Genetically modified (GM) crops first appeared commercially in the mid-1990s to what seemed a bright and promising future. Resistant to pests and the herbicides used to control weeds, these new crops were so popular with farmers that millions of acres were planted with them by the turn of the millennium. Today, GM crops are grown commercially by 8.25 million farmers on 200 million acres spread throughout 17 countries, reports the International Service for the Acquisition of Agri-Biotech Applications (ISAAA), an international nonprofit that advocates for the technology. The world’s top five producers—the United States, Argentina, Canada, Brazil, and China—account for 96% of global GM cultivation; of this, more than half is in the United States.
Yet these impressive numbers tell only part of the story. Fully as notable as the growth of GM agriculture is the relentless backlash that has developed against it. Although GM supporters insist the technology raises harvest yields, reduces agrochemical use, and will eventually even produce high-nutrition food that can grow in depleted soils, skeptics counter that the risks of GM foods—made with gene splicing methods from biotechnology—are unknown and poorly addressed by current testing methods. They also worry that the spread of GM crops, which are supplied mainly by a handful of multinational companies, fuels corporate ownership of the seed supply and threatens the purity of indigenous crops, with which GM varieties can breed by cross-pollination.
A Growing Backlash
The opposition’s attacks are generating sustained impacts. In April 2004, biotech companies including Novartis Seeds, Aventis CropScience, and Bayer CropScience abandoned GM field trials in England, citing challenges raised by British consumers. The next month, Monsanto dropped its new variety of herbicide-resistant wheat despite hundreds of millions reputedly spent on research and development. The product was shelved in part because of threatened boycotts by Europe and Japan, which together buy 45% of all U.S. wheat exports, according to the U.S. Department of Agriculture Economic Research Service (UDSA/ERS). And in November 2004, the world’s largest agrochemical company, the Swiss-based Syngenta, moved its European GM field trials to the United States, also citing public resistance.
Europe itself, where commercial GM crops are grown only in Spain—and there in small amounts—is politically gridlocked over the issue, says Geoffrey Lean, environment editor for The Independent on Sunday, a British newspaper. The European Commission lifted a six-year moratorium on GM food in Europe last year, but even so, no new crops have been granted entry, he says. The commission, which favors the technology, wants to allow more GM imports. However, a number of opposing countries—notably Austria, France, Portugal, Greece, Denmark, and Luxembourg—have so far prevented this from happening. “As far as opinions in Europe go, the public is heavily against GM, the scientific community is for it, and governments are split down the middle,” Lean says.
Developing countries are also heavily divided, even though they could arguably benefit the most from the technology. Some stakeholders worry that the introduction of GM seed in developing countries could threaten the purity of conventional crops, thus posing a risk to food exports bound for markets that reject the technology.
Meanwhile, a slew of “GM-free zones,” where all transgenic organisms are banned (including fish, other animals, and plants used to make drugs), are cropping up around the world. Three are in the United States, all in California. More than 3,000 are found throughout Europe, with others in Canada, Australia, and the Philippines, says Renata Brillinger, director of the citizens group Californians for GE [genetically engineered]-Free Agriculture.
GM crops also suffer a poor reputation among the general public, in part because they are made in ways that can sound scary when described to consumers. Biotechnology allows scientists to combine genes from totally unrelated species of plants, microbes, and animals. How is this possible? There are several methods. In one, bacteria and viruses—which are naturally able to penetrate cells—are deployed as delivery vehicles to shuttle genes directly into plant cell genomes. In another, tiny particles coated with a gene are propelled at high speeds into cells to deliver the gene. In still another, electric shocks are used to destabilize cell membranes, making them permeable to delivered genes. These and several other methods enable scientists to evade natural barriers that cells use to protect themselves from foreign DNA.
Thus, genes from bacteria can be introduced into a plant—or, as in one instance, a fish gene can be introduced into a tomato. Monsanto has made pest-resistant varieties with a gene from Bacillus thuringiensis (Bt), a bacterium that kills certain types of insects. The resultant varieties produce the Bt toxin, a protein that is lethal to these insects but safe for humans. DNA Plant Technology of Oakland, California (which has since gone out of business) was the company responsible for inserting a fish gene into a tomato. In that case, an “anti-freeze” gene that helps flounder survive frigid waters was spliced into tomato cells to enhance the plant’s resistance to cold. The fish-tomato didn’t swim, nor did it ever make it to market. But its memory lingers as a quintessential “frankenfood” that GM critics often refer to.
Dwindling Varieties
With growing opposition to GM crops has come a remarkable drop in new varieties being introduced by the agrobiotech industry. A 2 February 2005 report by the Center for Science in the Public Interest (CSPI), an environmental group, observes that three-quarters of federal approvals for GM crops in the United States were obtained between 1995 and 1999.
According to Gregory Jaffe, director of the Project on Biotechnology at CSPI, most of the new crops that drive GM agriculture’s growth now are cookie-cutter varieties that merely recycle the same genes for pest and herbicide resistance already used in existing products. Indeed, virtually all the GM crops grown today are different varieties of the same four crops that became available before 2000, mainly pest- or herbicide-resistant varieties of corn, cotton, soybeans, and canola.
These crops were made for and marketed specifically to farmers, who make up the industry’s key buyers. Farmers have embraced GM technology because it saves them time and money. Monsanto’s Roundup Ready crops, for instance, are resistant to the glyphosate-based herbicide Roundup. Farmers can eliminate weeds with one or two sprayings of the wide-spectrum herbicide without harming their crops.
Rob Rose, a spokesman for the Donald Danforth Plant Science Center, a nonprofit research facility funded partially by the agrobiotech industry, says companies barely considered the consumers who would buy and eat GM foods in their initial marketing efforts. This proved to be a mistake, he says. When the consumer backlash started, companies were caught off-guard. “Even now, as the backlash intensifies, they haven’t come up with an effective consumer marketing strategy,” Rose says.
To improve its public image, the agro-biotech industry has more recently begun promoting the concept of extra-nutritious, environmentally resilient crops to fight world hunger. But so far, none of these so-called second-generation crops have entered the marketplace, anywhere in the world.
The second-generation crops that are in the pipeline seem to be stuck there, mainly because of market uncertainties, insiders say. For example, Monsanto is developing grains to make cooking oils with lower saturated fats and higher levels of omega-3 fatty acids, which are thought to protect against heart disease. But Christopher Horner, director of public affairs for Monsanto, acknowledges that these grains have distant and unknown release dates.
Universities and small research centers also develop second-generation GM crops, but they lack the resources necessary to put them on the market. The Danforth Center, for instance, has developed numerous such crops, including grains enriched with vitamin E and vegetables with enhanced folate levels, a nutrient that protects against neural tube defects in newborns as well as cancer and cardiovascular disease in adults. Center scientists have also developed a nutritionally enhanced variety of cassava, a root vegetable that is a dietary staple for hundreds of millions worldwide.
At the University of California, Berkeley, Peggy Lemaux, a faculty member in the Department of Plant and Microbial Biology, and her colleague Bob Buchanan recently helped create a type of GM wheat that people with wheat allergies might eat more safely. She and her colleagues at Berkeley are now working on enhancing sorghum, another staple of the world’s poor, to make it more nutritionally complete and calorie-rich.
“I want to help people,” Lemaux says. “I work for a land-grant university, and our charge is to develop varieties that help agriculture and consumers. If I can do this for countries that really need it, then that’s what I want to do.”
But Lemaux and Karel Schubert, a Danforth Center principle investigator, both acknowledge that despite the potential benefits, the commercial value of these crops is limited. Without significant financial backing, universities and research centers can’t fund the extensive regulatory and patent reviews needed to bring the products to market. But as consumers increasingly turn against GM food, Lemaux adds, industry and federal funds for second-generation crop research and development are drying up.
“Second generation crops are developed in universities, and then those projects die,” Lemaux says. “There’s a pall hanging over GM and its products, so many companies have stopped supporting fundamental research.” Her grant from the U.S. Agency for International Development has been cut from a three-year to a one-year commitment.
The Question of Health Risks
Despite public fears, the health risks of eating commercialized GM foods on the market now appear to be negligible, experts say. Nearly 45% of the corn and 85% of the soybeans grown in the United States are transgenic, according to the USDA/ERS. Consumers are eating these foods without any apparent health effects, although some stakeholders caution that greater postmarket surveillance is needed to confirm this.
As part of research and development, GM foods are tested for safety, specifically to ensure they don’t contain compounds that might cause allergic reactions among those who eat them. How might this happen? Consider how biotechnology works: Scientists take genes from one species and incorporate them into the genome of another. The modified genes in the transgenic hybrid are designed to make proteins that ideally will do something useful, like deter pests or boost nutrition. But these same proteins might also be allergenic; in fact, most known allergens are protein molecules.
The only way to confirm that a transgenic protein is or is not an allergen is to test it in large numbers of people. But of course, large-scale human testing isn’t practical or ethically possible. Therefore, scientists resort to surrogate tests to predict whether the transgenic protein will elicit a human allergic response.
These tests have evolved considerably since GM crops were first introduced. In the early 1990s, scientists would test transgenic proteins with serum obtained from people known to be allergic to the gene sources of the modified plant. If a protein reacted with a serum antibody called IgE—which plays a role in nearly all allergies—it was flagged as an allergen. In 1993, scientists using this approach detected allergenicity in a transgenic soybean containing a gene from Brazil nuts. This soybean—created by Pioneer, now a subsidiary of Dupont—was to be used as a nutrition-enhanced poultry feed (Brazil nuts are high in methionine, an essential amino acid that soybeans lack). If commercialized, it could have posed serious health risks to farmers working with the feed: Brazil nuts can be fatal if you’re allergic to them. But the transgenic protein tested positive in the serum assay, so the soybean was pulled during early development and destroyed.
Steve Taylor, codirector of the Food Allergy Research and Resource Program at the University of Nebraska, discovered the soybean/Brazil nut problem while under contract to Pioneer. He says scientists took close note of the incident. Today, he adds, companies reduce the risk of similar problems by avoiding genes from known allergens, 90% of which are attributed to just eight foods (eggs, cow’s milk, peanuts, tree nuts, fish, shellfish, soybeans, and wheat).
The serum test would still be optimal for screening genes from known allergenic sources, Taylor says. But because no one uses genes from these sources anymore, the test is rarely used. Instead, companies now rely largely on initial screens that compare transgenic proteins to the structures and characteristics of known allergens.
In one such method, known as sequence homology, scientists compare a transgenic protein’s amino acid sequence with the sequences of known allergens in a database. If the protein shares a predetermined level of similarity with one or more allergens, then it is flagged for further study. Several databases have emerged to meet this need; one of these, developed by the Food Allergy Research and Resource Program, contains nearly 1,200 allergens and is growing steadily.
Another method exploits the fact that most allergens are large and resistant to stomach acids. Called the pepsin digestibility assay, this test exposes proteins to simulated stomach fluids for varying durations. Most allergens survive for up to an hour, whereas nonallergens degrade within 15–30 seconds.
If these initial screens suggest that a transgenic protein is allergenic, companies can use serum testing for further confirmation. If allergenicity is still indicated, then efforts to further develop the GM variety are typically abandoned.
Agronomists have long known that conventional plant breeding can produce allergenic compounds. For instance, the Chinese gooseberry, a small, somewhat bitter fruit, was conventionally modified in New Zealand to make kiwifruits, which produced allergic reactions among some consumers, although the modified fruits remain popular at produce markets. A key question is whether transgenic proteins have more allergenic potential than those produced by conventional plant breeding.
After more than a decade of testing and debate, the emerging consensus among scientists is that they do not. The National Academy of Sciences recently expressed this view in its 2004 report Safety of Genetically Engineered Foods: Approaches to Assessing Unintended Health Effects, which stated, “The process of genetic engineering has not been shown to be inherently dangerous but rather, evidence to date shows that any technique, including genetic engineering, carries the potential to result in unintended changes in the composition of the food.”
The U.S. Regulatory System
As far as U.S. regulatory agencies are concerned, agrobiotech companies need only demonstrate that—apart from the transgenic protein—a GM crop shares equivalent composition and nutritional status to its conventional counterpart. If this is shown to be the case, then the crop is said to be as safe as the conventional variety, and companies are free to sell it. Crops that contain a pesticidal protein such as Bt toxin must undergo mandatory allergenicity testing coordinated by the Environmental Protection Agency. All other GM traits are evaluated by voluntary consultations with the Food and Drug Administration (FDA). During these consultations, FDA and company representatives discuss procedures, and the companies disclose data and describe testing methods and results. The FDA recently introduced draft guidance on testing that encourages companies to come in at the very early stages of the process, when they are still in planning stages.
GM opponents have long argued that FDA consultations should be mandatory. But Jason Dietz, a consumer safety officer at the FDA’s Center for Food Safety and Applied Nutrition, says that in the administration’s view, the risks posed by transgenic crop breeding aren’t great enough to warrant mandatory testing. Moreover, he adds, companies are liable for the health risks of GM foods under the safety provisions of the Federal Food, Drug, and Cosmetic Act.
The best way for companies to ensure their compliance with the act, Dietz says, is to undergo a premarket consultation with the FDA. “To our knowledge, all [GM] foods intended to be commercialized in the United States have been through the consultation procedure,” he says.
An important and unresolved question is whether current testing methods will be adequate for second-generation crops. All the pest- and herbicide-resistance traits used now are found at minute levels in the plants, far below those likely to produce allergic responses, according to Taylor. But in some second-generation varieties, GM traits are intentionally expressed at high levels that change the nature of the food.
Taylor suggests that uncertainties about second-generation crop testing exacerbate the agrobiotech industry’s reluctance to develop these markets further. “Because [the plant’s] composition is significantly altered, and components are expressed at high levels, second-generation crops will probably require more extensive safety evaluation,” he says. “One of the key issues is that there is no international agreement on what will be required. The uncertainty is considerable, and that creates hesitancy on the part of companies. Regulatory approvals will be less certain, consumer acceptance is a hurdle, and scientific uncertainty about how to proceed with safety assessment causes worry.”
The Labeling Scene
In many countries, debates over GM foods have been accompanied by growing demands for an international labeling scheme to segregate transgenic and conventionally grown products. Labeling isn’t required in the United States because regulatory agencies here don’t view commercialized GM food as materially different from conventional varieties. However, the European Union does require it, and countries including Australia, Japan, and New Zealand, among others, have either established labeling systems or are in the process of doing so.
GM labeling is a tricky proposition that U.S. companies would rather avoid. Some surveys have shown that consumers are less likely to buy foods that they know are GM. Not only does labeling threaten markets, it could also be hard to implement, says Alan McHughen, a biotech specialist and geneticist at the University of California, Riverside. With few exceptions, most commodity crops grown in the United States aren’t segregated once they reach the supply chain. Thus, both GM and conventionally grown nonorganic crops can wind up in the same containers as they make their way through distribution channels.
McHughen says the challenge is to somehow guarantee that GM labeling is accurate and credible, which is no easy task. “From the farmer, to the county elevator, to the rail or barge that carries bulked grain to terminals, to the retailers—every step [in the labeling process] would have to be monitored and verified,” he says.
Even so, labeling is necessary because food distribution is increasingly globalized, says Juan Lopez, international coordinator for biosafety with Friends of the Earth, a nongovernmental organization. The problem, he emphasizes, is that without a comprehensive labeling system, GM products can wind up in countries that don’t want them.
Some recent high-profile episodes have heightened these concerns. In late 2004, Syngenta announced it had accidentally put a controversial type of GM corn on the market in the United States and Europe during the previous four years. The corn, known as Bt10, differs from a similar variety called Bt11 by only a few nucleotides. But whereas Bt11 has been approved in Europe, Bt10 never underwent review and thus is considered illegal in Europe. The accident produced no known illnesses, but many seized on it as further justification for labeling. Syngenta’s woes with Bt10 have only continued: in early summer 2005, large commodity corn shipments in Japan were found to be comingled with Bt10, and a similar comingled shipment was intercepted in Ireland.
While Syngenta was grappling with its botched shipments, the 119 signatories of the United Nations Cartagena Protocol on Biosafety (a supplementary agreement of the Convention on Biological Diversity) were deciding whether to create documentation requirements for bulk shipping of “living modified organisms,” which are the live GM organisms such as seeds (rather than milled forms such as flour). But this initiative failed during last-minute negotiations at a meeting in Montréal on 3 June 2005. Protocol rules require consensus for passage, which couldn’t be reached because Brazil and New Zealand refused to sign on, claiming the paperwork would be excessive and costly. (The United States is not a party to the Convention on Biological Diversity and therefore cannot be a party to the Cartagena protocol.) The failure means the burden of proof for ensuring GM-free shipments remains with importers, Lopez says.
“This would have been the first time a global system for the identification of [GM organisms] would have been in place,” he adds. “But countries at the national and regional level are working to implement identification and labeling schemes anyway.”
The Future
Today, GM agriculture’s future seems hard to predict. Its growth is undeniable—ISAAA figures indicate that global acreage of GM crops increased by 20% in 2004 with no sign of slowing. But the vast majority of this growth occurred in just a handful of countries planting just a handful of crop varieties. The new second-generation crops that comprise the bulk of the industry’s consumer marketing efforts appear to be largely stalled, held at bay by market uncertainty and the voracious attacks of environmental groups.
Consider the plight of Golden Rice, the product of a largely humanitarian effort led by Syngenta and a consortium of nonprofit research groups. Golden Rice was meant as a means to boost daily intakes of vitamin A; deficiency-related blindness and death currently afflicts nearly 2 million people annually, according to the United Nations Children’s Fund. However, Golden Rice is under sustained assault by Greenpeace, which claims that health effects have not been sufficiently addressed, that the rice could breed with and contaminate wild varieties, and that the whole effort is merely a ploy to gain acceptance for GM food in developing countries. Jorge Mayer, manager of the Golden Rice Project at the University of Freiburg in Germany, as quoted in the 2 April 2005 New Scientist, countered that Greenpeace’s blanket opposition to Golden Rice is impeding the very trials that will provide the answers the group demands. “It’s a catch-22,” he said.
So what is the truth of the matter? A conclusive answer isn’t easy to find. Biotech companies claim GM technology will help feed the world’s poor, but how do they intend to protect intellectual property in developing markets? Despite repeated questioning, sources for this article could not provide a clear answer to that question. Companies have sued farmers for saving seeds from their GM varieties and planting them without payment for intellectual property; Monsanto has more than 100 such lawsuits ongoing in the United States today, says Horner. Will farmers in developing countries also have to pay for GM seeds, year after year? What will that mean for traditional agriculture, which depends on the age-old practice of saving seeds for future planting?
While these questions remain, studies show that GM technology can produce important benefits. Carl Pray, a professor of agriculture, food, and resource economics at Rutgers University, recently concluded a study showing that growing Bt rice in China reduced by half the number of chemical pesticide poisonings among farmers. His research also showed that farmers who planted the rice saved money with increased crop yields and reduced chemical pesticide use. His results are published in the 29 April 2005 issue of Science. “I’m convinced [the crops] are a positive development for China,” Pray says.
Other farmers who grow GM crops echo these sentiments. Given that GM agriculture is here to stay, the optimal scenario for the future—and the likely eventual outcome—is a dual supply chain, one that clearly distinguishes GM from non-GM products. In the meantime, the rhetoric and spin that surrounds this most heated of environmental battles will go on.
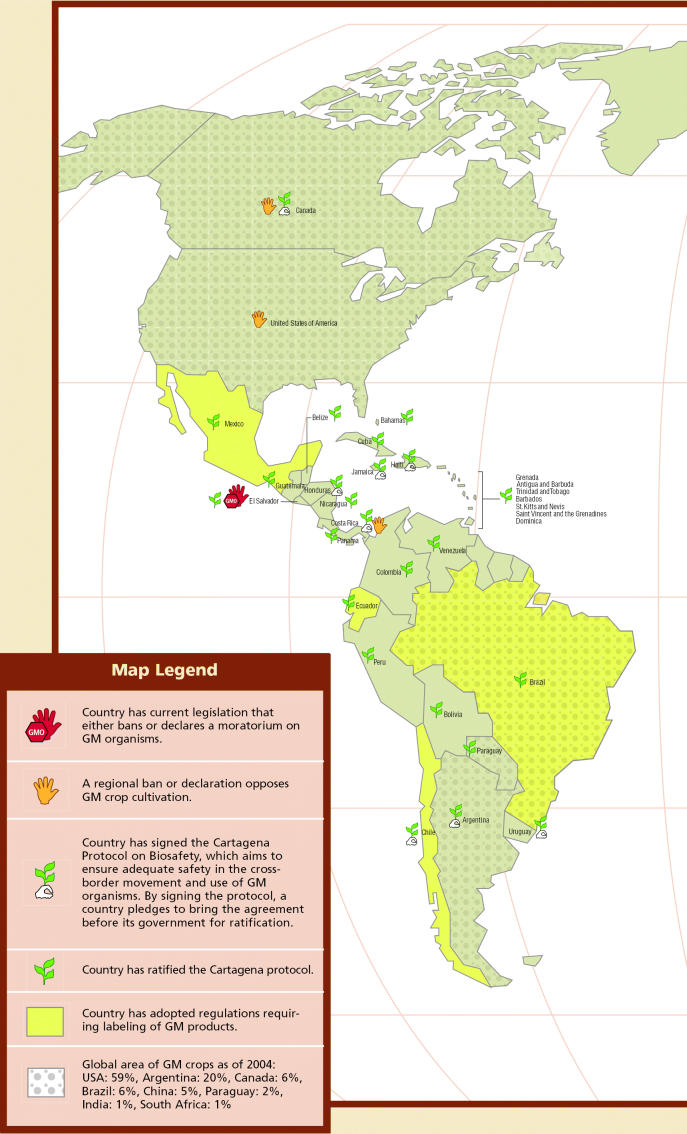
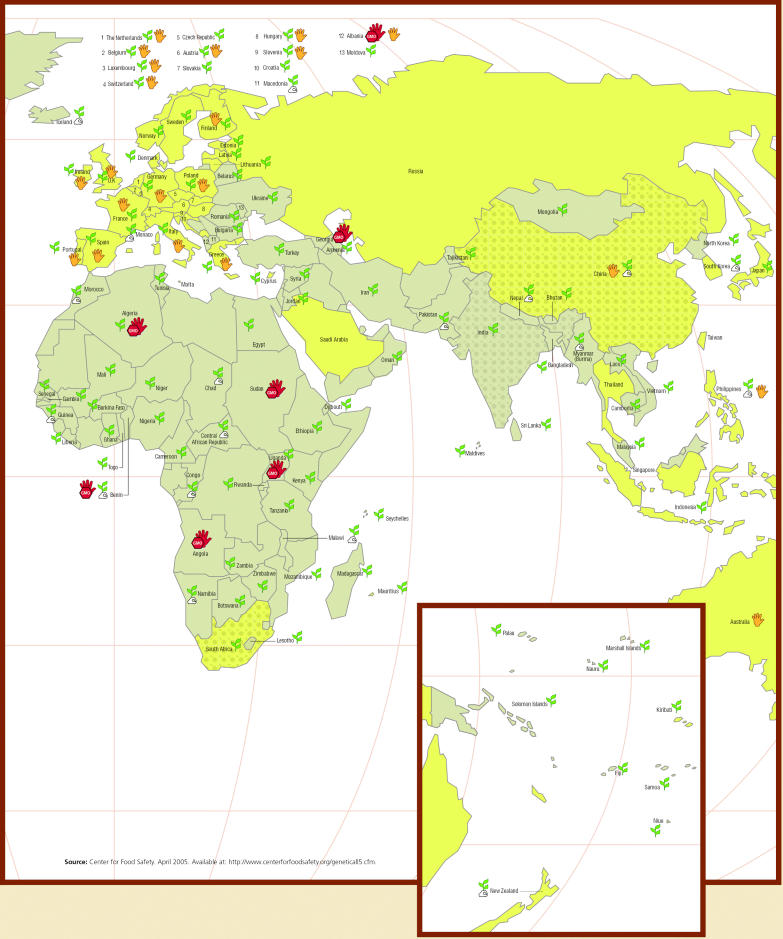
Genetically Modified Crops and Foods.
Worldwide Regulation, Prohibition, and Production
Crops and cops.
In Lincolnshire, England, a protester from the group Genetix Snowball digs up a GM sugar beet in protest as policemen intervene.
The labeling dilemma.
Some stakeholders claim that labeling of GM foods would go a long way toward assuring consumers that they have a choice in whether to consume such products, although studies have shown consumers are likely to avoid GM items labeled as such.
Boon or bane?

At the GMO Research Centre in Los Banos, Philippines, a scientist examines GM rice plants. New varieties of GM plants offer the promise of better yields and improved nutritional value; opponents contend that such benefits may come at too high a price. For example, Golden Rice (pictured above in two varieties, along with white conventional rice) could boost daily intakes of vitamin A and fight deficiency-related blindness and death. However, the activist organization Green-peace protests that the rice hasn’t been adequately tested for potential adverse health and environmental effects.