Abstract
A new tryptophan catabolic pathway is characterized from Burkholderia cepacia J2315. In this pathway, tryptophan is converted to 2-amino-3-carboxymuconate semialdehyde, which is enzymatically degraded to pyruvate and acetate via the intermediates 2-aminomuconate and 4-oxalocrotonate. This pathway differs from the proposed mammalian pathway which converts 2-aminomuconate to 2-ketoadipate and, ultimately, glutaryl-coenzyme A.
Tryptophan has a variety of metabolic functions within the cell. It is incorporated into the polypeptide chains of enzymes and proteins, and it is the biosynthetic precursor of the cofactor NAD (19), the antibiotics anthramycin (15) and actinomycin (16), the siderophore quinolobactin (22), and the neurotransmitters serotonin (2) and melatonin (10, 29). Tryptophan can also be fully catabolized. For example, Bacillus megaterium (1) and Rhodococcus erythropolis (27) can grow with tryptophan as their sole source of carbon and nitrogen, and several pseudomonads are capable of catabolizing tryptophan (26). Eukaryotes are also capable of breaking down excess tryptophan to CO2, NH3, and H2O. Labeling studies indicate that tryptophan degradation in mammals takes place via the kynurenine pathway, which is also used for NAD biosynthesis in all eukaryotic organisms and in a few bacterial species (9, 21, 24) (Fig. 1). On the kynurenine pathway, the branching point between NAD biosynthesis and complete tryptophan catabolism takes place at the intermediate 2-amino-3-carboxymuconate semialdehyde (ACMS) (Fig. 1). ACMS can cyclize nonenzymatically to yield quinolinate (5), the direct precursor to the pyridine ring of NAD, or it can be enzymatically decarboxylated by ACMS decarboxylase (ACMSD) (6, 7, 28). Although the biosynthesis of NAD via the kynurenine pathway is well understood, relatively little is known about the enzymology of tryptophan catabolism after ACMS. The recent discovery of the five enzymes necessary to biosynthesize ACMS from tryptophan in several prokaryotes (17) suggests that a complete tryptophan catabolic pathway, similar to the proposed human pathway, might also exist in bacteria.
FIG. 1.
KEGG pathway for tryptophan degradation in eukaryotes. CoA, coenzyme A.
To test this hypothesis, we searched for clusters of tryptophan catabolic genes in bacteria by using 3-hydroxyanthranilate-3,4-dioxygenase (HAD) (11, 20, 23) and ACMSD (14, 23, 28) sequences from the NCBI database (http://www.ncbi.nlm.nih.gov) and by using the SEED database (http://theseed.uchicago.edu/FIG/index.cgi) for comparative genome analysis. Several bacteria that contained likely gene candidates for further degradation of ACMS clustered with HAD and ACMSD were identified. In Burkholderia cepacia J2315, HAD and ACMSD orthologs occurred in a cluster with genes of unknown function. Sequence analysis suggested that one of the unknown genes might function as a 2-aminomuconate semialdehyde dehydrogenase (AMDH; EC 1.2.1.32) (12) and another as a 2-aminomuconate deaminase (AMD; EC 3.5.99.5) (13, 14).
A second related genomic cluster was identified immediately upstream of the HAD-AMD cluster (Fig. 2). Within the second cluster were putative homologs of 4-oxalocrotonate decarboxylase (4OCD; EC 4.1.1.77), 2-keto-pentenoate hydratase (KPH; EC 4.2.1.80), 2-keto-4-hydroxypentanoate aldolase (HOA; EC 4.2.1.-), and acetaldehyde dehydrogenase (ADH; EC 1.2.1.3). We later identified all eight genes within a single, uninterrupted cluster in the organism Bacillus cereus 10897, suggesting a shared metabolic function for these genes in tryptophan catabolism. This was further supported by the identification of B. cepacia J2315 homologs of the genes encoding tryptophan-2,3-dioxygenase (TDO; EC 1.13.11.11), kynurenine formamidase (KFA; EC 3.5.1.9), and kynureninase (KYN; EC 3.7.1.3). No gene encoding kynurenine-3-monooxygenase (KMO; EC 1.13.14.9) was found, suggesting the existence of a second nonorthologous form of KMO in Burkholderia.
FIG. 2.
Region of Burkholderia cepacia J2315 chromosomal DNA containing genomic clusters of tryptophan catabolic genes.
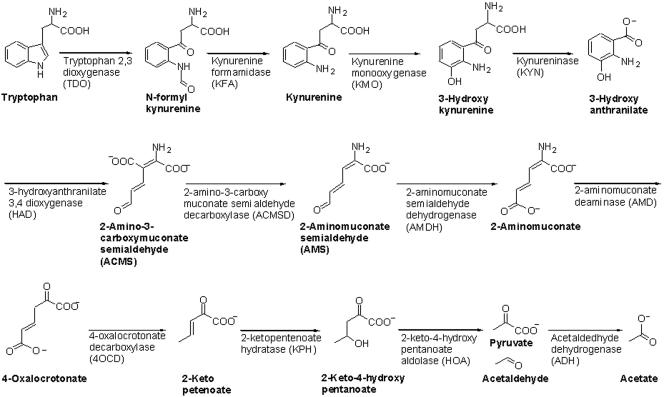
The identification of these putative enzymatic activities suggests the tryptophan catabolic pathway shown in Fig. 3. This pathway differs from the KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway previously proposed for eukaryotes (Fig. 1), where 2-aminomuconate is converted to 2-ketoadipate (Fig. 1) (25). However, the reported conversion of 2-aminomuconate to 2-ketoadipate may be regarded as a tentative assignment in the mammalian tryptophan catabolic pathway, since the only evidence is from radiolabeling studies performed with crude preparations of cat liver extract (8, 24). We propose that in B. cepacia J2315, 2-aminomuconate is instead deaminated to 4-oxalocrotonate (Fig. 3).
FIG. 3.
New tryptophan catabolic pathway in B. cepacia J2315.
To determine if B. cepacia J2315 utilized a catabolic pathway for tryptophan under normal growth conditions, the bacterial strain was plated on minimal medium containing 2% tryptophan as the sole carbon source. After incubation at 30°C, growth was observed at about half the rate at which colonies appeared on full medium.
To further test our proposed pathway, the putative HAD, ACMSD, AMDH, and AMD genes from B. cepacia J2315 were PCR amplified from genomic DNA and cloned into the plasmid pDESTF1, which encodes an N-terminal six-His tag and is under the control of the T7lac promoter. The resulting plasmids were named pBcHAD.XF1, pBcACD.XF1, pBcHMD.XF1, and pBcAMD.XF1, respectively, and were used to transform Escherichia coli Tuner (DE3). For overexpression, E. coli Tuner (DE3) cells transformed with one of these plasmids were grown at 37°C in Luria-Bertani medium containing 200 mg of ampicillin per liter. When the culture reached an optical density of 0.4 (absorbance at 600 nm), the temperature was lowered to 25°C. When the culture reached an optical density of 0.6, isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 0.1 mM, and the culture was incubated with shaking for 4 to 6 h at 25°C. The cells were then harvested and stored at −20°C until further use. Under these conditions, HAD, ACMSD, AMDH, and AMD all were overexpressed at a high level and were readily purified by nickel- nitrilotriacetic acid affinity chromatography according to the QIAGEN protocol for the purification of poly-His-tagged proteins (Fig. 4).
FIG. 4.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of tryptophan catabolic enzymes. Lane 1, molecular weight markers. Lane 2, cell extracts of the HAD overexpression strain. Lane 3, purified HAD. Lane 4, cell extracts of the ACMSD overexpression strain. Lane 5, purified ACMSD. Lane 6, cell extracts of the AMDH overexpression strain. Lane 7, purified AMDH. Lane 8, cell extracts of the AMD overexpression strain. Lane 9, purified AMD.
HAD is a soluble 23-kDa protein. The purified enzyme is oxidized as indicated by a pink color and a UV spectrum consistent with an oxidized rubredoxin Fe-S center (18). The enzyme was reconstituted with FeII in the presence of dithiothreitol; upon removal of reconstituting agents, the protein solution was colorless, consistent with a reduced rubredoxin Fe-S center. HAD catalyzes the oxidation of 3-hydroxyanthranilate to ACMS (λmax = 360 nm); the purified enzyme demonstrated a specific activity of 25 μmol/min/mg (4).
ACMSD was overexpressed as a soluble 41-kDa protein which catalyzed the decarboxylation of ACMS to 2-aminomuconate semialdehyde (AMS; λmax = 380 nm). AMS rapidly cyclizes nonenzymatically to picolinic acid (Fig. 1); therefore, enzymatic activity was monitored either by the decrease in absorbance of the substrate ACMS (λmax = 360 nm) or by the formation of picolinic acid. The identity of picolinic acid as the final reaction product was confirmed by high-pressure liquid chromatography and nuclear magnetic resonance (NMR) analysis (4).
AMDH was overexpressed as a soluble 57-kDa protein which utilized the cofactor NAD to catalyze the oxidation of AMS to 2-aminomuconate. The substrate AMS is unstable, cyclizing rapidly to picolinic acid; therefore, the AMDH reaction was coupled to the ACMSD-catalyzed decarboxylation of ACMS to generate AMS in situ. The formation of 2-aminomuconate was detected as a peak absorbing at 326 nm. At pH 7, 2-aminomuconate hydrolyzes nonenzymatically to the tautomers 2-hydroxymuconate and 4-oxalocrotonate with a half-life of 7 to 8 min. After acidification and extraction, the thermodynamically stable tautomer 2-hydroxymuconate was identified by NMR (4).
AMD was overexpressed as a soluble 18-kDa protein. The AMD-catalyzed deamination of 2-aminomuconate gave the same products as nonenzymatic hydrolysis, 2-hydroxymuconate and its tautomer, 4-oxalocrotonate. At pH 7.7, AMD accelerates the rate of deamination more than 70-fold over the nonenzymatic hydrolysis. The tautomeric products 4-oxalocrotonate and 2-hydroxymuconate were identified by high-pressure liquid chromatography and NMR (4).
This paper discusses the identification of likely candidates for the genes of a new tryptophan catabolic pathway in B. cepacia J2315. As illustrated in Fig. 3, tryptophan is converted to 3-hydroxyanthranilate using the first four enzymatic steps for NAD biosynthesis from tryptophan. 3-Hydroxyanthranilate is then cleaved to ACMS, which does not cyclize to quinolinate but instead is further degraded enzymatically to 2-aminomuconate and, finally, 4-oxalocrotonate. Enzymatic activities necessary to convert 4-oxalocrotonate to pyruvate and acetate are the same as those involved in the degradation of catechols and anthranilate (3). The formation of the intermediate 4-oxalocrotonate differentiates this pathway from the proposed mammalian pathway which converts 2-aminomuconate to 2-ketoadipate and, ultimately, glutaryl-coenzyme A.
Acknowledgments
We thank Cynthia Kinsland of the Cornell Protein Production Facility for cloning the Burkholderia genes and John McMurry for helpful discussions.
This work was supported by a grant from the NIH (DK44083).
REFERENCES
- 1.Bouknight, R. R., and H. L. Sadoff. 1975. Tryptophan catabolism in Bacillus megaterium. J. Bacteriol. 121:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, R. R. 1994. Tryptophan metabolism: a review, p. 17-30. In W. Kochen and H. Steinhart. l-Tryptophan: current prospects in medicine and drug safety. Walter de Gruyter, New York, N.Y.
- 3.Bugg, T. D. H., and C. J. Winfield. 1998. Enzymatic cleavage of aromatic rings: mechanistic aspects of the catechol dioxygenases and later enzymes of bacterial oxidative cleavage pathways. Nat. Prod. Rep. 15:513-530. [Google Scholar]
- 4.Colabroy, K. L. 2005. Mechanistic and structural studies in tryptophan catabolism. Ph.D. thesis. Cornell University, Ithaca, N.Y.
- 5.Colabroy, K. L., and T. P. Begley. 2005. The pyridine ring of NAD is formed by a nonenzymatic pericyclic reaction. J. Am. Chem. Soc. 127:840-841. [DOI] [PubMed] [Google Scholar]
- 6.Egashira, Y., H. Kouhashi, T. Ohta, and H. Sanada. 1996. Purification and properties of α-amino-β-carboxymuconate-ɛ-semialdehyde decarboxylase (ACMSD), key enzyme of niacin synthesis from tryptophan, from hog kidney. J. Nutr. Sci.Vitaminol. 42:173-183. [DOI] [PubMed] [Google Scholar]
- 7.Fukuoka, S.-I., K. Ishiguro, K. Yanagihara, A. Tanabe, Y. Egashira, H. Sanada, and K. Shibata. 2002. Identification and expression of a cDNA encoding human α-amino-β-carboxymuconate-ɛ-semialdehyde decarboxylase (ACMSD): a key enzyme for the tryptophan-niacine pathway and “quinolinate hypothesis.” J. Biol. Chem. 277:35162-35167. [DOI] [PubMed] [Google Scholar]
- 8.Gholson, R. K., Y. Nishizuka, A. Ichiyama, H. Kawai, S. Nakamura, and O. Hayaishi. 1962. New intermediates in the catabolism of tryptophan in mammalian liver. J. Biol. Chem. 237:2043-2045. [PubMed] [Google Scholar]
- 9.Gholson, R. K., D. C. Sanders, and L. M. Henderson. 1959. Glutaric acid: a product of tryptophan metabolism. Biochem. Biophys. Res. Commun. 1:98-100. [Google Scholar]
- 10.Guillemin, G. J., S. J. Kerr, G. A. Smythe, P. J. Armati, and B. J. Brew. 1999. Kynurenine pathway metabolism in human astrocytes. Adv. Exp. Med. Biol. 467:125-131. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa, Y., T. Muraki, T. Tokuyama, H. Iwaki, M. Tatsuno, and P. C. K. Lau. 2000. A novel degradative pathway of 2-nitrobenzoate via 3-hydroxyanthranilate in Pseudomonas fluorescens strain KU-7. FEMS Microbiol. Lett. 190:185-190. [DOI] [PubMed] [Google Scholar]
- 12.He, Z., J. K. Davis, and J. C. Spain. 1998. Purification, characterization, and sequence analysis of 2-aminomuconic 6-semialdehyde dehydrogenase from Pseudomonas pseudoalcaligenes JS45. J. Bacteriol. 180:4591-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, Z., and J. C. Spain. 1998. A novel 2-aminomuconate deaminase in the nitrobenzene degradation pathway of Pseudomonas pseudoalcaligenes JS45. J. Bacteriol. 180:2502-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He, Z., and J. C. Spain. 1997. Studies of the catabolic pathway of degradation of nitrobenzene by Pseudomonas pseudoalcaligenes JS45: removal of the amino group from 2-aminomuconic semialdehyde. Appl. Environ. Microbiol. 63:4839-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurley, L. H., W. L. Lasswell, J. M. Ostrander, and R. Parry. 1979. Pyrrolo[1,4]benzodiazepine antibiotics. Biosynthetic conversion of tyrosine to the C2- and C3-proline moieties of anthramycin, tomaymycin, and sibiromycin. Biochemistry 18:4230-4237. [DOI] [PubMed] [Google Scholar]
- 16.Jones, G., and U. Keller. 1997. Biochemistry and genetics of actinomycin production. Drugs Pharm. Sci. 82:335-361. [Google Scholar]
- 17.Kurnasov, O., V. Goral, K. Colabroy, S. Gerdes, S. Anantha, A. Osterman, and T. P. Begley. 2003. NAD biosynthesis: identification of the tryptophan to quinolinate pathway in bacteria. Chem. Biol. 10:1195-1204. [DOI] [PubMed] [Google Scholar]
- 18.Lovenberg, W., and B. E. Sobel. 1965. Rubredoxin: a new electron transfer protein from Clostridium pasteurianum. Proc. Natl. Acad. Sci. USA 54:193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magni, G., A. Amici, M. Emanuelli, N. Raffaelli, and S. Ruggieri. 1999. Enzymology of NAD+ synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 73:135-182. [DOI] [PubMed] [Google Scholar]
- 20.Malherbe, P., C. Koehler, M. Da Prada, G. Lang, V. Kiefer, R. Schwarcz, H.-W. Lahm, and A. M. Cesura. 1994. Molecular cloning and functional expression of human 3-hydroxyanthranilic-acid dioxygenase. J. Biol. Chem. 269:13792-13797. [PubMed] [Google Scholar]
- 21.Mathur, G. P., C. Y. Ng, and L. M. Henderson. 1964. The synthesis and metabolism of dl-tryptophan-5-14C. J. Biol. Chem. 239:2184-2188. [PubMed] [Google Scholar]
- 22.Matthijs, S., C. Baysse, N. Koedam, K. A. Tehrani, L. Verheyden, H. Budzikiewicz, M. Schaefer, B. Hoorelbeke, J.-M. Meyer, H. De Greve, and P. Cornelis. 2004. The Pseudomonas siderophore quinolobactin is synthesized from xanthurenic acid, an intermediate of the kynurenine pathway. Mol. Microbiol. 52:371-384. [DOI] [PubMed] [Google Scholar]
- 23.Muraki, T., M. Taki, Y. Hasegawa, H. Iwaki, and P. C. K. Lau. 2003. Prokaryotic homologs of the eukaryotic 3-hydroxyanthranilate 3,4-dioxygenase and 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase in the 2-nitrobenzoate degradation pathway of Pseudomonas fluorescens strain KU-7. Appl. Environ. Microbiol. 69:1564-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishizuka, Y., A. Ichiyama, R. K. Gholson, and O. Hayaishi. 1965. Metabolism of the benzene ring of tryptophan in mammalian tissues. I. Enzymic formation of glutaric acid from 3-hydroxyanthranilic acid. J. Biol. Chem. 240:733-739. [PubMed] [Google Scholar]
- 25.Nishizuka, Y., A. Ichiyama, and O. Hayaishi. 1970. Metabolism of the benzene ring of tryptophan (mammals), p. 463. In Methods in enzymology, vol. 17, pt. 1. Academic Press, New York, N.Y. [Google Scholar]
- 26.Stanier, R. Y., O. Hayaishi, and M. Tsuchida. 1951. The bacterial oxidation of tryptophan. I. A general survey of the pathways. J. Bacteriol. 62:355-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suemori, A., K. Nakajima, R. Kurane, and Y. Nakamura. 1995. Degradation of aromatic amino acids by Rhodococcus erythropolis. Lett. Appl. Microbiol. 21:55-59. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe, A., Y. Egashira, S.-I. Fukuoka, K. Shibata, and H. Sanada. 2002. Purification and molecular cloning of rat 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase. Biochem. J. 361:567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, L., H. Erlandsen, J. Haavik, P. M. Knappskog, and R. C. Stevens. 2002. Three-dimensional structure of human tryptophan hydroxylase and its implications for the biosynthesis of the neurotransmitters serotonin and melatonin. Biochemistry 41:12569-12574. [DOI] [PubMed] [Google Scholar]