Abstract
Type III secretion systems are used by many animal and plant interacting bacteria to colonize their host. These systems are often composed of at least 40 genes, making their temporal and spatial regulation very complex. Some type III chaperones of the translocator class are important regulatory molecules, such as the LcrH chaperone of Yersinia pseudotuberculosis. In contrast, the highly homologous PcrH chaperone has no regulatory effect in native Pseudomonas aeruginosa or when produced in Yersinia. In this study, we used LcrH-PcrH chaperone hybrids to identify a discrete region in the N terminus of LcrH that is necessary for YscY binding and regulatory control of the Yersinia type III secretion machinery. PcrH was unable to bind YscY and the homologue Pcr4 of P. aeruginosa. YscY and Pcr4 were both essential for type III secretion and reciprocally bound to both substrates YscX of Yersinia and Pcr3 of P. aeruginosa. Still, Pcr4 was unable to complement a ΔyscY null mutant defective for type III secretion and yop-regulatory control in Yersinia, despite the ability of YscY to function in P. aeruginosa. Taken together, we conclude that the cross-talk between the LcrH and YscY components represents a strategic regulatory pathway specific to Yersinia type III secretion.
Many animal and plant pathogenic bacteria utilize a common type III secretion system (T3SS) to cause disease (26, 41). A syringe-like translocon extending from a bacterium is thought to inject toxic proteins directly into host cells (38, 44). Infected cells become disarmed of their innate defenses, and this enables establishment of often-lethal infections (16, 65, 83). A unique feature of all T3SSs is their requirement for dedicated cytosolic accessory proteins (chaperones) to specifically bind one, or at most a few, cognate substrates to ensure their presecretory stabilization and/or efficient targeting to the type III secretion machinery (22, 53, 55). Recent high-resolution structural analysis suggests that these chaperones maintain their cargo in a partially nonfolded conformation, ensuring their efficient secretion (64). However, there is a clear structural demarcation between chaperones of the effector class (those that bind one or more substrates, which are destined for translocation into target cells) and chaperones of the translocator class (those that bind two substrates that are essential for translocation of the effectors), since only this latter class contains tetratricopeptide repeat (TPR) motifs (54). Not only are these TPRs required for chaperone function, but their inherent flexibility allows the chaperones to recognize the two cognate translocator substrates differently (21a).
LcrH (also termed SycD) of pathogenic Yersinia spp. is a translocator class chaperone responsible for the presecretory stabilization and efficient secretion of the translocator proteins YopB and YopD (24, 51, 75). YopD possesses two distinct LcrH binding domains, one spanning the N terminus and one encompassing the C-terminal amphipathic domain (24), while no discrete binding domains were observed in YopB (51). Interestingly, LcrH (2, 25) and other similar chaperones, like SicA of Salmonella enterica (14, 71), IpgC of Shigella flexneri (46), and SycB of Y. enterocolitica (73), are involved in regulation of gene expression and the ordered secretion of type III substrates. In Yersinia, LcrH-YopD complex formation is an important regulatory event (2, 25, 52), as is binding to the T3SS component YscY (25). However, another LcrH homologue, PcrH of Pseudomonas aeruginosa (1, 6), does not influence system regulation in this pathogen, nor can it complement the regulatory defect of an ΔlcrH null mutant of Yersinia, despite its capacity to bind, stabilize, and promote efficient secretion of the YopD regulatory element (6). This suggests that LcrH contains a distinct regulatory domain, not present in PcrH, which is required for maintenance of controlled type III secretion in Yersinia.
In this study we sought to locate key domains of LcrH that define it as an important T3SS regulator. We established chimeras between LcrH and PcrH in which fusion points were determined by the borders of the recently defined TPR sequences (54). This enabled the mapping of a distinct regulatory domain, independent of YopD binding, to the N terminus of LcrH, located just upstream of the first TPR. This region also contributed to the YscY binding ability of the chaperone. Moreover, since PcrH was unable to bind to either YscY or the homologue Pcr4 of P. aeruginosa, and since Pcr4 could not complement a ΔyscY null mutant, we envisage the LcrH-YscY complex to be a specific regulatory mechanism of type III secretion in pathogenic Yersinia.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Unless otherwise indicated, bacteria were routinely cultivated in Luria-Bertani (LB) agar or broth at either 26°C (Y. pseudotuberculosis) or 37°C (Escherichia coli, P. aeruginosa) with aeration. Where appropriate, antibiotics were added at the following final concentrations: ampicillin (Amp; 100 μg per ml), chloramphenicol (Cm; 25 μg per ml), gentamicin (Gm; 20 μg per ml), kanamycin (Km; 50 μg per ml), and tetracycline (Tet; 15 μg per ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| Strain | ||
| E. coli | ||
| DH5α | φ80dlacZΔm15 recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF) U169 | Stratagene |
| S17-1λpir | recA thi pro hsdRM+ Smr <RP4:2-Tc:Mu:Ku:Tn7>Tpr | 62 |
| P. aeruginosa | ||
| PAK | Wild-type clinical isolate | D. Bradley |
| PAKpcr3 | PAK, pcr3 in-frame deletion of codons 7-116 | This study |
| PAKpcr4 | PAK, pcr4 in-frame deletion of codons 7-101 | This study |
| Y. pseudotuberculosis | ||
| YPIII/pIB102 | yadA::Tn5, Kmr (wild type) | 4 |
| YPIII/pIB650 | pIB102, lcrH in-frame deletion of codons 2-157 | 25 |
| YPIII/pIB880 | pIB102, yscX in-frame deletion of codons 24-106 | This study |
| YPIII/pIB890 | pIB102, yscY in-frame deletion of codons 14-90 | This study |
| YPIII/pIB881 | pIB102, yscX yscY in-frame deletion spanning from codons 24 of yscX to 90 of yscY | This study |
| YPIII/pIB881-650 | pIB881, lcrH in-frame deletion of codons 2-157 | This study |
| S. cerevisiae | ||
| PJ69-4A | MATa, trp1-901 leu2-3 112 ura3-52 his3-200 gal4 gal80 GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ | 43 |
| Plasmids | ||
| pCR 4-TOPO | TA cloning vector, Kmr Ampr | Invitrogen |
| pALTER-Ex1 | Tetr Amps | Promega |
| pJEB85 | 522-bp EcoRI/BamHI fragment of pcrH on pALTER-Ex1, Tetr Amps | This study |
| pJEB87 | 522-bp EcoRI/BamHI fragment of pcrHRGLSE(30-34)NEISS on pALTER-Ex1, Tets Ampr | This study |
| pDM4 | Suicide plasmid carrying sacBR, Cmr | 49 |
| pEX18Gm | Suicide plasmid carrying sacBR, Gmr | 37 |
| pMF160 | 545-bp SpeI/XbaI PCR fragment of ΔlcrH2-157 on pDM4, Cmr | 24 |
| pMF535 | 640-bp XhoI/XbaI PCR fragment of ΔyscX24-106 on pDM4, Cmr | This study |
| pMF534 | 550-bp XhoI/XbaI PCR fragment of ΔyscY14-90 on pDM4, Cmr | This study |
| pJEB342 | 557-bp XhoI/XbaI PCR fragment of ΔyscX24-122yscY1-90 on pDM4, Cmr | This study |
| pJEB297 | 1,037-bp EcoRI/HindIII PCR fragment of Δpcr37-116 on pEX18Gm, Gmr | This study |
| pJEB298 | 1,026-bp EcoRI/HindIII PCR fragment of Δpcr47-101 on pEX18Gm, Gmr | This study |
| pEXT20 | Expression vector, Ampr | 21 |
| pPJE020 | 550-bp EcoRI/BamHI PCR fragment of lcrH on pEXT20, Ampr | This study |
| pKEC005 | 522-bp EcoRI/BamHI PCR fragment of pcrH on pEXT20, Ampr | This study |
| pMMB66HE | ptac expression vector, Ampr | 30 |
| pJEB121 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 1 on pMMB66HE, Ampr | This study |
| pJEB122 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 2 on pMMB66HE, Ampr | This study |
| pJEB123 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 3 on pMMB66HE, Ampr | This study |
| pJEB124 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 4 on pMMB66HE, Ampr | This study |
| pJEB125 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 5 on pMMB66HE, Ampr | This study |
| pJEB126 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 6 on pMMB66HE, Ampr | This study |
| pJEB127 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 7 on pMMB66HE, Ampr | This study |
| pJEB128 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 8 on pMMB66HE, Ampr | This study |
| pJEB129 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 9 on pMMB66HE, Ampr | This study |
| pJEB199 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged hybrid 10 on pMMB66HE, Ampr | This study |
| pJEB130 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged pcrH on pMMB66HE, Ampr | This study |
| pJEB132 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged pcrHRGLSE(30-34)NEISS on pMMB66HE, Ampr | This study |
| pJEB133 | HindIII/SalI PCR fragment of C-terminal FLAG-tagged lcrH on pMMB66HE, Ampr | This study |
| pMMB67EHgm | ptac expression vector, Gmr | 30 |
| pJEB291 | 385-bp EcoRI/HindIII PCR fragment of yscX on pMMB67EHgm, Gmr | This study |
| pJEB292 | 362-bp EcoRI/PstI PCR fragment of yscY on pMMB67EHgm, Gmr | This study |
| pJEB295 | 383-bp EcoRI/HindIII PCR fragment of pcr3 on pMMB67EHgm, Gmr | This study |
| pJEB296 | 343-bp EcoRI/HindIII fragment of pcr4 on pMMB67EHgm, Gmr | This study |
| pJEB335 | 720-bp EcoRI/HindIII PCR fragment of pcr3 and pcr4 on pMMB67EHgm, Gmr | This study |
| pJEB340 | 726-bp EcoRI/PstI PCR fragment of yscX and yscY on pMMB67EHgm, Gmr | This study |
| pGAD424 | LEU2, Ampr | Clontech Laboratories |
| pJEB338 | 120-bp PCR fragment of LcrH1-35 on pGAD424, LEU2, Ampr | This study |
| pMF095 | 550-bp EcoRI/PstI PCR fragment of lcrH on pGAD424, LEU2, Ampr | 24 |
| pJEB211 | EcoRI/BamHI PCR fragment of hybrid 1 on pGAD424, LEU2, Ampr | This study |
| pJEB212 | EcoRI/BamHI PCR fragment of hybrid 2 on pGAD424, LEU2, Ampr | This study |
| pJEB213 | EcoRI/BamHI PCR fragment of hybrid 3 on pGAD424, LEU2, Ampr | This study |
| pJEB214 | EcoRI/BamHI PCR fragment of hybrid 4 on pGAD424, LEU2, Ampr | This study |
| pJEB215 | EcoRI/BamHI PCR fragment of hybrid 5 on pGAD424, LEU2, Ampr | This study |
| pJEB216 | EcoRI/BamHI PCR fragment of hybrid 6 on pGAD424, LEU2, Ampr | This study |
| pJEB217 | EcoRI/BamHI PCR fragment of hybrid 7 on pGAD424, LEU2, Ampr | This study |
| pJEB218 | EcoRI/BamHI PCR fragment of hybrid 8 on pGAD424, LEU2, Ampr | This study |
| pJEB219 | EcoRI/BamHI PCR fragment of hybrid 9 on pGAD424, LEU2, Ampr | This study |
| pJEB220 | EcoRI/BamHI PCR fragment of hybrid 10 on pGAD424, LEU2, Ampr | This study |
| pJEB221 | EcoRI/BamHI PCR fragment of pcrH on pGAD424, LEU2, Ampr | This study |
| pJEB222 | EcoRI/BamHI PCR fragment of pcrHRGLSE(30-34)NEISS on pGAD424, LEU2, Ampr | This study |
| pGADT7 | LEU2 Ampr | Clontech Laboratories |
| pMF370 | 550-bp EcoRI/XhoI fragment of lcrH on pGADT7, LEU2, Ampr | 24 |
| pPJE026 | 370-bp EcoRI/BamHI fragment of yscX (from pSL122; unpublished) on pGADT7, LEU2, Ampr | This study |
| pPJE025 | 370-bp EcoRI/XhoI PCR fragment of pcr3 on pGADT7, LEU2, Ampr | This study |
| pJEB92 | EcoRI/BamHI PCR fragment of hybrid 1 on pGADT7, LEU2, Ampr | This study |
| pJEB93 | EcoRI/BamHI PCR fragment of hybrid 2 on pGADT7, LEU2, Ampr | This study |
| pJEB94 | EcoRI/BamHI PCR fragment of hybrid 3 on pGADT7, LEU2, Ampr | This study |
| pJEB95 | EcoRI/BamHI PCR fragment of hybrid 4 on pGADT7, LEU2, Ampr | This study |
| pJEB96 | EcoRI/BamHI PCR fragment of hybrid 5 on pGADT7, LEU2, Ampr | This study |
| pJEB97 | EcoRI/BamHI PCR fragment of hybrid 6 on pGADT7, LEU2, Ampr | This study |
| pJEB98 | EcoRI/BamHI PCR fragment of hybrid 7 on pGADT7, LEU2, Ampr | This study |
| pJEB99 | EcoRI/BamHI PCR fragment of hybrid 8 on pGADT7, LEU2, Ampr | This study |
| pJEB100 | EcoRI/BamHI PCR fragment of hybrid 9 on pGADT7, LEU2, Ampr | This study |
| pJEB234 | EcoRI/BamHI PCR fragment of hybrid 10 on pGADT7, LEU2, Ampr | This study |
| pJEB263 | EcoRI/BamHI PCR fragment of pcrHRGLSE(30-34)NEISS on pGADT7, LEU2, Ampr | This study |
| pJEB56 | EcoRI/XhoI PCR fragment of pcrH on pGADT7, LEU2, Ampr | 6 |
| pGBT9 | TRP1 Ampr | Clontech Laboratories |
| pSL114 | 350-bp EcoRI/PstI PCR fragment of yscY on pGBT9, TRP1, Ampr | 25 |
| pPJE065 | 340-bp BamHI/PstI PCR fragment of pcr4 on pGBT9, TRP1, Ampr | This study |
| pGBKT7 | TRP1 Kmr | Clontech Laboratories |
| pMF433 | 350-bp EcoRI/PstI of yscY (from pSL114) on pGBKT7, TRP1, Kmr | 25 |
| pPJE024 | 340-bp BamHI/PstI PCR fragment of pcr4 on pGBKT7, TRP1, Kmr | This study |
DNA amplification by PCR.
All primer combinations used to amplify wild-type Y. pseudotuberculosis YPIII/pIB102- or P. aeruginosa PAK-specific DNA are listed in Table 2. Amplified DNA was confirmed by sequence analysis using the DYEnamic ET terminator cycle sequencing kit (Amersham Biosciences, Uppsala, Sweden) by first cloning into the pCR4-TOPO TA cloning vector (Invitrogen AB, Stockholm, Sweden).
TABLE 2.
Oligonucleotides used in this study
| Activity and plasmid | Oligonucleotide pair(s)a |
|---|---|
| Deletion mutagenesis | |
| pJEB297 (Δpcr3) | pPcr3a: 5′-GCA TGT GAA TTC GAA CTG AAG CGT CTC TAC CG-3′ (EcoRI); pPcr3b: 5′-GGC ACC GAC CCG GCT CAT-3′ |
| pPcr3c: 5′-AGC CGG GTC GGT GCC CTG TTG CAC AAG GTA TGA ATC-3′; pPcr3d: 5′-ACT TCA AAG CTT TAC ACC GAC ATC ATC AGC AG-3′ (HindIII) | |
| pJEB298 (Δpcr4) | pPcr4a: 5′-GCA TGT GAA TTC GCT GCG TTG CGC CTG GAG C-3′; (EcoRI); pPcr4b: 5′-CGT CGG TTT CAA CGT CAT CG-3′ |
| pPcr4c: 5′-ACG TTG AAA CCG ACG GCG CTG GAG CTG GAG GCG CGG GAA TGA-3′; pPcr4d: 5′-ACT TCA AAG CTT CAT GTC GCC GTC GAT GCT CAT-3′ (HindIII) | |
| pMF535 (ΔyscX) | pyscXa: 5′-ACG TAC CTC GAG ACA CTG ACA TTG TGG T-3′ (XhoI); pyscXb: 5′- TAG CTC TTC CAG GCT AAT CG-3′ |
| pyscXc: 5′-TTA GCC TGG AAG AGC TAG ATG ATC GGT TAC TGC AA-3′; pyscXd: 5′-ACG TAC TCT AGA TCG CCG TTC AGT T-3′ (XbaI) | |
| pMF534 (ΔyscY) | pyscYa: 5′-ACG TAC CTC GAG TCG AAC CAC ATT-3′ (XhoI); pyscYb: 5′-CAA GAA CTC CTG TTG TCG TT-3′ |
| pyscYc: 5′-ACA ACA GGA GTT CTT GAA CGG CGA TCT TGA TA-3′; pyscYd: 5′-ACG TAC TCT AGA GGA GAG TTG ATA TAG-3′ (XbaI) | |
| pJEB342 (ΔyscX yscY) | pyscXa; pyscXb |
| pyscXYc: 5′-ATT AGC CTG GAA GAG CTA AAC GGC GAT CTT GAT AAG G-3′; pyscYd | |
| Site-directed mutagenesis | |
| pJEB85 (PcrH) | PcrH5F: 5′-ACT ACT GAA TTC GAT CTA GAG GTA TCC ATG AAC C-3′ (EcoRI); PcrH6R: 5′-GCA GCA GGA TCC TCA AGC GTT ATC GGA TTC ATA-3′ (BamHI) |
| pJEB87 (PcrHNEISS) | pMutH2: 5′-C GGC GGC ACC CTG GCG ATG CTT AAC GAA ATC AGC TCG GAC ACC CTG GAG CAG CTC TAT-3′ |
| Complementation | |
| pJEB121 (Hyb1-FLAG) | pLcrH3F: 5′-ACT ACT AAG CTT GA CAC GAG GTA ATT ATG CAA C-3′ (HindIII); pHybLcrH1: 5′-AGT GTC ACT TGA AAT TTC GTT GAG-3′ |
| pHybPcrH1: 5′-ATT TCA AGT GAC ACT CTG GAG CAG CTC TAT GCG CT-3′; pPcrHRFlag: 5′-ACT TCA GTC GAC TTA TTT ATC GTC ATC ATC TTT ATA ATC AGC GTT ATC GGA TTC ATA TGT TC-3′ (SalI) | |
| pJEB122 (Hyb2-FLAG) | pLcrH3F; pHybLcrH2: 5′-ATC ATA GTG GTC TAG CAC ACA GA-3′ |
| pHybPcrH2: 5′-CTA GAC CAC TAT GAT GCC CGC TAC TTT CTC GGC CTG-3′; pPcrHRFlag | |
| pJEB123 (Hyb3-FLAG) | pLcrH3F; pHybLcrH3: 5′-TTC TTT TAT ATC CAT TAT GGC GC-3′ |
| pHybPcrH3: 5′-ATG GAT ATA AAA GAA CCG CGC TTT CCC TTC CAT GCC-3′; pPcrHRFlag | |
| pJEB124 (Hyb4-FLAG) | pLcrH3F; pHybLcrH4: 5′-TTT GTC TGC GAT AAG CTC TTG A-3′ |
| pHybPcrH4: 5′-CTT ATC GCA GAC AAA CCG GCG CAC GAG GCC CTG GC-3′; pPcrHRFlag | |
| pJEB125 (Hyb5-FLAG) | pPcrHF: 5′-GCA TGT AAG CTT GAT CTA GAG GTA TCC ATG-3′ (HindIII); pHybPcrH5: 5-CTG TGC CGC GGC CAG GGC-3′ |
| pHybLcrH5: 5′-CTG GCC GCG GCA CAG CCT GAG TTT AAG GAG CTT TCC-3′; pLcrHRFlag: 5′-ACT TCA GTC GAC TTA TTT ATC GTC ATC ATC TTT ATA ATC TGG GTT ATC AAC GCA CTC ATG TTC-3′ (SalI) | |
| pJEB126 (Hyb6-FLAG) | pPcrHF; pHybPcrH6: 5′-CTC GTT GAT GTC CAT CAG CG-3′ |
| pHybLcrH6: 5′-ATG GAC ATC AAC GAG CCT CGT TTT CCG TTT CAT GCG-3′; pLcrHRFlag | |
| pJEB127 (Hyb7-FLAG) | pPcrHF; pHybPcrH7: 5′-GTC GTA GTG GTC GAG CAT GCA-3′ |
| pHybLcrH7: 5′-CTC GAC CAC TAC GAC TCA CGT TTC TTT TTA GGG CTA G-3′; pLcrHRFlag | |
| pJEB128 (Hyb8-FLAG) | pPcrHF; pHybPcrH8: 5′-GGT GTC CTC GCT GAG TCC G-3′ |
| pHybLcrH8: 5′-CTC AGC GAG GAC ACC TTA GAG CAA CTC TAC TCT CTT G-3′; pLcrHRFlag | |
| pJEB129 (Hyb9-FLAG) | pLcrH3F; pHybLcrH9: 5′-GGA TTC CAT TGC CAG CTG GTA TTC T-3′ |
| pHybPcrH9: 5′-CTG GCA ATG GAA TCC TTC CTG CGC GAC GGC GGC ACC CTG-3′; pPcrHRFlag | |
| pJEB130 (PcrH-FLAG) | pPcrHF; pPcrHRFlag |
| pJEB132 (PcrHNEISS-FLAG) | pPcrHF; pPcrHRFlag |
| pJEB133 (LcrH-FLAG) | pLcrH3F; pLcrHRFlag |
| pJEB199 (Hyb10-FLAG) | pLcrH3F; pHybLcrH10: 5′-GAG CAT GGC GAT AGT TCC CCC TCC-3′ |
| pHybPcrH10: 5′-GAA CTA TCG CCA TGC TC C GCG GAC TCA GCG AGG ACA CCC TG-3′; pPcrHRFlag | |
| pKEC005 (PcrH) | pPcrH5F: 5′-ACT ACT GAA TTC GAT CTA GAG GTA TCC ATG AAC C-3′ (EcoRI); pPcrH6R: 5′-GCA GCA GGA TCC TCA AGC GTT ATC GGA TTC ATA-3′ (BamHI) |
| pPJE020 (LcrH) | plcrH1: 5′-CTG GTG AAT TCC ACG AGG TAA TTA TGC-3′ (EcoRI); plcrH2: 5′-GTT ACT GCA GTT GAG CGG TCA T-3′ (PstI) |
| pJEB291 (YscX) | pYscXF: 5′-ACT ACT GAA TTC AAG AGG TGC TTG CGC CGT GAG-3′ (EcoRI); pYscXR: 5′-ACT ACT AAG CTT TCA TAC TTT GTG CAA CAG GTT-3′ (HindIII) |
| pJEB292 (YscY) | pYscY(20) for: 5′-GAA TTC AAC CTG TTG CAC AAA GTA TGA-3′ (EcoRI); pYscY(20)rev: 5′-CTG CAG TCA TGG GGA TTC ATT ATG ATC-3′ (PstI) |
| pJEB340 (YscX, YscY) | pYscXF; pYscY(20)rev |
| pJEB295 (Pcr3) | pPcr3F: 5′-ACT ACT GAA TTC AGC GAA GTG CTC GCC GCA TG-3′ (EcoRI); pPcr3R: 5′-ACT TCA AAG CTT TCA TAC CTT GTG CAA CAG GTT CAG-3′ (HindIII) |
| pJEB296 (Pcr4) | pPcr4F: 5′-ACT ACT GAA TTC CAC AAG GTA TGA ATC GAT GAC GTT-3′ (EcoRI); pPcr4R: 5′-ACT TCA AAG CTT CGT TCA TTC CCG CGC CTC CAG C-3′ (HindIII) |
| pJEB335 (Pcr3, Pcr4) | pPcr3F and pPcr4R |
| Yeast two-hybrid interaction studies | |
| pJEB211 (Hyb1) | pLcrH1: 5′-CTG GTG AAT TCC ACG AGG TAA TTA TGC-3′ (EcoRI); pHybLcrH1 |
| pHybPcrH1; pPcrH6R | |
| pJEB212 (Hyb2) | pLcrH1; pHybLcrH2 |
| pHybPcrH2; pPcrH6R | |
| pJEB213 (Hyb3) | pLcrH1; pHybLcrH3 |
| pHybPcrH3; pPcrH6R | |
| pJEB214 (Hyb4) | pLcrH1; pHybLcrH4 |
| pHybPcrH4; pPcrH6R | |
| pJEB215 (Hyb5) | pPcrH5F; pHybPcrH5 |
| pHybLcrH5; LcrH1R: 5′-GCA GCA GGA TCC TCA TCA TGG GTT ATC AAC GCA CT-3′ (BamHI) | |
| pJEB216 (Hyb6) | pPcrH5F; pHybPcrH6 |
| pHybLcrH6; LcrH1R | |
| pJEB217 (Hyb7) | pPcrH5F; pHybPcrH7 |
| pHybLcrH7; LcrH1R | |
| pJEB218 (Hyb8) | pPcrH5F; pHybPcrH8 |
| pHybLcrH8; LcrH1R | |
| pJEB219 (Hyb9) | pLcrH1; pHybLcrH9 |
| pHybPcrH9; pPcrH6R | |
| pJEB220 (Hyb10) | pLcrH1; pHybLcrH10 |
| pHybPcrH10; pPcrH6R | |
| pJEB221 (PcrH) | pPcrH5F; pPcrH6R |
| pJEB222 (PcrHNEISS) | pPcrH5F; pPcrH6R |
| pJEB338 (LcrH1-35) | pLcrH1; plcrH-Nterm(Pst): 5′-CTG CAG TCA AGT GTC ACT TGA AAT TTC GTT GAG-3′ (PstI) |
| pPJE025 (Pcr3) | pcr3-A: 5′-CCG GAA TTC ATG AGC CGG GTC GGT G-3′ (EcoRI); pcr3-C: 5′-CCG CTC GAG TCA TAC CTT GTG CAA CAG-3′ (XhoI) |
| pPJE024 (Pcr4) | pcr4-A: 5′-CGC CGC GGA TCC CAA TCG ATG ACG TTG AA-3′ (BamHI); pcr4-B: 5′-AAA ACT GCA GTC ATT CCC GCG CCT CCA-3′ (PstI) |
The nucleotide sequences in italics represent the incorporated HindIII, SalI, EcoRI, XhoI, PstI or BamHI restriction sites used for cloning of the PCR amplified DNA fragments. The underlined sequence indicates the complementary overlap between respective primers in the overlap PCR reactions. In the primer pMutH2, for generation of PcrHNEISS by site-directed mutagenesis, the codons substituted are indicated in boldface.
Construction of mutants in P. aeruginosa and Y. pseudotuberculosis.
Overlap PCR (39) was used in the construction of five suicide plasmids, pJEB297, pJEB298, pMF535, pMF534, and pJEB342, used to create the respective Δpcr3 and Δpcr4 mutations in wild-type P. aeruginosa PAK and the ΔyscX ΔyscY and ΔyscX yscY mutations in wild-type Y. pseudotuberculosis YPIII/pIB102. The resulting 1,037-, 1,026-, 640-, 550-, and 557-bp PCR fragments containing sequence flanking the pcr3, pcr4, yscX, and yscY genes, respectively, were introduced into EcoRI-HindIII-digested pEX18Gm (37) to give pJEB297 and pJEB298 or XhoI-XbaI-digested pDM4 (49) to give pMF535, pMF534, and pJEB342. Conjugal mating experiments using S17-1λpir as the donor strain allowed for the allelic exchange of the pJEB297 and pJEB298 suicide plasmids within regions of complementary sequences on the PAK chromosome and the pMF535, pMF534, and pJEB342 plasmids within regions of complementary sequences on the Yersinia virulence plasmid as described previously (49). The resulting mutants were denoted PAKpcr3 (a near-full-length in-frame deletion of codons 7 to 116 in Pcr3), PAKpcr4 (7 to 101 in Pcr4), YPIII/pIB880 (24 to 106 in YscX), YPIII/pIB890 (14 to 90 in YscY), and YPIII/pIB881 (from codon 24 of YscX to codon 90 of YscY). Finally, the ΔyscX yscY lcrH triple mutant (YPIII/pIB881-650) was constructed by the same allelic exchange procedure after introducing the mutagenesis vector pMF160, which contained a gene deletion of lcrH that removed codons 2 to 157 (24), into YPIII/pIB881.
Generation of trans-complementing expression plasmids.
DNA fragments encoding FLAG-tagged chaperone chimeras were generated by overlap PCR (39) and subsequently introduced into HindIII-SalI-digested pMMB66HE (30). For construction of the PcrHNEISS variant, the Altered Sites II in vitro mutagenesis system (Promega) was used as specified by the manufacturer. pcrH (pJEB85)-specific template for mutagenesis is a derivative of pALTER-Ex1. The resulting mutagenized gene pcrHRGLSE(30-34)NEISS in pALTER-Ex1 (pJEB87) was used as PCR template to generate a FLAG-tagged HindIII-SalI fragment for cloning into pMMB66HE. To clone yscX, yscY, pcr3, and pcr4 with their native ribosome binding sites, amplified products were introduced into EcoRI-HindIII- or EcoRI-PstI-digested pMMB67EHgm (30). Plasmids were transferred into Y. pseudotuberculosis and P. aeruginosa by conjugation.
Protein stability.
Intrabacterial protein stability was assessed by the method of Feldman and colleagues (23). Protein fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting using α-FLAG M2 monoclonal antiserum to detect chaperone chimeras (Sigma-Aldrich Sweden AB, Stockholm, Sweden) or a rabbit polyclonal antisera specifically recognizing YopD (AgriSera AB, Vännäs, Sweden) in combination with the enhanced chemiluminescence system (Amersham Biosciences).
Growth phenotypes and the MOX test.
Determination of Yersinia plating frequencies and subsequent growth phenotypes under high- and low-Ca2+ conditions at 37°C were assessed using the MOX (magnesium oxalate) test (3, 32). In some cases, the growth phenotype was assessed after growth at 37°C in liquid TMH medium under high- and low-Ca2+ conditions (25, 52, 67). For definitions of growth phenotypes, see Table 3.
TABLE 3.
Growth phenotypes and plating frequencies of Yersinia pseudotuberculosis strains
| Strain | Relevant genotype or phenotype | IPTG concn (mM) | Plating frequencya
|
Growth phenotypeb | |
|---|---|---|---|---|---|
| With Ca2+ | Without Ca2+ | ||||
| YPIII/pIB102 | Wild type | NA | 1 | 10−4 | CD |
| YPIII/pIB650 | ΔlcrH | NA | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB133 | ΔlcrH pLcrH+ | 0 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB133 | ΔlcrH pLcrH+ | 0.001 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB133 | ΔlcrH pLcrH+ | 0.002 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB133 | ΔlcrH pLcrH+ | 0.003 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB133 | ΔlcrH pLcrH+ | 0.004 | 1 | 10−4 | CD |
| YPIII/pIB650, pJEB133 | ΔlcrH pLcrH+ | 0.005 | 1 | 10−4 | CD |
| YPIII/pIB650, pJEB133 | ΔlcrH pLcrH+ | 0.01 | 1 | 10−4 | CD |
| YPIII/pIB650, pJEB133 | ΔlcrH pLcrH+ | 0.4 | 1 | 10−4 | CD |
| YPIII/pIB650, pJEB130 | ΔlcrH pPcrH+ | 0.4 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB130 | ΔlcrH pPcrH+ | 1.0 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB121 | ΔlcrH pHyb1+ | 0.4 | 1 | 10−4 | CD |
| YPIII/pIB650, pJEB122 | ΔlcrH pHyb2+ | 0.4 | 1 | 10−4 | CD |
| YPIII/pIB650, pJEB123 | ΔlcrH pHyb3+ | 0.4 | 1 | 10−4 | CD |
| YPIII/pIB650, pJEB124 | ΔlcrH pHyb4+ | 0.4 | 1 | 10−4 | CD |
| YPIII/pIB650, pJEB125 | ΔlcrH pHyb5+ | 0.4 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB126 | ΔlcrH pHyb6+ | 0.4 | 10−5 | 10−5 | TS |
| YPIII/pIB650, pJEB127 | ΔlcrH pHyb7+ | 0.4 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB128 | ΔlcrH pHyb8+ | 0.4 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB129 | ΔlcrH pHyb9+ | 0.4 | 10−5 | 10−5 | TS |
| YPIII/pIB650, pJEB199 | ΔlcrH pHyb10+ | 0.4 | 10−4 | 10−4 | TS |
| YPIII/pIB650, pJEB132 | ΔlcrH pPcrHNEISS+ | 0.4 | 10−4 | 10−4 | TS |
| YPIII/pIB880 | ΔyscX | NA | 1 | 1 | CI |
| YPIII/pIB880, pJEB291 | ΔyscX pYscX+ | 0.4 | 1 | 10−4 | CD |
| YPIII/pIB880, pJEB295 | ΔyscX pPcr3+ | 0.4 | 1 | 1 | CI |
| YPIII/pIB890 | ΔyscY | NA | 1 | 1 | CI |
| YPIII/pIB890, pJEB292 | ΔyscY pYscY+ | 0.4 | 1 | 10−4 | CD |
| YPIII/pIB890, pJEB296 | ΔyscY pPcr4+ | 0.4 | 1 | 1 | CI |
| YPIII/pIB881 | ΔyscX yscY | NA | 1 | 1 | CI |
| YPIII/pIB881, pJEB340 | ΔyscX yscY pYscX YscY+ | NA | NA | NA | CDc |
| YPIII/pIB881, pJEB335 | ΔyscX yscY pPcr3 Pcr4+ | NA | NA | NA | CIc |
| YPIII/pIB881-650 | ΔlcrH yscX yscY | NA | 10−4 | 10−4 | TS |
| YPIII/pIB881-650, pJEB020 | ΔlcrH yscX yscY pLcrH+ | 0.4 | 1 | 1 | CI |
| YPIII/pIB881-650, pKEC005 | ΔlcrH yscX yscY pPcrH+ | 0.4 | 10−4 | 10−4 | TS |
| YPIII/pIB881-650, pPJE020, pJEB340 | ΔlcrH yscX yscY pLcrH+ pYscX YscY+ | NA | NA | NA | CDc |
| YPIII/pIB881-650, pKEC005, pJEB335 | ΔlcrH yscX yscY pPcrH+ pPcr3 Pcr4+ | NA | NA | NA | CIc |
Plating frequency readout is derived from MOX analysis of Yersinia mutants trans-complemented with full-length yscX, yscY, pcr3, pcr4, lcrH, and pcrH or hybrid alleles of lcrH and pcrH under the control of an IPTG-inducible promoter. Determinations were made following at least two independent experiments according to the protocol of our established methods (5, 6, 25, 52).
CD, calcium dependent; bacteria grow only in the presence of Ca2+ at 37°C, reflecting wild-type regulatory control of Yop synthesis. TS, temperature sensitive; bacteria are growth restricted at 37°C, reflecting defective regulatory control whereby Yop synthesis is constitutive. CI, calcium independent; bacteria show calcium independent growth at 37°C, reflecting defective regulatory control whereby Yop synthesis is downregulated.
Phenotypic growth determinations for Yersinia strains were performed at 37°C in liquid TMH medium (minus Ca2+) or medium supplemented with 2.5 mM CaCl2 (plus Ca2+) (25, 52, 67). Determinations were made following at least two independent experiments.
NA, not applicable.
Synthesis and secretion of type III-secreted substrates.
Induction of type III substrate synthesis and secretion from Y. pseudotuberculosis and P. aeruginosa was performed as previously described (6, 24, 25). Total protein levels were assessed by sampling directly from the bacterial culture suspension containing a mix of proteins secreted to the culture medium and within intact bacteria. Sampling the cleared supernatant allowed assessment of secreted protein levels. All protein fractions were separated by SDS-PAGE and subjected to immunoblotting. Detection of specific proteins on membrane support was achieved by the use of rabbit polyclonal antisera raised against all secreted Yops or antisera specifically recognizing YopH, LcrV, or YopD (Yersinia substrates) as well as ExoS or PcrV (P. aeruginosa substrates) (AgriSera AB).
Cultivation and infection of HeLa cells.
The human epithelial cell line HeLa was used in all in vitro infection experiments. Culture maintenance and infections with Yersinia (68) or P. aeruginosa (69) followed our standard methods, except that isopropyl-β-d-thiogalactopyranoside (IPTG) (0.4 mM) was added to both bacteria and cell monolayers prior to infection. The cytotoxicity of infected HeLa cells was monitored by light microscopy, and images were collected at successive time points.
Yeast plasmid construction and transformation and the two-hybrid assay.
Interaction studies in yeast between YscY (25) and full-length or hybrid chaperones required plasmid construction of hybrid alleles essentially generated as described in the section above on generation of trans-complementing expression plasmids by cloning of amplified DNA without the C-terminal FLAG epitope into the EcoRI/BamHI-digested GAL4 activation domain plasmid pGAD424 (Clontech Laboratories, Palo Alto, CA). Generation of PcrHNEISS fused to the GAL4 activation domain required initial amplification by PCR using the template pJEB87. For YscY and YscX interaction studies, EcoRI/BamHI-digested yscX was lifted from plasmid pSL122 (unpublished data) and introduced into the GAL4 activation domain plasmid pGADT7 (Clontech Laboratories) to give pPJE026. To investigate Pcr4-Pcr3 binding, PCR-amplified pcr4 was cloned into BamHI/PstI-digested pGBKT7, forming pPJE024, while pcr3 was cloned into EcoRI/XhoI-digested pGADT7, giving pPJE025.
Transformation of the Saccharomyces cerevisiae reporter strain PJ69-4A was performed as described earlier (24). Protein interactions from multiple independent transformations were determined by measuring the activation of the ADE2 reporter gene and the HIS3 reporter gene. For the latter assays, 1 mM 3-aminotriazole was used in the growth medium to overcome any risk of false positives (43). Analysis of protein stability in yeast was performed as previously described (24). However, it necessitated the lifting of GAL4 activation domain (GAL4-AD) fusions from pGAD424 into pGADT7 and GAL4 DNA binding domain (GAL4-BD) fusions from pGBT9 to pGBKT7, since these vectors are more suitable for protein expression studies in yeast (6, 24, 25).
Nucleotide sequence accession number.
The nucleotide sequence incorporating pcr3 and pcr4 has been deposited in GenBank (accession number DQ000666).
RESULTS
PcrH is unable to restore regulatory control in the ΔlcrH null mutant of Y. pseudotuberculosis.
Although the PcrH chaperone of P. aeruginosa can substitute for LcrH in Yersinia to ensure correct assembly of a functional translocon, it is not able to restore yop-regulatory control, consistent with PcrH being dispensable for control of P. aeruginosa type III secretion (6). To investigate this difference in regulatory potential, we first compared the expression levels of C-terminally FLAG-tagged pcrH and lcrH under the control of the Ptac IPTG-inducible promoter from pMMB66HE. As detected by the anti-FLAG antibody, the PcrH levels produced in Yersinia when grown in media supplemented with 0.4 mM of IPTG was roughly equivalent to the level of LcrH recovered from 0.01 mM IPTG induction (Fig. 1). To determine if this low level of PcrH could explain the lack of complementation of the regulatory defect of the ΔlcrH null mutant (6), we examined the minimal level of LcrH required to complement this same mutant. As determined by a MOX plate analysis (3, 32), an ΔlcrH null mutant of Y. pseudotuberculosis did not grow at 37°C (termed the temperature-sensitive [TS] growth phenotype) regardless of Ca2+ concentration in the medium (Table 3). Reflecting a loss of regulatory control, this TS phenotype mirrored constitutive Yop synthesis during these same growth conditions (6, 25). In contrast, wild-type Y. pseudotuberculosis required Ca2+ to grow at 37°C (termed the calcium-dependent [CD] growth phenotype) (Table 3) and reflected normal yop-regulatory control (3, 56, 81). Significantly, as little as 0.004 mM IPTG was sufficient to induce expression of enough LcrH to restore regulatory control to the ΔlcrH null mutant by virtue of a change in growth phenotype from TS to CD (Fig. 1). However, the amount of LcrH was considerably less than the levels of noncomplementing PcrH resulting from 0.4 mM IPTG induction (Fig. 1). Therefore, we conclude that low expression levels do not account for the inability of PcrH to complement the regulatory defect of the ΔlcrH null mutant.
FIG. 1.
Comparative levels of PcrH and LcrH produced in trans by the Y. pseudotuberculosis ΔlcrH null mutant. Immunoblot of chaperone protein prepared from bacteria grown in Yop-inducing medium (BHI minus Ca2+) supplemented with a range of IPTG concentrations (0 to 0.4 mM). Both PcrH and LcrH were identified using monoclonal anti-FLAG antiserum in combination with enhanced chemiluminescence detection. Also indicated is the concentration of IPTG required to generate enough LcrH for trans-complementation of the ΔlcrH null mutant with respect to regulation. Definitions of TS (temperature sensitive) and CD (calcium dependent) can be found in Table 3.
Generation and expression analysis of LcrH/PcrH chaperone chimeras.
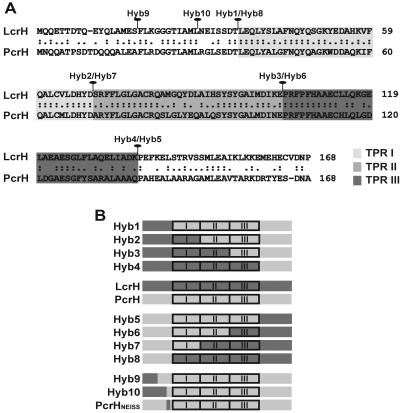
These findings indicate that LcrH might possess a distinct regulatory domain lacking in PcrH. We therefore generated a series of LcrH/PcrH chimeras to identify regulatory domains specific to LcrH. The fusion point for the chimeras was placed at junctions of the recurring TPR motif (Fig. 2A) (54), resulting in hybrid chaperones that initiated with LcrH and contained increasing amounts of LcrH extending from the N terminus (Hyb1 to Hyb4) or initiated with PcrH and contained increasing amounts of C-terminal LcrH (Hyb5 to Hyb8) (Fig. 2B). Alleles were cloned under the control of the Ptac promoter of pMMB66HE with a C-terminal FLAG tag to facilitate immunodetection. We used an intrabacterial protein stability assay (23, 52) to investigate the stability of each chaperone chimera when expressed in an ΔlcrH null mutant background grown in non-secretion-permissive medium (brain heart infusion medium [BHI] plus Ca2+) in the presence of IPTG. Although some hybrids were slightly more susceptible to endogenous proteases, all hybrids were essentially stable (Fig. 3A). Levels of synthesis of each hybrid in secretion-permissive medium (BHI minus Ca2+) were also examined. N-terminal PcrH chaperone variants behaved like full-length PcrH, in that they were produced at lower levels compared to those of variants initiated by LcrH (Fig. 3B). We interpret these results to indicate that the hybrid chaperones appear to behave in a manner similar to that of the parental chaperones.
FIG. 2.
Schematic representation of the chaperone hybrids used in this study. Shown is a sequence alignment between LcrH of Y. pseudotuberculosis and PcrH of P. aeruginosa (A), where the positions of the three tetratricopeptide repeat (TPR) regions are indicated by various shades of gray. The fusion points at positions Hyb1 to Hyb10 are indicated for chaperone hybrids derived from LcrH (dark gray) and PcrH (light gray) (B). Block diagrams are not drawn to scale.
FIG. 3.
Stability and expression analysis of chaperone hybrids and their effect on substrate stability. (A) The intrabacterial stability of chaperone hybrids (left panel) and YopD substrate (right panel) produced by Y. pseudotuberculosis grown at 37°C in BHI supplemented with 2.5 mM CaCl2 (type III secretion repressed) and 0.4 mM IPTG was examined. At time zero, chloramphenicol was added in order to stop de novo protein synthesis. Samples from pelleted bacteria were taken at different time intervals, and the amount of protein was detected by Western blot. (B) Immunoblot of synthesized chaperone hybrids prepared from pelleted bacteria grown in Yop inducing medium (BHI minus Ca2+) supplemented with 0.4 mM IPTG. Hybrids were identified using monoclonal anti-FLAG antiserum and YopD by a polyclonal rabbit anti-YopD antiserum.
The N terminus of LcrH contains a unique regulatory domain.
To examine the ability of these chaperone chimeras to restore yop-regulatory control in the ΔlcrH null mutant, a MOX test was performed in combination with a Western blot analysis of the total levels of Yops and LcrV synthesized (total samples) and secreted (cleared supernatants) during growth in inductive (BHI without Ca2+) and noninductive (BHI with Ca2+) media. Significantly, trans-production of full-length LcrH and chaperone chimeras Hyb1 to Hyb4 were all able to efficiently restore the growth phenotype of the null mutant from TS to a wild-type-like CD growth phenotype (Table 3) such that elevated levels of YopH and LcrV were produced and secreted only in inductive medium (Fig. 4). In contrast, the lcrH null mutant alone and that harboring full-length PcrH or the chaperone chimeras Hyb5 to Hyb8 all remained sensitive to the temperature up-shift (TS) (Table 3) and constitutively produced proteins in both inductive and noninductive media (Fig. 4, upper panel). In addition, these same strains also specifically secreted LcrV in non-secretion-competent medium (BHI with Ca2+) (Fig. 4, lower panel), which is a reproducible phenomenon observed in all mutants of lcrH or yopD exhibiting a TS phenotype (5, 6, 24, 25, 27, 63, 76).
FIG. 4.
Analysis of Yops and LcrV synthesis and secretion from Y. pseudotuberculosis strains grown either with (+) or without (−) Ca2+. Yops and LcrV in the total protein fraction (a mix of proteins secreted to the culture medium and contained within intact bacteria) (upper panel) or secreted to the extracellular medium (cleared culture supernatants) (lower panel) were separated by SDS-PAGE and identified by immunoblot analysis using polyclonal rabbit anti-YopH, anti-LcrV, and anti-YopD antiserum. Where indicated, IPTG was added at a final concentration of 0.4 mM upon temperature shift. Lanes: a and b, wild-type YPIII/pIB102; c and d, lcrH null mutant YPIII/pIB650; e and f, complemented YPIII/pIB650, pJEB133 (LcrH+); g and h, complemented YPIII/pIB650, pJEB121 (Hyb1+); i and j, complemented YPIII/pIB650, pJEB122 (Hyb2+); k and l, complemented YPIII/pIB650, pJEB123 (Hyb3+); m and n, complemented YPIII/pIB650, pJEB124 (Hyb4+); o and p, complemented YPIII/pIB650, pJEB125 (Hyb5+); q and r, complemented YPIII/pIB650, pJEB126 (Hyb6+); s and t, complemented YPIII/pIB650, pJEB127 (Hyb7+); u and v, complemented YPIII/pIB650, pJEB128 (Hyb8+); x and y, complemented YPIII/pIB650, pJEB129 (Hyb9+); z and aa, complemented YPIII/pIB650, pJEB199 (Hyb10+); bb and cc, complemented YPIII/pIB650, pJEB132 (PcrHNEISS+); dd and ee, complemented YPIII/pIB650, pJEB130 (PcrH+). Molecular masses shown in parentheses are deduced from primary sequences.
These data highlight a key regulatory domain specific to LcrH that resides within its N-terminal 35 amino acids (i.e., prior to the junction in Hyb1). To better define this regulatory region, two additional chaperone hybrids were generated (Hyb9 and Hyb10 in Fig. 2B). Hyb9 contained 17 and Hyb10 contained 28 N-terminal LcrH residues, followed by the remainder of PcrH. Both hybrids displayed a similar degree of stability (Fig. 3A) and were expressed at levels comparable to that of wild-type LcrH (Fig. 3B). However, when expressed in an ΔlcrH null mutant, neither hybrid could alter the temperature sensitivity of this mutant (Table 3) or its failure to regulate Yop synthesis and secretion (Fig. 4). Thus, the region of LcrH important for yop regulation is found between the boundaries of Hyb10 and Hyb1.
Closer inspection of this small region revealed that 5 residues, NEISS, located at positions 29 to 33 of LcrH, differ significantly from PcrH, which encodes RGLSE. We had previously identified the glutamate moiety at position 30 of LcrH as an important regulatory requirement (25). To further investigate this amino acid stretch, we replaced the RGLSE residues of PcrH with the NEISS residues of LcrH (Fig. 2B). This PcrHNEISS variant behaved like wild-type PcrH with respect to stability (Fig. 3A) and expression (Fig. 3B). In addition, it was unable to restore regulatory control when expressed in Yersinia defective for LcrH (Table 3, Fig. 4). We conclude that N-terminal LcrH is essential for controlled Yop synthesis; however, (an)other residue(s) in addition to NEISS at position 29 to 33 must also contribute to this function.
Loss of regulatory control is not due to alterations in YopD levels.
One consequence of manipulating LcrH might be altered stability of YopD (24, 75), another key regulatory element in Yop synthesis (27, 76). To investigate whether the N-terminal-dependent regulatory function of LcrH is independent of YopD, we analyzed the ability of each chaperone hybrid to maintain normal stability and secretion of YopD when expressed in the ΔlcrH null mutant of Y. pseudotuberculosis. Bacteria were grown in non-secretion-permissive medium (BHI with Ca2+) in the presence of IPTG, and protein synthesis was blocked by the addition of chloramphenicol after 1 h. No major difference in the stability of YopD over time was observed for strains expressing the different hybrid chaperones, although regulatory competent hybrids Hyb1 and Hyb4 resulted in slightly less stable YopD (Fig. 3A). Furthermore, growth in secretion-permissive conditions (BHI without Ca2+) resulted in a comparable level of YopD secretion from all strains, except for the noncomplemented ΔlcrH null mutant used as a control (Fig. 4, lower panel). Since YopD is also a key element of the Yersinia translocon (27, 52, 76), we used the HeLa cell infection assay to determine whether the chimera expressing mutants were competent for translocation of the cytotoxin YopE. All mutants efficiently translocated YopE into infected HeLa cells, as visualized by a rapid cell rounding up (data not shown), further supporting maintenance of functional YopD. Thus, the failure to regain regulatory control in the ΔlcrH null mutant harboring PcrH, PcrHNEISS, or Hyb5 to Hyb10 is not due to destabilization of the YopD regulatory element, confirming that the N terminus of LcrH does contain a unique YopD-independent regulatory domain(s) directly involved in yop-regulatory control.
LcrH specifically establishes a regulatory complex with YscY of the Yersinia type III secretion machine.
We have recently proposed a regulatory role for LcrH that involves binding a component of the Ysc (Yersinia secretion) secretion machine, YscY (25). Having defined an N-terminal region of LcrH that is important for yop regulation, we wondered whether this regulatory domain was related to the ability of LcrH to bind YscY. Using two independent promoter reporter assays (GAL2-ADE2 and LYS2::GAL1-HIS3) in the yeast two-hybrid system, we could confirm our earlier report that LcrH does interact with YscY (Table 4) (25). Furthermore, we observed that Hyb2 to Hyb4 and Hyb8 could also strongly bind to YscY, while Hyb1 displayed weaker binding. In contrast, PcrH, PcrHNEISS, and all remaining hybrids were all unable to form this complex as implied from a lack of growth on the dropout plates (Table 4). This was not due to instability of these chaperone variants in yeast, as most were readily detected in yeast protein lysates (Fig. 5A). Therefore, with one exception (Hyb8), these results highlight an intriguing correlation between the ability of a given chaperone hybrid to regulate Yop synthesis and its capacity to bind YscY of the type III secretion machine.
TABLE 4.
Protein-protein interactions in the yeast two-hybrid assaya
| Yeast two-hybrid construct
|
HIS3+ | ADE2+ | |
|---|---|---|---|
| DNA-binding domain | Activation domain | ||
| pSL114 (YscY+) | pMF095 (LcrH+) | ++ | ++ |
| pSL114 (YscY+) | pJEB221 (PcrH+) | − | − |
| pSL114 (YscY+) | None | − | − |
| None | pMF095 (LcrH+) | − | − |
| None | pJEB221 (PcrH+) | − | − |
| pSL114 (YscY+) | pJEB211 (Hyb1+) | + | + |
| pSL114 (YscY+) | pJEB212 (Hyb2+) | +++ | ++ |
| pSL114 (YscY+) | pJEB213 (Hyb3+) | +++ | ++ |
| pSL114 (YscY+) | pJEB214 (Hyb4+) | +++ | ++ |
| pSL114 (YscY+) | pJEB215 (Hyb5+) | − | − |
| pSL114 (YscY+) | pJEB216 (Hyb6+) | − | − |
| pSL114 (YscY+) | pJEB217 (Hyb7+) | − | − |
| pSL114 (YscY+) | pJEB218 (Hyb8+) | +++ | ++ |
| pSL114 (YscY+) | pJEB219 (Hyb9+) | − | − |
| pSL114 (YscY+) | pJEB220 (Hyb10+) | − | − |
| pSL114 (YscY+) | pJEB222 (PcrHNEISS+) | − | − |
| pSL114 (YscY+) | pJEB338 (LcrHN-term+) | − | − |
| pPJE065 (Pcr4+) | pJEB221 (PcrH+) | − | − |
| pPJE065 (Pcr4+) | pMF095 (LcrH+) | − | − |
| pPJE065 (Pcr4+) | None | − | − |
| pPJE024 (Pcr4+) | pPJE025 (Pcr3+) | +++ | ++ |
| pPJE024 (Pcr4+) | pPJE026 (YscX+) | +++ | +++ |
| pMF433 (YscY+) | pPJE025 (Pcr3+) | +++ | + |
| pMF433 (YscY+) | pPJE026 (YscX+) | +++ | +++ |
| None | pPJE025 (Pcr3+) | − | − |
| None | pPJE026 (YscX+) | − | − |
HIS3 and ADE2 are two reporter genes in S. cerevisiae PJ69-4A. HIS3+ or ADE2+ represents strong growth (+++) to no growth (−) on minimal medium devoid of histidine or adenine, respectively, recorded after day 4. Due to an intrinsic leakiness with the HIS3 reporter, 1 mM 3-aminotriazole was added to histidine dropout media to suppress false positives (43). Results reflect trends in growth from three independent experiments in which several individual transformants were tested on each occasion.
FIG. 5.
Expression of chaperone hybrids and other type III secretion components in Saccharomyces cerevisiae PJ69-4A. Protein extracts were generated from yeast harboring (A) lcrH (pLcrH+), hybrids (pHyb1+ to pHyb10+), and wild-type or mutated pcrH (pPcrH+ and pPcrHNEISS+) fused to the GAL4 activation domain of pGADT7, respectively; (B) yscX (pYscX+) and pcr3 (pPcr3+) fused to the GAL4 activation domain of pGADT7; and (C) yscY (pYscY+) and pcr4 (pPcr4+) fused to the GAL4 DNA binding domain plasmid pGBKT7. Samples were separated by SDS-PAGE, and recombinant proteins were identified by immunoblot analysis using a mouse hemagglutinin (HA) monoclonal antibody (mAb) (clone 12CA5) (Roche AB, Stockholm, Sweden) (A and B) or a GAL4-BD monoclonal antibody (Clontech Laboratories) (C). In each case, a protein extract from PJ69-4A harboring pGADT7 (A and B) or pGBKT7 (C) alone (vector) was included as a negative control. The arrowhead indicates the lower-molecular-weight protein band of interest.
The LcrH N terminus is obviously important for these functions. We therefore asked whether the first 35 residues of LcrH were sufficient to bind YscY in yeast. However, no interaction was observed (Table 4). This is likely explained by the phenotype of Hyb8, which could readily interact with YscY, even though control of Yop synthesis was not established when expressed in Yersinia. As Hyb8 lacks the NEISS domain required for yop-regulatory control, perhaps this indicates that full YscY binding actually requires two regions: the NEISS regulatory domain between the borders of Hyb10 and Hyb1 and also a flanking domain between the borders of Hyb8 and Hyb7 (encompassing the first TPR motif of LcrH). This is further supported by the phenotype of Hyb1, which lacks this latter domain and only moderately interacts with YscY.
The LcrH-YscY complex appears to be a strategic regulatory mechanism in Yersinia type III secretion. If so, one prediction would be that PcrH would not interact with the YscY homologue, Pcr4 of P. aeruginosa (80). Indeed, we could not detect this interaction using the two-hybrid system (Table 4), although both proteins were stably expressed (Fig. 5A and C). While this does not rule out the possibility of a weak interaction occurring in vivo, the LcrH-YscY complex does seem unique to Yersinia.
Functional analysis of pcr3 and pcr4 in P. aeruginosa type III secretion.
Little is known about the role of YscY in Yersinia type III secretion, except that it might function as a chaperone aiding in the presecretory stabilization and efficient secretion of YscX, a component promoting functional type III secretion (15, 42). The homologs of these two proteins in P. aeruginosa T3SS are 49% (Pcr4) and 48% (Pcr3) identical to their respective Yersinia counterparts (80). We generated Δpcr3 and Δpcr4 null mutants and found both Pcr3 and Pcr4 to be essential for P. aeruginosa type III secretion, since ExoS, ExoT, and PcrV were not secreted by mutant bacteria grown in low-calcium conditions (Fig. 6, compare lanes d and j with b). Accordingly, these mutants were also unable to deliver effector substrates (exoenzymes) into infected HeLa cells (Fig. 7, compare panels B and E with A). As expected, type III secretion (Fig. 6, compare lanes f and l with b) and effector translocation (Fig. 7, compare panels C and F with A) could be restored by providing Pcr3 or Pcr4 in trans under an IPTG-inducible promoter. Furthermore, Pcr4 specifically bound Pcr3 in the yeast two-hybrid system (Table 4). Therefore, Pcr4 probably chaperones Pcr3 for efficient secretion in P. aeruginosa, which in turn is necessary for building up a functional T3SS.
FIG. 6.
Analysis of type III protein secretion from P. aeruginosa strains grown either with (+) or without (−) Ca2+. Proteins secreted to the culture medium were separated by SDS-PAGE and identified by immunoblotting using polyclonal rabbit anti-ExoS (cross-reacts with ExoT) or anti-PcrV antisera. Where indicated, IPTG was added at a final concentration of 0.4 mM. Lanes: a and b, wild-type PAK; c and d, pcr3 null mutant PAKpcr3; e and f, complemented PAKpcr3, pJEB295 (Pcr3+); g and h, complemented PAKpcr3, pJEB291 (YscX+); i and j, pcr4 null mutant PAKpcr4; k and l, complemented PAKpcr4, pJEB296 (Pcr4+); m and n, complemented PAKpcr4, pJEB292 (YscY+). Molecular masses shown in parentheses are deduced from primary sequences.
FIG. 7.
Infection of HeLa cells by P. aeruginosa. Strains were allowed to infect a monolayer of growing HeLa cells. At 3 h after infection, the effect of the bacteria on the HeLa cells was recorded by phase-contrast microscopy. Note the extensive rounding up of the ExoS-dependent, cytotoxically affected HeLa cells (A, C, D, F, and G). HeLa cells infected with the Δpcr3 or Δpcr4 mutant show normal cell morphology (compare B and E with H), even after prolonged infection for up to 6 h (data not shown). Shown are phase-contrast images of cells infected with wild-type PAK (A), Δpcr3 mutant PAKpcr3 (B), trans-complemented PAKpcr3, pJEB295 (Pcr3+) (C), trans-complemented PAKpcr3, pJEB291 (YscX+) (D), Δpcr4 mutant PAKpcr4 (E), trans-complemented PAKpcr4, pJEB296 (Pcr4+) (F), trans-complemented PAKpcr4, pJEB292 (YscY+) (G), or cells left uninfected (H).
yscX and yscY trans-complement P. aeruginosa pcr3 and pcr4 mutants.
We further assessed the functional similarities between YscX/Pcr3 and YscY/Pcr4. Using the two-hybrid system, reciprocal binding for Pcr4 with YscX and YscY with Pcr3 was observed (Table 4). In agreement with these findings, YscX and YscY efficiently restored type III secretion (Fig. 6, compare lanes h and n with b) and effector translocation (Fig. 7, compare panels D and G with A) when expressed in P. aeruginosa pcr3 and pcr4 mutants, respectively. Intriguingly, however, expression of pcr3 and pcr4 could not restore the defects in yop regulation, secretion, or translocation caused by depletion of yscX or yscY in Yersinia. Like the noncomplemented mutants, these strains displayed calcium-independent growth phenotypes (Table 3), were severely down-regulated for yop expression and secretion (Fig. 8, compare lanes h and n with d and j), and consequently failed to translocate Yop effectors into HeLa cells during infection (Fig. 9, compare panels D and G with B and E). Importantly, these same mutants could be complemented by the native components (Table 3, Fig. 8, compare lanes f and l with b, and 9, compare panels C and F with A). Thus, the ability to efficiently bind YscY and YscX, respectively, is not sufficient to permit Pcr3 and Pcr4 to participate in Yersinia yop regulation.
FIG. 8.
Analysis of Yop synthesis and secretion from Y. pseudotuberculosis strains grown either with (+) or without (−) Ca2+. Yops in the total protein fraction (a mix of proteins secreted to the culture medium and contained within intact bacteria) (upper panel) or secreted to the extracellular medium (cleared culture supernatants) (lower panel) were separated by SDS-PAGE and identified by immunoblot analysis using a polyclonal rabbit antiserum recognizing secreted Yops. Where indicated, IPTG was added at a final concentration of 0.4 mM upon temperature shift. Lanes: a and b, wild-type YPIII/pIB102; c and d, yscX null mutant YPIII/pIB880; e and f, complemented YPIII/pIB880, pJEB291 (YscX+); g and h, complemented YPIII/pIB880, pJEB295 (Pcr3+); i and j, yscY null mutant YPIII/pIB890; k and l, complemented YPIII/pIB890, pJEB292 (YscY+); m and n, complemented YPIII/pIB890, pJEB296 (Pcr4+). Molecular masses shown in parentheses are deduced from primary sequences.
FIG. 9.
Infection of HeLa cells by Y. pseudotuberculosis. Strains were allowed to infect a monolayer of growing HeLa cells. At 3 h after infection, the effect of the bacteria on the HeLa cells was recorded by phase-contrast microscopy. Note the extensive rounding up of the YopE-dependent, cytotoxically affected HeLa cells (A, C, and F). HeLa cells infected with the ΔyscX or ΔyscY mutant show normal cell morphology (compare B and E with H), even after prolonged infection for up to 6 h (data not shown). Shown are phase-contrast images of cells infected with wild-type YPIII/pIB102 (A); yscX null mutant YPIII/pIB880 (B); complemented YPIII/pIB880, pJEB291 (YscX+) (C); complemented YPIII/pIB880, pJEB295 (Pcr3+) (D); yscY null mutant YPIII/pIB890 (E); complemented YPIII/pIB890, pJEB292 (YscY+) (F); complemented YPIII/pIB890, pJEB296 (Pcr4+) (G); and cells left uninfected (H).
Our earlier work determined that some P. aeruginosa components, such as PcrV and PopD, function poorly in Yersinia type III secretion unless they are coexpressed (5, 7). This made us wonder whether coexpression of Pcr3 and Pcr4 would make them able to complement the corresponding ΔyscX yscY double mutant of Yersinia. Not surprisingly, this mutant is unable to secrete or translocate Yops and is down-regulated for Yop synthesis (Table 3, Fig. 10, and data not shown). Although it could be trans-complemented by coexpression of native YscX and YscY, similarly expressed Pcr3 and Pcr4 still could not alter the mutant phenotypes (Table 3, Fig. 10, and data not shown). Importantly, this Pcr3 and Pcr4 coexpression construct is functional in P. aeruginosa, capable of complementing both pcr3 and pcr4 null mutants (data not shown). Furthermore, a ΔyscX yscY lcrH triple mutant in which Yop synthesis is constitutively up-regulated due to the loss of LcrH, even when in the absence of Yop secretion (Table 3, Fig. 10), could not be complemented by coexpression of PcrH, Pcr3, and Pcr4. Oddly, Yop synthesis was repressed in this strain (Fig. 10), which was consistent with a calcium-independent growth phenotype (Table 3). However, despite the technical challenges associated with two-plasmid expression in bacteria, controlled Yop synthesis, secretion and translocation, and also wild-type growth were restored in this mutant by the coexpression of native LcrH, YscX, and YscY (Table 3, Fig. 10, and data not shown). Thus, these collective data add further credence to our notion of different regulatory networks within these two pathogens. In Yersinia, a tight link between LcrH, YscY, and YscX is required for regulatory control, whereas PcrH-independent mechanisms appear to ensure regulatory control in P. aeruginosa.
FIG. 10.
Analysis of Yop synthesis and secretion from Y. pseudotuberculosis strains grown either with (+) or without (−) Ca2+. The protocol is essentially the same as that described in the legend to Fig. 8. Lanes: a and b as well as i and j, wild-type YPIII/pIB102; c and d, yscX yscY null mutant YPIII/pIB881; e and f, complemented YPIII/pIB881, pJEB340 (YscX, YscY+); g and h, complemented YPIII/pIB881, pJEB335 (Pcr3, Pcr4+); k and l, lcrH yscX yscY null mutant YPIII/pIB881-650; m and n, complemented YPIII/pIB881-650, pPJE020 (LcrH+), pJEB340 (YscX YscY+); o and p, complemented YPIII/pIB881-650, pKEC005 (PcrH+), pJEB335 (Pcr3 Pcr4+). Molecular masses shown in parentheses are deduced from primary sequences.
DISCUSSION
The recent discovery that type III chaperones are regulatory molecules is a key development, representing an ingenious mechanism by which bacteria tightly couple protein synthesis with an ordered secretion of substrates (28, 48). In Yersinia, the LcrH chaperone, when in complex with the YopD translocator substrate, represses synthesis of substrates predestined for type III secretion (25) by a posttranscriptional mechanism that involves binding directly to untranslated regions of yop gene mRNA (2, 10). This is consistent with Yop synthesis being derepressed in the absence of LcrH (3, 25, 56) or YopD (27, 52, 76). LcrH utilizes internal TPRs to engage both the YopB and YopD translocators, although this occurs differently (21a). This TPR-mediated binding also impacts on regulation via the formation of LcrH-YopD regulatory complexes. However, we have now identified a unique N-terminal region in LcrH, positioned outside of the TPRs, that is also required for system regulation. A complex with YscY is important for this effect, which therefore represents a new capacity in which LcrH regulates type III secretion. This is based upon several lines of evidence: (i) LcrH-PcrH chaperone hybrids lacking a domain of LcrH within the N terminus do not maintain regulatory control when expressed in an ΔlcrH null mutant of Yersinia, (ii) YopD function is unperturbed in these hybrids, (iii) hybrids with the capacity to regulate Yops in Yersinia also bind YscY, (iv) reciprocal binding between Pcr4 with Pcr3 or YscX and YscY with YscX or Pcr3 is not sufficient for Pcr4 or Pcr3 to complement the corresponding null mutants of Yersinia, and (v) PcrH bound neither to Pcr4 nor YscY. Taken together, these data suggest an important role for the LcrH-YscY complex in Yersinia T3SS regulation. It is therefore fascinating how a small molecule like LcrH combines different complexes with YopD and YscY to achieve this regulatory outcome.
Attempts to understand the molecular effect(s) of the LcrH-YscY regulatory complex are thwarted by our inability to pinpoint the role(s) of YscY and its cognate secreted substrate YscX in type III secretion (15, 42). Whatever this role, it appears other homologs may act similarly, since Pcr4 and Pcr3 were found to interact and both were required for functional type III secretion in P. aeruginosa. However, the fact that only YscY and YscX were functional in both bacterial backgrounds reinforces the necessary regulatory cross-talk between YscY, YscX, and LcrH in Yersinia. Furthermore, the role of other known YscY, YscX, and LcrH homologs found in Aeromonas spp. (8, 9, 82), Photorhabdus luminescens (18, 74), Vibrio spp. (36, 45), and Desulfovibrio vulgaris Hildenborough (35) remains unknown. Interestingly, as the regulatory role of LcrH involves LcrQ (10), a molecule unique to Yersinia (60), the LcrH-YscY-YscX regulatory loop is even more likely to be confined to Yersinia. Of interest is whether this newly discovered loop marks a branching from the established LcrH-YopD-LcrQ regulatory network (2, 10, 25). If this were true, perhaps LcrH could be dissected into functionally distinct domains, whereby the N terminus is linked to YscY and the remainder is linked to the function of YopD. However, our finding that the LcrH N terminus alone was unable to interact with YscY in yeast would indicate that this region still needs to function in the context of full-length LcrH to facilitate controlled Yop synthesis. This suggests a more complex picture and might imply that all components constitute the one regulatory network.
Given that YscX is secreted via the T3SS (15), perhaps this is one regulatory outcome of an LcrH-YscY association. It is therefore important to determine if secretion of YscX is required to permit LcrH-YscY complex formation. Another point to address is the final destination of secreted YscX, whether it is associated with the external type III needle or released directly into the extracellular milieu. In addition, the ratios of LcrH, YscY, and YscX might influence the regulatory status of Yop synthesis, although we did not detect any direct regulatory effect from overexpression of YscY or LcrH in wild-type, lcrH, or yopD null mutant backgrounds (J.E. Bröms, unpublished data). Moreover, just as our detailed mutagenesis of both LcrH and YopD revealed important functional information about the LcrH-YopD complex (24, 25), we are now using a similar approach to try and elucidate the mode of interaction for YscY with LcrH and YscX. Of interest is whether the TPR module of LcrH or YscY (54) is important for complex formation. Also noteworthy is the recent observation that some type III chaperones physically dock to the T3SS at the inner face of the cytoplasmic membrane via an interaction with the ATPase energizer (31, 70), an evolutionarily conserved core component of T3SSs (26, 41). Thus, docking of either LcrH or YscY with the Yersinia-specific ATPase YscN (77) could be another effect of LcrH-YscY complex formation.
It is the N terminus of LcrH that sets it apart from PcrH with respect to maintenance of yop-regulatory control in Yersinia. Interestingly, this appears to occur at two levels. First, a regulatory domain that also contributes to YscY binding lies within a region encompassing residues NEISS at positions 29 to 33 of LcrH. However, two pieces of evidence indicate that this region apparently does not operate alone: PcrH containing NEISS (PcrHNEISS) is unable to restore yop-regulatory control or engage YscY, and the first 35 LcrH residues did not appear to bind YscY. Thus, we propose a second functional level of the LcrH N terminus that makes it distinct from PcrH. This involves a second YscY binding domain that is present in Hyb2 to Hyb4 and Hyb8. The common domain in these four hybrids is the first TPR motif of LcrH located between positions 36 and 69. This site is intriguing, because alone (i.e., in the absence of the NEISS region, such as in Hyb8) it is unable to promote yop-regulatory control despite strong YscY binding. Therefore, it seems that two regions between 29 to 33 and 36 to 69 act in concert to promote strong YscY binding and establish LcrH-dependent control of Yop synthesis. In turn, this would also explain why Hyb1 binds YscY weakly, because it lacks the second YscY binding site incorporating the first TPR of LcrH.
The realization that a PcrH-Pcr4 complex is not required for regulation of P. aeruginosa type III secretion, despite their high identity to components of the Yersinia plasmid-borne system (6, 7, 80), was another fascinating outcome of this study. It is pertinent that the genome of P. aeruginosa is uniquely large, expanded by the presence of many genes coding for putative regulatory factors (66). This is now reflected by a number of independent studies which connect several of these factors with a complex pattern of type III secretion regulation in P. aeruginosa (13, 33, 34, 40, 47, 58, 59, 72, 78, 79). Therefore, the need for a regulatory complex involving PcrH and Pcr4 is likely bypassed by one or more of these numerous regulatory factors.
It is curious that PcrH is poorly expressed in Yersinia. We cannot exclude that this is due to instability of mRNA derived from the construct pJEB130 (pPcrH+) or the poor accessibility of translation initiation signals to the ribosomes. However, since PcrH was still poorly produced when expressed under the LcrH-derived leader sequence and ribosome binding site (data not shown) and transcription initiates from the same promoter (Ptac), this appears unlikely. More important could be that P. aeruginosa has a genomic G+C content of 66.6% (66), compared to 48.9% in Y. pseudotuberculosis (11). As this would impact on the codon usage preference of each organism, the pcrH translational efficiency in Y. pseudotuberculosis might account for these low expression levels. However, we have similarly expressed other components of the P. aeruginosa pcrGVH-popBD operon in Yersinia without any obvious expression restriction (5-7, 29). From our work with the chaperone hybrids, however, the cause of low PcrH expression must reside in the extreme N terminus, since Hyb9, which essentially contained all of PcrH except for the first 17 LcrH amino acids, was expressed at roughly LcrH-like levels in Yersinia. It has been established that the 5′ end of many E. coli genes is responsible for control of translation efficiency (12) as well as a wide variety of virulence determinants produced by numerous bacterial pathogens (17, 19, 20, 50, 57, 61). Therefore, minor codons in the extreme N terminus of LcrH might have evolved to control the translation efficiency of this important regulatory molecule, a concept we are currently exploring.
Acknowledgments
This work was supported by grants from the Carl Tryggers Foundation for Scientific Research (M.S.F.), Swedish Research Council (Å.F. and M.S.F.), Foundation for Medical Research at Umeå University (M.S.F.), Swedish Foundation for Strategic Research (Å.F.), Swedish Cystic Fibrosis Association Research Fund (M.S.F.), Swedish Medical Association (M.S.F.), and the J. C. Kempes Memorial Fund (J.E.B. and P.J.E.).
We thank Sara Eriksson, Yingqi Tang, Rose Cherry, Richard Kneeling, and Peter Steggo for valuable technical assistance.
REFERENCES
- 1.Allmond, L. R., T. J. Karaca, V. N. Nguyen, T. Nguyen, J. P. Wiener-Kronish, and T. Sawa. 2003. Protein binding between PcrG-PcrV and PcrH-PopB/PopD encoded by the pcrGVH-popBD operon of the Pseudomonas aeruginosa type III secretion system. Infect. Immun. 71:2230-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, D. M., K. S. Ramamurthi, C. Tam, and O. Schneewind. 2002. YopD and LcrH regulate expression of Yersinia enterocolitica YopQ by a posttranscriptional mechanism and bind to yopQ RNA. J. Bacteriol. 184:1287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman, T., S. Håkansson, Å. Forsberg, L. Norlander, A. Macellaro, A. Bäckman, I. Bölin, and H. Wolf-Watz. 1991. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bölin, I., and H. Wolf-Watz. 1984. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect. Immun. 43:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bröms, J. E., A.-L. Forslund, Å. Forsberg, and M. S. Francis. 2003. Dissection of homologous translocon operons reveals a distinct role for YopD in type III secretion by Yersinia pseudotuberculosis. Microbiology 149:2615-2626. [DOI] [PubMed] [Google Scholar]
- 6.Bröms, J. E., A.-L. Forslund, Å. Forsberg, and M. S. Francis. 2003. PcrH of Pseudomonas aeruginosa is essential for secretion and assembly of the type III translocon. J. Infect. Dis. 188:1910-1922. [DOI] [PubMed] [Google Scholar]
- 7.Bröms, J. E., C. Sundin, M. S. Francis, and Å. Forsberg. 2003. Comparative analysis of type III effector translocation by Yersinia pseudotuberculosis expressing native LcrV or PcrV from Pseudomonas aeruginosa. J. Infect. Dis. 188:239-249. [DOI] [PubMed] [Google Scholar]
- 8.Burr, S. E., K. Stuber, T. Wahli, and J. Frey. 2002. Evidence for a type III secretion system in Aeromonas salmonicida subsp. salmonicida. J. Bacteriol. 184:5966-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burr, S. E., T. Wahli, H. Segner, D. Pugovkin, and J. Frey. 2003. Association of type III secretion genes with virulence of Aeromonas salmonicida subsp. salmonicida. Dis. Aquat. Organ. 57:167-171. [DOI] [PubMed] [Google Scholar]
- 10.Cambronne, E. D., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: yscM1 and yscM2 regulate yop gene expression by a posttranscriptional mechanism that targets the 5′ untranslated region of yop mRNA. J. Bacteriol. 184:5880-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 101:13826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, G. F., and M. Inouye. 1990. Suppression of the negative effect of minor arginine codons on gene expression; preferential usage of minor codons within the first 25 codons of the Escherichia coli genes. Nucleic Acids Res. 18:1465-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dacheux, D., O. Epaulard, A. de Groot, B. Guery, R. Leberre, I. Attree, B. Polack, and B. Toussaint. 2002. Activation of the Pseudomonas aeruginosa type III secretion system requires an intact pyruvate dehydrogenase aceAB operon. Infect. Immun. 70:3973-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darwin, K. H., and V. L. Miller. 2001. Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J. 20:1850-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day, J. B., and G. V. Plano. 2000. The Yersinia pestis YscY protein directly binds YscX, a secreted component of the type III secretion machinery. J. Bacteriol. 182:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeVinney, I., I. Steele-Mortimer, and B. B. Finlay. 2000. Phosphatases and kinases delivered to the host cell by bacterial pathogens. Trends Microbiol. 8:29-33. [DOI] [PubMed] [Google Scholar]
- 17.Dobrindt, U., L. Emody, I. Gentschev, W. Goebel, and J. Hacker. 2002. Efficient expression of the alpha-haemolysin determinant in the uropathogenic Escherichia coli strain 536 requires the leuX-encoded tRNA(5)(Leu). Mol. Genet. Genomics 267:370-379. [DOI] [PubMed] [Google Scholar]
- 18.Duchaud, E., C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. F. Charles, E. Dassa, R. Derose, S. Derzelle, G. Freyssinet, S. Gaudriault, C. Medigue, A. Lanois, K. Powell, P. Siguier, R. Vincent, V. Wingate, M. Zouine, P. Glaser, N. Boemare, A. Danchin, and F. Kunst. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 21:1307-1313. [DOI] [PubMed] [Google Scholar]
- 19.Durand, J. M., and G. R. Björk. 2003. Putrescine or a combination of methionine and arginine restores virulence gene expression in a tRNA modification-deficient mutant of Shigella flexneri: a possible role in adaptation of virulence. Mol. Microbiol. 47:519-527. [DOI] [PubMed] [Google Scholar]
- 20.Durand, J. M., B. Dagberg, B. E. Uhlin, and G. R. Björk. 2000. Transfer RNA modification, temperature and DNA superhelicity have a common target in the regulatory network of the virulence of Shigella flexneri: the expression of the virF gene. Mol. Microbiol. 35:924-935. [DOI] [PubMed] [Google Scholar]
- 21.Dykxhoorn, D. M., R. St. Pierre, and T. Linn. 1996. A set of compatible tac promoter expression vectors. Gene 177:133-136. [DOI] [PubMed] [Google Scholar]
- 21a.Edqvist, P. J., J. E. Bröms, H. J. Betts, Å. Forsberg, M. J. Pallen, and M. S. Francis. Tetratricopeptide repeats in the type-III-secretion chaperone, LcrH: their role in substrate binding and secretion. Mol. Microbiol., in press. [DOI] [PubMed]
- 22.Feldman, M. F., and G. R. Cornelis. 2003. The multitalented type III chaperones: all you can do with 15 kDa. FEMS Microbiol. Lett. 219:151-158. [DOI] [PubMed] [Google Scholar]
- 23.Feldman, M. F., S. Muller, E. Wuest, and G. R. Cornelis. 2002. SycE allows secretion of YopE-DHFR hybrids by the Yersinia enterocolitica type III Ysc system. Mol. Microbiol. 46:1183-1197. [DOI] [PubMed] [Google Scholar]
- 24.Francis, M. S., M. Aili, M. L. Wiklund, and H. Wolf-Watz. 2000. A study of the YopD-LcrH interaction from Yersinia pseudotuberculosis reveals a role for hydrophobic residues within the amphipathic domain of YopD. Mol. Microbiol. 38:85-102. [DOI] [PubMed] [Google Scholar]
- 25.Francis, M. S., S. A. Lloyd, and H. Wolf-Watz. 2001. The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis. Mol. Microbiol. 42:1075-1093. [DOI] [PubMed] [Google Scholar]
- 26.Francis, M. S., K. Schesser, Å. Forsberg, and H. Wolf-Watz. 2004. Type III secretion systems in animal- and plant-interacting bacteria, p. 361-392. In P. Cossart, P. Boquet, S. Normark, and R. Rappuoli (ed.), Cellular microbiology, 2nd ed. American Society for Microbiology Press, Washington, D.C.
- 27.Francis, M. S., and H. Wolf-Watz. 1998. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol. 29:799-813. [DOI] [PubMed] [Google Scholar]
- 28.Francis, M. S., H. Wolf-Watz, and Å. Forsberg. 2002. Regulation of type III secretion systems. Curr. Opin. Microbiol. 5:166-172. [DOI] [PubMed] [Google Scholar]
- 29.Frithz-Lindsten, E., A. Holmström, L. Jacobsson, M. Soltani, J. Olsson, R. Rosqvist, and Å. Forsberg. 1998. Functional conservation of the effector protein translocators PopB/YopB and PopD/YopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol. Microbiol. 29:1155-1165. [DOI] [PubMed] [Google Scholar]
- 30.Fürste, J. P., W. Pansegrau, R. Frank, H. Blöcker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier, A., and B. B. Finlay. 2003. Translocated intimin receptor and its chaperone interact with ATPase of the type III secretion apparatus of enteropathogenic Escherichia coli. J. Bacteriol. 185:6747-6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gemski, P., J. R. Lazere, and T. Casey. 1980. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect. Immun. 27:682-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goranson, J., A. K. Hovey, and D. W. Frank. 1997. Functional analysis of exsC and exsB in regulation of exoenzyme S production by Pseudomonas aeruginosa. J. Bacteriol. 179:1646-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha, U. H., Y. Wang, and S. Jin. 2003. DsbA of Pseudomonas aeruginosa is essential for multiple virulence factors. Infect. Immun. 71:1590-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heidelberg, J. F., R. Seshadri, S. A. Haveman, C. L. Hemme, I. T. Paulsen, J. F. Kolonay, J. A. Eisen, N. Ward, B. Methe, L. M. Brinkac, S. C. Daugherty, R. T. Deboy, R. J. Dodson, A. S. Durkin, R. Madupu, W. C. Nelson, S. A. Sullivan, D. Fouts, D. H. Haft, J. Selengut, J. D. Peterson, T. M. Davidsen, N. Zafar, L. Zhou, D. Radune, G. Dimitrov, M. Hance, K. Tran, H. Khouri, J. Gill, T. R. Utterback, T. V. Feldblyum, J. D. Wall, G. Voordouw, and C. M. Fraser. 2004. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat. Biotechnol. 22:554-559. [DOI] [PubMed] [Google Scholar]
- 36.Henke, J. M., and B. L. Bassler. 2004. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186:3794-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 38.Hoiczyk, E., and G. Blobel. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. USA 98:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horton, R. M., and L. R. Pease. 1991. Recombination and mutagenesis of DNA sequences using PCR, p. 217-247. In M. J. McPherson (ed.), Directed mutagenesis: a practical approach. Oxford University Press, New York, N.Y.
- 40.Hovey, A. K., and D. W. Frank. 1995. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 177:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iriarte, M., and G. R. Cornelis. 1999. Identification of SycN, YscX, and YscY, three new elements of the Yersinia yop virulon. J. Bacteriol. 181:675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin, Q., and S. Y. He. 2001. Role of the Hrp pilus in type III protein secretion in Pseudomonas syringae. Science 294:2556-2558. [DOI] [PubMed] [Google Scholar]
- 45.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori, and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 46.Mavris, M., A. L. Page, R. Tournebize, B. Demers, P. Sansonetti, and C. Parsot. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43:1543-1553. [DOI] [PubMed] [Google Scholar]
- 47.McCaw, M. L., G. L. Lykken, P. K. Singh, and T. L. Yahr. 2002. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol. Microbiol. 46:1123-1133. [DOI] [PubMed] [Google Scholar]
- 48.Miller, V. L. 2002. Connections between transcriptional regulation and type III secretion? Curr. Opin. Microbiol. 5:211-215. [DOI] [PubMed] [Google Scholar]
- 49.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morona, R., M. Mavris, A. Fallarino, and P. A. Manning. 1994. Characterization of the rfc region of Shigella flexneri. J. Bacteriol. 176:733-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neyt, C., and G. R. Cornelis. 1999. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopD. Mol. Microbiol. 31:143-156. [DOI] [PubMed] [Google Scholar]
- 52.Olsson, J., P. J. Edqvist, J. E. Bröms, Å. Forsberg, H. Wolf-Watz, and M. S. Francis. 2004. The YopD translocator of Yersinia pseudotuberculosis is a multifunctional protein comprised of discrete domains. J. Bacteriol. 186:4110-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page, A. L., and C. Parsot. 2002. Chaperones of the type III secretion pathway: jacks of all trades. Mol. Microbiol. 46:1-11. [DOI] [PubMed] [Google Scholar]
- 54.Pallen, M. J., M. S. Francis, and K. Futterer. 2003. Tetratricopeptide-like repeats in type-III-secretion chaperones and regulators. FEMS Microbiol. Lett. 223:53-60. [DOI] [PubMed] [Google Scholar]
- 55.Parsot, C., C. Hamiaux, and A. L. Page. 2003. The various and varying roles of specific chaperones in type III secretion systems. Curr. Opin. Microbiol. 6:7-14. [DOI] [PubMed] [Google Scholar]
- 56.Price, S. B., and S. C. Straley. 1989. lcrH, a gene necessary for virulence of Yersinia pestis and for the normal response of Y. pestis to ATP and calcium. Infect. Immun. 57:1491-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramamurthi, K. S., and O. Schneewind. 2003. Yersinia yopQ mRNA encodes a bipartite type III secretion signal in the first 15 codons. Mol. Microbiol. 50:1189-1198. [DOI] [PubMed] [Google Scholar]
- 58.Rietsch, A., I. Vallet-Gely, S. L. Dove, and J. J. Mekalanos. 2005. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 102:8006-8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rietsch, A., M. C. Wolfgang, and J. J. Mekalanos. 2004. Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infect. Immun. 72:1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rimpiläinen, M., Å. Forsberg, and H. Wolf-Watz. 1992. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J. Bacteriol. 174:3355-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saier, M. H., Jr. 1995. Differential codon usage: a safeguard against inappropriate expression of specialized genes? FEBS Lett. 362:1-4. [DOI] [PubMed] [Google Scholar]
- 62.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1:787-796. [Google Scholar]
- 63.Skrzypek, E., and S. C. Straley. 1995. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J. Bacteriol. 177:2530-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stebbins, C. E., and J. E. Galan. 2003. Priming virulence factors for delivery into the host. Nat. Rev. Mol. Cell Biol. 4:738-743. [DOI] [PubMed] [Google Scholar]
- 65.Stebbins, C. E., and J. E. Galan. 2001. Structural mimicry in bacterial virulence. Nature 412:701-705. [DOI] [PubMed] [Google Scholar]
- 66.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 67.Straley, S. C., and W. S. Bowmer. 1986. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sundin, C., M. L. Henriksson, B. Hallberg, Å. Forsberg, and E. Frithz-Lindsten. 2001. Exoenzyme T of Pseudomonas aeruginosa elicits cytotoxicity without interfering with Ras signal transduction. Cell Microbiol. 3:237-246. [DOI] [PubMed] [Google Scholar]
- 69.Sundin, C., M. C. Wolfgang, S. Lory, Å. Forsberg, and E. Frithz-Lindsten. 2002. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33:265-277. [DOI] [PubMed] [Google Scholar]
- 70.Thomas, J., G. P. Stafford, and C. Hughes. 2004. Docking of cytosolic chaperone-substrate complexes at the membrane ATPase during flagellar type III protein export. Proc. Natl. Acad. Sci. USA 101:3945-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tucker, S. C., and J. E. Galán. 2000. Complex function for SicA, a Salmonella enterica serovar Typhimurium type III secretion-associated chaperone. J. Bacteriol. 182:2262-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Urbanowski, M. L., G. L. Lykken, and T. L. Yahr. 2005. A secreted regulatory protein couples transcription to the secretory activity of the Pseudomonas aeruginosa type III secretion system. Proc. Natl. Acad. Sci. USA 102:9930-9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker, K. A., and V. L. Miller. 2004. Regulation of the Ysa type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J. Bacteriol. 186:4056-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waterfield, N. R., P. J. Daborn, and R. H. French-Constant. 2002. Genomic islands in Photorhabdus. Trends Microbiol. 10:541-545. [DOI] [PubMed] [Google Scholar]
- 75.Wattiau, P., B. Bernier, P. Deslee, T. Michiels, and G. R. Cornelis. 1994. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl. Acad. Sci. USA 91:10493-10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams, A. W., and S. C. Straley. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 180:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woestyn, S., A. Allaoui, P. Wattiau, and G. R. Cornelis. 1994. YscN, the putative energizer of the Yersinia Yop secretion machinery. J. Bacteriol. 176:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolfgang, M. C., V. T. Lee, M. E. Gilmore, and S. Lory. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell. 4:253-263. [DOI] [PubMed] [Google Scholar]
- 79.Yahr, T. L., and D. W. Frank. 1994. Transcriptional organization of the trans-regulatory locus which controls exoenzyme S synthesis in Pseudomonas aeruginosa. J. Bacteriol. 176:3832-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yother, J., and J. D. Goguen. 1985. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J. Bacteriol. 164:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu, H. B., P. S. Rao, H. C. Lee, S. Vilches, S. Merino, J. M. Tomas, and K. Y. Leung. 2004. A type III secretion system is required for Aeromonas hydrophila AH-1 pathogenesis. Infect. Immun. 72:1248-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaharik, M. L., S. Gruenheid, A. J. Perrin, and B. B. Finlay. 2002. Delivery of dangerous goods: type III secretion in enteric pathogens. Int. J. Med. Microbiol. 291:593-603. [DOI] [PubMed] [Google Scholar]