Abstract
Health effects associated with particulate matter (PM) show seasonal variations. We hypothesized that these heterogeneous effects may be attributed partly to the differences in the elemental composition of PM. Normal human bronchial epithelial (NHBE) cells and alveolar macrophages (AMs) were exposed to equal mass of coarse [PM with aerodynamic diameter of 2.5–10 μm (PM2.5–10)], fine (PM2.5), and ultrafine (PM < 0.1) ambient PM from Chapel Hill, North Carolina, during October 2001 (fall) and January (winter), April (spring), and July (summer) 2002. Production of interleukin (IL)-8, IL-6, and reactive oxygen species (ROS) was measured. Coarse PM was more potent in inducing cytokines, but not ROSs, than was fine or ultrafine PM. In AMs, the October coarse PM was the most potent stimulator for IL-6 release, whereas the July PM consistently stimulated the highest ROS production measured by dichlorofluorescein acetate and dihydrorhodamine 123 (DHR). In NHBE cells, the January and the October PM were consistently the strongest stimulators for IL-8 and ROS, respectively. The July PM increased only ROS measured by DHR. PM had minimal effects on chemiluminescence. Principal-component analysis on elemental constituents of PM of all size fractions identified two factors, Cr/Al/Si/Ti/Fe/Cu and Zn/As/V/Ni/Pb/Se, with only the first factor correlating with IL-6/IL-8 release. Among the elements in the first factor, Fe and Si correlated with IL-6 release, whereas Cr correlated with IL-8 release. These positive correlations were confirmed in additional experiments with PM from all 12 months. These results indicate that elemental constituents of PM may in part account for the seasonal variations in PM-induced adverse health effects related to lung inflammation.
Keywords: air pollutant, interleukin-6, interleukin-8, reactive oxygen species
Particulate pollution has been linked to increased rates of morbidity and mortality from cardiopulmonary diseases in both developed and developing countries (Borja-Aburto et al. 1998; Dockery et al. 1993; Michelozzi et al. 1998; Pope et al. 2002; Samet et al. 2000; Schwartz et al. 2002; Thurston et al. 1994). Although the association between particulate matter (PM) exposure and adverse health effects is generally positive, the magnitude of associations among different geographic locations varies (Samet et al. 2000). Such variability has been attributed partly to the different composition of PM in different geographic locations (Artinano et al. 2003; Claiborn et al. 2000; Roemer et al. 2000; Roosli et al. 2000; Vega et al. 2002).
PM from different seasons also may have different toxicity profiles. One study examining two major metropolitan areas in the United States showed that the associations of air pollution with mortality had considerable heterogeneity with seasonal variability (Moolgavkar 2003). Another study in Germany found a greater decrease in peak flow rate in patients with chronic bronchitis, emphysema, and asthma for May and June compared with December (Franke et al. 1992). Particle-induced increases in mortality are greater in the summer months than in the winter months (Goldberg et al. 2003). A single-city analysis showed that the PM effect was the highest in spring and summer when the anthropogenic concentration of coarse PM is the lowest (Smith et al. 2000). One hypothesis for such variable associations is that particles from different seasons consist of different proportion of specific size fractions and therefore have different toxicity profiles. Epidemiologic studies have indicated that fine particles may be more toxic because these particles are derived from industrial, domestic heating, and traffic-related emission sources and contain abundant transition metals (Anderson et al. 2001; Klemm et al. 2000; Schwartz et al. 2002; von Klot et al. 2002). On the other hand, ultrafine particles also have been suggested to be more toxic because of the large surface area available for biologic interactions with lung cells (Utell and Frampton 2000).
Alternatively, the composition of PM in different seasons may account for the different toxicity (Clarke et al. 1999; Huang et al. 2003a; Obot et al. 2002). During the disastrous London smog episodes in December of 1952, smoke and sulfur dioxide levels were 10 times higher than those of other months of the same year (Lioy and Zhang 1999). Although the high mortality had been linked to the high pollutant mass, the composition of the particles of that month was likely vastly different because there was increased combustion of bituminous coal and local atmospheric stagnation. In modern days, ambient particles do not reach such a high level, but the composition of particles in different seasons can still vary many-fold as the contributions from the natural and anthropogenic sources change from season to season (Lioy and Zhang 1999).
In this study, we hypothesized that differences in the elemental constituents of PM may account partly for the variable health effects induced by PM from different seasons. We exposed human alveolar macrophages (AMs) and normal human bronchial epithelial (NHBE) cells to the same mass of PM of different size fractions collected in different seasons in Chapel Hill, North Carolina. The release of interleukin (IL)-6/IL-8 and the production of reactive oxygen species (ROSs) were assessed. We used principal-component analysis in conjunction with multiple linear regression analysis to determine the contribution of particle components to changes in these end points.
Materials and Methods
Particle collection and extraction.
Particles were collected monthly from October 2001 to September 2002 in Chapel Hill, North Carolina, outside the U.S. Environmental Protection Agency (EPA) Human Studies Facility using a ChemVol model 2400 high-volume cascade impactor (Rupprecht & Patashnick Co., Albany, NY) (Figure 1). Coarse PM [PM 2.5–10 μm (PM2.5–10)] and fine PM [PM < 2.5 μm (PM2.5)] particles were collected for 1 week onto polyurethane foam (PUF; McMaster-Carr, Atlanta, GA), which was previously cleaned with methanol and water and dried under sterile conditions. Ultrafine particles [PM < 0.1 μm (PM<0.1)] were collected onto G5300 filters (Monandock Non-Wovens LLC, Mt. Pocono, PA).
Figure 1.
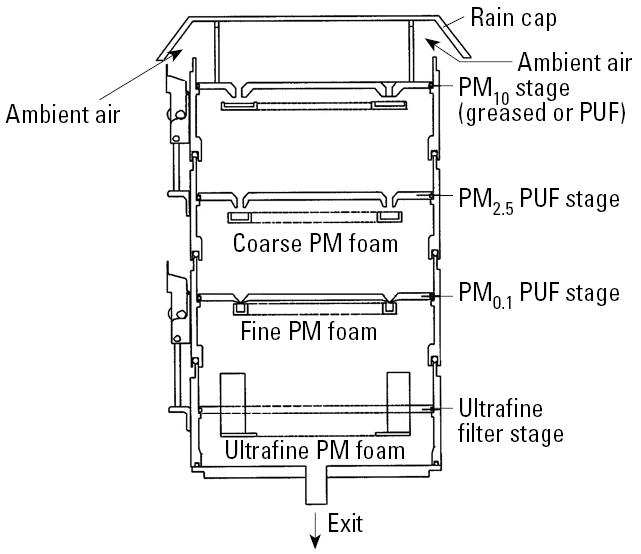
A schematic for the ChemVol model 2400 high-volume cascade impactor. The ambient air sample stream enters the sampler from the top between the rain cap and the PM10 stage and exits from the bottom. The cascade impactor contains a PM10 stage for collecting particles > 10 μm in diameter, a PM2.5 stage for collecting coarse PM (PM2.5–10), a PM0.1 stage for collecting fine PM (PM0.1–2.5), and an ultrafine filter for collecting ultrafine PM (PM< 0.1). The figure has been modified from the original schematic provided by Thermo Electron Corporation (R&P Products) with permission.
The foam or filter was prewetted with a small amount of 70% ethanol, and endotoxin-free water was added to a total volume of 40 mL. The particles were removed from the foam or filter by sonication for 1 hr in a water bath (FS220; Fisher Scientific, Pittsburgh, PA). The foam was removed, and particles were then lyophilized.
Analysis of particle constituents.
Five milligrams of particles of different size fractions were mixed with 15 mL sterile water to produce particle solutions at 0.33 mg/mL. The constituent elements in these particle solutions for each fraction of PM from each month were analyzed by Research Triangle Institute (Research Triangle Park, NC) using inductively coupled plasma mass spectroscopy and inductively coupled plasma atomic emission spectroscopy and are expressed as nanograms per milliter. The detection limits (nanograms per milliter) for each element are as follows: aluminum, 10; arsenic, 0.1; chromium, 0.5; copper, 1.0; iron, 10; lead, 0.2; nickel, 0.05; selenium, 0.8; silicon, 20; titanium, 0.5; vanadium, 0.5; zinc, 0.8. The elemental constituents of the particles were expressed relative to the particle mass in ng/mg of particle.
Culture of NHBE cells and AMs.
We obtained NHBE cells and AMs from normal individuals through bronchial brushings and cultured them as described previously (Ghio et al. 2000; Huang et al. 2003a). Subjects were informed of the procedures and potential risks, and each signed an informed consent. The protocol was approved by the University of North Carolina School of Medicine Committee on Protection of the Rights of Human Subjects and by the U.S. EPA. Bronchoscopic brushings were performed following the same operation guidelines. NHBE cells adherent to the brushes were treated for 15 min at room temperature with 1 mM dithiothreitol (Sigma, St. Louis, MO) in bronchial epithelial cell growth medium (BEGM; Clonetics, San Diego CA) supplemented with bovine pituitary extract, insulin 5 μg/mL, hydrocortisone 0.5 μg/mL, gentamicin 50 μg/mL, retinoic acid 0.1 ng/mL, transferrin 10 μg/mL, triiodothyrodine 6.5 ng/mL, epinephrine 0.5 μg/mL, and human epidermal growth factor 0.5 ng/mL, and dislodged from the brushes by pipetting. The cells were then plated at a density of 1 × 105 viable cells/cm2 in 12-mm cluster plates that were precoated with 50 mg/mL human placental collagen type IV (Sigma) and used after passage 2 or 3 when the cells were judged to be 75–95% confluent. Typically at confluency, NHBE cells in the 12-well plate (catalog no. 3512; Costar, Corning, NY) had a density of 300,000–500,000 cells/well in 2 mL of BEGM. NHBE cells were then exposed to equal concentrations of coarse and fine PM in BEGM for 18–24 hr. Supernatants were collected, centrifuged at 1,200 rpm for 10 min to pellet any nonadherent cells, and then transferred to cryovials for storage at –80°C until analyzed.
For AMs, bronchoalveolar lavage (BAL) was performed in the right middle lobe and lingular segment of the left upper lobe. BAL samples were put on ice immediately and centrifuged at 300 × g for 10 min at 4°C. The lavaged cells were washed once with ice-cold RPMI-1640 medium with 20 mg/mL gentamicin (Life Technologies, Rockville, MD). Cell counts were performed using a hemacytometer. Cytocentrifuge slides were prepared and stained with Diff Quick (Leukostat Solution; Fisher Scientific) to check for AM purity. The cell preparation consisted of 85–95% AMs. The viability of AMs was determined by try-pan blue exclusion. Viability exceeded 85% in all samples. AMs at 2–3 × 105 cells in 1 mL of RPMI-1640 supplemented with 2.5% fetal bovine serum (Life Technologies) were exposed to PM for 18 hr in 5-mL polypropylene tubes. Supernatants were collected and stored frozen until assayed for cytokines.
ELISA assays.
We measured IL-6 and IL-8 in the medium by ELISA kits (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s recommendations.
Detection of production of ROSs.
Dihydrorhodamine 123 (DHR) at 5 μM (Molecular Probes, Eugene, OR; catalog no. D-632) or 2′,7′- dichlorofluorescein diacetate (DCF) at 20 μM (Molecular Probes; catalog no. D-399) was preloaded into cells at 37°C for 20 min. Cell suspension (100 μL) was pipetted into individual wells of a 96-well plate (catalog no. 3610; Costar) in triplicate. The signals were read every 5 min for 1 hr using a Bioassay HTS7000 plate reader (Perkin Elmer, Inc., Wellesley, MA) with HTSoft software (version 1.0; PE Applied Biosystems, Weiterstadt, Germany). The excitation and emission wave lengths were 485 nm and 595 nm, respectively, for DHR, and 485 nm and 535 nm, respectively, for DCF. Generation of ROSs over 1 hr was calculated by subtracting the optical density at baseline from that at 1 hr. Data are expressed as fold change over control cells. DCF measures primarily intracellular hydrogen peroxide (Yuan et al. 1993) and nitric oxide (Rao et al. 1992). DHR is the uncharged and nonfluorescent reduction product of the mitochondrion-selective dye rhodamine 123. The dye passively diffuses across the cell membranes, where it is oxidized to cationic rhodamine 123, which localizes in the mitochondria. DHR reacts with hydrogen peroxide in the presence of peroxidase, cytochrome c, or Fe2+ (Royall and Ischiropoulos 1993), and peroxynitrite (Kooy et al. 1994).
Chemiluminescence.
AMs and NHBE cells were pipetted in triplicate into 96-well plates (catalog no. 3610; Costar). Particles were then added at desired concentrations followed by the addition of 200 μL luminol reagent (0.4 mM in Gey’s balanced salt solution with no bicarbonate). The plate was read using a Tropix TR717 microplate luminometer (PE Applied Biosystems) and WinGlow (version 1.24; Berthold Technologies GmbH & Co. KG, Bad Wildbad, German) software package for 1–2 hr. Luminol can detect peroxidase- or metal-mediated oxidative events.
Statistical analysis.
We compared data from different months using one-way analysis of variance (ANOVA) followed by the Tukey’s multiple comparison post-test. This was performed using Statview (version 5.0.1; SAS Institute Inc., Cary, NC).
We used exploratory principal-component analysis to examine how components of PM vary together (Stevens 2002). Although the sample size available for analysis was small, the results of Preacher and MacCallum (2002) suggest that good factor recovery can be achieved with very small sample sizes. Data from all 4 months and all three particle fractions were included. The number of factors retained was determined using the Kaiser’s stopping rule and scree plots. For interpretation, principal components were rotated using the varimax rotation method, an orthogonal rotation procedure. We interpreted the individual principal components using factor loadings (i.e., the Pearson correlation between each variable and the factor). Variables with a factor loading score > 0.6 were used to assist in interpretation of rotated factors. Factor scores obtained from this analysis were used in linear regression analyses to determine the association between factors and cytokine and ROS measurements.
Forward stepwise linear regression analysis (with entry criteria of p < 0.10) was also performed to determine the association between individual particle elements (independent variables) and the end points that were associated with the factors identified above (dependent variables). Only linear terms of the independent variables were considered. Both R2 values and p-values indicating overall model significance are reported. The general goal of this analysis was to identify independent variables showing significant association with the dependent variable.
Results
Elemental composition of PM fractions.
The months of October 2001, January 2002, April 2002, and July 2002 were selected to represent fall, winter, spring, and summer, respectively. The elemental compositions of PM of different size fractions from these four months are shown in Table 1. The coarse fraction contained more Al, Fe, Si, Cu, and Ti than did other fractions, whereas the ultrafine fraction contained more As, Pb, and Zn. There was no correlation between season and PM elements. Table 2 shows the correlations between each pair of elemental components of PM.
Table 1.
Elemental constituents of coarse (C), fine (F), and ultrafine (UF) fractions of PM (ng/mg).
| Particles (ng/mg)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| October 2001
|
January 2002
|
April 2002
|
July 2002
|
||||||||||
| Element | C | F | UF | C | F | UF | C | F | UF | C | F | UF | ROFA |
| Al | 5700.0 | 1665.0 | 1677.0 | 14430.0 | 891.0 | 714.0 | 5850.0 | 930.0 | 1224.0 | 2610.0 | 1380.0 | 948.0 | 9400.0 |
| As | 7.2 | 59.1 | 111.0 | 14.4 | 76.5 | 125.1 | 10.2 | 44.7 | 75.9 | 9.0 | 29.1 | 58.2 | — |
| Cr | 16.5 | 10.8 | 12.9 | 31.5 | 9.9 | 10.2 | 15.9 | 10.2 | 14.4 | 18.3 | 6.0 | 10.8 | — |
| Cu | 194.7 | 146.4 | 192.0 | 197.1 | 102.3 | 112.5 | 230.1 | 77.7 | 94.2 | 241.5 | 80.7 | 150.0 | — |
| Fe | 9060.0 | 3396.0 | 834.0 | 14430.0 | 2064.0 | 717.0 | 8190.0 | 1713.0 | 981.0 | 10620.0 | 1590.0 | 879.0 | 51200.0 |
| Pb | 32.1 | 138.6 | 136.5 | 70.2 | 224.1 | 236.7 | 39.6 | 133.2 | 181.2 | 59.1 | 103.5 | 181.2 | 6700.0 |
| Ni | 17.7 | 7.8 | 32.7 | 30.3 | 27.6 | 29.4 | 22.8 | 26.7 | 30.9 | 17.4 | 11.4 | 36.3 | 36600.0 |
| Se | 8.7 | 63.6 | 72.6 | 11.1 | 105.0 | 149.4 | 17.1 | 70.8 | 62.4 | 19.8 | 55.2 | 51.3 | — |
| Si | 4980.0 | 1206.0 | 840.0 | 14370.0 | 915.0 | 849.0 | 6450.0 | 729.0 | 924.0 | 9360.0 | 1602.0 | 1098.0 | 26100.0 |
| Ti | 122.1 | 34.2 | 7.5 | 276.9 | 33.6 | 14.1 | 116.4 | 21.0 | 18.9 | 164.1 | 29.4 | 16.5 | 1300.0 |
| V | 21.0 | 58.8 | 57.0 | 39.6 | 55.5 | 65.7 | 27.0 | 66.0 | 54.6 | 24.3 | 23.1 | 27.9 | 58200.0 |
| Zn | 348.0 | 933.0 | 1731.0 | 714.0 | 1116.0 | 2532.0 | 522.0 | 663.0 | NA | 381.0 | 387.0 | 669.0 | — |
Abbreviations: —, no data; NA, not applicable. The constituents of ROFA were from Kadiiska et al. (1997).
Table 2.
Correlation coefficients for each pair of elemental components in all three size fractions of PM.
| Al | As | Cr | Cu | Fe | Pb | Ni | Se | Si | Ti | V | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1.000 | 0.661 | 0.951 | 0.705 | 0.978 | 0.705 | 0.142 | 0.726 | 0.992 | 0.992 | 0.457 | 0.402 |
| As | 0.661 | 1.000 | 0.468 | 0.424 | 0.742 | 0.851 | 0.570 | 0.892 | 0.654 | 0.679 | 0.748 | 0.922 |
| Cr | 0.951 | 0.468 | 1.000 | 0.659 | 0.900 | 0.535 | 0.144 | 0.600 | 0.936 | 0.942 | 0.249 | 0.219 |
| Cu | 0.705 | 0.424 | 0.659 | 1.000 | 0.714 | 0.659 | 0.023 | 0.669 | 0.672 | 0.637 | 0.493 | 0.245 |
| Fe | 0.978 | 0.742 | 0.900 | 0.714 | 1.000 | 0.754 | 0.255 | 0.762 | 0.963 | 0.979 | 0.497 | 0.245 |
| Pb | 0.705 | 0.851 | 0.535 | 0.659 | 0.754 | 1.000 | 0.541 | 0.919 | 0.657 | 0.663 | 0.675 | 0.714 |
| Ni | 0.142 | 0.570 | 0.144 | 0.023 | 0.255 | 0.541 | 1.000 | 0.340 | 0.136 | 0.159 | 0.493 | 0.516 |
| Se | 0.726 | 0.892 | 0.600 | 0.669 | 0.762 | 0.919 | 0.340 | 1.000 | 0.689 | 0.698 | 0.755 | 0.833 |
| Si | 0.992 | 0.654 | 0.936 | 0.672 | 0.963 | 0.657 | 0.136 | 0.689 | 1.000 | 0.990 | 0.455 | 0.394 |
| Ti | 0.992 | 0.679 | 0.942 | 0.637 | 0.979 | 0.663 | 0.159 | 0.698 | 0.990 | 1.000 | 0.445 | 0.421 |
| V | 0.457 | 0.748 | 0.249 | 0.493 | 0.497 | 0.675 | 0.493 | 0.755 | 0.455 | 0.445 | 1.000 | 0.719 |
| Zn | 0.402 | 0.922 | 0.219 | 0.245 | 0.485 | 0.714 | 0.516 | 0.833 | 0.394 | 0.421 | 0.719 | 1.000 |
Cytokine induction by PM.
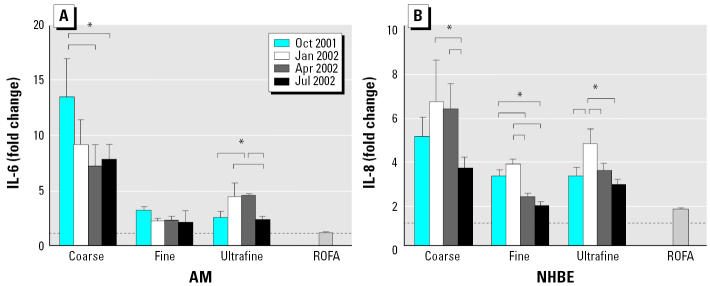
In general, coarse PM induced a greater increase in IL-6 release compared with fine and ultrafine PM in AMs (Figure 2). Coarse PM also stimulated a greater increase in the release of IL-8 than fine and ultrafine PM in NHBE cells (Figure 2).
Figure 2.
Production of (A) IL-6 by AM and (B) IL-8 by NHBE cells stimulated with coarse, fine, and ultrafine Chapel Hill pollution particles collected in four different months. The control IL-8 and IL-6 concentrations were 1.7 ± 0.2 ng/mL and 198 ± 5 pg/mL, respectively. Particles were added to NHBE cells at 11 μg/mL and to AMs at 50 μg/mL.
*Tukey adjusted p-value < 0.05; n = 3–4 each. The dashed line denotes 1.0 (no change over control). The brackets indicate the groups that are significantly different by ANOVA and the Tukey subtest.
The potency for PM from different seasons to induce these inflammatory mediators, however, varied. For coarse particles, the October PM were the most potent in inducing IL-6 in AMs, whereas for ultrafine particles, the ultrafine PM for January and April were more potent than particles from the other two months. In NHBE cells, the January PM consistently had stronger effects on IL-8 release than did PM from other months for all three size fractions. The only exception was the April coarse PM, which was as potent as the January coarse PM. The differences in potency were as much as several-fold.
ROS production stimulated by PM.
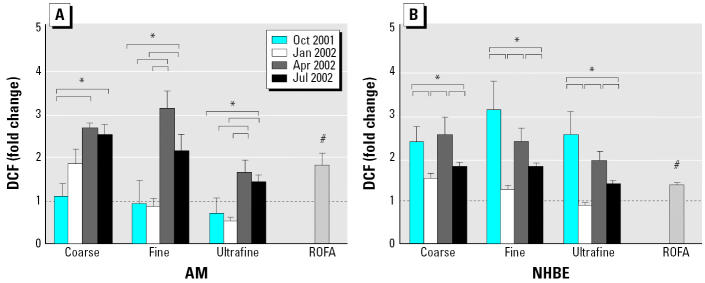
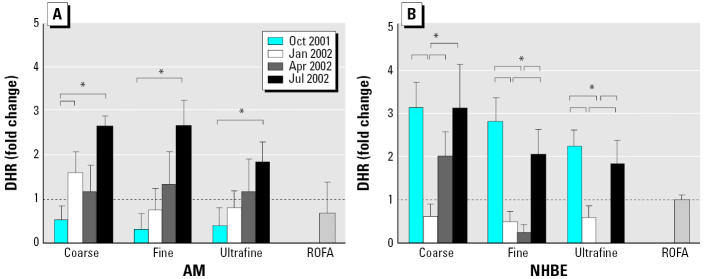
ROS production was assessed by three different probes: DCF, DHR, and chemiluminescence. The April and July PM were more potent in increasing DCF signals than was PM from other months regardless of their size fractions in AMs, whereas the October and April PM were more potent in NHBE cells (Figure 3). For DHR signals, the July PM was consistently the most potent stimulator in AMs, whereas the October and July PM were more potent in NHBE cells (Figure 4). The April coarse PM also increased DHR signals by 2-fold. The differences in potency to stimulate ROS detectable by DCF and DHR were as much as several-fold. For chemiluminescence, only coarse PM (1.2- to 1.7-fold of control) and residual oil fly ash (ROFA; 1.8-fold of control) had mild stimulatory effects in AMs (Huang et al. 2003b). There were no increases in chemiluminescence in NHBE cells by any of the particles.
Figure 3.
Production of ROSs measured by DCF in (A) AM and (B) NHBE cells stimulated with coarse, fine, and ultrafine Chapel Hill pollution particles collected in four different months. Particles were added to NHBE cells at 11 μg/mL and to AMs at 50 μg/mL. In general, NHBE cells appeared to be more responsive to PM than the AMs because increases in DCF signals were similar between the two cell types even though NHBE cells were exposed to lower doses of PM.
*Tukey adjusted p-value < 0.05; #p < 0.05 versus control (ratio = 1.0) by the one-group Student t-test; n = 3–4 each. The dashed line denotes 1.0 (no change over control). The brackets indicate groups that are significantly different by the Tukey subtest.
Figure 4.
Production of ROSs measured by DHR in (A) AM and (B) NHBE cells stimulated with coarse, fine, and ultrafine Chapel Hill pollution particles collected in four different months. Particles were added to NHBE cells at 11 μg/mL and to AMs at 50 μg/mL. In general, NHBE cells appeared more responsive to PM than were AMs because increases in DHR signals were similar between the two cell types even though NHBE cells were exposed to lower doses of PM.
*Tukey adjusted p-value < 0.05; n = 3–4 each. The dashed line denotes 1.0 (no change over control). The brackets indicate groups that are significantly different by ANOVA and the Tukey subtest.
Association of source factors with cytokine and ROS end points.
We determined whether or not differences in particle constituents may account for the variable potency for PM to induce cytokine and ROS production. Using principal-component analysis, we identified two factors in PM elements. The first factor consisted of Cr (factor load = 0.99), Al (factor load = 0.96), Si (factor load = 0.94), Ti (factor load = 0.94), Fe (factor load = 0.91), and Cu (factor load = 0.74) as the significant loading variables. The second factor consisted of Zn (factor load = 0.90), As (factor load = 0.85), V (factor load = 0.80), Ni (factor load = 0.78), Pb (factor load = 0.75), and Se (factor load = 0.73) as the significant loading variables. The factor regression analyses indicated that only factor 1 was associated with the release of IL-8 (adjusted R2 = 0.548, p = 0.017) and IL-6 (adjusted R2 = 0.532, p = 0.020). There was a trend for factor 2 in association with ROS end points, but the associations were not statistically significant.
Association of individual PM elements with cytokine release.
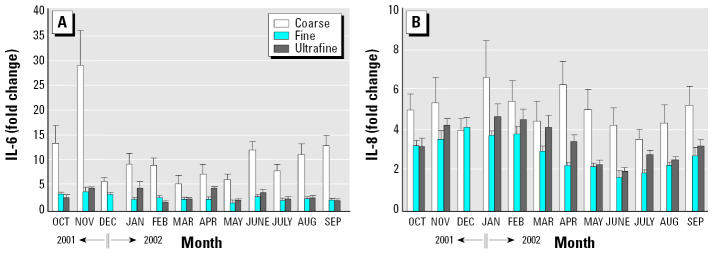
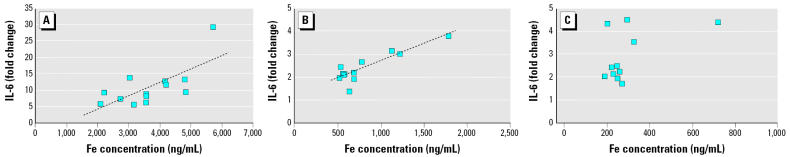
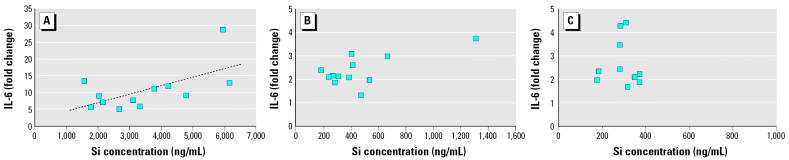
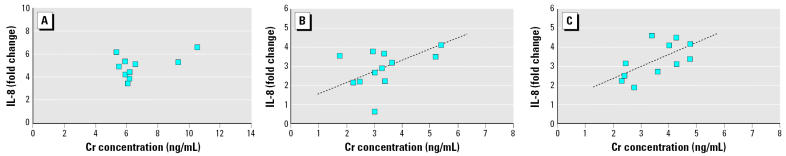
We also performed forward stepwise linear regression analysis using the individual PM elements to determine which element in factor 1 was associated with the release of IL-6 and IL-8. For IL-6, Fe and Si had positive association (model R 2 = 0.625, p = 0.001). For IL-8, Cr had a positive association (model R 2 = 0.638, p = 0.006). Because the above analyses combined all data from PM of different-size fractions, we further determined whether the association between these elements with IL-6 and IL-8 would be retained within PM of the same size fraction. We analyzed the release of IL-6 and IL-8 from AMs and NHBE cells incubated with PM from all 12 months, respectively. Again, coarse particles in general were more potent than fine and ultrafine particles regardless of the month, and the potency for PM to induce IL-6/IL-8 production varied significantly (Figure 5). Fe and Si were positively associated with IL-6 release in AMs incubated with the coarse but not the fine or ultrafine fraction (Figures 6, 7). Cr was positively associated with IL-8 release in NHBE cells incubated with fine and ultrafine fractions but not the coarse fraction (Figure 8).
Figure 5.
Effects of PM from 12 different months on the release of (A) IL-6 in AMs and (B) IL-8 in NHBE cells. Particles were added to AMs at 50 μg/mL and to NHBE cells at 11 μg/mL. The data for December ultrafine PM were not available and were omitted in the figure; n = 3–4 each.
Figure 6.
Correlations between Fe and IL-6 release in AMs incubated with (A) coarse, (B) fine, and (C) ultrafine PM from 12 months. The dashed line represents the linear regression line. The overall model p-values for coarse, fine, and ultrafine PM were 0.012, 0.0002, and 0.103, respectively. R2 = 0.4862 and 0.7704 for coarse and fine particles, respectively.
Figure 7.
Association between Si and IL-6 release in AMs incubated with (A) coarse, (B) fine, and (C) ultrafine PM from 12 months. The dashed line represents the linear regression line. The overall model p-values for coarse, fine, and ultrafine PM were 0.034, 0.016 (0.507 if the data with the largest Si concentration are excluded), and 0.182, respectively. R2 = 0.3747 for coarse PM.
Figure 8.
Correlations between Cr and IL-8 release in NHBE cells incubated with (A) coarse, (B) fine, and (C) ultrafine PM from 12 months. The dashed line represents the linear regression line. The overall model p-values for coarse, fine, and ultrafine PM were 0.106, 0.003, and 0.036, respectively. R2 = 0.6082 and 0.401 for fine and ultrafine PM, respectively.
Discussion
Our study incubated human AMs and NHBE cells with the same mass of PM of different size fractions collected from different seasons in an attempt to address the mechanism of seasonal variations in the PM-associated health effects. We confirmed that PM of all size fractions induced the release of IL-6 and IL-8 from AMs and NHBE cells, respectively (Alfaro-Moreno et al. 2002; Becker et al. 2003; Steerenberg et al. 1998; Tao et al. 2003; Tao and Kobzik 2002), and the coarse fraction was in general more potent than the fine and ultrafine fractions regardless of the month (Becker et al. 2003). For the production of ROSs, however, all three fractions of PM had similar potency when PM from the same month was compared. These results indicate that PM containing more particles in the coarse fraction may have greater proinflammatory potential. ROFA, which is a prototypical particle with high metal content, was in general less potent in stimulating the inflammatory mediators and ROS production than was the Chapel Hill PM. The smaller effects of ROFA on DCF and DHR may reflect the inability of these dyes to detect some of the ROSs stimulated by ROFA (e.g., hydroxyl radical-like species). Previous studies have shown that oil fly ashes with high metal content produce oxidation of DNA characteristic of hydroxyl radical formation (Prahalad et al. 2000, 2001). The smaller effects on the production of IL-6 and IL-8 by ROFA indicate that other nonmetal components of particles may be required for enhancing the effects. One such component is endotoxin, which is more abundant in PM with aerodynamic diameter of 10 μm (PM10) (Becker et al. 1996, 2003; Soukup and Becker 2001).
When PM from different months were compared within their size fractions, the effects on IL-6, IL-8, and ROS production show significant heterogeneity, and the differences between the most and the least potent were several-fold. For example, the October coarse PM was twice as potent as was the April coarse PM for IL-6 release in AMs, and the January coarse PM was twice as potent as the July coarse PM for IL-8 release in NHBE cells. For DCF, the April fine PM was three times as potent as the January fine PM in AMs, whereas the October ultrafine PM was two and a half times as potent as the January ultrafine PM in NHBE cells. On the other hand, the July PM regardless of the size fraction was two to three times as potent as the October PM in increasing the DHR signal in AMs, whereas the October and July PM were at least three times as potent as the January PM in NHBE cells. DCF measures intracellular oxidants (e.g., H2O2 and NO), whereas DHR measures reactive species (e.g., H2O2 and peroxynitrite) produced in the mitochondria. Thus, the different patterns of DCF and DHR induced by the particles indicate that PM is capable of enhancing the production of ROSs from different enzymatic sources in different subcellular compartments. These results also raise the hypothesis that ROSs produced from different sources may be associated with different PM-induced health effects.
Several previous studies have shown that the association of PM with mortality was stronger for the warmer months. A meta-analysis of time-series studies of air pollution and mortality showed that the effect size for PM10-associated respiratory mortality was greater for summer months (Stieb et al. 2002). Another study examining the daily mortality from congestive heart failure in Canada showed stronger association with PM from the warmer season (Goldberg et al. 2003). One other study analyzed air pollution and daily mortality in two U.S. counties and showed that the exposure-response relationship was stronger for the warmer season (Moolgavkar 2003). In our study, PM from the warmest month (July) was consistently a potent inducer for ROS production in both cell types. Although these results tend to support the observations in the above epidemiologic studies, it should be noted that in our study we investigated PM collected only over a 1-year cycle. Whether or not these results can be generalized will require studies using PM from multiple years. If PM from the warmer months from multiple years does have similar potency, it would raise the hypothesis that the capacity for PM to induce oxidative stress may be an important determinant for PM-induced mortality.
In contrast, the cytokine-inducing capability of PM did not show seasonal predilection. Instead, the size of the PM seems to determine the potential for cytokine induction; that is, the coarse PM is in general a more potent inducer of IL-6 and IL-8 than is the fine or the ultrafine PM. PM < 10 μm in diameter is more likely to enter the lung during inhalation. In a resting person breathing nasally, up to 20% of the inhaled coarse PM (PM2.5–10) may be deposited in the distal lung (Raabe 1999). This fraction can increase to 60% if the person is breathing orally. Our in vitro findings provided a mechanistic support for previous epidemiologic studies in which exposure to PM10 is linked to increased respiratory illnesses (Schwartz 1996). PM containing more of the coarse fraction may produce more intense pulmonary inflammation.
The heterogeneous effects of PM from different months within the size fraction on cytokine release and ROS production also suggest that factors other than particle size may be important. In our study, we hypothesized that particle components may contribute to this heterogeneity. We correlated the elemental components of PM with the cytokine and ROS end points using the principal-component analysis. We found that a factor consisting of Cr/Al/Si/Ti/Fe/Cu was associated with IL-6 and IL-8 release. Further analyses of our data showed that of the elements in this factor, Fe and Si correlated positively with IL-6 release whereas Cr correlated with IL-8 release. These positive associations were largely confirmed when data from additional experiments using PM from all 12 months were analyzed (Figures 6–8). A factor containing Fe was associated with pulmonary neutrophilic inflammation in our previous study that exposed normal human volunteers to Chapel Hill concentrated ambient particles (Huang et al. 2003a). The fine crustal factor (Si), however, was not associated with increased daily mortality in a study of six U.S. cities (Laden et al. 2000).
Another factor (Zn/As/V/Ni/Pb/Se) was also identified. Our factor regression analysis, however, showed only a trend toward associations between this factor and ROS production. Elements in this factor derive primarily from various burning processes (coal, oil) (Nriagu and Pacyna 1988). A trend toward increased daily mortality has been linked to vanadium in some cities such as Boston and Steubenville, Ohio (Laden et al. 2000). Note that PM from the warmest month (July) was the strongest stimulator for ROS production, and this coincides with the increased mortality in the warmer months observed in several epidemiologic studies (Goldberg et al. 2003; Smith et al. 2000). How the ROS-stimulating potential of PM may be linked mechanistically to the increased mortality is unclear, but it seems that the contribution of factors other than the elemental composition would need to be considered.
One such factor may be the content of organic compounds, which may vary in PM from different seasons. Ethanol used in our study to extract PM would have removed most organic compounds. The potency for the ethanol-extracted PM of all three size fractions to stimulate IL-8 release from NHBE cells, however, did not change compared with PM extracted by water (data not shown). The surface area of PM may also be an important factor. Our experiment exposed the cells to the same mass of PM. The ultrafine fraction of PM would have the greatest surface area, but the ultrafine PM was not the most potent inducer for IL-6, IL-8, and ROS production in our study. The ultrafine PM may have conglomerated when added to the culture medium and thus decreased its effective surface area. More microbial products (e.g., endotoxin) are associated with coarse PM and may partially explain the greater effects on inflammatory mediators by the coarse PM (Becker et al. 1996, 2003; Soukup and Becker 2001).
The concentrations of particles to which NHBE cells and AMs were exposed were higher than the one-time exposure in most ambient settings in humans. This concentration, however, may be reached in highly polluted metropolitan areas (Calderon-Garciduenas et al. 2001a, 2001b) or during natural disasters, such as a forest fire (Tan et al. 2000). Under these conditions, PM concentrations may reach ≥ 50 μg/m3. If a person exercises at 30 L/min of ventilation for 1 hr under this condition, assuming the volume of epithelial lining fluid is 20–40 mL (Rennard et al. 1986), the concentration of PM to which AMs and bronchial epithelial cells are exposed in vivo may be as high as 50 μg/mL.
In summary, our study demonstrated that PM from different seasons had different potency for stimulating the production of proinflammatory mediators and ROSs in human AMs and NHBE cells. The potency for inducing the release of proinflammatory mediators may be related in part to the content of PM elements of crustal origin, but the mechanisms for the variable potency for stimulating ROS production remain unclear and likely involve factors in addition to elemental constituents. PM from different seasons and geographical locations contain different elemental compositions that are affected by changes in local environment and weather conditions. Our study identified several elemental biomarkers that may help improve the prediction of health risks associated with PM exposure regardless of where and when the exposure occurs. This hypothesis will need to be tested in future studies.
Footnotes
This report has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Alfaro-Moreno E, Martinez L, Garcia-Cuellar C, Bonner JC, Murray JC, Rosas I, et al. Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ Health Perspect. 2002;110:715–720. doi: 10.1289/ehp.02110715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HR, Bremner SA, Atkinson RW, Harrison RM, Walters S. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom: associations with fine and coarse particles, black smoke and sulphate. Occup Environ Med. 2001;58:504–510. doi: 10.1136/oem.58.8.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artinano B, Salvador P, Alonso DG, Querol X, Alastuey A. Anthropogenic and natural influence on the PM(10) and PM(2.5) aerosol in Madrid (Spain). Analysis of high concentration episodes. Environ Pollut. 2003;125:453–465. doi: 10.1016/s0269-7491(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Gilmour MI, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–648. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Sioutas C, Cassee FR. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Exp Lung Res. 2003;29:29–44. doi: 10.1080/01902140303762. [DOI] [PubMed] [Google Scholar]
- Borja-Aburto VH, Castillejos M, Gold DR, Bierzwinski S, Loomis D. Mortality and ambient fine particles in southwest Mexico City, 1993–1995. Environ Health Perspect. 1998;106:849–855. doi: 10.1289/ehp.106-1533229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Gambling TM, Acuna H, Garcia R, Osnaya N, Monroy S, et al. Canines as sentinel species for assessing chronic exposures to air pollutants: part 2. Cardiac pathology. Toxicol Sci. 2001a;61:356–367. doi: 10.1093/toxsci/61.2.356. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Fordham LA, Chung CJ, Garcia R, Osnaya N, et al. Canines as sentinel species for assessing chronic exposures to air pollutants: part 1. Respiratory pathology. Toxicol Sci. 2001b;61:342–355. doi: 10.1093/toxsci/61.2.342. [DOI] [PubMed] [Google Scholar]
- Claiborn CS, Finn D, Larson TV, Koenig JQ. Windblown dust contributes to high PM2.5 concentrations. J Air Waste Manag Assoc. 2000;50:1440–1445. doi: 10.1080/10473289.2000.10464179. [DOI] [PubMed] [Google Scholar]
- Clarke RW, Catalano PJ, Koutrakis P, Murthy GG, Sioutas C, Paulauskis J, et al. Urban air particulate inhalation alters pulmonary function and induces pulmonary inflammation in a rodent model of chronic bronchitis. Inhal Toxicol. 1999;11:637–656. doi: 10.1080/089583799196781. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, III, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Franke K, Boeriu A, Degens P, Dresler R, Fritsch H, Haake D, et al. A 3-year cohort study on short-term effects of air pollution in Germany. 1. Influences of medication and season. Sci Total Environ. 1992;127:69–78. doi: 10.1016/0048-9697(92)90470-d. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med. 2000;162:981–988. doi: 10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Burnett RT, Valois MF, Flegel K, Bailar JC, III, Brook J, et al. Associations between ambient air pollution and daily mortality among persons with congestive heart failure. Environ Res. 2003;91:8–20. doi: 10.1016/s0013-9351(02)00022-1. [DOI] [PubMed] [Google Scholar]
- Huang YC, Ghio AJ, Stonehuerner J, McGee J, Carter JD, Grambow SC, et al. The role of soluble components in ambient fine particles-induced changes in human lungs and blood. Inhal Toxicol. 2003a;15:327–342. doi: 10.1080/08958370304460. [DOI] [PubMed] [Google Scholar]
- Huang YC, Soukup J, Harder S, Becker S. Mitochondrial oxidant production by a pollutant dust and NO-mediated apoptosis in human alveolar macrophage. Am J Physiol Cell Physiol. 2003b;284:C24–C32. doi: 10.1152/ajpcell.00139.2002. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Mason RP, Dreher KL, Costa DL, Ghio AJ. In vivo evidence of free radical formation in the rat lung after exposure to an emission source air pollution particle. Chem Res Toxicol. 1997;10:1104–1108. doi: 10.1021/tx970049r. [DOI] [PubMed] [Google Scholar]
- Klemm RJ, Mason RM, Jr, Heilig CM, Neas LM, Dockery DW. Is daily mortality associated specifically with fine particles? Data reconstruction and replication of analyses. J Air Waste Manag Assoc. 2000;50:1215–1222. doi: 10.1080/10473289.2000.10464149. [DOI] [PubMed] [Google Scholar]
- Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med. 1994;16:149–156. doi: 10.1016/0891-5849(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy PJ, Zhang J. 1999. Air pollution. In: Air Pollutants and the Respiratory Tract (Swift DL, Foster WM, eds). Vol 128. New York:Marcel Dekker, 1–38.
- Michelozzi P, Forastiere F, Fusco D, Perucci CA, Ostro B, Ancona C, et al. Air pollution and daily mortality in Rome, Italy. Occup Environ Med. 1998;55:605–610. doi: 10.1136/oem.55.9.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolgavkar SH. Air pollution and daily mortality in two U.S. counties: season-specific analyses and exposure-response relationships. Inhal Toxicol. 2003;15:877–907. doi: 10.1080/08958370390215767. [DOI] [PubMed] [Google Scholar]
- Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;333:134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- Obot CJ, Morandi MT, Beebe TP, Hamilton RF, Holian A. Surface components of airborne particulate matter induce macrophage apoptosis through scavenger receptors. Toxicol Appl Pharmacol. 2002;184:98–106. [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahalad AK, Inmon J, Dailey LA, Madden MC, Ghio AJ, Gallagher JE. Air pollution particles mediated oxidative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bioreactivity. Chem Res Toxicol. 2001;14:879–887. doi: 10.1021/tx010022e. [DOI] [PubMed] [Google Scholar]
- Prahalad AK, Inmon J, Ghio AJ, Gallagher JE. Enhancement of 2′-deoxyguanosine hydroxylation and DNA damage by coal and oil fly ash in relation to particulate metal content and availability. Chem Res Toxicol. 2000;13:1011–1019. doi: 10.1021/tx000110j. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, MacCallum RC. Exploratory factor analysis in behavior genetics research: factor recovery with small sample sizes. Behav Genet. 2002;32:153–161. doi: 10.1023/a:1015210025234. [DOI] [PubMed] [Google Scholar]
- Raabe OG. 1999. Respiratory exposure to air pollutants. In: Air Pollutants and the Respiratory Tract (Swift DL, Foster WM, eds). Vol 128. New York:Marcel Dekker, 39–73.
- Rao KM, Padmanabhan J, Kilby DL, Cohen HJ, Currie MS, Weinberg JB. Flow cytometric analysis of nitric oxide production in human neutrophils using dichlorofluorescein diacetate in the presence of a calmodulin inhibitor. J Leukoc Biol. 1992;51:496–500. doi: 10.1002/jlb.51.5.496. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Roemer W, Hoek G, Brunekreef B, Clench-Aas J, Forsberg B, Pekkanen J, et al. PM10 elemental composition and acute respiratory health effects in European children (PEACE project). Pollution Effects on Asthmatic Children in Europe. Eur Respir J. 2000;15:553–559. doi: 10.1034/j.1399-3003.2000.15.21.x. [DOI] [PubMed] [Google Scholar]
- Roosli M, Braun-Fahrlander C, Kunzli N, Oglesby L, Theis G, Camenzind M, et al. Spatial variability of different fractions of particulate matter within an urban environment and between urban and rural sites. J Air Waste Manag Assoc. 2000;50:1115–1124. doi: 10.1080/10473289.2000.10464161. [DOI] [PubMed] [Google Scholar]
- Royall JA, Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and hospital admissions for respiratory disease. Epidemiology. 1996;7:20–28. doi: 10.1097/00001648-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Laden F, Zanobetti A. The concentration-response relation between PM2.5 and daily deaths. Environ Health Perspect. 2002;110:1025–1029. doi: 10.1289/ehp.021101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Spitzner D, Kim Y, Fuentes M. Threshold dependence of mortality effects for fine and coarse particles in Phoenix, Arizona. J Air Waste Manag Assoc. 2000;50:1367–1379. doi: 10.1080/10473289.2000.10464172. [DOI] [PubMed] [Google Scholar]
- Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171:20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, et al. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp Lung Res. 1998;24:85–100. doi: 10.3109/01902149809046056. [DOI] [PubMed] [Google Scholar]
- Stevens JP. 2002. Applied Multivariate Statistics for the Social Sciences. Mahwah, NJ:Lawrence Erlbaum Associates.
- Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc. 2002;52:470–484. doi: 10.1080/10473289.2002.10470794. [DOI] [PubMed] [Google Scholar]
- Tan WC, Qiu D, Liam BL, Ng TP, Lee SH, van Eeden SF, et al. The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med. 2000;161:1213–1217. doi: 10.1164/ajrccm.161.4.9904084. [DOI] [PubMed] [Google Scholar]
- Tao F, Gonzalez-Flecha B, Kobzik L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic Biol Med. 2003;35:327–340. doi: 10.1016/s0891-5849(03)00280-6. [DOI] [PubMed] [Google Scholar]
- Tao F, Kobzik L. Lung macrophage-epithelial cell interactions amplify particle-mediated cytokine release. Am J Respir Cell Mol Biol. 2002;26:499–505. doi: 10.1165/ajrcmb.26.4.4749. [DOI] [PubMed] [Google Scholar]
- Thurston GD, Ito K, Hayes CG, Bates DV, Lippmann M. Respiratory hospital admissions and summertime haze air pollution in Toronto, Ontario: consideration of the role of acid aerosols. Environ Res. 1994;65:271–290. doi: 10.1006/enrs.1994.1037. [DOI] [PubMed] [Google Scholar]
- Utell MJ, Frampton MW. Acute health effects of ambient air pollution: the ultrafine particle hypothesis. J Aerosol Med. 2000;13:355–359. doi: 10.1089/jam.2000.13.355. [DOI] [PubMed] [Google Scholar]
- Vega E, Reyes E, Sanchez G, Ortiz E, Ruiz M, Chow J, et al. Basic statistics of PM2.5 and PM10 in the atmosphere of Mexico City. Sci Total Environ. 2002;287:167–176. doi: 10.1016/s0048-9697(01)00980-9. [DOI] [PubMed] [Google Scholar]
- von Klot S, Wolke G, Tuch T, Heinrich J, Dockery DW, Schwartz J, et al. Increased asthma medication use in association with ambient fine and ultrafine particles. Eur Respir J. 2002;20:691–702. doi: 10.1183/09031936.02.01402001. [DOI] [PubMed] [Google Scholar]
- Yuan L, Inoue S, Saito Y, Nakajima O. An evaluation of the effects of cytokines on intracellular oxidative production in normal neutrophils by flow cytometry. Exp Cell Res. 1993;209:375–381. doi: 10.1006/excr.1993.1323. [DOI] [PubMed] [Google Scholar]