Abstract
Mycoplasmas can cause interstitial pneumonias inducing critical illness in humans and animals. Mycoplasma infections are characterized by an influx of neutrophils, followed by an accumulation of macrophages and lymphocytes. The present study deals with the question of which mycoplasmal components cause this host reaction. The mycoplasma-derived, macrophage-activating lipopeptide 2S-MALP-2 was used to mimic the sequelae of a mycoplasma infection. To this end, 2S-MALP-2 was intratracheally instilled into the lungs of Lewis rats, and the bronchoalveolar lavage cells were examined at different times after different doses of 2S-MALP-2. Application of 2.5 μg induced a pronounced leukocyte accumulation in the bronchoalveolar space. At 24 h after 2S-MALP-2 administration, the majority of leukocytes consisted of neutrophils, followed by macrophages, peaking on days 2 and 3. Lymphocyte numbers, although amounting to only a few percent of the total bronchoalveolar lavage cells, also increased significantly, with maximal lymphocyte accumulation occurring by 72 h after instillation. The leukocyte count of the lung interstitium was increased on day 3 after treatment. After 10 days all investigated cell populations returned to control levels. Transient chemotactic activity for neutrophils was detected in the bronchoalveolar lavage fluid early after 2S-MALP-2 application, followed by monocyte chemoattractant protein-1 activity (MCP-1) in lung homogenates. MCP-1 was produced by bronchoalveolar lavage cells upon stimulation with 2S-MALP-2. Our data indicate that mycoplasmal lipoproteins and lipopeptides are probably the most relevant mycoplasmal components for the early host reaction. The primary target cells are likely to be the alveolar macrophages liberating chemokines, which attract further leukocytes.
Mycoplasmas can cause interstitial pneumonias inducing critical illness in patients (17, 21). The lung-specific host responses to mycoplasmas, however, are not well understood. Mycoplasmas are the smallest organisms capable of autonomous growth. They have an extremely simple but pleiomorphic cell structure and a minimal genome. The lack of a cell wall is their main characteristic. When mycoplasmas colonize an organism, they do not necessarily induce an infection. Depending on the species, they are either harmless commensals or cause diseases, for example, atypical pneumonia and nongonococcal urethritis (1, 7, 13). Mycoplasma pneumoniae preferentially infects the human respiratory tract causing atypical pneumonia, but this mycoplasma species has also been isolated from the urogenital tract (12, 30). Generally, mycoplasma infection is characterized by an influx of polymorphonuclear leukocytes (PMN), followed by an accumulation of macrophages and lymphocytes (16, 19, 38).
Until recently the molecular entities and mechanisms inducing this infiltration remained unclear, since mycoplasmas lack a cell wall and the classical, cell wall-derived stimulators of the innate immune system, such as lipopolysaccharide, lipoteichoic acids, or peptidoglycan. However, Deiters and Mühlradt (9) have shown in a murine experimental system that intraperitoneal injection of the mycoplasma component 2S-MALP-2 (2-kDa macrophage-activating lipopeptide), be it in soluble or liposome encapsulated form can, like heat-killed mycoplasmas, induce leukocyte infiltration into the peritoneal cavity. The effects are thought to be due to in vivo, as well as in vitro, 2S-MALP-2-dependent synthesis of chemokines such as macrophage inflammatory protein 1α (MIP-1α), monocyte chemoattractant protein 1 (MCP-1), and MIP-2 (9).
2S-MALP-2 was originally isolated from Mycoplasma fermentans. The structure of this compound was elucidated and corroborated by chemical synthesis (25). 2S-MALP-2 differs from conventional bacterial lipopeptides in that the lipid moiety carries two ester-linked fatty acids only, lacking the N-terminal third fatty acid, a feature that conveys an exceptionally high biological activity (25). Studies in mice have shown that only the natural stereoisomer of MALP-2, namely, 2S-MALP-2, is biologically active and that the 2S-MALP-2-mediated response is dependent on the signaling molecules Toll-like receptor 2 (36) and Toll-like receptor 6 (37).
Whereas the earlier studies by Deiters and Mühlradt (25) were done in the peritoneal cavity, an experimental system for which no corresponding natural mycoplasma infection is known, the aim of the present study was to investigate the influence of 2S-MALP-2 in the pulmonary immune environment. Mycoplasma infections of the respiratory tract are well documented as, for example, in the case of M. pneumoniae in humans and Mycoplasma pulmonis in rodents, and both are associated with leukocyte infiltration in the lung (17, 19, 23, 38). The present study focuses on the effects of 2S-MALP-2 after application in rat lungs in order to test whether symptoms such as those caused by a natural mycoplasma pneumonia can be induced without using replicating bacteria. The rat was taken because it is a well-established animal model and because it was feasible to simultaneously investigate different compartments of the lung (8, 23). In addition to the bronchoalveolar lavage (BAL), the marginal pool of the lung and the lung interstitium were studied. Finally, chemotactic activity and chemokines were determined in the BAL and in homogenates of the MALP-treated lungs.
MATERIALS AND METHODS
Animals.
Male Lewis rats and Brown Norway rats with a mean body weight of 259 ± 56 g were obtained from the central animal laboratory (Medical School of Hannover, Hannover, Germany). They were maintained under specific-pathogen-free conditions until the experiments. The following numbers of animals were used per group. In the experiments with different 2S-MALP-2 doses, 3 Lewis rats were used; in the kinetic study, 5 Lewis rats were used; in the different cell count analysis, 6 to 11 animals were used; and in the chemotactic experiment 1 untreated and 4 treated Lewis rats were used. In the chemokine application, 3 to 8 Brown Norway rats were used because this is the only strain of rats for an asthma model in this species (34). In future studies, functional consequences of singular cytokines will be studied, e.g., in the asthma model. C3H/HeJ endotoxin low-responder mice were supplied by Jackson Laboratories (Bar Harbor, Maine), and C57BL/6 mice were provided by Charles River Laboratories (Sulzfeld, Germany).
2S-MALP-2.
In the majority of experiments, the biologically active, chemically synthesized 2S-MALP-2 stereoisomer, corresponding to the natural compound, was used for the intratracheal application. In some experiments, the inactive 2R-MALP-2 was used to check for nonspecific effects of lipopeptides. Both compounds were synthesized and purified as described before (36) and kept as stock solutions in a 33% 2-propanol-water mixture. The stock solution was diluted with pyrogen-free NaCl 0.9% (Braun, Melsungen, Germany) shortly before application. Solvent controls consisted of corresponding mixtures without 2S-MALP-2. The comparability of the doses used in the in vivo infection will be outlined in the Discussion section below.
Intrapulmonary application.
The intratracheal instillation was performed under short ether anesthesia. For this process, rats were suspended in a hanging position by a rubber band fixed to the teeth of the upper jaw. After intubation of the trachea via the oral cavity, a total volume of 500 μl was instilled. Before application, the correct position of the tube was checked by blowing air into the lung.
Determination of the effective 2S-MALP-2 concentration in the lung.
Rats were sacrificed at different times after intratracheal instillation of 2.5 μg of 2S-MALP-2. The lungs were immediately frozen and then pulverized in liquid nitrogen with a pestle and mortar. The powder was suspended in 2 ml of 0.9% NaCl containing 5% protease inhibitor cocktail for mammalian cells (Sigma, Deisenhofen, Germany), and 0.5 ml of each homogenate was mixed with 0.5 ml octyl glucoside (50 mM in 0.9% NaCl). 2S-MALP-2 was extracted by heating the mixture for 5 min at 100°C. After centrifugation (15 min, 11,000 × g, 4°C), the supernatants were frozen at −20°C until the following assay was carried out.
Determination of the remaining 2S-MALP-2 activity in the rat lung by nitric oxide release assay.
2S-MALP-2 activity was determined by nitric oxide release assay as described previously (24). Briefly, resident peritoneal macrophages from C3H/HeJ endotoxin low-responder mice were stimulated with 25 ng of recombinant gamma interferon (a generous gift of G. R. Adolf, Ernst Boehringer Institut für Arzneimittelforschung, Vienna, Austria)/ml and a serial 1:2-dilution of octyl glucoside-extracts of lung homogenates. After incubation at 37°C in 7.5% CO2 for 45 to 48 h, nitrate was reduced with nitrate reductase (Hoffmann-La Roche, Mannheim, Germany), and the nitric oxide formed was calculated from the sum of nitrate and nitrite after a staining reaction with Griess reagent. We defined one unit of 2S-MALP-2 activity/milliliter by the dilution yielding half-maximal nitric oxide release. One unit corresponds to 2.5 pg of 2S-MALP-2.
Determination of MCP-1 in lung homogenates.
MALP was instilled into rat lungs as described above. Rats were killed, and the lungs were removed and immediately frozen. The right and left lungs of each rat were separately pulverized in liquid nitrogen as described above. Each preparation was suspended in 2 ml of saline containing 5% (vol/vol) protease inhibitor cocktail. Insoluble material was removed by centrifugation for 15 min at 11,000 × g. MCP-1 was determined in the supernatant by enzyme-linked immunosorbent assay (ELISA; Pharmingen, San Diego, Calif.,) according to the supplier's protocol.
Isolation of blood leukocytes.
The cells from the blood, BAL, and marginal vascular pool were isolated as described previously (34). Animals were killed by aortic exsanguination in deep ether anesthesia. The blood was collected in heparinized tubes, and erythrocytes were lysed. Cells were washed and resuspended in 1 ml of phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.1% sodium azide.
Preparation of bronchoalveolar cells.
The lung was lavaged via a tracheal cannula 10 times with 5 ml of cold 0.9% NaCl. The lavage fluid was pooled, and an average of > 90% of the volume was recovered. The BAL cells were processed as described for the blood leukocytes.
Chemokine release from BAL cells.
Lewis rats, aged 6 to 8 weeks, were killed with an overdose of halothane. Lungs were washed 10 times with 5 ml of physiological saline by opening the trachea and injecting the saline with a blunt syringe. BAL cells were sedimented at 400 × g for 10 min at 4°C. BAL cells were adjusted to 2.4 × 105 cells/ml in Dulbecco modified Eagle medium, 5% fetal calf serum, 2 mM glutamine, 2.5 × 10−5 M 2-mercaptoethanol, 5 U of penicillin G/ml, and 5 μg of streptomycin sulfate/ml. Then, 3 × 105 cells were cultured in 1.25-ml volumes in 24-well cell culture plates for 24 h with or without 0.3 ng of MALP-2/ml. The MCP-1 content was determined in the culture medium by ELISA as described above.
Preparation of marginal vascular pool cells.
The lung was rinsed by perfusion of the pulmonary arteries via the right ventricle with 20 ml of cold (4°C) perfusate medium (RPMI with 35 g of dextran [Biochrom, Berlin, Germany]/liter) to remove the adhering blood cells. The perfusion was performed 10 times with 10 ml of perfusate, and the effluent was collected. The cells were processed as described for the blood leukocytes.
Preparation of interstitial cells.
The left lung was passed through a metal sieve with two rounded tweezers. The sieve was rinsed with 40 ml of PBS, and the suspension was centrifuged at 400 × g for 10 min. The interstitial cells were processed as described for the blood leukocytes. The right lung was used for histology. After fixation in 4% buffered formaline, the tissue was embedded in paraffin, and sections were prepared and stained with hematoxylin and eosin.
Staining of lymphocyte subsets, neutrophils, monocytes/macrophages, and dendritic cells.
The total cell numbers in the lung compartments (BAL, marginal pool, and interstitium) and in the blood were determined in a Neubauer counting chamber. Differential cell counts were assessed on cytospins. The slides were fixed in acetone for 10 min and washed with Tris-buffered saline containing 0.1% Tween 20. Cells were stained with a monoclonal antibody cocktail against lymphocytes (R73 for T cells, Ox12 for B cells, and 3.2.3 for natural killer cells [all from Serotec, Oxford, United Kingdom]) as described previously (34). At least 400 cells were differentiated on each slide. In addition, neutrophils and macrophages were morphologically identified by May-Grünwald and/or Giemsa staining.
For flow cytometry, cells were transferred into microtiter plates (106 cells/well) and washed twice in PBS containing 1% BSA and 0.1% NaN3. The staining was performed as described elsewhere (34), with the antibodies described above. After the cells were washed twice, the secondary antibody was incubated for 30 min at 4°C. The cells were analyzed by using a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.), focusing on the lymphocyte cluster. CD8+ T cells were identified as R73+ Ox8+; CD4+ T cells were identified as R73+ Ox8−; memory CD4+ T cells were identified as Ox22− W3/25high; naive T cells were identified as Ox22+ W3/25high; and monocytes were identified as Ox22− W3/25low. Dendritic cells were identified as low-autofluorescence cells, non-T and non-B lymphocytes, and double positive for Ox6 and Ox62 (18). All primary antibodies were monoclonal mouse anti-rat (Serotec). Unconjugated antibodies were detected by using phycoerythrin-conjugated secondary antibody (κPE; Dianova, Hamburg, Germany) and biotinylated primary antibodies with Red 670-streptavidin (Gibco, Gaithersburg, Md.). Isotype-matched antibodies served as a control.
Determination of chemotactic activity in the BAL fluid.
Chemotactic activity was measured as described previously (5). PMN from the bone marrow of C57BL/6 mice were suspended at 7.5 × 106 cells per ml of RPMI 1640 medium containing 0.5% BSA. Aliquots of 100 μl of this suspension were placed into the insert of a Transwell chemotaxis chamber (6.5 mm in diameter, 3-μm-pore-size polycarbonate filter; Corning Life Science, Schiphol, Netherlands). The bottom well was filled with 600 μl of RPMI-0.5% BSA (control) or 600 μl of sample (BAL fluid diluted 1:2 in RPMI-1% BSA). Inserts were put into the lower chambers and incubated at 37°C and 5% CO2 for 2 h. To release adherent cells from the lower surface of the membrane and from the bottom of the wells, 50 μl of 70 mM EDTA solution was added to the lower chambers. The chambers were incubated at 4°C for 30 min. Afterward, the inserts were removed and the transmigrated neutrophils were resuspended and counted with a FACScalibur (Becton Dickinson, Mountain View, Calif.) for 1 min at 60 μl/min with gating on forward and side scatter. Migration of neutrophils from the insert to the bottom well was quantitated as a percentage of total neutrophils loaded into the upper chamber.
Instillation of IL-16 and RANTES.
Brown Norway rats were treated with recombinant mouse cytokines (R&D Systems, Wiesbaden, Germany). Interleukin-16 (IL-16) was applied at concentrations of 0.03, 0.3, and 2.5 μg, whereas RANTES was used at a concentration of 2.5 μg. The chemokines were diluted in 500 μl of NaCl and were applied intratracheally. At least three rats were used for each dose, and the animals were killed after 24 or 48 h. Leukocyte subsets were determined in the BAL fluid by flow cytometry.
Data analysis.
Means, standard errors, and the level of significance were determined by SPSS-Windows (SPSS, Inc., Chicago, Ill.). The Mann-Whitney U test was used to compare the results of two experimental groups. P values of ≤0.05 were taken as statistically significant.
RESULTS
Leukocyte infiltration in response to two stereoisomers of MALP-2.
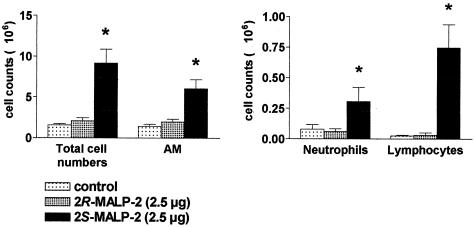
To investigate whether the prototypical mycoplasmal lipopeptide MALP-2 would be able to exert effects in the lungs similar to those observed during a mycoplasma infection in an atypical pneumonia, 2.5 μg of the natural stereoisomer 2S-MALP-2 was instilled into the lungs of anesthetized rats. 2R-MALP-2, which had shown poor in vitro activity (36), as well as the vehicle (33% 2-propanol appropriately diluted with saline) served as controls. Three days after intratracheal application, the total cell number in the BAL was clearly increased only in the group of animals that had received 2S-MALP-2 compared to those that had received 2R-MALP-2 or vehicle control (Fig. 1). Closer investigation of leukocyte subsets demonstrated a significant increase of alveolar macrophages (AM), neutrophils, and lymphocytes in the 2S-MALP-2 treated animals (Fig. 1). Taken together, only the natural 2S stereoisomer of MALP-2 specifically affected the cell counts and distribution of subsets in the BAL. There was no nonspecific response to lipopeptide or to the traces of organic solvent in the vehicle control.
FIG. 1.
Leukocyte infiltration in response to the two stereoisomers, 2S-MALP-2 and 2R-MALP-2. A total of 2.5 μg of either 2S-MALP-2, 2R-MALP-2, or control vehicle was intratracheally instilled into the lungs of Lewis rats. BAL and cell counting were performed 3 days later. Macrophages/monocytes, neutrophils, and lymphocytes were identified after May-Grünwald-Giemsa staining. Data are means ± the standard error of the mean (SEM; n = 6 per group). Asterisks indicate significant differences (P < 0.01) from the diluent control (2-propanol) and the inactive stereoisomer 2R-MALP-2 as calculated by the Mann-Whitney U test.
Effective 2S-MALP-2 activity in the lung tissue.
When the animals came out of the anesthesia, some of the instilled solution was expectorated. To determine the remaining amount of 2S-MALP-2 in the lung after intratracheal instillation of 2.5 μg of 2S-MALP-2, the lungs were homogenized and tested for 2S-MALP-2 by assaying the macrophage stimulatory activity. At time point zero, when lungs were excised immediately after instillation, 10.5% ± 2.1% of the originally applied amount of 2S-MALP-2 was recovered from the whole lung. This corresponds to ca. 250 ng. To obtain additional information about the persistence of 2S-MALP-2 activity in the lung, macrophage stimulatory activity was also measured 24 h after instillation. Significant activity was no longer detectable at this time point (0.3% ± 0.2%).
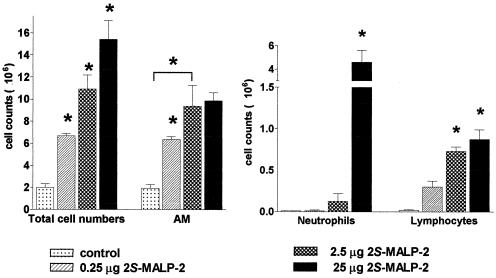
Dose-dependent leukocyte accumulation in the bronchoalveolar space in response to 2S-MALP-2.
Three days after application of 0.25, 2.5, or 25 μg of 2S-MALP-2 the total cell numbers as well as numbers of macrophages, neutrophils, and lymphocytes were increased in a dose-dependent manner (Fig. 2). AM and lymphocytes reached maximal cell counts with 2.5 μg of 2S-MALP-2, whereas neutrophil accumulation required higher doses at this time point (but see below).
FIG. 2.
Dose-dependent leukocyte accumulation in the BAL fluid in response to 2S-MALP-2. The indicated doses of 2S-MALP-2 or control vehicle were intratracheally instilled into the lungs of Lewis rats. Rats were sacrificed 3 days later, and the numbers of cells from the BAL were determined as described in Fig. 1. Data are means ± the SEM (n = 3 per group). Asterisks indicate significant differences (P < 0.01) from the control vehicle (2-propanol) as calculated by the Mann-Whitney U test.
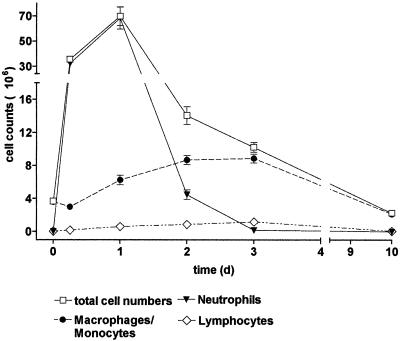
Time dependency of leukocyte accumulation in response to 2S-MALP-2.
The maximal cell count in the BAL after the application of 2.5 μg of 2S-MALP-2 was found after 24 h (Fig. 3), but already 6 h after treatment the total cell number increased ca. 10-fold. The cellular accumulation in the bronchoalveolar space at this early time point was mainly caused by neutrophils. After 24 h the cell numbers doubled compared to 6 h and reached the maximum, still mainly consisting of neutrophils but now with a significant percentage of AM. In the following days neutrophil counts decreased, although the AM count continued to increase. Lymphocyte numbers, although they amounted to only a few percent of the total BAL cells, were already significantly increased at 24 and 48 h (0 h, [0.04 ± 0.01] × 106; 24 h, [0.6 ± 0.1] × 106; 48 h, [0.9 ± 0.1] × 106), but maximal lymphocyte accumulation (sixfold) was achieved 72 h after instillation ([1.2 ± 0.1] × 106). After 10 days, all investigated cell types returned to control levels.
FIG. 3.
Time dependency of leukocyte infiltration in response to 2S-MALP-2. Rats were sacrificed at the indicated time points after instillation of 2.5 μg of 2S-MALP-2, and leukocytes in the BAL fluid were determined as described in Fig. 1. Data are means ± the SEM (n = 5 per group).
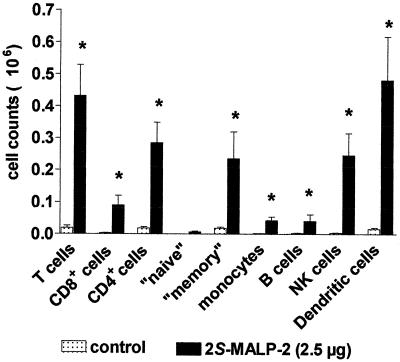
Differential cell counts in the BAL fluid and other compartments of the lung after 2S-MALP-2 treatment.
Three days after 2S-MALP-2 application, when neutrophil counts had subsided, minor leukocyte subsets in the BAL were determined by flow cytometry with specific antibody staining. An increase of all subpopulations was significant after 2S-MALP-2, the only exception being the “naive” CD4+ lymphocyte subset (Fig. 4). The highest relative increase in cell numbers was found in T lymphocytes and dendritic cells (>10-fold), whereas the increase in B cells and monocytes was lower (>5-fold). In addition, leukocytes were investigated in other compartments, such as the blood, the lung vascular marginal pool perfusate, and the interstitium of the lung (Table 1). Only in the lung interstitium were the total cell count and the number of macrophages/monocytes significantly increased after treatment with 2S-MALP-2. No significant differences in the number of neutrophils or lymphocytes were detected between 2S-MALP-2-treated and control animals.
FIG. 4.
Differential cell counts in the BAL fluid after 2S-MALP-2 treatment. At 3 days after instillation of 2.5 μg of 2S-MALP-2, rats were sacrificed, and leukocytes in the BAL fluid were determined as described in Fig. 1. Differentiation of leukocytes was performed by flow cytometry with subset-specific antibodies. Data are means ± the SEM (n = 8 per group). Asterisks indicate significant differences (P < 0.01) from the control vehicle (2-propanol) as calculated by the Mann-Whitney U test.
TABLE 1.
Leukocyte yields from blood, marginal pool, and lung interstitium after intratracheal instillation of 2S-MALP-2
| Treatment group | Mean leukocyte yield (106) ± SEMa from:
|
|||||
|---|---|---|---|---|---|---|
| Blood (cells/ml)
|
Marginal pool (total cells recovered)
|
Lung interstitium (total cells recovered from left lung)
|
||||
| Total leukocytes | Monocytes | Total leukocytes | Macrophages/monocytes | Total leukocytes | Macrophages/monocytes | |
| Controlb | 5.9 ± 0.6 | 0.1 ± 0.07 | 11.6 ± 1.7 | 0.3 ± 0.02 | 7.6 ± 1.1 | 2.2 ± 0.4 |
| 2S-MALP-2c | 7.9 ± 0.9 | 0.2 ± 0.05 | 13.4 ± 0.8 | 0.6 ± 0.2 | 15.8 ± 1.6d | 6.0 ± 0.4d |
Cells were enumerated by immunocytology. Data are means (n = 7 per group) ± the SEM.
Control rats were treated with vehicle control (2-propanol) in a final volume of 250 μl of 0.9% NaCl and sacrificed 3 days after intratracheal instillation.
Rats were treated with 2.5 μg of 2S-MALP-2 in a final volume of 250 μl of 0.9% NaCl and sacrificed 3 days after intratracheal instillation.
Significantly different (P < 0.05) from value for control group as calculated by the Mann-Whitney U test.
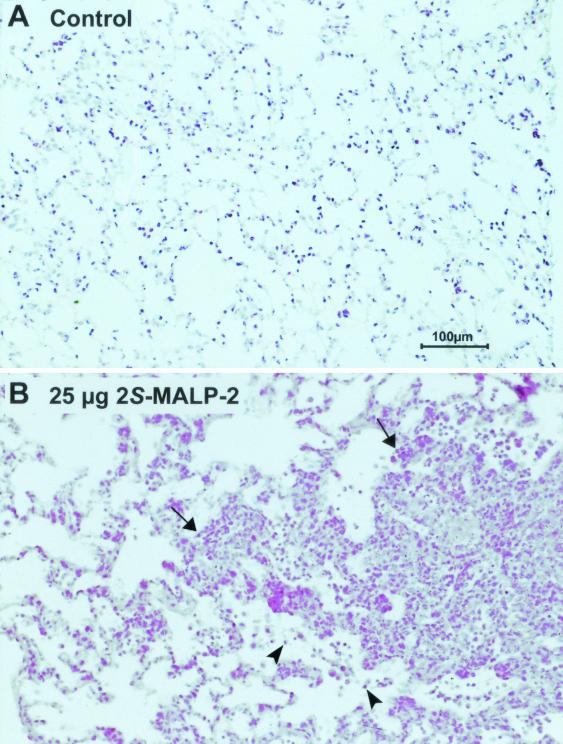
Histological investigation showed that 3 days after intratracheal instillation of 25 μg of 2S-MALP-2 there was a multifocal, pneumonia-like infiltration consisting of neutrophils and mononuclear cells (Fig. 5B). Inflammatory cells were found around most of the larger and the smaller vessels and bronchi. In addition, neutrophils were present in many alveoli, indicating alveolitis (Fig. 5B). After application of 2.5 μg, a mild inflammation was observed (not shown). Neither the lung morphology of the control vehicle (Fig. 5A) nor the R-isomer (not shown) was altered.
FIG. 5.
Histology of the lungs after intratracheal instillation of 2S-MALP-2. At 3 days after intratracheal instillation of 0.5 ml of control vehicle (A) or 25 μg of 2S-MALP-2 (B), rats were sacrificed and the lungs were removed. The right lungs were fixed in 4% formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin. Combined intraluminal and interstitial cell accumulations (arrows) consisted mainly of neutrophils but also of mononuclear cells. In many alveoli neutrophils (arrowheads) were found, demonstrating alveolitis.
Chemotactic activity of the BAL fluid.
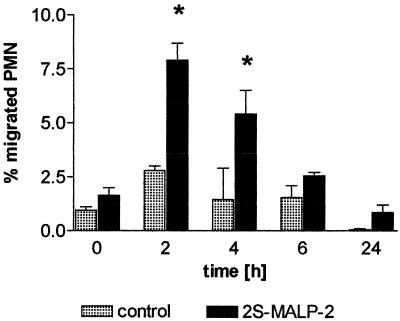
Earlier studies in a different experimental system and species had shown that leukocyte infiltration could be explained on the basis of chemokines liberated by activated macrophages (9). To examine a possible involvement of chemotactic factors set free by 2S-MALP-2 in the lung, a chemotactic effect of the BAL fluid on mouse neutrophils was tested in a chemotactic assay. The chemotactic activity was significantly increased 2 and 4 h after 2S-MALP-2 application, the maximum being observed 2 h after application (Fig. 6). In the following 4 h the activity continually decreased to finally reach the control level at 24 h. Using the BAL from stimulated rat lungs in a mouse neutrophil migration assay, a characteristic migration kinetic was found, demonstrating the chemotactic activity of rat chemokines (5) (e.g., MCP-1).
FIG. 6.
Chemotactic activity in the BAL fluid. Rats (n = 4) were treated with 2.5 μg of 2S-MALP-2 and sacrificed at the times indicated. One vehicle-treated rat served as a control. Cells were removed from the BAL fluid (50 ml) by centrifugation. The supernatant solution was diluted 1:2. The chemotactic activity was assessed from 300-μl aliquots in a chemotaxis chamber in duplicate. Data are means ± the SEM (n = 1 per vehicle control, n = 4 per treated group). Asterisks indicate significant differences (P < 0.01) in comparison to the time point at 0 h as calculated by the Mann-Whitney U test.
MCP-1 in the lungs of 2S-MALP-2-treated rats and in cultures of 2S-MALP-2-stimulated BAL cells.
Since the second major effect of 2S-MALP-2 besides PMN influx was the infiltration of AM, we examined the presence of the macrophage chemoattractant factor MCP-1 in the lungs of 2S-MALP-2-treated versus untreated animals 2 and 24 h after treatment. MCP-1 content was determined by ELISA in lung homogenates. The data in Table 2 show that significantly elevated MCP-1 levels were measured after 2 h, which were still increasing after 24 h. To further clarify which cells were primarily producing this chemokine, BAL cells were isolated from untreated rats. These cells, consisting of 97% AM, were cultured with optimal concentrations of 2S-MALP-2, and the MCP-1 content was determined in the culture fluid (Table 3). Whereas unstimulated AM produced very little MCP-1, stimulation with 2S-MALP-2 led to significant levels of MCP-1.
TABLE 2.
MCP-1 in rat lungs after 2S-MALP-2 instillation
| Time (h) after treatment with 2.5 μg of 2S-MALP-2a | Rat no. | MCP-1 in lung tissue (ng/g)b |
|---|---|---|
| 0 | 1 | 8.6 |
| 2 | 8.1 | |
| 3 | 7.2 | |
| Mean ± SD | 8.0 ± 0.7 | |
| 2 | 4 | 199.1 |
| 5 | 183.1 | |
| 6 | 197.6 | |
| Mean ± SD | 193.0 ± 9.0 | |
| 24 | 7 | 1,050.6 |
| 8 | 166.3 | |
| 9 | 243.3 | |
| Mean ± SD | 487.0 ± 490.0 |
2S-MALP-2 was administered by intratracheal instillation of 0.5-ml volumes into the lungs of anesthetized Lewis rats. “0 h” means rats were killed immediately after recovery from anesthesia.
Left and right lungs from three rats in each group were removed, weighed, and frozen until homogenization.
TABLE 3.
2S-MALP-2-dependent MCP-1 production by BAL cells
| Rat no. | MCP-1 release from cultured BAL cells (ng/ml/24 h)a ± SD
|
|
|---|---|---|
| Control (no 2S-MALP-2) | 0.3 ng of 2S-MALP-2/ml | |
| 1 | 2.8 ± 0.1 | 38.9 ± 2.4 |
| 2 | 1.5 ± 0 | 16.4 ± 1.1 |
| 3 | 2.0 ± 0.1 | 24.1 ± 1.0 |
Values are triplicates ± the standard deviation from 3 × 105 cells cultured in 1.25-ml volumes.
Intratracheal application of recombinant RANTES and IL-16.
To investigate whether some of the effects of 2S-MALP-2 could be mimicked by the instillation of the single recombinant chemokines RANTES and IL-16, expected to attract monocytes and memory T cells (33) and CD4+ T cells (11), respectively, doses of up to 2.5 μg were applied intratracheally. However, no significant cell recruitment was observed at any of the different time points or doses. For example, the total cell counts from IL-16 versus control ([2.5 ± 0.3] × 106 versus [2.5 ± 0.4] × 106) or RANTES versus control ([2.5 ± 0.3] × 106 versus [2.5 ± 0.2] × 106) were similiar at 48 h after instillation.
DISCUSSION
Respiratory mycoplasmosis caused by M. pulmonis is a well-studied model in rats and mice (3, 8, 10, 14, 23, 29, 35). An influx of neutrophils (3) and mononuclear cells (8, 35) is observed early after infection, whereas at later chronic stages angiogenesis (8) and airway fibrosis (23) are noted. AM were identified as early effector cells in fighting the infection (14). The early effects of mycoplasmal infection could be mimicked by intratracheal application of the 2S-MALP-2 stereoisomer which, although synthetic, is the natural stereoisomer (36). The 2R-stereoisomer, which does not occur in nature, was ineffective. Leukocyte influx into the BAL fluid was elicited by as little as 2.5 μg of 2S-MALP-2 per animal. Of this amount only ca. 10%, i.e., 250 ng, remained in the lung, because most of the material was lost, probably when the rats cleared their respiratory tract of the instilled liquid by coughing immediately after the instillation process.
A precise correlation of the 250 ng of 2S-MALP-2 actually remaining in the lung with the number of mycoplasmas containing this amount of lipopeptide is extremely difficult because (i) depending on the growth conditions, mycoplasmas may grow as filaments or as short cells, i.e., the same amount of mycoplasmas may give rise to greatly differing numbers of CFU, and (ii) the content of lipopeptide or protein, as measured by macrophage stimulatory activity, varies from species to species and even within one species between different clones (see, for example, reference 25). However, a very rough estimate may be given based on the following data: we measured macrophage stimulatory activity equivalent to 40 to 90 ng of 2S-MALP-2/mg of mycoplasma protein in M. fermentans strains isolated from patients, and 800 to 2,300 ng/mg of protein in laboratory strains of Mycoplasma hyorhinis, a species that is sometimes associated with pneumonia or polyserositis in swine. On the assumption that 1 mg of mycoplasma protein corresponds to 109 CFU, it can be estimated that 250 ng of 2S-MALP-2 corresponds to 3 × 109 to 6 × 109 M. fermentans cells or 2 × 108 to 5 × 108 M. hyorhinis cells.
Lipoproteins and lipopeptides, of which 2S-MALP-2 is just one example, are ubiquitously expressed in mycoplasmas and are the most relevant mycoplasmal components for the early host reaction (see, for example, reference 4). Thus, in M. pneumoniae the genes for >30 different lipoproteins were detected (15). It may be important in this context to emphasize that it is the lipid portion that is responsible for the reaction of 2S-MALP-2 and other lipopeptides with the innate immune system (see, for example, reference 26), whereas the peptide moiety is the target of the subsequent specific immune response (6). Although our results were obtained with a single lipopeptide from a mycoplasma species that is not commonly thought of as a pathogen of the respiratory tract, we are convinced that our findings are of general relevance for the processes set in motion by mycoplasmal respiratory pathogens. We hypothesize that it is not just one lipoprotein or peptide in these mycoplasmas but the natural mixture of them that causes the observed effects in an infection, just as a molecular mixture of lipopolysaccharides isolated from gram-negative bacteria will have similar endotoxic effects, as does purified or synthetic lipid A.
The kinetics of influx of the various cell subpopulations after intratracheal administration of 2S-MALP-2 were reminiscent of those observed by Deiters and Mühlradt after intraperitoneal 2S-MALP-2 application (9). During the actual infection process (35), chemoattractant cytokines appear to be involved, as was also found in our present and preceding studies (9). Transient chemotactic activity for neutrophils was measured in the BAL fluid early after 2S-MALP-2 application (Fig. 6), followed later by more persistent MCP-1 activity in lung homogenates, a chemoattractant protein for monocytes/macrophages (Table 2). This mirrors the kinetics of the observed leukocyte appearance in the BAL fluid, with the neutrophils preceding the AM. The primary target cell of 2S-MALP-2 is in all probability the AM, since it has been shown that 2S-MALP-2, as its name implies, activates macrophages. Our data support this notion, because AM reacted strongly to 2S-MALP-2 by liberation of MCP-1 (Table 3) and TNF-α (data not shown but see references 10 and 29).
Although not obvious at first sight, but measurable when absolute cell numbers rather than percentages were calculated, there was a significant increase of T cells, natural killer cells, and dendritic cells 3 days after instillation of 2S-MALP-2 (Fig. 4). The appearance of specific immune cells in pulmonary immune reactions has often been reported (20) and occurred regularly after the influx of innate immune cells. This was again confirmed in the present study. The preferential influx of CD4 memory-type T cells into the bronchoalveolar space is a general observation in asthma models, e.g., after OVA exposure (34).
It was of interest to determine whether pure recombinant mouse chemokines such as RANTES and IL-16 would be capable of attracting T cells in our intratracheal model. Both proved ineffective. It had been previously shown that RANTES does not contribute to mononuclear cell infiltration associated with mycoplasma infection (35), but it was detected as an early chemotactic activity in a rat model of immunocomplex-mediated alveolitis (2). However, other explanations should be considered, e.g., a stringent species specificity.
Lymphocyte traffic and that of other leukocytes is controlled by various chemokines (31, 32). A complex cascade of chemotactic factors released by innate immune cells (32) may subsequently attract specific immune cells. In in vitro studies in humans Yang et al. (39, 40) showed that β-defensins secreted from neutrophils, macrophages, and epithelial cells were chemotactic for immature dendritic cells and memory T cells. Another mechanism of lymphocyte accumulation could be local proliferation, which was not investigated in the present study. It is of interest in this context that mycoplasmas have a mitogenic effect on lymphocytes, causing nonspecific proliferation of T and B cells (27, 28). Lastly, an additional mechanisms of mycoplasma-mediated leukocyte accumulation, besides the liberation of chemokines, could activate the alternative pathway of the complement system. Matsumoto et al. (22) have shown that an M. fermentans-derived lipoprotein, M161Ag, which has an N-terminal portion identical to that of 2S-MALP-2, can activate human complement. However, this mechanism is unlikely to occur in our experimental system, since soluble 2S-MALP-2 did not activate complement (Deiters and Mühlradt, unpublished data).
In conclusion, the mycoplasmal lipopeptide 2S-MALP-2, in such small amounts as may occur in live mycoplasmas, was found to be capable of raising a reaction in the rat lung which is similar to an infection with replicating mycoplasmas.
Acknowledgments
The synthesis of 2S- and 2R-MALP-2 by M. Morr is gratefully acknowledged. We also thank M. Heuer, K. Westermann, and D. Stelte for skillful technical help and S. Fryk for correction of the English.
This research was supported by the German Research Foundation (Pa 240/8-2 and Mu 672/2-5).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Baseman, J. B., and J. G. Tully. 1997. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg. Infect. Dis. 3:21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bless, N. M., M. Huber-Lang, R. F. Guo, R. L. Warner, H. Schmal, B. J. Czermak, T. P. Shanley, L. D. Crouch, A. B. Lentsch, V. Sarma, M. S. Mulligan, H. P. Friedl, and P. A. Ward. 2000. Role of CC chemokines (macrophage inflammatory protein-1β, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J. Immunol. 164:2650-2659. [DOI] [PubMed] [Google Scholar]
- 3.Cassell, G. H., J. R. Lindsey, and H. J. Baker. 1974. Immune response of pathogen-free mice inoculated intranasally with Mycoplasma pulmonis. J. Immunol. 112:124-136. [PubMed] [Google Scholar]
- 4.Chambaud, I., H. Wroblewski, and A. Blanchard. 1999. Interactions between mycoplasma lipoproteins and the host immune system. Trends Microbiol. 7:493-499. [DOI] [PubMed] [Google Scholar]
- 5.Chouchakova, N., J. Skokowa, U. Baumann, T. Tschernig, K. Philippens, B. Nieswandt, R. E. Schmidt, and J. E. Gessner. 2001. FcγRIII-mediated production of TNF-α induces immune complex-alveolitis independent of CXC chemokine generation. J. Immunol. 166:193-200. [DOI] [PubMed] [Google Scholar]
- 6.Citti, C., M. F. Kim, and K. S. Wise. 1997. Elongated versions of Vlp surface lipoproteins protect Mycoplasma hyorhinis escape variants from growth-inhibiting host antibodies. Infect. Immun. 65:1773-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, B. C. 1999. Mycoplasma-induced arthritis in animals: relevance to understanding the etiologies of the human rheumatic diseases. Rev. Rheum. 66:45S-49S. [PubMed] [Google Scholar]
- 8.Dahlqvist, K., E. Y. Umemoto, J. J. Brokaw, M. Dupuis, and D. M. McDonald. 1999. Tissue macrophages associated with angiogenesis in chronic airway inflammation in rats. Am. J. Respir. Cell Mol. Biol. 20:237-247. [DOI] [PubMed] [Google Scholar]
- 9.Deiters, U., and P. F. Muhlradt. 1999. Mycoplasmal lipopeptide MALP-2 induces the chemoattractant proteins macrophage inflammatory protein 1α (MIP-1α), monocyte chemoattractant protein 1, and MIP-2 and promotes leukocyte infiltration in mice. Infect. Immun. 67:3390-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulkner, C. B., J. W. Simecka, M. K. Davidson, J. K. Davis, T. R. Schoeb, J. R. Lindsey, and M. P. Everson. 1995. Gene expression and production of tumor necrosis factor alpha, interleukin 1, interleukin 6, and gamma interferon in C3H/HeN and C57BL/6N mice in acute Mycoplasma pulmonis disease. Infect. Immun. 63:4084-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franz, J. K., S. A. Kolb, K. M. Hummel, F. Lahrtz, M. Neidhart, W. K. Aicher, T. Pap, R. E. Gay, A. Fontana, and S. Gay. 1998. Interleukin-16, produced by synovial fibroblasts, mediates chemoattraction for CD4+ T lymphocytes in rheumatoid arthritis. Eur. J. Immunol. 28:2661-2671. [DOI] [PubMed] [Google Scholar]
- 12.Goulet, M., R. Dular, J. G. Tully, G. Billowes, and S. Kasatiya. 1995. Isolation of Mycoplasma pneumoniae from the human urogenital tract. J. Clin. Microbiol. 33:2823-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hentschel, J., and P. F. Muhlradt. 1998. Mycoplasma, infection, and immunity, p. 1798-1802. In P. J. Delves and I. Roitt (ed.), Encyclopedia of immunology, 2nd ed. Harcourt Brace and Co./Academic Press, San Diego, Calif.
- 14.Hickman-Davis, J. M., S. M. Michalek, J. Gibbs-Erwin, and J. R. Lindsey. 1997. Depletion of alveolar macrophages exacerbates respiratory mycoplasmosis in mycoplasma-resistant C57BL mice but not mycoplasma-susceptible C3H mice. Infect. Immun. 65:2278-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Himmelreich, R., H. Plagens, H. Hilbert, B. Reiner, and R. Herrmann. 1997. Comparative analysis of the genomes of the bacteria Mycoplasma pneumoniae and Mycoplasma genitalium. Nucleic Acids Res. 25:701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard, C. J., J. C. Anderson, R. N. Gourlay, and D. Taylor-Robinson. 1975. Production of mastitis in mice with human and bovine ureaplasmas (T-mycoplasmas). J. Med. Microbiol. 8:523-529. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, E. 1997. Mycoplasma infections of the human respiratory tract. Wien. Klin. Wochenschr. 109:574-577. [PubMed] [Google Scholar]
- 18.Lambrecht, B. N., I. Carro-Muino, K. Vermaelen, and R. A. Pauwels. 1999. Allergen-induced changes in bone-marrow progenitor and airway dendritic cells in sensitized rats. Am. J. Respir. Cell Mol. Biol. 20:1165-1174. [DOI] [PubMed] [Google Scholar]
- 19.Lindsey, J. R., and H. Cassell. 1973. Experimental Mycoplasma pulmonis infection in pathogen-free mice: models for studying mycoplasmosis of the respiratory tract. Am. J. Pathol. 72:63-90. [PMC free article] [PubMed] [Google Scholar]
- 20.Lipscomb, M. F., D. E. Bice, C. R. Lyons, M. R. Schuyler, and D. Wilkes. 1995. The regulation of pulmonary immunity. Adv. Immunol. 59:369-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmion, B. P. 1990. Eaton agent-science and scientific acceptance: a historical commentary. Rev. Infect. Dis. 12:338-353. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto, M., M. Nishiguchi, S. Kikkawa, H. Nishimura, S. Nagasawa, and T. Seya. 1998. Structural and functional properties of complement-activating protein M161Ag, a Mycoplasma fermentans gene product that induces cytokine production by human monocytes. J. Biol. Chem. 273:12407-12414. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh, J. C., J. W. Simecka, S. E. Ross, J. K. Davis, E. J. Miller, and G. H. Cassell. 1992. Infection-induced airway fibrosis in two rat strains with differential susceptibility. Infect. Immun. 60:2936-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mühlradt, P. F., and M. Frisch. 1994. Purification and partial biochemical characterization of a Mycoplasma fermentans-derived substance that activates macrophages to release nitric oxide, tumor necrosis factor, and interleukin-6. Infect. Immun. 62:3801-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mühlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at a picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mühlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1998. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect. Immun. 66:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naot, Y., S. Davidson, and E. S. Lindenbaum. 1981. Mitogenicity and pathogenicity of Mycoplasma pulmonis in rats. I. Atypical interstitial pneumonia induced by mitogenic myeoplasmal membranes. J. Infect. Dis. 143:55-62. [DOI] [PubMed] [Google Scholar]
- 28.Naot, Y., J. G. Tully, and H. Ginsburg. 1977. Lymphocyte activation by various Mycoplasma strains and species. Infect. Immun. 18:310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishiguchi, M., M. Matsumoto, T. Takao, M. Hoshino, Y. Shimonishi, S. Tsuji, N. A. Begum, O. Takeuchi, S. Akira, K. Toyoshima, and T. Seya. 2001. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J. Immunol. 166:2610-2616. [DOI] [PubMed] [Google Scholar]
- 30.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 32.Scapini, P., J. A. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. A. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177:195-203. [DOI] [PubMed] [Google Scholar]
- 33.Schall, T. J., K. Bacon, K. J. Toy, and D. V. Goeddel. 1990. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 347:669-671. [DOI] [PubMed] [Google Scholar]
- 34.Schuster, M., T. Tschernig, N. Krug, and R. Pabst. 2000. Lymphocytes migrate from the blood into the bronchoalveolar lavage and lung parenchyma in the asthma model of the Brown Norway rat. Am. J. Respir. Crit. Care Med. 161:558-566. [DOI] [PubMed] [Google Scholar]
- 35.Simecka, J. W. 1999. Beta-chemokines are produced in lungs of mice with mycoplasma respiratory disease. Curr. Microbiol. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Muhlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. (Author’s correction, 165:5995.) [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 38.Tuffrey, M. A., P. M. Furr, P. Falder, and D. Taylor-Robinson. 1984. The anti-chlamydial effect of experimental Mycoplasma pulmonis infection in the murine genital tract. J. Med. Microbiol. 17:357-362. [DOI] [PubMed] [Google Scholar]
- 39.Yang, D., Q. Chen, O. Chertov, and J. J. Oppenheim. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68:9-14. [PubMed] [Google Scholar]
- 40.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T-cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]