. I. Lehrer, and W. Lu. 2005. Human neutrophil alpha-defensin 4 inhibits HIV-1 infection in vitro. FEBS Lett. 579:162-166. [DOI] [PubMed] [Google Scholar]
- 59.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]
- 60.Yang, D., Q. Chen, O. Chertov, and J. J. Oppenheim. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68:9-14. [PubMed] [Google Scholar]
- 61.Younai, F. S. 2001. Oral HIV transmission. J. Calif. Dent. Assoc. 29:142-148. [PubMed] [Google Scholar]
- 62.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, L., W. Yu, T. He, J. Yu, R. E. Caffrey, E. A. Dalmasso, S. Fu, T. Pham, J. Mei, J. J. Ho, W. Zhang, P. Lopez, and D. D. Ho. 2002. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science 298:995-1000. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, C., I. Wang, and R. I. Lehrer. 1996. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 396:319-322. [DOI] [PubMed] [Google Scholar]