Abstract
The presynaptic protein α-synuclein, implicated in Parkinson disease (PD), binds phospholipids and has a role in brain fatty acid (FA) metabolism. In mice lacking α-synuclein (Snca−/−), total brain steady-state mass of the mitochondria-specific phospholipid, cardiolipin, is reduced 22% and its acyl side chains show a 51% increase in saturated FAs and a 25% reduction in essential n-6, but not n-3, polyunsaturated FAs. Additionally, 23% reduction in phosphatidylglycerol content, the immediate biosynthetic precursor of cardiolipin, was observed without alterations in the content of other brain phospholipids. Consistent with these changes, more ordered lipid head group and acyl chain packing with enhanced rotational motion of diphenylhexatriene (DPH) about its long axis were demonstrated in time-resolved DPH fluorescence lifetime experiments. These abnormalities in mitochondrial membrane properties were associated with a 15% reduction in linked complex I/III activity of the electron transport chain, without reductions in mitochondrial number, complex II/III activity, or individual complex I, II, III, or IV activity. Reduced complex I activity is thought to be a critical factor in the development of PD. Thus, altered membrane composition and structure and impaired complex I/III function in Snca−/− brain suggest a relationship between α-synuclein's role in brain lipid metabolism, mitochondrial function, and PD.
α-Synuclein is a 140-amino-acid protein of unknown function that is most highly expressed in the central nervous system (CNS) and is particularly enriched in presynaptic nerve terminals. Mutation or overexpression of the human α-synuclein gene (Snca) causes early-onset autosomal dominant Parkinson disease (PD). The α-synuclein protein is a major component of Lewy bodies, the cytoplasmic protein aggregates pathognomonic for PD, as well as the protein aggregates seen in axons and dendrites of PD patients, referred to as Lewy neurites (as reviewed in reference 17). However, the mechanisms by which an abnormality in structure or expression of α-synuclein causes PD have not been elucidated, nor is it known if these pathognomonic aggregates play a toxic, protective, or coincidental role in the pathogenesis of the disease.
Although natively unfolded in solution, the α-synuclein protein has an amino-terminal domain containing seven 11-amino-acid repeat motifs similar to class A2 exchangeable apolipoproteins and, also like these apolipoproteins, adopts an amphipathic α-helical structure when it binds acidic phospholipid-containing membranes (as reviewed in reference 11). Class A2 apolipoproteins function by transient association and transport of lipid in the bloodstream, by regulating various lipid metabolic enzymes, and are thought to stabilize lipid membranes (as reviewed in reference 64). These structural similarities suggest that α-synuclein could serve as an intracellular functional equivalent of apolipoproteins in the brain and so similarly may function in binding and intracellular transport of lipids, stabilizing membranes, and regulating lipid metabolism. Results to support this hypothesis include the observations that α-synuclein alters both phospholipase C and D activities in vitro, while its inhibitory effect on phospholipase D may be regulated by α-synuclein phosphorylation (31, 49, 52). Additionally, α-synuclein binds to phospholipid vesicles containing stored triglycerides in cultured cells and protects them from hydrolysis (12), suggesting that α-synuclein may have a role in stabilizing lipid membrane. Possible involvement of α-synuclein in lipid metabolism was also suggested by experiments demonstrating that α-synuclein may have fatty acid (FA) binding activity and that Snca−/− mice have decreased cytosolic polyunsaturated FA (PUFA) content, while α-synuclein overexpression in cultured neurons results in increased PUFA content (68, 69). Furthermore, PUFA and saturated FA (SFA) uptake kinetics and their incorporation into phospholipids are reduced in astrocytes cultured from Snca−/− mice, while SFA uptake into the brain and incorporation into brain lipids in vivo are reduced in Snca−/− mice (8, 25), also consistent with a function for α-synuclein in brain lipid metabolism.
In an attempt to elucidate further the normal function of α-synuclein, we and others have reported studies on mice lacking α-synuclein and have found that Snca−/− mice lack an overt neurological phenotype (1, 7, 10). Given this relationship between α-synuclein, FA uptake and distribution, phospholipid association, and lipid metabolism, we undertook an in-depth reexamination of the physiological consequences of loss of Snca function. We found Snca−/− mice have reduced total brain cardiolipin content with an altered acyl side chain composition characterized by decreased n-6 PUFAs and increased SFAs without alterations in n-3 PUFA and monounsaturated FA (MUFA) composition. Brain content of other phospholipids was unchanged with the exception of a precursor of cardiolipin, phosphatidylglycerol (PtdGro), which was also reduced. Changes in phospholipid content and composition correlated with more ordered lipid head group and acyl chain packing in nonsynaptic mitochondrial membranes, along with similar changes in synaptosomal membranes. Since cardiolipin is a mitochondria-specific phospholipid required for assembly and function of the electron transport chain (ETC) (51, 67), we examined ETC function of whole brain lysates and observed a reduction of complex I activity only when measured by linked assay to complex III. Given that abnormalities in complex I have been implicated in the pathogenesis of PD, a physiological connection between α-synuclein, mitochondrial lipid composition and structure, and ETC function points to a previously unrecognized, pathogenic pathway that may be involved in this disorder.
MATERIALS AND METHODS
Mice.
Age- and sex-matched wild-type (WT) and Snca-ablated inbred 129S6/SvEv mice (7) aged 3 to 6 months were used in all experiments. The diet contained 5.5% fat, including 2.5% PUFA with an n-6 to n-3 ratio of 4:1. Control Snca+/+ and gene-ablated Snca−/− mice were derived by crossing littermates of the appropriate genotypes derived from Snca+/− × Snca+/− intercrosses. Animals were euthanized according to the guidelines “Public Health Service Policy on Humane Care and Use of Laboratory Animals” approved by the NHGRI Institutional Animal Care and Use Committee or the IACUC at the University of North Dakota (protocol number 0110-1).
Enzyme activity assays.
Assays were performed in two laboratories (F. Scaglia and R. L. Nussbaum) with slightly different procedures. In the Scaglia laboratory, homogenates were prepared from liquid N2-frozen mouse brain in nine volumes of buffer (150 mM sucrose, 10 mM Tris-HCl [pH 7.45], and 2 mM EDTA) using a motorized Glas-Col homogenizer with Teflon pestle and centrifuged at 700 × g for 20 min, and the supernatant was assayed immediately. A temperature-controlled Pharmacia spectrophotometer was used for all assays. Enzyme complexes were assayed in triplicate for five mice per genotype using standard spectrophotometric analyses as follows. Succinate cytochrome c reductase was assayed in buffer (55 mM potassium phosphate [pH 7.4], 3 mM succinate, 0.5 mM KCN, and 0.1 mM cytochrome c) by following the reduction of cytochrome c at 550 nm (34). Succinate dehydrogenase was assayed in reaction buffer (50 mM potassium phosphate [pH 7], 16 mM succinate, 1.5 mM KCN, and 0.003% 2,6-dichloroindophenol [2,6-DCIP]) by following the reduction of 2,6-DCIP at 600 nm (34). The reaction was initiated by addition of DCIP. Cytochrome c oxidase was assayed in buffer (9 mM potassium phosphate [pH 7] and 0.1% reduced cytochrome c [prepared with 5% sodium hydroxysulfite]) by monitoring oxidation of cytochrome c at 550 nm (77). Citrate synthase was assayed at 30°C in buffer (0.1 mM 5,5′-dithio-bis-2-nitrobenzoic acid [DTNB], 0.3 mM acetyl coenzyme A [CoA], and 0.5 mM oxaloacetate) and absorbance at 412 nm was monitored (72). For fumarase assays, homogenates were prepared from mouse brain in nine volumes of buffer (250 mM sucrose, 5 mM Tris-HCl [pH 7.4], and 1 mM EDTA) as outlined above and centrifuged at 600 × g for 10 min, the supernatant was centrifuged at 14,000 × g for 10 min, and the pellet was assayed immediately. The fumarase reaction was initiated with disodium malate and assayed at 30°C in buffer (50 mM sodium phosphate [pH 7.6] and 50 mM disodium malate) by monitoring an increase in absorbance at 240 nm (30). Enzyme activities were calculated as μmol/min/g (wet weight) of brain tissue.
In the Nussbaum laboratory, enzyme assays were performed on a temperature-controlled Cary50 UV/Vis spectrophotometer (Varian) using a “crude mitochondrial fraction” isolated from liquid N2-frozen mouse brain (36) that was freeze-thawed three times to allow substrate access to the inner mitochondrial membrane. Protein concentration was determined using the BCA protein assay kit (Pierce) with bovine serum albumin (BSA) as a standard. NADH oxidase and NADH ubiquinone oxidoreductase activities were measured in quadruplicate on four pairs of WT and Snca−/− animals. Enzyme activities were measured by determining the decrease in concentration of NADH spectrophotometrically at 340 nm. The NADH oxidase assay was performed in reaction buffer (25 mM potassium phosphate [pH 7.4], 2 mM NaN3, 5 mM EDTA, and 0.3 mM K2NADH [Sigma N4505]) without detergent (51). The reaction was initiated with ∼150 μg of “crude mitochondrial” protein. The NADH-ubiquinone oxidoreductase assay was performed in buffer (25 mM potassium phosphate [pH 7.4], 2 mM KCN, 5 mM MgCl2, 2.5 mg/ml BSA, 2 μM antimycin, 100 μM decyl-ubiquinone [Sigma D7911], and 0.3 mM K2NADH) without detergent (5, 35). The reaction was initiated with ∼50 μg of protein, and specific activities were determined from the difference in slopes of the linear portion of assays performed in the absence or presence of 1 μM rotenone. NADH cytochrome c reductase activity was assayed in quadruplicate from four pairs of mice with reaction buffer (50 mM potassium phosphate [pH 7.4], 105 μM NADH, 2 mM KCN, 1 mM EDTA, and 32 μM cytochrome c) by following the reduction of cytochrome c at 550 nm (5, 18). The reaction was initiated by addition of ∼40 μg of protein. Rotenone-sensitive NADH cytochrome c reductase activity was then calculated. Ubiquinol-cytochrome c reductase was assayed in quadruplicate using three pairs of mice and reaction buffer (25 mM potassium phosphate [pH 7.4], 1 mM EDTA, 1 mM KCN, 16 μM cytochrome c, 28 μM decyl-ubiquinol, and 0.6 mM dodecylmaltoside) by following the reduction of cytochrome c at 550 nm (5, 18). The absorption was monitored after addition of decyl-ubiquinol for 1 min, and the reaction was initiated with ∼2.5 μg of protein. Activity was calculated by determining the difference in slopes of the linear portion of assays performed in the absence or presence of 2 μM antimycin. Decyl-ubiquinol was prepared by reducing decyl-ubiquinone with sodium dithionite (58). Citrate synthase was assayed at 25°C as described above (72). Enzyme activities were calculated as nmol/min/mg of protein.
Lipid extraction, separation, and quantification.
Brain lipids were extracted quantitatively from pulverized frozen brain with hexane-2-propanol (3:2, vol/vol) (28) and phospholipids, including cardiolipin, separated by thin-layer chromatography (TLC) on Whatman LK6 silica gel 60 plates using chloroform-methanol-acetic acid-water (55:37.5:3:2 by volume) (32). Chromatographic analysis revealed no evidence for oxidation and breakdown of PUFAs during sample preparation. Cardiolipin and other phospholipids were quantified by lipid phosphorus analysis (62).
Phospholipid FA composition was determined using a base-catalyzed transesterification, converting the phospholipid acyl chains to fatty acid methyl esters (FAME) (48). FAME were separated using a trace gas chromatograph (Thermo Finnigan) equipped with an SP-2330 capillary column (0.32-mm inner diameter by 30-m length; Supelco) and quantified using flame ionization detection using an internal standard method. Detector response was linear within the sample concentration range for all FAs. Data were collected using an analog-to-digital interface and converted to peak area using ChromQuest (Thermo Finnigan).
Kinetic analysis.
Surgery and [1-14C]16:0 or [1-14C]20:4 n-6 (Moravek Biochemical) infusion was performed on seven and five pairs of male mice, respectively, as described previously (47). Briefly, fasted mice (25 to 30 g) were anesthetized with halothane (1 to 3%), and PE-10 catheters were inserted into the femoral artery and vein. Using an infusion pump (BS-8000; Braintree Scientific, Inc.), awake mice were infused with ∼170 μCi/kg of body weight of [1-14C]16:0 or [1-14C]20:4 n-6, solubilized in 5 mM HEPES (pH 7.4) buffer containing 50 mg/ml “essentially FA-free” BSA (Sigma) into the femoral vein over 10 min at a constant rate of 30 μl/min to achieve steady-state plasma radioactivity. Prior to and during infusion, arterial blood samples (∼20 μl) were taken to determine plasma radioactivity. Following infusion, each mouse was sacrificed using pentobarbital (100 mg/kg, intravenously) and immediately subjected to head-focused microwave radiation (2.8 kW, 1.35 s; Cober Electronics Inc.). The whole brain was removed, frozen in liquid nitrogen, and pulverized under liquid nitrogen temperatures to homogeneity.
Lipids from tissue, plasma, and blood were extracted using a two-phase extraction procedure (24). Radioactivity in the aqueous and organic fractions was determined by liquid scintillation counting using a Beckman LS5000 CE liquid scintillation counter. Extracts were concentrated with N2 at 40°C and dissolved in n-hexane-2-propanol-water (56.7:37.8:5.5 by volume).
Cardiolipin was separated from brain phospholipids, and cardiolipin radioactivity was determined by liquid scintillation counting. Plasma neutral lipids were separated by TLC on heat-activated Whatman silica gel 60 plates (20 by 20 cm; 250 μm) and developed in petroleum ether-diethyl ether-acetic acid (75:25:1.3 by volume) (40). Radioactivity of the free FA fraction was determined by liquid scintillation counting. Unesterified plasma FA mass was determined by gas-liquid chromatography after TLC separation and conversion to FAME using an acid-catalyzed esterification (2).
Acyl-CoA from brain was extracted and purified using a solid-phase extraction procedure and separated by high-performance liquid chromatography on a C18 (2) column (Luna; Phenomenex) (26). The eluent was monitored at 260 nm using a Beckman 166 UV/Vis detector. The 16:0-CoA mass was determined using a standard curve from commercially purchased standards (Sigma), and 17:0-CoA was the internal standard. The 16:0-CoA radioactivity was determined by liquid scintillation counting.
This study was performed under steady-state conditions, and calculations were performed as described elsewhere (25, 59-61).
Fluorescence measurements.
Synaptosomes and nonsynaptic mitochondria were isolated from a “crude mitochondrial fraction” from liquid N2-frozen mouse brain, utilizing a 5.4 to 10% (wt/wt) discontinuous Ficoll gradient, essentially as described previously (36). Preparation purity and yield were confirmed by citrate synthase assay and Western analysis using antibodies against the 39-kDa subunit of complex I, core II protein (complex III), and cytochrome c oxidase subunit IV of complex IV. The crude mitochondrial fraction contained ∼95% of the mitochondria present in whole mouse brain. Mitochondrial content in synaptosomes was similar to that of the crude mitochondrial fraction, while nonsynaptic mitochondria were three- to fourfold enriched over the crude mitochondrial fraction. Additionally, no protein composition differences were observed in synaptosomes, based on use of various mitochondrial and synaptic protein markers (7) (data not shown), or nonsynaptic mitochondria, using mitochondrial markers (data not shown), in Snca−/− mice compared to controls. All analyses were performed in at least triplicate on fractions isolated from three (crude mitochondrial fraction referred to as “crude brain lysate” in the text) or six (synaptosome and nonsynaptic mitochondria) pairs of mice. The repeated measurements on each individual sample from a single animal were averaged, and the means of these averaged measurements were compared between the two genotypes. Membrane fractions were suspended in buffer [60 mM KCl, 30 mM NaCl, 10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 7.0)] at a phospholipid concentration of 0.1 mM, as determined previously (62). 1,6-Diphenyl-1,3,5-hexatriene (DPH; Molecular Probes) dissolved in THF was added to membrane suspensions at a phospholipid-to-DPH ratio of 300 to 1. Fluorescence lifetime and differential polarization measurements were performed at 37°C with a K2 multifrequency cross-correlation phase fluorometer (ISS) with excitation at 351 nm by an Innova 307 argon ion laser (Coherent) as described elsewhere (46). Total fluorescence intensity decays were analyzed with the sum of two exponential decays, and the intensity-weighted average lifetime, τ, is reported. Fluorescence anisotropy decays were analyzed using the Brownian rotational diffusion model as reported previously (46). In this model, DPH motion is characterized by Drot, the diffusion constant for rotation about the long axis of DPH. The orientational freedom of DPH was quantified using the relative free volume parameter ƒv, calculated from the DPH angular distribution function, ƒ(θ) (46). Fluorescence anisotropy decays were also analyzed using an empirical sum-of-three-exponentials model, and the weighted average correlation time, φ, is reported.
Real-time PCR.
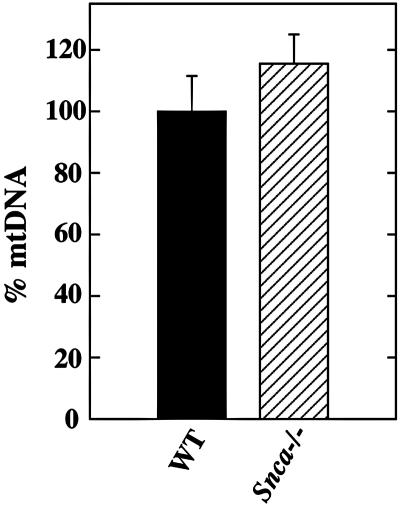
Genomic DNA was isolated from mouse brain by standard methods (45). DNA from five mice per genotype was analyzed in triplicate by real-time PCR using the ABI Prism 7000 sequence detection system (Applied Biosystems) and the Invitrogen Platinum SYBR green kit (11733-046) according to the manufacturers' standard instructions. Primers specific for mitochondrial DNA (mtDNA) (cytochrome c oxidase subunit I) and nuclear DNA (H19) were utilized as published previously (9). Dissociation curves were plotted for each primer pair, confirming a single amplification product. Additionally, relative efficiencies of both sets of primers, calculated according to standard guidelines (ABI Prism user bulletin 2), were determined to be equivalent (absolute value of <0.01). Results shown were determined by calculating the average ΔCt (mitochondria normalized to nuclear) for each mouse using mean Ct values obtained from triplicate amplifications, and the average change in normalized mtDNA content was calculated (2ave. ΔCt). The mean of this value was determined for each genotype and plotted as the percent mtDNA ± the standard error of the mean in Snca−/− mice relative to controls (arbitrarily set to 100%).
BN-PAGE, 2D-BN/SDS-PAGE, and Western analysis.
Samples were prepared and separated by blue native-polyacrylamide gel electrophoresis (BN-PAGE) as described previously (33), except mitochondria were purified from mouse brain using a “crude mitochondrial fraction” isolation procedure (36). Briefly, the mitochondrial pellets that were frozen previously at −80°C were suspended in sample buffer (1 M aminocaproic acid, 50 mM bis-Tris-HCl, and Sigma protease cocktail [P8340]), and supernatant was obtained after dissolving membrane proteins in dodecylmaltoside (Sigma) as described elsewhere (33). Protein was quantified using the BCA protein assay kit (Pierce) with BSA as a standard. Coomassie brilliant blue G-250 (Sigma) was added to the samples to a final concentration of 0.36%. Samples were separated by either BN-PAGE (7%) or two-dimensional (2D) BN/sodium dodecyl sulfate (SDS)-PAGE using 7 and 12% gels, respectively, and transferred to polyvinylidene difluoride (PVDF) membrane (performed by Kendrick Labs Inc.). Membranes were blocked in 5% nonfat milk-PBS-Tween (PBS-T) and immunoblotted using complex I (39, 30, 20, and/or 17 kDa) or III (core II) mouse monoclonal antibodies (Molecular Probes), and protein was detected by enhanced chemiluminescence (Amersham). Laser densitometry was performed on multiple exposures of several experiments and analyzed using ImageQuant 5.2 software (Molecular Dynamics).
Statistical analyses.
Significance testing of differences between means was performed using Student's t test as implemented in SSP.1 software (http://www.economics.pomona.edu/StatSite/SSP.html) under the assumption of unequal population variances. Correction for multiple hypothesis testing was according to the conservative Bonferroni correction (E. W. Weisstein, MathWorld [http://mathworld.wolfram.com/BonferroniCorrection.html]).
RESULTS
Steady-state brain lipid measurements.
Lipids were isolated and quantified from brains of six age- and sex-matched pairs of WT and Snca−/− mice fed ad libitum a diet containing 5.5% total fat with 2.5% PUFA at an n-6 to n-3 ratio of 4:1. Total brain lipid membrane mass and the levels of all major classes of phospholipids (Table 1) were unchanged in Snca−/− mice compared to controls, with the notable exception of cardiolipin (Ptd2Gro), which was reduced 22% as measured by mass of phosphorus in cardiolipin. This 22% reduction in total cardiolipin mass represented a 28% reduction in the mole percentage of cardiolipin relative to total membrane lipids and correlated well with a 28% reduction in the fraction of total brain FA contained in cardiolipin in Snca−/− brain compared to controls (data not shown). The decrease in cardiolipin was not due to a reduction in brain mitochondrial content, since the amount of mtDNA relative to nuclear DNA, used as a surrogate measure of mitochondrial number, was not different between WT and Snca−/− brains (Fig. 1). The steady-state mass of PtdGro, a precursor of cardiolipin, was reduced 23%, representing a 28% reduction in its mole percent relative to other membrane lipids. Although this reduction did not reach statistical significance due to measurement variability, likely reflecting the difficulty in measuring a low-abundance biosynthetic intermediate, lipids were isolated and quantified from a second cohort of mice maintained in a separate laboratory (E. J. Murphy). These data not only confirmed the significant reduction in cardiolipin content but also had a 25% reduction in PtdGro mass that did reach statistical significance (P = 0.0334) without changes in other major phospholipids.
TABLE 1.
Cardiolipin content is reduced in Snca−/− brain in the absence of changes in other major phospholipids and sphingolipids
| Lipid | Lipid content (nmol/g) ± SD
|
P valuea | |
|---|---|---|---|
| WT (n = 6) | Snca−/− (n = 6) | ||
| Cardiolipin | 995 ± 76 | 776 ± 87 | 0.001b |
| Phosphatidylglycerol | 544 ± 191 | 419 ± 169 | 0.26c |
| Phosphatidic acid | 664 ± 156 | 601 ± 211 | 0.57c |
| Phosphatidylethanolamine | 17,502 ± 3,760 | 19,955 ± 3,289 | 0.26c |
| Phosphatidylinositol | 2,067 ± 111 | 2,250 ± 242 | 0.14c |
| Phosphatidylserine | 5,577 ± 1,240 | 6,061 ± 1,149 | 0.50c |
| Phosphatidylcholine | 23,338 ± 1,937 | 24,945 ± 1,345 | 0.13c |
| Sphingomyelin | 6,208 ± 830 | 6,040 ± 744 | 0.72c |
| Total | 56,895 ± 5,666 | 61,048 ± 3,933 | 0.17c |
Two-sided t test, unequal population variance.
Statistical significance at the P < 0.00625 level, corresponding to a nominal significance of P < 0.05 under a conservative Bonferroni correction for multiple hypothesis testing involving eight phospholipid classes.
Not significant (P > 0.00625).
FIG. 1.
The amount of mtDNA is not altered in Snca−/− mouse brain. Real-time PCR was performed in triplicate on DNA isolated from WT (n = 5) and Snca−/− (n = 5) mouse brains using primers specific for nuclear and mtDNA as outlined in Materials and Methods. Results shown are plotted as percent mtDNA ± the standard error of the mean in Snca−/− relative to control (arbitrarily set to 100%). No significant difference was observed between the two genotypes (Student's t test).
We then analyzed the acyl side chain composition of cardiolipin and other phospholipids in Snca−/− mice. Phosphatidylethanolamine and phosphatidylinositol had statistically significant decreases in n-3 PUFA composition, while n-3 PUFA content of phosphatidylcholine and phosphatidylserine were also reduced but did not reach significance (summarized in Table 2, with details published as supporting information in Table S1 in the supplemental material). Additionally, total MUFA content was reduced in phosphatidylcholine only. The reduction in phospholipid n-3 PUFA, specifically 22:6 n-3, is consistent with its reduced incorporation into several major phospholipids in cultured astrocytes isolated from Snca−/− mice (8) and in additional reports (68, 69). We did not, however, observe similar changes in n-3 PUFA composition in cardiolipin. Instead, we observed a 25% reduction in the mole percentage of n-6 PUFA and a 51% increase of SFA side chains in cardiolipin in Snca−/− mice, while total n-3 PUFAs and MUFAs were unchanged. Changes in n-6 PUFAs were due primarily to reductions in several less abundant n-6 PUFAs in brain cardiolipin, such as osmondic (22:5 n-6), linoleic (18:2 n-6), and dihomo-γ-linoleic (20:3 n-6) acids, while the increase in SFA was due primarily to an increase in the most abundant SFA in cardiolipin, stearic acid (18:0), although palmitic acid (16:0) was also increased significantly. The reduction in steady-state n-6 PUFA in cardiolipin occurred despite the fact that mice were maintained on a typical mouse chow containing a high PUFA content. Thus, we conclude that ablation of α-synuclein has a distinct effect on the steady-state content and acyl side chain composition of brain cardiolipin compared to other phospholipids.
TABLE 2.
Acyl side chain content of brain phospholipids from WT and Snca−/− mice
| Phospholipid | Fatty acid | Side chain content (mol% ± SD)
|
P valuea | |
|---|---|---|---|---|
| WTd | Snca−/−e | |||
| Cardiolipin | Total SFA | 20.2 ± 3.2 | 30.6 ± 2.6 | <0.0001b |
| Total MUFA | 25.6 ± 1.5 | 23.2 ± 2.0 | 0.04c | |
| Total n-3 PUFA | 23.5 ± 1.6 | 23.3 ± 3.9 | 0.91c | |
| Total n-6 PUFA | 30.7 ± 3.0 | 23.0 ± 0.8 | <0.0001b | |
| Phosphatidylcholine | Total SFA | 62.2 ± 1.8 | 56.7 ± 4.5 | 0.087c |
| Total MUFA | 20.9 ± 0.8 | 27.7 ± 1.0 | <0.0001b | |
| Total n-3 PUFA | 7.5 ± 0.5 | 6.2 ± 0.8 | 0.038c | |
| Total n-6 PUFA | 9.6 ± 0.4 | 9.5 ± 1.35 | 0.89c | |
| Phosphatidylethanolamine | Total SFA | 24.6 ± 0.4 | 27.1 ± 1.0 | 0.011b |
| Total MUFA | 16.7 ± 1.6 | 17.1 ± 0.6 | 0.63c | |
| Total n-3 PUFA | 37.0 ± 1.5 | 32.7 ± 1.7 | 0.007b | |
| Total n-6 PUFA | 21.6 ± 1.0 | 23.3 ± 0.5 | 0.016c | |
| Phosphatidylserine | Total SFA | 44.2 ± 1.2 | 45.9 ± 1.6 | 0.13c |
| Total MUFA | 16.4 ± 0.5 | 16.8 ± 3.0 | 0.81c | |
| Total n-3 PUFA | 32.7 ± 0.6 | 28.7 ± 2.8 | 0.062c | |
| Total n-6 PUFA | 6.5 ± 0.26 | 7.4 ± 0.72 | 0.083c | |
| Phosphatidylinositol | Total SFA | 42.4 ± 1.2 | 44.5 ± 2.2 | 0.15c |
| Total MUFA | 10.7 ± 0.95 | 12.8 ± 1.5 | 0.06c | |
| Total n-3 PUFA | 6.3 ± 0.51 | 4.6 ± 0.73 | 0.01b | |
| Total n-6 PUFA | 40.8 ± 1.8 | 37.9 ± 2.8 | 0.13c | |
Nominal P value under two-sided t test (not assuming equal population variances).
Indicates statistical significance at the P < 0.0125 level, corresponding to a nominal significance of P < 0.05 under a conservative Bonferroni correction for multiple hypothesis testing for each of the four types of fatty acids.
Indicates not significant (P > 0.0125).
For cardiolipin, n = 6 WT mice; n = 5 WT mice for other phospholipids.
For Snca−/− mice, n = 6 for cardiolipin and n = 4 for the other phospholipids.
Uptake and turnover kinetics of FAs in brain cardiolipin.
SFA and n-6 PUFA uptake and turnover kinetics into brain cardiolipin were then assessed in awake WT or Snca−/− mice infused intravenously with [1-14C]palmitic acid (16:0) or [1-14C]arachidonic acid (20:4 n-6) (Table 3). The incorporation rate of 16:0-CoA and 20:4 n-6-CoA into brain cardiolipin from infused 16:0 and 20:4 n-6 was decreased 52% and 46%, respectively, while fractional turnover of 16:0 and 20:4 n-6 was diminished 52% and 44% in Snca−/− versus controls, consistent with 84% and 86% increases, respectively, in half-lives of the two FAs in cardiolipin. We conclude that loss of α-synuclein expression in brain is associated with a profound reduction in the incorporation of exogenously supplied SFA and n-6 PUFA as well as in their turnover in cardiolipin. This difference between the kinetic measurements, in which both SFA and n-6 PUFA incorporation into cardiolipin were reduced following FA infusion, and the steady-state levels of SFA and n-6 PUFA in cardiolipin, where SFA was increased markedly while n-6 PUFA was reduced, is consistent with the dominant role that de novo synthesis plays in providing SFAs to the brain, in contrast to the situation with PUFAs, which are essential dietary nutrients that cannot be synthesized endogenously and must be transported into the brain of rodents and other mammals from the bloodstream (reviewed in reference 74).
TABLE 3.
Reduced incorporation and turnover of palmitic and arachidonic acids into brain cardiolipin in mice lacking α-synuclein
| Parameter and FA | Mean response ± SD
|
% Change | P value | |
|---|---|---|---|---|
| WT | Snca−/− | |||
| Incorporation rate (nmol/h) | ||||
| Palmitic acid (16:0)a | 4.4 ± 2.3 | 2.1 ± 1.4 | −52 | 0.048 |
| Arachidonic acid (20:4 n-6)b | 76.7 ± 20.0 | 41.5 ± 12.7 | −46 | 0.013 |
| Fractional turnover (% h) | ||||
| Palmitic acid (16:0)a | 7.1 ± 2.7 | 3.4 ± 2.1 | −52 | 0.015 |
| Arachidonic acid (20:4 n-6)b | 54.1 ± 14.2 | 28.0 ± 7.3 | −48 | 0.010 |
| Half-life (h) | ||||
| Palmitic acid (16:0)a | 11.6 ± 5.3 | 21.3 ± 10.0 | 84 | 0.049 |
| Arachidonic acid (20:4 n-6)b | 1.4 ± 0.4 | 2.6 ± 0.7 | 86 | 0.014 |
Studies performed with seven pairs of mice.
Studies performed with five pairs of mice.
Lipid head group and acyl chain packing in mitochondrial and other membranes.
Next, we examined Snca−/− mice for consequences of these lipid composition alterations on the structural properties of brain membranes, including those in mitochondria. Previous experiments performed using model membranes indicated that decreasing cardiolipin content results in an increased order of both the lipid head groups and acyl side chains of membranes, while decreasing the PUFA/SFA ratio in the hydrophobic core of membranes results in increased order and thereby decreased fluidity of the acyl side chains only (43, 46). Rather than using pyrene-labeled probes, which are limited to measuring lateral diffusion in membranes of a crude Snca−/− brain preparation (68), we used time-resolved DPH fluorescence lifetime measurements to determine whether changes in steady-state levels and acyl side chain composition of cardiolipin affect membrane acyl chain packing and dynamics as well as lipid head group order in brain fractions enriched for mitochondria. We examined the physical properties of three membrane fractions: a fraction enriched highly for nonsynaptic mitochondria, a synaptosomal membrane preparation rich in synaptic mitochondria, and a crude brain lysate (Table 4). The fraction most highly enriched for mitochondria, the nonsynaptic mitochondrial fraction, showed a 14% increase in fluorescent lifetime of DPH at 37°C in Snca−/− versus control mice, indicating that water is less able to penetrate this membrane and suggesting that the lipid head group packing is tighter in these Snca−/− mitochondrial membranes. There were also significant reductions in the relative free volume (fv), an acyl chain packing parameter, in all three membrane preparations from Snca−/− brains at 37°C, indicating that the equilibrium orientation of DPH is more constrained and that acyl chains are more tightly packed throughout the hydrophobic core of the lipid bilayer. In addition to the increase in acyl chain packing in Snca−/− membrane, the rotational diffusion coefficient, Drot, derived from the Brownian rotational diffusion or P2-P4 model of DPH fluorescent probe behavior, was increased 78% and 43% in Snca−/− synaptosome and nonsynaptic mitochondrial membranes, respectively, indicating a more rapid rotation of DPH about its long axis. This increase was confirmed in both membranes by ∼16% decreases in the rotational correlation time, φ, a model-independent but less sensitive characterization of generalized DPH rotation. So, although fv was reduced, indicating a significant decrease in inner membrane fluidity, DPH rotational movement about its axis was actually less constrained in both synaptosome and nonsynaptic mitochondrial membranes isolated from Snca−/− animals. Interestingly, this combination of decreased membrane fluidity and increased rotational motion has been observed in membranes with a reduced amount of n-6 PUFAs relative to n-3 PUFAs and SFAs (46), precisely what was observed in cardiolipin from Snca−/− mice. Thus, significant increases of both lipid head group and acyl chain order were observed only in the most highly enriched mitochondrial fraction and the changes are consistent with abnormalities in steady-state content and acyl chain composition of the mitochondria-specific phospholipid, cardiolipin. Furthermore, these changes in the biophysical properties of membranes in the synaptosome and crude brain fractions, both of which are complex mixtures of many types of cellular membranes, indicate that mitochondrial membranes are not the only membranes altered in these mice and is consistent with steady-state and kinetic data that show abnormalities in FA composition, incorporation, and turnover of acyl side chains in phospholipids in addition to cardiolipin (25) (Table 2). These data suggest that α-synuclein may function in the production and/or proper maintenance of brain membranes, including synaptic and mitochondrial membranes.
TABLE 4.
Membrane order is altered in Snca−/− brain fractions as determined by time-resolved DPH lifetime measurements
| Parameter and fraction analyzed | WTe | Snca−/−e | % Change | P value |
|---|---|---|---|---|
| DPH fluorescence lifetime (τ), ns | ||||
| Crude brain lysatea | 10.46 ± 0.05 | 10.51 ± 0.07 | 0.5 | 0.37d |
| Synaptosomesb | 9.53 ± 0.32 | 9.76 ± 0.07 | 2 | 0.14d |
| Nonsynaptic mitochondriab | 7.50 ± 0.19 | 8.08 ± 0.15 | 14 | 0.0002c |
| Relative free volume (fv, acyl chain packing parameter) | ||||
| Crude brain lysatea | 0.064 ± 0.002 | 0.048 ± 0.002 | −25 | 0.0006c |
| Synaptosomesb | 0.045 ± 0.001 | 0.037 ± 0.001 | −18 | 7 × 10−8c |
| Nonsynaptic mitochondriab | 0.065 ± 0.002 | 0.052 ± 0.001 | −20 | 10−6c |
| Rotational diffusion coefficient for DPH (Drot), 1/ns | ||||
| Crude brain lysatea | 0.092 ± 0.001 | 0.092 ± 0.003 | 0 | 1.0d |
| Synaptosomesb | 0.069 ± 0.002 | 0.123 ± 0.007 | 78 | 2 × 10−6c |
| Nonsynaptic mitochondriab | 0.103 ± 0.004 | 0.147 ± 0.003 | 43 | 3 × 10−9c |
| Avg rotational correlation time for DPH (φ), ns | ||||
| Crude brain lysatea | 1.21 ± 0.03 | 1.32 ± 0.04 | 9 | 0.02d |
| Synaptosomesb | 1.77 ± 0.03 | 1.48 ± 0.04 | −16 | 10−7c |
| Nonsynaptic mitochondriab | 1.97 ± 0.16 | 1.67 ± 0.06 | −15 | 0.0044c |
Studies performed with three pairs of mice.
Studies performed with six pairs of mice.
Significant at the P < 0.01 level.
Not significant (P ≥ 0.01).
Values are means ± SEM.
ETC function in Snca−/− mice.
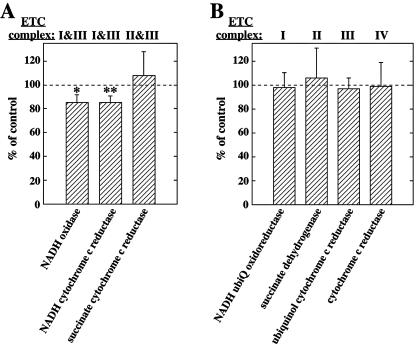
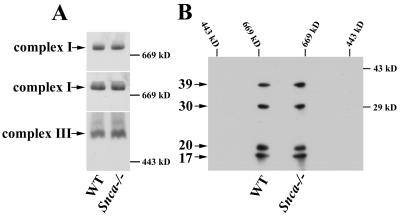
Because cardiolipin, a phospholipid specific to the inner mitochondrial membrane, is important for efficient oxidative phosphorylation (OxPhos) in yeast and mammals (51, 67), we next asked whether the lipid membrane abnormalities observed in Snca−/− mice have physiological consequences on mitochondrial function. Linked enzyme activity assays that require flow of reducing equivalents from NADH or FADH2 in complexes I and II, respectively, to cytochrome c in complex III were performed on crude Snca−/− mouse brain lysates, prepared by a gentle freeze-thaw technique that allowed access of substrates to the inner mitochondrial membrane without disrupting the capacity of membranes to transfer electrons between the various complexes. The results are plotted as a percentage of WT control (Fig. 2A). Statistically significant 15% reductions of NADH oxidase and NADH cytochrome c reductase activities, two different but related linked assays specific for complex I to III, were observed; however, succinate cytochrome c reductase activity, a measure of complex II to III, was unchanged. Assays for citrate synthase or fumarase, Kreb cycle enzymes serving as mitochondrial matrix markers, were also unchanged (data not shown). Given the impairment of linked complex I/III activity, we performed individual (unlinked) activity assays of complexes I, II, III, and IV (Fig. 2B). Surprisingly, no difference in any individual enzyme activity, including complex I or III, was observed in Snca−/− mouse brain compared to control. Additionally, Western blot analysis of brain lysates from WT and Snca−/− mice separated by one-dimensional BN-PAGE, SDS-PAGE, and two-dimensional BN-PAGE/SDS-PAGE demonstrated no differences in expression or size of complex I or III, nor were any abnormalities in expression of particular subunits of complex I or III observed or in the amount or size of complex I or III subcomplexes (Fig. 3 and data not shown). Thus, the reduction of linked complex I/III activity observed in Snca−/− brain (Fig. 2A) is likely due to a direct effect of changes in mitochondrial lipid membrane composition and structure on the functional interaction between complex I and III and not to a reduction in number or functional integrity of mitochondria, an alteration of individual complex I or III assembly, or impairment of individual complex I or III activity.
FIG. 2.
Complex I/III activity is reduced in Snca−/− mouse brain in the absence of complex II/III and individual complex activity impairment. Linked (A) and unlinked (B) enzyme assays representing various ETC complex activities were performed on mouse brain lysates as described in Materials and Methods. Relative enzymatic activities observed in Snca−/− mouse brain are plotted as a percentage of the WT control ± the standard deviation. Each enzyme activity with the corresponding ETC complex(es) assayed is labeled. Significant differences were observed between the two genotypes (Student's t test), as indicated: *, P < 0.02; **, P < 0.01.
FIG. 3.
No alterations in assembly of the ∼950-kDa complex I or dimeric ∼500-kDa complex III were observed in Snca−/− mouse brain. Mitochondria were isolated as described in Materials and Methods. (A) Fifty micrograms of protein was separated by BN-PAGE (7%) and transferred to PVDF. Western analysis was performed using the 20- (upper panel) and 39-kDa (middle panel) complex I antibodies or the core II (lower panel) complex III antibody as indicated (arrows). (B) A 100-μg aliquot of protein was separated in the first dimension by BN-PAGE (7%) and SDS-PAGE (12%) in the second dimension and transferred to PVDF. Western analysis was performed using the 39-, 30-, 20-, and 17-kDa complex I antibodies as indicated (arrows). No significant differences in complex I or III expression or size were observed in multiple experiments.
DISCUSSION
Cardiolipin biosynthesis.
The brains of Snca−/− mice have reduced steady-state levels of both cardiolipin and its precursor, PtdGro, as well as decreased steady-state n-6 PUFA and increased SFA content of acyl chains in cardiolipin, without alterations in n-3 PUFA and MUFA composition. Reductions of cardiolipin and PtdGro were of similar relative magnitudes, but a further upstream precursor, phosphatidic acid, was unchanged. Decreased cardiolipin could be explained formally either by decreased synthesis or increased degradation. We believe that reduced synthesis and not increased degradation likely accounts for the decrease in cardiolipin content for two reasons. First, the level of PtdGro, an immediate precursor of cardiolipin, was decreased. Second, kinetic data demonstrate increased half-lives of both the n-6 PUFA and SFA acyl side chains of cardiolipin, suggesting that the cardiolipin backbone itself is unlikely to be undergoing increased degradation, although kinetic experiments measuring cardiolipin backbone synthesis and turnover directly are necessary to confirm this possibility.
The enzymes involved in cardiolipin biosynthesis, from the production of phosphatidic acid in the outer membrane to the final step, catalyzed by cardiolipin synthase within the inner membrane, are located in mitochondria. The rate-limiting step of cardiolipin biosynthesis is production of CDP-diacylglycerol, one of two substrates for cardiolipin synthase (reviewed in reference 67) within the inner mitochondrial membrane. This step is regulated by the specific activity of phosphatidate cytidylyl transferase and the availabilities of CTP and phosphatidic acid. Of the enzymes in this biosynthetic pathway, only cardiolipin synthase is localized exclusively to mitochondria. Thus, multiple pools of unknown relative size of the precursors of cardiolipin biosynthesis exist inside and outside of mitochondria in brain and contribute to our whole-brain phospholipid measurements. Although PtdGro can be synthesized in the inner mitochondrial membrane, it has been demonstrated that PtdGro from nonmitochondrial sources can contribute to cardiolipin biosynthesis. However, the relative contributions of mitochondrial versus nonmitochondrial sources of PtdGro to cardiolipin biosynthesis in brain mitochondria in vivo are unknown. Because α-synuclein is not localized to the inner mitochondrial membrane, it is doubtful that the primary abnormality in Snca−/− mouse brain is due to inhibition of the mitochondrial biosynthetic enzymes directly. Thus, we hypothesize that the mechanism for the reductions in steady-state cardiolipin and PtdGro are due to extramitochondrial substrate limitation, thereby reducing substrate availability for mitochondrial phospholipid biosynthesis. Interestingly, both phosphatidic acid and PtdGro are acidic phospholipids that can bind α-synuclein (16, 57). Thus, it is conceivable that α-synuclein may have a role in targeting phosphatidic acid and/or PtdGro to mitochondria for subsequent cardiolipin biosynthesis.
PUFA uptake into brain.
Both SFA and n-6 PUFA incorporation into cardiolipin were reduced following FA infusion, while steady-state measurements showed that SFA levels were increased markedly while n-6 PUFA was reduced. This difference between kinetic and steady-state measurements can be explained by our published data and that of others (25, 70) that indicate that while intravenously infused SFA can be taken up by the brain, endogenous synthesis appears far more important than dietary intake in supplying SFA to the brain under normal physiological conditions (22). In contrast to SFAs, PUFAs cannot be synthesized endogenously and are supplied to the mammalian brain entirely by dietary intake. We hypothesize that the reduced incorporation of FA into cardiolipin is due to a direct effect of lack of α-synuclein on mitochondrial FA availability and that the observed reduction in FA turnover is a compensatory mechanism due to the reduced FA incorporation, possibly to maintain FA and/or cardiolipin levels.
Pathways by which essential PUFAs are taken up into the brain are characterized incompletely and may be different for different classes of PUFA. Brain n-6 PUFAs are derived from the diet either as preformed longer-chain PUFAs, such as arachidonic acid (20:4 n-6), or as shorter-chain PUFAs, such as linoleic acid (18:2 n-6), which must then be elongated to longer chain series. These n-6 PUFAs are transported in the blood in the form of free FA bound to albumin and are brought across the endothelium and blood brain barrier by monocarboxylic acid transporters or FA transport proteins. In contrast, the supply of n-3 PUFA as free FA in the bloodstream is probably inadequate to satisfy brain requirements and may be supplied in an esterified form, transported across the endothelium through lipoprotein-mediated receptor endocytosis, and then processed in the endothelium for transport into the brain (21, 71). Additionally, simple diffusion may contribute to n-6 and n-3 PUFA uptake as well (56). However, it is unlikely that α-synuclein affects monocarboxylic acid transporters or FA transport proteins directly, because they are plasma membrane-associated proteins while α-synuclein is a cytoplasmic protein that associates loosely with vesicles and not the plasma membrane (11).
In addition to mechanisms that affect PUFA uptake directly, there are many other intracellular processes that affect uptake indirectly either by direct effects on intracellular FA trafficking and/or on metabolism, including phospholipid biosynthesis and β-oxidation. For example, the function of FA binding proteins is to distribute FA to various intracellular compartments, thereby indirectly increasing FA uptake into the cell (73). Although an initial report suggested that α-synuclein can bind MUFAs (69), we reported recently that α-synuclein cannot bind to SFA or MUFA based on the more sensitive and specific technique of titration microcalorimetry, indicating that the effect of loss of α-synuclein on SFA uptake kinetics is not likely due to it acting as an FA binding protein (25). Acyl-CoA synthetases also increase FA uptake indirectly by esterification of free FAs, thereby creating the more-water-soluble acyl-CoAs, which are then available for cell metabolism (41). Interestingly, several FA binding protein and acyl-CoA synthetase isoforms expressed in brain bind preferentially to PUFA relative to SFA (41, 73), and thus changes in their activities due to loss of α-synuclein could result in altered PUFA relative to SFA metabolism and/or trafficking.
Dietary PUFA content affects phospholipid acyl chain composition, including that of cardiolipin, in rodents (3, 44). In fact, brain mitochondria isolated from rodents fed an experimental diet for 6 weeks containing “low” n-6 and n-3 PUFA content (although still well within the normal dietary requirements), consisting of a 30 to 1 ratio of linoleic (18:2 n-6) and linolenic (18:3 n-3) acids, respectively, compared to a relatively “high” PUFA control diet consisting of ∼3.5-fold more PUFA, demonstrated a 50% reduction in the amount of the n-6 PUFA linoleic acid in cardiolipin without changes in the two most abundant n-6 and n-3 PUFAs, arachidonic and cervonic (DHA) acids, respectively (20). Rodents fed this experimental diet also had a variable increase in palmitic acid (16:0) in cardiolipin relative to animals on control diet. These data suggest that altering the amount of essential PUFA in the diet results in steady-state acyl chain composition changes in rodent brain cardiolipin similar to what we observe in mice lacking α-synuclein. Additionally, mitochondria isolated from rodents fed this experimental diet also demonstrated reduced linoleic acid composition, without changes in arachidonic and cervonic (DHA) acids, in the two most abundant mitochondrial phospholipids, phosphatidylcholine and phosphatidylethanolamine, indicative of the specific importance of dietary n-6 PUFA intake on the acyl chain composition of brain mitochondrial phospholipids. Thus, the increased abundance, relative to other tissues, and the critical functions of long-chain PUFAs in the brain (50) make it likely that the brain has special mechanisms for the uptake and metabolism of PUFAs. Therefore, the defect in the incorporation of exogenously supplied SFA and PUFA into brain cardiolipin, seen in Snca−/− mice (Fig. 3), would be reflected in the n-6 PUFA steady-state content rather than the SFA content, since SFA can be synthesized endogenously.
Mechanisms for regulating acyl chain composition and steady-state cardiolipin content in mitochondria.
Cardiolipin has a very unique acyl chain composition that is quite distinct from its immediate precursor, PtdGro. Although the acyl side chain composition of cardiolipin is determined initially by acyl chains present in its precursors, CDP-diacylglycerol and PtdGro, the steady-state acyl chain composition of cardiolipin is determined ultimately by ongoing mitochondrial membrane remodeling. This remodeling takes place either through acyl-specific deacylation/reacylation reactions involving phospholipase A2 and putative acyltransferases (as reviewed in reference 67) or by transacylation reactions, in which acyl groups are transferred directly from phospholipids to cardiolipin in the inner mitochondrial membrane (76). The source of n-6 PUFAs for the transacylation reaction is thought to be inner mitochondrial phosphatidylcholine and phosphatidylethanolamine, which are very sensitive to dietary intake of n-6 PUFA (more so than n-3 PUFA) (20). If transacylation is the predominant mechanism for cardiolipin remodeling in the brain and if phosphatidylcholine and phosphatidylethanolamine in the inner mitochondrial membrane are n-6 PUFA deficient, then this could help explain the n-6 PUFA deficiency in cardiolipin. The identity of brain-specific acyltransferases and/or transacylases with long-chain PUFA specificity necessary to account for cardiolipin's unique acyl-chain composition remains elusive.
Maintaining normal steady-state levels of cardiolipin may require that its side chains have the correct PUFA composition. This supposition comes, in part, from the study of patients with Barth syndrome, a rare X-linked disorder characterized by heart and skeletal muscle myopathies, growth retardation, neutropenia, and high morbidity and mortality but little CNS impairment (as reviewed in reference 4). Patients show severe reductions in steady-state cardiolipin and PtdGro resulting in a major defect in mitochondrial energy production in affected tissues. There are also abnormalities in the acyl chain composition of cardiolipin, with a marked deficiency of the n-6 PUFA linoleic acid, normally the major constituent of cardiolipin outside the CNS. Linoleic acid is the most abundant FA in cardiolipin from heart, kidney, and liver, constituting 60 to 80% of the acyl side chains in cardiolipin from these tissues, and is the second most abundant FA in skeletal muscle, constituting ∼25% (reviewed in reference 75). In contrast to heart and skeletal muscle, linoleic acid is a minor constituent of brain cardiolipin, comprising only 4 to 10% of its acyl chains (75) (see Table S1 in the supplemental material), with the majority of acyl chains consisting of longer-chain PUFAs, possibly accounting for the lack of severe neurological impairment in Barth syndrome.
Barth syndrome is caused by mutations affecting an intramitochondrial protein, tafazzin, expressed most abundantly in heart, skeletal muscle, liver, and kidney, with far lower levels of expression in the brain (39). Tafazzin has sequence homology to the family of acyltransferases and is thought to be involved in remodeling acyl side chains of cardiolipin and possibly PtdGro directly with n-6 PUFA (4, 76). The reduction in PtdGro and cardiolipin in Barth syndrome occurs despite a normal capacity to synthesize PtdGro, as determined by studies in Barth syndrome fibroblasts. Thus, it was concluded that a failure to remodel cardiolipin with the n-6 PUFA linoleic acid in Barth syndrome results in an abnormal acyl chain composition, leading to reduced steady-state cardiolipin content due to reduced stability or increased degradation of cardiolipin with abnormal side chain composition. The reduced PtdGro level may be the result of either decreased stability (or increased degradation) due to abnormal acyl chain composition or could be due to a compensatory increase in cardiolipin synthase activity thereby decreasing the PtdGro pool.
Although reduction in cardiolipin and in PUFA side chain content is observed both in Snca−/− mice and patients with Barth syndrome, the phenotype and mechanisms of the alterations are likely to be different. First, α-synuclein is not an intramitochondrial protein, and so a deficiency of this protein is likely to have an indirect effect on intramitochondrial cardiolipin content and acyl chain composition, while tafazzin is intramitochondrial and may be involved in cardiolipin remodeling directly. Thus, it is unlikely that reduced activity of an intramitochondrial acyltransferase or transacylase, responsible for remodeling brain cardiolipin with long-chain n-6 PUFAs, alters the acyl chain composition and thereby reduces the steady-state content of cardiolipin in Snca−/− mice, because the increased half-lives of both n-6 PUFA and SFA in brain cardiolipin in Snca−/− mice are not compatible with increased degradation of cardiolipin containing abnormal acyl side chains. Second, α-synuclein, unlike tafazzin, is expressed primarily in the CNS and not in liver or muscle, and so deficiency of α-synuclein in mice is expected to have an effect in the CNS and not be seen as a systemic illness.
Effect of cardiolipin content and membrane structure on complex I/III activity.
We have shown that a reduction in the steady-state amount and n-6 PUFA composition of cardiolipin and subsequent mitochondrial membrane structural abnormalities caused by Snca ablation correlates with a significant and distinctive reduction in one component of ETC function, as measured by linked assays that require the transfer of electrons from complex I to III. Precedence for a direct effect of cardiolipin deficiency on coupling between otherwise normal complex I and III is found in studies of a CHO cell line carrying a temperature-sensitive mutation in PtdGro synthase (51). At the nonpermissive temperature, biosynthesis of PtdGro is impaired and leads to reduced steady-state cardiolipin levels and complex I/III activity, but not of other ETC complexes. This reduction of complex I/III activity could be corrected completely by addition of a less lipophilic analog of endogenous ubiquinone, the electron acceptor for complexes I and II, indicating complex I was intrinsically normal. We observed a similar phenomenon, with reduced activity of complex I when the endogenous electron acceptors were present versus the normal activity of complex I when excess exogenous decylubiquinone was provided. The extent of complex I/III reduction (60%) relative to cardiolipin reduction (67%) in these cells was comparable to the 15 and 22% reductions observed in complex I/III and cardiolipin in Snca−/− brain. The ETC abnormality in Snca−/− mice is therefore similar, albeit less dramatic, than the abnormality observed in the severely cardiolipin-deficient CHO cells and suggests that the cardiolipin abnormality in Snca−/− brain is sufficient to account for reduced complex I/III activity. Alterations in cardiolipin content have been examined in hearts and livers, where it has been shown to also affect activities of non-ETC mitochondrial proteins, including many transporters (67). We believe that the current study is the first to examine the effect of changes in cardiolipin on ETC function in mouse brain. Interestingly, a previous experiment compared mitochondrial phospholipid content between young and old rodents and concluded that cardiolipin content is reduced significantly during aging; however, mitochondrial enzymatic measurements were not performed (63). Thus, it is possible that additional mitochondrial enzymatic activities not measured in these experiments could be affected.
Abnormalities in lipid order of the inner mitochondrial membrane may reduce linked I/III activity without altering I or III activity individually by a number of mechanisms, such as impaired association of complex I and III into a supercomplex, restricted ubiquinone movement within the membrane, and decreased accessibility of ubiquinone to its site of reduction in complex I (29, 42, 54, 55). Lenaz and coworkers (as reviewed in reference 37) have suggested that the concentration of ubiquinone available locally to complexes I and/or III in the membrane, and not ubiquinone movement between complex I and III per se, is rate limiting in complex I/III activity. They determined in kinetic experiments that the ubiquinone concentration in heart mitochondrial membrane is sufficient for only half-maximal I/III activity, while less than 20% of the normal ubiquinone concentration is necessary to achieve full II/III activity (23), indicating that subtle changes in the local concentration of ubiquinone can affect I/III activity significantly without affecting II/III activity. Formation of I/III and I/III/IV supercomplexes in heart mitochondria has also been proposed to be rate limiting for I/III activity based on the observation that experimental conditions that eliminate supercomplex formation also eliminate I/III activity but not individual I and III activity (65). Interestingly, in the yeast Saccharomyces cerevisiae, which lacks a complex similar to mammalian complex I, cardiolipin associates with and stabilizes complex III/IV supercomplexes, demonstrating that cardiolipin has a role in supercomplex stability and function (53, 67, 78).
How significant is the observed complex I/III activity impairment in Snca−/− brain?
OxPhos accounts for ∼90% of brain ATP (27), and complex I is the major control point for OxPhos in synaptic mitochondria (15). Synaptic mitochondria have a reduced complex I threshold compared to nonsynaptic mitochondria, so that only a 25% reduction in complex I activity was shown in vitro to be sufficient in synaptic mitochondria to diminish ATP production significantly, while a 60% reduction of complex I activity was necessary for significant impairment of ATP production in nonsynaptic mitochondria (14). The increased sensitivity of synaptic mitochondria to reduced complex I function is interesting given the synaptic localization of α-synuclein and the pronounced synaptic membrane structural abnormalities observed in Snca−/− mice. Despite the increased sensitivity of synaptic mitochondria to complex I inhibition and the potential for synaptic energy deficiencies developing during times of high metabolic activity, Snca−/− mice lack an overt neurological phenotype. A 50% reduction of reserve pool synaptic vesicles associated with impairment of synaptic response to prolonged repetitive stimulation was observed in hippocampal synapses from Snca−/− mice (7), although others have reported little or no change in synaptic function (1, 10). Whether discrepancies in these studies are due to mouse strain-specific genetic background differences or to variable levels of dietary PUFA fed to Snca−/− mice, thereby leading to inconsistencies in maintenance of cardiolipin content and acyl chain composition required to produce consistent neurological impairment, will be the subject of future investigation.
We recognize that the 15% reduction of complex I/III activity observed in Snca−/− mice, although significant, is modest. However, the change observed represents an average of complex I/III dysfunction over the entire brain, and it is likely that alterations in mitochondrial membrane composition and structure, energy production, and the extent of reactive oxygen species generation (thought to be produced by complex I and possibly complex III, with production enhanced during conditions of reduced OxPhos efficiency [38]) in some brain regions, cell types, and/or cellular subcompartments are more or less severely impacted. Thus, identification and characterization of subpopulations of more defective mitochondria will also be the subject of future investigation.
Could the observed mitochondrial membrane abnormalities in Snca−/− brain help expand our understanding of PD pathogenesis?
Elucidating the normal function(s) of α-synuclein, in part through an analysis of Snca−/− mice, is important for furthering our understanding of PD pathogenesis. Complex I defects are thought to be a critical factor in PD, because exposure to complex I inhibitors, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone, result in dopaminergic cell death and parkinsonism (17), and 30% and 39% reductions in complex I and I/III activities, without reduction in II/III activity, have been reported in the substantia nigra of PD patient brains (66). Interestingly, Dauer and coworkers showed that Snca−/− mice and neuronal cultures isolated from these mice were more resistant to toxic affects of the complex I inhibitor, MPTP, than WT mice (13). Although this observation might, at first, be interpreted to argue against there being a physiologically significant reduction in complex I/III function in Snca−/− mice, the mechanism for resistance is in fact unknown and could be a reflection of altered intraneuronal transport or availability of 1-methyl-4-phenylpyridinium, the toxic metabolite of MPTP, to mitochondria rather than a change in ETC function. This possibility gains some support from the observation that dopaminergic neurons in primary ventral midbrain cultures isolated from Snca−/− mice actually appear more sensitive than control cultures to complex I inhibition by lipophilic rotenone (13), which inhibits the complete reduction of ubiquinone within complex I. This increased rotenone sensitivity could indicate that complex I/III is not only impaired in whole Snca−/− brain (Fig. 2A), but also in midbrain dopaminergic neurons, the very neuronal cell-type most affected in PD.
Although α-synuclein aggregate formation, as observed with oxidative and nitrative modifications, mutations, or overexpression of α-synuclein, is thought to be toxic to neurons directly (17), we can speculate that α-synuclein containing aggregates may also decrease its functional concentration and alter its intracellular localization. This could lead to aberrant lipid metabolism, progressive mitochondrial membrane abnormalities, and decreased I/III activity, thereby contributing to PD pathogenesis. Of interest, we have shown that a line of transgenic mice overexpressing α-synuclein in the spinal cord develop a synucleinopathy only when the transgene is crossed onto a Snca−/− background, suggesting that deficiency of endogenous α-synuclein contributes to the development of a severe neurological phenotype (6). Furthermore, these data also raise the interesting possibility that low dietary intake of essential PUFAs could be a contributory factor in PD, as has been suggested in a population-based association study (19). Discovery of a relationship between α-synuclein and mitochondrial complex I/III function in brain through its effect on the lipid composition, essential FA content, and structure of mitochondrial membranes may serve as a critical link between genetic and environmental factors in PD pathogenesis.
Supplementary Material
Acknowledgments
We thank Bill Craigen for helping to support our studies, Douglass Turnbull for helpful advice, Anna Sirota for excellent technical assistance, and Douglas Wallace and Vincent Procaccio for helpful discussions and exploratory experiments.
This work was supported, in part, by the Intramural Research Program of the National Human Genome Research Institute, NIH, and NIH grants 1R21 NS043697-01A and 5P20R01-7699-02 to E.J.M.
We declare that we have no competing financial interests.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abeliovich, A., Y. Schmitz, I. Farinas, D. Choi-Lundberg, W. H. Ho, P. E. Castillo, N. Shinsky, J. M. Verdugo, M. Armanini, A. Ryan, M. Hynes, H. Phillips, D. Sulzer, and A. Rosenthal. 2000. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239-252. [DOI] [PubMed] [Google Scholar]
- 2.Akesson, B., J. Elovsson, and G. Arvidsson. 1970. Initial incorporation into rat liver glycerolipids of intraportally injected [3H] glycerol. Biochim. Biophys. Acta 210:15-27. [DOI] [PubMed] [Google Scholar]
- 3.Barcelo-Coblijn, G., K. Kitajka, L. G. Puskas, E. Hogyes, A. Zvara, L. Hackler, Jr., and T. Farkas. 2003. Gene expression and molecular composition of phospholipids in rat brain in relation to dietary n-6 to n-3 fatty acid ratio. Biochim. Biophys. Acta 1632:72-79. [DOI] [PubMed] [Google Scholar]
- 4.Barth, P. G., F. Valianpour, V. M. Bowen, J. Lam, M. Duran, F. M. Vaz, and R. J. A. Wanders. 2004. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am. J. Med. Genet. 126A:349-354. [DOI] [PubMed] [Google Scholar]
- 5.Birch-Machin, M. A., and D. M. Turnbull. 2001. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Mitochondria 65:97-117. [DOI] [PubMed] [Google Scholar]
- 6.Cabin, D. E., S. Gispert-Sanchez, D. Murphy, G. Auburger, R. R. Myer, and R. L. Nussbaum. 2005. Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neurobiol. Aging 26:25-35. [DOI] [PubMed] [Google Scholar]
- 7.Cabin, D. E., K. Shimazu, D. Murphy, N. B. Cole, W. Gottschalk, K. L. McIlwain, B. Orrison, A. Chen, C. E. Ellis, R. Paylor, B. Lu, and R. L. Nussbaum. 2002. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 22:8797-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castagnet, P. I., M. Y. Golovko, G. C. Barceló-Coblijn, R. L. Nussbaum, and E. J. Murphy. 2005. Fatty acid incorporation is decreased in astrocytes cultured from alpha-synuclein gene-ablated mice. J. Neurochem. 94:839-849. [DOI] [PubMed] [Google Scholar]
- 9.Cerritelli, S. M., E. G. Frolova, C. Feng, A. Grinberg, P. E. Love, and R. J. Crouch. 2003. Failure to produce mitochondrial DNA results in embryonic lethality in Rnaseh1 null mice. Mol. Cell 11:807-815. [DOI] [PubMed] [Google Scholar]
- 10.Chandra, S., F. Fornai, H. B. Kwon, U. Yazdani, D. Atasoy, X. Liu, R. E. Hammer, G. Battaglia, D. C. German, P. E. Castillo, and T. C. Sudhof. 2004. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc. Natl. Acad. Sci. USA 101:14966-14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton, D. F., and J. M. George. 1998. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 21:249-254. [DOI] [PubMed] [Google Scholar]
- 12.Cole, N. B., D. D. Murphy, T. Grider, S. Rueter, D. Brasaemle, and R. L. Nussbaum. 2002. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J. Biol. Chem. 277:6344-6352. [DOI] [PubMed] [Google Scholar]
- 13.Dauer, W., N. Kholodilov, M. Vila, A. C. Trillat, R. Goodchild, K. E. Larsen, R. Staal, K. Tieu, Y. Schmitz, C. A. Yuan, M. Rocha, V. Jackson-Lewis, S. Hersch, D. Sulzer, S. Przedborski, R. Burke, and R. Hen. 2002. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc. Natl. Acad. Sci. USA 99:14524-14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davey, G. P., L. Canevari, and J. B. Clark. 1997. Threshold effects in synaptosomal and nonsynaptic mitochondria from hippocampal CA1 and paramedian neocortex brain regions. J. Neurochem. 69:2564-2570. [DOI] [PubMed] [Google Scholar]
- 15.Davey, G. P., S. Peuchen, and J. B. Clark. 1998. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J. Biol. Chem. 273:12753-12757. [DOI] [PubMed] [Google Scholar]
- 16.Davidson, W. S., A. Jonas, D. F. Clayton, and J. M. George. 1998. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273:9443-9449. [DOI] [PubMed] [Google Scholar]
- 17.Dawson, T. M., and V. L. Dawson. 2003. Molecular pathways of neurodegeneration in Parkinson's disease. Science 302:819-822. [DOI] [PubMed] [Google Scholar]
- 18.Degli-Esposti, M. 2001. Assessing functional integrity of mitochondria in vitro and in vivo. Mitochondria 65:75-96. [DOI] [PubMed] [Google Scholar]
- 19.de Lau, L. M. L., M. Bornebroek, J. C. M. Witteman, A. Hofman, P. J. Koudstaal, and M. M. B. Breteler. 2005. Dietary fatty acids and the risk of Parkinson disease. Neurology 64:2040-2045. [DOI] [PubMed] [Google Scholar]
- 20.Dyer, J. R., and C. E. Greenwood. 1991. Dietary essential fatty acids change the fatty acid profile of rat neural mitochondria over time. J. Nutr. 121:1548-1553. [DOI] [PubMed] [Google Scholar]
- 21.Edmond, J. 2001. Essential polyunsaturated fatty acids and the barrier to the brain. J. Mol. Neurosci. 16:181-193. [DOI] [PubMed] [Google Scholar]
- 22.Edmond, J., T. A. Higa, R. A. Korsak, E. A. Bergner, and W. N. Lee. 1998. Fatty acid transport and utilization for the developing brain. J. Neurochem. 70:1227-1234. [DOI] [PubMed] [Google Scholar]
- 23.Estornell, E., R. Fato, C. Castelluccio, M. Cavazzoni, G. Parenti Castelli, and G. Lenaz. 1992. Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett. 311:107-109. [DOI] [PubMed] [Google Scholar]
- 24.Folch, J., M. Lees, and G. H. Sloane-Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 25.Golovko, M. Y., N. J. Faergeman, N. B. Cole, P. I. Castagnet, R. L. Nussbaum, and E. J. Murphy. 2005. Alpha-synuclein gene-deletion decreases brain palmitate uptake and alters palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry 44:8251-8259. [DOI] [PubMed] [Google Scholar]
- 26.Golovko, M. Y., and E. J. Murphy. 2004. An improved method for tissue long chain acyl-CoA extraction and analysis. J. Lipid Res. 45:1777-1782. [DOI] [PubMed] [Google Scholar]
- 27.Gunter, T. E., K. K. Gunter, S.-S. Sheu, and C. E. Gavin. 1994. Mitochondrial calcium transport: physiological and pathological relevance. Am. J. Physiol. 267:C313-C339. [DOI] [PubMed] [Google Scholar]
- 28.Hara, A., and N. S. Radin. 1978. Lipid extraction of tissues using low-toxicity solvents. Anal. Biochem. 90:420-426. [DOI] [PubMed] [Google Scholar]
- 29.Heron, C., M. G. Gore, and C. I. Ragan. 1979. The effects of lipid phase transitions on the interaction of mitochondrial NADH-ubiquinone oxidoreductase with ubiquinol-cytochrome c oxidoreductase. Biochem. J. 178:415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill, R. L., and R. H. Bradshaw. 1969. Fumarase. Methods Enzymol. 1969:91-99. [Google Scholar]
- 31.Jenco, J. M., A. Rawlingson, B. Daniels, and A. J. Morris. 1998. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry 37:4901-4909. [DOI] [PubMed] [Google Scholar]
- 32.Jolly, C. A., T. Hubbell, W. D. Behnke, and F. Schroeder. 1997. Fatty acid binding protein: stimulation of microsomal phosphatidic acid formation. Arch. Biochem. Biophys. 341:112-121. [DOI] [PubMed] [Google Scholar]
- 33.Jung, C., C. M. Higgins, and Z. Xu. 2000. Measuring the quantity and activity of mitochondrial electron transport chain complexes in tissues of central nervous system using blue native polyacrylamide gel electrophoresis. Anal. Biochem. 286:214-223. [DOI] [PubMed] [Google Scholar]
- 34.King, T. E. 1967. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol. 1967:322-331. [Google Scholar]
- 35.Kuznetsov, A. V., and E. Gnaiger. 2003. Complex I laboratory protocol. Mitochondrial Physiol. Network 8:15.1-15.18. [Google Scholar]
- 36.Lai, C. K., and J. B. Clark. 1989. Isolation and characterization of synaptic and nonsynaptic mitochondria from mammalian brain. Neuromethods 11:43-97. [Google Scholar]
- 37.Lenaz, G. 2001. A critical appraisal of the mitochondrial coenzyme Q pool. FEBS Lett. 509:151-155. [DOI] [PubMed] [Google Scholar]
- 38.Liu, Y., G. Fiskum, and D. Schubert. 2002. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 80:780-787. [DOI] [PubMed] [Google Scholar]
- 39.Lu, B., M. R. Kelher, D. P. Lee, T. M. Lewin, R. A. Coleman, P. C. Choy, and G. M. Hatch. 2004. Complex expression pattern of the Barth syndrome gene product tafazzin in human cell lines and murine tissues. Biochem. Cell Biol. 82:569-576. [DOI] [PubMed] [Google Scholar]
- 40.Marcheselli, V. L., B. L. Scott, T. S. Reddy, and N. G. Bazan. 1988. Quantitative analysis of acyl group composition of brain phospholipids, neutral lipids, and free fatty acids, p. 83-110. In A. A. Boulton, G. B. Baker, and L. A. Horrocks (ed.), Neuromethods 7: lipids and related compounds. Humana Press, Clifton, N.J.
- 41.Marszalek, J. R., C. Kitidis, A. Dararutana, and H. F. Lodish. 2004. Acyl-CoA synthetase 2 overexpression enhances fatty acid internalization and neurite outgrowth. J. Biol. Chem. 279:23882-23891. [DOI] [PubMed] [Google Scholar]
- 42.Mathai, J. C., Z. E. Sauna, O. John, and V. Sitaramam. 1993. Rate-limiting step in electron transport. J. Biol. Chem. 268:15442-15454. [PubMed] [Google Scholar]
- 43.Mathai, J. C., and V. Sitaramam. 1994. Stretch sensitivity of transmembrane mobility of hydrogen peroxide through voids in the bilayer. J. Biol. Chem. 269:17784-17793. [PubMed] [Google Scholar]
- 44.McGee, C. D., P. Lieberman, and C. E. Greenwood. 1996. Dietary fatty acid composition induces comparable changes in cardiolipin fatty acid profile of heart and brain mitochondria. Lipids 31:611-616. [DOI] [PubMed] [Google Scholar]
- 45.Miller, S. A., D. D. Dykes, and H. F. Polesky. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell, D. C., and B. J. Litman. 1998. Molecular order and dynamics in bilayers consisting of highly polyunsaturated phospholipids. Biophys. J. 74:879-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy, E. J., G. Barcelo-Coblijn, B. Binas, and J. F. Glatz. 2004. Heart fatty acid uptake is decreased in heart-fatty acid binding protein gene-ablated mice. J. Biol. Chem. 279:34481-34488. [DOI] [PubMed] [Google Scholar]
- 48.Murphy, E. J., T. Sitles, and F. Schroeder. 2000. Sterol carrier protein-2 expression alters phospholipid content and fatty acid acyl composition of L-cell fibroblasts. J. Lipid Res. 41:788-796. [PubMed] [Google Scholar]
- 49.Narayanan, V., Y. Guo, and S. Scarlata. 2005. Fluorescence studies suggest a role for alpha-synuclein in the phosphatidylinositol lipid signaling pathway. Biochemistry 44:462-470. [DOI] [PubMed] [Google Scholar]
- 50.Neuringer, M., G. J. Anderson, and W. E. Conner. 1988. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annu. Rev. Nutr. 8:517-541. [DOI] [PubMed] [Google Scholar]
- 51.Ohtsuka, T., M. Nishijima, K. Suzuki, and Y. Akamatsu. 1993. Mitochondrial dysfunction of a cultured Chinese hamster ovary cell mutant deficient in cardiolipin. J. Biol. Chem. 268:22914-22919. [PubMed] [Google Scholar]
- 52.Payton, J. E., R. J. Perrin, W. S. Woods, and J. M. George. 2004. Structural determinants of PLD2 inhibition by alpha-synuclein. J. Mol. Biol. 337:1001-1009. [DOI] [PubMed] [Google Scholar]
- 53.Pfeiffer, K., V. Gohil, R. A. Stuart, C. Hunte, U. Brandt, M. L. Greenberg, and H. Schagger. 2003. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 278:52873-52880. [DOI] [PubMed] [Google Scholar]
- 54.Poore, V. M., J. T. R. Fitzsimons, and C. I. Ragan. 1982. The effects of lipid fluidity on the rotational diffusion of complex I and complex III in reconstituted NADH-cytochrome c oxidoreductase. Biochim. Biophys. Acta 693:113-124. [DOI] [PubMed] [Google Scholar]
- 55.Poore, V. M., and C. I. Ragan. 1982. A spin label study of the lipid boundary layer of mitochondrial NADH-ubiquione oxidoreductase. Biochim. Biophys. Acta 693:105-112. [DOI] [PubMed] [Google Scholar]
- 56.Pownall, H. J. 2001. Cellular transport of nonesterified fatty acids. J. Mol. Neurosci 16:109-115. [DOI] [PubMed] [Google Scholar]
- 57.Ramakrishnan, M., P. H. Jensen, and D. Marsh. 2003. Alpha-synuclein association with phosphatidylglycerol probed by lipid spin labels. Biochemistry 42:12919-12926. [DOI] [PubMed] [Google Scholar]
- 58.Rieske, J. S. 1967. Preparation and properties of reduced coenzyme Q-cytochrome c reductase (complex III) of the respiratory chain. Methods Enzymol. 10:239-245. [Google Scholar]
- 59.Robinson, P. J., J. Noronha, J. J. DeGeorge, L. M. Freed, T. Nariai, and S. I. Rapoport. 1992. A quantitative method for measuring regional in vivo fatty acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res. Rev. 17:187-214. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberger, T. A., N. E. Villacreses, M. A. Contreras, J. V. Bonventre, and S. I. Rapoport. 2003. Brain lipid metabolism in the cPLA2 knockout mouse. J. Lipid Res. 44:109-117. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberger, T. A., N. E. Villacreses, J. T. Hoyda, F. Boestti, G. Weerasinghe, R. N. Wine, G. J. Harry, and S. I. Rapoport. 2004. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J. Neurochem. 88:1168-1178. [DOI] [PubMed] [Google Scholar]
- 62.Rouser, G., A. Siakotos, and S. Fleischer. 1969. Quantitative analysis of phospholipids by thin layer chromatography and phosphorus analysis of spots. Lipids 1:85-86. [DOI] [PubMed] [Google Scholar]
- 63.Ruggiero, F. M., F. Cafagna, V. Petruzzella, M. N. Gadaleta, and E. Quagliariello. 1992. Lipid composition in synaptic and nonsynaptic mitochondria from rat brains and effect of aging. J. Neurochem. 59:487-491. [DOI] [PubMed] [Google Scholar]
- 64.Saito, H., S. Lund-Katz, and M. C. Phillips. 2004. Contributions of domain structure and lipid interaction to the functionality of exchangable human apolipoproteins. Prog. Lipid Res. 43:350-380. [DOI] [PubMed] [Google Scholar]
- 65.Schagger, H., and K. Pfeiffer. 2000. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 19:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schapira, A. H., J. M. Cooper, D. Dexter, J. B. Clark, P. Jenner, and C. D. Marsden. 1990. Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 54:823-827. [DOI] [PubMed] [Google Scholar]
- 67.Schlame, M., D. Rua, and M. L. Greenberg. 2000. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 39:257-288. [DOI] [PubMed] [Google Scholar]
- 68.Sharon, R., I. Bar-Joseph, G. E. Mirick, C. N. Serhan, and D. J. Selkoe. 2003. Altered fatty acid composition of dopaminergic neurons expressing alpha-synuclein and human brains with alpha-synucleinopathies. J. Biol. Chem. 278:49874-49881. [DOI] [PubMed] [Google Scholar]
- 69.Sharon, R., M. S. Goldberg, I. Bar-Josef, R. A. Betensky, J Shen, and D. J. Selkoe. 2001. α-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc. Natl. Acad. Sci. USA 98:9110-9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith, Q. R., and H. Nagura. 2001. Fatty acid uptake and incorporation in brain: studies with the perfusion model. J. Mol. Neurosci. 16:167-172. [DOI] [PubMed] [Google Scholar]
- 71.Spector, A. A. 2001. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J. Mol. Neurosci. 16:159-165. [DOI] [PubMed] [Google Scholar]
- 72.Srere, P. A. 1969. Citrate synthase. Methods Enzymol. 13:3-11. [Google Scholar]
- 73.Veerkamp, J. H., and A. W. Zimmerman. 2001. Fatty acid-binding proteins of nervous tissue. J. Mol. Neurosci. 16:133-142. [DOI] [PubMed] [Google Scholar]
- 74.Watkins, P. A., J. A. Hamilton, A. Leaf, A. A. Spector, S. A. Moore, R. E. Anderson, H. W. Moser, M. J. Noetzel, and R. Katz. 2001. Brain uptake and utilization of fatty acids: applications to peroxisomal biogenesis diseases. J. Mol. Neurosci. 16:87-92. [DOI] [PubMed] [Google Scholar]
- 75.White, D. A. 1973. The phospholipid composition of mammalian tissues, p. 441-479. In G. B. Ansell, J. N. Hawthorne, and R. M. C. Dawson (ed.), Form and function of phospholipids, vol. 3. Elsevier Scientific Publishing Company, Amsterdam, The Netherlands. [Google Scholar]
- 76.Xu, Y., R. I. Kelley, T. J. Blanck, and M. Schlame. 2003. Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 278:51380-51385. [DOI] [PubMed] [Google Scholar]
- 77.Yonetan, T. 1967. Cytochrome oxidase: beef heart. Methods Enzymol. 1967:332-335. [Google Scholar]
- 78.Zhang, M., E. Mileykovskaya, and W. Dowhan. 2002. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 277:43553-43556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.