Abstract
Epidemiologic research has shown increases in adverse cardiovascular and respiratory outcomes in relation to mass concentrations of particulate matter (PM) ≤2.5 or ≤10 μm in diameter (PM2.5, PM10, respectively). In a companion article [Delfino RJ, Sioutas C, Malik S. 2005. Environ Health Perspect 113(8):934–946]), we discuss epidemiologic evidence pointing to underlying components linked to fossil fuel combustion. The causal components driving the PM associations remain to be identified, but emerging evidence on particle size and chemistry has led to some clues. There is sufficient reason to believe that ultrafine particles < 0.1 μm (UFPs) are important because when compared with larger particles, they have order of magnitudes higher particle number concentration and surface area, and larger concentrations of adsorbed or condensed toxic air pollutants (oxidant gases, organic compounds, transition metals) per unit mass. This is supported by evidence of significantly higher in vitro redox activity by UFPs than by larger PM. Although epidemiologic research is needed, exposure assessment issues for UFPs are complex and need to be considered before undertaking investigations of UFP health effects. These issues include high spatial variability, indoor sources, variable infiltration of UFPs from a variety of outside sources, and meteorologic factors leading to high seasonal variability in concentration and composition, including volatility. To address these issues, investigators need to develop as well as validate the analytic technologies required to characterize the physical/chemical nature of UFPs in various environments. In the present review, we provide a detailed discussion of key characteristics of UFPs, their sources and formation mechanisms, and methodologic approaches to assessing population exposures.
Keywords: cardiovascular diseases, cytokines, diesel, epidemiology, oxidative stress, particle size, photochemistry, respiratory diseases, toxic air pollutants, ultrafine particles
Ambient aerosols are defined as suspensions of relatively stable solid or liquid particles in ambient air. Ambient particles range from a few nanometers to 100 μm in diameter. Ambient particulate matter (PM) has been described in three size distributions (Hinds 1999): ultrafine particles (UFPs), approximately < 0.1 μm in diameter and largely consisting of primary combustion products; accumulation-mode particles between 0.1 and 2.5 μm in diameter, from aggregation of UFPs and vapors; and coarse-mode particles > 2.5 μm in diameter, largely mechanically generated particles.
Most ambient PM mass is distributed in the last two size ranges (or modes), that is, the coarse and accumulation modes. Typically, the mass-based size distribution of ambient PM is bimodal, with a “saddle” point in the size range of 1–3 μm, which distinguishes the coarse and accumulation modes: by convention, the coarse mode consists of particles > 2.5 μm in aerodynamic diameter, whereas the fine (or accumulation) mode consists of particles ≤2.5 μm in aerodynamic diameter (PM2.5). The particle size range < 0.1 μm, known as the ultrafine mode, contains the majority (in numbers) of the ambient particles and an appreciable portion of total surface area (Hinds 1999).
These three particle modes have distinctly different chemical composition, sources, and lifetimes in the atmosphere. Particles in the coarse mode are produced by mechanical processes (grinding, erosion, and resuspension by the wind). Because of their relatively larger size, they have higher gravitational settling velocities and are thus removed from the atmosphere within hours. Particles in the accumulation mode are mostly anthropogenic in origin; they are generated through gas-to-particle conversion mechanisms, including homogeneous and heterogeneous nucleation, and by condensation onto preexisting particles in the accumulation-size mode. Because they are too small to settle out, particles of the accumulation mode have lifetimes in the atmosphere on the order of days (Hinds 1999), and they can be transported over long distances. The major chemical constituents of fine particles are sulfate, nitrate, ammonium, organic carbon, and elemental carbon (EC), as well as a variety of trace metals formed in combustion processes.
Because of their increased number and surface area as well as their high pulmonary deposition efficiency, UFPs are particularly important in atmospheric chemistry and environmental health. For example, the UFP’s surface can carry large amounts of adsorbed or condensed toxic air pollutants (oxidant gases, organic compounds, and transition metals) (Oberdörster 2001). Many of these toxic air pollutants have been identified as having pro-inflammatory effects, yet relevant exposure data are rarely available to epidemiologists. Results from several recent studies in mostly urban areas (Jones et al. 2000; Kim et al. 2002; Morawska et al. 1998; Shi et al. 2001; Woo et al. 2001) showed that a large proportion of urban UFPs consists of primary combustion products from mobile source emissions (particularly diesel and automobile exhaust) and includes organic compounds, EC, and metals. Because exposure to mobile emissions can vary across short distances and depends on personal activity patterns, assessing such exposures requires methods that go beyond the use of government monitoring data alone.
In this review article we provide a detailed discussion of key characteristics of UFPs, their sources, and formation mechanisms and discuss methodologic approaches to the assessment of population exposures.
Characteristics and Potential Importance of UFPs
Unlike coarse and fine particles, which, as discussed above, are naturally divided by a cut-point of 2.5 μm, there is no clear cut-point that separates UFPs from accumulation-mode PM. This is because, unlike coarse and fine (accumulation plus ultrafine) particles, which have distinctly different origins, a major fraction of accumulation-mode PM originates from the ultrafine mode. The distinction between the ultrafine and accumulation modes has varied from 0.1 to 0.2 μm, depending on location and season for the following reasons. “Ultrafine PM” is traditionally defined as particulates originating mostly from “fresh” emission sources and accounting for > 90% of the number-based particle concentrations. Recent studies in the Los Angeles Basin have shown that median mobility diameters in the inland valleys (downwind receptor areas) of the basin are in the 90–150 nm range in the summer months (Fine et al. 2004; Kim et al. 2002). This finding implies that a simple 100-nm cut-point does not always ensure that the vast majority of ambient particles by number concentration will fall below that size. The definition of UFPs is complicated further because unlike coarse- or accumulation-mode PM, for which the assumption that they have a perfectly spherical shape is reasonable, a significant fraction of directly emitted soot particles in the ultrafine range has a fractal or agglomerate-like structure (Friedlander 2000). These particles are generated primarily from high-temperature combustion sources such as motor vehicles. By their very nature, agglomerate structures have higher surface areas than spherical particles with the same equivalent diameter and are generally less dense. This deviation from a spherical shape creates a significant deviation between the aerodynamic diameter (typically measured by means of inertial classifiers, e.g., impactors) and mobility diameter (typically measured by means of electrical differential mobility analyzers). Because of their low density, a substantial fraction of these particles would be classified by an inertial separator as UFPs, whereas an electrical mobility classifier would classify these irregular particles in larger size ranges because of their high surface area and, hence, mobility. These arguments have been further supported by observations in a recent study by McMurry et al. (2002) in which the effective density of diesel particles was measured by relating the mobility-measured diameter of combustion particles to their aerodynamic diameter. This study demonstrated that as the mobility size increases, the effective density tends to decrease, presumably because of the surface irregularities of the larger particles. It should be noted, however, that the relative abundance of these fractal-like agglomerates is highly variable, depending on the sampling location(s) as well as the time of day in order to account for the effect of vehicular emissions.
To date, there has been rapidly increasing epidemiologic evidence linking respiratory health effects and exposures to UFPs. Epidemiologic studies conducted by Peters et al. (1997) have demonstrated a stronger association between respiratory health in asthmatic adults and exposure to UFPs compared with fine or coarse particles. A study by Pekkanen et al. (1997) showed associations of fine particles and UFPs with deficits in peak expiratory flow among asthmatic children. A study by Penttinen et al. (2001) also demonstrated a negative association between daily mean number concentrations (dominated by UFPs) and peak expiratory flow. Wichmann et al. (2000) showed that positive associations of cardiovascular mortality with both UFPs and fine ambient particles were comparable and seemed to be largely independent of each other in two-pollutant models. It has been proposed that UFPs have contributed to other epidemiologic findings of adverse effects of particulate air pollution on cardiovascular health (Oberdörster et al. 1995; Seaton et al. 1999). In a companion article (Delfino et al. 2005), we discuss epidemiologic evidence indirectly pointing to the potential adverse cardiovascular effects of pollutant components of fossil fuel combustion that dominate the ultrafine fraction.
There is sufficient toxicologic basis for believing that UFPs are capable of inducing the greatest amount of inflammation per unit PM mass because of high particle number (PN), high lung deposition efficiency, and surface chemistry. UFPs have very low mass but magnitudes higher PNs and therefore a high surface area relative to fine or coarse particles. For example, a study in Pasadena, California, by Hughes et al. (1998) found that UFP PNs were consistently in the range of 1.3–8.9 × 104 particles/cm3 air, and UFP mass was only in the range of 0.80–1.58 μg/m3. Chalupa et al. (2004) found the deposition efficiency of carbon UFPs in human subjects was > 60% and increased with exercise and in subjects with asthma. As discussed below, toxic air pollutants carried by UFPs are expected to induce inflammatory responses through reactive oxygen species (ROSs) or other mechanisms.
Evidence now supports the view that UFPs carry considerable amounts of air toxics. Kim et al. (2002) studied composition of size-fractionated particulate air pollution in urban sites of Los Angeles, California, with considerable mobile source emissions. They found a large proportion of UFPs are made up of organic carbon, followed by EC as primary products from mobile source emissions, particularly diesel and automobile exhaust. Other studies showed that UFPs contain the largest fraction of polycyclic aromatic hydrocarbons (PAHs) by mass (Eiguren-Fernandez et al. 2003; Li et al. 2003). Overall PAH concentrations are likely to be higher where there is a greater traffic density, such as downtown Los Angeles where diesel exhaust was found to make up 32.7% of the fine particle mass (Glovsky et al. 1997). Given these findings, if PAHs and other organic compounds are major causal components of the inflammatory response to PM, then greater responses from UFPs compared with larger particle fractions are expected at urban areas in the proximity of mobile sources. This expectation is further supported by the greater PN and surface area of UFPs, and greater internal doses due to the higher respiratory deposition of UFPs (Kim and Jaques 2000, 2004).
Experimental data show that compared with larger particles, UFPs are capable of avoiding phagocytosis by alveolar macrophages and gain entry to pulmonary interstitial sites, including vascular endothelium. Therefore, UFPs may induce pulmonary inflammation at both epithelial and interstitial sites, as well as enter the circulation to reach other target sites, including the cardiovascular system (Nemmar et al. 2002, 2004; Oberdörster 2001; Oberdörster et al. 2002). Additionally, organic components of PM such as PAHs, which comprise a large proportion of both freshly emitted exhaust and secondary aerosols, have been shown to induce a broad polyclonal expression of cytokines and chemokines in respiratory epithelium. As discussed, this effect may be due to the action of metals, PAHs, and related compounds that lead to the production of cytotoxic ROSs (Nel et al. 1998, 2001). ROSs induce oxidant injury and inflammatory responses (Pritchard et al. 1996), including the production of nuclear transcription factor κB, which increases the transcription of cytokines and acute phase proteins (Nel et al. 2001). These inflammatory and oxidant stress responses are expected to occur at extra-pulmonary sites as well, including the vascular endothelium of the heart. Evidence for the importance of oxidant stress responses to cardiovascular effects is that antioxidant therapy is protective against the development of hypertension, aetherosclerosis, cardiomyopathies, coronary heart disease, and congestive heart failure (Dhalla et al. 2000). Li et al. (2003) showed that UFPs in Los Angeles were most potent toward inducing cellular heme oxygenase-1 (HO-1) expression and depleting intracellular glutathione, both important in oxidant stress responses. A separate study in Los Angeles by Cho et al. (in press) used the dithiothreitol assay as a quantitative measure of in vitro ROS formation. That study also showed that UFPs had the highest ROS activity. Li et al. (2003) also showed that UFPs and, to a lesser extent, accumulation-mode particles localize in mitochondria where they induce major structural damage, which may contribute to oxidative stress. Xia et al. (2004) showed that suspensions of urban UFPs as well as diesel exhaust particles on epithelial cells decreased membrane potential and induced both loss of mitochondrial membrane mass and apoptosis. Interestingly, commercial polystyrene nanoparticles failed to exert a mitochondrial effect. Together, these studies provide strong evidence that the increased biologic potency of UFPs is related to the content of redox cycling organic chemicals and ability to damage mitochondria.
Sources and Formation Mechanisms of UFPs
UFPs may be formed in the atmosphere by at least three processes: a) UFPs may be formed during combustion processes associated mostly with traffic or industrial sources, and emitted directly to the atmosphere as UFPs (Kittelson 1998); b) these combustion processes may also emit hot supersaturated vapors, which undergo nucleation and condensation while being cooled to ambient temperatures; and c) chemical reactions in the atmosphere may lead to chemical species with low vapor pressure at ambient temperature. The former sources produce UFPs that are more “localized,” with their concentrations decreasing with distance to their emissions sources, whereas the latter processes tend to produce UFPs that are more regionally (thus homogeneously) dispersed over an urban or rural area. UFPs sources and their impact on human exposure are discussed in the following sections.
Emission inventories suggest that motor vehicles are the primary direct emission sources of fine and UFPs to the atmosphere in urban areas (Hitchins et al. 2000; Zhu et al. 2002b). Most PNs from vehicle exhaust are in the size range of 20–130 nm for diesel engines (Morawska et al. 1998) and 20–60 nm for gasoline engines (Ristovski et al. 1998). In addition to UFP formation by direct emission, recent studies show that photochemically driven atmospheric reactions lead to the formation of low-volatility species at ambient temperature. These chemical species may form UFPs by a variety of nucleation processes (Kulmala et al. 2004; Stanier et al. 2004). Nucleation may sometimes occur on ions and probably involves more than one species (i.e., is a multicomponent process). There is strong evidence that sulfuric acid vapor sometimes participates in nucleation, and there is growing consensus that ammonia and water vapor are also involved. However, the atmosphere probably also contains other trace gases, including organic compounds, that either participate in the nucleation process or react in the atmosphere to form compounds that nucleate. Because they may be present at extremely low concentrations, the identity and concentrations of those gases are not yet known. Kulmala et al. (2004) have written an excellent review on this topic. A variety of different nucleation mechanisms have been proposed for the atmosphere, including binary water–sulfuric acid nucleation (Kulmala and Laaksonen 1990), ternary water–sulfuric acid–ammonia nucleation (Kulmala et al. 2000), and ion-induced nucleation (Yu and Turco 2000).
In urban areas, nucleation events have been observed in Atlanta, Georgia (Woo et al. 2001), Pittsburgh, Pennsylvania (Stanier et al. 2004), and Los Angeles (Fine et al. 2004). Stanier et al. (2004) showed in their study in Pittsburgh that the nucleation events are fairly well correlated with the product of ultraviolet intensity and sulfur dioxide concentration and can depend on the effective area available for condensation. This indicates that sulfuric acid (H2SO4) is a component of the new particles. However, they noted that published correlations for nucleation by binary H2SO4–H2O could not explain the observed nucleation frequency and intensity, suggesting that an additional component (perhaps ammonia) is participating in the particle formation, thus supporting the notion of a ternary process. Kulmala et al. (2004) indicate that after nucleation, UFPs grow at rates ranging from 1 to 20 nm/hr (increase in physical diameter), depending on season and locale. It is of particular note that recent evidence supports the notion that the species that dominate growth may be different from the species responsible for particle formation, with particle growth being attributed to the concentration of “nonvolatile” vapors. Our current understanding of atmospheric nanoparticle processes suggests that growth of these particles to larger sizes within the UFP mode occurs by condensation of low-volatility organic species. These species are products of photochemical oxidation of volatile organic precursors on these preexisting nuclei (Kulmala et al. 2004; O’Dowd et al. 1999). Recent studies by Zhang et al. (2004) showed that nucleation rates of sulfuric acid are greatly increased in the presence of organic acids (including products of atmospheric photochemical reactions), by forming unusually stable organic–sulfuric acid complexes, thereby reducing the nucleation barrier of sulfuric acid.
Exposure to UFPs
Because of the importance of traffic sources in the overall emission rates of UFPs and the resulting human exposure, it has been essential to determine UFP behavior after emission as they are transported away from the emission source, namely, busy roads and freeways.
The most comprehensive studies of PM in the proximity of roadways were initiated in the late 1990s. Hitchins et al. (2000) measured the horizontal and vertical profiles of submicrometer particulates (16–626 nm) near a major arterial route in the urban area of Brisbane, Australia. The study found that, with the exception of measurements in close proximity to the road (~ 15 m), the horizontal profile measurements did not show statistically significant differences in fine PN concentration at ground-level distances up to 200 m away from the road. The same study examined particle size distribution and concentration in the size range of 15–200 nm and at distances from a road ranging from 15 to 375 m at two sites in Australia and found that when the wind blew directly from the road, the concentration of the fine particles and UFPs decreased to about half their maximum at a distance of 100–150 m from the road.
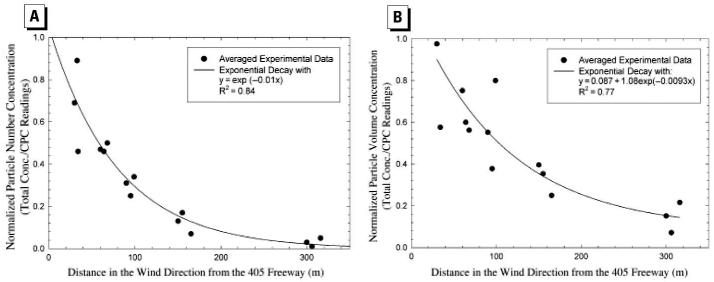
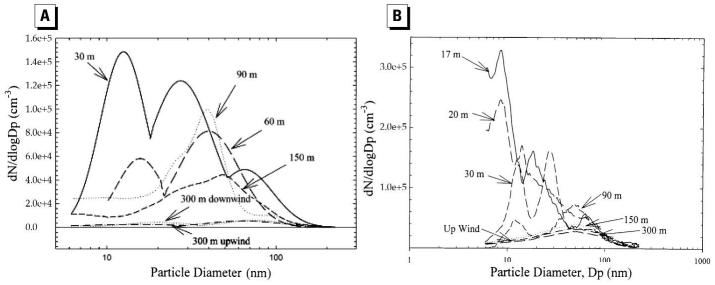
Almost simultaneously, Zhu et al. (2002b) measured the size distributions of PM and concentrations of gaseous co-pollutants in the proximity of a freeway mostly affected by gasoline vehicles [Interstate 405 (I-405)] in west Los Angeles. Measurements were taken at distances varying from 30 to 300 m downwind and 300 m upwind from freeway I-405. At each sampling location, concentrations of carbon monoxide (CO), black carbon (BC), and particle mass concentrations were also measured. The ranges of average concentration of CO, BC, total PN, and mass concentration at nearest location (30 m) to the freeway were 1.7–2.2 ppm, 3.4–10.0 μg/m3, 1.3–2.0 × 105/cm3, and 30.2–64.6 μg/m3, respectively. For the conditions of these measurements, the decrease in concentration of CO, BC, and PN tracked each other well with increasing distance from the freeway. PN concentration (6–220 nm) decreased exponentially with downwind distance from the freeway (Figure 1). Zhu at al. (2002a) attributed the rapid decrease in PN concentration and change in particle size distribution to atmospheric dispersion, evaporation of volatile PM, and possibly some coagulation. Measured number concentrations tracked traffic flow well (Figure 2). Figure 2 also indicates that PN concentrations on the roadway increase with vehicle speed and decrease with idling. Nearest the freeway site, three distinct ultrafine modes were observed with geometric mean diameters of 12.6 nm, 27.3 nm, and 65.3 nm. The smallest mode, with a peak concentration of 1.6 × 105 particles/cm3, disappeared at distances > 90 m from the freeway. Ultrafine PN concentration measured at 300 m downwind from the freeway was indistinguishable from upwind background concentration (Figure 3A).
Figure 1.
Normalized total particle (A) number and (B) volume concentration, in the size range of 6 –220 nm, as a function of distance from the I-405 freeway. CPC, condensation particle counter (TSI Inc., Shoreview, MN). Reprinted with permission from Zhu et al. (2002b). Copyright 2002 Air and Waste Management Association.
Figure 2.
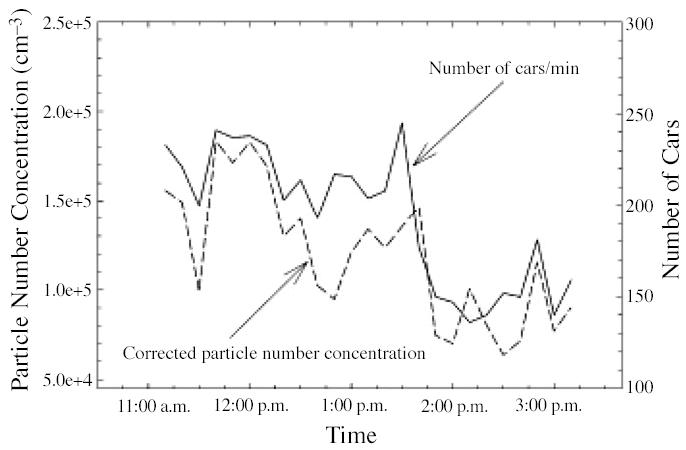
Correlation between traffic density and measured total PN concentration, corrected for wind velocity, 30 m downwind from the freeway. Reprinted with permission from Zhu et al. (2002b). Copyright 2002 Air and Waste Management Association.
Figure 3.
UFP size distribution at different sampling locations near (A) the I-405 freeway [reprinted with permission from Zhu et al. (2002b); copyright 2002 Air and Waste Management Association] and (B) the I-710 freeway [reprinted from Zhu et al. (2002a); copyright 2002, with permission from Elsevier].
In a companion study, Zhu et al. (2002b) obtained similar data from sites in the vicinity of the I-710 freeway in Los Angeles—an area affected primarily by heavy-duty diesel traffic—with the most notable difference from the I-405 study, as BC levels were substantially more elevated in the I-710 freeway (Figures 3B, 4A,B). Similar to the study in the vicinity of the I-405 freeway, the sharpest decrease in particle concentrations with distance from freeways was observed for the <20 nm particles (Figure 4A) for I-710.
Figure 4.
(A) Normalized PN concentration for different size ranges as a function of distance to the I-710 freeway. (B) Concentrations of BC as a function of distance from the I-405 and I-710 freeways. Reprinted from Zhu et al. (2002b). Copyright 2002, with permission from Elsevier.
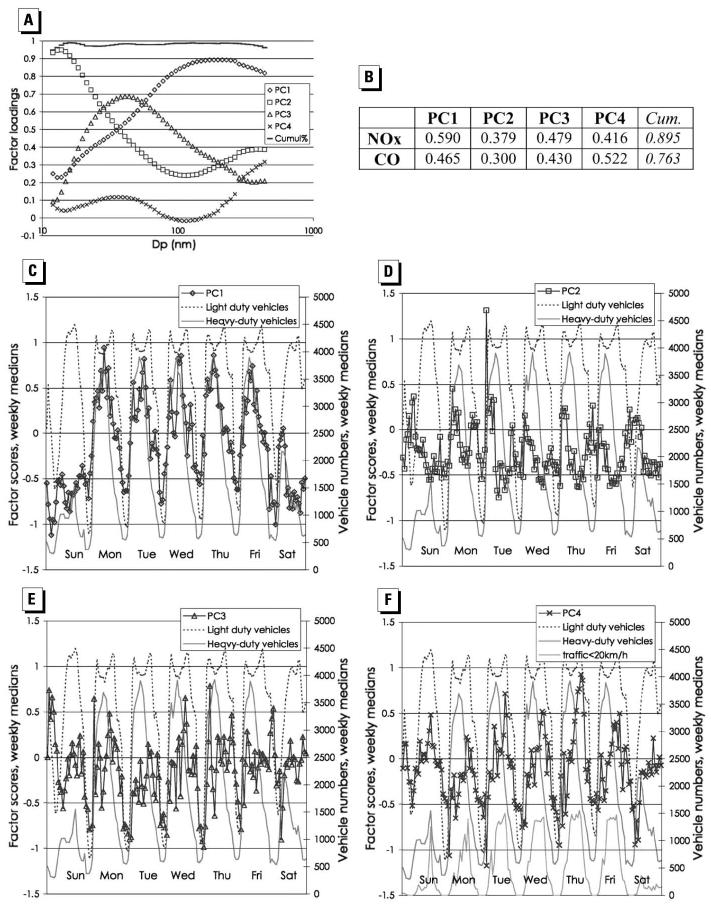
Measurements of PN size distribution in the range of 11–452 nm were conducted on the side of a busy road in central London, over a period from April 1998 to August 2001 by Charron and Harrison (2003). The data were analyzed to demonstrate the influences of meteorologic factors on the overall size distribution. The relationship to traffic volumes indicated that accumulation-mode particles are associated with emissions from heavy-duty traffic (mainly diesel vehicles), whereas particles in the range of 30–60 nm showed a stronger association with light-duty traffic (Figure 5). The concentrations of both these particle size fractions decreased with increasing wind speed as a result of increased atmospheric dilution. Meteorologic parameters such as low temperatures and high relative humidity were shown to favor the formation of new particles. The relative number of particles ranging in size from 11 to 30 nm measured during the morning rush hour is strongly influenced by the prevailing temperature and humidity conditions. The strong dependence on the temperature corroborates the idea that these nanoparticles are not primarily emitted but formed during the cooling and dilution of the vehicle exhausts. By contrast no obvious relation was found between PN concentrations and relative humidity, despite the inverse correlation of temperature and relative humidity. Higher relative humidity is expected to favor homogeneous binary nucleation of sulfuric acid and water; ternary nucleation involving ammonia is expected to be independent of relative humidity, whereas the potential nucleation from organic compounds should be independent of relative humidity. The lack of a dependence on the relative humidity suggests that the binary nucleation from sulfuric acid and water is not a major factor in particle production.
Figure 5.
Results of a principal component (PC) analysis from the work of Charron and Harrison (2003): (A) factor loadings for PNs; (B) factor loadings for NOx and CO—median weekly factor scores for (C) PC1, (D) PC2, (E) PC3, (F) PC4. Reprinted from Charron and Harrison (2003). Copyright 2003, with permission from Elsevier.
The effect of season on freeway PM characteristics was also investigated in a study by Zhu et al. (2004). The decay rates of CO and BC were slightly greater in summer than in winter for both freeways, suggesting a weaker atmospheric dilution effect in winter (Figure 6). PN concentration in the size range of 6–12 nm is significantly higher in winter than in summer. The associated concentration in that size range decreased at a slower rate in winter than in summer. The surface area concentrations in the size range of 6–220 nm are consistently higher in summer for all sampling locations. These results suggest that wintertime conditions favor greater particle formation, possibly because of increased condensation of organic vapors coupled with decreased atmospheric mixing depth. These results are consistent with the observations made by Charron and Harrison (2003). Higher UFP concentrations in winter-time were also observed in recent studies by Jeong et al. (2004) and Stanier et al. (2004).
Figure 6.
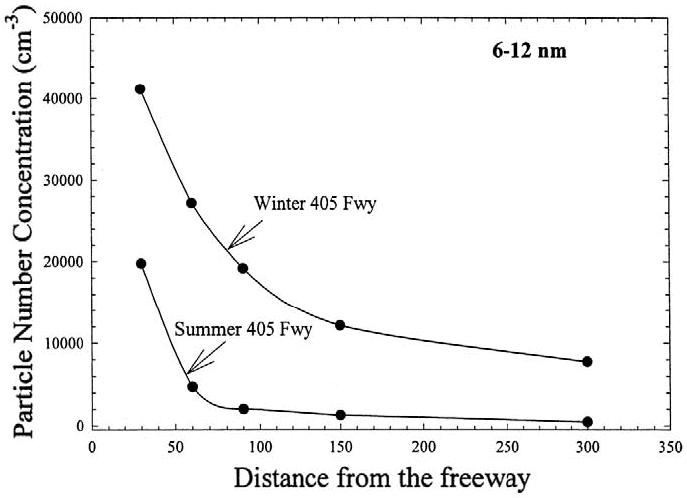
Comparison of decay of PN concentrations in summer and winter in the size range of 6–12 nm near the I-405 freeway. Reprinted from Zhu et al. 2004: “Aerosol Science & Technology: Seasonal Trends of Concentration and Size Distribution of Ultrafine Particles Near Major Highways in Los Angeles.” 38(suppl 1):5–13. Copyright 2004. Mount Laurel, NJ. Reprinted with permission.
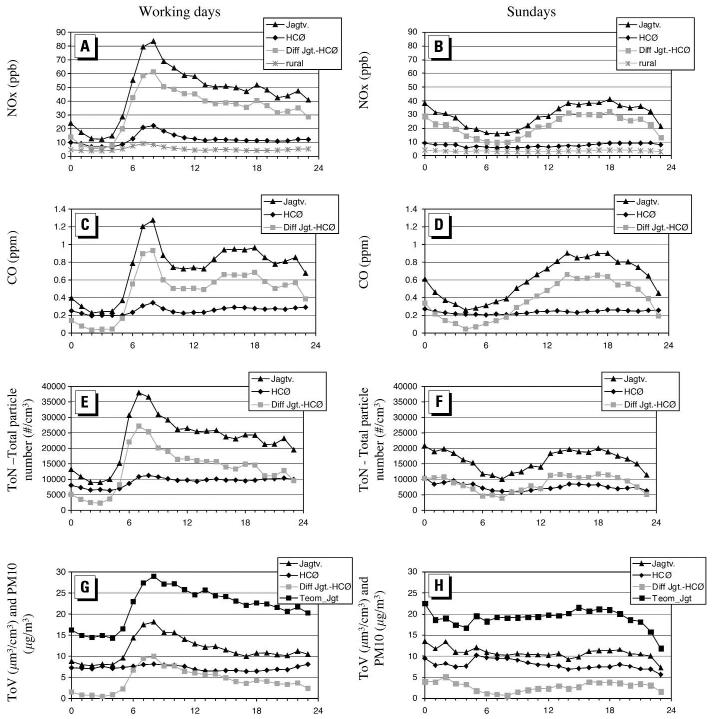
Ketzel et al. (2003) performed simultaneous measurements of particle size distribution (size range, 10–700 nm) inside an urban street canyon and a nearby urban background location in Copenhagen, Denmark, in May–November 2001, attempting to separate the traffic source contribution in the street canyon from the background levels. The background concentrations were highly variable due to changing contributions from long-range transport and local sources, showing a diurnal pattern with a shift to smaller particle sizes during midday hours. As expected, higher concentrations were measured at the street site, with the average ratio of background-to-street concentration being 0.26 for nitrogen oxides (NOx) and 0.35, 0.42, 0.60, and 0.64 for CO, total PN, surface, and volume, respectively (Figure 7). During daytime and evening hours, a maximum of particle sizes of 20–30 nm was observed in the particle size distribution, independent of the changing heavy-duty vehicle traffic during the same time interval.
Figure 7.
Average diurnal variation of NOx, CO, PN and particle volume, (A) working days, and (B) Sundays in various locations in Copenhagen, Denmark. Abbreviations: Diff Jgt.-HCØ, difference between street and urban background sites; Teom_Jgt, tapered element oscillating microbalance (R&P Inc., Albany, New York) measurements of PM10 in the street canyon. Jagtvej (Jagtv.) is located in a street canyon, The second station is located at the roof of the 20 m high H.C. Ørsted Institute (HCØ) and is measuring the urban background concentration. Reprinted from Ketzel et al. (2003). Copyright 2003, with permission from Elsevier.
All the aforementioned studies mostly, if not exclusively, reported measured aerosol size distributions with little attempt to interpret the observed results. Conversely, fundamental insight into the “road-to-ambient” evolution of PN distributions near the I-405 and I-710 freeways in Los Angeles in both summer and winter was developed by a multicomponent sectional aerosol dynamic model developed by Zhang et al. (2004). The model used CO as a marker of atmospheric dilution and examined the change in particle size due to evaporation and recondensation onto larger particles of volatile material because of dilution. As the vehicle exhaust leaves the tailpipe, the sharp drop in temperature and relatively high concentrations leads to significant condensation of vapor emissions, making particle composition a complex mixture. As exhaust disperses from roadways, the gas-phase concentration decreases, and some compounds may continue condensing, whereas others may begin evaporating, depending on the relative magnitude of their partial pressure and vapor pressure. Because of the atmospheric dilution process, volatile gases may evaporate from particles to achieve gas-particle equilibrium. The dynamics of volatilization are even more pronounced for the smaller particles of the UFP range, because a higher vapor pressure is required to prevent these particles from volatilizing compared with particles larger than 100 nm (in classic aerosol theory, this phenomenon is also known as the “Kelvin effect”; Hinds 1999). Smaller particles thus have to grow fast enough to minimize their Kelvin effect before the concentration of the condensing materials drops to a level that makes their growth improbable.
Zhang et al. (2004) demonstrated that condensation, evaporation, and dilution are the major mechanisms in altering aerosol size distribution, whereas coagulation and deposition play minor roles. Seasonal effects were significant with winters generally less dynamic than summers. A large number of particles grow into the > 10 nm range around 30–90 m downwind of the freeways. Beyond 90 m, some particles shrink to sizes < 10 nm, whereas others continue growing to > 100 nm as result of competition between partial pressure and vapor pressure. As a result, people who live within about 90 m of roadways are exposed to particles of very different size and chemical composition than are others who live farther away from busy streets and roadways. Particle compositions probably change dramatically as components adapt to decreasing gas-phase concentration due to dilution, so number distribution evolution is also an evolution of composition.
The high concentrations of PM and gaseous co-pollutants in the proximity of freeways raise concerns on population exposure level (and its health implications) during commute. Time spent in and near vehicles is an important route of exposure to air pollution, but few studies of UFP concentrations in vehicle-related settings have been conducted, especially inside moving vehicles. Investigators in southern California used an electric vehicle to house and power a suite of particle and gaseous pollutant measurements (Westerdahl et al., in press). Measurements were conducted on a variety of streets and freeways in Los Angeles from February through April 2003. Diesel-powered vehicles, as expected, were often a major source of high UFP count concentrations, especially when being directly followed. However, gasoline-powered vehicles were also often observed to produce comparably high UFP counts, particularly when the vehicles were older; when vehicles were accelerating hard or from a standing start, such as after waiting at a stop light; and when vehicles were driven and/or accelerated at high speeds (Figure 8A). Because of the ubiquitous nature of gasoline-powered vehicles and the frequency of such types of driving, they may be the predominate source of in-vehicle, roadway, and near-roadway UFP concentrations. Figure 8B presents a comparison of in-vehicle and roadway UFP number–based concentrations and estimates of overall UFP contributions by vehicle type for various types of roads, driving conditions, and meteorology. As evident from Figure 8, the UFP concentrations are higher than the urban background measurements by at least an order of magnitude in the freeway affected by diesel traffic (I-710) or the mostly gasoline engine freeway (I-110).
Figure 8.
In-vehicle measurements of (A) PN concentrations (B) number-based particle size distributions, in freeways and urban areas in Los Angeles, CA. PIU, particle instrumentation unit, located at the southern California Supersite in downtown Los Angeles. Long Beach and Pasadena are two other urban areas in Los Angeles. Arrows indicate when the vehicle was off road at these three urban locations. From Westerdahl et al. (2005). Copyright 2005, with permission from Elsevier.
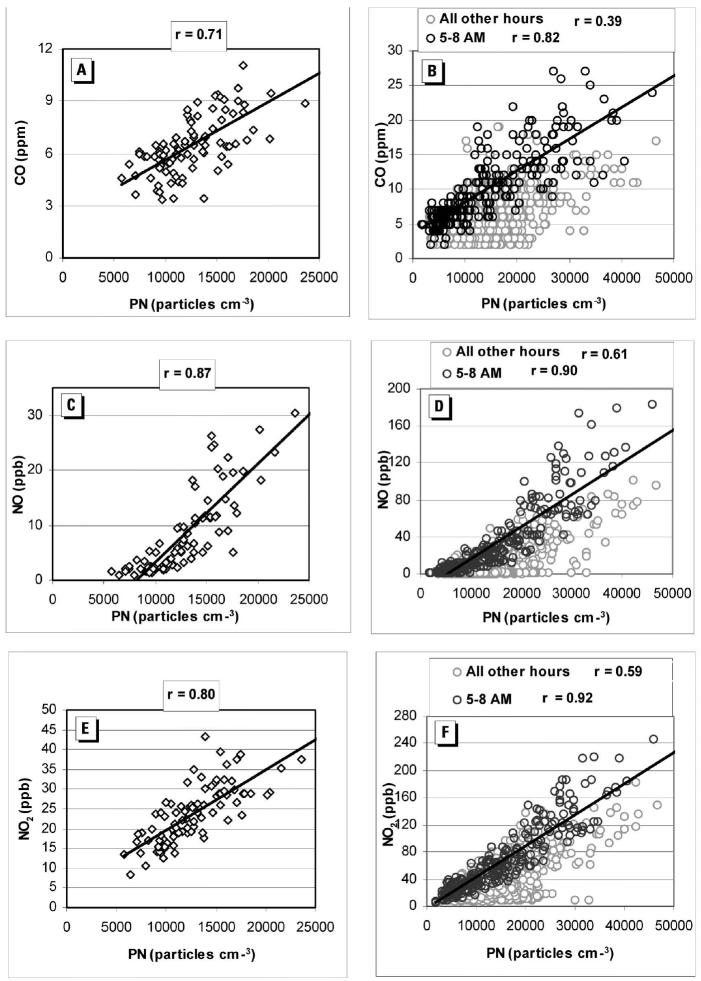
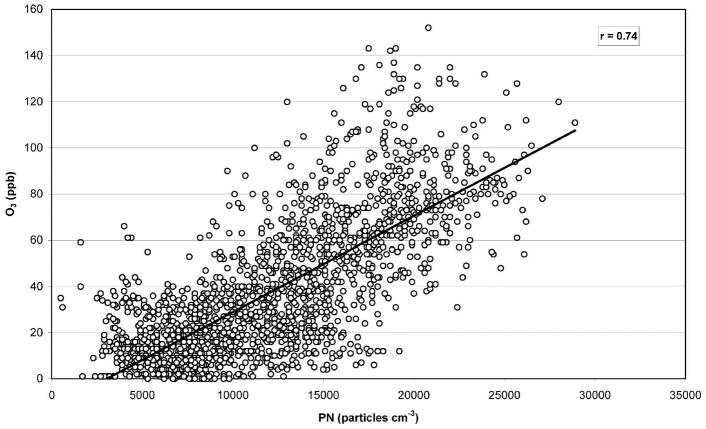
The argument that concentrations of UFPs (or, equivalently, PN concentrations) should be highly correlated with those of gaseous co-pollutants used as markers for vehicular emission, such as CO and nitrogen oxide (NO), cannot be made unilaterally. Sardar et al. (2004) performed continuous measurements of PN, PM ≤10 μm in aerodynamic diameter (PM10), and gaseous pollutants [CO, NO, NOx, and ozone (O3)] at urban (“source”) and inland (“receptor”) sites of the Los Angeles Basin for 2002 in support of the University of Southern California (USC) Children’s Health Study. As indicated in Table 1, the degree of correlation between hourly PN and co-pollutant concentrations at each site over the entire year was generally low (i.e., Pearson correlation coefficient, r < 0.4). Similar analyses of the 24-hr averaged data also resulted in generally low levels of correlation (Table 2). Some correlations between PN and both CO and NO were strengthened during morning rush-hour periods, indicating a common vehicular source, but when integrated over the entire 24-hr day period, they decreased dramatically (Figure 9). In a location near Los Angeles (Glendora, California) but not directly affected by vehicular emissions, PN concentrations were moderately to highly correlated with O3 in the summer, indicating that photochemical formation of PM is a more prominent source than traffic (Figure 10). From the standpoint of classic nucleation theory, the photochemical production of UFPs in highly polluted areas such as Los Angeles is a somewhat surprising finding, considering that the preexisting high surface area of the urban aerosol would act as a condensational sink for precursors that would be responsible for the formation of UFPs. In addition to Los Angeles, recent studies in polluted urban areas such as Detroit, Michigan (Young and Keeler 2004); Leipzig, Germany (Wehner and Wiedensohler 2003); and Mexico City (Baumgardner et al. 2004) also demonstrated a striking similarity of diurnal patterns between PNs in the smaller fractions (< 56 nm) and O3 only during the summer period. Pearson correlation coefficients (r) between the concentrations of UFPs in various size ranges and gaseous co-pollutants measured in the study by Sardar et al. (2005) are shown in Table 3. The r-values for the smaller particles are significantly high in the summer for both Long Beach and USC sites, with r-values of 0.62–0.64 and 0.68–0.69 for particles in the size range of 0–32 and 32–56 nm, respectively. As argued above, in addition to primary emissions, photochemical reactions in the atmosphere can form new particles via nucleation (Kulmala et al. 2004) or form new particle mass by condensation of low-volatility reaction products onto existing particle surfaces (Pandis et al. 1992; Shi et al. 2001). It is expected that nucleation is more likely to occur during the summer with elevated photo-chemical activity due to larger solar zenith angles. The similarity in diurnal patterns of PNs in the < 56 nm ranges and O3 observed for this period suggests a photochemical origin for these particles.
Table 1.
Hourly Pearson correlation coefficient, r, of PN vs. co-pollutant concentrations for the entire calendar year 2002, all sites.
| Glendora | Long Beach | Mira Loma | Riverside | Upland | |
|---|---|---|---|---|---|
| CO | 0.13 | 0.46 | 0.47 | 0.52 | 0.66 |
| NO | 0.06 | 0.44 | 0.60 | 0.59 | 0.65 |
| NO2 | 0.21 | 0.50 | 0.24 | 0.32 | 0.17 |
| PM10 | 0.18 | 0.27 | 0.00 | 0.16 | 0.14 |
| O3 | 0.30 | 0.22 | 0.34 | 0.04 | 0.26 |
Reprinted with permission from Sardar et al. (2004). Copyright 2004 Air and Waste Management Association.
Table 2.
24-hr average Pearson correlation coefficient, r, of PN vs. co-pollutant concentrations for the entire calendar year 2002, all sites.
| Glendora | Long Beach | Mira Loma | Riverside | Upland | |
|---|---|---|---|---|---|
| CO | 0.00 | 0.50 | 0.44 | 0.39 | 0.63 |
| NO | 0.30 | 0.48 | 0.34 | 0.32 | 0.66 |
| NO2 | 0.07 | 0.68 | 0.11 | 0.23 | 0.08 |
| PM10 | 0.18 | 0.10 | 0.17 | 0.32 | 0.19 |
| O3 | 0.31 | 0.63 | 0.33 | 0.26 | 0.54 |
Reprinted with permission from Sardar et al. (2004). Copyright 2004 Air and Waste Management Association.
Figure 9.
PN concentrations vs. CO, NO, and NO2 in Riverside, California, during summer 2002. (A) 24-hr average PN vs. CO, (B) hourly PN vs. CO, (C) 24-hr average PN vs. NO, (D) hourly PN vs. NO, (E) 24-hr aver- age PN vs. NO2, (F) hourly PN vs. NO2. Reprinted with permission from Sardar et al. (2004). Copyright 2004, Air and Waste Management Association.
Figure 10.
Hourly PN vs. O3 concentrations in Glendora, California, during summer 2002, with a lag time of 2 hr (PN vs. O3 2 hr earlier). Reprinted with permission from Sardar et al. (2004). Copyright 2004, Air and Waste Management Association.
Table 3.
Size fractionated PN vs. co-pollutant correlation coefficients at source (Long Beach, CA; USC) and receptor (Riverside, CA) sites.
| Size range (nm) | CO | NOx | O3 |
|---|---|---|---|
| Fall, Long Beach | |||
| 0–32 | −0.26 | −0.03 | 0.26 |
| 32–56 | 0.20 | 0.31 | −0.51 |
| 56–100 | 0.49 | 0.52 | −0.38 |
| 100–180 | 0.66 | 0.66 | −0.50 |
| 180–320 | 0.68 | 0.70 | −0.47 |
| 320–1,000 | 0.48 | 0.56 | −0.30 |
| USC, Winter | |||
| 0–32 | 0.09 | 0.23 | −0.03 |
| 32–56 | 0.38 | 0.54 | −0.10 |
| 56–100 | 0.65 | 0.78 | −0.13 |
| 100–180 | 0.65 | 0.75 | −0.05 |
| 180–320 | 0.64 | 0.62 | −0.06 |
| 320–1,000 | 0.53 | 0.45 | 0.01 |
| USC, Summer | |||
| 0–32 | 0.25 | 0.28 | 0.62 |
| 32–56 | 0.16 | 0.16 | 0.68 |
| 56–100 | 0.19 | 0.21 | 0.59 |
| 100–180 | 0.35 | 0.41 | 0.44 |
| 180–320 | 0.26 | 0.31 | 0.39 |
| 320–1,000 | 0.29 | 0.36 | 0.21 |
| Fall, Riverside | |||
| 0–32 | 0.48 | 0.66 | −0.45 |
| 32–56 | 0.67 | 0.84 | −0.50 |
| 56–100 | 0.78 | 0.80 | −0.51 |
| 100–180 | 0.75 | 0.60 | −0.37 |
| 180–320 | 0.69 | 0.46 | −0.18 |
| 320–1,000 | 0.59 | 0.32 | −0.04 |
| Summer, Long Beach | |||
| 0–32 | 0.25 | 0.28 | 0.64 |
| 32–56 | 0.22 | 0.24 | 0.69 |
| 56–100 | 0.33 | 0.40 | 0.54 |
| 100–180 | 0.46 | 0.63 | 0.40 |
| 180–320 | 0.47 | 0.63 | 0.25 |
| 320–1,000 | 0.32 | 0.61 | 0.14 |
Reprinted with permission from Sardar et al. (2004). Copyright 2004 American Chemical Society.
All the aforementioned studies indicate clearly that, besides contributions from vehicular sources, photochemical secondary formations are also a source of PM in urban atmosphere. What is important from the standpoint of human exposure is that, unlike vehicular emissions, which by nature have a strong local character whereby concentration decreases rapidly with increasing distance from their source, secondary formation of UFPs is more regional in nature, which implies a more uniform population exposure to UFPs generated by this mechanism.
Remaining Challenges in UFP Exposure Assessment
Despite the increasing concerns about the health impacts of UFPs, very little information is available on their concentrations or physical/chemical properties in places where people live and work, such as in community air, homes, schools, workplaces, restaurants, or vehicles. It is therefore essential to develop and deploy technologies that can assess the nature and extent to which people are exposed to these particles in these microenvironments. The complexity of the sources and nature of UFPs suggest that considerable characterization efforts will be needed to either discover and/or refine our understanding of linkages between exposures and various types of health outcomes. At one extreme is the need to determine how large, spatially dispersed populations experience ultrafine exposures over prolonged periods of study. At the other extreme is the need to determine how individuals experience the hour-to-hour or minute-to-minute dynamics of ambient UFPs as they move within the aforementioned microenvironments. The analytic technologies required to characterize the physical and chemical nature of UFPs in these various microenvironments are largely unavailable or untested outside the controlled environment of the laboratory.
The information needs of health and exposure assessors suggests that various approaches should be considered to meet these needs. Air monitoring needed in support of long-term population-based or cohort studies typically must be expected to operate with very little operator intervention for periods of up to several years. Siting of such monitoring operations should be made with care to represent the overall community exposures. In time-series studies where citywide populations are often represented by central site PM monitoring, Pekkanen and Kulmala (2004) concluded that such data might be a worse proxy for human exposure to UFPs than to PM2.5. In panel studies where small groups of individuals are followed for days or weeks, it may prove practical to perform enhanced monitoring of UFPs. Enhancement might include technologies that report particle size distributions, the chemical nature of UFPs, and information on physical parameters such as shape and density. Panel studies offer the opportunity for individuals under study to carry instruments and record their locations and activities. Data from these sources have been shown to be essential when modeling overall exposures as well as in identifying the microenvironments of most concern. As with large-scale studies, careful consideration should be given to the siting of monitoring in order to provide robust data that may be related to observed effects. Although these studies offer the opportunity to determine or model exposures to individuals in the study population, it is important to develop and employ monitoring instrumentation suited to placement in homes, in classrooms, and in cars and that can be carried by children and adults. Data collected in these studies should be augmented with assessments of the activities of participants and descriptions of the microenvironments monitored.
When exposure models are developed and employed to estimate UFP exposures, it is important to consider both indoor and outdoor sources. For the purpose of estimating exposures related to time spent indoors, it is useful to gather data on infiltration of UFPs from outside sources. The extent of particle penetration into indoor environments is governed by indoor and outdoor sources, exchange rates, and particle physicochemical characteristics. Indoor particle concentrations, therefore, depend on the dynamics of the transport and fate of outdoor particles in indoor environments. Previous research in this area has focused on PM2.5 and PM10 properties and behavior (Jones et al. 2000; Thatcher and Layton 1995). These studies indicated that concentrations of indoor particles of outdoor origin are significant. In addition, the building shell was found to be ineffective in removing infiltrating particles. Considering health implications of UFP exposure, it is important to assess particle penetration characteristics into indoor environments and the relationship between their physical and chemical properties and infiltration.
Although experiments have been performed to investigate penetration properties of submicrometer particles, these laboratory-based studies have assumed that particles are spherical and rigid (Liu and Nazaroff 2003). Results indicated that particle size and building gap dimensions were most important factors determining particle penetration. Furthermore, real-world UFP penetration studies conducted thus far have examined infiltration properties for a limited set of conditions. For example, Long et al. (2001) evaluated penetration efficiencies in only suburban neighborhoods. Franck et al. (2003) studied indoor and outdoor UFP size distributions at one location. Vette et al. (2001) measured indoor and outdoor particle size distributions of a single residence at urban background concentrations. However, characterization of urban particle infiltration should consider recent studies showing that UFPs exhibit great spatial variations near sources (Zhu et al. 2002a). Sakurai et al. (2003) studied the chemical composition and volatility of nanoparticles emitted from diesel vehicles and found that these aerosols consist of residual species, which may represent nonvolatile cores or low-volatility organic compounds as well as more volatile, smaller particles thought to be products of condensation of hot supersaturated organic vapors associated with fuel and lube oils. The volatile fraction constitutes about 90% of the total aerosol emitted by vehicles based on number concentrations. Such findings suggest that at least the volatile particles of outdoor origin can experience substantial changes and may be lost to building walls during indoor penetration. This notion is further supported by a recent study investigating the transformation of labile ambient ammonium nitrate aerosols in indoor environments, which has shown that measured indoor concentrations were considerably lower than the values predicted based only on penetration and deposition losses (Lunden et al. 2003). Because of the public health implications of UFPs and their spatial variations near pollutant sources such as freeways, it is therefore important to evaluate outdoor UFP size distributions, volatility properties, and penetration efficiencies into indoor environments.
In conclusion, exposure assessment issues for UFPs are complex and need to be considered before undertaking epidemiologic investigations of their health effects. For instance, because of the high spatial variability of UFPs, the use of central site concentration data alone may not reveal its relative importance compared with other PM-size fractions or compared with gaseous pollutants. Particular attention needs to be given to indoor sources and infiltration of UFPs from outside sources, as well as meteorology because of the potentially high seasonal variability in UFP PN concentrations, sources, and chemical composition, including volatility.
Footnotes
This work was supported by grant ES-12243 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH); the contents of this article are solely the responsibility of the author and do not necessarily represent the official views of the NIEHS, NIH. This work was also supported by the Southern California Particle Center and Supersite (SCPCS) funded by the U.S. Environmental Protecton Agency (U.S. EPA; STAR award R82735201).
This manuscript has not been subjected to the U.S. EPA peer and policy review and therefore does not necessarily reflect the views of the agencies. No official endorsement should be inferred.
References
- Baumgardner D, Raga GB, Muhlia A. Evidence for the formation of CCN by photochemical processes in Mexico City. Atmos Environ. 2004;38:357–367. [Google Scholar]
- Chalupa DC, Morrow PE, Oberdörster G, Utell MJ, Frampton MW. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect. 2004;112:879–82. doi: 10.1289/ehp.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron A, Harrison RM. Primary particle formation from vehicle emissions during exhaust dilution in the roadside atmosphere. Atmos Environ. 2003;37:4109–4119. [Google Scholar]
- Cho AK, Sioutas C, Schmitz DA, Kumagai Y, Singh M, Miguel AH, et al. In press. Redox activity of airborne particulate matter (PM) at different sites in the Los Angeles Basin. Environ Res. [DOI] [PubMed]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113(8):934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Eiguren-Fernandez A, Miguel AH, Jaques P, Sioutas C. Evaluation of a denuder-MOUDI-PUF sampling system to determine the size distribution of semivolatile polycyclic aromatic hydrocarbons in the Atmosphere. Aerosol Sci Technol. 2003;37:201–209. [Google Scholar]
- Fine PM, Si S, Geller MG, Sioutas C. Inferring the sources of fine and ultrafine PM at downwind receptor areas in the Los Angeles basin using multiple continuous monitors. Aerosol Sci Technol. 2004;38:182–195. [Google Scholar]
- Franck U, Herbarth O, Wehner B, Wiedensohler A, Manjarrez M. How do the indoor size distributions of airborne submicron and ultrafine particles in the absence of significant indoor sources depend on outdoor distributions? Indoor Air. 2003;13(2):174–181. doi: 10.1034/j.1600-0668.2003.00177.x. [DOI] [PubMed] [Google Scholar]
- Friedlander SK. 2000. Dynamics of Agglomerate Formation and Restructuring in Smoke, Dust and Haze. New York:Oxford University Press.
- Glovsky MM, Miguel AG, Cass GR. Particulate air pollution: possible relevance in asthma. Allergy Asthma Proc. 1997;18:163–166. doi: 10.2500/108854197778984392. [DOI] [PubMed] [Google Scholar]
- Hinds WC. 1999. Aerosol Technology. 2nd ed. New York:John Wiley & Sons.
- Hitchins J, Morawska L, Wolff R, Gilbert D. Concentrations of sub-micrometer particles from vehicle emissions near a major road. Atmos Environ. 2000;34:51–59. [Google Scholar]
- Hughes L, Cass GR, Gone J, Ames M, Olmez I. Physical and chemical characterization of atmospheric ultrafine particles in the Los Angeles Area. Environ Sci Technol. 1998;32(9):1153–1161. [Google Scholar]
- Jeong CH, Hopke PK, Chalupa D, Utell M. Characteristics of nucleation and growth events of ultrafine particles measured in Rochester, NY. Environ Sci Technol. 2004;38 (7):1933–1940. doi: 10.1021/es034811p. [DOI] [PubMed] [Google Scholar]
- Jones NC, Thornton CA, Mark D, Harrison RM. Indoor/outdoor relationships of particulate matter in domestic homes with roadside, urban and rural locations. Atmos Environ. 2000;34(16):2603–2612. [Google Scholar]
- Ketzel M, Wåhlin P, Berkowicz R, Palmgren F. Particle and trace gas emission factors under urban driving conditions in Copenhagen based on street and roof-level observations. Atmos Environ. 2003;37:2735–2749. [Google Scholar]
- Kim CS, Jaques PA. Respiratory dose of inhaled ultrafine particles in healthy adults. Philos Trans R Soc Lond A. 2000;358(1775):2693–2705. [Google Scholar]
- Kim CS, Jaques PA. Analysis of total respiratory deposition of inhaled ultrafine particles in adult subjects as various breathing patterns. Aerosol Sci Technol. 2004;38 (6):525–540. [Google Scholar]
- Kim S, Shen S, Sioutas C. Size distribution and diurnal and seasonal trends of ultrafine particles in source and receptor sites of the Los Angeles basin. J Air Waste Manage Assoc. 2002;52:297–307. doi: 10.1080/10473289.2002.10470781. [DOI] [PubMed] [Google Scholar]
- Kittelson DB. Engines and nanoparticles: a review. J Aerosol Sci. 1998;29:575–588. [Google Scholar]
- Kulmala M, Laaksonen A. Binary nucleation of water-sulfuric acid system: comparison of classical theories with different H2SO4 saturation vapor pressures. J Chem Physics. 1990;93:696–701. [Google Scholar]
- Kulmala M, Pirjola L, Mäkelä JM. Stable sulphate clusters as a source of new atmospheric particles. Nature. 2000;404:66–69. doi: 10.1038/35003550. [DOI] [PubMed] [Google Scholar]
- Kulmala M, Vehkamäki H, Petäjä T, Dal Maso M, Lauri A, Kerminen VM, et al. Formation and growth rates of ultrafine atmospheric particles: a review of observations. J Aerosol Sci. 2004;35:143–176. [Google Scholar]
- Li N, Sioutas C, Froines JR, Cho A, Misra C, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DL, Nazaroff WW. Particle penetration through building cracks. Aerosol Sci Technol. 2003;37(7):565–573. [Google Scholar]
- Long CM, Suh HH, Catalano PJ, Koutrakis P. Using time- and size-resolved particulate data to quantify indoor penetration and deposition behavior. Environ Sci Technol. 2001;35:2089–2099. doi: 10.1021/es001477d. [DOI] [PubMed] [Google Scholar]
- Lunden MM, Revzan KL, Fischer ML, Thatcher TL, Littlejohn D, Hering SV, et al. The transformation of outdoor ammonium nitrate aerosols in the indoor environment. Atmos Environ. 2003;37(39–40):5633–5644. [Google Scholar]
- McMurry PH, Wang X, Park K, Ehara K. The relationship between mass and mobility for atmospheric particles: a new technique for measuring particle density. Aerosol Sci Technol. 2002;36:227–238. [Google Scholar]
- Morawska L, Bofinger ND, Kocis L, Nwankwoala A. Submicrometer and super micrometer particles from diesel vehicle emissions. Environ Sci Technol. 1998;32:2033–2042. [Google Scholar]
- Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001;7:20–26. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol. 1998;102:539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PHM, Thomeer M, Nemery B, Vanquickenborne B, Vanbilloen H, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicol Lett. 2004;149:243–253. doi: 10.1016/j.toxlet.2003.12.061. [DOI] [PubMed] [Google Scholar]
- Oberdörster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Oberdörster OG, Gelein RM, Ferin J, Weiss B. Association of particulate air pollution and acute mortality: involvement of ultrafine particles? Inhal Toxicol. 1995;7:111–124. doi: 10.3109/08958379509014275. [DOI] [PubMed] [Google Scholar]
- O’Dowd C, McFiggans G, Creasey DJ, Pirjola L, Hoell C, Smith MH, et al. On the photochemical production of new particles in the coastal boundary layer. Geophys Res Lett. 1999;26 (12):1707–1710. [Google Scholar]
- Pandis SN, Harley RA, Cass GR, Seinfeld JH. Secondary organic aerosol formation and transport. Atmos Environ A. 1992;26(13):2269–2282. [Google Scholar]
- Pekkanen J, Kulmala M. Exposure assessment of ultrafine particles in epidemiologic time-series studies. Scand J Work Environ Health. 2004;30(suppl 2):9–18. [PubMed] [Google Scholar]
- Pekkanen J, Timonen KL, Ruuskanen J, Reponen A, Mirme A. Effects of ultrafine and fine particles in urban air on peak flow expiratory flow among children with asthmatic symptoms. Environ Res. 1997;74:24–33. doi: 10.1006/enrs.1997.3750. [DOI] [PubMed] [Google Scholar]
- Penttinen P, Timonen KL, Tiittanen P, Mirme A, Ruuskanen J, Pekkanen J. Number concentration and size of particles in urban air: effects on spirometric lung function in adult asthmatic subjects. Environ Health Perspect. 2001;109:319–323. doi: 10.1289/ehp.01109319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultra-fine particles. Am J Respir Crit Care Med. 1997;155:1376–1383. doi: 10.1164/ajrccm.155.4.9105082. [DOI] [PubMed] [Google Scholar]
- Pritchard RJ, Ghio AJ, Lehmann JR, Winsett DW, Tepper JS, Park P, et al. Oxidant generation and lung injury after particulate air pollutant exposure increase with the concentrations of associated metals. Inhal Toxicol. 1996;8:457–477. [Google Scholar]
- Ristovski ZD, Morawska L, Bofinger ND, Hitchins J. Submicrometer and supermicrometer particles from spark ignition vehicles. Environ Sci Technol. 1998;32:3845–3852. [Google Scholar]
- Sakurai H, Tobias HJ, Park K, Zarling D, Docherty S, Kittelson DB, et al. On-line measurements of diesel nanoparticle composition and volatility. Atmos Environ. 2003;37(9–10):1199–1210. [Google Scholar]
- Sardar S, Fine M, Mayo PR, Sioutas C. Size fractionated measurements of ambient ultrafine particle chemical composition in Los Angeles using the NanoMOUDI. Environ Sci Technol. 2005;39:923–944. doi: 10.1021/es049478j. [DOI] [PubMed] [Google Scholar]
- Sardar SB, Fine PM, Sioutas C. The relationship between particle number and co-pollutant concentrations in the Los Angeles basin. J Air Waste Manag Assoc. 2004;54:992–105. doi: 10.1080/10473289.2004.10470970. [DOI] [PubMed] [Google Scholar]
- Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, et al. Particulate air pollution and the blood. Thorax. 1999;54:1027–1032. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi JP, Evans DE, Khan AA, Harrison RM. Source and concentration of nanoparticles (< 10 nm diameter) in the urban atmosphere. Atmos Environ. 2001;35:1193–1202. [Google Scholar]
- Stanier CO, Khlystov AY, Pandis SN. Nucleation events during the Pittsburgh Air Quality Study: description and relation to key meteorological, gas phase, and aerosol parameters. Aerosol Sci Technol. 2004;38(suppl 1):253–264. [Google Scholar]
- Thatcher TL, Layton DW. Deposition, resuspension, and penetration of particles within a residence. Atmos Environ. 1995;29(13):1487–1497. [Google Scholar]
- Utell MJ, Frampton MW. Acute health effects of ambient air pollution: the ultrafine particle hypothesis. J Aerosol Med. 2000;13:355–359. doi: 10.1089/jam.2000.13.355. [DOI] [PubMed] [Google Scholar]
- Vette AF, Rea AW, Lawless PA, Rodes CE, Evans G, Highsmith VR, et al. Characterization of indoor-outdoor aerosol concentration relationships during the Fresno PM exposure studies. Aerosol Sci Technol. 2001;34(1):118–126. [Google Scholar]
- Wehner B, Wiedensohler A. Long term measurements of submicrometer urban aerosols: statistical analysis for correlations with meteorological conditions and trace gases. Atmos Chem Phys Disc. 2003;3:867–879. [Google Scholar]
- Westerdahl D, Fruin S, Sax T, Fine PM, Sioutas C. Mobile platform measurements of ultrafine particles and associated pollutant concentrations on freeways and residential streets in Los Angeles. Atmos Environ. 2005;39(20):3597–3610. [Google Scholar]
- Wichmann HE, Spix C, Tuch T, Wolke G, Peters A, Heinrich J, et al. Daily mortality and fine and ultrafine particles in Erfurt, Germany. Part I: Role of particle number and particle mass. Res Rep Health Eff Inst. 2000;98:5–86. [PubMed] [Google Scholar]
- Woo KS, Chen DR, Pui DYH, McMurry PH. Measurement of Atlanta aerosol size distributions: observations of ultra-fine particle events. Aerosol Sci Technol. 2001;34:75–87. [Google Scholar]
- Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, Nel A. Quinones and aromatic chemical compounds in particulate matter (PM) induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112(14):1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LH, Keeler GJ. Characterization of ultrafine particle number concentration and size distribution during a summer campaign in southwest Detroit. J Air Waste Manage Assoc. 2004;54 (9):1079–1090. doi: 10.1080/10473289.2004.10470987. [DOI] [PubMed] [Google Scholar]
- Yu F, Turco RP. Ultrafine aerosol formation via ion-mediated nucleation. Geophys Res Lett. 2000;27:883–886. [Google Scholar]
- Zhang KM, Wexler AS, Zhu Y, Hinds WC, Sioutas C. Evolution of particle number distributions near roadways. Part II: The “road-to-ambient process. Atmos Environ. 2004;38:6655–6665. [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Shen S, Sioutas C. Study on ultrafine particles and other vehicular pollutants near a major highway with heavy duty diesel traffic. Atmos Environ. 2002a;36:4323–4335. [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manage Assoc. 2002b;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hinds WC, Shen S, Sioutas C. Seasonal trends of concentration and size distributions of ultrafine particles near major highways in Los Angeles. Aerosol Sci Technol. 2004;38(suppl 1):5–13. [Google Scholar]