Abstract
Exposure to chlorpyrifos (CPF) alters neuronal development of serotonin (5HT) and dopamine systems, and we recently found long-term alterations in behaviors related to 5HT function. To characterize the synaptic mechanisms underlying these effects, we exposed developing rats to CPF regimens below the threshold for systemic toxicity, in three treatment windows: gestational days (GD) 17–20, postnatal days (PN) 1–4, or PN11–14. In early adulthood (PN60), we assessed basal neurotransmitter content and synaptic activity (turnover) in brain regions containing the major 5HT and dopamine projections. CPF exposure on GD17–20 or PN1–4 evoked long-term increases in 5HT turnover across multiple regions; the effects were not secondary to changes in neurotransmitter content, which was unaffected or even decreased. When the treatment window was shifted to PN11–14, there were no long-term effects. Dopamine turnover also showed significant increases after CPF exposure on GD17–20, but only when the dose was raised above the threshold for overt toxicity; however, hippocampal dopamine content was profoundly subnormal after exposures below or above the acute, toxic threshold, suggesting outright neurotoxicity. These results indicate that, in a critical developmental period, apparently nontoxic exposures to CPF produce lasting activation of 5HT systems in association with 5HT-associated behavioral anomalies.
Keywords: brain development, chlorpyrifos, dopamine, organophosphate pesticides, serotonin
The organophosphorus pesticide chlorpyrifos (CPF) remains in use throughout the world despite its recent curtailment in the United States [U.S. Environmental Protection Agency (EPA) 2000, 2002]. The major concern for CPF is its propensity to elicit developmental neurotoxicity at exposure levels below the threshold for systemic toxicity, such that damaging exposures of pregnant women and children may go undetected because of the lack of symptoms (Landrigan 2001; Landrigan et al. 1999; May 2000; Physicians for Social Responsibility 1995; Pope 1999; Slotkin 1999, 2004; Weiss et al. 2004). CPF was originally thought to exert adverse effects on brain development through the same mechanism by which it elicits systemic toxicity, namely, inhibition of cholinesterase through its active metabolite, CPF oxon, but it is now evident that other, noncholinergic mechanisms participate in its developmental neurotoxicity (Barone et al. 2000; Clegg and van Gemert 1999a, 1999b; Gupta 2004; Mileson et al. 1998; Pope 1999; Slotkin 1999, 2004). Even at exposures below the threshold for cholinesterase inhibition, CPF itself disrupts the patterns of neural cell replication and differentiation, axonogenesis and synaptogenesis, and the functional development of neurotransmitter and neurotrophin systems, culminating in aberrant behavioral performance (Barone et al. 2000; Casida and Quistad 2004; Gupta 2004; Pope 1999; Qiao et al. 2002, 2003; Yanai et al. 2002). Consequently, CPF-induced damage extends beyond cholinergic pathways to include other neurotransmitter systems, notably the monoamines, norepinephrine, dopamine (DA), and serotonin (5HT), and recent studies show that 5HT systems are especially sensitive (Aldridge et al. 2003, 2004, 2005a, 2005b; Raines et al. 2001; Sachana et al. 2001).
In recent work we found that, during a critical developmental window centered around the immediate perinatal period, CPF elicits immediate and persistent effects on expression of 5HT receptors and the 5HT transporter, biomarkers for 5HT synaptic development and integrity (Aldridge et al. 2003, 2004, 2005a; Raines et al. 2001). Additionally, we found that the same exposures elicited lasting changes in 5HT-related behaviors, resembling animal models of depression (Aldridge et al. 2005a). Notably, these studies were conducted spanning CPF exposures below the threshold for observable toxicity and, more important, could be detected at doses devoid of cholinesterase inhibition in the developing brain (Qiao et al. 2002). In the present study, we assessed 5HT levels and turnover, a measure of synaptic activity, in adulthood after prenatal or neonatal CPF exposure in order to provide a mechanistic link between effects on static biomarkers of 5HT synaptic development and altered behavior. Measurements were conducted using the metabolite ratio method (Slotkin et al. 2000; Xu et al. 2001), assessing the relative amounts of 5HT and its metabolite 5-hydroxyindole-acetic acid (5HIAA), with turnover calculated as the 5HIAA:5HT ratio. We examined brain regions with prominent 5HT innervation that are known to be targets for CPF (Aldridge et al. 2003, 2004, 2005b; Raines et al. 2001). CPF exposure was carried out during the immediate perinatal period, the phase in which the receptor and transporter biomarkers indicate high susceptibility, or at a later postnatal development period in which these effects wane: exposure on gestational days (GD) 17–20 and postnatal days (PN) 1–4 and PN11–14 (Aldridge et al. 2003, 2004, 2005b; Raines et al. 2001). Finally, effects on 5HT systems were contrasted to those on DA levels and turnover, again using the metabolite ratio method (Slotkin et al. 2000; Xu et al. 2001), assessing dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), calculating the ratio as [DOPAC + HVA]:DA.
Materials and Methods
Animal treatments.
All experiments using live animals were carried out in accordance with the Declaration of Helsinki (World Medical Association 2004) and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Institute of Laboratory Animal Resources 1996). Timed-pregnant Sprague-Dawley rats (Zivic Laboratories, Pittsburgh, PA, USA) were housed in breeding cages, with a 12-hr light/dark cycle and with free access to food and water. CPF (Chem Service, West Chester, PA, USA) was dissolved in dimethyl sulfoxide to provide consistent absorption (Whitney et al. 1995) and was injected subcutaneously in a volume of 1 mL/kg body weight; control animals received equivalent dimethyl sulfoxide vehicle injections.
For exposure on GD17–20, dams were injected daily with CPF at 1 and 5 mg/kg body weight, doses that span the threshold for inhibition of fetal brain cholinesterase activity, fetal growth impairment, and reduced maternal weight gain, all of which become evident at ≥5 mg/kg (Garcia et al. 2003; Qiao et al. 2002). On the day of birth, all pups were randomized within their respective treatment groups and redistributed to the dams with a litter size of 10 to maintain a standard nutritional status. Randomization was repeated at intervals of 3 days, and in addition, within each treatment group, dams were rotated among litters to distribute any maternal caretaking differences randomly. Animals were weaned on PN21. On PN60, one male and one female were selected from each litter and were decapitated. The cerebellum, including flocculi, was removed, and the forebrain was separated from the midbrain and brainstem by a cut made rostral to the thalamus. The fore-brain was then further dissected into the regions containing major 5HT projections (cerebral cortex, hippocampus, striatum), and the midbrain and brainstem, which contain the preponderance of 5HT cell bodies, were separated from each other. The cerebellum, which is sparse in 5HT neurons or projections, was not evaluated. Tissues were frozen with liquid nitrogen and stored at −45°C.
For studies of CPF effects in the first few days after birth, animals were given a subcutaneous injection of 1 mg/kg daily on PN1–4; for studies in older animals, which tolerate higher doses (Campbell et al. 1997; Pope and Chakraborti 1992; Pope et al. 1991; Whitney et al. 1995), daily treatment with 5 mg/kg was given on PN11–14. The same randomization procedure was followed as described for prenatal CPF treatment. Neither regimen evoked weight loss or mortality (Campbell et al. 1997; Dam et al. 1998; Johnson et al. 1998; Song et al. 1997). Samples were obtained on PN60 as described above.
Assays.
Tissues were thawed and homogenized in ice-cold 0.1 M perchloric acid and sedimented for 20 min at 18,000 × g. The supernatant solution was collected, and aliquots were used for analysis of 5HT, 5HIAA, DA, HVA, and DOPAC by HPLC with electrochemical detection (Slotkin et al. 2000; Xu et al. 2001). Concurrently run standards, containing each of the neurotransmitters and metabolites (Sigma Chemical Co., St. Louis, MO, USA), were used to calculate the regional content of each neurochemical. DOPAC and HVA levels in the hippocampus were too low for accurate measurement. Transmitter levels were calculated as nanograms per region, and, because there were no treatment-related differences in brain region weights, the same effects were obtained with calculation as nanograms per gram of tissue or per milligram of protein (data not shown). Similarly, because 5HT and DA turnover were calculated as ratios of metabolites to transmitter, the values are independent of whether region, tissue weight, or protein concentration is used as the denominator term.
Data analysis.
Each treatment group comprised six males and six females. We compiled data as means ± SEs, and treatment-related differences were assessed by analysis of variance (ANOVA), with data log-transformed because of heterogeneous variance, incorporating factors of treatment (control, CPF), brain region, and sex. Where the initial test identified interactions of CPF treatment with region, we subdivided the effects into the individual brain regions and conducted lower-order tests to examine treatment and sex differences. Similarly, where ANOVA identified interactions of CPF with sex, we subdivided data to examine males and females separately. After subdivisions, we used Fisher’s protected least significant difference to evaluate differences between individual groups. However, where CPF treatment did not interact with other variables, we assessed only the main treatment effect. Significance was assumed at the level of p < 0.05 for main effects; however, for interactions at p < 0.1, we examined lower-order main effects after subdivision of the interactive variables (Snedecor and Cochran 1967).
To facilitate comparison of treatment effects across regions with widely differing transmitter levels or turnovers, some results are presented as the percentage change from control values. However, statistical evaluations were always conducted on the original data. Similarly, for convenience, control values for the three different treatment regimens were combined for presentation, but comparisons of CPF effects were conducted only with the appropriately matched control cohort. For reference, control values appear in Table 1.
Table 1.
Neurotransmitter and metabolite levels (ng/region) in control rat brain regions.
| Brain region | Sex | 5HT | 5HIAA | DA | DOPAC | HVA |
|---|---|---|---|---|---|---|
| Cerebral cortex | Male | 267 ± 6 | 186 ± 5 | 243 ± 9 | 59 ± 2 | 42 ± 2 |
| Female | 255 ± 5 | 197 ± 5 | 230 ± 12 | 56 ± 2 | 42 ± 2 | |
| Hippocampus | Male | 113 ± 3 | 190 ± 5 | 1.3 ± 0.2 | BD | BD |
| Female | 114 ± 3 | 202 ± 5 | 1.2 ± 0.2 | BD | BD | |
| Striatum | Male | 165 ± 4 | 210 ± 5 | 541 ± 13 | 203 ± 7 | 55 ± 2 |
| Female | 173 ± 4 | 231 ± 5* | 470 ± 13* | 189 ± 6 | 50 ± 2 | |
| Midbrain | Male | 487 ± 15 | 299 ± 9 | 31 ± 1 | 16.1± 0.8 | BD |
| Female | 461 ± 13 | 324 ± 7* | 26 ± 1* | 14.1 ± 0.5 | BD | |
| Brainstem | Male | 189 ± 10 | 91 ± 6 | 4.0 ± 0.2 | 2.2 ± 0.2 | BD |
| Female | 188 ± 8 | 97 ± 7 | 3.4 ± 0.1* | 2.2 ± 0.2 | BD |
BD, below detection limit.
*Significant sex differences.
Results
In brain regions of control rats, levels of 5HT, DA, and their metabolites showed few sex differences (Table 1). 5HIAA was slightly higher in the striatum and midbrain of females, whereas DA levels were lower in midbrain and brainstem. None of the prenatal or postnatal treatment regimens evoked significant deficits in brain region weights (data not shown).
CPF exposure on GD17–20.
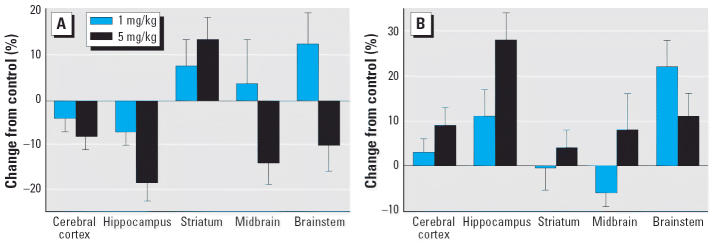
Across all brain regions, late gestational exposure to CPF elicited a significant net overall reduction (main treatment effect) in 5HT levels (Figure 1A). However, the effect was seen only at the higher dose (5 mg/kg), which elicits maternal weight deficits and significant fetal brain cholinesterase inhibition (Garcia et al. 2003; Qiao et al. 2002). Superimposed on the main treatment effect, there were regional differences: most regions showed a decrease in content, but values increased in the striatum. The pattern of effects on 5HT turnover was unrelated to the effects on 5HT content, with a main treatment effect representing a net increase (Figure 1B). In this case, however, effects were statistically significant at both 1 and 5 mg/kg, thus indicating increased 5HT turnover even at CPF exposures below the threshold for maternal toxicity or fetal brain cholinesterase inhibition. Again, some regions (hippocampus, brainstem) showed much larger changes than the others, particularly compared with the striatum.
Figure 1.
Effects of GD17–20 CPF exposure on (A) 5HT content and (B) turnover. ANOVA across treatment, region, and sex: (A), treatment, p < 0.03; control versus 5 mg/kg, p < 0.03; (B), treatment, p < 0.007; control versus 1 mg/kg, p < 0.05; control versus 5 mg/kg, p < 0.002. Separate ANOVAs were not conducted for each region because of the absence of treatment × region interactions. Similarly, values are shown combined for males and females because of the lack of treatment × sex interactions.
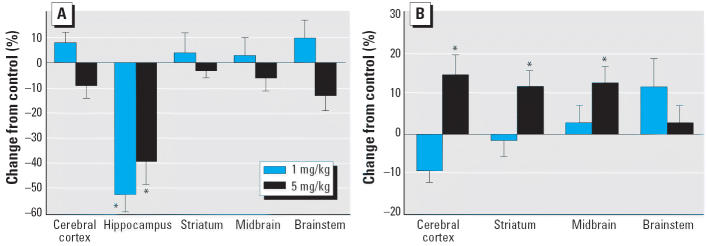
The pattern of effects on DA levels and turnover differed substantially from those seen for 5HT. Although DA content was unaffected in most brain regions, there were massive deficits in the hippocampus with either the low or high dose of CPF (Figure 2A). DA turnover was affected only at the higher dose, with significant elevations in cerebral cortex, striatum, and midbrain (Figure 2B); DA turnover could not be assessed in the hippocampus because of the low levels of DA metabolites.
Figure 2.
Effects of GD17–20 CPF exposure on (A) DA content and (B) turnover. ANOVA across treatment, region, and sex: (A), treatment, p < 0.04; treatment × region, p < 0.02; (B), treatment, p < 0.007; treatment × region, p < 0.02.
*Individual treatments and regions are significantly different; values are shown combined for males and females because of the lack of treatment × sex interactions.
CPF exposure on PN1–4.
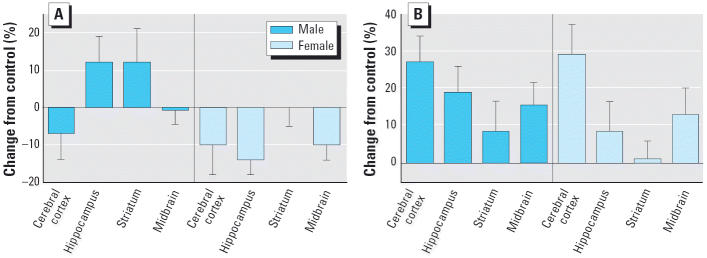
With early postnatal treatment, CPF elicited sex-selective alterations in 5HT content (Figure 3A). Males showed no significant change, whereas females showed a small but statistically significant net decrease. Again, the effects on 5HT turnover appeared to be unrelated to changes in 5HT content, displaying a net increase that was not sex selective and with the smallest magnitude of effect in the striatum (Figure 3B).
Figure 3.
Effects of PN1–4 CPF (1 mg/kg) exposure on (A) 5HT content and (B) turnover. ANOVA across treatment, region, and sex: (A), treatment × sex, p < 0.04; male, not significant; female, p < 0.03; (B), treatment, p < 0.0001 (separate ANOVAs for males and females were not evaluated because of the lack of a treatment × sex interaction). Separate ANOVAs were not conducted for each region because of the absence of treatment × region interactions.
Effects on DA levels and turnover after CPF exposure on PN1–4 have appeared previously (Slotkin et al. 2002), exhibiting decreases in cerebrocortical DA content and turnover, increases in both parameters in the striatum, and increased turnover in the midbrain.
CPF exposure on PN11–14.
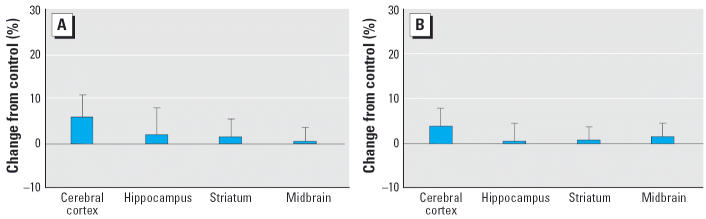
Despite the use of a higher dose (5 mg/kg compared with 1 mg/kg on PN1–4) and the resultant greater degree of cholinesterase inhibition (Qiao et al. 2002; Song et al. 1997), CPF exposure during this later postnatal period had no significant effect on either 5HT content (Figure 4A) or turnover (Figure 4B). Earlier work for DA similarly found no alterations in either levels or turnover (Slotkin et al. 2002).
Figure 4.
Effects of PN11–14 CPF (5 mg/kg) exposure on (A) 5HT content and (B) turnover. ANOVA across treatment, region, and sex: not significant. Separate ANOVAs were not conducted for each region because of the absence of treatment × region interactions; similarly, values are shown combined for males and females because of the lack of treatment × sex interactions.
Discussion
In earlier work, we found that CPF exposure during a discrete developmental period in late gestation and early neonatal life elicits lasting up-regulation of the expression of 5HT synaptic proteins, including 5HT1A and 5HT2 receptors and the presynaptic, high-affinity 5HT transporter (Aldridge et al. 2004). The present results indicate that these changes in static 5HT biomarkers are associated with functional changes in 5HT levels, and, more important, in 5HT turnover, a dynamic index of synaptic activity. Moreover, the critical period for the alterations of transmitter turnover seen here corresponds precisely to that identified previously for the protein biomarkers, with closure of the window of vulnerability by the second postnatal week. In both cases, it is of critical importance that the lasting effects on 5HT function are elicited at CPF exposures below the threshold for systemic toxicity and even below that required for inhibition of fetal brain cholinesterase. The main findings here thus reinforce the idea that cholinesterase inhibition per se is an inadequate indicator of thresholds for CPF-induced developmental neurotoxicity.
The increase in 5HT turnover evoked by CPF exposure on either GD17–20 or PN1–4 is indicative of presynaptic hyperactivity, a particularly striking finding in light of the up-regulation of 5HT receptors evoked by the same treatments (Aldridge et al. 2004). Ordinarily, increased 5HT concentrations in the synapse can be expected to evoke receptor down-regulation as a compensation for over-stimulation. As an initial impression, our finding of global presynaptic/postsynaptic up-regulation (receptors, transporter, turnover) might then suggest an overwhelming increase in 5HT function. However, evaluations of 5HT-linked behaviors and cell signaling both indicate quite the opposite, namely, deficiencies in 5HT function (Aldridge et al. 2004, 2005a). Accordingly, “miswiring” of 5HT circuits provides the most likely explanation for the effects of CPF on developing 5HT systems. If 5HT projections do not innervate the appropriate postsynaptic cells, then receptor up-regulation will occur because of the lack of neurotransmitter stimulation, and at the same, presynaptic neurons will be hyperactive as an attempt to compensate for the de facto lack of 5HT effect. With miswiring, these compensations cannot overcome the underlying architectural problem, leading to behavioral deficits resembling 5HT deficiencies as seen in animal models of depression (Aldridge et al. 2005a). Indeed, these types of circuitry defects also occur with prenatal xenobiotic insults that target cholinergic systems. Both phenobarbital and heroin disrupt the regional architecture of acetylcholine projections in the hippocampus, leading to parallel increases in cholinergic receptors, acetylcholine release, and expression of the presynaptic choline transporter, accompanied by behavioral and signaling defects that are not rectified by the combined presynaptic/ postsynaptic up-regulation (Steingart et al. 1998; Vatury et al. 2004; Yanai et al. 2004). If this interpretation is correct, deficits of 5HT function elicited by developmental exposure to CPF will also prove to be refractory to drugs that typically restore 5HT function, such as the 5HT reuptake inhibitors, resulting in drug-resistant depression. This possibility certainly deserves future examination.
Our findings also support the idea that CPF is frankly neurotoxic in the developing brain. We found decrements of 5HT levels in both males and females given the higher CPF dose on GD17–20 and also for the PN1–4 regimen in females. Nevertheless, the effects on 5HT turnover occurred at a dose below that required for loss of transmitter, suggesting that the miswiring effect is distinct from outright neurotoxicity. The effects on 5HT levels were also evident in the hippocampus, a region that we found in earlier work to be highly targeted for CPF-induced cellular damage and similar defects in cholinergic projections (Qiao et al. 2003, 2004; Slotkin et al. 2001). In keeping with this, the present results showed prominent loss of hippocampal DA even at 1 mg/kg, a dose devoid of maternal or fetal toxicity or fetal cholinesterase inhibition (Garcia et al. 2003; Qiao et al. 2002). In previous work we found only minor effects on DA levels with CPF treatment on PN1–4, and no such effects with the PN11–14 regimen (Slotkin et al. 2002), indicating early neonatal closing of the window of vulnerability for these particular neurotoxic events. Although hippocampal DA levels are lower than in the other regions, the loss of these projections is likely to play an important role in behavioral alterations. DA projections provide important regulatory inputs to hippocampal cholinergic synapses, with consequent effects on learning and memory (Yanai et al. 1993). Like the effects on 5HT turnover, we also found increased DA turnover after prenatal CPF exposure, with closure of the window of vulnerability in the second postnatal week (Slotkin et al. 2002). The effects on 5HT thus represent one component of a larger spectrum of CPF-induced disruption of synaptic development and function that ultimately contribute to behavioral anomalies.
There are two additional features of the present results that are worth noting. First, with gestational CPF exposure, the loss of 5HT spared the striatum and similarly, whereas the hippocampus showed 40–50% loss of DA, there was no corresponding striatal decrement. Effectively, this regional difference de-emphasizes the importance of oxidative stress as a contributory mechanism for the neurotoxic actions of CPF because the striatum is typically more sensitive to such effects than other regions, largely due to its high content of DA, an oxidative neurotransmitter (Bagchi et al. 1995; Crumpton et al. 2000; Gupta 2004; Karen et al. 2001; Qiao et al. 2005). Indeed, we recently evaluated lipid peroxidation with each of the CPF regimens used here and found little or no evidence for oxidative damage with the GD17–20 or PN1–4 regimens, although lipid peroxidation was readily detected after exposure on PN11–14 (Slotkin et al. 2005). Apparently, other, non-cholinesterase-related actions of CPF are more important contributors to neurobehavioral teratogenicity in the critical perinatal period in which 5HT systems are especially vulnerable (Barone et al. 2000; Casida and Quistad 2004; Gupta 2004; Pope 1999; Qiao et al. 2002, 2003; Yanai et al. 2002), although oxidative stress may be relatively more important for later postnatal exposures. The second interesting characteristic is the relative lack of sex selectivity to the effects on 5HT turnover, whereas effects on behavior and 5HT receptor expression show distinct sex differences (Aldridge et al. 2004, 2005a). In fact, many of the functional consequences of CPF exposure represent the elimination of normal sex differences in synaptic function and behavior, partially masculinizing the patterns in females but feminizing males (Aldridge et al. 2004, 2005a). Accordingly, CPF may interfere with sexual differentiation of the brain, which peaks during the vulnerable perinatal period found here (MacLusky and Naftolin 1981; McCarthy 1994); as a consequence, even where CPF effects on males and females might be the same in regard to alterations of 5HT synaptic activity and/or miswiring of 5HT circuits, the outcome nevertheless can be expressed differently for males and females because of CPF’s effects on sexual differentiation. Again, future studies should enable us to establish whether this explanation is correct.
In conclusion, the present results reinforce the concept that developmental CPF exposure, at doses below the threshold for maternal or fetal/neonatal toxicity, and below that required for inhibition of fetal brain cholinesterase, nevertheless causes lasting disruption of 5HT synaptic activity when exposure occurs in a critical developmental window centered around the immediate perinatal period. In combination with earlier studies of static synaptic protein biomarkers and 5HT-related behaviors (Aldridge et al. 2004, 2005a), the increase in 5HT turnover seen here suggests strongly that CPF acts through architectural miswiring of 5HT circuits, resulting in functional deficits despite global up-regulation of presynaptic and postsynaptic sensitivity. The developmental neuroteratogenicity of CPF thus encompasses neurotransmitter systems beyond those involving acetylcholine, and the effects seen here for 5HT and DA are likely to contribute to the emergence of behavioral alterations in adolescence and adulthood (Aldridge et al. 2005a; Carr et al. 2001; Dam et al. 2000; Icenogle et al. 2004; Levin et al. 2001, 2002; Ricceri et al. 2003).
Footnotes
This work was supported by National Institutes of Health grants ES10387, ES10356, and ES07031.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005a;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmaco-therapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005b;203:132–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- Barone S, Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology. 2000;21:15–36. [PubMed] [Google Scholar]
- Campbell CG, Seidler FJ, Slotkin TA. Chlorpyrifos interferes with cell development in rat brain regions. Brain Res Bull. 1997;43:179–189. doi: 10.1016/s0361-9230(96)00436-4. [DOI] [PubMed] [Google Scholar]
- Carr RL, Chambers HW, Guarisco JA, Richardson JR, Tang J, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyrifos on open-field behavior in juvenile rats. Toxicol Sci. 2001;59:260–267. doi: 10.1093/toxsci/59.2.260. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, van Gemert M. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J Toxicol Environ Health. 1999a;2:211–255. doi: 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, van Gemert M. Expert panel report of human studies on chlorpyrifos and or other organophosphate exposures. J Toxicol Environ Health. 1999b;2:257–279. doi: 10.1080/109374099281188. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res. 2000;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: delayed targeting of DNA synthesis after repeated administration. Dev Brain Res. 1998;108:39–45. doi: 10.1016/s0165-3806(98)00028-5. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA. Developmental neuro-toxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environ Health Perspect. 2003;111:297–303. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RC. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol Mech Meth. 2004;14:103–143. doi: 10.1080/15376520490429175. [DOI] [PubMed] [Google Scholar]
- Icenogle LM, Christopher C, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, et al. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol Teratol. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources 1996. Guide for the Care and Use of Laboratory Animals. 7th ed. Washington, DC:National Academy Press.
- Johnson DE, Seidler FJ, Slotkin TA. Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chlorpyrifos. Brain Res Bull. 1998;45:143–147. doi: 10.1016/s0361-9230(97)00329-8. [DOI] [PubMed] [Google Scholar]
- Karen DJ, Li W, Harp PR, Gillette JS, Bloomquist JR. Striatal dopaminergic pathways as a target for the insecticides permethrin and chlorpyrifos. Neurotoxicology. 2001;22:811–817. doi: 10.1016/s0161-813x(01)00063-8. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. Pesticides and polychlorinated biphenyls (PCBs): an analysis of the evidence that they impair children’s neurobehavioral development. Mol Genet Metab. 2001;73:11–17. doi: 10.1006/mgme.2001.3177. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, et al. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107(suppl 3):431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, et al. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- May M. Disturbing behavior: neurotoxic effects in children. Environ Health Perspect. 2000;108:A262–A267. doi: 10.1289/ehp.108-a262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Molecular aspects of sexual differentiation of the rodent brain. Psychoneuroendocrinology. 1994;19:415–427. doi: 10.1016/0306-4530(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, et al. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Physicians for Social Responsibility 1995. Pesticides and Children. Washington, DC:Physicians for Social Responsibility.
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK. Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures. Toxicology. 1992;73:35–43. doi: 10.1016/0300-483x(92)90168-e. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD, Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Abreu-Villaça Y, Tate CA, Cousins MM, Slotkin TA. Chlorpyrifos exposure during neurulation: cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood. Dev Brain Res. 2004;148:43–52. doi: 10.1016/j.devbrainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: what is the vulnerable period? Environ Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect. 2003;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines KW, Seidler FJ, Slotkin TA. Alterations in serotonin transporter expression in brain regions of rats exposed neonatally to chlorpyrifos. Dev Brain Res. 2001;130:65–72. doi: 10.1016/s0165-3806(01)00211-5. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, et al. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicol Appl Pharmacol. 2003;191:189–201. doi: 10.1016/s0041-008x(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Sachana M, Flaskos J, Nikolaidis E, Hargreaves A, Alexaki-Tzivanidou E. Inhibition of rat platelet 5-hydroxy-tryptamine uptake by chlorpyrifos and carbaryl. Pharmacol Toxicol. 2001;89:195–200. doi: 10.1111/j.0901-9928.2001.890409.x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Oliver CA, Seidler FJ. Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Dev Brain Res. 2005;157:172–180. doi: 10.1016/j.devbrainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Ali SF. Cellular determinants of reduced adaptability of the aging brain: neurotransmitter utilization and cell signaling responses after MDMA lesions. Brain Res. 2000;879:163–173. doi: 10.1016/s0006-8993(00)02767-0. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Dev Brain Res. 2002;133:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. 1967. Statistical Methods. Ames, IA:Iowa State University Press.
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Steingart RA, Barg J, Maslaton J, Nesher M, Yanai J. Pre- and postsynaptic alterations in the septohippocampal cholinergic innervations after prenatal exposure to drugs. Brain Res Bull. 1998;46:203–209. doi: 10.1016/s0361-9230(97)00454-1. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency) 2000. Administrator’s Announcement. Available: http://www.epa.gov/pesticides/announcement6800.htm [accessed 13 October 2003].
- U.S. EPA (Environmental Protection Agency) 2002. Chlorpyrifos: End-Use Products Cancellation Order. Available: http://www.epa.gov/fedrgstr/EPA-PEST/2002/January/Day-25/p1764.htm [accessed 6 December 2004].
- Vatury O, Barg J, Slotkin TA, Yanai J. Altered localization of choline transporter sites in the mouse hippocampus after prenatal heroin exposure. Brain Res Bull. 2004;53:25–32. doi: 10.1016/j.brainresbull.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Weiss B, Amler S, Amler RW. Pesticides. Pediatrics. 2004;113:1030–1036. [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- World Medical Association 2004. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available: http://www.wma.net/e/policy/b3.htm [accessed 23 June 2005].
- Xu Z, Seidler FJ, Ali SF, Slikker W, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- Yanai J, Beer A, Huleihel R, Izrael M, Katz S, Levi Y, et al. Convergent effects on cell signaling mechanisms mediate the actions of different neurobehavioral teratogens: alterations in cholinergic regulation of PKC in chick and avian models. Ann NY Acad Sci. 2004;1025:595–601. doi: 10.1196/annals.1316.074. [DOI] [PubMed] [Google Scholar]
- Yanai J, Rogel-Fuchs Y, Pick CG, Slotkin T, Seidler FJ, Zahalka EA, et al. Septohippocampal cholinergic changes after destruction of the A10-septal dopaminergic pathways. Neuropharmacology. 1993;32:113–117. doi: 10.1016/0028-3908(93)90090-p. [DOI] [PubMed] [Google Scholar]
- Yanai J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann NY Acad Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]