Abstract
Mycobacteria are the etiologic agents of numerous diseases which account for significant morbidity and mortality in humans and other animal species. Many mycobacteria are intramacrophage pathogens and therefore the macrophage response to infection, which includes synthesis of cytokines such as tumor necrosis factor alpha (TNF-α) and production of nitric oxide, has important consequences for host immunity. However, very little is known about the macrophage cell signaling pathways initiated upon infection or how pathogenic mycobacteria may modulate the macrophage responses. Using primary murine bone marrow macrophages, we established that p38 and extracellular signal-regulated kinases 1 and 2 of the mitogen-activated protein kinase (MAPK) pathways are activated upon infection with different species of mycobacteria. However, we observed decreased MAPK activity over time in macrophages infected with pathogenic Mycobacterium avium strains relative to infections with nonpathogenic mycobacteria. Furthermore, macrophages infected with M. avium produced lower levels of TNF-α, interleukin 1β, and inducible nitric oxide synthase 2 than macrophages infected with nonpathogenic species. Inhibitor studies indicate that the MAPKs are required for the Mycobacterium-mediated induction of these effector proteins. Our data indicate that MAPKs are activated in macrophages upon invasion by mycobacteria and that this activation is diminished in macrophages infected with pathogenic strains of M. avium, resulting in decreased production of important immune effector proteins. The decreased MAPK activation associated with M. avium infections suggests a novel point of immune intervention by this mycobacterial species.
Mycobacterium spp. are intramacrophage pathogens and therefore the engagement of macrophage receptors by mycobacteria is one of the initial steps in the infection process. A macrophage may respond to a mycobacterial infection by secreting cytokines such as tumor necrosis factor alpha (TNF-α) or interleukin 1β (IL-1β) and by producing reactive oxygen and nitrogen intermediates. These effects, among others, are the end result of activating various macrophage-signaling pathways. However, questions remain regarding which pathways are initiated and/or modulated by a mycobacterial infection. Previous studies have shown that mycobacterial components such as lipoarabinomannan (LAM) can stimulate a macrophage response, resulting in the production and secretion of TNF-α and IL-1β (1). Studies have also indicated that arabinofuranosyl-terminated LAM isolated from nonpathogenic mycobacteria stimulates a stronger cytokine response in treated macrophages than does mannose-capped LAM (ManLAM) from pathogenic mycobacteria (50, 52). Furthermore, recent work by Ting et al. indicates that human macrophages infected with Mycobacterium tuberculosis are less responsive to gamma interferon, due to a disruption in STAT1 binding to the transcription factor CREB (55). These experiments suggest that mycobacteria and mycobacterial components can modulate macrophage-signaling pathways. A more complete analysis of these macrophage responses to a mycobacterial infection may help elucidate the mechanisms underlying mycobacterial pathogenesis and host response.
We have focused our initial studies on the mitogen-activated protein kinases (MAPK) because this kinase family is activated upon engagement of macrophage growth factor and cytokine receptors and is important in the activation of cytokine gene transcription. The MAPK family is composed of the extracellular signal-regulated kinase 1 and 2 (ERK1/2), p38, and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) pathways. Although distinct in their activation (20), there is considerable cooperation between these kinases, and many substrates are shared between pathways (14). This family of kinases is important in a wide spectrum of cellular functions, including proliferation (43), apoptosis (16), cytokine biosynthesis (34), and cytoskeletal reorganization (27). All MAPKs are highly conserved serine-threonine kinases that are activated by upstream MAPK kinases through a Thr-XXX-Tyr phosphorylation motif (39). The stimuli that activate these signaling cascades are distinct, and the downstream effects can vary greatly between cell types. Studies have shown that the ERK pathway is activated primarily by growth factors, mitogenic stimuli, and tumor promoters (15), whereas environmental stress and inflammatory cytokines stimulate the p38 and SAPK/JNK pathways (5, 34, 58).
Recent studies have implicated the MAPKs as important cellular targets for infectious organisms. Yersinia enterocolitica was shown to suppress TNF-α production by inhibiting ERK1/2, p38, and JNK kinase activities (54). Treating macrophages with Leishmania lipophosphoglycans, which are known to promote parasite survival, resulted in ERK1/2 stimulation and subsequent inhibition of IL-12 production (24). In neutrophils, the activation of p38 was shown to be critical for the generation of reactive oxygen intermediates, following infection with the attenuated M. tuberculosis H37Ra strain (44). Additional studies have demonstrated the important regulatory role MAPKs play in nitric oxide synthase 2 (NOS2) production, following RAW 264.7 cell stimulation with M. tuberculosis-derived ManLAM (12). More recent studies have shown that Mycobacterium avium infection of human monocyte-derived macrophages results in p38, JNK, and ERK1/2 activation (49). This activation was inhibited by anti-CD14 antibodies (49). In this study, we found that macrophages infected with M. avium show decreased MAPK activation relative to cells infected with nonpathogenic mycobacteria, suggesting that the MAPKs are a target for immune intercession by pathogenic mycobacteria.
MATERIALS AND METHODS
BMM isolation and culture.
Bone marrow macrophages (BMMs), used in all experiments, were isolated from 6- to 8-week-old BALB/c mice as previously described (51). The isolated macrophages were cultured on 100-mm non-tissue culture plates in Dulbecco's modified Eagle's medium (GIBCO BRL, Grand Island, N.Y.) supplemented with 20 mM HEPES (Fisher Scientific, Fair Lawn, N.J.), 10% fetal bovine serum (FBS) (GIBCO BRL), 100-U/ml penicillin and 100-μg/ml streptomycin (both from BioWhittaker, Walkersville, Md.), 1× l-glutamine, and 20% l-cell supernatant as a source of macrophage colony-stimulating factor (BMM medum). The macrophages were used 7 to 14 days after isolation or frozen after 7 days of culture in freezing media (50% Dulbecco's modified Eagle's medium, 40% FBS, and 10% endotoxin-tested dimethyl sulfoxide (DMSO; Sigma, St. Louis, Mo.). Thawed or fresh macrophages were cultured on non-tissue culture plates for 3 to 7 days and then replated at approximately 3 × 105 cells per 35-mm tissue culture plate. The cells were allowed to adhere for 24 h prior to treatments with bacteria, zymosan (from Saccharomyces cerevisiae; Sigma), or 200-ng/ml lipopolysaccharide (LPS) (from Escherichia coli serotype O55:B5; Sigma). The plates were kept on ice for 10 min after the bacteria, zymosan, or LPS was added. The plates were then incubated at 37°C in 5% CO2 for various times. For the time points beyond 4 h, the macrophages were incubated for 4 h with the mycobacteria or other reagents and washed with phosphate-buffered saline (PBS), and then 2 ml of fresh media was added and incubated for the additional times. All tissue culture reagents were found to be negative for endotoxin contamination by the E-Toxate assay (Sigma).
Bacteria culture.
To generate M. avium 101 and 724 stocks, the bacteria (generously provided by David Russell at Cornell University, Ithaca, N.Y., and Andrea Cooper at Colorado State University, Fort Collins, respectively) were passaged through a mouse to ensure virulence. A single colony was used to produce frozen stocks as described (7). Frozen stocks were quantitated by serial dilution. Mycobacterium phlei ATCC11758, Mycobacterium smegmatis strain MC2155 (generously provided by Rich Groger, Washington University, St. Louis, Mo.), and Mycobacterium bovis BCG ATCC 35734 were grown for 4 days (M. phlei and M. smegmatis) or 10 days (M. bovis BCG) as previously described (7). Frozen stocks were prepared as described for M. avium. Infection assays evaluated by fluorescence microscopy were performed on each stock of mycobacteria to determine the infection ratio needed to obtain approximately 80% of the macrophages infected. The amount of mycobacteria required to obtain the 80% infection rate varied between species and batch preps of mycobacteria. The ratios extended from 8:1 to 60:1, and the variation observed is likely due to differences in the amount of clumping from batch to batch. However, the results were consistent across the different mycobacteria-to-macrophage ratios when the infection level was kept constant.
Complement opsonization.
Appropriate concentrations of mycobacteria were suspended in macrophage culture media containing 10% normal human serum as a source of complement components (37) and incubated for 2 h at 37°C as described (7). The normal human serum came from the same donor for all experiments. Zymosan was complement opsonized as described previously (42). Complement-opsonized zymosan was washed three times in PBS before being added to the macrophages. Ingestion assays were performed to determine the concentration of zymosan required to obtain approximately 80% of the macrophages containing one or more zymosan particles.
Western blot analysis.
At designated times, the treated macrophages were removed from the incubator and placed on ice. The conditioned media were collected, centrifuged for 15 min at 14 krpm (Eppendorf model 5415C centrifuge) to remove nonadherent macrophages and any free mycobacteria, and saved for subsequent enzyme-linked immunosorbent assays (ELISAs). The cells were washed three times with ice-cold PBS containing 1 mM pervanadate. The cells were then treated for 20 min with ice-cold lysis buffer (150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1-μg/ml aprotinin, 1-μg/ml leupeptin, 1-μg/ml pepstatin, 1 mM pervanadate, 1 mM EDTA, 1% Igepal, 0.25% deoxycholic acid, 1 mM NaF, and 50 mM Tris-HCl [pH 7.4]). The cell lysates were removed from the plates and centrifuged at 14 krpm for 15 min at 4°C. The clarified lysates were stored at −20°C. Equal amounts of protein, as defined by the Micro BCA Protein assay (Pierce, Rockford, Ill.), were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, electrophoresed, and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). The membranes were blocked in Tris-buffered saline with 0.05% Tween 20 (TBST) supplemented with 5% powdered milk and then incubated with primary antibodies phospho-p38, total p38, phospho-ERK1/2, or total ERK1/2 from Cell Signaling (Beverly, Mass.) or with NOS2 primary antibody from Santa Cruz Biotechnology (Santa Cruz, Calif.) as indicated by the manufacturer. The blots were washed with TBST and incubated with a secondary antibody, either horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin (Ig) (Pierce) in TBST plus 5% powdered milk. The bound antibodies were detected using SuperSignal West Femto-enhanced chemiluminescence reagents (Pierce). Densitometry was performed with some blots with the LKB Bromma Ultroscan XL Enhanced Laser densitometer with GelScan XL software (Pharmacia LKB Biotechnology, Uppsala, Sweden).
TNF-α neutralization.
Thirty micrograms of a rat anti-mouse monoclonal IgG TNF-α blocking antibody (Upstate Biotechnology, Lake Placid, N.Y.) was added to macrophages immediately following treatment with LPS or infection by M. avium 101 or M. smegmatis. Ten micrograms of additional antibody was added to the 24-h samples following the 4-h infection. Equivalent concentrations of normal rat IgG antibody (Santa Cruz Biotechnology) were added to the control samples. The culture supernatants and cell lysates were collected for Western blot and ELISA analysis as described above.
ELISA.
The levels of TNF-α and IL-1β secreted into the culture media by infected macrophages were measured using the OptEia mouse TNF-α ELISA kit (PharMingen, San Diego, Calif.) and the Biotrak IL-1β mouse ELISA kit (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). Culture media collected from the macrophages were analyzed for cytokines according to the manufacturer's instructions, and the cytokine concentrations were determined against TNF-α and IL-1β standard curves. For the experiments with the TNF-α neutralizing antibody, a large excess of UltraLink Immobilized Protein G (Pierce) was incubated with the culture media to remove the anti-TNF-α antibody and bound TNF-α. Media alone and culture media with IgG control antibody were also treated with Protein G. The Protein G was removed from the culture supernatants by centrifugation.
Statistical analysis.
Data were analyzed by a one-tailed Student t test and a one-way analysis of variance. Statistical significance was assumed at a P value of <0.05. For all TNF-α ELISAs, n = 2 for each time point; error bars represent standard deviation.
RESULTS
Activation of p38 MAPK in M. avium-infected mouse macrophages.
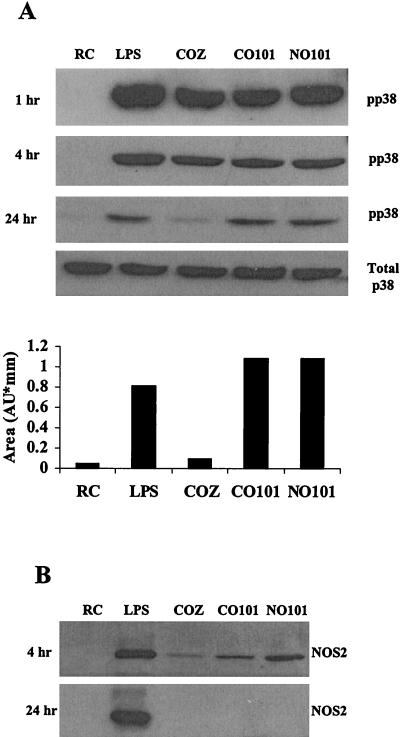
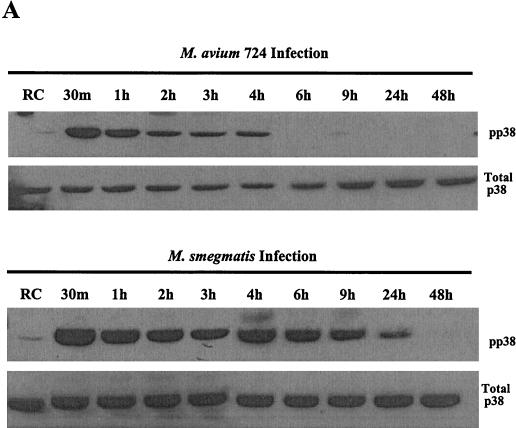
In our initial experiments we examined whether MAPK p38 was activated in mouse BMMs following infection with M. avium. We were also interested in defining the activation state of macrophage p38 MAPK upon infection with complement-opsonized and nonopsonized mycobacteria to determine if the mechanism of invasion results in a differential regulation of this kinase. LPS and zymosan, an inert yeast particle that is phagocytosed by macrophages, are known activators of MAPK p38 at early time points (13, 29) and were used as controls. BMMs were incubated with either complement-opsonized or nonopsonized M. avium 101 or complement-opsonized zymosan or treated with LPS. Prior studies were performed to determine the concentration required to obtain equivalent numbers of ingested mycobacteria and zymosan (data not shown; see Materials and Methods). The treated BMMs were cultured at 37°C for 1, 4, and 24 h. The cells were harvested, and protein lysates were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, blotted, and probed with antibodies to the MAPKs as described in Materials and Methods. Numerous studies have shown that phosphorylation of the MAPK at a Thr-Tyr motif define their activation (10, 47), and we used MAPK antibodies specific for these phosphorylated residues. As shown in Fig. 1, there is strong phosphorylation of p38 with all BMM treatments at 1 and 4 h, indicating that attachment and phagocytosis of complement opsonized and nonopsonized M. avium 101 or zymosan leads to increased activation of p38 compared to untreated cells. After 24 h of infection a considerable decline in the level of p38 phosphorylation is seen for all treatments. However, the M. avium-infected cells had significantly greater p38 phosphorylation after 24 h than zymosan-treated cells (Fig. 1A). Densitometry of the bands at the 24-h time point indicates similar levels of p38 phosphorylation in LPS-treated and M. avium 101-infected cells, but the zymosan-treated cells have levels of p38 activation similar to those of resting cells (Fig. 1A).
FIG. 1.
Infection of murine bone marrow macrophages with M. avium 101 activates p38 MAPK. (A) Western blot analysis of p38 phosphorylation (pp38) was performed following treatment of bone marrow macrophages with LPS or infection with complement-opsonized zymosan (COZ), complement-opsonized M. avium 101 (CO101) or nonopsonized M. avium 101 (NO101) or left untreated as resting cells (RC). Equal protein, as determined by the Micro BCA assay, was loaded in each lane. All blots were stripped and reprobed with a p38 antibody to show equal amounts of this protein, but only the 24-h total p38 blot is shown here. The relative densities of the 24-h phosphorylated p38 bands were analyzed by densitometry. AU, arbitrary units. (B) NOS2 expression from cell lysates was detected by Western blotting. Results are representative of three separate experiments.
Previous studies have shown that the MAPKs are required for the LPS-mediated increase in the expression of inducible NOS2 (13), a protein known to be important for controlling mycobacterial infections in mice. Therefore, we tested whether NOS2 expression was up-regulated in macrophages infected with M. avium. As shown in Fig. 1B, LPS stimulation of mouse macrophages led to a marked increase in NOS2 expression at both early and late time points. However, macrophages infected with M. avium 101 show NOS2 expression at 4 h postinfection, but no detectable expression after 24 h. Macrophages incubated with zymosan show only a small increase in NOS2 expression after 4 h and no detectable expression after 24 h.
Decreased p38 phosphorylation in macrophages infected with pathogenic compared to nonpathogenic mycobacteria.
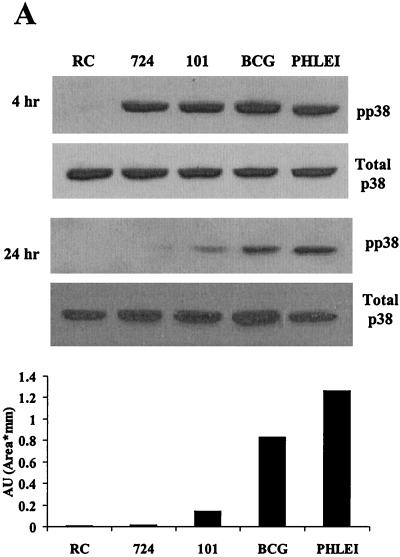
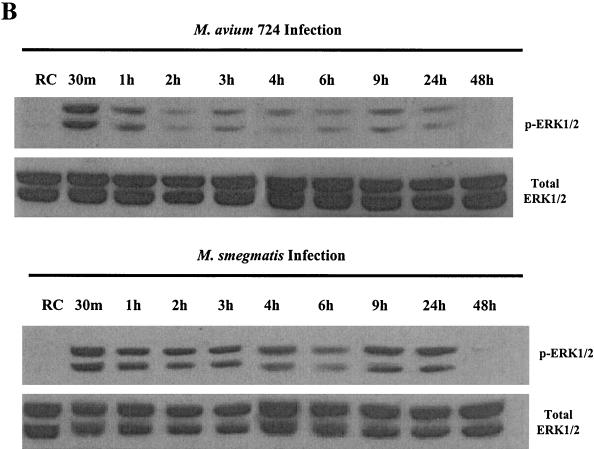
Previous studies have shown that infectious organisms of differing virulence can modulate MAPK activity (46, 54). We therefore initiated experiments to determine whether pathogenic and nonpathogenic mycobacteria differ in their ability to induce p38 and ERK1/2 activity in infected BMMs. We infected BMMs with M. avium strain 101 or 724. Infection studies indicate that M. avium 724 is approximately 100 times more virulent than strain 101 in a mouse infection model (data not shown). We also used the attenuated M. bovis BCG and the nonpathogenic M. phlei for the infection studies. Initial experiments were performed to determine the number of mycobacteria needed to obtain equal infection levels (see Materials and Methods). BMMs were infected for 4 and 24 h as described above and analyzed for phosphorylated p38, NOS2 production, and TNF-α and IL-1β secretion. As shown in Fig. 2A, all 4-h infection samples showed increased p38 phosphorylation compared to resting cells, but only M. bovis BCG- and M. phlei-infected BMMs showed high levels of p38 phosphorylation after 24 h. At this 24-h time point, no detectable p38 phosphorylation was seen in BMMs infected with the highly virulent M. avium 724. Densitometry of the phosphorylated p38 24-h autoradiograph confirmed the differences in band intensity (Fig. 2A).
FIG. 2.
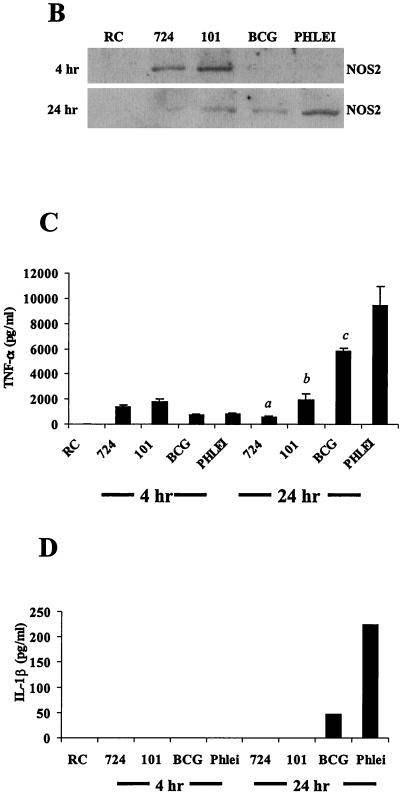
Macrophages infected with pathogenic mycobacteria show decreased MAPK activation and downstream production of inflammatory mediators compared to macrophages infected with nonpathogenic mycobacteria. (A) Western blot analysis of p38 phosphorylation (pp38) following infection with M. avium 724, M. avium 101, M. bovis BCG, and M. phlei. Noninfected macrophages (RC) were treated the same as infected macrophages for all incubations. Blots were stripped and reprobed with a p38 antibody. Densitometry was performed with the 24-h phospho-p38 blot. AU, arbitrary units. (B) NOS2 expression was detected by Western blotting. (C and D) TNF-α and IL-1β present in the culture supernatants at each time point were detected by ELISA. a, P < 0.001 compared to M. bovis BCG and to M. phlei; b, P < 0.01 compared to M. bovis BCG and P < 0.001 compared to M. phlei; c, P < 0.01 compared to M. phlei. Results are representative of three separate experiments.
We also tested whether NOS2 expression was up-regulated in BMMs infected with the different mycobacteria. The pattern of NOS2 production for BMMs infected with both M. avium strains showed expression at 4 h but no detectable NOS2 after 24 h. This was in contrast to M. phlei-infected BMMs that showed detectable NOS2 only at the 24-h time point (Fig. 2B). TNF-α production is also a downstream effect of MAPK activation in BMMs treated with LPS (18, 40). As seen previously, infection of BMMs with M. avium 101 results in TNF-α production (3, 11). TNF-α secretion was highest for M. phlei-infected BMMs after 24 h, followed by M. bovis BCG, M. avium 101, and M. avium 724 (Fig. 2C). Interestingly, this inversely correlates with the virulence of the different mycobacteria in mice (data not shown and references 3 and 21). The macrophage-conditioned media were also assayed by ELISA for secreted IL-1β, another proinflammatory cytokine downstream of p38. IL-1β secretion was observed only with BMMs infected with M. phlei and, to a lesser extent, M. bovis BCG (Fig. 2D).
Neutralization of secreted TNF-α does not inhibit the prolonged p38 MAPK activation in macrophages infected with M. avium 101 or M. smegmatis.
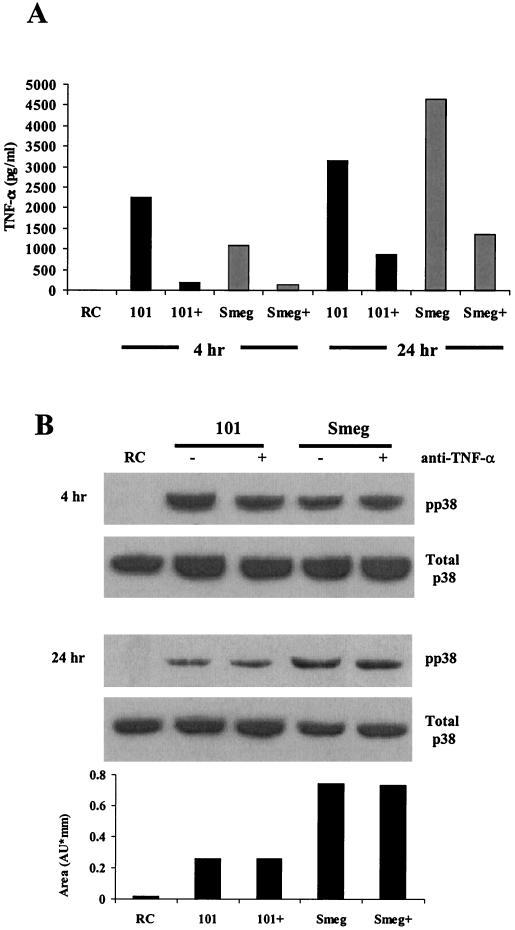
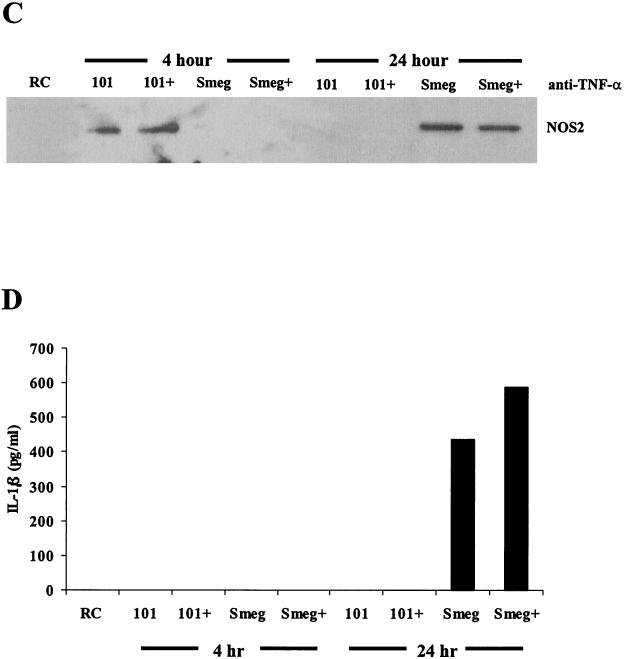
Previous studies indicate that BMMs treated with TNF-α can induce p38 phosphorylation (35, 57). However it is unknown whether BMMs infected with mycobacteria are capable of p38 activation via the TNF-α receptor. To determine whether the sustained phosphorylation of p38 in infected BMMs was due to an autocrine or paracrine effect of TNF-α secretion, we incubated M. avium 101- and nonpathogenic M. smegmatis-infected BMMs with a TNF-α neutralizing antibody. As shown in Fig. 3A, the neutralizing antibody decreased the available TNF-α by 70 to 80%, but this had no effect on the levels of p38 phosphorylation or NOS2 production (Fig. 3B and C), suggesting that TNF-α in the media is not responsible for the prolonged p38 phosphorylation.
FIG. 3.
An autocrine effect of TNF-α is not the cause for the extended p38 phosphorylation seen after 24 h. (A) TNF-α in the culture supernatants was detected by ELISA for the 4- and 24-h time points. Each supernatant was incubated with Protein G to remove any antibodies and bound TNF-α prior to the ELISA. (B) Western blot analysis of p38 phosphorylation (pp38) following treatment with LPS or infection with M. avium 101 or M. smegmatis. Cells were treated with either anti-TNF-α neutralizing antibody (+) or antibody control (−) during the 24-h infection. Noninfected macrophages, i.e., resting cells (RC), were incubated with the IgG control antibody. Blots were stripped and reprobed for total p38. Densitometry was performed with the 24-h phospho-p38 blot. (C) NOS2 expression from the cell lysates was detected by Western blotting. (D) IL-1β in the culture supernatants was detected by ELISA. Results are representative of two separate experiments. AU, arbitrary units; Smeg, M. smegmatis.
Similar to our results with M. phlei, BMMs infected with M. smegmatis, another nonpathogenic mycobacterium, showed increased p38 phosphorylation compared to BMMs infected with M. avium 101 at the 24-h time point (Fig. 3B). Densitometry of these bands confirmed this difference (Fig. 3B). Similarities were also observed between M. phlei and M. smegmatis in the kinetics of NOS2 production (Fig. 3C) and in the secretion of cytokines TNF-α and IL-1β compared to BMMs infected with M. avium 101 (Fig. 3A and D).
Kinetic analysis of p38 and ERK1/2 phosphorylation in macrophages infected with M. avium 724 and M. smegmatis.
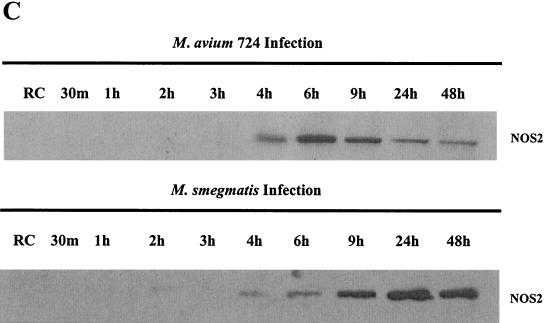
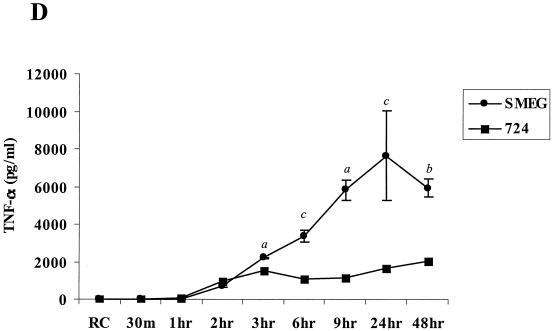
The phosphorylation of the MAPK may be transient; therefore, a more careful kinetic analysis may reveal additional differences between the ability of pathogenic and nonpathogenic mycobacteria to activate the MAPK pathways. Therefore, we infected BMMs with M. avium 724 and M. smegmatis and harvested the BMMs at 30 min and 1, 2, 3, 4, 6, 9, 24, and 48 h as described above. Phosphorylation of p38 and ERK1/2 was observed within 30 min of infection with either M. avium or M. smegmatis (Fig. 4A and B). However, p38 phosphorylation gradually decreased in M. avium 724-infected BMMs and was undetectable after 6 h. In contrast, following an M. smegmatis infection, BMMs maintained p38 phosphorylation even 24 h postinfection (Fig. 4A). Similarly, ERK1/2 phosphorylation peaked at 30 min in M. avium 724-infected BMMs and rapidly decreased to slightly above that of noninfected cells by 2 h, while ERK1/2 phosphorylation remained high in BMMs even 24 h after an M. smegmatis infection (Fig. 4B). Again, the data illustrate that BMMs infected with nonpathogenic mycobacteria maintain a prolonged activation of the MAPKs compared to BMMs infected with a highly virulent strain of M. avium.
FIG. 4.
Kinetics of MAPK activation and inflammatory responses differ between M. avium 724 and M. smegmatis infections. Western blot analyses of phosphorylated p38 (A) and ERK1/2 (B) following infections with either M. avium 724 or M. smegmatis over 48 h are shown. Blots were stripped and reprobed for total p38 and ERK1/2. (C) Western blot of NOS2 production. (D) The amount of TNF-α in the supernatants was analyzed by ELISA. a, P < 0.001; b, P < 0.01; c, P < 0.05. The results are representative of two separate experiments.
These differences extend to the production of effector molecules as well. As described above, the kinetics of NOS2 production differed in BMMs infected with M. avium and M. smegmatis. As shown in Fig. 4C, NOS2 production peaked after 6 h in M. avium 724-infected BMMs, while its expression peaked after 24 h following M. smegmatis infection, with little expression at 4 or 6 h. Finally, the M. avium- and M. smegmatis-infected BMMs showed detectable TNF-α in the culture supernatants 2 h postinfection, and the cytokine was still detectable after 48 h (Fig. 4C). For both M. smegmatis and M. avium-infected BMMs, the media were replaced after the 4-h infection; therefore, at later time points we measured only the TNF-α released from 4 h postinfection onwards. The kinetics of TNF-α secretion were similar for BMMs infected with M. smegmatis and M. avium, but the levels were considerably higher in M. smegmatis-infected BMMs (Fig. 4D). IL-1β was detected only in the culture supernatants from M. smegmatis-infected BMMs 24 and 48 h postinfection (data not shown).
TNF-α secretion and NOS2 production are MAPK dependent in mycobacterium-infected macrophages.
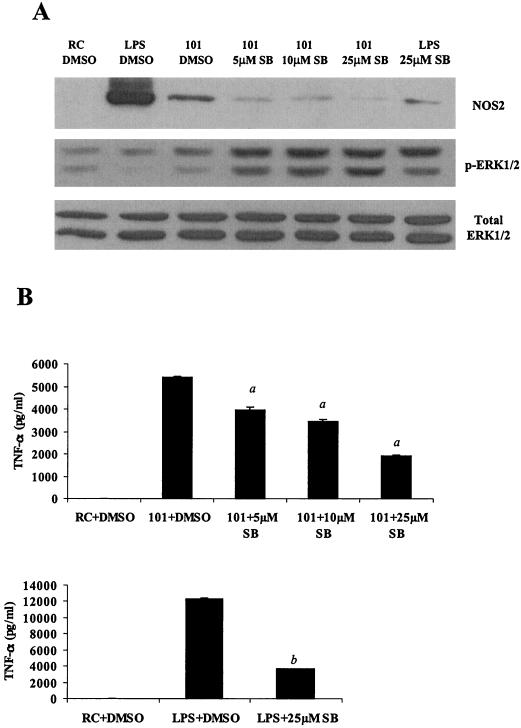
It is well established that early responses to mycobacterial infection in BMMs are the production of TNF-α and NOS2 (25). The results above show a potential association between MAPK activation and the production of immune effector proteins following a mycobacterial infection. To determine if p38 or ERK1/2 MAPK activation is indeed necessary for the BMM response to a mycobacterial infection, we used the p38-specific inhibitor SB203580 and the MEK1-specific inhibitor PD98059. Both inhibitors have been well characterized and have been shown to act with considerable specificity (2, 28, 38). Preliminary experiments using varying concentrations of both inhibitors were performed. Treatment of BMMs with SB203580, followed by a 4-h M. avium 101 infection, showed nearly complete inhibition of NOS2 production with 5 μM SB203580 (Fig. 5A), indicating that NOS2 expression was dependent on the p38 MAPK pathway. Although M. avium 101 shows little stimulation of ERK1/2 phosphorylation at this 4-h time point (Fig. 4A), we observed increased ERK1/2 phosphorylation in M. avium-infected BMMs treated with the p38 inhibitor SB203580 (Fig. 5A). TNF-α production was decreased significantly with 5 μM SB203580, which is the concentration which showed almost complete inhibition of NOS2 production. At a 25 μM concentration of the p38 inhibitor we observed more than 50% inhibition of TNF-α secretion (Fig. 5B). LPS-stimulated release of TNF-α in BMMs was also significantly inhibited with 25 μM SB203580.
FIG. 5.
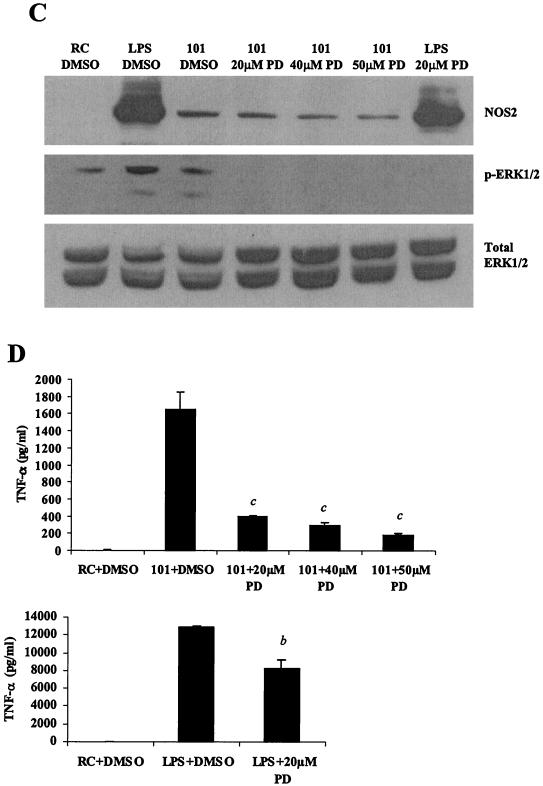
p38 and ERK1/2 MAPKs are essential for NOS2 and TNF-α production during M. avium infection. (A and C) Western blot analysis of NOS2 production and ERK1/2 phosphorylation in cell lysates. Cells were treated with varying concentrations of the p38 inhibitor SB203580 (A) or the MEK1 inhibitor PD98059 (C) (Calbiochem, San Diego, Calif.) at 37°C for 1 h before LPS treatment or M. avium 101 for a 4-h infection. DMSO vehicle control was added to resting cells (RC) and LPS treated- and M. avium 101-infected cells. Blots were probed with total ERK1/2 antibody to show equal protein loading. (B and D) TNF-α in the respective culture supernatants was analyzed by ELISA. a, P < 0.001compared to M. avium 101 plus DMSO; b, P < 0.001 compared to LPS plus DMSO; c, P < 0.01 compared to M. avium 101 plus DMSO. The results are representative of two separate experiments.
Treatment of M. avium 101-infected BMMs with 20 μM MEK1 inhibitor PD98059 resulted in no detectable ERK1/2 phosphorylation (Fig. 5C). However, treatment with the MEK1 inhibitor did not affect NOS2 expression levels, signifying that NOS2 production is not dependent on ERK1/2 activation (Fig. 5C). Conversely, a marked inhibition of TNF-α secretion was observed with 20 μM PD98059, indicating that TNF-α secretion was primarily dependent on ERK1/2 activation (Fig. 5D). Treatment of macrophages with 20 μM PD98059 also resulted in a slight but significant inhibition of TNF-α secretion following LPS stimulation (Fig. 5D).
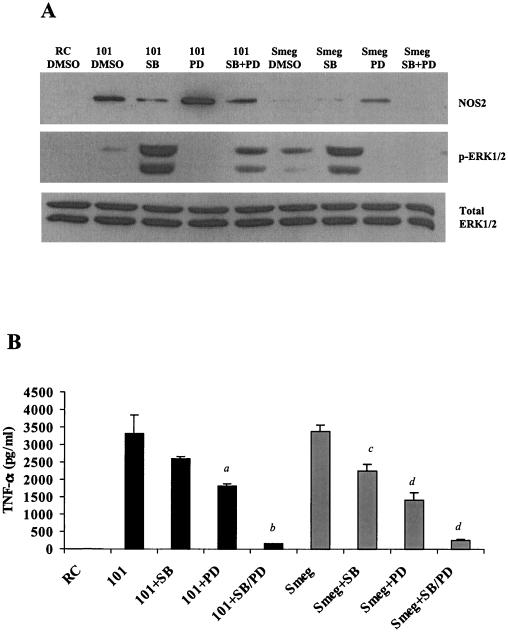
We next compared the effect of these MAPK inhibitors on the BMM response to an infection with M. avium 101 and M. smegmatis. As described above, NOS2 production was markedly decreased in M. avium 101-infected BMMs treated with the p38 inhibitor (Fig. 6A). No expression of NOS2 in M. smegmatis-infected BMMs was observed at this 4-h infection time, as previously noted (Fig. 4C). TNF-α secretion was inhibited following both M. avium and M. smegmatis infections when BMMs were treated with either inhibitor. The combination of both inhibitors resulted in almost a complete loss of TNF-α secretion (Fig. 6B). Furthermore, as was observed with M. avium, ERK1/2 phosphorylation was significantly increased in M. smegmatis-infected BMMs treated with the p38 inhibitor SB203580 (Fig. 6A). This suggests some cross talk between the two MAPK pathways in BMMs infected with mycobacteria, as has been shown previously in human umbilical vein endothelial cells after IL-1α stimulation (30) and in THP-1 cells treated with bufalin (36).
FIG. 6.
p38 and ERK1/2 MAPKs are required for NOS2 and TNF-α production by pathogenic and nonpathogenic mycobacteria. (A) Western blot analysis of NOS2 production and phosphorylated ERK1/2 in macrophages pretreated with 25 μM SB203580 and/or 50 μM PD98059. The treated and control macrophages were infected for 4 h with M. avium 101 or M. smegmatis. Blots were probed for total ERK1/2 to show equal protein loading. (B) TNF-α in the culture supernatant was analyzed by ELISA. a, P < 0.01 compared to M. avium 101 plus DMSO; b, P < 0.001 compared to M. avium 101 plus DMSO; c, P < 0.05 compared to M. smegmatis. plus DMSO; d, P < 0.001 compared to M. smegmatis. Results are representative of three separate experiments.
DISCUSSION
Pathogenic mycobacteria have evolved mechanisms to undermine the host immune response, thereby aiding their intracellular survival. Numerous studies have indicated differences between pathogenic and nonpathogenic mycobacteria in their stimulation of host macrophages to produce immune effector molecules (6, 21, 23). However, the signaling cascades initiated in macrophages upon mycobacterial invasion, which result in the production of these important effector molecules, remain poorly defined. Furthermore, how these processes are differentially regulated in BMMs infected with various species of mycobacteria is unclear. We have focused our studies on the MAPKs, since this kinase family is known to be activated by various bacterial components, including LAM (12).
Our results indicate that the MAPKs p38 and ERK1/2 are activated in BMMs upon attachment and ingestion of all mycobacteria tested. The activation of p38 may not be specific to a particular BMM receptor, since both complement-opsonized and nonopsonized M. avium induced equivalent p38 phosphorylation in infected BMMs. Our studies did not directly address which receptors induce p38 activation. However, recent studies by Reiling et al. indicate that M. avium activation of p38, ERK1/2, and JNK in human macrophages requires CD14, illustrating the importance of this molecule in BMM signaling during a mycobacterial infection (49).
Following this initial MAPK activation in macrophages infected with mycobacteria, we found that the p38 and ERK1/2 activity differed depending on the mycobacteria used for the infection. BMMs infected with nonpathogenic mycobacteria showed significantly higher p38 and ERK1/2 phosphorylation rates over time then that observed for M. avium-infected cells (Fig. 2 to 4). This suggests that either M. avium suppresses the p38 and ERK phosphorylation in BMMs relative to the nonpathogenic mycobacteria or the nonpathogenic mycobacteria are more active in their stimulation of the MAPKs. It is interesting that LAM isolated from M. tuberculosis suppresses MAPK activity in THP-1 cells and that this is dependent on the activation of the phosphatase SHP-1 (33). Perhaps M. avium suppresses p38 and ERK1/2 phosphorylation in mouse primary macrophages in a comparable manner, since the composition of LAM is similar in M. avium and M. tuberculosis (i.e., both are mannose capped) (32). However, this cannot be the only explanation, since M. bovis BCG also has ManLAM (45). How this continued p38 and ERK1/2 phosphorylation is maintained in macrophages infected with nonpathogenic mycobacteria is unclear. We are presently investigating whether this is through the direct effect of a mycobacterial component or the induction of a macrophage response (e.g., cytokine production) that subsequently induces MAPK activity. Nevertheless, our results indicate that it is not an autocrine effect of TNF-α release that triggers this response, since inhibitor antibodies to TNF-α had no effect on p38 activity (Fig. 3A). Furthermore, differences in ERK1/2 activation were observed with macrophages 1 h after the M. avium 724 and M. smegmatis infections, which is prior to detectable TNF-α secretion. In addition, 48 h after an M. avium 724 infection BMMs released more than 1,500 pg of TNF-α/ml, but this did not result in measurable p38 phosphorylation. However, the biological activity of this secreted TNF-α was not determined. Interestingly, a previous study by Balcewicz-Sablinska et al. has shown that macrophages infected with virulent M. tuberculosis strain H37Rv release TNF-α receptor, which functions to bind and inhibit the activity of secreted TNF-α (4).
To measure the potential effect of MAPK activation on the production of various immune mediators, we analyzed the infected BMMs for production of NOS2 and secretion of the cytokines TNF-α and IL-1β. We chose these particular proteins since previous studies have shown their production in macrophages to be MAPK dependent after treatment with various stimuli, including LPS (9, 13). In addition, all three proteins are known to serve important roles in controlling a mycobacterial infection (25). We found that TNF-α secretion occurred in BMMs following each of the different infections, but as has been shown previously, the levels were highest for the macrophages infected with the nonpathogenic mycobacteria M. smegmatis and M. phlei (Figs. 2 to 4) (6). These differences in TNF-α production were noticeable 6 h after infection, which is similar to what has been described previously in human monocytes treated with ManLAM and AraLAM, with AraLAM-treated cells inducing a greater TNF-α response (17).
IL-1β was also detected in BMM culture supernatants 24 and 48 h after an M. smegmatis and M. phlei infection and to a lesser extent after an M. bovis BCG infection but was not detected after the M. avium infections (Fig. 2D and 3D). These results are similar to those seen by Fattorini et al., where smooth transparent colonial variants (pathogenic) of a clinical isolate of M. avium were less capable of inducing the release of IL-1β than their smooth opaque counterparts (less pathogenic) (22). Other studies have shown IL-1β to play a protective role for the host during mycobacterial infections (19, 31). It has been previously reported that M. avium infection induces an IL-1β response; however, this response has been seen only after many days of infection (19, 23). Our results show that nonpathogenic mycobacteria are capable of inducing a much faster IL-1β response in macrophages than is observed with M. avium, which might partially account for the delayed clearance of M. avium during an active infection.
NOS2 expression was not observed or observed at low levels in BMMs 24 h post-M. avium infection, contrary to BMMs treated with LPS, which led to a continued production of NOS2 (Fig. 1B). NOS2 expression in M. smegmatis- and M. phlei-infected BMMs was also observed at this 24-h time point. Interestingly, the kinetics of NOS2 production differed for the various BMM infections (Fig. 2B, 3C, and 4C), suggesting that the signaling pathways surrounding p38 activation differ. A number of signaling pathways can lead to activation of the MAPK cascade in macrophages, including Ras-dependent (8, 26), phosphatidylinositol kinase/protein kinase C-dependent (56), and ceramide-dependent (41) pathways. However, at present we do not know what upstream pathways lead to MAPK activation.
Overall, we observed a diminished response in BMMs infected with M. avium 724 in regards to p38 and ERK1/2 activation and production of NOS2, IL-1β, and TNF-α (Fig. 4). This limited response by BMMs infected with M. avium 724 suggests that this strain has evolved to minimize its inflammatory response during an infection. This may be one of the reasons for the high pathogenicity of strain 724 in mice compared to that of other M. avium strains (data not shown).
Our results indicate that NOS2 production is dependent on p38; however, TNF-α release is dependent on both p38 and ERK1/2 activation (Fig. 5 and 6). This is contrary to the results for RAW 264.7γNO(−) cells, which showed only the requirement for ERK activation in the induction of NOS2 expression following treatment of the cells with M. tuberculosis LAM (12). The observed differences between our results and those of Chan et al. (12) may be the use of intact mycobacteria compared to an individual component or differences between primary macrophages and the RAW 264.7 cell line. Differences in MAPK activation between macrophage cell lines and primary macrophages have been noted previously (48). Interestingly, we found that the addition of the p38 inhibitor markedly enhanced the phosphorylation of ERK1/2. This may be due to a p38 inhibitory effect on the protein kinase B/Akt pathway, resulting in increased Raf1 activity and subsequent activation of the MEK-ERK pathway (53).
In summary, our results indicate that the p38 and ERK1/2 MAPKs are activated in mouse macrophages upon infection with mycobacteria. Furthermore, prolonged activation of p38 and ERK1/2 is observed with BMMs infected with nonpathogenic mycobacteria but not with BMMs infected with M. avium 724. The activation of BMM MAPKs upon mycobacterial infection is required for TNF-α secretion and NOS2 production. These results define the MAPKs as important signaling molecules in the BMM reaction to mycobacterial infection. Furthermore, the decreased MAPK activity associated with M. avium infections suggests a unique mechanism by which this mycobacteria can modulate an immune response.
Acknowledgments
This work was supported, in part, through a grant from the American Heart Association. Shannon K. Roach is a Walther Cancer Institute Pre-Doctoral Fellow.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Adams, J. L., and C. J. Czuprynski. 1994. Mycobacterial cell wall components induce the production of TNF-alpha, IL-1, and IL-6 by bovine monocytes and the murine macrophage cell line RAW 264.7. Microb. Pathog. 16:401-411. [DOI] [PubMed] [Google Scholar]
- 2.Ajizian, S. J., B. K. English, and E. A. Meals. 1999. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J. Infect. Dis. 179:939-944. [DOI] [PubMed] [Google Scholar]
- 3.Appelberg, R., A. Sarmento, and A. G. Castro. 1995. Tumour necrosis factor-alpha (TNF-alpha) in the host resistance to mycobacteria of distinct virulence. Clin. Exp. Immunol. 101:308-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balcewicz-Sablinska, M. K., J. Keane, H. Kornfeld, and H. G. Remold. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 161:2636-2641. [PubMed] [Google Scholar]
- 5.Bellmann, K., V. Burkart, J. Bruckhoff, H. Kolb, and J. Landry. 2000. p38-dependent enhancement of cytokine-induced nitric-oxide synthase gene expression by heat shock protein 70. J. Biol. Chem. 275:18172-18179. [DOI] [PubMed] [Google Scholar]
- 6.Beltan, E., L. Horgen, and N. Rastogi. 2000. Secretion of cytokines by human macrophages upon infection by pathogenic and non-pathogenic mycobacteria. Microb. Pathog. 28:313-318. [DOI] [PubMed] [Google Scholar]
- 7.Bohlson, S. S., J. A. Strasser, J. J. Bower, and J. S. Schorey. 2001. Role of complement in Mycobacterium avium pathogenesis: in vivo and in vitro analyses of the host response to infection in the absence of complement component C3. Infect. Immun. 69:7729-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buscher, D., R. A. Hipskind, S. Krautwald, T. Reimann, and M. Baccarini. 1995. Ras-dependent and -independent pathways target the mitogen-activated protein kinase network in macrophages. Mol. Cell. Biol. 15:466-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caivano, M., and P. Cohen. 2000. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1 beta in RAW264 macrophages. J. Immunol. 164:3018-3025. [DOI] [PubMed] [Google Scholar]
- 10.Canagarajah, B. J., A. Khokhlatchev, M. H. Cobb, and E. J. Goldsmith. 1997. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90:859-869. [DOI] [PubMed] [Google Scholar]
- 11.Champsi, J., L. S. Young, and L. E. Bermudez. 1995. Production of TNF-alpha, IL-6 and TGF-beta, and expression of receptors for TNF-alpha and IL-6, during murine Mycobacterium avium infection. Immunology 84:549-554. [PMC free article] [PubMed] [Google Scholar]
- 12.Chan, E. D., K. R. Morris, J. T. Belisle, P. Hill, L. K. Remigio, P. J. Brennan, and D. W. Riches. 2001. Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-êB signaling pathways. Infect. Immun. 69:2001-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, C., Y. H. Chen, and W. W. Lin. 1999. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology 97:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobb, M. H. 1999. MAP kinase pathways. Prog. Biophys. Mol. Biol. 71:479-500. [DOI] [PubMed] [Google Scholar]
- 15.Cobb, M. H., and E. Goldsmith. 1995. How MAP kinases are regulated. J. Biol. Chem. 270:14843-14846. [DOI] [PubMed] [Google Scholar]
- 16.Cross, T. G., D. Scheel-Toellner, N. V. Henriquez, E. Deacon, M. Salmon, and J. M. Lord. 2000. Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 256:34-41. [DOI] [PubMed] [Google Scholar]
- 17.Dahl, K. E., H. Shiratsuchi, B. D. Hamilton, J. J. Ellner, and Z. Toossi. 1996. Selective induction of transforming growth factor beta in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis. Infect. Immun. 64:399-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFranco, A. L., J. Hambleton, M. McMahon, and S. L. Weinstein. 1995. Examination of the role of MAP kinase in the response of macrophages to lipopolysaccharide. Prog. Clin. Biol. Res. 392:407-420. [PubMed] [Google Scholar]
- 19.Denis, M., and E. Ghadirian. 1994. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect. Immun. 62:457-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. (Erratum, 269:17.) [DOI] [PubMed]
- 21.Falcone, V., E. B. Bassey, A. Toniolo, P. G. Conaldi, and F. M. Collins. 1994. Differential release of tumor necrosis factor-alpha from murine peritoneal macrophages stimulated with virulent and avirulent species of mycobacteria. FEMS Immunol. Med. Microbiol. 8:225-232. [DOI] [PubMed] [Google Scholar]
- 22.Fattorini, L., Y. Xiao, C. M. Ausiello, F. Urbani, A. laSala, M. Mattei, and G. Orefici. 1996. Late acquisition of hyporesponsiveness to lipopolysaccharide by Mycobacterium avium-infected human macrophages in producing tumor necrosis factor-alpha but not interleukin-1 beta and -6. J. Infect. Dis. 173:1030-1034. [DOI] [PubMed] [Google Scholar]
- 23.Fattorini, L., Y. Xiao, B. Li, C. Santoro, F. Ippoliti, and G. Orefici. 1994. Induction of IL-1 beta, IL-6, TNF-alpha, GM-CSF and G-CSF in human macrophages by smooth transparent and smooth opaque colonial variants of Mycobacterium avium. J. Med. Microbiol. 40:129-133. [DOI] [PubMed] [Google Scholar]
- 24.Feng, G. J., H. S. Goodridge, M. M. Harnett, X. Q. Wei, A. V. Nikolaev, A. P. Higson, and F. Y. Liew. 1999. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 163:6403-6412. [PubMed] [Google Scholar]
- 25.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 26.Geppert, T. D., C. E. Whitehurst, P. Thompson, and B. Beutler. 1994. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol. Med. 1:93-103. [PMC free article] [PubMed] [Google Scholar]
- 27.Guay, J., H. Lambert, G. Gingras-Breton, J. N. Lavoie, J. Huot, and J. Landry. 1997. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J. Cell Sci. 110:357-368. [DOI] [PubMed] [Google Scholar]
- 28.He, H., X. Wang, M. Gorospe, N. J. Holbrook, and M. A. Trush. 1999. Phorbol ester-induced mononuclear cell differentiation is blocked by the mitogen-activated protein kinase kinase (MEK) inhibitor PD98059. Cell Growth Differ. 10:307-315. [PubMed] [Google Scholar]
- 29.Hiller, G., and R. Sundler. 1999. Activation of arachidonate release and cytosolic phospholipase A2 via extracellular signal-regulated kinase and p38 mitogen-activated protein kinase in macrophages stimulated by bacteria or zymosan. Cell. Signal. 11:863-869. [DOI] [PubMed] [Google Scholar]
- 30.Houliston, R. A., J. D. Pearson, and C. P. Wheeler-Jones. 2001. Agonist-specific cross talk between ERKs and p38(mapk) regulates PGI(2) synthesis in endothelium. Am. J. Physiol. Cell Physiol. 281:C1266-C1276. [DOI] [PubMed]
- 31.Juffermans, N. P., A. Verbon, J. T. Belisle, P. J. Hill, P. Speelman, S. J. van Deventer, and T. van der Poll. 2000. Mycobacterial lipoarabinomannan induces an inflammatory response in the mouse lung. A role for interleukin-1. Am. J. Respir. Crit. Care Med. 162:486-489. [DOI] [PubMed] [Google Scholar]
- 32.Khoo, K. H., J. B. Tang, and D. Chatterjee. 2001. Variation in mannose-capped terminal arabinan motifs of lipoarabinomannans from clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium complex. J. Biol. Chem. 276:3863-3871. [DOI] [PubMed] [Google Scholar]
- 33.Knutson, K. L., Z. Hmama, P. Herrera-Velit, R. Rochford, and N. E. Reiner. 1998. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes. Role of the Src homology 2 containing tyrosine phosphatase 1. J. Biol. Chem. 273:645-652. [DOI] [PubMed] [Google Scholar]
- 34.Kovalovsky, D., D. Refojo, F. Holsboer, and E. Arzt. 2000. Molecular mechanisms and Th1/Th2 pathways in corticosteroid regulation of cytokine production. J. Neuroimmunol. 109:23-29. [DOI] [PubMed] [Google Scholar]
- 35.Kovarik, P., D. Stoiber, P. A. Eyers, R. Menghini, A. Neininger, M. Gaestel, P. Cohen, and T. Decker. 1999. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway. Proc. Natl. Acad. Sci. USA 96:13956-13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurosawa, M., S. Numazawa, Y. Tani, and T. Yoshida. 2000. ERK signaling mediates the induction of inflammatory cytokines by bufalin in human monocytic cells. Am. J. Physiol. Cell Physiol. 278:C500-C508. [DOI] [PubMed]
- 37.Law, S. K. A., K. B. M. Reid, and British Society for Immunology. 1988. Complement. IRL Press, Oxford, United Kingdom.
- 38.Lee, J. C., S. Kassis, S. Kumar, A. Badger, and J. L. Adams. 1999. p38 mitogen-activated protein kinase inhibitors—mechanisms and therapeutic potentials. Pharmacol. Ther. 82:389-397. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Blanco, E. 2000. p38 MAPK signalling cascades: ancient roles and new functions. Bioessays 22:637-645. [DOI] [PubMed] [Google Scholar]
- 40.Means, T. K., R. P. Pavlovich, D. Roca, M. W. Vermeulen, and M. J. Fenton. 2000. Activation of TNF-alpha transcription utilizes distinct MAP kinase pathways in different macrophage populations. J. Leukoc. Biol. 67:885-893. [DOI] [PubMed] [Google Scholar]
- 41.Medvedev, A. E., J. C. Blanco, N. Qureshi, and S. N. Vogel. 1999. Limited role of ceramide in lipopolysaccharide-mediated mitogen- activated protein kinase activation, transcription factor induction, and cytokine release. J. Biol. Chem. 274:9342-9350. [DOI] [PubMed] [Google Scholar]
- 42.Newman, S. L., and L. K. Mikus. 1985. Deposition of C3b and iC3b onto particulate activators of the human complement system. Quantitation with monoclonal antibodies to human C3. J. Exp. Med. 161:1414-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pages, G., P. Lenormand, G. L'Allemain, J. C. Chambard, S. Meloche, and J. Pouyssegur. 1993. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA 90:8319-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perskvist, N., L. Zheng, and O. Stendahl. 2000. Activation of human neutrophils by Mycobacterium tuberculosis H37Ra involves phospholipase C gamma 2, Shc adapter protein, and p38 mitogen-activated protein kinase. J. Immunol. 164:959-965. [DOI] [PubMed] [Google Scholar]
- 45.Prinzis, S., D. Chatterjee, and P. J. Brennan. 1993. Structure and antigenicity of lipoarabinomannan from Mycobacterium bovis BCG. J. Gen. Microbiol. 139:2649-2658. [DOI] [PubMed] [Google Scholar]
- 46.Prive, C., and A. Descoteaux. 2000. Leishmania donovani promastigotes evade the activation of mitogen-activated protein kinases p38, c-Jun N-terminal kinase, and extracellular signal-regulated kinase-1/2 during infection of naive macrophages. Eur J. Immunol. 30:2235-2244. [DOI] [PubMed] [Google Scholar]
- 47.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen- activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 48.Rao, K. M. 2001. MAP kinase activation in macrophages. J. Leukoc. Biol. 69:3-10. [PubMed] [Google Scholar]
- 49.Reiling, N., A. Blumenthal, H. D. Flad, M. Ernst, and S. Ehlers. 2001. Mycobacteria-induced TNF-alpha and IL-10 formation by human macrophages is differentially regulated at the level of mitogen-activated protein kinase activity. J. Immunol. 167:3339-3345. [DOI] [PubMed] [Google Scholar]
- 50.Riedel, D. D., and S. H. Kaufmann. 2000. Differential tolerance induction by lipoarabinomannan and lipopolysaccharide in human macrophages. Microbes Infect. 2:463-471. [DOI] [PubMed] [Google Scholar]
- 51.Roach, T., S. Slater, M. Koval, L. White, E. C. McFarland, M. Okumura, M. Thomas, and E. Brown. 1997. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr. Biol. 7:408-417. [DOI] [PubMed] [Google Scholar]
- 52.Roach, T. I., C. H. Barton, D. Chatterjee, and J. M. Blackwell. 1993. Macrophage activation: lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, KC, JE, and tumor necrosis factor-alpha. J. Immunol. 150:1886-1896. [PubMed] [Google Scholar]
- 53.Rommel, C., B. Clark, S. Zimmermann, L. Nunez, R. Rossman, K. Reid, K. Moelling, G. Yancopoulos, and D. Glass. 1999. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway. Science 286:1741-1743. [DOI] [PubMed] [Google Scholar]
- 54.Ruckdeschel, K., J. Machold, A. Roggenkamp, S. Schubert, J. Pierre, R. Zumbihl, J. P. Liautard, J. Heesemann, and B. Rouot. 1997. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. Correlation with its inhibitory effect on tumor necrosis factor-alpha production. J. Biol. Chem. 272:15920-15927. [DOI] [PubMed] [Google Scholar]
- 55.Ting, L. M., A. C. Kim, A. Cattamanchi, and J. D. Ernst. 1999. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163:3898-3906. [PubMed] [Google Scholar]
- 56.Vernhet, L., J. Y. Petit, and F. Lang. 1997. An anti-inflammatory benzamide derivative inhibits the protein kinase C (PKC)-dependent pathway of ERK2 phosphorylation in murine macrophages. J. Pharmacol. Exp. Ther. 283:358-365. [PubMed] [Google Scholar]
- 57.Winston, B. W., E. D. Chan, G. L. Johnson, and D. W. Riches. 1997. Activation of p38mapk, MKK3, and MKK4 by TNF-alpha in mouse bone marrow-derived macrophages. J. Immunol. 159:4491-4497. [PubMed] [Google Scholar]
- 58.Zu, Y. L., J. Qi, A. Gilchrist, G. A. Fernandez, D. Vazquez-Abad, D. L. Kreutzer, C. K. Huang, and R. I. Sha'afi. 1998. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-alpha or FMLP stimulation. J. Immunol. 160:1982-1989. [PubMed] [Google Scholar]