Abstract
The profile of cytokines induced by soluble leishmania antigen (SLA) and the Leishmania homologue of the mammalian receptor for activated C kinase (LACK), a candidate vaccine against leishmaniasis, and the cellular source of the cytokines produced in response to these antigens were analyzed in patients infected with Leishmania guyanensis. Gamma interferon (IFN-γ) and interleukin-10 (IL-10) were produced in response to LACK. Although LACK-specific CD4+ cells producing IFN-γ were isolated only during the early phase of infection (less than 30 days following the onset of infection), cells producing IL-10 in response to LACK were detected in all patients. CD4+ T cells producing IFN-γ and IL-13 were produced in response to SLA in all patients. SLA- and LACK-specific T cells are effector memory cells, as they are CD45RA− CCR7− CD4+ T cells. CD4+ T cells producing IFN-γ are CD62L−, and CD4+ T cells producing IL-10 are CD62L+, indicating that these cells have different tissue-homing capacities. These findings show that SLA and LACK induce both type 1 (IFN-γ) and type 2 (IL-10 or IL-13) cell responses.
The clinical outcomes of human infection with Leishmania spp. depend on the infective agent and the specific immune responses to leishmania antigens. CD4+ T cells can be divided into two functionally distinct T-helper (Th) cell subsets: Th1 cells, which produce gamma interferon (IFN-γ) and interleukin-2 (IL-2), and Th2 cells, which produce IL-4, IL-5, IL-10, and IL-13 (1, 19). In mice, resistance and susceptibility to infection with Leishmania major are associated with the development of polarized Th1 and Th2 responses, respectively (22). Although production of IFN-γ has been associated with the successful treatment of human leishmaniasis (8, 14), the recent demonstration that IL-13 is produced in response to specific Leishmania antigens by peripheral blood mononuclear cells (PBMC) from patients with American localized cutaneous leishmaniasis (LCL) due to Leishmania guyanensis suggests that the T-cell response to human leishmaniasis is in fact a mixture of type 1 and type 2 responses (5). Furthermore, the detection of IL-13 mRNA in human leishmaniasis lesions shows that some Th2 cells may develop at the site of infection (5). The role of IL-13, which induces IL-12 unresponsiveness in specific CD4+ T cells and consequently a type 2 cell response, may be to maintain the infection (6). Another Th2 cytokine, IL-10, has been shown to down-regulate IFN-γ production in patients with human cutaneous leishmaniasis due to Leishmania braziliensis and consequently to promote the disease (23). If the immunologic milieu rather the antigen itself is more important in determining the development of an immune response, then identification of the antigen(s) responsible either for the induction of a protective immune response (IFN-γ production) or for the induction of pathological processes (IL-10 and IL-13) would be an important step in the development of vaccines against Leishmania infection.
In a murine model of infection with L. major, a single epitope of the Leishmania homologue of the mammalian receptor for activated C kinase (LACK) was shown to induce Th2 cell development and susceptibility to infection in BALB/c mice (15). Studies showing that immunization with DNA (12), altered peptides (21), or protein in the presence of IL-12 (20) rendered susceptible mice resistant to infection with L. major also suggest that LACK is a possible candidate as a vaccine against leishmaniasis.
Although IFN-γ-producing CD8+ T cells and natural killer (NK) cells have been found to be produced in response to stimulation with LACK in persons who had never been exposed to Leishmania (7, 17), the role of these cells in the outcome of infection is not known. Furthermore, some IFN-γ production by LACK-specific cells has been detected in LCL patients, but inconsistent results regarding the exact cellular source of this cytokine have been obtained (4, 18). Finally, the demonstration that LACK can induce IL-10 in cells from LCL patients indicates that this antigen may play some role in the pathological immune responses that develop during infection with Leishmania (18). The aim of this study was to analyze the cytokine profile induced by LACK and to describe the cellular source of the cytokines produced in LCL patients infected with L. guyanensis.
MATERIALS AND METHODS
Samples.
Blood samples were obtained by venipuncture from 29 patients with active American LCL due to L. guyanensis before specific treatment and collected into sterile tubes (Veinoject; Terumo, Leuven, Belgium). The diagnoses for all patients were confirmed by the isolation of Leishmania parasites from their lesions. Identification of L. guyanensis was made on the basis of isoenzyme polymorphisms or the reactivity of monoclonal antibodies (a generous gift from F. Modabber, World Health Organization Special Programme for Research and Training in Tropical Diseases). All the patients were seronegative for human immunodeficiency virus. Informed consent was obtained from the patients, and the human experimentation guidelines of the Centre Hospitalier Andrée Rosemon in Cayenne, French Guiana, were followed in the conduct of this research.
Antigens.
L. guyanensis promastigotes (M4147) were cultured in biphasic rabbit blood agar with Dulbecco's minimal essential medium complemented with 10% fetal calf serum (Sigma, l'Isle d'Abeau, France) as described previously (16). Extracellular proteins were removed from the pellets by three washes with phosphate-buffered saline. The soluble leishmania antigen (SLA) lysate was prepared by freezing (at −70°C) and thawing (at 37°C) the Leishmania preparation 10 times. The SLA lysate was then centrifuged and maintained at a final concentration equivalent to 106 parasites/ml (protein concentration, 1 μg/ml). Recombinant LACK protein, the 41-amino-acid deletion construct (ΔLACK), was obtained as described previously (15) and used at a concentration of 1.5 μg/ml. The LACK peptide comprised of amino acids (aa) 156 to 173 (ICFSPSLEHPIVVSGSWD) (peptide aa 153-176) was synthesized by Sigma and purified by reversed-phase high-performance liquid chromatography. Because LACK was produced in Escherichia coli by a method of protein purification involving the addition of an epitope (hemagglutinin of the influenza virus) (10), we ensured that bacterial contamination and the epitope used in the purification of the protein were not responsible for the cytokine production induced by recombinant LACK antigen. The finding that treatment of LACK protein with polymyxin B (20 mg/ml) to neutralize the endotoxin did not change IFN-γ and IL-10 production by PBMC showed that the cytokine production by PBMC in response to LACK protein is not an endotoxin process (data not shown). Furthermore, the Limulus amebocyte lysate test (E-Toxate; Sigma) showed that endotoxin levels were 0.05 endotoxin unit (EU)/ml for LACK, 0.04 EU/ml for ΔLACK, and <0.015 EU/ml for the peptide aa 153-176.
Reagents.
The reagents for the magnetic separation and the anti-CD4-, anti-CD8-, and anti-mouse immunoglobulin G (IgG)-coated magnetic beads were obtained from Dynal (Compiègne, France). Mouse anti-human CD3 (UTCHT1, IgG1), anti-human CD45RA (HI100, IgG2b), and anti-human CD62L (Dreg 56, IgG1) were obtained from Pharmingen (San Diego, Calif.). Mouse anti-human CCR7 was kindly provided by Hitoshi Hasagewa, Ehime University School of Medicine, Shigenobu, Japan.
Separation of cells.
CD3+ and CD3− cells were purified in a magnetic-activated cell sorter obtained from Dynal. Briefly, cells conjugated with an anti-CD3 monoclonal antibody were suspended with anti-mouse IgG-coated magnetic microbeads and isolated after exposure to a magnetic field. The purity was 95%, as determined by fluorescence-activated cell sorter (FACS) analysis. CD4+ and CD8+ cells were purified and depleted with anti-CD4 and anti-CD8 magnetic beads according to the instructions of the manufacturer (Dynal). This resulted in a T-cell population that was 90% CD8+, 93% CD4+, and 95% depleted as determined by FACS analysis. The CD8 and CD4 monoclonal antibodies were released with DETACHaBEADs according to the instructions of the manufacturer (Dynal). The purified CD8+ and CD4+ T cells were then incubated with various mouse anti-human monoclonal antibodies (anti-CD45RA, anti-CD62L, and anti-CCR7) and purified with magnetic beads conjugated with anti-mouse IgG monoclonal antibodies. The purity in all cases was >90%, as determined by FACS analysis.
Lymphocyte culture and detection of cytokines.
PBMC were obtained by venipuncture, isolated on Ficoll-Hypaque gradient (d = 1.077), and resuspended in RPMI medium supplemented with 2 mM l-glutamine, 100 U of penicillin/ml, 0.1 mg of streptomycin/ml (all from Sigma), and 10% human heat-inactivated serum from red blood cell group AB. Cultures for cytokine production (106 cells/ml of culture medium) were plated on flat-bottom 24-well plates with or without antigens. T cells positively and negatively purified by magnetic procedures (104 cells) were stimulated in the presence of autologous PBMC treated with mitomycin (106 cells) for use as antigen-presenting cells in flat-bottom 24-well plates with or without antigens. The culture supernatants were harvested after 7 days for IL-4, IL-13, IL-10, and IFN-γ and stored at −20°C. We analyzed cytokine production by using specific IL-4, IL-10, and IFN-γ enzyme-linked immunosorbent assays (ELISAs) (Pharmingen) and a specific IL-13 ELISA (Immunotech, Marseille, France). The ELISAs had a sensitivity of 10 pg/ml.
Statistical analysis.
The data were subjected to statistical analysis by the nonparametric Kruskal-Wallis test.
RESULTS
LACK-stimulated PBMC released IL-10 in all LCL patients but IFN-γ only in patients with early lesions.
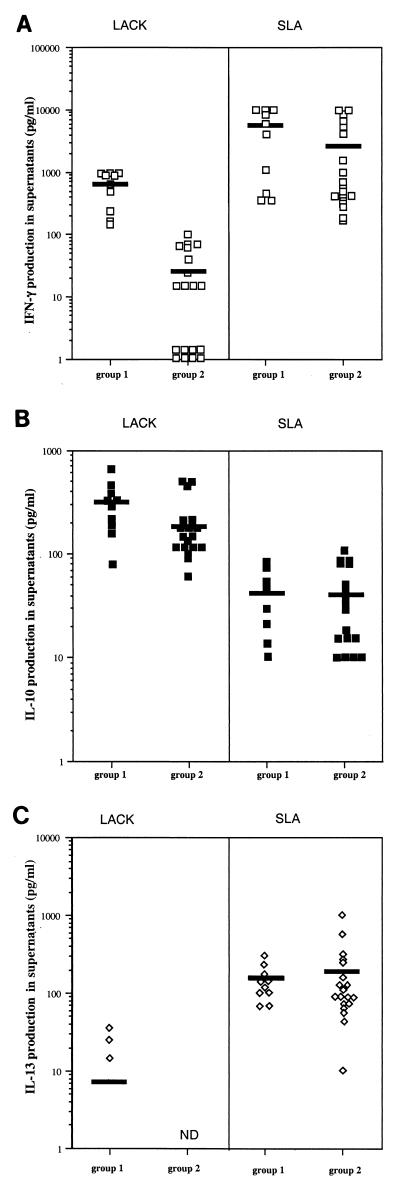
IL-4, IL-10, IL-13, and IFN-γ production in PBMC was analyzed for 29 patients with LCL responding to LACK or SLA stimulation. Ten patients reacted to LACK by producing IFN-γ (range, 144 to 1,000 pg/ml; mean, 668 ± 118 pg/ml), and these patients were assigned to group 1. In 19 of the 29 patients with LCL, only low levels of IFN-γ production (range, 0 to 100 pg/ml; mean, 26 ± 7 pg/ml) were detected in response to LACK stimulation, and these patients were assigned to group 2 (Fig. 1 ). Clinical and parasitological data for patients from groups 1 and 2 are given in Table 1. The correlations between IFN-γ reactivity towards LACK and clinical and/or parasitological data were then analyzed. No correlation was found between reactivity or lack of response to LACK stimulation and the number of parasites per lesion, the number and location of lesions, or the presence or absence of adenopathy or lymphangitis. However, high levels of IFN-γ production in response to LACK correlated with the early phase of infection, as patients in group 1 developed lesions within 30 days of infection and patients in group 2 developed lesions after more than 40 days (median of 21 days, range of 10 to 45 days, and interquartile range of 9 days for group 1; median of 60 days, range of 40 to 90 days, and interquartile range of 15 days for group 2; P < 0.001 [Kruskal-Wallis test]). The levels of IFN-γ in PBMC from patients in the two groups in response to SLA were not significantly different (5,153 ± 1,413 pg/ml for group 1 and 2,698 ± 808 pg/ml for group 2; P = 0.22).
FIG. 1.
Production of IFN-γ (A), IL-10 (B), and IL-13 (C) in cells restimulated with LACK or SLA from LCL patients who developed lesions within 30 days of (group 1) or more than 30 days after (group 2) the onset of infection. Cytokine production by PBMC in culture for 7 days in the presence of antigens was measured by ELISA, as described in Materials and Methods. The mean levels are indicated by horizontal bars. ND, not detected.
TABLE 1.
Clinical and parasitological data for LCL patients
| Patient no.a | Days to development of lesion(s)b | No. of lesions | Location of lesion(s) | Adenopathy | Lymphangitis | No. of parasitesc |
|---|---|---|---|---|---|---|
| Group 1 | ||||||
| 1 | 10 | 3 | Upper limbs | Yes | Yes | 1-5 |
| 2 | 15 | 1 | Lower limb | No | No | <1 |
| 3 | 21 | 1 | Upper limb | Yes | No | <1 |
| 4 | 21 | 1 | Upper limb | No | No | 1-5 |
| 5 | 21 | 1 | Upper limb | No | No | <1 |
| 6 | 21 | 2 | Lower limbs | No | No | <1 |
| 7 | 21 | 1 | Lower limb | No | No | 1-5 |
| 8 | 30 | 1 | Upper limb | No | No | <1 |
| 9 | 30 | 6 | Head, upper limbs | No | No | <1 |
| 10 | 30 | 1 | Upper limb | Yes | Yes | <1 |
| Group 2 | ||||||
| 11 | 40 | 1 | Upper limb | Yes | No | 1-5 |
| 12 | 45 | 1 | Thorax | No | No | <1 |
| 13 | 45 | 4 | Neck, thorax, upper and lower limbs | No | No | <1 |
| 14 | 45 | 1 | Thorax | No | No | <1 |
| 15 | 45 | 3 | Upper and lower limbs | Yes | No | 1-5 |
| 16 | 60 | 6 | Lower limbs | Yes | No | 1-5 |
| 17 | 60 | 3 | Lower limbs | Yes | Yes | 1-5 |
| 18 | 60 | 1 | Upper limb | Yes | No | 1-5 |
| 19 | 60 | 3 | Lower limbs | Yes | No | 1-5 |
| 20 | 60 | 3 | Head, upper limbs | No | No | 1-5 |
| 21 | 60 | 1 | Lower limb | No | No | <1 |
| 22 | 60 | 1 | Upper limb | Yes | Yes | 1-5 |
| 23 | 60 | 1 | Lower limb | Yes | Yes | 1-5 |
| 24 | 75 | 2 | Upper limbs | Yes | Yes | 1-5 |
| 25 | 75 | 5 | Head, upper limbs | No | Yes | <1 |
| 26 | 90 | 6 | Head, upper and lower limbs | No | No | 10 |
| 27 | 90 | 1 | Upper limb | Yes | No | <1 |
| 28 | 90 | 1 | Lower limb | Yes | No | 1-5 |
| 29 | 90 | 1 | Lower limb | Yes | No | 1-5 |
Group 1, patients who react with IFN-γ production of >100 pg/ml in response to LACK; group 2, patients who react with IFN-γ production of <100 pg/ml in response to LACK.
Duration of lesion before patient sought care.
Number of parasites per 50 fields in May-Grünwald- and Giemsa-stained smears.
IL-10 production was detected in LACK-stimulated PBMC from all 29 patients (Fig. 1). In contrast, IL-10 was detected at very low levels in supernatants of SLA-stimulated PBMC, confirming that this antigen induces less IL-10 production than does LACK (P of <0.001 for both groups) (Fig. 1) (7). No difference in the levels of IL-10 production was detected between the groups in response to either LACK (304 ± 52 pg/ml for group 1 and 197 ± 31 pg/ml for group 2; P = 0.14) or SLA (45 ± 8 pg/ml for group 1 and 40 ± 7 pg/ml for group 2; P = 0.70).
IL-13 was detected in SLA-stimulated PBMC from all patients regardless of the time of lesion development (147 ± 24 pg/ml for group 1 and 192 ± 54 pg/ml for group 2; P = 0.30). IL-13 production was detected in PBMC after stimulation with LACK in only three patients in group 1 (25, 35, and 15 pg/ml) and was completely absent in patients in group 2. The absence of IL-13 production in response to LACK stimulation was confirmed with a semiquantitative reverse transcription-PCR on LACK-stimulated PBMC (data not shown).
IL-4 was not detected in supernatants of LACK- or SLA-stimulated cells from any patients.
LACK peptide aa 156-173 induced IFN-γ and IL-10 production in PBMC from LCL patients.
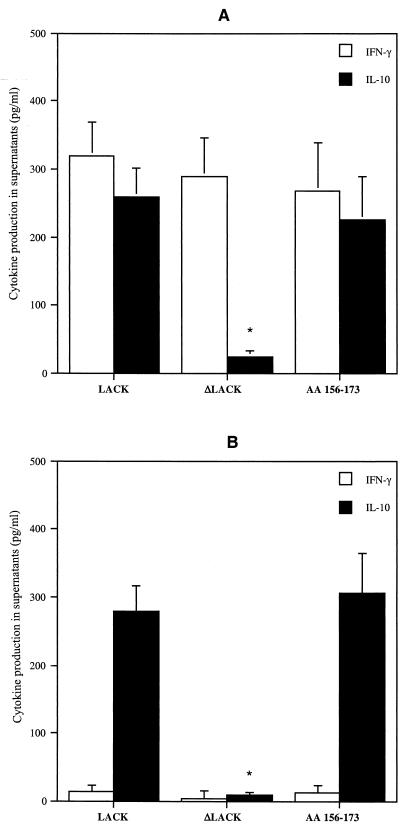
Recombinant LACK in BALB/c mice showed a single dominant major histocompatibility complex class II-restricted T-cell epitope in the fourth domain (peptide aa 156-173) which could induce IL-4 mRNA expression after infection (15). Furthermore, this T-cell epitope is a potent inducer of IL-10 in persons who have never been exposed to Leishmania (7). We therefore evaluated whether the release of IL-10 was dependent on this epitope. We first analyzed IL-10 production by PBMC from LCL patients in the early phase of infection (group 1) in response to recombinant LACK in which 41 aa were deleted, including the 18 aa making up the epitope (ΔLACK). As shown in Fig. 2A, this construct did not induce IL-10 production in PBMC from LCL patients (P, <0.01); however, no statistically significant difference in the levels of IFN-γ production was seen between PBMC stimulated with complete LACK and those stimulated with ΔLACK. In order to confirm that IL-10 production depends on this 18-amino-acid epitope, we measured IL-10 production in response to the purified peptide. As expected, in the presence of peptide aa 156-173, PBMC released IL-10 (Fig. 2). In contrast, no detectable IL-10 or IFN-γ production was detected in PBMC from LCL patients stimulated with an unrelated peptide (YDYDVPDVYA) from the hemagglutinin of the influenza virus (HA1) (data not shown). Of note is the fact that both IL-10 and IFN-γ were released by PBMC cultured in the presence of the parasite-derived peptide aa 156-173.
FIG. 2.
IFN-γ and IL-10 production in response to LACK, ΔLACK, and peptide aa 153-176 by PBMC from LCL patients who developed lesions within 30 days of (group 1) (A) or more than 30 days after (group 2) (B) the onset of infection. Cytokine production by PBMC in culture for 7 days in the presence of LACK, ΔLACK, and peptide aa 156-173 was measured by ELISA, as described in Materials and Methods. The data are means + standard errors of the means (SEM) from results for 10 subjects in each group. ∗, P of <0.01, as determined by the Kruskal-Wallis test, for cytokine production in response to LACK versus that in response to ΔLACK or peptide aa 156-173.
As expected, PBMC from patients who developed lesions for more than 40 days (group 2) did not produce IFN-γ in response to LACK, ΔLACK, or peptide aa 156-173. However IL-10 was detected in supernatants of PBMC from these patients in response to LACK and peptide aa 156-173 (Fig. 2B).
In response to LACK, CD45RA− CD62L− CCR7− CD4+ T cells produce IFN-γ while CD45RA− CD62L+ CCR7− CD4+ T cells produce IL-10
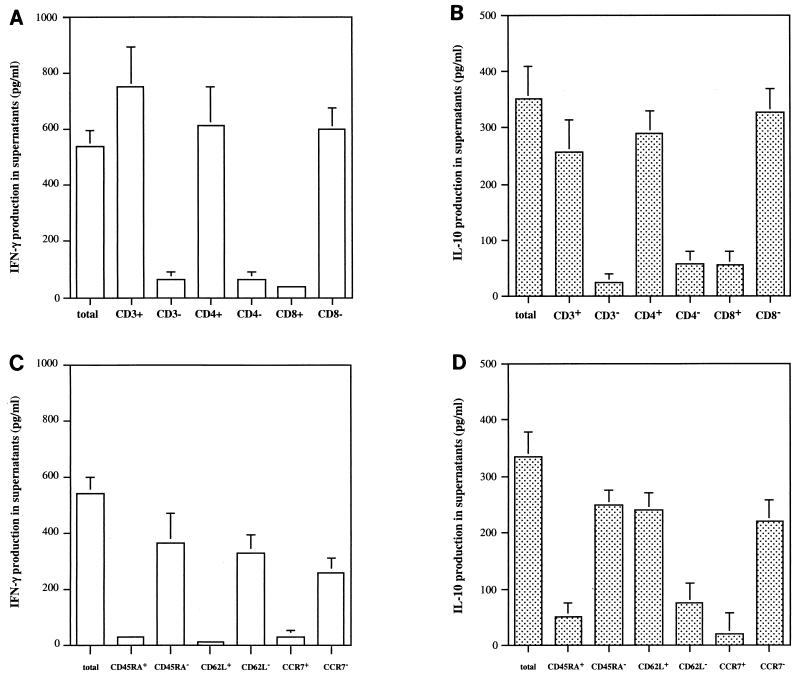
In order to identify the cells responsible for IFN-γ and IL-10 production in response to LACK in patients from group 1, we analyzed the secretion of these cytokines in supernatants from stimulated, unfractionated, positively and negatively selected cells before exposure to antigen. The results with anti-CD4 and anti-CD8 monoclonal antibodies showed that IFN-γ and IL-10 are produced in response to LACK by CD4+ T cells. Analysis of the phenotype of the CD4+ T cells that produced IFN-γ in response to LACK showed that they are memory CD4+ T cells, because IFN-γ was detected in CD4+ T cells that were CD45RA−. Sallusto et al. (24) showed that memory T cells can be divided into central memory and effector memory cells on the basis of the expression of CCR7 (CCR7+ and CCR7− phenotypes for central and effector memory T cells, respectively). We stimulated the positive and negative populations of CCR7 cells obtained from CD4+ T cells with LACK to determine the CCR7 phenotype of the IFN-γ-producing cells. As shown in Fig. 3, IFN-γ production in response to LACK was detected in CCR7− CD4+ T cells, suggesting that CD4+ T cells producing IFN-γ in response to LACK in LCL patients are effector memory cells. As CD62L molecules must be expressed on T cells in order for them to migrate to lymph nodes, IFN-γ production in response to LACK was analyzed in CD62L+ and CD62L− CD4+ T cells. Figure 3 shows that the IFN-γ-producing CD4+ T cells stimulated by LACK are CD62L−.
FIG. 3.
Phenotype of IFN-γ- and IL-10-producing cells stimulated with LACK in LCL patients who developed lesions within 30 days of the onset of infection (group 1). IFN-γ and IL-10 production levels (A and B, respectively) were analyzed in supernatants of LACK-stimulated unfractionated cells and negatively and positively selected CD3, CD4, and CD8 cells before antigen exposure. Positively and negatively purified CD45RA, CD62L, CCR7, and CLA CD4+ T cells were then stimulated with LACK, and after 7 days of culture, the supernatants were harvested for determination of IFN-γ and IL-10 production levels (C and D, respectively). The data are mean levels + SEM from results for 10 LCL patients.
By analyzing IL-10 production in unfractionated positively and negatively purified CD4+ T cells for the expression of CD45RA, CD62L, and CCR7, we demonstrated that the IL-10-producing CD4+ T cells are memory cells, as they do not express CD45RA and are CD62L+ and CCR7−.
In conclusion, if cells that produce IFN-γ and IL-10 in response to LACK are effector memory CD4+ T cells, then IL-10-producing cells but not IFN-γ-producing cells express the CD62L antigen. Furthermore, the same phenotype was found in IL-10-producing cells in patients from group 2, who developed lesions for more than 40 days (data not shown).
Cellular origin of IFN-γ and IL-13 produced in response to SLA in LCL patients.
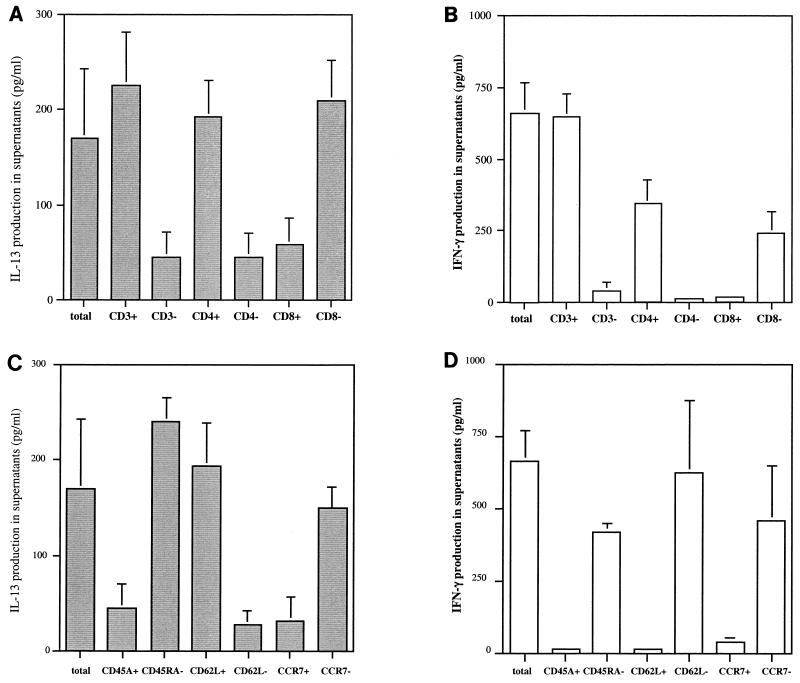
Since PBMC from LCL patients produced IFN-γ and IL-13 in the presence of SLA, we analyzed the phenotype of the cells producing IFN-γ and IL-13 in response to SLA in patients from group 1. As shown in Fig. 4, the IFN-γ-producing cells are memory CD4+ T cells, as all the IFN-γ production was detected in CD45RA− CD4+ T cells. Furthermore, analysis of IFN-γ secretion in CCR7 and CD62L positively and negatively selected T cells showed that the cells producing IFN-γ in response to SLA are CCR7− and CD62L− CD4+ T cells.
FIG. 4.
Phenotype of IL-13- and IFN-γ-producing cells stimulated with SLA in LCL patients who developed lesions within 30 days of the onset of infection (group 1). IL-13 and IFN-γ production levels (A and B, respectively) were analyzed in supernatants of SLA-stimulated unfractionated cells and negatively and positively selected CD3, CD4, and CD8 cells before antigen exposure. Positively and negatively purified CD45RA, CD62L, CCR7, and CLA CD4+ T cells were then stimulated with SLA, and after 7 days of culture, the supernatants were harvested for determination of IL-13 and IFN-γ production levels (C and D, respectively). The data are mean levels + SEM from results for 10 LCL patients.
The T cells that produce IL-13 in response to SLA are memory CD45RA− CD4+ T cells that do not express the CCR7 molecule. Further experiments showed that, in contrast to IFN-γ-producing cells, these cells express CD62L, as IL-13 production in response to SLA was detected in CD62L+ CD4+ T cells.
In conclusion, if cells producing IFN-γ and IL-13 in response to SLA are effector memory CD4+ T cells, then IL-13-producing cells but not IFN-γ-producing cells express the CD62L antigen. Furthermore, the same phenotype of the cells producing IFN-γ and IL-13 was detected in LCL patients from group 2, who developed lesions more than 40 days after infection.
DISCUSSION
We have shown that IFN-γ is produced in response to SLA and LACK in LCL patients. However, although IFN-γ was produced at high levels in response to SLA stimulation in all the patients, IFN-γ was produced in response to LACK stimulation only during the early phase of infection. This was shown by the finding that IFN-γ was produced in response to LACK only in patients who developed lesions within 30 days of the onset of infection. As LACK has been shown to induce an early immune response in L. major-infected mice (15), it is not surprising to find an early response to LACK in humans. Previous studies showed that patients with LCL due to Leishmania braziliensis who developed lesions in less than 60 days or with LCL due to Leishmania aethiopica responded to LACK by producing IFN-γ (4, 18); however, the results are heterogeneous and should be reevaluated in terms of time to lesion development, as was done in the present study. In this study and in a previous one, the IFN-γ response to LACK was found to be approximately 10 to 15% of that to SLA (668 ± 118 and 5,153 ± 1,413 pg/ml in response to LACK and SLA, respectively) (4). As LACK represents only 0.03 to 0.05% of the total protein (15), we consider responses to LACK rather than responses to SLA to be the predominant ones in humans.
When we analyzed the cellular source of IFN-γ produced in response to LACK and to SLA in stimulated, positively and negatively selected cells before antigen exposure in patients with leishmaniasis due to L. guyanensis, we found that the cells were memory CD45RA− CD4+ T cells. Our finding that IFN-γ-producing CD4+ T cells do not express the CCR7 molecule suggests that they are effector cells. It is not clear why CD4+ T cells that produce IFN-γ in response to LACK are no longer detectable in the blood 30 days after lesion development. They may die due to apoptosis or be recruited locally in the lesions to perform effector functions, as they do not express the CD62L molecules responsible for the migration of T cells to lymph nodes (3). Thus, these cells may provide an immediate antiparasitic function with a consequent local protection against leishmaniasis in the skin. In accordance with this hypothesis, memory CD4+ T cells occur more frequently than naive CD4+ T cells in lesions due to L. guyanensis (9). We are currently analyzing the expression of molecules, such as cutaneous lymphocyte antigen (11), on skin-homing T cells to analyze the homing of the LACK-specific CD4+ T cells.
Although LACK has been shown to induce IFN-γ in CD8+ T cells from persons who have never been exposed to Leishmania (7), we did not detect IFN-γ production in CD8+ T cells in LCL patients. In contrast, Maasho et al. described NK and CD8+ T cells that produced IFN-γ in response to LACK (17). The reasons for these findings are not understood, but we are currently analyzing IFN-γ-producing CD8+ T cells in a follow-up of naive subjects who were exposed to Leishmania in a rain forest.
In contrast to IFN-γ, IL-10 is produced in response to LACK regardless of the time to lesion development, but it is produced at very low levels in response to SLA stimulation. In healthy persons, IL-10 production in response to LACK was found to be due to a specific unique peptide (peptide aa 156-173) (7). In patients with LCL, regardless of the time of lesion development, cells that secrete IL-10 in response to LACK are CD4+ memory cells that are CD62L+ but CCR7−. Interestingly, the phenotype of these cells is identical to that of IL-10-producing T cells in naive subjects (7). The role of these IL-10-producing cells in the development and spread of infection is under analysis.
As previously described, IL-13 is produced by T cells in response to SLA (5) but not to LACK in LCL patients regardless of the time to lesion development. Cells producing IL-13 in response to SLA stimulation in LCL patients are memory CD4+ T cells that are CD62L+ and CCR7−. As IL-13 causes IL-12 unresponsiveness in specific CD4+ T cells that induce Th2 cell development in LCL patients (5), IL-13 may be responsible for the maintenance of infection (6).
As it has been clearly demonstrated that effector memory CCR7− CD4+ T cells produce cytokines such as IFN-γ, IL-4, and IL-5 (24), our finding that Th2 cytokines (IL-13 and IL-10) are produced by effector memory T cells that express the CD62L molecule while a Th1 cytokine (IFN-γ) is produced by effector memory T cells that do not express CD62L suggests that the expression of L-selectin (CD62L) may discriminate Th1 from Th2 cytokine effector memory CCR7− CD4+ T cells. This hypothesis has already been evaluated (13), but the conclusions are not clear.
Recently, some data from murine models have demonstrated that the onset of lesions relies on the recruitment of IFN-γ-secreting cells and suggested that counterinflammatory cytokines (e.g., IL-13, IL-10, and transforming growth factor β) may contribute to the healing process in the cutaneous site (2). There is no clear evidence of a similar situation in humans. However, the fact that high IFN-γ production in response to LACK is found only in LCL patients with early lesion development may suggest that this cytokine is detrimental and causes lesion development. Such a hypothesis is now being evaluated.
In conclusion, LACK can induce not only IFN-γ but also IL-10 in LCL patients during early infection (less than 30 days). Thus, LACK can be either a protective or an immunoregulatory antigen. Use of LACK as an antigen vaccine to induce a strong Th1 response is therefore questionable. However, effective protection can be induced with an adequate adjuvant which surpasses the IL-10 production induced by LACK. In addition to and in agreement with the results obtained with altered peptide ligands in L. major-infected BALB/c mice which rendered these susceptible mice resistant to infection (21) is the finding that the use of modified LACK in the IL-10 epitope (peptide aa 156-173) or of the LACK protein with this epitope deleted can be an alternative to LACK vaccination against Leishmania.
Acknowledgments
We are especially grateful to J. A. Louis (University of Lausanne, Lausanne, Switzerland) for helpful comments during this project and to R. M. Locksley for providing the LACK constructs.
The study was supported by grants from the Institut Pasteur and the French Ministry of Research (Programme de Recherche Fondamentale, Microbiologie Maladies Infectieuses et Parasitaires du Ministère de l'Enseignement Supérieur, de la Recherche et de la Technologie).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 3.Butcher, E. C., and L. J. Picker. 1996. Lymphocyte homing and homeostasis. Science 272:60-66. [DOI] [PubMed] [Google Scholar]
- 4.Bottrel, R. L. A., W. O. Dutra, F. A. Martins, B. Gontijo, E. Carvalho, M. Barral-Netto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourreau, E., G. Prévot, R. Pradinaud, and P. Launois. 2001. Interleukin (IL)-13 is the predominant Th2 cytokine in localized cutaneous leishmaniasis lesions and renders specific CD4+ T cells unresponsive to IL-12. J. Infect. Dis. 183:953-959. [DOI] [PubMed] [Google Scholar]
- 6.Bourreau, E., G. Prévot, R. Pradinaud, and P. Launois. 2001. Unresponsiveness of specific T cells to interleukin 12 is associated with active cutaneous leishmaniasis owing to Leishmania guyanensis. Scand. J. Immunol. 54:335-339. [DOI] [PubMed] [Google Scholar]
- 7.Bourreau, E., M. Collet, G. Prévot, G. Milon, D. Ashimoff, H. Hasegawa, C. Parra-Lopez, and P. Launois. 2002. IFN-γ producing CD45RA+ CD8+ and IL-10 producing CD45RA− CD4+ T cells generated in response to LACK in naive subjects never exposed to Leishmania. Eur. J. Immunol. 32:510-520. [DOI] [PubMed] [Google Scholar]
- 8.Da-Cruz, A. M., F. Conceicao-Silva, A. L. Bertho, and S. G. Coutinho. 1994. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect. Immun. 62:2614-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esterre, P., J. P. Dedet, C. Frenay, M. Chevallier, and J. A. Grimaud. 1992. Cell populations in the lesion of human cutaneous leishmaniasis: light microscopical, immunohistochemical and ultrastructural study. Virchows Arch. A 421:239-247. [DOI] [PubMed] [Google Scholar]
- 10.Field, J., J. Nikawa, D. Broek, B. MacDonald, L. Rodgers, I. A. Wilson, R. A. Lerner, and M. Wigler. 1988. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol. Cell. Biol. 8:2159-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuhlbrigge, R. C., J. D. Kieffer, D. Armerding, and T. S. Kupper. 1997. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 389:978-981. [DOI] [PubMed] [Google Scholar]
- 12.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanegane, H., Y. Kasahara, Y. Niida, A. Yachie, S. Sughii, K. Takatsu, N. Taniguchi, and T. Miyawaki. 1996. Expression of L-selectin (CD62L) discriminates Th1- and Th2-like cytokine-producing memory CD4+ T cells. Immunology 87:186-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp, K., T. G. Theander, L. Hviid, A. Garfar, A. Kharazami, and M. Kemp. 1999. Interferon gamma and tumor necrosis factor-alpha-producing cells in humans who are immune to cutaneous leishmaniasis. Scand. J. Immunol. 49:655-659. [DOI] [PubMed] [Google Scholar]
- 15.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by Vβ4 Vα8 CD4+ T cells instructs Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 16.Louis, J. A., E. Moedder, R. Behin, and H. D. Engers. 1985. A limiting dilution assay for quantifying Leishmania major in tissue of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 17.Maasho, K., I. Satti, S. Nylèn, G. Guzman, F. Koning, and H. Akuffo. 2000. A leishmania homologue of receptors for activated C-kinase (LACK) induces both interferon-γ and interleukin-10 in natural killer cells of healthy blood donors. J. Infect. Dis. 182:570-578. [DOI] [PubMed] [Google Scholar]
- 18.Maasho, K., D. Wolday, M. Edjigu, K. Soderstrom, S. Britton, and H. Akuffo. 2001. Induction and abrogation of LACK reactive cells in the evolution of human leishmaniasis. Clin. Exp. Immunol. 124:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mossmann, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 20.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppola, Z.-E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563-566. [DOI] [PubMed] [Google Scholar]
- 21.Pingel, S., P. Launois, D. J. Fowell, C. Turck, S. Southwood, A. Sette, N. Glaichenhaus, J. A. Louis, and R. M. Locksley. 1999. Altered ligands reveal limited plasticity in the T cell response to a pathogenic epitope. J. Exp. Med. 189:1111-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 23.Rocha, P. N., R. P. Almeida, O. Bacellar, A. R. de Jesus, D. C. Filho, A. C. Filho, A. Barral, R. L. Coffman, and E. M. Carvalho. 1999. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J. Infect. Dis. 180:1731-1734. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto, F., D. Lenig, R. Förster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Science 401:708-712. [DOI] [PubMed] [Google Scholar]