Abstract
Mycoplasmal lipopeptides S-(2,3-bispalmitoyloxypropyl)-CGDPKHSPKSF and S-(2,3-bispalmitoyloxypropyl)-CGNNDESNISFKEK activated a monocytic cell line, THP-1 cells, to produce tumor necrosis factor alpha. The activity of the lipopeptides was augmented by ATP in a dose-dependent manner. In addition, the level of expression of mRNAs for tumor necrosis factor alpha and interleukin-1β, -6, and -8 was also upregulated by the lipopeptides and/or extracellular ATP, but that of interleukin-10 was not. The P2X purinergic receptor antagonists pyridoxal phosphate 6-azophenyl 2′,4′-disulfonic acid and periodate-oxidized ATP suppressed the activity of ATP to augment the activation of THP-1 cells by the lipopeptides, suggesting that P2X receptors play important roles in the activity of ATP. The nuclear factor κB inhibitor dexamethasone also suppressed the activity, suggesting that the activity of ATP is dependent upon the nuclear factor κB. Thus, these results suggest that the interaction of extracellular ATP with the P2X receptors is attributed to the activity of ATP to augment the activation of THP-1 cells by mycoplasmal lipopeptides.
Mycoplasmas are cell wall-less and the smallest self-replicating microorganisms. Although they do not possess bacterial modulins such as lipopolysaccharides (LPSs), lipoteichoic acids, or peptidoglycans, they are capable of activating macrophages/monocytes and fibroblasts (11, 39). Recently, the membrane-bound lipoproteins of mycoplasmas were found to play important roles in activation of monocytes/macrophages and fibroblasts by mycoplasmas (11, 18, 24, 30, 31, 40). Furthermore, the active site of mycoplasmal lipoproteins was identified as the N-terminal lipopeptide moieties (31, 40).
S-(2,3-Bispalmitoyloxypropyl)-CGNNDESNISFKEK (MALP-2) purified from Mycoplasma fermentans was first characterized as a macrophage-activating lipopeptide (31). We have also characterized S-(2,3-bispalmitoyloxypropyl)-CGDPKHSPKSF, called FSL-1, synthesized on the basis of the structure of a 44-kDa lipoprotein of Mycoplasma salivarium responsible for activation of human gingival fibroblasts (40). FSL-1 can activate monocytes/macrophages as well as human gingival fibroblasts (40). Thus, mycoplasmal lipopeptides possess several biological activities which are shared by LPS, although the activities of the lipopeptides are not inhibited by polymyxin B, which abrogates the activities of LPS (11).
Recently we found that signaling by FSL-1 was mediated by Toll-like receptor 2 (TLR2) (unpublished data), in accordance with previous data that TLR2 is a receptor for bacterial lipoproteins and MALP-2 (7, 17, 23, 44, 45). Members of the TLR family recently emerged as candidate receptors capable of transmitting signaling by pathogen-associated molecular patterns such as LPS, lipoteichoic acid, peptidoglycans, bacterial CpG DNA, and lipoproteins (2, 27). TLR4 has been well characterized as a receptor for LPS (5, 8, 46, 49). These findings also suggest that mycoplasmal lipopeptides activate mammalian cells by a mechanism different from that of LPS.
Several studies have also suggested that activation of macrophages by LPS is regulated by extracellular ATP and purinergic receptors, P2X7, and periodate-oxidized ATP (oATP) (4, 14, 19, 20, 28, 37). In addition, ATP-gated ionotropic (P2X) receptors, including the P2X7 receptor, were confirmed to be expressed on the cell surface of macrophages (21, 22, 35).
In this study, therefore, experiments were carried out to examine the effects of extracellular ATP on activation of monocytes/macrophages by mycoplasmal lipopeptides.
MATERIALS AND METHODS
Reagents.
ATP was obtained from Molecular Probes (Eugene, Oreg.). Pyridoxal phosphate 6-azophenyl 2′,4′-disulfonic acid (PPADS), oATP, and dexamethasone (DEX) were purchased from Sigma-Aldrich (St. Louis, Mo.). All other chemicals were obtained from commercial sources and were of analytical or reagent grade.
Synthesis of FSL-1 and MALP-2.
FSL-1 and MALP-2 were synthesized as follows. The side chain-protected peptide GDPKHPKSF or GNNDESNISFKEK was built up with an automated peptide synthesizer, model 433 (Applied Biosystems, Foster City, Calif.). 9-Fluorenylmethoxy carbonyl (Fmoc)-S-(2,3-bispalmitoyloxypropyl)-cysteine (Novabiochem, Laeufelfingen, Switzerland) was manually coupled to the peptide resin using a solvent system of 1-hydroxy-7-azabenzotriazole-1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide/CH2Cl2-DMF. The Fmoc and resin were removed from the lipopeptide by trifluoroacetic acid. The lipopeptides were purified by preparative high-pressure liquid chromatography (HPLC) with a reverse-phase C18 column (30 by 250 mm). The purity of FSL-1 and MALP-2 was confirmed by analytical HPLC with a reverse-phase C18 column (4.6 by 150 mm) to be 97 and 98%, respectively.
Determination of TNF-α in the culture supernatant.
A human acute monocytic leukemia cell line, THP-1 (47), was purchased from Health Science Research Resources Bank (Osaka, Japan). Cells were grown at 37°C in a humidified atmosphere of 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin G (100 U/ml), and streptomycin (100 μg/ml). A 1-ml volume of cell suspension (106 cells/ml) of THP-1 cells was seeded in each well of a 24-well tissue culture plate. The cells were stimulated at 37°C for 1 h with 10 nM FSL-1 or 50 nM MALP-2 or unstimulated and incubated at 37°C for 8 h after the addition of various concentrations of ATP. The amount of tumor necrosis factor alpha (TNF-α) in the culture supernatant was measured by an enzyme-linked immunosorbent assay (ELISA) using a human TNF-α Cytoset (Biosource, Camarillo, Calif.) according to the manufacturer's instructions.
Expression of mRNAs of cytokines and purinergic receptors in THP-1 cells by reverse transcription-coupled PCR.
Oligonucleotide primers for interleukin-1β (IL-1β), IL-6, IL-8, IL-10, TNF-α, and β-actin for PCR amplification were synthesized according to the sequences described previously (25). Primers specific for TLR2, TLR6, and the purinergic P2 receptors P2X1, P2X7, P2Y2, and P2Y11 were synthesized on the basis of the sequences described by Zhang et al. (49) and Adrian et al. (1), respectively. The specificity of these primers was confirmed by Southern hybridization with a probe coding for internal sequence.
THP-1 cells (3 × 106) in 3-ml volumes of RPMI 1640 medium were stimulated at 37°C for 1 h with 10 nM FSL-1 or unstimulated and then incubated at 37°C for various periods of time after the addition of 500 μM ATP. Total RNA from THP-1 cells was prepared by using an RNeasy kit (Qiagen Inc., Valencia, Calif.) according to the manufacturer's instructions. The quantity of mRNA was determined photometrically at 260 nm. RNA (approximately 0.8 μg) was reverse transcribed to cDNA in a 20-μl reaction volume containing a 1 μM concentration of each of the antisense primers using an RNA PCR kit (AMV version 2.1; Takara Biochemicals, Shiga, Japan) according to the manufacturer's instructions.
The PCRs were performed in 50-μl final volumes containing 10 μl of cDNA, 2.5 mM MgCl2, and 20 pmol of each sense primer. After initial denaturation at 94°C for 30 s, amplifications were carried out for 28 cycles as follows: denaturation at 94°C for 30 s, annealing at primer-specific temperatures (see below) for 30 s, and extension at 72°C for 90 s. After the final PCR cycle, extension was allowed to proceed at 72°C for 2 min. The annealing temperatures were 54°C for P2X7, 55°C for β-actin, TNF-α, IL-1β, IL-6, IL-8, IL-10, P2Y11, TLR2, and TLR6, 64°C for P2X1, and 67°C for P2Y2.
The PCR products were electrophoresed on a 2% gel of NuSieve 3:1 agarose in 0.5× Tris-borate-EDTA buffer containing 5 μg of ethidium bromide per ml. The stained PCR products were photographed under UV light. The net intensity of gene-specific PCR products was analyzed by using a Kodak IS 440 CF image analyzer (Kodak, Rochester, N.Y.).
Determination of ATP in the culture supernatant.
A 1-ml volume of cell suspensions (106 cells/ml) of THP-1 cells was seeded in each well of a 24-well tissue culture plate. Cells were stimulated at 37°C for 8 h with various concentrations of mycoplasmal lipopeptides. The amount of ATP in the culture supernatant was measured with an ATP determination kit (Molecular Probes) according to the manufacturer's instructions.
RESULTS
Augmentation of mycoplasmal lipopeptide-induced activation of THP-1 cells by ATP added extracellularly.
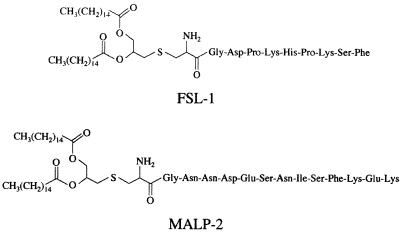
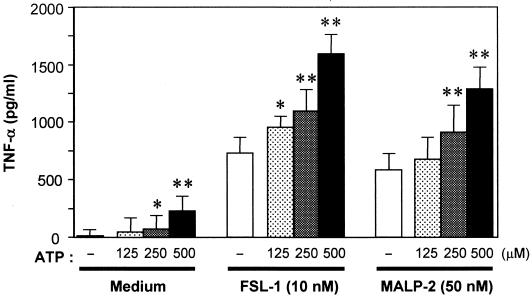
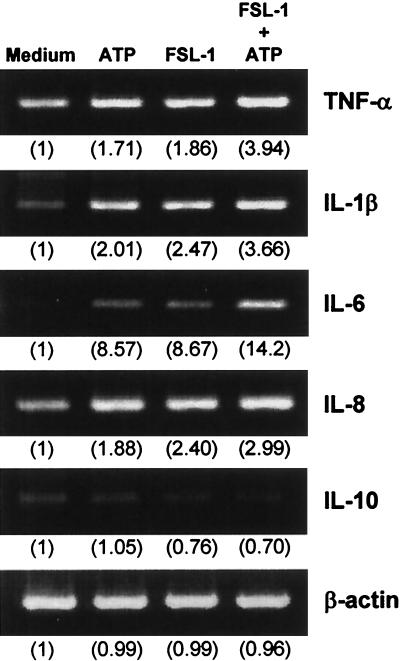
The synthetic mycoplasmal lipopeptides FSL-1 and MALP-2 (Fig. 1) are potent activators of monocytes/macrophages (18, 24, 31, 40). The differences in the structures of FSL-1 and MALP-2 are the amino acid sequence and length of the peptide portion (Fig. 1). An experiment was carried out to determine whether ATP added extracellularly promotes FSL-1- or MALP-2-induced activation of THP-1 cells, because activation of macrophages by LPS is upregulated by ATP (4, 14, 19, 20, 28, 37). The activation of THP-1 cells by FSL-1 or MALP-2 assessed by TNF-α production was augmented by ATP in a dose-dependent manner (Fig. 2). The TNF-α production-inducing activity of FSL-1 and MALP-2 was enhanced approximately twofold by 500 μM ATP. In the absence of lipopeptides, ATP activated THP-1 cells (Fig. 2). In addition, RT-PCR analysis demonstrated that the expression level of IL-1β, IL-6, and IL-8 as well as TNF-α mRNAs was also enhanced by ATP, whereas that of IL-10 was not (Fig. 3).
FIG. 1.
Structures of FSL-1 and MALP-2.
FIG. 2.
Effects of extracellular ATP on the activity of mycoplasmal lipopeptides to induce TNF-α production by THP-1 cells. THP-1 cells (106 cells/ml) were treated at 37°C for 1 h with 10 nM FSL-1 or 50 nM MALP-2 or not treated, and then ATP was added to the culture at the indicated concentrations. After an 8-h incubation period, the culture supernatant was collected and assayed for the amount of TNF-α by ELISA. Results, expressed as the means ± standard deviations (SD) of values for triplicate wells, are representative of three separate experiments. Statistical significance of augmentation by ATP, compared with the ATP-nontreated cells (open bars), was analyzed by t test. ∗, P < 0.05; ∗∗, P < 0.01.
FIG. 3.
Transcription of cytokine genes in THP-1 cells stimulated with FSL-1 and/or ATP or not. THP-1 cells (3 × 106) were stimulated at 37°C for 1 h with 10 nM FSL-1 or not and incubated for 1 h after the addition of 500 μM ATP. The expression of TNF-α, IL-1β, IL-6, IL-8, and IL-10 mRNAs was analyzed by RT-PCR. The identity of the PCR products was confirmed by Southern hybridization with a probe coding for the internal sequence (data not shown). Values in parentheses indicate the intensities of the signals of the mRNAs obtained by densitometric analysis, with the intensity of the signal of the mRNAs of β-actin or various cytokines in the cells in the absence of the stimulators (medium) being taken as 1.
Thus, these results suggest that ATP augments mycoplasmal lipopeptide-induced activation of macrophages by its interaction with some ATP receptors.
mRNA expression of ATP-sensitive P2 purinergic receptors on THP-1 cells.
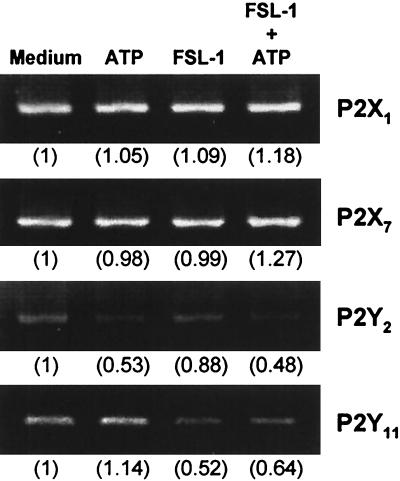
Extracellular nucleotides bind to cell surface receptors, which are designated purinergic P2 receptors (12, 16). Several P2 receptors are divided into two groups: P2X receptors, ligand-gated cation channels, and P2Y receptors coupled to G proteins (16, 35, 48). It was reported that P2X and P2Y receptor subtypes, including P2X1, P2X7, P2Y2, and P2Y11, which are sensitive to ATP (26, 35, 42, 48), regulate differentiation, activation, or proliferation of immunocytes (1, 3, 10, 12, 35, 48). Therefore, the expression of these ATP-sensitive P2 receptor mRNAs was examined by RT-PCR in THP-1 cells stimulated with FSL-1 and/or ATP or unstimulated. P2X1, P2X7, P2Y2, and P2Y11 genes were transcribed in THP-1 cells irrespective of stimulation (Fig. 4).
FIG. 4.
Transcription of ATP-sensitive purinergic receptor genes in THP-1 cells stimulated with FSL-1 and/or ATP or not. THP-1 cells (3 × 106) were stimulated at 37°C for 1 h with 10 nM FSL-1 or not and incubated for 8 h after the addition of 500 μM ATP. The expression of mRNAs of the ATP-sensitive P2 receptors P2X1, P2X7, P2Y2, and P2Y11 was analyzed by RT-PCR. The identity of the PCR products was confirmed by Southern hybridization with a probe coding for the internal sequence (data not shown). Values in parentheses indicate intensities of the signals of the mRNAs obtained by densitometric analysis, taking the intensity of the signal of the mRNAs of various P2 receptors in the cells in the absence of the stimulators (medium) as 1.
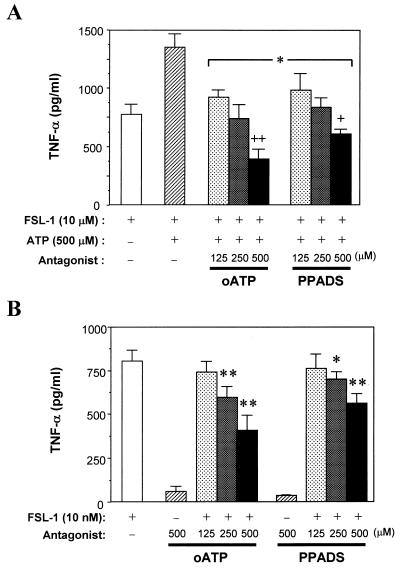
Therefore, the effects of PPADS and oATP on the enhancement of the TNF-α production-inducing activity of the lipopeptides by extracellular ATP were examined, because PPADS is an antagonist for P2X receptors (P2X1 to P2X7) and some P2Y receptors except for P2Y2 and P2Y11 (26, 29) and oATP is an antagonist for P2X receptors, especially P2X7 (4, 14, 19, 28, 29, 32). Both PPADS and oATP suppressed the enhancement by ATP in a dose-dependent manner (Fig. 5A). Furthermore, oATP suppressed the activity more strongly than PPADS. The activity obtained by oATP treatment was significantly lower than that of FSL-1 in the absence of extracellular ATP (Fig. 5A). Therefore, the effects of oATP on the TNF-α production-inducing activity of FSL-1 were examined. As a result, it was found that oATP inhibited the activity of FSL-1 even in the absence of extracellular ATP (Fig. 5B).
FIG. 5.
Effects oATP and PPADS on TNF-α production by THP-1 cells stimulated with FSL-1 and/or ATP. (A) THP-1 cells (106cells/ml) were treated at 37°C for 1 h with oATP or PPADS at 125, 250, and 500 μM and then incubated for 8 h after the addition of 10 nM FSL-1 and 500 μM ATP. The culture supernatant was collected and assayed for the amount of TNF-α by ELISA. Results, expressed as the means ± SD of triplicate wells, are representative of three separate experiments. Statistical significance was analyzed by t test. ∗, P < 0.01 versus hatched bar; +, P < 0.05 versus open bar; ++, P < 0.01 versus open bar. (B) THP-1 cells (106 cells/ml) were treated at 37°C for 1 h with 125, 250, and 500 μM oATP or PPADS and then incubated for 8 h after the addition of 10 nM FSL-1. The culture supernatant was collected and assayed for the amount of TNF-α by ELISA. Results, expressed as the means ± SD of triplicate wells, are representative of three separate experiments. Statistical significance was analyzed by t test. ∗, P < 0.05 versus open bar; ∗∗, P < 0.01 versus open bar.
These results suggest that P2X receptors, especially a P2X7 receptor, are involved in the modulation of FSL-1-induced activation of macrophages by extracellular ATP.
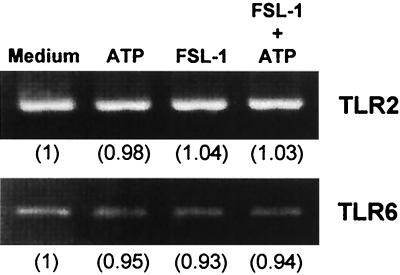
Effects of extracellular ATP and FSL-1 on the mRNA expression of TLR2 or TLR6 in THP-1 cells.
It was reported that signaling by MALP-2 is mediated by TLR2 (2, 44). We have also found that a receptor for FSL-1 is TLR2 (unpublished data). Recently, mycoplasmal lipopeptides were shown to be discriminated by TLR6 (45). Therefore, the effects of extracellular ATP or FSL-1 on the expression of TLR2 and -6 were examined. It was confirmed that TLR2 and TLR6 mRNAs were expressed in the cells, but the expression level was not enhanced by stimulation with ATP and/or FSL-1 (Fig. 6).
FIG. 6.
Effects of FSL-1 and/or ATP on the transcription of TLR2 or TLR6 mRNA in THP-1 cells. THP-1 cells (3 × 106) were stimulated at 37°C for 1 h with 10 nM FSL-1 or not and incubated for 8 h after the addition of 500 μM ATP. The expression of TLR2 mRNA was analyzed by RT-PCR. The identity of the PCR products was confirmed by Southern hybridization with a probe coding for the internal sequence (data not shown). Values in parentheses indicate intensities of the signals of the mRNAs obtained by densitometric analysis, taking the intensity of the signal of the mRNA of TLR2 or TLR6 in the cells in the absence of the stimulators (medium) as 1.
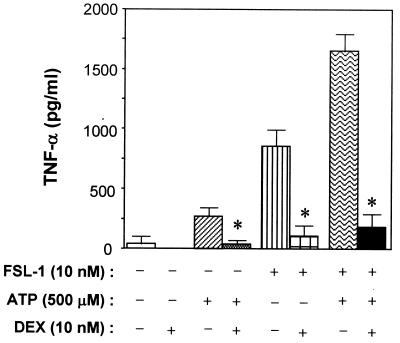
Effects of DEX on TNF-α production-inducing activity of FSL-1 and/or ATP.
TLRs activate kinase cascades which result in nuclear translocation of NF-κB (2, 5, 27, 41, 49). It was demonstrated that extracellular ATP is capable of inducing activation of the NF-κB subunit p65 (RelA) via the P2X7 receptor (15). Therefore, the effects of the NF-κB inhibitor DEX on the TNF-α production-inducing activity of FSL-1 and/or ATP on THP-1 cells were examined. DEX markedly inhibited the activity of FSL-1 and/or ATP (Fig. 7). The same result was also obtained when MALP-2 was used as a stimulator (data not shown). This result suggests that TNF-α production by THP-1 cells induced by mycoplasmal lipopeptides or ATP is mediated by NF-κB activation.
FIG. 7.
Effects of DEX on TNF-α production by THP-1 cells induced by FSL-1 and/or ATP. THP-1 cells (106 cells/ml) were treated at 37°C for 1 h with 10 nM DEX and then incubated for 8 h after the addition of 10 nM FSL-1 and/or 500 μM ATP. The culture supernatant was collected and assayed for the amount of TNF-α by ELISA. Results, expressed as the means ± SD of triplicate wells, are representative of three separate experiments. Statistical significance was analyzed by t test. ∗, P < 0.005 versus the absence of DEX.
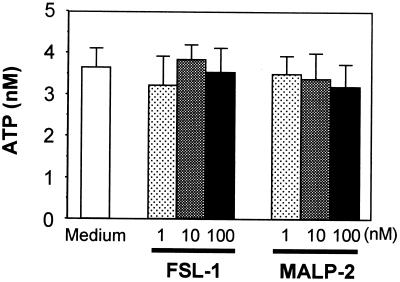
Effects of lipopeptides on extracellular ATP concentrations.
The findings obtained in this study suggest that extracellular ATP plays important roles in the enhancement of FSL-1-induced activation of THP-1 cells. Therefore, an experiment was carried out to test whether lipopeptide stimulation causes the release of ATP from THP-1 cells. THP-1 cells constitutively released ATP, producing extracellular concentrations of approximately 4 nM when assayed at 106 cells in 1 ml. However, THP-1 cells did not release additional ATP during an 8-h stimulation period with FSL-1 or MALP-2 (Fig. 8).
FIG. 8.
Determination of ATP in the culture of THP-1 cells stimulated with lipopeptides or not. THP-1 cells (106 cells/ml) were treated at 37°C for 8 h with 1 to 100 nM FSL-1 or MALP-2. The culture supernatant was collected and assayed for the amount of ATP. Results, expressed as the means ± SD of triplicate wells, are representative of three separate experiments.
DISCUSSION
This is the first study to report the effects of extracellular ATP on mycoplasmal lipopeptide-induced activation of macrophages. FSL-1 is capable of inducing production of TNF-α and IL-6 by THP-1 cells and ICAM-1 expression on the cell surface of human gingival fibroblasts (40). MALP-2 is capable of activating macrophages to release nitric oxide and produce TNF-α, IL-1, and IL-6 (18, 24, 31). It was demonstrated that signaling by MALP-2 and FSL-1 is mediated by TLR2. More recently, Takeuchi et al. demonstrated that macrophages prepared from TLR6 knockout (TLR6−/−) as well as TLR2−/− mice are unresponsive to MALP-2, suggesting that mycoplasmal lipopeptide is recognized by dual receptors consisting of TLR2 and TLR6 (45).
A similar result was obtained when FSL-1 was used as a stimulator of macrophages prepared from these knockout mice (unpublished data). Furthermore, the expression of TLR2 and TLR6 mRNAs was confirmed in THP-1 cells (Fig. 6). Taken together, our findings that mycoplasmal lipopeptide-induced activation of macrophages is augmented by the addition of ATP suggest that there is cross talk between the signaling pathways triggered by TLR2 and -6 and ATP receptors, especially P2X7, which leads to TNF-α production. Indeed, signaling transmitted by both TLRs and P2X7 leads to translocation of NF-κB, which regulates production of TNF-α (2, 5, 15, 27, 41, 49).
It was previously demonstrated that TLR signaling involves steps that are similar to those used by the IL-1 receptor (2, 27, 49). These common steps include the involvement of the adaptor molecule MyD88 and the serine kinase IL-1R-associated kinase, which interacts with an adaptor known as TRAF6 (43, 49). TRAF6 links to the mitogen-activated protein (MAP) kinase kinase kinase TAK1 through an adaptor, TAB (34, 41). TAK1 is involved in activation of the transcription factor NF-κB through the activation IκB kinase and in the activation of AP-1 transcription family members Jun and Fos via additional MAP kinases (34, 41, 43, 49). We also confirmed that mycoplasmal lipopeptides FSL-1 and MALP-2 also activate NF-κB and AP-1 (33). Furthermore, it was reported that extracellular ATP also activates NF-κB through the P2X7 receptor by selectively targeting NF-κB subunit p65 (RelA) (15).
In the present study, we demonstrated that DEX treatment significantly attenuated the secretion of TNF-α by macrophages induced by mycoplasmal lipopeptides and/or ATP (Fig. 7). DEX is one of the synthetic glucocorticoids that selectively inhibit NF-κB/Rel and AP-1 (13, 38). Therefore, it is suggested that the inhibitory activity of DEX is attributed to inhibition of NF-κB activation induced by signaling through TLR2/6 and/or the ATP receptor, which results in the repression of TNF-α production by macrophages.
The TNF-α production-inducing activity of FSL-1 obtained by oATP treatment was significantly lower than that of FSL-1 in the presence of extracellular ATP (Fig. 5A). Therefore, the effects of oATP on the activity of FSL-1 were examined, and it was found that oATP inhibited the activity of FSL-1 even in the absence of extracellular ATP (Fig. 5B). These results suggest two possibilities: one is that a small amount of ATP is included in the culture medium and the ATP enhanced the activity of FSL-1; and the other is that FSL-1 is able to interact with the receptors such as P2X7 to which oATP binds. However, the former possibility seems less possible, because approximately 4 nM ATP in the culture medium (Fig. 8) is too weak to induce the same amount of TNF-α as that suppressed by oATP, judging from the result shown in Fig. 2.
Recently it has been demonstrated that the C-terminal part of the P2X7 receptor forms a domain that has the potential to bind LPS in a manner similar to that observed with the LPS-binding domain of LPS-binding protein, and indeed, LPS can bind the peptide synthesized on the basis of the amino acid sequence of the C-terminal part of P2X7 in vitro (9). Therefore, the latter possibility may be more likely, that FSL-1 interacts with P2X7 receptor, because both LPS and FSL-1 have common lipid moieties responsible for the expression of their biological activities.
It was found that the macrophage response to LPS is modulated by extracellular ATP (4, 14, 19, 20, 28, 37). Beigi and Dubyak reported that activation of macrophages by LPS does not induce ATP release and autocrine stimulation of P2 receptors (4). The present study also showed that extracellular ATP enhanced mycoplasmal lipopeptide-induced activation of macrophages, and the macrophages did not induce additional ATP release during stimulation with these lipopeptides (Fig. 8). Thus, mycoplasmal lipopeptides function as an activator of macrophages without induction of ATP release in the same way as LPS. Many studies have demonstrated that under acute inflammatory condition or mechanical stress, various types of cells induce release of ATP (6, 36), although the mechanism remains unknown. Therefore, it is very likely that an inflammatory response induced by bacterial products such as LPS or lipopeptide is enhanced in vivo by ATP released in the lesions.
Acknowledgments
This work was partially supported by a Grant-in-Aid for Science Research (no. 13671891) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Editor: J. D. Clements
REFERENCES
- 1.Adrian, K., M. K. Bernhard, H. G. Breitinger, and A. Ogilvie. 2000. Expression of purinergic receptors (ionotropic P2X1-7 and metabotropic P2Y1-11) during myeloid differentiation of HL60 cells. Biochim. Biophys. Acta 1492:127-138. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Baricordi, O. R., L. Melchiorri, E. Adinolfi, S. Falzoni, P. Chiozzi, G. Buell, and F. Di Virgilio. 1999. Increased proliferation rate of lymphoid cells transfected with the P2X7 ATP receptor. J. Biol. Chem. 274:33206-33208. [DOI] [PubMed] [Google Scholar]
- 4.Beigi, R. D., and G. R. Dubyak. 2000. Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J. Immunol. 165:7189-7198. [DOI] [PubMed] [Google Scholar]
- 5.Beutler, B. 2000. Endotoxin, Toll-like receptor 4, and the afferent limb of innate immunity. Curr. Opin. Microbiol. 3:23-28. [DOI] [PubMed] [Google Scholar]
- 6.Bodin, P., and G. Burnstock. 1998. Increased release of ATP from endothelial cells during acute inflammation. Inflamm. Res. 47:351-354. [DOI] [PubMed] [Google Scholar]
- 7.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 8.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovski. 1999. Toll-like-receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 9.Denlinger, L. C., P. L. Fisette, J. A. Sommer, J. J. Watters, U. Prabhu, G. R. Dubyak, R. A. Proctor, and P. J. Bertics. 2001. The nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J. Immunol. 167:1871-1876. [DOI] [PubMed] [Google Scholar]
- 10.Di Virgilio, F. 1995. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol. Today 16:524-528. [DOI] [PubMed] [Google Scholar]
- 11.Dong, L., K. I. Shibata, Y. Sawa, A. Hasebe, Y. Yamaoka, S. Yoshida, and T. Watanabe. 1999. Transcriptional activation of mRNA of intercellular adhesion molecule 1 and induction of its cell surface expression in normal gingival fibroblasts by Mycoplasma salivarium and Mycoplasma fermentans. Infect. Immun. 67:3061-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubyak, G. R., and C. el Moatassim. 1993. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 265:C577-C606. [DOI] [PubMed] [Google Scholar]
- 13.Dumont, A., S. P. Hehner, M. L. Schmitz, J. Å. Gustafsson, J. Lidén, S. Okret, P. T. Van Der Saag, S. Wissink, B. Van Der Burg, P. Herrlich, et al. 1998. Cross-talk between steroids and NF-κB: what language? Trends Biochem. Sci. 23:233-235. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari, D., P. Chiozzi, S. Falzoni, S. Hanau, and F. Di Virgilio. 1997. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 185:579-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari, D., S. Wesselborg, M. K. A. Bauer, and K. Schulze-Osthoff. 1997. Extracellular ATP activates transcriptional factor NF-κB through the P2Z purinoreceptor by selective targeting NF-κB p65 (RelA). J. Cell Biol. 139:1635-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredholm, B. B., M. P. Abbracchio, G. Burnstock, J. W. Daly, T. K. Harden, K. A. Jacobson, P. Leff, and M. Williams. 1994. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 46:143-156. [PMC free article] [PubMed] [Google Scholar]
- 17.Galanos, C., M. Gumenscheimer, P. F. Mühlradt, E. Jirillo, and M. A. Freudenberg. 2000. MALP-2, a mycoplasma lipopeptide with classical endotoxic properties: end of an era of LPS monopoly? J. Endotoxin Res. 6:471-476. [PubMed] [Google Scholar]
- 18.Garcia, J., B. Lemercier, S. Roman-Roman, and G. Rawadi. 1998. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-κB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273:34391-34398. [DOI] [PubMed] [Google Scholar]
- 19.Grahams, C. B., A. D. Michel, I. P. Chessell, and P. P. Humphrey. 1999. Pharmacological characterization of ATP- and LPS-induced IL-1β release in human monocytes. Br. J. Pharmacol. 127:1915-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths, R. J., E. J. Stam, J. T. Downs, and I. G. Otterness. 1995. ATP induces the release of IL-1 from LPS-primed cells in vivo. J. Immunol. 154:2821-2828. [PubMed] [Google Scholar]
- 21.Humphreys, B. D., and G. R. Dubyak. 1996. Induction of the P2z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-γ in the human THP-1 monocytic cell line. J. Immunol. 157:5627-5637. [PubMed] [Google Scholar]
- 22.Humphreys, B. D., and G. R. Dubyak. 1998. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J. Leukoc. Biol. 64:265-273. [DOI] [PubMed] [Google Scholar]
- 23.Janeway, C. A., Jr., and R. Medzhitov. 1999. Lipoproteins take their Toll on the host. Curr. Biol. 9:R879-R882. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann, A., P. F. Mühlradt, D. Gemsa, and H. Sprenger. 1999. Induction of cytokines and chemokines in human monocytes by Mycoplasma fermentans-derived lipoprotein MALP-2. Infect. Immun. 67:6303-6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk, A. D., R. R. Bollinger, and O. J. Finn. 1995. Rapid, comprehensive analysis of human cytokine mRNA and its application to the study of acute renal allograft rejection. Hum. Immunol. 43:113-128. [DOI] [PubMed] [Google Scholar]
- 26.Lambrecht, G. 2000. Agonists and antagonists acting at P2X receptors: selectivity profiles and functional implications. Naunyn-Schmiedeberg's Arch. Pharmacol. 362:340-350. [DOI] [PubMed] [Google Scholar]
- 27.Means, T. K., D. T. Golenbock, and M. J. Fenton. 2000. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 11:219-232. [DOI] [PubMed] [Google Scholar]
- 28.Mehta, V. B., J. Hart, and M. D. Wewers. 2001. ATP-stimulated release of interleukin (IL)-1β and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J. Biol. Chem. 276:3820-3826. [DOI] [PubMed] [Google Scholar]
- 29.Michel, A. D., R. Kaur, I. P. Chessell, and P. P. Humphrey. 2000. Antagonist effects on human P2X (7) receptor-mediated cellular accumulation of YO-PRO-1. Br. J. Pharmacol. 130:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mühlradt, P. F., and M. Frisch. 1994. Purification and partial biochemical characterization of a Mycoplasma fermentans-derived substance that activates macrophages to release nitric oxide, tumor necrosis factor, and interleukin-6. Infect. Immun. 62:3801-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mühlradt, P. F., M. Kieβ, H. Meyer, R. Süssmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murgia, M., S. Hanau, P. Pizzo, M. Rippa, and F. Di Virgilio. 1993. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J. Biol. Chem. 268:8199-8203. [PubMed] [Google Scholar]
- 33.Nakamura, J. I., K. I. Shibata, A. Hasebe, T. Into, T. Watanabe, and N. Ohata. 2002. Signaling pathway induced by lipoproteins derived from Mycoplasma salivarium and a synthetic lipopeptide (FSL-1) in normal human gingival fibroblasts. Microbiol. Immunol. 46:151-158. [DOI] [PubMed] [Google Scholar]
- 34.Ninomiya-tsuji, J., K. Kishimoto, A. Hiyama, J. I. Inoue, Z. Cao, and K. Matsumoto. 1999. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398:252-256. [DOI] [PubMed] [Google Scholar]
- 35.North, R. A., and A. Surprenant. 2000. Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol. 40:563-580. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen, S., S. F. Pedersen, B. Nilius, I. H. Lambert, and E. K. Hoffmann. 1999. Mechanical stress induces release of ATP from Ehrlich ascites tumor cells. Biochim. Biophys. Acta 1416:271-284. [DOI] [PubMed] [Google Scholar]
- 37.Perregaux, D. G., P. McNiff, R. Lalibert, M. Conklyn, and C. A. Gabel. 2000. ATP acts as an agonist to promote stimulus-inducted secretion of IL-1β and IL-18 in human blood. J. Immunol. 165:4615-4623. [DOI] [PubMed] [Google Scholar]
- 38.Ray, A., D. H. Zhang, and P. Ray. 1995. Antagonism of nuclear factor-kappa B functions by steroid hormone receptors. Biochem. Soc. Trans. 23:952-958. [DOI] [PubMed] [Google Scholar]
- 39.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata, K. I., A. Hasebe, T. Into, M. Yamada, and T. Watanabe. 2000. The N-terminal lipopeptide of 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal gingival fibroblasts. J. Immunol. 165:6538-6544. [DOI] [PubMed] [Google Scholar]
- 41.Shuto, T., H. Xu, B. Wang, J. Han, H. Kai, X. X. Gu, T. F. Murphy, D. J. Lim, and J. D. Li. 2001. Activation of NF-κB by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKKα/β-IκBα and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc. Natl. Acad. Sci. USA 98:8774-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surprenant, A., F. Rassendren, E. Kawashima, R. A. North, and G. Buell. 1996. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735-738. [DOI] [PubMed] [Google Scholar]
- 43.Takaesu, G., S. Kishida, A. Hiyama, K. Yamaguchi, H. Shibuya, K. Irie, J. Ninomiya-tsuji, and K. Matsumoto. 2000. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol. Cell 5:649-658. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Mühlradt, and S. Akira. 2000. Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi, O., T. Kawai, P. F. Mühlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 46.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165:5780-5787. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 48.Von Kügelgen, I., and A. Wetter. 2000. Molecular pharmacology of P2Y-receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 362:310-323. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, F. X., C. J. Kirschning, R. Mancinelli, X. P. Xu, Y. Jin, E. Faure, A. Mantovani, M. Rothe, M. Muzio, and M. Arditi. 1999. Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J. Biol. Chem. 274:7611-7617. [DOI] [PubMed] [Google Scholar]