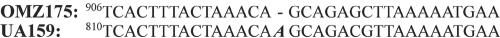Abstract
Allelic replacement of the C terminus of a Streptococcus mutans surface protein affects murein hydrolase activity. The targeted open reading frame encodes a 67-kDa protein (SmaA) with an N-terminal signal sequence and cleavage site, three 46-amino-acid (aa) direct repeats, and two 88-aa direct repeats. The identical autolytic profile was obtained using a sortase mutant (SrtA−).
Streptococcus mutans is the major pathogenic agent causing dental caries (3, 5, 6). The unidentified 65-kDa cell surface protein (SP) of S. mutans is immunodominant (2) and is present in SP preparations from all S. mutans c isolates examined (8). Crude SPs were obtained from 9 liters of S. mutans A32-2 (8) grown in Todd-Hewitt broth (THB) plus 1% dextrose for 18 h at 37°C in 5% CO2 and 95% air. Cells were pelleted at 16,000 × g at 4°C for 10 min, washed once gently in buffer A (10 mM phosphate-buffered saline, 1 mM CaCl2, and 1 mM phenylmethylsulfonyl fluoride, pH 7.2), and stored as a pellet at −20°C overnight. Frozen cells were thawed and then suspended in buffer A, and SP was removed with three 1-min cycles at high speed in a Waring blender. After blending, the sample was centrifuged as above to remove intact cells and cell debris. The proteins in the supernatant were isolated by centrifugation (110,000 × g, 4°C, 2.5 h). The pellet containing the SP was resuspended in buffer A and centrifuged (16,000 × g, 4°C, 10 min) to remove cellular debris. The supernatant was divided into aliquots and stored at −80°C. The protein concentration was determined using the Quantipro bicinchoninic acid protein assay (Sigma Chemical Co., St. Louis, MO).
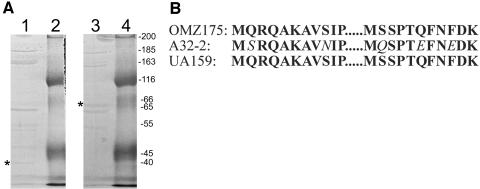
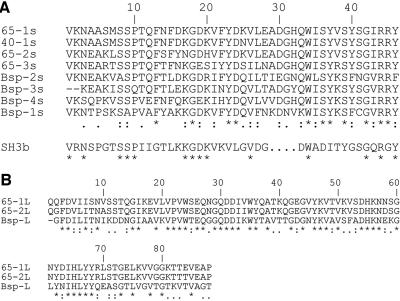
CNBr sequencing (Biochemistry Biotechnology Facility, Indiana University Purdue University at Indianapolis) of the 65-kDa band recovered from separation of SP by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis resulted in two 11-amino-acid (aa) peptides (MSRQAKAVNIP and MQSPTEFNEDK) (Fig. 1). The gene encoding this SP (SmaA) was localized to the open reading frame (ORF) at position SMU.609 (1) on the S. mutans UA159 genome. This ORF is designated as a “putative 40K cell wall protein precursor” based on sequence homology to the 40-kDa protein (p40f) from S. mutans OMZ175 (7). Visual comparison of the sequences of these homologous ORFs (Fig. 2) revealed that the predicted size difference is actually due to a divergence at a single base, absent in the OMZ175 genome, which produces a reading frameshift and subsequent earlier stop codon in OMZ175. Additional analysis of SMU.609 revealed three copies of a 46-aa repeat region with homology to SH3b, a domain associated with murein hydrolase function (13), and two 88-aa direct repeats of unknown function (Fig. 3).
FIG. 1.
Protein sequence analysis of the 65-kDa surface protein. A. Coomassie blue-stained 10% SDS-polyacrylamide gel of S. mutans surface proteins. Lane 1, OMZ175; lane 3, A32-2; lanes 2 and 4, molecular mass markers (size in kilodaltons indicated on right). Asterisks indicate positions of the 40-kDa and 65-kDa homologs. The 65-kDa band from A32-2 was excised from the gel and sequenced. B. Two 11-aa sequences were obtained by CNBr sequencing of the 65-kDa surface protein from S. mutans A32-2 and were found to contain 78% identity to the homologous sequences from both OMZ175 and UA159. Changes from the UA159 sequence are in italics.
FIG. 2.
Site of OMZ175 deletion mutation. The sequence surrounding the deletion mutation in the OMZ175 gene encoding p40f is compared to the homologous sequence from UA159 encoding SmaA. This deletion leads to an early stop codon in p40f compared to SmaA. The numbering differences reflect different predicted start sites for the two ORFs (not shown).
FIG. 3.
Comparison of direct repeat regions. A. Short repeats (46 aa), three from S. mutans UA159 SmaA (65-1s, 65-2s, and 65-3s), one from S. mutans OMZ175 p40f (40-1s), and four from homologous Streptococcus agalactiae 60-kDa murein hydrolase protein Bsp (9) (Bsp-1s, Bsp-2s, Bsp-3s, and Bsp-4s), compared to the consensus sequence for the SH3b repeat motif. B. Long repeats (88 aa) of S. mutans UA159 SmaA (65-1L and 65-2L) compared to the single homologous sequence from Bsp (Bsp-L). Asterisk, exact match; period, weakly similar; colon, strongly similar.
The OMZ175 deletion mutation was recreated in UA159 by allelic replacement. The Em resistance marker PcEm (860 bp) was kindly provided by D. A. Morrison, University of Illinois at Chicago. Briefly, a 1,920-bp fragment, 40-UP, was amplified from OMZ175 genomic DNA by using primers p65-up-P1 (5′-TTAAAGGGTGAGCTGAG-3′) and p40-up-P2 (5′-GGCGCGCCTGTTTAGTAAAGTGATAGG-3′) (containing an AscI site at the 5′ end). This fragment includes 1,096 bp of sequence immediately upstream from the start site, to the nucleotide which corresponds to the site of the deletion mutation in the homologous gene from OMZ175. Another fragment (65-DN) containing the 960-bp sequence immediately downstream from SMU.609 was amplified from UA159 DNA using primers p65-dn-P3 (5′-GCTGGCCGGCCAGGTTAGGATGACAAAATCCTGAC-3′) (with an FseI site at the 5′ end) and p65-dn-P4 (5′-T GGCGGCTGCATTAGCTCCTGAAC-3′). PcEm (860 bp) was amplified using primers Em-P1 (5′-GGCGCGCCCCGGGCCCAAAATTTGTTTGAT-3′) and Em-P2 (5′-GCTGGCCGGCCAGTCGGCAGCGA-3′) with AscI and FseI sites engineered into their 5′ ends, respectively. The amplicons were subjected to restriction enzyme digestion and subsequent ligation to produce a 40-UP::PcEm::65-DN fragment. The ligated product was directly transformed into S. mutans wild-type UA159 using the biofilm transformation technique (4). Following double-crossover homologous recombination, the region of SMU.609 from nucleotide 825 to the 3′ end was replaced by the Em cassette (PcEm). The transformant UA159D65::fPcEm was selected on THB agar plus Em (10 μg/ml). Confirmation of the Emr marker at the desired locus was determined by PCR.
Murein hydrolase activity of the SP of S. mutans was examined using the peptidoglycan zymogram assay (Fig. 4). Crude SP-enriched fractions of S. mutans A32-2, UA159, UA159D65::fPcEm, NG8, SrtA− (a sortase-knockout mutant of NG8 obtained from Song Lee, Dalhousie University, Halifax, Nova Scotia, Canada), PC3370 (an antigen I/II-knockout mutant of NG8 obtained from L. J. Brady, University of Florida), and OMZ175 (obtained from Suzanne Michalek, University of Alabama at Birmingham) were used as the enzyme sources. The peptidoglycan was obtained from a 3-liter (THB plus 1% dextrose) overnight culture of S. mutans washed in 10 mM HEPES, pH 6.8, and boiled in 4% SDS and 10 mM HEPES for 45 min (12). The mixture was cooled to room temperature and centrifuged at 70,000 × g for 40 min at 20°C, and the pellet was washed with 2 M NaCl. The pellet was weighed and resuspended in 2 M NaCl to a concentration of 185 mg/ml and sonicated for 5 min, on ice, at the maximum setting. The peptidoglycan was treated with 48% hydrofluoric acid (HF) at 4°C overnight and then washed 3× with distilled H2O and resuspended in the original volume of 2 M NaCl. The HF-treated peptidoglycan (0.5 ml) was added to a 7.5% SDS-polyacrylamide gel prior to solidification. The nondenatured SPs were separated on the gel at 150 V for 1 h.
FIG. 4.
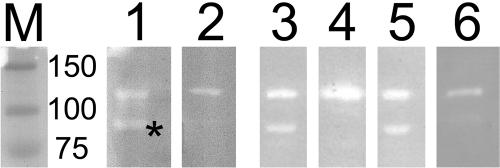
Zymogram activity gel. HF-treated peptidoglycan from S. mutans UA159 was incorporated into a 7.5% SDS-polyacrylamide gel. Nondenatured surface proteins were separated on the gel. Lane 1, UA159; lane 2, UA159D65::fPcEm; lane 3, NG8; lane 4, SrtA−; lane 5, PC3370; lane 6, OMZ175; lane M, prestained molecular mass markers (kilodaltons). The asterisk indicates the band associated with SmaA.
Two bands of activity, corresponding to 125 and 90 kDa, respectively, were present in SPs from A32-2, NG8, UA159, and PC3370. UA159D65::fPcEm, OMZ175, and SrtA− SP preparations contain the upper (125-kDa equivalent band) but not the lower (90-kDa) band of autolytic activity. The autolytic activity associated with the lower band but not the upper band is present in the supernatant of SrtA− cultures (data not shown). The loss of the 90-kDa autolytic band in the sortase mutant SrtA− suggests that the enzyme or enzyme complex uses this transpeptidase cell wall anchoring system (10). Sequence analysis suggests a 5-aa sequence, LPAQG, at nucleotide 296 as a possible candidate for the LPXTG canonical recognition sequence for sortase.
Two bacteriolytic bands of similar sizes (107 and 79 kDa) were recently reported using whole-cell S. mutans extracts (14) separated on HF-treated peptidoglycan. Subsequently, the upper band was reported to be associated with AltA (homologous to SMU.689), while the lower band was predicted to be a proteolytic product of AltA (11). Our data suggest that there are two separate autolytic activities present in the SP fractions of S. mutans, one of which is inactivated by a mutation in SMU.609 and is linked to the cell surface via the sortase system.
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontana, M. 1996. Streptococcus mutans fimbriae: role in dental caries formation and prevention. Ph.D. thesis. Indiana University School of Dentistry, Indianapolis.
- 3.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li, Y.-H., P. C. Y. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183:897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loesche, W. J., and L. H. Straffon. 1979. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect. Immun. 26:498-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGhee, J. R., and S. M. Michalek. 1981. Immunobiology of dental caries: microbial aspects and local immunity. Annu. Rev. Microbiol. 35:595-638. [DOI] [PubMed] [Google Scholar]
- 7.Ogier, J. A., M. Scholler, Y. Lepoivre, S. Gangloff, R. M'Zoughi, and J. P. Klein. 1991. A 40-kilodalton cell wall protein-coding sequence upstream of the sr gene of Streptococcus mutans OMZ175 (serotype f). Infect. Immun. 59:1620-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrone, M., L. E. Gfell, M. Fontana, and R. L. Gregory. 1997. Antigenic characterization of fimbria preparations from Streptococcus mutans isolates from caries-free and caries-susceptible subjects. Clin. Diagn. Lab. Immunol. 4:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinscheid, D. J., C. Stosser, K. Ehlert, R. W. Jack, K. Moller, B. J. Eikmanns, and G. S. Chhatwal. 2002. Influence of proteins Bsp and FemH on cell shape and peptidoglycan composition in group B streptococcus. Microbiology 148:3245-3254. [DOI] [PubMed] [Google Scholar]
- 10.Schneewind, O., P. Model, and V. A. Fischetti. 1992. Sorting of protein A to the staphylococcal cell wall. Cell 70:267-281. [DOI] [PubMed] [Google Scholar]
- 11.Shibata, Y., M. Kawada, Y. Nakano, K. Toyoshima, and Y. Yamashita. 2005. Identification and characterization of an autolysin-encoding gene of Streptococcus mutans. Infect. Immun. 73:3512-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugai, M., T. Akiyama, H. Komatsuzawa, Y. Miyake, and H. Suginaka. 1990. Characterization of sodium dodecyl sulfate-stable Staphylococcus aureus bacteriolytic enzymes by polyacrylamide gel electrophoresis. J. Bacteriol. 172:6494-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whisstock, J. C., and A. M. Lesk. 1999. SH3 domains in prokaryotes. Trends Biochem. Sci. 24:132-133. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura, G., H. Homatsuzawa, J. Kajimura, T. Fukiwara, M. Ohara, K. Kozai, and M. Sugai. 2004. Zymographic characterization of bacteriolytic enzymes produced by oral streptococci. Microbiol. Immunol. 48:465-469. [DOI] [PubMed] [Google Scholar]