Abstract
Polyglutamate is found in various bacteria, but displays different functions depending on the species and their environment. Here, we describe a minimal polyglutamate synthesis system in Bacillus anthracis. In addition to the three genes previously described as sufficient for polyglutamate synthesis, this system includes a small open reading frame, capE, belonging to the cap operon. The polyglutamate system's requirement for the five cap genes, for capsulation and anchoring, was assayed in nonpolar mutants. The capA, capB, capC, and capE genes are all necessary and are sufficient for polyglutamate synthesis by B. anthracis. capD is required for polyglutamate anchoring to the peptidoglycan. The 47-amino-acid peptide encoded by capE is localized in the B. anthracis membrane. It is not a regulator and it is required for polyglutamate synthesis, suggesting that it has a structural role in polyglutamate synthesis. CapE appears to interact with CapA. Bacillus subtilis ywtC is similar to capE and we named it pgsE. Genes similar to capE or pgsE were found in B. subtilis natto, Bacillus licheniformis, and Staphylococcus epidermidis, species that produce polyglutamate. All the bacterial polyglutamate synthesis systems analyzed show a similar genetic organization and, we suggest, the same protein requirements.
Poly-γ-glutamate (PGA) is a natural polymer. It has diverse functions that vary according to the species and their environment. For example, because it is anionic, PGA has a high affinity for metal ions (27). Planococcus halophilus, Sporosarcina halophila, and probably the hallophilic archaeon Natrialba asiatica produce PGA that allows them to resist high salt concentrations (16, 17). PGA is also produced in the nematocyst of the common freshwater polyp Hydra vulgaris that belongs to the cnidarian species. PGA is also very poorly immunogenic, and bacilli, such as Bacillus anthracis, that are covered by a capsule of PGA do not elicit anticapsule antibodies in the host (4, 14, 32, 34). When present, bacterial capsules are the outermost structure of the bacterial surface. Capsules can prevent desiccation and can also promote adherence. They have mainly been studied because many capsulated bacteria are pathogenic and the capsule is involved in the first contact between the bacterium and the host. However, very little is known about PGA synthesis.
B. anthracis, the etiological agent of anthrax, is a gram-positive sporeforming bacterium that expresses two major virulence factors, toxins and capsule. Anthrax disease is a lethal infection that involves both toxemia and septicemia. The toxin genes are carried on a 186-kb plasmid, pXO1, and the capsule synthetic operon (capBCAD) is carried on a 90-kb plasmid, pXO2. The B. anthracis capsule is composed of poly-γ-d-glutamate and is covalently linked to the peptidoglycan (4). Three B. anthracis genes, capB, capC, and capA, were shown to be necessary and sufficient to drive the production of polyglutamate in Escherichia coli (24, 25). However, Makino et al. reported that polyglutamate production was weak in E. coli. Moreover, these three genes are not sufficient to promote PGA synthesis in Bacillus subtilis (24). Therefore, there may be an essential element that is absent in B. subtilis.
CapB, CapC, and CapA have not been studied in B. anthracis. Their functions have been predicted or deduced by similarity to the corresponding proteins in B. subtilis. Other gram-positive bacteria, including bacilli, also produce PGA: over the last 10 years, genes similar to B. anthracis capB, capC, and capA have been found in Bacillus subtilis 168 IFO3336 (named ywsC, ywtA, and ywtB, respectively), Staphylococcus epidermidis ATCC 12228 (capB, capC, and capA), Bacillus licheniformis ATCC 14580, and B. subtilis natto IFO16449 (pgsB, pgsC, pgsAA, and pgsA for both) genomes (1, 12, 22, 31, 45). PGA synthesis systems have been studied in B. licheniformis (18, 19, 43), and more recently in B. subtilis (1, 44). However, the PGAs in these species are composed of d- and l-glutamates whereas the B. anthracis PGA is composed of the d-isomer only. Furthermore, the B. anthracis PGA is anchored, whereas the B. subtilis and some B. licheniformis PGAs are secreted or released into the culture supernatant.
A B. licheniformis membranous enzymatic complex was shown to be involved in PGA synthesis using l-glutamate as substrate and ATP as the energy source, but its components were not described (23, 43). YwsC, YwtA, and YwtB in B. subtilis 168 and the similar PgsB, PgsC, and PgsA in B. subtilis natto may also be organized in complexes (3, 44). It is assumed that PgsB, PgsC, and PgsA have similar roles to CapB, CapC, and CapA, respectively. CapB/PgsB harbors a Walker sequence and appears to be the polymerase, able to use ATP (10, 44). Ashuishi et al. reported that PgsB, PgsC, and PgsA together had a higher ATPase activity than either the isolated proteins or the PgsB PgsC pair of proteins, and therefore the three proteins might be organized in a complex (3). PgsB and PgsC together possess activity, albeit lower than that of the three proteins, suggesting that PgsB and PgsC form a tight complex (3).
Contradictory data have been published. PgsA may be necessary for PGA synthesis (3), or PgsA may be required to improve PGA production, indicating that PgsB and PgsC could have the polymerase role (44). The complex must also transport the substrate or the PGA through the plasma membrane, and PgsC (YwtB) has similarity with a transport protein (44). In B. anthracis, a fourth protein encoded by the capBCAD operon, CapD, belongs to the γ-glutamyl-transpeptidase family. It is involved in anchoring of the capsule (4), and it may well catalyze the covalent anchoring of PGA to the peptidoglycan.
Here, we defined a minimum PGA-synthesizing system in B. anthracis. Our data indicate that capB, capC, and capA are not sufficient to produce PGA in B. anthracis. A previously undescribed open reading frame (ORF), capE, encoding a 47-amino-acid peptide, is necessary for PGA synthesis. The protein encoded, CapE, appears to interact with CapA. A genetic analysis suggests that PGA synthesis in other bacteria also requires a similar gene.
MATERIALS AND METHODS
Bacterial strain, cloning vectors, and culture conditions.
Escherichia coli TG1 (33) was used as a host for derivatives of pUC19 (New England Laboratories), pAT113, pAT18 and pAT28 (40, 41, 42), pGemT-easy (Promega), pSPCH + 1, pSPCH + 2 (28,) and pBACP1 (4, 11). HB101(pRK24) was used for mating experiments (39). Two B. anthracis strains were used during the study, 9131 (pXO1− and pXO2−) and RPG1 (pXO1+, pXO2+, Tox−) (9, 13). B. anthracis strains were grown in brain-heart infusion (BHI; Difco Laboratories). To observe capsule, B. anthracis was grown in R-medium with 0.6% sodium bicarbonate (35) or on CAP medium under a 5% CO2 atmosphere (11). Antibiotics were used as previously described (37).
DNA manipulation.
Plasmid extraction, endonuclease digestion, ligation and agarose and polyacrylamide gel electrophoreses were carried out as described by Maniatis et al. (26). pXO2 was purified by a method derived from the classical alkaline purification method. The lysis step is carried out in 20% sucrose and the protoplast formation is followed by optical microscopy. PCR amplifications were carried out with long-range high fidelity Taq DNA polymerase according to the manufacturer's instructions (Roche).
Genetic constructions.
The capB, capA, capC, and capE genes were inactivated with non polar spectinomycin cassettes (28). A BglII and PstI fragment from pBACP1 was inserted into pUC19 digested with BamHI and PstI giving pUCBC10. pUCBC20 was constructed by replacing XbaI-HincII fragment of pUCBC10 with a HincII fragment of pSPCH+2 containing a nonpolar spectinomycin cassette. The SphI-SacI fragment of pUCBC20 was inserted into pAT113 digested with the same endonucleases, giving p113capB.
A DNA fragment was amplified by PCR with oligonucleotides capBinterne (GGTGGTGTCGACGTGAAGGAGGAGCATTATGTCAGAAGAATTCTT ACGAAAATTTGATTACATGGTCTTCCC) and capA2a (TCCCCCGGGCATTTTGATACACAATATTTTTTACATCTTTGAAAT) using B. anthracis pXO2 as the template. The fragment was inserted into pUC19 digested with SmaI and HincII giving pUCCA10. pUCCA20 was constructed by replacing the StuI/EcoNI fragment with a HincII fragment of pSPCH+1 containing a nonpolar spectinomycin cassette. The SphI-SacI fragment of pUCCA20 was inserted into pAT113 digested with the same endonucleases, giving p113capA.
The capC open reading frame was amplified by PCR with oligonucleotides capCs (CGCGGATCCATGATCTTCATAATAGGTATATGTACAGTGTTTTTG) and capCa (TCCCCCGGGTTAAAATAAGTAATAAATATTCATGATTGCAAATG) using B. anthracis pXO2 as the template. This fragment was inserted into pUC19 digested with SmaI giving pCAPC10. A HincII fragment of pSPH+2 was inserted into pCAPC10 digested by SnaBI to give pCAPC20. The BamHI-SmaI fragment of pUCCA20 was inserted into pAT113 digested with the same endonucleases, giving p113capC.
An SphI-NheI fragment of pBACP1 was inserted into pUC19 digested by SmaI giving pUCE10. A HincII fragment of pSPCH+1 was inserted into pUCE10 digested by SnaBI giving pUCE20. The KpnI-SphI fragment of pUCE20 was inserted into pAT113 digested with the same endonucleases, giving p113capE.
To overexpress CapA2, a peptide contained within CapA (amino acid residues 46 to 191), we inserted the corresponding DNA into an expression vector. A DNA fragment was amplified with oligonucleotides capA2s (CGCGGATCCGAAGCAGTAGCACCAGTAAAACATCGTGAGAACGAAAAATT) and capA2a using B. anthracis pXO2 as the template. This fragment was inserted into pGEMT-easy and moved to pQE30 digested by BamHI-SmaI, giving rise to pQcapA2.
Plasmids containing capE or ywtC were constructed, using a replicative plasmid containing the pag promoter named pPPA40 (constructed as described below). First, an integrative plasmid harboring a NdeI cloning site 3′ to the pag promoter was constructed. To do so, an NdeI site was introduced overlapping the pag ATG codon by directed mutagenesis of the 6-kb BamHI fragment contained in pACP1 (5). The 3.4-kb XhoI-BamHI fragment was transferred to the replicative vector pAT28, giving pACP50. pPPA40 was constructed by inserting the SphI 2-kb fragment of pACP50 into pAT18 digested by SphI.
All open reading frames were amplified with capEs (CATATGGTTAAAAAAGTTTTTGGATGGATTATGCC) and HindIII-48a (AAGCTTTTAGGGGTTAGCCTGTAGATAATCACTAATC) for capE, and with 55s (CATATGAAATTTGTCAAAGCCATCTGGCCGTTTG) and 55a (AAGCTTTTTATTGGCGTTTACCGGTTCTTCCTGCTGC). All fragments were inserted into pGemT-easy and moved into pPPA40 by NdeI and SacI digestion, giving rise to pPAGE and pPAG55, respectively. The same strategy was used to obtain ywtB, ywtB-ywtC, pgsA and pgsA-pgsE. The following oligonucleotides were used, harboring NdeI and EcoRI sites (in uppercase letters): pgsAls (CATATGaaaaaacaactgaactttcaggaaaaactgc), pgsAla (GAATTCtcatttgttcaccactccgtttttattatttttcagc), pgsEls (CATATGaaatttgtcagagccatttggccgttcg), pgEla (GAATTCttattgcttgttttctgttgtttgatcctgctg), pgsAs (CATATGatgaaaaaagaactgagctttcatgaaaagctgctaaagc), pgsAa (GAATTCttagattttagtttgtcactatgatcaatatcaaacgtc), 55s, and 55a.
The SacI fragment of pBACP1 was inserted into pAT28 digested with SacI giving rise to pMINI10. The SpeI fragment of pMINI10 was deleted, giving pMINI20. The Bsu36I fragment of pMINI20 was deleted, giving rise to pMINI21 (Table 1).
TABLE 1.
Plasmids and B. anthracis strains used in this study
| Plasmid or strain | Relevant characteristics or genotype | Source or reference |
|---|---|---|
| Conjugative plasmids | ||
| pAT18 | Conjugative plasmid, replicative in B. anthracis, 20 to 50 copies per cell | 41 |
| pAT28 | Conjugative plasmid, replicative in B. anthracis, 20 to 50 copies per cell | 40 |
| pAT113 | Conjugative suicide plasmid used for gene inactivation in B. anthracis | 9 |
| p113capA | pAT113 carrying the inactivated capA gene | This work |
| p113capB | pAT113 carrying the inactivated capB gene | This work |
| p113capC | pAT113 carrying the inactivated capC gene | This work |
| pDSP20 | pAT113 carrying the inactivated capD gene | 4 |
| p113capE | pAT113 carrying the inactivated capE gene | This work |
| pPPA40 | pAT18 carrying Ppag | This work |
| pPAGE | pPPA40 carrying capE | This work |
| pPAG55 | pPPA40 carrying B. subtilis ywtD | This work |
| pPGSA | pPPA40 carrying B. subtilis ywtB | This work |
| pPGSAE | pPPA40 carrying B. subtilis ywtB and ywtD | This work |
| pPGSA1 | pPPA40 carrying B. licheniformis ywtB | This work |
| pPGSE1 | pPPA40 carrying B. licheniformis ywtD | This work |
| pPGSAE1 | pPPA40 carrying B. licheniformis ywtB and ywtD | This work |
| pMini20 | pAT28 carrying Pcap, capB, capC, capA, capD, and capE | This work |
| pMini21 | pAT28 carrying Pcap, capB, capC, and capA | This work |
| B. anthracis strains | ||
| 9131 | pXO1−, pXO2− | 9 |
| RPG1 | pXO1+ (cya lef), pXO2+ | 13 |
| RTC10 | ΔcapB derivative of RPG1 | This work |
| RTC20 | ΔcapC derivative of RPG1 | This work |
| RTC30 | ΔcapA derivative of RPG1 | This work |
| RTC40 | ΔcapD derivative of RPG1 | 4 |
| RTC50 | ΔcapE derivative of RPG1 | This work |
All recombinant plasmids were transferred from E. coli to B. anthracis by a heterogramic conjugation procedure (39).
RNA extraction and RT-PCR.
Total RNA extraction from B. anthracis RTC50 was adapted from the protocol described by Guillouard et al. for B. subtilis and using the Trizol reagent (Invitrogen) (15). The quality of RNA preparations was analyzed on an RNA NanoLabChip (Agilent Technologies). Five μg of RNA was reverse transcribed using an Invitrogen M-MLV reverse transcriptase kit and random hexamers from Amersham, according to the manufacturer's instructions. Ten ng of cDNA was then amplified by reaction with the Expand high-fidelity PCR system from Roche. Negative controls included PCR amplification using 10 ng of RNA without a reverse transcription step. Reverse transcription (RT)-PCR was performed with capD-RT2s (GGGGAAACGATCGGCATTGGGTCACCAGGTGG), capDa, capEs, capE-internea (AAGCTTGTTTCCGAACGTTTAAAGGTCCCCATTGTTAC), and capEa.
Phenotypic studies.
To visualize B. anthracis capsule, bacilli were incubated in the presence of India ink. The samples were then observed by light microscopy at a magnification of ×400. To observe capsule attachment, bacilli were incubated at 65°C for 30 min and then similarly examined by light microscopy (4).
Cell fraction separation.
Pellets from B. anthracis liquid culture were sonicated and the soluble fraction (corresponding to the cytoplasm) and insoluble fraction (corresponding to the cell wall) were separated. After solubilization in 2% Triton X-100, membrane and insoluble fractions (corresponding mostly to peptidoglycan) were further separated, by ultracentrifugation (20,000 × g for 1 h) and tested as described previously (4); 10 μg of each fraction were loaded on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) for analysis.
Antibody production.
CapA2 (a peptide contained in CapA, amino-acid residues 46 to 191) was overexpressed and purified under denaturing conditions as described in Candela and Fouet (4). CapA2 serum was obtained by subcutaneously injecting 10 μg of CapA2 in Freund incomplete adjuvant into OF1 mice (Charles River) four times at 2-week intervals. CapB and CapE antibodies were raised by Neosystem (Strasbourg, France).
Immunodetection.
Dot blotting for PGA was performed as described in Candela and Fouet (4). Briefly, bacteria were loaded directly onto nitrocellulose membranes and these were probed with specific anti-PGA antibodies. The following antibodies were used for Western blotting: anti-CapD antibodies (mouse) diluted 1:1,000 (4), CapA2 antibodies (mouse) diluted 1:1,000, CapB antibodies (rabbit) diluted 1:10,000 and CapE antibodies (rabbit) diluted 1:5,000. Antibody binding was revealed with anti-mouse, or anti-rabbit, peroxidase-conjugated secondary antibodies. Blots were developed using the ECL Western blotting analysis system (Amersham).
Virulence assays.
OF/1 outbred mice (6 to 8 weeks old, females; Charles River, Arbresle, France), were used. The 50% lethal dose (LD50) was determined by subcutaneous injection of spores of the capE mutant or parental strain, as described previously (30).
Bioinformatic.
We used TopPred as membranous domain prediction program (6). Sequences were aligned using ClustalW and Boxshade programs (38). The similarity (identical plus accepted replacement amino acid residues) scores were deduced from the Boxshade figure.
RESULTS
In B. anthracis CapB, CapC, and CapA require an additional small peptide to synthesize PGA.
In E. coli, capB, capC, and capA are necessary and sufficient for a weak PGA synthesis, but are not sufficient in B. subtilis (24). We tested if capB, capC, and capA are necessary and sufficient for PGA synthesis in B. anthracis. We constructed a replicative multicopy plasmid, pMINI21, carrying capB, capC and capA genes preceded by the cap operon promoter (Fig. 1A) and introduced it into strain 9131 (pXO1−, pXO2−). No PGA synthesis was revealed by dot blotting (Fig. 1B). The absence of PGA was confirmed by optical microscopy (capsule can be easily observed under the light microscope as a white halo in presence of India ink [8]) (Fig. 1C). CapB, CapC, and CapA were therefore, as in B. subtilis, not sufficient for PGA production in B. anthracis.
FIG. 1.
The minimal synthesis and anchoring system includes a small ORF, capE. Bacilli containing pMINI20 or pMINI21 were tested for PGA production. (A) Schematic representation of the plasmid content (pMINI21 and pMINI20). (B) PGA was detected by dot blotting using anti-PGA specific antibodies. (C) PGA around the bacilli was visualized by light microscopy with India ink without further treatment. Covalent anchoring of capsule was tested by visualizing the PGA after heat treating the bacteria at 65°C.
The cap locus sequence on pXO2 was examined thoroughly. It includes a small ORF just downstream of capD. This ORF is described in the pXO2 sequence annotation as ORF pXO2-54 (ID AAF13659) (accession number AF188935) (29). pXO2-54 is 144 base pairs long, coding a 47-amino-acid peptide. A new plasmid was constructed, pMINI20, containing capB, capC, capA, capD and ORF pXO2-54, and was transferred into strain 9131 (Fig. 1A). Bacilli containing pMINI20 synthesized PGA (Fig. 1B and C). Strain 9131 contains no plasmids, and the transcriptional activating elements required for cap transcription, AcpA, AcpB and AtxA, are absent (7). Nevertheless, our results indicated that there was sufficient transcriptional readthrough for expression of the cap genes on a multicopy plasmid to obtain capsulated bacteria.
CapD is involved in the anchoring, not in the synthesis, of PGA (4). We also tested if CapB, CapC, CapA, CapD, and the product of ORF pXO2-54 are sufficient for the covalent anchoring of the capsule to the peptidoglycan (4). The PGA was not released from the 9131 bacteria containing pMINI20 by heat treatment (Fig. 1C). The capsule was therefore covalently anchored to the peptidoglycan as in a wild-type strain (4). The plasmid harboring capB, capC, capA, capD, and pXO2-54 therefore defines a system that is sufficient for synthesis and anchoring of B. anthracis PGA.
CapB, CapC, CapA, and CapE are necessary for PGA synthesis.
We studied the involvement of pXO2-54 in PGA synthesis when on its natural genetic element, pXO2. We inactivated this ORF in the RPG1 (pXO1+, pXO2+, Tox−) strain and PGA production was tested by dot blot. The parental strain, RPG1, produced PGA whereas the mutant strain, RTC50 (Table 1), did not (Fig. 2A). This is consistent with the results obtained in strains 9131(pMINI21) and 9131(pMINI20) (Fig. 1). We named the pXO2-54 ORF capE. The PGA-producing phenotype was restored to strain RTC50 by complementation with a plasmid containing a wild-type capE (Fig. 2A). capE is therefore necessary for PGA synthesis in B. anthracis.
FIG. 2.
capA, capB, capC, and capE are each necessary for PGA synthesis. (A and C) PGA was detected by dot blot. Bacteria were loaded directly on a nitrocellulose membrane and the membranes were probed with specific anti-PGA antibodies. (A) Strains RPG1, RTC50 (capE), and RTC50 complemented with capE. (B) Testing for CapD in RPG1, RTC10 (capB), RTC20 (capC), RTC30 (capA) and RTC40 (capD) strains. The same quantity of each sample was loaded. The Western blot membrane was probed with anti-CapD antibodies. (C) Strains RPG1, RTC10, RTC20, RTC30, and RTC40.
To test whether capB, capC, capA, and capD genes are all necessary for PGA synthesis, nonpolar mutants of each of these genes (see below) were constructed in the RPG1 genetic background, giving RTC10, RTC20, RT30, and RTC40, respectively (Table 1). The presence of CapD, product of the most downstream of the four genes, was assayed by loading the same quantity of the insoluble fraction proteins (see Materials and Methods) on SDS-PAGE and it was revealed with anti-CapD antibodies (Fig. 2B). CapD was found in the parental strain and in the capB, capC, and, although in a lower abundance, in the capA mutants, indicating that all mutants are nonpolar. The corresponding strains were assayed for PGA synthesis by dot blotting (Fig. 2C). The capD mutant produced PGA, indicating that the PGA synthesis complex is active in that mutant. All the other three nonpolar cap mutants were deficient for PGA synthesis (Fig. 2C). All nondeleted genes are expressed; thus, the absence of PGA in the mutants confirmed that CapB, CapC, and CapA, as well as CapE (Fig. 2A), are necessary for PGA synthesis.
capE is the fifth ORF of the cap operon and encodes a membranous protein.
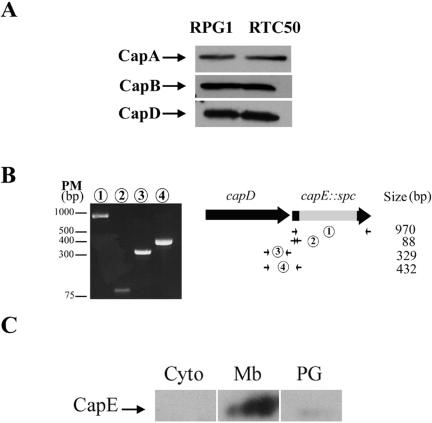
Many small peptides are regulators. We therefore tested for the presence of CapB, CapA, and CapD in the capE-deficient strain RTC50 by Western blotting (Fig. 3A). We did not test for CapC because antibodies directed against it were not obtained. Proteins CapB, CapA, and CapD were found in similar amounts in strains RTC50 and RPG1. Therefore CapE is not required for the synthesis of these proteins, and it is not a regulator of the cap operon. Moreover, the membranous localization of both CapB and CapA was the same in a capE mutant strain and in a wild-type strain (data not shown).
FIG. 3.
CapE, the product of the 5th gene of the cap operon, is a membranous peptide. (A) Strains RPG1 and RTC50 were tested for CapB, CapA, and CapD. The same quantities of protein were loaded for each sample. CapB, CapA, and CapD were revealed by the corresponding specific antibodies. (B) RT-PCR was carried out on RNA from strain RTC50 using capEs/capEa (1), capEs/capEinternea (2), capD-RT2s/capDa (3), and capD-RT2s/capEinternea (4) primers. (C) Localization of the CapE peptide: cytoplasm (Cyto), membranous (Mb), and peptidoglycan (PG)-enriched fractions were separated. CapE was revealed by Western blot with specific anti-CapE antibodies.
Only 14 nucleotides separate the capD stop codon from the capE translation initiation codon. An RT-PCR experiment was performed to test if capE belongs to the cap operon. Because capsule interferes with RNA extraction, RNAs were extracted from RTC50 and not RPG1. mRNA for the capD gene and that for the cassette harboring capE were detected (Fig. 3B, lanes 1, 2, and 3). Moreover, RT-PCR with the fourth pair of oligonucleotides indicated that the transcript encompasses the end of capD and the beginning of capE (Fig. 3A, lane 4). Therefore, the capE gene belongs to the cap operon.
The predicted amino acid sequence of CapE suggests that it may be associated with membranes. This was tested. The crude bacterial extract was separated into three fractions: the cytoplasmic protein fraction, the membranous protein fraction, and the peptidoglycan-associated protein fraction (4). Only one specific band was found in the membranous protein fraction corresponding to the size of CapE. This band is slightly smeared because of Triton X-100 contained in the sample that disturbed the membranous protein fraction migration (Fig. 3C). Therefore, CapE is a membranous peptide.
The LD50 of the capE mutant was determined. The LD50 of RPG1 was 5 × 105 spores/mouse, whereas no mice were killed when 8 × 108 spores of strain RTC50 were injected. The capE mutant strain is therefore avirulent, as expected for a nontoxinogenic and noncapsulated strain (46).
CapE and CapA functionally interact.
The polyglutamate synthetic (pgs) operon has been described to be responsible for the PGA synthesis in B. subtilis, and the yw(s/t) operon in B. licheniformis may have the same function, on the basis of sequence similarity. In silico analysis of these operons, pgsB, pgsC, and pgsA in B. subtilis and ywsC, ywtA, and ywtB in B. licheniformis, shows that they are both followed by a small ORF, called ywtC (Fig. 4A) (22, 45). ywtC encodes a 55-amino-acid peptide and we investigated whether it has the same function as CapE. We therefore complemented RTC50, a capE mutant strain, with the B. subtilis and B. licheniformis ywtC genes. There was no PGA production (Table 2): YwtC, on its own, does not fulfill CapE function.
FIG. 4.
Genetic comparison of polyglutamate synthesis operons. Proposed standardized genetic nomenclature (A). The nomenclatures previously published for the PGA synthesis systems for other PGA-producing Bacillus and Staphylococcus species are indicated on the schematic representation of the genetic organization of the operons. The bottom line is the proposed nomenclature. Protein sequence alignments of CapA equivalents (B) or CapE equivalents (C). ClustalW and Boxshade programs were used. B.s., B. subtilis; B.l., B. licheniformis; B.a., B. anthracis.
TABLE 2.
pgsA and pgsE are necessary to complement a capE mutant straina
| Complementing gene | RTC50 (ΔcapE)
|
RTC30 (ΔcapA)
|
||
|---|---|---|---|---|
| Light microscopy | Petri dishes | Light microscopy | Petri dishes | |
| capE | + | + | ND | ND |
| ywtC | − | − | ND | ND |
| ywtB or pgsA | − | − | + | + |
| ywtB-ywtC or pgsA-ywtC | + | − | + | + |
Colonies on petri dishes were smooth (+) or rough (−), indicative of capsulation. The presence of PGA surrounding the bacteria was also determined by light microscopy in the presence of India ink. Capsulated bacteria present a white halo (+); others do not (−). The ywtC and pgsA genes are from B. subtilis, and the ywtC and ywtB genes are from B. licheniformis. ND, not done.
We compared the Cap/Pgs/Yw(s/t) protein sequences, deduced from the available DNA sequences, for B. subtilis, B. licheniformis, and B. anthracis. CapB/PgsB/YwsC and CapC/PgsC/YwtA were all very similar (above 80% similarity). Interestingly, CapA/PgsA/YwtB were more divergent, with large gaps and insertions, although PgsA and YwtB were more similar to each other than either were to CapA (Fig. 4B). CapE/YwtC also differed, and similarly, CapE was the most different (Fig. 4C). It is possible that CapA and CapE function together, and this would explain why YwtC could not complement the B. anthracis capE mutant (Table 2; also, see above). Alternatively, YwtC could have a role entirely different from that of CapE and be inessential for PgsA/YwtB activity. RTC30, a capA mutant strain, was complemented with PgsA and with YwtB. In both cases, the complemented strain had a smooth aspect on plates as expected for capsulated strains (Table 2).
To check whether PgsA or YwtB were also able to replace CapE functionally, RTC50 was complemented by each pgsA and ywtB, separately. Neither PgsA nor YwtB complemented the capE mutant strain (Table 2). This suggested that PgsA and YwtB require a CapE equivalent. Both RTC50 and RTC30 were also complemented with either pgsA and ywtC (pgsA-ywtC) or with ywtB and ywtC (ywtB-ywtC). All the complemented bacilli were surrounded by a capsule as assessed by light microscopy (Table 2). However, complemented RTC50 had a rough aspect on petri dishes, similar to noncapsulated, or weakly capsulated strains (Table 2). That suggests that PGA was produced at a lower level. Interestingly, pgsA-ywtC- and ywtB-ywtC-complemented RTC30 strains had a smooth aspect, and were as capsulated as a wild-type strain (Table 2). In all complemented and PGA-producing strains, the capsule was anchored as determined by heat treatment (data not shown).
DISCUSSION
We conducted the first detailed genetic studies of the cap operon in B. anthracis. CapB, CapC, and CapA are each necessary for PGA synthesis but they are not sufficient in B. anthracis: an additional peptide, CapE, is required. The genetic region determining PGA synthesis appears to be well conserved between B. anthracis, B. subtilis, B. licheniformis, and Staphylococcus epidermidis. All possess all four synthetic genes: capB, capC, capA, and capE or their equivalents. We therefore propose a simplified nomenclature (Fig. 4A). pgsA could not be used because a gene termed pgsA already exists in B. subtilis 168. It encodes a phosphatidylglycerophosphate synthase (21). We propose to keep the nomenclature capB, capC, capA, capD, and capE for genes from bacteria in which the polyglutamate is the constituent of an anchored capsule, i.e., for B. anthracis (see below for S. epidermidis). For B. subtilis and B. licheniformis, which possess nonanchored polyglutamate, we propose the nomenclature pgsB, pgsC, pgsAA, and pgsE in place of ywsC, ywtA, ywtB, and ywtC, respectively (Fig. 4A). In fact, pgsAA has already been used for this gene (NC_006270) in B. licheniformis ATCC 14580; DSM 13.
There is one notable difference between B. anthracis and the three other organisms. The capE gene is separated from capA by capD in B. anthracis, whereas the others harbor pgsE (also called ywtC) immediately downstream from pgsAA (also called capA) (Fig. 4A). This indicates that the B. anthracis cap operon is less related to the others than they are to each other. This is confirmed by the analysis of sequence divergence between the CapA and CapE proteins and their equivalents (Fig. 4A).
All previous studies with the B. subtilis pgs operon included ywtC (also called pgsE) (3, 44). The minimal region of B. subtilis genome required to synthesize PGA in E. coli was found by screening a plasmid library (3). One plasmid in 10,000 was able to direct PGA synthesis in E. coli (3). These authors described a DNA fragment composed of about 3 kb containing the pgsB, pgsC, and pgsAA genes. However, pgsE is also present on this fragment. This result is strongly in favor of pgsE being necessary for PGA synthesis. Independently, Urushibata et al. directly cloned the four genes and obtained PGA synthesis in B. subtilis (44). They suggested that these genes form an operon but did not discuss the role of ywtC (also called pgsE). Both studies are in agreement with our finding that ywtC (also called pgsE), like capE in B. anthracis, is indispensable for PGA synthesis. Also consistent with our study, the minimal system defined for E. coli by Makino et al. did not function in B. subtilis, showing that an element was missing (24). Moreover, the PGA synthesis was weak in E. coli and in B. anthracis, probably due to the absence of the same element (24). This element was capE. It is not excluded that when the CapB, CapC, and CapA proteins are overproduced, rare complexes can be formed in the bacterium even in absence of CapE. They would lead to some PGA synthesis, which can be detected in E. coli and B. anthracis boiled extracts, as described by Makino et al. (24). However, our results showed that the PGA synthesis yield is insufficient to give rise to properly capsulated bacilli.
CapE is a very small peptide, 47 amino acids. Small peptides are generally involved in regulation. However, this is not the case for CapE, our results suggesting that CapE has a structural protein-interaction role. CapE is involved in the synthesis function, and probably interacts with CapA. Indeed, the phenotype of the capE mutant was only complemented in the presence of both pgsAA and pgsE. Whereas PgsAA fully replaces CapA in RTC30, PgsAA and PgsE did not fully complement RTC50: the strain was less capsulated than the wild type. This suggested that PgsE may also interact, but not as efficiently as CapE, with one or the other two components of the enzymatic complex, probably CapB and/or CapC. Indeed, CapE may need to interact with the entire complex and not only with CapA.
We propose two possible roles for CapE. It could interact functionally with CapA. Alternatively, through its interaction with CapB, CapC, and CapA, it could contribute to the formation of the complex. However, CapE is not involved in stabilization or in membrane localization of CapB and CapA. pgsE was unsuccessfully used for complementation. PgsE interacting with at least PgsAA may have the same function as CapE, and by extrapolation, this may also be the case for B. licheniformis PgsE and S. epidermidis YwtC.
The replicative plasmid pMINI20 harbors all elements required to produce PGA in a strain devoid of pXO1 and pXO2. Moreover, the PGA produced is anchored to the peptidoglycan. This demonstrated that there is no enzyme encoded by pXO2, other than capD, involved in PGA anchoring, reinforcing the suggestion that CapD alone is responsible for catalysis of this anchoring. Differences between bacteria in which PGA is not anchored and those that harbor a PGA that is anchored to the peptidoglycan, constituting a capsule, appear therefore to be due to a single gene belonging to the pgs and cap operons. In the absence of a γ-glutamyltranspeptidase, encoded by capD, PGA is secreted. Noteworthy, the pgs operons encode a γ-glutamyl-hydrolase. This is the case for B. subtilis natto and B. licheniformis ATCC 14580, which possess the γ-glutamyl-hydrolase genes pgsS and ywtD, respectively (2, 36). The only well-characterized example of PGA-capsulated bacteria is B. anthracis, which possesses CapD, a γ-glutamyltranspeptidase. S. epidermidis CapD is predicted to be a glutamyltranspeptidase precursor (12). It is very different from B. anthracis CapD (less than 35% similarity). PGA anchoring in S. epidermidis has not been studied, but PGA was observed on the surface by immunoscanning electron microscopy (20). This result is in agreement with PGA anchoring involving a glutamyltranspeptidase.
The virulence of the B. anthracis RPG1 parental strain is due to its ability to multiply within the host, causing septicemia. The capE mutant strain was fully avirulent in mice. The requirement for the small 47-amino-acid CapE peptide in B. anthracis capsule synthesis is absolute, and therefore it is a potential therapeutic target for the control of anthrax septicemia.
Acknowledgments
V. Viare is thanked for plasmid constructions. We are grateful to O. Mary-Possot for the supply of RNA.
T.C. was funded by MNRT and by CANAM.
REFERENCES
- 1.Ashiuchi, M., and H. Misono. 2002. Biochemistry and molecular genetics of poly-gamma-glutamate synthesis. Appl. Microbiol. Biotechnol. 59:9-14. [DOI] [PubMed] [Google Scholar]
- 2.Ashiuchi, M., H. Nakamura, T. Yamamoto, T. Kamei, K. Soda, C. Park, M. H. Sung, T. Yagi, and H. Misono. 2003. Poly-γ-glutamate depolymerase of Bacillus subtilis: production, simple purification and substrate selectivity. J. Mol. Catalysis B Enzymatic 23:249-255. [Google Scholar]
- 3.Ashiuchi, M., K. Soda, and H. Misono. 1999. A poly-γ-glutamate synthetic system of Bacillus subtilis IFO 3336: gene cloning and biochemical analysis of poly-γ-glutamate produced by Escherichia coli clone cells. Biochem. Biophys. Res. Commun. 263:6-12. [DOI] [PubMed] [Google Scholar]
- 4.Candela, T., and A. Fouet. 2005. Bacillus anthracis CapD, belonging to the gamma-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol. Microbiol. 57:717-726. [DOI] [PubMed] [Google Scholar]
- 5.Cataldi, A., E. Labruyère, and M. Mock. 1990. Construction and characterization of a protective antigen-deficient Bacillus anthracis strain. Mol. Microbiol. 4:1111-1117. [DOI] [PubMed] [Google Scholar]
- 6.Claros, M. G., and G. von Heijne. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685-686. [DOI] [PubMed] [Google Scholar]
- 7.Drysdale, M., A. Bourgogne, S. G. Hilsenbeck, and T. M. Koehler. 2004. atxA controls Bacillus anthracis capsule synthesis via acpA and a newly discovered regulator, acpB. J. Bacteriology 186:307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duguid, J. P. 1951. The demonstration of bacterial capsules and slime. J. Pathol. Bacteriol. 63:673-685. [DOI] [PubMed] [Google Scholar]
- 9.Etienne-Toumelin, I., J.-C. Sirard, E. Duflot, M. Mock, and A. Fouet. 1995. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J. Bacteriol. 177:614-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eveland, S. S., D. L. Pompliano, and M. S. Anderson. 1997. Conditionally lethal Escherichia coli murein mutants contain point defects that map to regions conserved among murein and folyl poly-gamma-glutamate ligases: identification of a ligase superfamily. Biochemistry 36:6223-6229. [DOI] [PubMed] [Google Scholar]
- 11.Fouet, A., and M. Mock. 1996. Differential influence of the two Bacillus anthracis plasmids on regulation of virulence gene expression. Infect. Immun. 64:4928-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimenez, A. P., Y. Z. Wu, M. Paya, C. Delclaux, L. Touqui, and P. L. Goossens. 2004. High bactericidal efficiency of type IIA phospholipase A(2) against Bacillus anthracis and inhibition of its secretion by the lethal toxin. J. Immunology 173:521-530. [DOI] [PubMed] [Google Scholar]
- 14.Goodman, J. W., and D. E. Nitecki. 1967. Studies on the relation of a prior immune response to immunogenicity. Immunology 13:577-583. [PMC free article] [PubMed] [Google Scholar]
- 15.Guillouard, I., S. Auger, M. F. Hullo, F. Chetouani, A. Danchin, and I. Martin-Verstraete. 2002. Identification of Bacillus subtilis CysL, a regulator of the cysJI operon, which encodes sulfite reductase. J. Bacteriol. 184:4681-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hezayen, F. F., B. H. Rehm, B. J. Tindall, A. Steinbuchel, and R. Eberhardt. 2001. Transfer of Natrialba asiatica B1T to Natrialba taiwanensis sp. nov. and description of Natrialba aegyptiaca sp. nov., a novel extremely halophilic, aerobic, nonpigmented member of the Archaea from Egypt that produces extracellular poly(glutamic acid). Polymer production by two newly isolated extremely halophilic Archaea: application of a novel corrosion-resistant bioreactor. Int. J. Syst. Evol Microbiol. 51:1133-1142. [DOI] [PubMed] [Google Scholar]
- 17.Kandler, O., H. König, J. Wiegel, and D. Claus. 1983. Occurence of poly-γ-D-glutamic acid and poly-α-l-glutamine in the genera Xanthobacter, Flexithrix, Sporosarcina and Planococcus. Syst. Appl. Microbiol. 4:34-41. [DOI] [PubMed] [Google Scholar]
- 18.King, E. C., A. J. Blacker, and T. D. H. Bugg. 2000. Enzymatic breakdown of poly-γ-D-glutamic acid in Bacillus licheniformis: identification of a polyglutamyl γ-hydrolase enzyme. Biomacromolecules 1:75-83. [DOI] [PubMed] [Google Scholar]
- 19.Ko, Y. H., and R. A. Gross. 1998. Effects of glucose and glycerol on γ-poly(glutamic acid) formation by Bacillus licheniformis ATCC 9945a. Biotechnol. Bioeng. 57:430-437. [DOI] [PubMed] [Google Scholar]
- 20.Kocianova, S., C. Vuong, Y. Yao, J. M. Voyich, E. R. Fischer, F. R. DeLeo, and M. Otto. 2005. Key role of poly-gamma-dl-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J. Clin. Investig. 115:688-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontinen, V. P., and H. Tokuda. 1995. Overexpression of phosphatidylglycerophosphate synthase restores protein translocation in a secG deletion mutant of Escherichia coli at low temperature. FEBS Lett. 364:157-160. [DOI] [PubMed] [Google Scholar]
- 22.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, and et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 23.Leonard, C. G., R. D. Housewright, and C. B. Thorne. 1958. Effects of some metallic ions on glutamyl polypeptide synthesis by Bacillus subtilis. J. Bacteriol. 76:499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makino, S., C. Sasakawa, I. Uchida, N. Terakado, and M. Yoshikawa. 1988. Cloning and CO2-dependent expression of the genetic region for encapsulation from Bacillus anthracis. Mol. Microbiol. 2:371-376. [DOI] [PubMed] [Google Scholar]
- 25.Makino, S.-I., I. Uchida, N. Terakado, C. Sasakawa, and M. Yoshikawa. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.McLean, R. J., D. Beauchemin, L. Clapham, and T. J. Beveridge. 1990. Metal-binding characteristics of the gamma-glutamyl capsular polymer of Bacillus licheniformis ATCC 9945. Appl. Environ. Microbiol. 56:3671-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesnage, S., T. Fontaine, T. Mignot, M. Delepierre, M. Mock, and A. Fouet. 2000. Bacterial SLH domain proteins are noncovalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 19:4473-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okinaka, R., K. Cloud, O. Hampton, A. Hoffmaster, K. Hill, P. Keim, T. Koehler, G. Lamke, S. Kumano, D. Manter, Y. Martinez, D. Ricke, R. Svensson, and P. Jackson. 1999. Sequence, assembly and analysis of pX01 and pX02. J. Appl. Microbiol. 87:261-262. [DOI] [PubMed] [Google Scholar]
- 30.Pezard, C., P. Berche, and M. Mock. 1991. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 59:3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rey, M. W., P. Ramaiya, B. A. Nelson, S. D. Brody-Karpin, E. J. Zaretsky, M. Tang, A. Lopez de Leon, H. Xiang, V. Gusti, I. G. Clausen, P. B. Olsen, M. D. Rasmussen, J. T. Andersen, P. L. Jorgensen, T. S. Larsen, A. Sorokin, A. Bolotin, A. Lapidus, N. Galleron, S. D. Ehrlich, R. M. Berka, B. Veith, C. Herzberg, S. Steckel, J. Feesche, K. H. Maurer, P. Ehrenreich, S. Baumer, A. Henne, H. Liesegang, R. Merkl, A. Ehrenreich, and G. Gottschalk. 2004. Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. Genome Biol. 5:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roelants, G. E., and J. W. Goodman. 1968. Immunochemical Studies on the poly-gamma-D-glutamyl capsule of Bacillus anthracis. IV. The association with peritoneal exudate cell ribonucleic acid of the polypeptide in immunogenic and nonimmunogenic forms. Biochemistry 7:1432-1440. [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Schneerson, R., J. Kubler-Kielb, T. Y. Liu, Z. D. Dai, S. H. Leppla, A. Yergey, P. Backlund, J. Shiloach, F. Majadly, and J. B. Robbins. 2003. Poly(gamma-D-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc. Natl. Acad. Sci. USA 100:8945-8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sirard, J.-C., M. Mock, and A. Fouet. 1994. The three Bacillus anthracis toxin genes are coordinately regulated by bicarbonate and temperature. J. Bacteriol. 176:5188-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki, T., and Y. Tahara. 2003. Characterization of the Bacillus subtilis ywtD gene, whose product is involved in γ-polyglutamic acid degradation. J. Bacteriol. 185:2379-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sylvestre, P., E. Couture-Tosi, and M. Mock. 2002. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol. Microbiol. 45:169-178. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trieu-Cuot, P., C. Carlier, P. Martin, and P. Courvalin. 1987. Plasmid transfer by conjugation from Escherichia coli to Gram-positive bacteria. FEMS Microbiol. Lett. 48:289-294. [Google Scholar]
- 40.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1990. A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and Gram-positive bacteria. Nucleic Acids Res. 18:4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1991. Shuttle vectors containing a multiple cloning site an lacZα a gene for conjugal transfer of DNA from Escherichia coli to Gram-positive bacteria. Gene 102:99-104. [DOI] [PubMed] [Google Scholar]
- 42.Trieu-Cuot, P., C. Poyart-Salmeron, C. Carlier, and P. Courvalin. 1990. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 18:3660-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troy, F. A. 1973. Chemistry and biosynthesis of the poly (γ-D-glutamyl) capsule in Bacillus licheniformis. I. Properties of the membrane mediated biosynthetic reaction. J. Biol. Chem. 248:305-315. [PubMed] [Google Scholar]
- 44.Urushibata, Y., S. Tokuyama, and Y. Tahara. 2002. Characterization of the Bacillus subtilis ywsC gene, involved in γ-polyglutamic acid production. J. Bacteriol. 184:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veith, B., C. Herzberg, S. Steckel, J. Feesche, K. H. Maurer, P. Ehrenreich, S. Baumer, A. Henne, H. Liesegang, R. Merkl, A. Ehrenreich, and G. Gottschalk. 2004. The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. J. Mol. Microbiol. Biotechnol. 7:204-211. [DOI] [PubMed] [Google Scholar]
- 46.Welkos, S. L. 1991. Plasmid-associated virulence factors of non-toxigenic (pXO1−) Bacillus anthracis. Microb. Pathog. 10:183-198. [DOI] [PubMed] [Google Scholar]