Abstract
The twin-arginine translocation (Tat) pathway exports folded proteins across the bacterial cytoplasmic membrane and is responsible for the proper extracytoplasmic localization of proteins involved in a variety of cellular functions, including pathogenesis. The Mycobacterium tuberculosis and Mycobacterium smegmatis genomes contain open reading frames with homology to components of the Tat export system (TatABC) as well as potential Tat-exported proteins possessing N-terminal signal sequences with the characteristic twin-arginine motif. Due to the importance of exported virulence factors in the pathogenesis of M. tuberculosis and the limited understanding of mycobacterial protein export systems, we sought to determine the functional nature of the Tat export pathway in mycobacteria. Here we describe phenotypic analyses of ΔtatA and ΔtatC deletion mutants of M. smegmatis, which demonstrated that tatA and tatC encode components of a functional Tat system capable of exporting characteristic Tat substrates. Both mutants displayed a growth defect on agar medium and hypersensitivity to sodium dodecyl sulfate. The mutants were also defective in the export of active β-lactamases of M. smegmatis (BlaS) and M. tuberculosis (BlaC), both of which possess twin-arginine signal sequences. The Tat-dependent nature of BlaC was further revealed by mutation of the twin-arginine motif. Finally, we demonstrated that replacement of the native signal sequence of BlaC with the predicted Tat signal sequences of M. tuberculosis phospholipase C proteins (PlcA and PlcB) resulted in the Tat-dependent export of an enzymatically active ′BlaC. Thus, ′BlaC can be used as a genetic reporter for Tat-dependent export in mycobacteria.
Tuberculosis, caused by the bacillus Mycobacterium tuberculosis, remains a global health problem accounting for over two million deaths annually (23). The ability to survive in host phagocytes is a poorly understood virulence property of M. tuberculosis. The M. tuberculosis proteins exported to the bacterial cell surface or secreted by the bacillus are ideally positioned to interact with host cell components and include virulence factors that promote intracellular survival.
Our understanding of how exported mycobacterial proteins are transported beyond the cytoplasmic membrane is limited (42). In bacteria, the well-characterized general secretion (Sec) pathway transports unfolded proteins across the cytoplasmic membrane to their final destination (for reviews, see references 19 and 56). Proteins targeted to this system contain specific N-terminal signal sequences (ss) that consist of a positively charged region followed by a stretch of hydrophobic residues and end with a short uncharged polar region. Upon export, the signal sequence is cleaved, yielding the mature protein. The Sec pathway is also involved in the insertion of some integral membrane proteins, and in these cases the signal sequences are not always cleaved (18). Numerous Sec proteins are required for the operation of this pathway, including SecA, which provides the energy to move unfolded proteins through the Sec pore (19, 56). Mycobacteria possess a functional Sec pathway and the unusual property of having two SecA proteins (11, 12).
Recent studies reveal that many bacteria also utilize a twin-arginine translocation (Tat) pathway to transport proteins across the cytoplasmic membrane. The Tat pathway operates independently of the Sec pathway and is distinguished by the ability to translocate proteins in a folded state. Although studies of Escherichia coli provide the majority of information regarding the Tat pathway (for reviews, see references 13 and 59), the list of prokaryotic organisms experimentally shown to possess a functional Tat export pathway is expanding and includes gram-positive bacteria such as Bacillus subtilis and Streptomyces spp. (40, 66, 67). In addition, the Tat pathway was shown to function in some bacterial pathogens and to contribute to virulence in Pseudomonas aeruginosa, Agrobacterium tumefaciens, enterohemorrhagic E. coli O157:H7, and Legionella pneumophila (22, 55, 60, 79).
Substrates of the Tat pathway are synthesized as precursor proteins containing N-terminal signal sequences with the same overall structure as Sec signal sequences. The major distinction between Sec and Tat signal sequences is the presence of a characteristic twin-arginine motif in the charged region of Tat signal sequences, defined as R-R-X-φ-φ (where φ is an uncharged residue) (4, 6). The importance of the twin arginines “RR” to Tat export was shown by conservative substitution with twin lysines “KK” in Tat signal sequences, which eliminates the export of many native Tat substrates and Tat-dependent reporter proteins (17, 30, 73). However, the requirement for twin arginines in a Tat-exported protein is not absolute since there are reported examples of Tat substrates that lack a characteristic “RR” signature (33, 36). Additional elements, such as the degree of hydrophobicity in the region following the R-R-X-φ-φ motif and the presence of a positively charged residue near the end of the signal sequence, may also play a role in Tat-dependent export (17).
In E. coli, the Tat translocation apparatus is composed of the TatA, TatB, and TatC proteins (63). E. coli has an additional TatA homologue, designated TatE, which has overlapping functions with TatA. TatA and TatB are similar in structure, with each having a single transmembrane domain and a cytoplasmic tail (64). A number of GC-rich gram-positive bacteria lack tatB (21), and in these bacteria TatA likely fulfills the function of both TatA and TatB. TatC is a more complex protein containing four to six transmembrane domains (9, 28). Current models suggest that TatC and TatB are involved in the initial recognition and delivery of Tat substrates to the protein conducting channel, composed of an oligomer of TatA (5, 27). In all reported cases, deletions of tatA, tatB, or tatC yield viable mutants with defects in the export of Tat signal sequence-containing proteins (9, 22, 40, 63, 66, 79).
We describe here an analysis of the Tat pathway in the fast-growing nonpathogen Mycobacterium smegmatis, often used as a genetic model for investigating physiologic properties of mycobacteria. We demonstrate that the Tat pathway is functional in M. smegmatis. Furthermore, we show that an intact Tat system is required for optimal growth of M. smegmatis in vitro and for the export of active mycobacterial β-lactamases. We also demonstrate the potential for using a truncated β-lactamase (′BlaC) lacking its native Tat signal sequence as a reporter for Tat-dependent exported proteins, including virulence factors of M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains and culture methods.
The strains used during this work are listed in Table 1. E. coli strains DH5α, TOP10 (Invitrogen), EP1300 (Epicentre), and EZ (Qiagen) were used for DNA cloning procedures. Luria-Bertani (LB) medium (Fisher) was used for culturing of E. coli, and antibiotics were added at the following concentrations: 150 μg/ml, hygromycin B (Roche Applied Science); 100 μg/ml, ampicillin; or 40 μg/ml, kanamycin (Acros Chemicals). Middlebrook 7H9 or 7H10 medium (Difco; BD Biosciences) was used for the culturing of M. smegmatis and M. tuberculosis. For growth of M. smegmatis, Middlebrook medium was supplemented with 0.5% glycerol and 0.2% dextrose. For growth of M. tuberculosis, Middlebrook medium was supplemented with 0.5% glycerol and 1× ADS (albumin dextrose salt; 0.5% bovine serum albumin, fraction V [Roche]; 0.2% dextrose; and 0.85% NaCl). When necessary, media were supplemented with 0.05 to 0.1% Tween 80 (Fisher). Antibiotics were added to Middlebrook media at the following concentrations: 50 μg/ml, hygromycin B; 50 μg/ml, carbenicillin; or 20 μg/ml, kanamycin. When necessary, sucrose was added to 7H10 plates at a concentration of 4.5%. l-Lysine at 40 and 80 μg/ml was added to agar and liquid media, respectively, for growth of M. smegmatis strains PM759 and JM578.
TABLE 1.
Strains used in this study
| Strain | Description | Reference or source |
|---|---|---|
| E. coli | ||
| K-12 DH5α | F− [φ80dΔlacZM15] Δ(lacZYA-argF)U169 deoR recA1 endA1 hsd R17 glnV44 thi-1 gyrA96 relA1 | Gibco-BRL |
| TOP10 | F−mcrA [φ80dΔlacZM15] Δ(mrr-hsdRMS-mcrBC) ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK rpsL nupG | Invitrogen |
| EP1300 | F−mcrA [φ80dΔlacZM15] Δ(mrr-hsdRMS-mcrBC) ΔlacX74 recA1 endA1 araD139 Δ(ara leu)7697 galU galK λ−rpsL nupG trfA | Epicentre |
| Qiagen EZ | [F′::Tn10(Tcr) proA+B+lacIqZΔM15] recA1 end A1 hsdR17 (rK-12− mK-12+) lac glnV44 thi-1 gyrA96 relA1 | Qiagen |
| M. smegmatis | ||
| mc2155 | ept-1 | 70 |
| MB679 | mc2155::pKEH4, tatA single-crossover strain | This work |
| JM499 | mc2155::pJM112, tatC single-crossover strain | This work |
| MB692 | mc2155, ΔtatA | This work |
| JM567 | mc2155, ΔtatC | This work |
| PM759 | mc2155, ΔblaS1 ΔlysA4 rpsL6 | 25 |
| JM575 | PM759::pKEH4, tatA single-crossover strain | This work |
| JM578 | PM759, ΔtatA | This work |
| M. tuberculosis | ||
| H37Rv | Virulent | 2 |
| PM638 | H37Rv, ΔblaC1 | 25 |
Molecular biology procedures.
Standard molecular biology techniques were employed as previously described (61). The Expand high-fidelity PCR system (Roche) was used for all PCRs from chromosomal DNA, and dimethyl sulfoxide at 5.0% was included in select PCRs. DNA sequencing was performed at the UNC-CH Automated DNA Sequencing Facility.
Construction of Δtat suicide plasmids. (i) ΔtatA suicide plasmid pKEH4.
Genomic sequences immediately upstream and downstream of the tatA gene, including the 5′ and 3′ termini of tatA, were amplified from M. smegmatis genomic DNA by using the following primers: tatAUpstream1 (5′-AGATATCCTCCGCAGGCGTGGATCAT), tatAUpstream2 (5′-ACATATGGGTTACCTCCAGAACGTCGGTG), tatADownstream1 (5′-TCATATGCAGTCCACGGACCGGCACAC), and tatADownstream2 (5′-CGACTTCCAGCAGCACCGTCA) (underlined residues denote the NdeI-XbaI restriction sites). The PCR products were cloned into the pCR2.1 vector (Invitrogen) to generate pKEH1 and pKEH2. The NdeI-XbaI fragment from pKEH2 containing the downstream tatA flank was cloned into NdeI-XbaI cut pKEH1. The resulting plasmid, pKEH3, contained a 221-bp unmarked in-frame deletion of tatA. The 1.7-kbp tatA deletion fragment was then cut out of pKEH3 by EcoRV and cloned into the EcoRV site on counterselectable suicide vector pYUB657 (51), generating pKEH4. DNA inserts in the above plasmids were sequenced and determined to be error free.
(ii) ΔtatC suicide plasmid pJM112.
Genomic sequences immediately upstream and downstream of the tatC gene, including the 5′ and 3′ termini of tatC, were amplified from M. smegmatis genomic DNA by using the following primers: SmTatC1 (5′-GCGTGGATGTTCGACTACTACC), SmTatC2 (5′-AAGCTTGTTGCGAAGCTCGTGGAGGTG), SmTatC3 (5′-AAGCTTCTCAACGACCGCAGGAAGGC), and SmTatC4 (5′-GCAGGTGCAGGATGACCTCTTC) (underlined residues denote the HindIII restriction sites). The PCR products were cloned into the pCC1 vector by using a CopyControl PCR cloning kit (Epicentre) to generate pJM104 and pJM105. A 753-bp HindIII fragment from pJM104 containing the upstream tatC flank was cloned into pJM105 cut with HindIII. The resulting plasmid, pJM110, contained a 690-bp unmarked in-frame deletion of the tatC gene. The 1.3-kbp tatC deletion fragment was then cut out of pJM110 by BamHI and cloned into the BglII site on counterselectable suicide vector pYUB657 (51), producing pJM112. DNA inserts were sequenced and determined to be error free.
Two-step allelic exchange to create Δtat mutants of M. smegmatis.
To construct the ΔtatA mutants, pKEH4 was electroporated into M. smegmatis strain mc2155 as described previously (50, 51), and hygromycin-resistant transformants were selected. Individual transformants were subjected to Southern analysis and shown to contain the suicide vector integrated into the tatA region of the chromosome by means of a single-crossover event. One of these strains, MB679, was employed in the subsequent steps. For the second homologous recombination event, MB679 was grown to saturation in 7H9 medium with hygromycin. This culture was then subcultured at a 1:100 dilution into the same medium lacking hygromycin and incubated overnight. Dilutions were plated onto 7H10 plates containing 4.5% sucrose to select for sucrose-resistant colonies. Sucrose-resistant strains were patched onto 7H10 plates containing hygromycin. Strains that were both sucrose resistant and hygromycin sensitive were analyzed by PCR and Southern analysis for a deletion at the tatA locus. The same procedure was performed in the ΔblaS PM759 background to generate a ΔblaS ΔtatA deletion strain. To obtain ΔtatC mutants, pJM112 was electroporated into mc2155 and hygromycin-resistant transformants were confirmed by Southern analysis to be the result of a single-crossover event. One of these clones, JM499, was used for the subsequent work presented in this report. The second homologous recombination event was selected by using the protocol described above for MB679.
Southern analysis.
To analyze tatA recombinants, genomic DNA was isolated from M. smegmatis strains as previously described (10) and digested with NdeI-FspI. The probe used was a 910-bp SalI fragment of pJM120 containing tatC of M. smegmatis. To analyze tatC recombinants, genomic DNA was isolated and digested with FspI. The probe used was a 753-bp HindIII fragment obtained from pJM104 that contains tatA of M. smegmatis. Southern analysis was performed as previously described (61), and probes were labeled with [32P]dCTP by using a Ready-to-Go labeling kit (Amersham).
Antimicrobial susceptibility testing.
The replica-inoculating MIC method used was adapted from a previously described protocol (71). Mid-exponential-phase M. smegmatis cultures were diluted 10-fold in 7H9 liquid medium and spotted using a multipronged replica-inoculating device onto 7H10 agar plates containing twofold dilutions of carbenicillin. Culture dilutions containing an estimated 102 to 104 CFU of M. smegmatis resulted in confluent growth on agar plates lacking carbenicillin. For plates containing antibiotic, the lowest concentration of carbenicillin that inhibited growth completely after incubation for 4 days was designated the MIC. Each MIC determination was performed in triplicate. For determining sensitivity to sodium dodecyl sulfate (SDS), mid-log-phase M. smegmatis cultures were diluted 10-fold in 7H9 liquid medium and spotted using a multipronged replica-inoculating device onto 7H10 agar plates containing 0.0025% SDS.
Preparation of culture filtrates and whole-cell extracts from M. smegmatis for nitrocefin analysis.
To obtain cell-free culture filtrates, exponential-phase M. smegmatis cultures were diluted to an optical density at 600 nm (OD600) of 0.1 and allowed to grow at 37°C for two generations. When the culture reached an OD600 of 0.4, 500 μl was applied to a 0.2-μm Nanosep centrifugal filter (Filtron; Pall) and the culture filtrate was harvested by centrifugation at 13,000 rpm. Alternatively, culture filtrates were harvested using a 0.22-μm Millex-GV syringe-driven filter unit (Millipore). Whole-cell extracts were prepared from the remaining culture by harvesting 10 ml of culture by centrifugation, and the pellets were stored at −20°C. At a later time, the pellets were resuspended in 200 μl of 1× phosphate-buffered saline (PBS) with protease inhibitors (10 μg/ml aprotinin, 10 μg/ml E-64, 10 μg/ml leupeptin, 500 μg/ml Pefabloc SC, and 10 μg/ml pepstatin A), to which 0.4 g of 106-μm glass beads (Sigma) was added. The cells and beads were then vortexed twice for 5 min at 4°C, with a 5-min rest on ice. Two hundred microliters of 1× PBS with protease inhibitors was added, followed by vortexing and then centrifugation for 10 min at 4°C. Culture filtrates and cell extracts were assayed for total protein content by using a bicinchoninic acid protein quantification kit (Pierce).
Nitrocefin assays.
The β-lactamase activities of M. smegmatis whole-cell extracts and culture filtrates were determined by using the chromogenic β-lactam nitrocefin (46), as previously described (25). Assays were performed on triplicate wells set up from a single culture, and β-lactamase activities, expressed as A486 min−1 mg total protein−1, were reported as averages with standard deviations. Each experiment was repeated three times, and a representative experiment is presented.
Construction of ′blaC fusion plasmids. (i) ss-plcA.
The genomic sequence encoding the predicted signal sequence of M. tuberculosis plcA was amplified from M. tuberculosis genomic DNA by using the following primers: plcA-1 (5′-TGGCCAGC-CGTCGAGAGTTTTTGACAAAG) (underlined residues denote the MscI restriction site) and plcA-3 (5′-GGATC-CGCCGTAGGCCTTTTCAATCA) (underlined residues denote the BamHI restriction site). The 104-bp PCR product was cloned into the pCR2.1 vector (Invitrogen) to generate pJM128. A 96-bp MscI-BamHI fragment from pJM128 was cloned into pMV261 cut with MscI-BamHI. The resulting plasmid, pJM129, contained the predicted signal sequence of plcA cloned downstream of the hsp60 promoter.
(ii) ss-plcB.
The genomic sequence encoding the predicted signal sequence of M. tuberculosis plcB was amplified from M. tuberculosis genomic DNA by using the following primers: plcB-1 (5′-ATGGCC-ACCCGCCGACAATTTTTTGC) (underlined residues denote the MscI restriction site) and plcB-3 (5′-GGATCC-TCCGTAGGCTTTTTCGATA) (underlined residues denote the BamHI restriction site). The 104-bp PCR product was cloned into the pCR2.1 vector (Invitrogen) to generate pMB221. A 104-bp MscI-BamHI fragment from pMB221 was cloned into pMV261 cut with MscI-BamHI. The resulting plasmid, pMB222, contained the predicted signal sequence of plcB cloned downstream of the hsp60 promoter.
(iii) ss-mpt63.
The genomic sequence containing the 5′ region of the mpt63-Rv1926c gene was amplified from M. tuberculosis genomic DNA by using the following primers: Rv1926c-5 (5′-CTGCGCAT-GAAGCTCACCACAATGATCAAG) (underlined residues denote the FspI restriction site) and Rv1926c-6 (5′-AGGATCC-AGCCAACGCGACCGGTGCCGCAAAG) (underlined residues denote the BamHI restriction site). The 94-bp PCR product was cloned into the pDRIVE vector (Qiagen) to generate pMB226. A 94-bp FspI-BamHI fragment from pMB226 was cloned into pMV261 cut with MscI-BamHI. The resulting plasmid, pMB228, contained the predicted signal sequence of mpt63 cloned downstream of the hsp60 promoter.
(iv) ′blaC.
The sequence encoding a truncated version of M. tuberculosis blaC lacking its predicted signal sequence (′blaC) was amplified from pMP159 (25) by using the following primers: blaCfor1 (5′-AGATCTGCG-CGTCCGGCATCGACAACCTTGC) and blaCrev (5′-AGATCT-CCACGAGCCTATGCAAGCACAC) (underlined residues denote the BglII restriction sites). The 854-bp PCR product was cloned into the pCC1 vector (Epicentre) to generate pJM106. An 848-bp BglII fragment from pJM106 was end filled with Klenow and cloned into pMV261 cut with MscI. The resulting plasmid, pJM113, contained the predicted mature sequence of blaC cloned downstream of the hsp60 promoter. All other plasmids used in this work are described in Table 2. Translational fusion plasmids were sequenced and shown to be error free.
TABLE 2.
Plasmids used in this studya
| Plasmid | Genotype | Description | Source or reference |
|---|---|---|---|
| pCC1 | cat oriV ori2 | CopyControl (single-copy) blunt cloning vector | Epicentre |
| pCR2.1 | bla aph ColE1 | TA cloning vector | Invitrogen |
| pDRIVE | bla aph ColE1 | UA cloning vector | Qiagen |
| pMV261.kan | aph Phsp60 oriM ColE1 | Multicopy mycobacterial shuttle plasmid | 75 |
| pJSC77 | aph Phsp60-HA oriM ColE1 | HA tag cloned into pMV261 | 26 |
| pMB198 | hyg int attP ColE1 | Single-copy mycobacterial shuttle plasmid | 11 |
| pYUB657 | bla hyg Phsp60-sacB ColE1 | Counterselectable suicide plasmid for mycobacteria | 51 |
| pJM104 | cat oriV ori2 | M. smegmatis tatC upstream flank cloned into pCC1 | This work |
| pJM105 | cat oriV ori2 | M. smegmatis tatC downstream flank cloned into pCC1 | This work |
| pJM110 | cat ΔtatC (M. smegmatis) oriV ori2 | tatC upstream flank from pJM104 cloned adjacent to tatC downstream flank in pJM105 | This work |
| pJM112 | bla hyg Phsp60-sacB ΔtatC (M. smegmatis) ColE1 | 1.3-kbp BamHI fragment containing ΔtatC from pJM110 cloned into pYUB657 | This work |
| pKEH1 | bla aph ColE1 | M. smegmatis tatA upstream flank cloned into pCR2.1 | This work |
| pKEH2 | bla aph ColE1 | M. smegmatis tatA downstream flank cloned into pCR2.1 | This work |
| pKEH3 | bla aph ColE1 ΔtatA (M. smegmatis) | tatA downstream flank from pKEH2 cloned adjacent to tatA upstream flank in pKEH1 | This work |
| pKEH4 | bla hyg Phsp60-sacB ΔtatA (M. smegmatis) ColE1 | 1.7-kbp EcoRV fragment containing ΔtatA from pKEH3 cloned into pYUB657 | This work |
| pJM119 | cat tatC (M. smegmatis) oriV ori2 | M. smegmatis tatC ORF cloned into pCC1 | This work |
| pJM120 | aph Phsp60-tatC-HA (M. smegmatis) oriM ColE1 | M. smegmatis tatC ORF from pJM119 cloned into pJSC77; HA-tagged M. smegmatis tatC under control of hsp60 promoter | This work |
| pMB234 | bla aph tatA (M. smegmatis) ColE1 | M. smegmatis tatA ORF cloned into pCR2.1 | This work |
| pMB236 | aph Phsp60-tatA (M. smegmatis) oriM ColE1 | M. smegmatis tatA ORF from pMB234 cloned into pMV261; M. smegmatis tatA under control of hsp60 promoter | This work |
| pJM124 | hyg Phsp60-tatA (M. smegmatis) int attP ColE1 | Phsp60-tatA from pMB236 cloned into pMB198 | This work |
| pMP159 | bla blaC (M. tuberculosis) ColE1 | Fragment containing blaC from MTCY49 in pKS+ | 25 |
| pMP327 | aph PblaC-blaC (M. tuberculosis) oriM ColE1 | M. tuberculosis blaC from pMP159 cloned into pMV261; M. tuberculosis blaC under control of endogenous promoter | This work |
| pJM106 | cat ′blaC (M. tuberculosis) oriV ori2 | Predicted M. tuberculosis ′blaC mature sequence cloned into pCC1 | This work |
| pMM7-2 | aph Phsp60-ss-fbpB (M. tuberculosis) oriM ColE1 | M. tuberculosis fbpB signal sequence in pMV261 under control of hsp60 promoter | 82 |
| pJM109 | aph Phsp60-ss-fbpB-′blaC (M. tuberculosis) oriM ColE1 | ′blaC from pJM106 cloned into pMM7-2 | This work |
| pJM128 | bla aph ColE1 | Predicted M. tuberculosis plcA signal sequence cloned into pCR2.1 | This work |
| pJM129 | aph Phsp60-ss-plcA (M. tuberculosis) oriM ColE1 | M. tuberculosis plcA signal sequence from pJM128 cloned into MscI-BamHI cut pMV261 | This work |
| pJM130 | aph Phsp60-ss-plcA-′blaC (M. tuberculosis) oriM ColE1 | ′blaC from pJM106 cloned into pJM129 | This work |
| pMB221 | bla aph ColE1 | Predicted M. tuberculosis plcB signal sequence cloned into pCR2.1 | This work |
| pMB222 | aph Phsp60-ss-plcB (M. tuberculosis) oriM ColE1 | M. tuberculosis plcB signal sequence from pMB221 cloned into MscI-BamHI cut pMV261 | This work |
| pJM111 | aph Phsp60-ss-plcB-′blaC (M. tuberculosis) oriM ColE1 | ′blaC from pJM106 cloned into pMB222 | This work |
| pMB226 | bla aph ColE1 | M. tuberculosis mpt63 signal sequence cloned into pDRIVE | This work |
| pMB227 | aph Phsp60-ss-mpt63 (M. tuberculosis) oriM ColE1 | M. tuberculosis mpt63 signal sequence from pMB226 cloned into MscI-BamHI cut pMV261 | This work |
| pMB228 | aph Phsp60-ss-mpt63-′blaC (M. tuberculosis) oriM ColE1 | ′blaC from pJM106 cloned into pMB227 | This work |
| pJM113 | aph Phsp60-′blaC (M. tuberculosis) oriM ColE1 | ′blaC from pJM106 cloned into MscI-linearized pMV261 | This work |
| pJM117 | aph PblaC-(KK)blaC (M. tuberculosis) oriM ColE1 | M. tuberculosis ss-blaC(KK) under control of endogenous promoter | This work |
| pJM118 | aph Phsp60-ss-plcB(KK)-′blaC (M. tuberculosis) oriM ColE1 | M. tuberculosis ss-plcB(KK)-′blaC under control of hsp60 promoter | This work |
HA, hemagglutinin.
Site-directed mutagenesis of ′blaC fusion plasmids.
Site-directed mutagenesis was employed to replace the twin-arginine residues of the M. tuberculosis PlcB signal sequence with twin-lysine residues. pJM111 was used as the template in an inverse PCR using primers plcB-RR2KKfor (5′-AAGCAATTTTTTGCCAAAGCCGCC) and plcB-RR2KKrev (5′-CTTGGTGGCCATTGCGAAGTGATT) (underlined residues denote mutated bases). The resulting PCR product was gel purified, 5′-phosphorylated using an End-It DNA end repair kit (Epicentre), and self ligated. The resulting plasmid, pJM118, was sequenced to verify the presence of the mutated bases. Likewise, to replace the twin-arginine residues of the BlaC signal sequence with twin-lysine residues, pMP327 was used as the template in an inverse PCR using primers blaC-RR2KKfor (5′-AAGGAACTGCTGGTAGCGATGGCAATG) and blaC-RR2KKrev (5′-CTTACCGAATCCTCTGTTGCGCATGCC). The resulting plasmid, pJM117, was sequenced to verify the presence of the mutated bases.
Nucleotide sequence accession number.
The DNA sequence of a 2,018-bp cloned product containing the M. smegmatis tatA and tatC open reading frames (ORFs) was submitted to GenBank and given the accession number AY998985.
RESULTS
Identification of a putative Tat system in mycobacteria.
The genome of M. tuberculosis strain H37Rv contains two distinct loci with ORFs homologous to components of the bacterial Tat system. M. tuberculosis TatA (Rv2094c), TatB (Rv1224), and TatC (Rv2093c) are 44%, 38%, and 43% similar at the amino acid level to TatA, TatB, and TatC of E. coli (16). The proximity of tatA and tatC (17 bp between ORFs) suggests that these genes are located in an operon. The tatB gene is located approximately 1 Mbp away from tatAC. Using sequence data obtained from The Institute for Genomic Research (http://www.tigr.org), we identified homologues of tatABC in the genome of M. smegmatis strain mc2155. The organization of the tat genes is conserved in all of the available mycobacterial genome sequences, including for M. tuberculosis, M. bovis, M. leprae, M. avium, and M. smegmatis (http://www.tigr.org).
The M. tuberculosis genome also contains 31 ORFs with predicted Tat signal sequences, as identified by the TATFIND search algorithm (21). The TATFIND algorithm is based on experimentally demonstrated Tat substrates and sequences of putative Tat substrates in halophilic archaea. The list of TATFIND-predicted M. tuberculosis Tat substrates is available at http://www.sas.upenn.edu/∼pohlschr/tatprok.html. Notable among the predicted M. tuberculosis Tat substrates are the virulence factor phospholipase C (PlcB) (58) and the major β-lactamase BlaC (25, 78). Our inspection of the amino terminus of the major β-lactamase of M. smegmatis, BlaS (25, 44), also revealed a potential Tat signal sequence. The identification of putative Tat components and Tat-exported substrates in mycobacteria strongly suggested the existence of a functional Tat pathway.
M. smegmatis tatA and tatC deletion mutants have growth defects.
To examine the functional nature of the predicted Tat pathway in mycobacteria, in-frame and unmarked deletion alleles of the tatA and tatC genes were generated in M. smegmatis by using a two-step allelic-exchange strategy (10, 50).
To construct the ΔtatA mutant, we electroporated the suicide plasmid pKEH4, containing an in-frame unmarked deletion within M. smegmatis tatA, into wild-type M. smegmatis mc2155. This ΔtatA deletion allele is predicted to produce a truncated protein of 34 amino acids (in comparison to the wild-type protein of 106 amino acids). Integration of pKEH4 at the tatA chromosomal locus via a single homologous recombination event yielded the single-crossover strain MB679. This single-crossover strain contained both a wild-type copy and a deleted copy of tatA along with the intervening pKEH4 vector sequence carrying the selectable hygromycin resistance gene and the counterselectable sacB marker. Southern blot analysis confirmed successful construction of the single-crossover strain (Fig. 1).
FIG. 1.
Southern analysis of tatA and tatC recombinants from M. smegmatis. (A) Diagram of the mc2155 (wild-type) chromosomal tatAC locus. (B) Construction of M. smegmatis ΔtatA allele. (i) tatA single-crossover strain MB679, (ii) ΔtatA allele, and (iii) Southern blot of FspI-NdeI-digested genomic DNA. Lanes: 1, mc2155; 2, MB679; 3, MB692 (ΔtatA); 4, wild-type recombinant. (C) Construction of M. smegmatis ΔtatC allele. (i) tatC single-crossover strain JM499, (ii) ΔtatC allele, (iii) Southern blot of FspI-digested genomic DNA. Lanes: 1, mc2155; 2, JM499; 3, JM567 (ΔtatC); 4, wild-type recombinant. The FspI-NdeI restriction endonuclease sites are indicated, as well as the sizes of the DNA fragments detected by hybridization with each probe used (bold lines). Diagrams are not drawn to scale.
We employed the same strategy to construct a single-crossover strain for generation of a ΔtatC mutant by using the suicide plasmid pJM112, which contained an in-frame deletion within M. smegmatis tatC. This tatC deletion is predicted to produce a truncated protein of 87 amino acids (in comparison to the wild-type protein of 317 amino acids) which lacks the six transmembrane domains. Southern blot analysis confirmed construction of the tatC single-crossover strain JM499 (Fig. 1).
Our initial attempts to isolate secondary recombinants with tat deletions from the single-crossover strains yielded only wild-type alleles at both the tatA and tatC loci. This strongly suggested that, under the growth conditions tested, a tat deletion in M. smegmatis was lethal and that the Tat pathway was essential for growth. However, further incubation for 2 additional days at 37°C yielded a population of slow-growing secondary recombinants. These small colonies were subsequently proven by PCR (data not shown) and Southern blot analysis (Fig. 1) to be tatA or tatC deletion mutants. The ΔtatA and ΔtatC mutants represented 65% and 32% of the total secondary recombinants, respectively. All of the ΔtatA and ΔtatC mutants obtained formed single colonies on both minimal 7H10 and rich LB agar media that were smaller than those of wild-type M. smegmatis (Fig. 2A). The M. smegmatis ΔtatA mutant strain MB692 and ΔtatC mutant strain JM567 were used for all subsequent analyses (Table 1).
FIG. 2.
ΔtatA and ΔtatC mutants have growth defects. (A) Single colonies of wild-type (mc2155, pMB198), ΔtatA mutant (MB692, pMB198), and complemented ΔtatA attB::tatA (MB692, pJM124) strains are shown on 7H10 agar medium containing 0.1% Tween 80, grown at 37°C. Not shown are the ΔtatC mutant and the complemented ΔtatC strain, which display the same growth phenotypes. (B) Representative growth curves for wild-type (WT) (mc2155, pMV261), ΔtatC mutant (JM567, pMV261), and complemented ΔtatC (JM567, pJM120) strains in 7H9 liquid medium containing 0.1% Tween 80, grown at 37°C, are shown as OD600. (C) Viable-count measurements from the same cultures shown in panel B, shown here as the log10 of viable CFU/ml.
We compared growth of the Δtat mutants to that of wild-type M. smegmatis in Middlebrook 7H9 liquid medium. Growth of the mutants over time was determined by measuring the OD600 and by plating for CFU. The ΔtatC mutant had lower OD600 readings than the wild-type strain throughout the growth curve (Fig. 2B). However, there was no difference in the numbers of counted CFU during liquid growth between the ΔtatC mutant and wild-type M. smegmatis strains (Fig. 2C). The same phenotypes were observed for the ΔtatA mutant (data not shown). Thus, the Δtat mutants appeared to replicate at the same rate as wild-type M. smegmatis in liquid medium, even though slower-growing colonies were observed with agar medium. The basis for the discrepancy between OD600 readings and viable CFU in culture is not clear.
Complementation analysis of the ΔtatA mutant (MB692) was performed with a single-copy plasmid, pJM124, that expresses M. smegmatis tatA from the constitutive hsp60 promoter. For the ΔtatC mutant (JM567), the multicopy plasmid pJM120, which constitutively expresses M. smegmatis tatC from the hsp60 promoter, was used. Introduction of these tat expression constructs into the respective Δtat mutants successfully rescued the mutant phenotypes: small-colony growth on agar and reduced OD600 readings during liquid culture (Fig. 2). The complementation experiments demonstrated that the Δtat mutations are responsible for the observed phenotypes and indicated that both TatA and TatC of M. smegmatis are functional and required for optimal in vitro growth.
M. smegmatis tat mutants are sensitive to SDS.
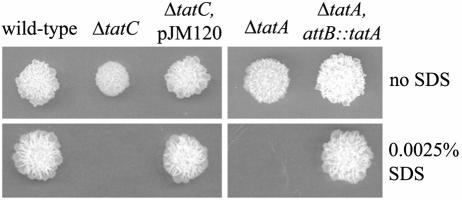
Tat-deficient mutants of E. coli are hypersensitive to the detergent SDS (38, 72). To determine if the M. smegmatis ΔtatA and ΔtatC mutants were similarly hypersensitive to SDS, we compared the abilities of the mutant strains to grow on agar media containing SDS by using a replica-inoculating protocol (described in Materials and Methods) (71). The ΔtatA and ΔtatC mutants failed to grow in the presence of 0.0025% SDS, in contrast to growth observed for the wild-type strain (Fig. 3). This was true even after extended incubation (data not shown). Growth of the Δtat mutants on 0.0025% SDS was restored to wild-type levels when complemented with Tat proteins expressed in trans. The failure of the Δtat mutants to grow on medium containing SDS was not a result of the general growth defect on agar medium, as the Δtat mutants grew on agar media lacking SDS, albeit more slowly (Fig. 3).
FIG. 3.
ΔtatA and ΔtatC mutants are hypersensitive to SDS. Strains were grown in liquid media, and approximately 103 CFU was spotted onto plates with and without 0.0025% SDS by using a multipronged replica-inoculating device as described in Materials and Methods. The following strains were used: wild-type (mc2155, pMV261), ΔtatC mutant (JM567, pMV261), complemented ΔtatC strain (JM567, pJM120), ΔtatA mutant (MB692, pMB198), and complemented ΔtatA attB::tatA strain (MB692, pJM124).
M. smegmatis tat mutants are sensitive to β-lactam antibiotics.
Tat-deficient mutants of E. coli are also hypersusceptible to multiple antibiotics, including the β-lactam ampicillin (72). We similarly tested the ΔtatA and ΔtatC mutants of M. smegmatis for drug susceptibility. β-Lactam susceptibility was assayed by determining the MIC of carbenicillin, using a replica-inoculating protocol on 7H10 agar medium (described in Materials and Methods) (71). In contrast to wild-type M. smegmatis, which is intrinsically resistant to β-lactam antibiotics and had an MIC of >200 μg/ml for carbenicillin, the MIC for the ΔtatA and ΔtatC mutants was 5 μg/ml. The complemented strains were resistant to >200 μg/ml carbenicillin, indicating that increased susceptibility of the mutants to carbenicillin was due to the Δtat mutations. Similar results were obtained using a disk diffusion assay to test susceptibility to the β-lactams carbenicillin, ampicillin, and amoxicillin (data not shown). In both the MIC and disk diffusion assays, the sensitivity of the Δtat mutants was comparable to the sensitivity of a ΔblaS mutant lacking the major M. smegmatis β-lactamase. In contrast to the results with the β-lactam antibiotics, the ΔtatA and ΔtatC mutants exhibited no increased sensitivity to the other antibiotics tested (5 μg isoniazid, 25 μg rifampin, 25 μg ethambutol, 12.5 μg streptomycin, 20 μg tetracycline, and 20 μg vancomycin). Thus, the β-lactam-sensitive phenotype of the M. smegmatis Δtat mutants is a specific phenomenon and not the result of a general increase in drug susceptibility.
β-Lactamase export requires the Tat pathway of M. smegmatis.
The major β-lactamases BlaC and BlaS are required for the β-lactam resistance of M. tuberculosis and M. smegmatis, respectively (25, 44, 78). Since β-lactam antibiotics target extracytoplasmic cell wall biosynthetic enzymes, a β-lactamase must be exported to the cell envelope or extracellular environment in order to protect the bacterium. Inspection of the N termini of BlaC and BlaS revealed a twin-arginine motif, suggesting that both enzymes are exported by the Tat pathway. We hypothesized that the β-lactam sensitivity phenotype of the ΔtatA and ΔtatC mutants of M. smegmatis was due to a defect in export of the putative Tat substrate BlaS.
It was previously reported that during liquid growth M. smegmatis releases β-lactamase into the medium (57). To test whether M. smegmatis β-lactamase export depends upon the Tat pathway, we assayed short-term cell-free culture filtrates from the Δtat mutants for β-lactamase activity by using the chromogenic β-lactam nitrocefin.
Substantially less β-lactamase activity was observed for culture filtrates produced from the ΔtatA and ΔtatC mutants than for wild-type M. smegmatis (Fig. 4A). The Δtat mutant levels were comparable to that observed with the ΔblaS mutant. The low levels of nitrocefin hydrolyzing activity detected for the ΔblaS and Δtat strains were largely attributable to background from the 7H9 medium (Fig. 4A). To rule out the possibility that the reduced β-lactamase activity in culture filtrates of the Δtat mutants was due to reduced expression, we measured the nitrocefin hydrolyzing activity of whole-cell extracts of the strains. This analysis revealed β-lactamase expression with more cell-associated activity for the Δtat mutants than for the ΔblaS mutant (Fig. 4B). Further, a fivefold higher level of β-lactamase activity was associated with whole-cell extracts of the Δtat mutants than with the wild-type strain. This suggests that in the absence of Tat export β-lactamase accumulates with the cells of the Δtat mutants and that β-lactamase activity is specifically reduced in the culture filtrates of the Δtat mutants. As seen in Fig. 4B, whole-cell extracts of the ΔblaS mutant did not exhibit a complete lack of nitrocefin hydrolyzing activity, presumably due to the expression of the BlaE protein. BlaE was recently shown to have a minor contribution to the total β-lactamase activity of M. smegmatis, without contributing significantly to resistance (25). Examination of the BlaE protein sequence revealed a Sec-like signal sequence. When complemented, the ΔtatC mutant showed wild-type β-lactamase activity in the culture filtrate. Complementation of the ΔtatA mutant also resulted in an increase in culture filtrate β-lactamase activity, although to a reduced degree. These results indicate a defect in β-lactamase export by the Δtat mutants and serve to explain the increased β-lactam sensitivity observed in the absence of TatA and TatC.
FIG. 4.
Nitrocefin assays with culture filtrates and whole-cell extracts of M. smegmatis wild type and ΔtatA and ΔtatC mutants. Short-term culture filtrates (A) and whole-cell extracts (B) of log-phase M. smegmatis cultures were assayed for the ability to hydrolyze nitrocefin. Strains used were as follows: wild type (mc2155, pMV261), ΔblaS (PM759, pMV261), ΔtatC mutant (JM567, pMV261), complemented ΔtatC strain (JM567, pJM120), ΔtatA mutant (MB692, pMB198), and complemented ΔtatA attB::tatA strain (MB692, pJM124). Liquid culture medium (7H9) was used. Nitrocefin hydrolyzing activity was expressed as A486 min−1 mg total protein−1 and was standardized to PBS. Shown is a representative assay, reported as averages ± standard deviations.
Export of active M. tuberculosis BlaC depends on the Tat pathway and a twin-arginine signal sequence.
The above results strongly suggest that the M. smegmatis β-lactamase is a Tat substrate. Like BlaS, the major β-lactamase of M. tuberculosis (BlaC) has a predicted Tat signal sequence (Table 3). To determine if BlaC is a Tat substrate, we tested for Tat-dependent export of BlaC expressed in M. smegmatis ΔblaS, M. smegmatis ΔtatA ΔblaS, and M. tuberculosis ΔblaC mutants.
TABLE 3.
M. tuberculosis signal sequences fused to ′BlaC
| Signal sequence origin | Amino acid sequencea |
|---|---|
| BlaC | MRNRGFGRRELLVAMAMLVSVTGCARHASGARP |
| BlaC(KK) | MRNRGFGKKELLVAMAMLVSVTGCARHASGARP |
| PlcA | MASRREFLTKLTGAGAAAFLMDWAAPVIEKAYGGSARP |
| PlcB | MATRRQFFAKAAAATTAGAFMSLAGPIIEKAYGGSARP |
| PlcB(KK) | MATKKQFFAKAAAATTAGAFMSLAGPIIEKAYGGSARP |
| Mpt63 | MGMKLTTMIKTAVAVVAMAAIATFAAPVALAGSARP |
| FbpB | MATDVSRKIRAWGRRLMIGTAAAVVLPGLVGLAGGAATAGAGSARP |
Signal sequences of PlcA and PlcB were predicted by SignalP (3). Signal sequences of Mpt63 and FbpB were determined previously (35). Underlined residues denote the start of ′BlaC in the fusion constructs, residues in italics are derived from the Hsp60 coding region, and boldface indicates the predicted twin-arginine motif.
Unlike wild-type M. smegmatis and M. tuberculosis, the corresponding Δbla mutants are sensitive to β-lactams, failing to grow on 7H10 agar medium containing 50 μg/ml of carbenicillin. When full-length M. tuberculosis BlaC was expressed from plasmid pMP327 in M. smegmatis ΔblaS and M. tuberculosis ΔblaC mutants, the strains were resistant to carbenicillin (Table 4). This β-lactam resistance was dependent on BlaC export, since expression of a truncated ′BlaC lacking the native signal sequence, from plasmid pJM113, in either M. smegmatis ΔblaS or M. tuberculosis ΔblaC did not allow growth on carbenicillin containing agar medium (Table 4). Whole-cell extracts of M. smegmatis ΔblaS carrying pJM113 assayed for nitrocefin activity revealed a β-lactamase activity 20-fold higher than that of M. smegmatis ΔblaS, confirming that truncated ′BlaC was expressed in this strain (Fig. 5A).
TABLE 4.
Export of a functional ′BlaC is dependent on a twin-arginine signal sequence and an intact Tat pathway
| Strain | Genotypeb | Carbenicillin resistance for mycobacterial vectora
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Empty vector | pMP327 (BlaC) | pJM113 (′BlaC) | pJM117 [BlaC(KK)] | pJM130 (ss-PlcA- ′BlaC) | pJM111 (ss-PlcB- ′BlaC) | pJM118 [ss-PlcB(KK)- ′BlaC] | pMB228 (ss-Mpt63- ′BlaC) | pJM109 (ss-FbpB- ′BlaC) | ||
| M. smegmatis | ||||||||||
| mc2155 | WT | + | + | + | + | ND | + | + | + | + |
| PM759 | ΔblaS | − | + | − | − | + | + | − | − | − |
| JM578 | ΔtatA ΔblaS | − | − | − | − | − | − | − | − | − |
| M. tuberculosis | ||||||||||
| H37Rv | WT | + | + | + | + | ND | + | + | + | + |
| PM638 | ΔblaC | − | + | − | − | + | + | − | − | − |
All strains carrying a vector were resistant to 20 μg/ml kanamycin as a result of the vector resistance marker. Carbenicillin resistance and sensitivity were determined by the presence (+) or absence (−) of single-colony growth on 7H10 plates containing 50 μg/ml carbenicillin and 20 μg/ml kanamycin. ND, not done.
WT, wild type.
FIG. 5.
Nitrocefin assays with whole-cell extracts of M. smegmatis strains carrying ′BlaC fusion constructs. Whole-cell extracts of M. smegmatis cultures were assayed for the ability to hydrolyze nitrocefin. (A) Assays were performed with M. smegmatis ΔblaS (strain PM759) strains carrying the following vectors: pMV261 (empty), pMP327 (BlaC), pJM113 (′BlaC), pJM117 [BlaC(KK)], pJM111 (ss-PlcB-′BlaC), pJM118 [ss-PlcB(KK)-′BlaC], pJM130 (ss-PlcA-′BlaC), pMB228 (ss-Mpt63-′BlaC), and pJM109 (ss-FbpB-′BlaC). (B) Assays were performed with M. smegmatis ΔtatA ΔblaS (strain JM578) carrying pMV261 (empty), pMP327 (BlaC), pJM111 (ss-PlcB-′BlaC), or pJM130 (ss-PlcA-′BlaC). Nitrocefin hydrolyzing activity was expressed as A486 min−1 mg total protein−1 and was standardized to PBS. Shown is a representative assay reported as averages ± standard deviations.
Based on the above results, we used β-lactam resistance as an indicator of the export of the functional BlaC enzyme in the following experiments. Unlike the results obtained when we expressed full-length BlaC in the ΔblaS M. smegmatis mutant, expression of full-length BlaC in an M. smegmatis ΔtatA ΔblaS double mutant did not protect against β-lactams. Nitrocefin analysis of whole-cell extracts of M. smegmatis ΔtatA ΔblaS carrying pMP327 demonstrated that BlaC was expressed in this strain (Fig. 5B). Thus, the Tat pathway also appears to function in BlaC export.
In addition, we tested the significance of the twin-arginine motif in the BlaC signal sequence to the export of BlaC by using mutants in which the twin-arginine “RR” residues were substituted with conservative twin-lysine “KK” residues [BlaC(KK)] (Table 3). Similar substitutions in Tat substrates from other bacteria have revealed the importance of the “RR” dipeptide in Tat export (1, 20, 37, 73, 80). Expression of BlaC(KK) from plasmid pJM117 did not protect the Δbla mutants of M. smegmatis and M. tuberculosis from carbenicillin (Table 4). Nitrocefin analysis of M. smegmatis whole-cell extracts confirmed that BlaC(KK) was expressed from plasmid pJM117 in the ΔblaS background (Fig. 5A). This result further demonstrates that M. tuberculosis BlaC is a Tat substrate.
Truncated ′BlaC can be used as a reporter of Tat export in mycobacteria.
Since BlaC activity was dependent on the Tat pathway and an intact twin-arginine signal sequence, we tested whether truncated ′BlaC could report on the export of other Tat substrates. Plasmids pJM130 and pJM111, expressing ss-PlcA-′BlaC and ss-PlcB-′BlaC fusion proteins, in which the putative Tat signal sequences of M. tuberculosis PlcA and PlcB were fused in-frame and upstream of truncated ′BlaC (Table 3), respectively, were electroporated into ΔblaS M. smegmatis and ΔblaC M. tuberculosis. All of the resulting strains were β-lactam resistant (i.e., grew in the presence of carbenicillin) (Table 4). This indicates that the PlcA and PlcB signal sequences direct export of functional ′BlaC in M. smegmatis and M. tuberculosis.
Additional experiments demonstrated that the β-lactam protection observed with the ss-PlcA-′BlaC and ss-PlcB-′BlaC fusions was dependent on Tat export. First, TatA was required for β-lactam resistance, as shown by the failure of either ss-PlcA-′BlaC or ss-PlcB-′BlaC to protect against carbenicillin when expressed in the M. smegmatis ΔtatA ΔblaS double mutant (Table 4). Second, a ss-PlcB-′BlaC fusion engineered to have a twin-lysine (KK) substitution for the twin arginines (RR) in the signal sequence (expressed from plasmid pJM118) did not protect against carbenicillin when expressed in the ΔblaS and ΔblaC strains of M. smegmatis and M. tuberculosis (Tables 3 and 4). Cell lysates from all of these strains possessed β-lactamase activity by nitrocefin analysis (Fig. 5). These results reveal a role for the Tat pathway and the “RR” motif in PlcB export. They further demonstrate that ′BlaC can be used as a reporter of protein export by the Tat pathway.
The ′BlaC reporter does not function with Sec-dependent signal sequences.
Having demonstrated that native BlaC is a Tat substrate that could report on Tat export, we asked whether the ′BlaC reporter also functions when targeted to the Sec pathway. MPT63/Rv1926c and antigen 85b/FbpB/Rv1886c are well-characterized secreted proteins of M. tuberculosis (35), and both of these proteins have putative Sec signal sequences (Table 3) (43). Plasmids pMB228 (ss-Mpt63-′BlaC) and pJM109 (ss-FbpB-′BlaC) were tested in M. smegmatis ΔblaS and M. tuberculosis ΔblaC. Both ss-Mpt63-′BlaC and ss-FbpB-′BlaC failed to protect the bla mutants from carbenicillin (Table 4). Nitrocefin analysis of whole-cell lysates of ΔblaS M. smegmatis carrying pMB228 (ss-Mpt63-′BlaC) revealed a very low level of β-lactamase activity, while ΔblaS M. smegmatis carrying pJM109 (ss-FbpB-′BlaC) did not exhibit any detectable activity (Fig. 5A). The different outcomes of testing these ss-Sec-′BlaC fusions suggests that, unlike Tat signal sequences, Sec signal sequences are unable to promote export of a functionally active ′BlaC.
DISCUSSION
Here we report the functional characterization of the Tat pathway in mycobacteria. Our study shows that deletion of the tatA and tatC genes in M. smegmatis results in slow growth on agar medium, hypersensitivity to SDS, and sensitivity to β-lactam antibiotics. The diverse phenotypes we observed suggest that the mycobacterial Tat pathway exports multiple substrates.
Our first attempts to construct tatA and tatC deletion mutants from merodiploid single-crossover strains were problematic and suggested that the Tat pathway was essential in M. smegmatis. This was consistent with transposon site hybridization (TraSH) analysis of M. tuberculosis, which defined tatC as an essential gene for optimal growth (65). However, with extended incubation we recovered deletion mutants of tatA and tatC. Currently, the basis of the Δtat mutant growth defect is not clear. We hypothesize that mislocalization of a Tat substrate involved in nutrient uptake is responsible, since a number of M. tuberculosis Tat substrates, predicted by TATFIND, are homologous to periplasmic substrate-binding proteins of ABC transporters.
The M. smegmatis ΔtatA and ΔtatC mutants exhibited identical phenotypes, consistent with TatA and TatC being components of the same system. Research with other bacteria suggests that a functional Tat pathway requires a minimum of one TatA and one TatC homologue (7, 54). A mycobacterial ORF with similarity to E. coli TatB and conserved residues important to TatB function is present in the M. smegmatis and M. tuberculosis genomes (J. A. McDonough and M. Braunstein, unpublished results; also TubercuList, Institut Pasteur [http://genolist.pasteur.fr/TubercuList/]) (32, 81). However, the role of TatB in mycobacteria remains to be established. Unlike tatC, tatB of M. tuberculosis was described as nonessential by TraSH analysis (65). Furthermore, mycobacterial tatB is located distantly from tatA and tatC in the chromosome. Instead, tatB is located with genes encoding σE and potential regulators of σE (sigE, rseA, and htrA). This has led to the proposal that TatB functions in σE regulation, possibly as a specialized component of the Tat translocation system (45).
Some of the phenotypes reported for tat mutants of other bacteria were also exhibited by the M. smegmatis Δtat mutants. Interestingly, the basis of these tat phenotypes is not necessarily conserved. For E. coli Δtat mutants, the SDS hypersensitivity phenotype was linked to impairment of cell division, as indicated by cell filamentation, and attributed to mislocalization of amidases carrying Tat signal sequences (38, 72). Although the M. smegmatis Δtat mutants were also hypersensitive to SDS, no filamentation, morphological defect, or size difference was observed by light microscopy (data not shown). Furthermore, analysis of the protein sequences of putative mycobacterial amidases did not reveal recognizable Tat signal sequences. Consequently, we believe the basis of the SDS sensitivity phenotype for M. smegmatis Δtat mutants differs from that for E. coli. The drug sensitivity phenotype of the M. smegmatis Δtat mutants also differs from that for E. coli in being specific for β-lactams as opposed to a general increase in drug susceptibility due to a defect in the integrity of the cell envelope (72).
Using multiple approaches, we demonstrated that mycobacterial β-lactamases are native Tat substrates. The ΔtatA and ΔtatC mutants of M. smegmatis failed to grow in the presence of 50 μg/ml carbenicillin and exhibited a significant reduction in β-lactamase activity in the culture filtrate that was similar to the level observed with the β-lactam-sensitive ΔblaS β-lactamase mutant. We further showed that expression of M. tuberculosis BlaC in the M. smegmatis ΔblaS background protected against β-lactams in a Tat-dependent manner and that protection was dependent on the “RR” dipeptide in the twin-arginine motif. The latter experiments were performed with both M. smegmatis ΔblaS and M. tuberculosis ΔblaC strains.
To our knowledge, BlaS and BlaC are the first examples of Tat-exported β-lactamases. Since the Tat pathway exports folded proteins, the Tat-dependent nature of the mycobacterial β-lactamases may reflect rapid intracellular folding of the proteins prior to export. Cofactor-bound proteins are a common category of Tat substrates (48), but BlaC and BlaS are homologous to cofactorless class A β-lactamases (25, 29, 44). The primary amino acid sequences of BlaC and BlaS are only 37% identical, which suggests that the Tat dependence reflects a common requirement for Tat export as opposed to simple conservation among orthologues. Evaluation of bacterial genome databases reveals that Mycobacterium leprae and Mycobacterium fortuitum also have β-lactamases with predicted Tat signal sequences, as do a small number of additional nonmycobacterial species, including Burkholderia cepacia (77), Burkholderia pseudomallei (15), Nocardia asteroides (53), Streptomyces clavuligerus (52), and Yersinia enterocolitica (68).
Because β-lactams target proteins in the cell wall, β-lactamases must be exported to protect the cell. This feature of β-lactamase function was previously exploited in the development of TEM β-lactamase as a genetic reporter of protein export in E. coli and other bacteria (14, 49, 69). Despite the appearance of a Sec-like signal sequence on the native TEM β-lactamase, the truncated reporter was shown to work with both Sec and Tat signal sequences (69, 74). In an effort to develop a reporter of protein export that works directly in M. tuberculosis, we tested truncated ′BlaC and protein fusions in which signal sequences were placed in-frame and upstream of ′BlaC in ΔblaS or ΔblaC mycobacteria for the ability to protect against β-lactams. Of the four ′BlaC fusions tested, only those with the putative Tat signal sequences (ss-PlcA-′BlaC and ss-PlcB-′BlaC) promoted growth on carbenicillin plates; the ss-Sec-′BlaC fusions (Mpt63 and FbpB) did not. These results indicate that Tat signal sequences can direct export of a functional ′BlaC reporter but that the Sec signal sequences we tested cannot. This suggests that ′BlaC will be a useful tool for studying the Tat pathway in mycobacteria and for identifying exported Tat substrates in M. tuberculosis by selecting active ′BlaC fusions from expression libraries.
The plasmid pMM7-2 we used to express the ss-FbpB-′BlaC fusion was successful in expressing an active exported ss-FbpB-′PhoA alkaline phosphatase fusion in M. smegmatis (data not shown). Thus, it appears that this FbpB signal sequence construct can drive export of functional Sec substrates, such as PhoA, but not of Tat substrates, like BlaC. Interestingly, the signal sequence of FbpB contains consecutive arginines (R13R14L15M16I17) (Table 3). However, it possesses a region of high hydrophobicity following the R-R-X-φ-φ motif, which is not characteristic of Tat substrates, and TATFIND did not predict FbpB to be a Tat substrate (21). Our results with the ′BlaC reporter are consistent with FbpB not being a Tat substrate.
Our inability to detect significant levels of ss-Sec-′BlaC fusion proteins by nitrocefin analysis of whole-cell extracts may reflect rapid degradation of fusions incompatible with export from the cytoplasm, as this is a common fate of nonexported Tat substrates (8, 17). An alternate possibility is that the ss-Sec-′BlaC fusions are translocated by the Sec pathway in an unfolded state and that the resulting exported ′BlaC is enzymatically inactive due to improper folding. In this latter scenario, ′BlaC reports on Tat export because it is only functional if exported by the Tat pathway. A precedent for this type of Tat dependence is the export of green fluorescent protein by E. coli. When exported by the Tat pathway green fluorescent protein is active, but when exported by the Sec pathway it is inactive (24, 62, 76).
We used the nitrocefin assay with whole-cell extracts as a way to assess fusion protein expression in carbenicillin-sensitive strains (Fig. 5). Interestingly, this analysis also revealed substantial cell-associated β-lactamase activity in the ΔblaS strain expressing full-length BlaC. This was surprising since our analysis of native M. smegmatis BlaS revealed a relatively low level of β-lactamase activity in the wild-type cell extract (Fig. 4B). Comparison of BlaC and BlaS revealed a potential lipoprotein lipid attachment site at cysteine-24 of the BlaC predicted signal sequence (Table 3) (41). Thus, lipid modification of BlaC may tether the protein to the cell wall, accounting for the different localizations of exported M. smegmatis and M. tuberculosis β-lactamases.
Examination of the PlcA, PlcB, and PlcC proteins of M. tuberculosis reveals the presence of an N-terminal twin-arginine motif for each enzyme, and empirical evidence with M. tuberculosis demonstrates that these proteins are cell wall localized (39, 58). There is a precedent for phospholipase C proteins being Tat substrates in Pseudomonas aeruginosa (79). Using ss-PlcA-′BlaC and ss-PlcB-′BlaC fusions, we demonstrated Tat dependence for the signal sequences of the M. tuberculosis phospholipase C proteins. These ′BlaC fusion proteins were active when expressed in a ΔblaS background but not in a ΔblaS ΔtatA background. Furthermore, the “RR” dipeptide in the PlcB signal sequence was required for the export of an active ′BlaC fusion, as shown by using the ss-PlcB(KK)-′BlaC fusion protein. Interestingly, PlcA was not identified by the TATFIND program, presumably due to the presence of a negatively charged aspartic acid in the C-terminal region of the predicted signal sequence. In contrast, PlcB lacks this residue and was predicted by TATFIND to be a Tat substrate (21). Our results with the PlcA signal sequence may reflect mycobacterial-specific properties of Tat signal sequences that are not incorporated into the TATFIND algorithm. Because the ′BlaC reporter is a native mycobacterial protein dependent on a Tat signal sequence and Tat pathway for export, it may be able to identify mycobacterial Tat substrates overlooked by a bioinformatic approach.
Using M. tuberculosis phospholipase C mutants, Raynaud et al. demonstrated the contribution of PlcA, PlcB, and PlcC to the full virulence of M. tuberculosis in a mouse model of infection (58). Similarly, a P. aeruginosa ΔtatC mutant unable to export two phospholipase C proteins, PlcH and PlcN, is attenuated for virulence (47). Additional bacterial pathogens (Agrobacterium tumefaciens, enterohemorrhagic E. coli O157:H7, and Legionella pneumophila) require a functional Tat pathway for full virulence, although the exported factors involved are not clearly defined (22, 55, 60). We expect that the M. tuberculosis Tat pathway also contributes to pathogenesis by exporting virulence factors, including Plc enzymes. M. tuberculosis Δtat mutants are required to directly test the role of the Tat pathway in M. tuberculosis virulence; however, the potential for an M. tuberculosis Δtat mutant to exhibit a growth defect in vitro may complicate such analysis.
Our demonstration of the existence of a functional Tat pathway in mycobacteria brings us closer to understanding the protein export systems that function in mycobacterial physiology and pathogenesis. Knowledge of the Tat pathway may also benefit the development of novel tuberculosis vaccines. Recombinant Mycobacterium bovis BCG strains with improved secretion capability and/or the ability to export foreign antigens have been proposed as a vaccine strategy (31, 34). Since the Tat pathway is capable of exporting folded proteins, it could be the ideal mechanism for secreting highly expressed proteins and foreign antigens. Finally, the ′BlaC reporter system should serve as a powerful genetic tool for studying protein export directly with M. tuberculosis and help us identify the complete set of M. tuberculosis Tat-dependent exported proteins.
Acknowledgments
This research was funded in part by grants from the National Institute of Allergy and Infectious Disease of the NIH (AI54540 to M.B. and AI47311 to M.S.P.), a developmental grant from the University of North Carolina at Chapel Hill Center for AIDS Research (NIH no. 9P30 AI 50410-04), and a Burroughs Wellcome Fund Career Award in the Biomedical Sciences (M.S.P.). J.A.M. was also supported by the Training in Sexually Transmitted Diseases and AIDS NIH NIAID training grant 5T32 AI07001-28. A.R.F. was also supported by the Molecular Pathogenesis of Bacteria and Viruses NIH training grant T32 AI07362 and the Medical Scientist Training Program funded by the NIH grant T32 GM07356.
We thank M. Pohlschröder for providing us with the M. tuberculosis TATFIND list prior to public release of the program and the members of the Braunstein laboratory for review of the manuscript.
REFERENCES
- 1.Alami, M., D. Trescher, L. F. Wu, and M. Muller. 2002. Separate analysis of twin-arginine translocation (Tat)-specific membrane binding and translocation in Escherichia coli. J. Biol. Chem. 277:20499-20503. [DOI] [PubMed] [Google Scholar]
- 2.Bange, F. C., F. M. Collins, and W. R. Jacobs, Jr. 1999. Survival of mice infected with Mycobacterium smegmatis containing large DNA fragments from Mycobacterium tuberculosis. Tuber. Lung Dis. 79:171-180. [DOI] [PubMed] [Google Scholar]
- 3.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 4.Berks, B. C. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22:393-404. [DOI] [PubMed] [Google Scholar]
- 5.Berks, B. C., T. Palmer, and F. Sargent. 2005. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr. Opin. Microbiol. 8:174-181. [DOI] [PubMed] [Google Scholar]
- 6.Berks, B. C., F. Sargent, and T. Palmer. 2000. The Tat protein export pathway. Mol. Microbiol. 35:260-274. [DOI] [PubMed] [Google Scholar]
- 7.Blaudeck, N., P. Kreutzenbeck, M. Muller, G. A. Sprenger, and R. Freudl. 2005. Isolation and characterization of bifunctional Escherichia coli TatA mutant proteins that allow efficient Tat-dependent protein translocation in the absence of TatB. J. Biol. Chem. 280:3426-3432. [DOI] [PubMed] [Google Scholar]
- 8.Blaudeck, N., G. A. Sprenger, R. Freudl, and T. Wiegert. 2001. Specificity of signal peptide recognition in Tat-dependent bacterial protein translocation. J. Bacteriol. 183:604-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogsch, E. G., F. Sargent, N. R. Stanley, B. C. Berks, C. Robinson, and T. Palmer. 1998. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J. Biol. Chem. 273:18003-18006. [DOI] [PubMed] [Google Scholar]
- 10.Braunstein, M., S. S. Bardarov, and W. R. Jacobs, Jr. 2002. Genetic methods for deciphering virulence determinants of Mycobacterium tuberculosis. Methods Enzymol. 358:67-99. [DOI] [PubMed] [Google Scholar]
- 11.Braunstein, M., A. M. Brown, S. Kurtz, and W. R. Jacobs, Jr. 2001. Two nonredundant SecA homologues function in mycobacteria. J. Bacteriol. 183:6979-6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunstein, M., B. J. Espinosa, J. Chan, J. T. Belisle, and W. R. Jacobs, Jr. 2003. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 48:453-464. [DOI] [PubMed] [Google Scholar]
- 13.Bronstein, P., M. Marrichi, and M. P. DeLisa. 2004. Dissecting the twin-arginine translocation pathway using genome-wide analysis. Res. Microbiol. 155:803-810. [DOI] [PubMed] [Google Scholar]
- 14.Broome-Smith, J. K., M. Tadayyon, and Y. Zhang. 1990. Beta-lactamase as a probe of membrane protein assembly and protein export. Mol. Microbiol. 4:1637-1644. [DOI] [PubMed] [Google Scholar]
- 15.Cheung, T. K. M., P. L. Ho, P. C. Y. Woo, K. Y. Yuen, and P. Y. Chau. 2002. Cloning and expression of class A β-lactamase gene blaABPS in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 46:1132-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 17.Cristóbal, S., J.-W. de Gier, H. Nielsen, and G. von Heijne. 1999. Competition between Sec- and TAT-dependent protein translocation in Escherichia coli. EMBO J. 18:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalbey, R. E., and M. Chen. 2004. Sec-translocase mediated membrane protein biogenesis. Biochim. Biophys. Acta 1694:37-53. [DOI] [PubMed] [Google Scholar]
- 19.de Keyzer, J., C. van der Does, and A. J. Driessen. 2003. The bacterial translocase: a dynamic protein channel complex. Cell. Mol. Life Sci. 60:2034-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLisa, M. P., P. Samuelson, T. Palmer, and G. Georgiou. 2002. Genetic analysis of the twin arginine translocator secretion pathway in bacteria. J. Biol. Chem. 277:29825-29831. [DOI] [PubMed] [Google Scholar]
- 21.Dilks, K., R. W. Rose, E. Hartmann, and M. Pohlschröder. 2003. Prokaryotic utilization of the twin-arginine translocation pathway: a genomic survey. J. Bacteriol. 185:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding, Z., and P. J. Christie. 2003. Agrobacterium tumefaciens twin-arginine-dependent translocation is important for virulence, flagellation, and chemotaxis but not type IV secretion. J. Bacteriol. 185:760-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dye, C., S. Scheele, P. Dolin, V. Pathania, M. C. Raviglione, et al. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 24.Feilmeier, B. J., G. Iseminger, D. Schroeder, H. Webber, and G. J. Phillips. 2000. Green fluorescent protein functions as a reporter for protein localization in Escherichia coli. J. Bacteriol. 182:4068-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flores, A. R., L. M. Parsons, and M. S. Pavelka, Jr. 2005. Genetic analysis of the β-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to β-lactam antibiotics. Microbiology 151:521-532. [DOI] [PubMed] [Google Scholar]
- 26.Glickman, M. S., J. S. Cox, and W. R. Jacobs, Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 27.Gohlke, U., L. Pullan, C. A. McDevitt, I. Porcelli, E. de Leeuw, T. Palmer, H. R. Saibil, and B. C. Berks. 2005. The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc. Natl. Acad. Sci. USA 102:10482-10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gouffi, K., C. L. Santini, and L. F. Wu. 2002. Topology determination and functional analysis of the Escherichia coli TatC protein. FEBS Lett. 525:65-70. [DOI] [PubMed] [Google Scholar]
- 29.Hackbarth, C. J., I. Unsal, and H. F. Chambers. 1997. Cloning and sequence analysis of a class A β-lactamase from Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 41:1182-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halbig, D., T. Wiegert, N. Blaudeck, R. Freudl, and G. A. Sprenger. 1999. The efficient export of NADP-containing glucose-fructose oxidoreductase to the periplasm of Zymomonas mobilis depends both on an intact twin-arginine motif in the signal peptide and on the generation of a structural export signal induced by cofactor binding. Eur. J. Biochem. 263:543-551. [DOI] [PubMed] [Google Scholar]
- 31.Hess, J., D. Miko, A. Catic, V. Lehmensiek, D. G. Russell, and S. H. E. Kaufmann. 1998. Mycobacterium bovis bacille Calmette-Guérin strains secreting listeriolysin of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 95:5299-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks, M. G., E. de Leeuw, I. Porcelli, G. Buchanan, B. C. Berks, and T. Palmer. 2003. The Escherichia coli twin-arginine translocase: conserved residues of TatA and TatB family components involved in protein transport. FEBS Lett. 539:61-67. [DOI] [PubMed] [Google Scholar]
- 33.Hinsley, A. P., N. R. Stanley, T. Palmer, and B. C. Berks. 2001. A naturally occurring bacterial Tat signal peptide lacking one of the ‘invariant’ arginine residues of the consensus targeting motif. FEBS Lett. 497:45-49. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Masleša-Galić. 2000. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horwitz, M. A., B. W. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ignatova, Z., C. Hornle, A. Nurk, and V. Kasche. 2002. Unusual signal peptide directs penicillin amidase from Escherichia coli to the Tat translocation machinery. Biochem. Biophys. Res. Commun. 291:146-149. [DOI] [PubMed] [Google Scholar]
- 37.Ize, B., F. Gerard, and L. F. Wu. 2002. In vivo assessment of the Tat signal peptide specificity in Escherichia coli. Arch. Microbiol. 178:548-553. [DOI] [PubMed] [Google Scholar]
- 38.Ize, B., N. R. Stanley, G. Buchanan, and T. Palmer. 2003. Role of the Escherichia coli Tat pathway in outer membrane integrity. Mol. Microbiol. 48:1183-1193. [DOI] [PubMed] [Google Scholar]
- 39.Johansen, K. A., R. E. Gill, and M. L. Vasil. 1996. Biochemical and molecular analysis of phospholipase C and phospholipase D activity in mycobacteria. Infect. Immun. 64:3259-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jongbloed, J. D., U. Martin, H. Antelmann, M. Hecker, H. Tjalsma, G. Venema, S. Bron, J. M. van Dijl, and J. Muller. 2000. TatC is a specificity determinant for protein secretion via the twin-arginine translocation pathway. J. Biol. Chem. 275:41350-41357. [DOI] [PubMed] [Google Scholar]
- 41.Juncker, A. S., H. Willenbrock, G. Von Heijne, S. Brunak, H. Nielsen, and A. Krogh. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12:1652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurtz, S., and M. Braunstein. 2005. Protein secretion and export in Mycobacterium tuberculosis, p. 71-138. In T. Parish (ed.), Mycobacterium molecular microbiology. Horizon Bioscience, London, United Kingdom.
- 43.Lee, B. Y., and M. A. Horwitz. 1999. T-cell epitope mapping of the three most abundant extracellular proteins of Mycobacterium tuberculosis in outbred guinea pigs. Infect. Immun. 67:2665-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, X.-Z., L. Zhang, and H. Nikaido. 2004. Efflux pump-mediated intrinsic drug resistance in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 48:2415-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manganelli, R., R. Provvedi, S. Rodrigue, J. Beaucher, L. Gaudreau, and I. Smith. 2004. Sigma factors and global gene regulation in Mycobacterium tuberculosis. J. Bacteriol. 186:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Callaghan, C. H., A. Morris, S. M. Kirby, and A. H. Shingler. 1972. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochsner, U. A., A. Snyder, A. I. Vasil, and M. L. Vasil. 2002. Effects of the twin-arginine translocase on secretion of virulence factors, stress response, and pathogenesis. Proc. Natl. Acad. Sci. USA 99:8312-8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer, T., F. Sargent, and B. C. Berks. 2005. Export of complex cofactor-containing proteins by the bacterial Tat pathway. Trends Microbiol. 13:175-180. [DOI] [PubMed] [Google Scholar]
- 49.Palva, I., M. Sarvas, P. Lehtovaara, M. Sibakov, and L. Kaariainen. 1982. Secretion of Escherichia coli beta-lactamase from Bacillus subtilis by the aid of alpha-amylase signal sequence. Proc. Natl. Acad. Sci. USA 79:5582-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavelka, M. S., Jr., and W. R. Jacobs, Jr. 1996. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J. Bacteriol. 178:6496-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavelka, M. S., Jr., and W. R. Jacobs, Jr. 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guérin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 181:4780-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pérez-Llarena, F., J. F. Martín, M. Galleni, J. J. R. Coque, J. L. Fuente, J.-M. Frère, and P. Liras. 1997. The bla gene of the cephamycin cluster of Streptomyces clavuligerus encodes a class A β-lactamase of low enzymatic activity. J. Bacteriol. 179:6035-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poirel, L., F. Laurent, T. Naas, R. Labia, P. Boiron, and P. Nordmann. 2001. Molecular and biochemical analysis of AST-1, a class A β-lactamase from Nocardia asteroides sensu stricto. Antimicrob. Agents Chemother. 45:878-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pop, O., U. Martin, C. Abel, and J. P. Muller. 2002. The twin-arginine signal peptide of PhoD and the TatAd/Cd proteins of Bacillus subtilis form an autonomous Tat translocation system. J. Biol. Chem. 277:3268-3273. [DOI] [PubMed] [Google Scholar]
- 55.Pradel, N., C. Ye, V. Livrelli, J. Xu, B. Joly, and L. F. Wu. 2003. Contribution of the twin arginine translocation system to the virulence of enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 71:4908-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raynaud, C., G. Etienne, P. Peyron, M. A. Laneelle, and M. Daffe. 1998. Extracellular enzyme activities potentially involved in the pathogenicity of Mycobacterium tuberculosis. Microbiology 144:577-587. [DOI] [PubMed] [Google Scholar]
- 58.Raynaud, C., C. Guilhot, J. Rauzier, Y. Bordat, V. Pelicic, R. Manganelli, I. Smith, B. Gicquel, and M. Jackson. 2002. Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 45:203-217. [DOI] [PubMed] [Google Scholar]
- 59.Robinson, C., and A. Bolhuis. 2004. Tat-dependent protein targeting in prokaryotes and chloroplasts. Biochim. Biophys. Acta 1694:135-147. [DOI] [PubMed] [Google Scholar]
- 60.Rossier, O., and N. P. Cianciotto. 2005. The Legionella pneumophila tatB gene facilitates secretion of phospholipase C, growth under iron-limiting conditions, and intracellular infection. Infect. Immun. 73:2020-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 62.Santini, C. L., A. Bernadac, M. Zhang, A. Chanal, B. Ize, C. Blanco, and L. F. Wu. 2001. Translocation of jellyfish green fluorescent protein via the Tat system of Escherichia coli and change of its periplasmic localization in response to osmotic up-shock. J. Biol. Chem. 276:8159-8164. [DOI] [PubMed] [Google Scholar]
- 63.Sargent, F., E. G. Bogsch, N. R. Stanley, M. Wexler, C. Robinson, B. C. Berks, and T. Palmer. 1998. Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J. 17:3640-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sargent, F., N. R. Stanley, B. C. Berks, and T. Palmer. 1999. Sec-independent protein translocation in Escherichia coli. A distinct and pivotal role for the TatB protein. J. Biol. Chem. 274:36073-36082. [DOI] [PubMed] [Google Scholar]
- 65.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 66.Schaerlaekens, K., M. Schierová, E. Lammertyn, N. Geukens, J. Anné, and L. Van Mellaert. 2001. Twin-arginine translocation pathway in Streptomyces lividans. J. Bacteriol. 183:6727-6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaerlaekens, K., L. Van Mellaert, E. Lammertyn, N. Geukens, and J. Anné. 2004. The importance of the Tat-dependent protein secretion pathway in Streptomyces as revealed by phenotypic changes in tat deletion mutants and genome analysis. Microbiology 150:21-31. [DOI] [PubMed] [Google Scholar]
- 68.Seoane, A., and J. M. Garcia Lobo. 1991. Nucleotide sequence of a new class A beta-lactamase gene from the chromosome of Yersinia enterocolitica: implications for the evolution of class A beta-lactamases. Mol. Gen. Genet. 228:215-220. [DOI] [PubMed] [Google Scholar]
- 69.Smith, H., S. Bron, J. Van Ee, and G. Venema. 1987. Construction and use of signal sequence selection vectors in Escherichia coli and Bacillus subtilis. J. Bacteriol. 169:3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 71.Sparling, P. F., F. A. Sarubbi, Jr., and E. Blackman. 1975. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J. Bacteriol. 124:740-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanley, N. R., K. Findlay, B. C. Berks, and T. Palmer. 2001. Escherichia coli strains blocked in Tat-dependent protein export exhibit pleiotropic defects in the cell envelope. J. Bacteriol. 183:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanley, N. R., T. Palmer, and B. C. Berks. 2000. The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli. J. Biol. Chem. 275:11591-11596. [DOI] [PubMed] [Google Scholar]
- 74.Stanley, N. R., F. Sargent, G. Buchanan, J. Shi, V. Stewart, T. Palmer, and B. C. Berks. 2002. Behaviour of topological marker proteins targeted to the Tat protein transport pathway. Mol. Microbiol. 43:1005-1021. [DOI] [PubMed] [Google Scholar]
- 75.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, and G. F. Hatfull. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 76.Thomas, J. D., R. A. Daniel, J. Errington, and C. Robinson. 2001. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 39:47-53. [DOI] [PubMed] [Google Scholar]
- 77.Trépanier, S., A. Prince, and A. Huletsky. 1997. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrob. Agents Chemother. 41:2399-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voladri, R. K., D. L. Lakey, S. H. Hennigan, B. E. Menzies, K. M. Edwards, and D. S. Kernodle. 1998. Recombinant expression and characterization of the major beta-lactamase of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voulhoux, R., G. Ball, B. Ize, M. L. Vasil, A. Lazdunski, L. F. Wu, and A. Filloux. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20:6735-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yahr, T. L., and W. T. Wickner. 2001. Functional reconstitution of bacterial Tat translocation in vitro. EMBO J. 20:2472-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yen, M. R., Y. H. Tseng, E. H. Nguyen, L. F. Wu, and M. H. Saier, Jr. 2002. Sequence and phylogenetic analyses of the twin-arginine targeting (Tat) protein export system. Arch. Microbiol. 177:441-450. [DOI] [PubMed] [Google Scholar]
- 82.Zeoli, C., M. Maitland, H. Rose, B. Bloom, A. Mittelman, and J. Geliebter. 2001. Expression of prostate specific molecules in bacille Calmette-Guérin: an immunotherapeutic approach to prostate cancer. Prostate J. 3:92-97. [Google Scholar]