Abstract
Individuals who are latently infected with Mycobacterium tuberculosis can develop active disease via either endogenous reactivation of the latent bacilli or exogenous reinfection with a second mycobacterial strain. In this study, we investigated whether immunization with a tuberculosis DNA vaccine cocktail that induces significant protective responses in mice could prevent reactivation of disease in a murine latent-tuberculosis model. In addition, we assessed whether DNA vaccination could retard the growth of a secondary aerogenic infection with M. tuberculosis (exogenous reinfection) in latently infected mice. In the reactivation studies, administration of the DNA vaccine combination did not prevent recrudescence of the latent infection after injection of dexamethasone. Moreover, for the reinfection experiments, only a modest decrease in the growth of a secondary M. tuberculosis challenge in DNA-vaccinated animals, compared to controls, was observed 14 and 28 days after the reinfection of previously exposed mice. Interestingly, although proliferation of the secondary challenge was reduced significantly in a nonvaccinated chronic-infection group relative to the naïve controls, the number of bacilli still increased by 500-fold 1 month after the secondary challenge in mice with active tuberculosis. These results indicate that novel immunotherapeutic approaches will be required to prevent reactivation of infection or reinfection of individuals with latent tuberculosis.
Pulmonary tuberculosis is the most important life-threatening bacterial disease in the world today. The World Health Organization has estimated that about 1.7 billion people are infected with Mycobacterium tuberculosis, and approximately 2 million individuals die annually as a result of this highly infectious disease (10). The pathogenesis of tuberculosis is unusual because, unlike many other infections, the development of active disease can involve a delay between infection and clinical disease ranging from several weeks to several decades (11). While active disease may arise almost immediately after infection in about 5% of exposed individuals, most of the others will develop a latent infection in which the tubercle bacilli can persist in vivo without causing any clinical symptoms. Active disease may develop decades later either as a recrudescence of the initial infection (endogenous reactivation) or because of a secondary infection with a different tuberculosis strain (exogenous reinfection). Although most recurrent tuberculosis cases in adults probably result from reactivation of the latent infection, several recent studies using DNA fingerprinting methodologies indicate that exogenous reinfection may contribute significantly to the total tuberculosis burden in areas of high endemicity (4, 5, 24, 28). Reports which show that immunocompetent hosts can have simultaneous infections with multiple M. tuberculosis strains provide further support for the exogenous-reinfection mode of disease transmission (2, 3).
The development of a more effective vaccine against pulmonary tuberculosis represents the best long-term strategy for controlling the worldwide epidemic. The existing vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG), has been used throughout the world for nearly 70 years, but its variable efficacy, seen in a number of carefully controlled clinical trials, has suggested that the capacity of BCG to prevent adult primary tuberculosis is severely limited (6). In recent years, many new candidate tuberculosis vaccines, including recombinant BCG strains, attenuated tuberculosis auxotrophs, various subunit preparations, and DNA vaccines, have been developed and are being actively tested in animals (for a review, see reference 20). Several of these immunogenic preparations have been shown to elicit protective responses that approach the protective efficacy of BCG when tested in primary-infection models (1, 8, 19). However, the therapeutic effectiveness of these new tuberculosis vaccines in postexposure models is much less certain. Recently, Lowrie et al. reported that vaccination of mice with a DNA construct expressing the hsp60 protein of Mycobacterium leprae completely prevented bacterial reactivation in mice with latent tuberculous infections (14). In contrast to this hsp60 data, several other tuberculosis vaccines that have been shown to be protective in primary-challenge models have failed to protect the host when administered immunotherapeutically (26).
Our laboratory has recently developed a tuberculosis DNA vaccine combination (consisting of 10 different single DNA vaccines) which was shown to effectively protect naïve mice against a low-dose aerogenic challenge with virulent M. tuberculosis (8). In survival experiments, the mean time to death for mice immunized with the DNA vaccine combination was extended by as much as sevenfold relative to the naïve controls, and the mean survival time for the DNA-vaccinated mice was statistically equivalent to the mean survival time of BCG-vaccinated mice. Given the effectiveness of this combination vaccine in a primary-infection model, we tested whether postexposure immunization with this DNA vaccine cocktail protected against the development of tuberculosis in a murine latent-tuberculosis model. Our results suggest that the DNA vaccination had a modest impact on the growth of a secondary challenge strain in exogenous-reinfection studies. However, DNA immunization with this vaccine cocktail had little effect on the endogenous reactivation of the disease in a mouse latent-tuberculosis model.
MATERIALS AND METHODS
Animals.
Pathogen-free C57BL/6 female mice were obtained from the Jackson Laboratories (Bar Harbor, Maine). The mice were 6 to 8 weeks old when the studies were initiated. They were maintained under barrier conditions and fed commercial mouse chow and water ad libitum.
Microorganisms.
M. tuberculosis H37Rv and the acriflavin-resistant (Acr) M. tuberculosis Erdman strain were obtained from the Trudeau Mycobacterial Culture Collection, Saranac Lake, N.Y.
Preparation of the DNA vaccine combination.
The cloning of the tuberculosis genes into the pJW4303 vector and the testing of the constructs have been described previously (7, 8, 13, 18). The DNA vaccine combination was comprised of a plasmid cocktail which contained equal amounts of the 10 individual DNA vaccines listed in Table 1. In previous studies, each of these single vaccines induced substantial protective responses in the primary mouse challenge model. For this study, endotoxin-free plasmid DNA was prepared and purified with the EndoFree Plasmid Maxi and Mega kits (Qiagen, Chatsworth, Calif.).
TABLE 1.
M. tuberculosis antigens encoded by combination vaccine DNA constructs
| Antigen | Function | Antigen format |
|---|---|---|
| Esat-6 | CFPa | TPA fusion |
| Ag85B | CFP | Native |
| MTB8.4 | CFP | TPA fusion |
| MTB12 | CFP | TPA fusion |
| MPT63 | CFP | TPA fusion |
| MPT64 | CFP | TPA fusion |
| MPT83 | CFP | TPA fusion |
| KatG | Virulence factor | TPA fusion |
| MTB39A | PPE family | TPA fusion |
| Rv_1818 | PE family | Native |
CFP, culture filtrate protein.
Reinfection of DNA-vaccinated mice with latent tuberculosis infections.
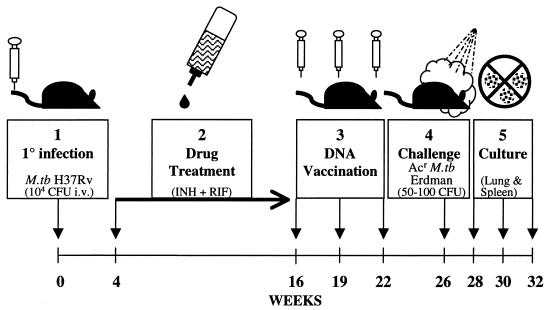
The protocol for the exogenous-reinfection studies is outlined in Fig. 1. To establish a latent tuberculous infection, C57BL/6 mice were injected intravenously with 104 CFU of the M. tuberculosis H37Rv strain. Four weeks after primary infection, most of the animals were treated for 12 weeks with isoniazid (INH)- and rifampin (RIF)-enriched water (85 μg of INH plus 50 μg of RIF per liter). Grosset et al. previously showed that this therapeutic regimen is as effective as regimens using pyrazinamide in the treatment of murine tuberculous infections (12). Importantly, following chemotherapy (drug treatment), no viable tubercle bacilli could be detected in homogenates of the lungs and spleens of the initially infected mice by a standard CFU plating procedure. As a control, one group of mice with the primary infection was not drug treated prior to DNA vaccination and therefore maintained a chronic M. tuberculosis H37Rv infection.
FIG. 1.
Protocol for DNA vaccination of mice with latent tuberculosis infection. Mice were infected intravenously with 104 CFU of M. tuberculosis H37Rv. After 4 weeks, INH and RIF chemotherapy was administered for 3 months. Three doses of DNA combination vaccine were given 3 weeks apart. One month after the last vaccination, the mice were challenged aerogenically with 50 to 100 CFU of Acr M. tuberculosis (M.tb) Erdman. The growth of Acr bacteria from lung and spleen homogenates was monitored at 2, 4, and 6 weeks postchallenge.
DNA vaccination was initiated with three intramuscular injections of 200 μg of the 10-component tissue plasminogen activator signal sequence (TPA) DNA vaccine combination given at 3-week intervals as described previously (8). The plasmid cocktail contained 20 μg of each of the 10 individual DNA vaccines. As a control, three injections of 200 μg of the empty vector were given to the drug-treated mice. In parallel, a group of naïve mice were injected with the TPA DNA vaccine combination using the same immunization protocol. The effect of DNA vaccination on exogenous reinfection was evaluated in two separate studies. In each experiment, mice were challenged with a low dose of Acr M. tuberculosis Erdman (50 to 100 CFU) given 4 weeks after the final injection of either the DNA vaccine combination or the vector control. Subsequently, the growth of the secondary challenge in the lungs and spleens of the infected mice was assessed 2, 4, and 6 weeks postchallenge by plating serial dilutions of organ homogenates on Middlebrook 7H11 plates supplemented with 10% oleic acid-albumin-dextrose-catalase and 10 μg of acriflavin/ml. The use of this virulent drug-resistant strain permitted the differentiation of the secondary challenge from the residual primary H37Rv infection. In all cases, five mice per group were used for each time point.
Reactivation of latent tuberculosis in DNA-vaccinated mice.
The infection model described above was also used to investigate the effect of immunization with our DNA vaccine combination on the reactivation of disease. For these studies, the mice were infected and then treated with INH and RIF and vaccinated with DNA as described above. The drug-treated mice were then immunosuppressed with dexamethasone (120 μg of dexamethasone was injected intramuscularly every other day for 14 days) 4 weeks after the final DNA vaccination. Classic studies by McCune et al. had shown that glucocorticoid injections can reactivate murine latent tuberculosis infections which had been reduced to nondetectable levels by antibiotic therapy (15, 16). At specified intervals after the dexamethasone administration, mice were sacrificed to assess the mycobacterial burden in relevant organs. The numbers of CFU in the lungs and spleens of the infected mice were determined by plating serial dilutions of organ homogenates on Middlebrook 7H11 agar. In all cases, five mice per group were used for each time point.
Statistical evaluation of the CFU results.
The mycobacterial CFU numbers of experimental and control groups were compared using the one-way analysis of variables of the In Stat program version 3.0 (GraphPad Software, San Diego, Calif.).
RESULTS
Effect of DNA vaccination on exogenous reinfection.
The tuberculosis vaccine preparation utilized in these studies consists of 10 different single DNA vaccines (Table 1). Eight of these vaccines encode proteins fused at the N terminus to the TPA, while the other two express native tuberculosis proteins. The protocol for investigating the impact of immunization with the vaccine cocktail on the growth of a secondary challenge is outlined in Fig. 1, and the results of the reinfection experiments are shown in Table 2. In the initial experiments, the following groups were aerogenically infected with the secondary Acr M. tuberculosis Erdman challenge strain: naïve controls, DNA-vaccinated naïve mice (DNA), H37Rv chronically infected animals (chronic group), drug-treated mice, and drug-treated animals immunotherapeutically treated with either the vaccine preparation (drug treated plus DNA) or the vector alone (drug treated plus vector). Surprisingly, the secondary challenge grew extensively in the chronically infected mice that had not been subjected to the antibiotic treatment (chronic group) and that still had a substantial mycobacterial burden at the time of reinfection (4.6 log10 CFU of H37Rv in the lung). Although highly significant reductions in lung CFU (0.91 to 1.17 log10; P < 0.001) were seen compared to naïve controls at each assay time point in this chronic-infection group, the bacterial numbers of the secondary Acr M. tuberculosis Erdman challenge still increased by 500-fold during the first month. In the nonvaccinated drug-treated animals, decreased growth of the secondary challenge relative to that in naïve controls (P < 0.001) was also observed at 2, 4, and 6 weeks. However, as with the chronic-infection group, the CFU for Acr M. tuberculosis Erdman secondary challenge also increased 500-fold during the first month. When the drug-treated mice were vaccinated with the DNA vaccine cocktail (drug treated plus DNA) prior to the second infection, modest but significant reductions (P < 0.05) in the growth of the Acr M. tuberculosis Erdman strain compared to that in the unvaccinated drug-treated group were detected 2 and 4 weeks after the secondary challenge. In contrast, injection of the vector alone (drug treated plus vector) into the antibiotic-treated animals did not reduce the proliferation of the secondary infection compared to the drug-treated-only group. While DNA vaccination had only a modest effect in drug-treated mice, immunization with the DNA vaccine combination significantly reduced the growth of a primary infection in naïve mice (DNA group; P < 0.01). In this study, an ∼10-fold decrease (−0.97 log10 CFU) was detected in the DNA-vaccinated mice relative to naïve controls 28 days after a challenge with the Acr M. tuberculosis Erdman organisms.
TABLE 2.
Reinfection studya
| Expt | Site | Experimental groupb | No. of CFUc
|
||
|---|---|---|---|---|---|
| Day 14 | Day 28 | Day 42 | |||
| 1 | Lung | Naive | 5.21 ± 0.17 | 5.36 ± 0.15 | 4.90 ± 0.07 |
| DNA | 4.61 ± 0.19 (−0.60) | 4.39 ± 0.18 (−0.97) | 4.50 ± 0.13 (−0.40) | ||
| Chronic | 4.04 ± 0.11 (−1.17) | 4.33 ± 0.21 (−1.03) | 3.99 ± 0.22 (−0.91) | ||
| Drug treated | 4.61 ± 0.13 (−0.60) | 4.47 ± 0.15 (−0.89) | 4.22 ± 0.18 (−0.68) | ||
| Drug treated + DNA | 4.21 ± 0.05 (−1.00)d | 4.14 ± 0.12 (−1.22)d | 4.10 ± 0.14 (−0.80) | ||
| Drug treated + vector | 4.52 ± 0.11 (−0.69) | 4.50 ± 0.29 (−0.85) | 4.36 ± 0.20 (−0.54) | ||
| Spleen | Naive | 4.60 ± 0.36 | 4.25 ± 0.10 | ||
| DNA | 3.81 ± 0.52 (−0.79) | 3.61 ± 0.35 (−0.64) | |||
| Chronic | <2e | <2 (−2.25) | |||
| Drug treated | <2 | 2.96 ± 0.15 (−1.29) | |||
| Drug treated + DNA | <2 | 2.46 ± 0.45 (−1.77) | |||
| Drug treated + vector | <2 | 2.32 ± 0.33 (−1.93) | |||
| 2 | Lung | Naive | 4.50 ± 0.34 | 4.74 ± 0.17 | 4.56 ± 0.11 |
| Chronic | 3.28 ± 0.06 (−1.22) | 3.34 ± 0.12 (−1.40) | 3.52 ± 0.30 (−1.04) | ||
| Drug treated | 3.95 ± 0.28 (−0.55) | 4.01 ± 0.05 (−0.73) | 4.18 ± 0.10 (−0.36) | ||
| Drug treated + DNA | 3.57 ± 0.07 (−0.93)d | 3.65 ± 0.16 (−1.09)d | 3.91 ± 0.43 (−0.65) | ||
| Spleen | Naive | 3.99 ± 0.17 | 4.19 ± 0.24 | ||
| Chronic | <2 | <2 (−2.19) | |||
| Drug treated | <2 | 2.12 ± 0.23 (−2.07) | |||
| Drug treated + DNA | <2 | 2.08 ± 0.15 (−2.11) | |||
The naïve and DNA groups of mice were aerogenically challenged with an Acr M. tuberculosis Erdman strain as a primary infection. The other groups of mice were aerogenically challenged with the same acriflavin resistant strain following the initial intravenous M. tuberculosis H37Rv infection and the subsequent DNA vaccinations. The CFU numbers represent only the growth of the acriflavin-resistant challenge strain on Middlebrook 7H11 agar containing 10 μg of acriflavin/ml.
DNA, naïve mice immunized with the DNA vaccine combination; chronic, H37Rv primary infection only; drug treated, H37Rv primary infection followed by INH and RIF chemotherapy; drug treated + DNA, primary infection followed by chemotherapy and DNA vaccination; drug treated + vector, primary infection followed by chemotherapy and injection of the vector control.
Mean CFU log10 ± standard deviation; the numbers in parentheses represent the log10 reduction in CFU in experimental mice relative to that in naïve mice.
Drug-treated + DNA group is significantly less than drug-treated group (P < 0.05).
Limit of detection of the assay is 100 CFU/organ (2 log10).
For the naïve controls, typical bacterial numbers (4.60 log10) were detected in the spleens 28 days after the low-dose challenge with the Acr M. tuberculosis Erdman strain. As shown in Table 2, significant reductions in spleen CFU relative to controls were seen in naïve animals that had been only DNA immunized (DNA group; 0.79 log10 decrease; P < 0.05). However, the impact of DNA vaccination on dissemination of the lung infection to the spleen and other lymphoreticular organs was difficult to assess because bacterial dissemination was substantially delayed in all test animals that received the primary H37Rv infection. At this time point, the mycobacterial CFU numbers for all mice given the primary H37Rv infection were below the level of detection of this assay (100 CFU/organ). By 42 days after the secondary challenge, low bacterial CFU numbers were detected in the spleen homogenates of the drug-treated, drug-treated plus DNA, and drug-treated plus vector groups, but dissemination to the spleen was still not observed in the group with chronic tuberculosis infection. Although the splenic CFU for the drug-treated plus DNA vaccine and drug-treated plus vector control groups were lower at this time point than for the drug-treated-only mice, these differences were not significant.
The overall results of a second reinfection experiment (Table 1) paralleled the data from the initial study despite a lower secondary-challenge dose (as demonstrated by 4.60 log10 CFU at day 14). In this second experiment, the DNA vaccination only (DNA) group was not repeated because the effectiveness of the DNA vaccine combination had been consistently demonstrated in experiment 1 and other, earlier studies (8). A vector control was also not included in the second trial because we have repeatedly shown that injection with the vector alone does not elicit a memory response against M. tuberculosis. In this experiment, the active H37Rv infection (chronic group) again substantially limited, but did not prevent, the growth of the secondary Acr M. tuberculosis Erdman challenge in the lung (P < 0.001). Growth of the Acr M. tuberculosis Erdman strain was also reduced in the drug-treated control group at all time points tested (P < 0.01). Similar to the first study, at 2 and 4 weeks postaerogenic challenge, moderate but significantly decreased proliferation of the secondary Acr M. tuberculosis Erdman infection within the lung was detected in the drug-cured mice that had been immunized with the DNA vaccine combination (drug-treated plus DNA) compared to the drug-treated-only controls (P < 0.05). In addition, dissemination to the spleen was substantially retarded in all mice receiving the primary H37Rv infection. Again, no spread of the secondary infection to the spleen was detected until 42 days after the aerosol challenge.
Impact of DNA vaccination on reactivation of disease.
The effect of immunization with our DNA vaccine combination on reactivation of disease was investigated using the same latent tuberculosis infection model shown in Fig. 1. The mice were infected with the M. tuberculosis H37Rv strain, treated with INH and RIF, and vaccinated with DNA as described above. However, for these studies, the drug-treated mice were immunosuppressed with dexamethasone to reactivate the infection 4 weeks after the final DNA vaccination. At the time of the dexamethasone injections (10 weeks after the completion of INH and RIF therapy), no tubercle bacilli were detected in the lungs or spleens of the drug-treated mice. However, 3 weeks after the mice received the dexamethasone treatment, about 105 mycobacterial CFU per lung were present in all experimental groups (drug treated, drug treated plus DNA, and drug treated plus vector). No differences in CFU numbers were detected among the various groups (Table 3). Furthermore, the CFU numbers at 6 and 9 weeks after dexamethasone treatment were essentially the same, and again, no significant differences in bacterial CFU numbers were found among the three treatment groups.
TABLE 3.
Reactivation study
| Expt | Site | Experimental groupa | No. of CFUb
|
|||
|---|---|---|---|---|---|---|
| Day 21 | Day 42 | Day 52 | Day 63 | |||
| 1 | Lung | Drug treated | 4.98 ± 0.07 | 4.98 ± 0.30 | 5.28 ± 0.48 | |
| Drug treated + DNA | 5.12 ± 0.11 | 4.88 ± 0.10 | 5.08 ± 0.39 | |||
| Drug treated + vector | 5.41 ± 0.44 | 5.24 ± 0.13 | 5.10 ± 0.39 | |||
| Spleen | Drug treated | 3.87 ± 0.25 | 4.25 ± 0.49 | 4.15 ± 0.75 | ||
| Drug treated + DNA | 3.37 ± 0.22 (−0.50)c | 3.88 ± 0.30 | 3.90 ± 0.45 | |||
| Drug treated + vector | 4.28 ± 0.20 | 4.38 ± 0.47 | 4.26 ± 0.49 | |||
| 2 | Lung | Drug treated | 5.45 ± 0.35 | 5.36 ± 0.30 | ||
| Drug treated + DNA | 5.13 ± 0.23 | 5.07 ± 0.24 | ||||
| Spleen | Drug treated | 5.63 ± 0.21 | 4.87 ± 0.28 | |||
| Drug treated + DNA | 5.32 ± 0.16 | 4.89 ± 0.37 | ||||
Drug treated, H37Rv primary infection followed by INH and RIF chemotherapy; drug treated + DNA, primary infection followed by chemotherapy and DNA vaccination; drug treated + vector, primary infection followed by chemotherapy and injection of the vector control.
Mean CFU ± standard deviation; the number in parentheses represents a log10 reduction in CFU in experimental mice relative to that in naïve mice.
Drug-treated + DNA group is significantly less than drug-treated-only group (P < 0.05).
DNA vaccination had a modest effect in the spleen after dexamethasone administration. At 21 days following the steroid treatment, a small but significant reduction in mycobacterial CFU (0.50 log10 decrease; P < 0.05) was detected in the spleens of drug-treated mice immunized with the DNA cocktail (drug treated plus DNA) relative to the unvaccinated drug-treated controls. Although this moderate decrease in bacterial CFU for the drug-treated plus DNA vaccine group persisted for 6 weeks, the significance of the 42-day result was obscured by high counting errors in the assay. Again at 9 weeks, no significant differences in splenic CFU could be detected for any of the experimental groups.
In a second reactivation experiment, bacterial CFU in the lungs and spleens of drug-treated mice were assessed 3 and 7 weeks after the initial dexamethasone injection. Although the mycobacterial CFU numbers in the lungs and the spleens were moderately reduced in the drug-treated plus DNA-vaccinated group relative to controls at 3 weeks, these differences were not statistically significant. At 7 weeks post-dexamethasone treatment, no significant differences were seen in the organ CFU numbers of the two treatment groups.
DISCUSSION
In these studies, we have demonstrated that a tuberculosis DNA vaccine cocktail which is effective in protecting naïve mice against a primary aerogenic tuberculosis challenge showed only modest activity when used in a postexposure model. In exogenous-reinfection studies, immunization with this combination plasmid vaccine had a moderate effect on the growth of a secondary challenge. However, in complementary experiments, vaccination with the DNA cocktail had virtually no effect on the dexamethasone-induced endogenous reactivation of latent tuberculosis infections. Our results are consistent with several other reports which strongly suggest that postexposure vaccination does not prevent relapse in mice with latent tuberculosis. For example, Turner et al. (26) showed that a DNA vaccine that was effective against a primary infection did not protect when administered immunotherapeutically in a postexposure model. In addition, Dhillon and Mitchison found that immunization with either BCG or a heat-killed Mycobacterium vaccae vaccine did not alter the relapse rate in a murine model of dormant tuberculosis (9).
It is not clear why a DNA vaccine combination that effectively protects naïve animals against tuberculosis infections does not efficiently protect mice in a latency model. This apparent inconsistency may be due to the refractory nature of stimulated immune cells in mice with latent tuberculosis infections. In the chronically infected host, the continued stimulation of immune cells seems to be the basis for the maintenance of the tuberculosis lesion. The reactivation of tuberculosis seen after treatments with anti-cytokine antibodies that reduce tumor necrosis factor alpha or gamma interferon levels or anti-T-cell antibodies that eliminate CD4+- or CD8+-cell populations supports the conclusion that a strong and active immune response is required to retain the latently infected state (17, 23, 27). Thus, it is not surprising that DNA vaccination has only a modest impact on a host that is already effectively stimulated by the presence of mycobacterial immunogens. Consistent with these observations, we and others have shown that it is difficult to boost the antimycobacterial immunoreactivity of mice that have concurrent mycobacterial infections with DNA vaccines (26; S. Morris, unpublished observations). At the molecular level, chronically infected animals control the tuberculosis infection largely by forming granulomas (21). To reduce or eliminate reactivation of disease, a vaccine must potentiate antimycobacterial activity within the lung granulomas. Thus far, there is little evidence to indicate that postexposure vaccination can further stimulate the immunoreactivity in the preexisting chronic granulomas. Therefore, the failure of DNA vaccination to decrease recrudescent disease in our reactivation studies probably occurs because DNA immunization does not effectively augment preexisting antituberculous activity within these granulomas. In contrast, immunization with our DNA vaccine combination does seem to accelerate the formation of lymphocyte-rich infiltrates after aerogenic challenge of naïve mice (8). Consequently, the moderate protective effect seen in our postexposure reinfection studies may result because DNA vaccination stimulates the newly formed granulomas needed to contain the secondary-challenge population.
The modest immunotherapeutic effect of the 10-component DNA cocktail vaccine seen in these postexposure studies is difficult to reconcile with the spectacular results of Lowrie et al. (14). Lowrie and colleagues reported that latently infected mice that had been immunized with a DNA vaccine expressing the M. leprae hsp60 protein did not develop reactivation of disease following glucocorticoid immunosuppression. Obviously, the difference in vaccine preparations utilized in these experiments is the simplest explanation for these divergent results. In particular, overwhelming recent evidence suggests that heat shock proteins can uniquely activate the immune system (25, 29). Among the novel immune properties of heat shock proteins are their ability to chaperone antigenic peptides to relevant immune sites, to stimulate antigen-presenting cells to secrete inflammatory cytokines, and to mediate maturation of dendritic cells. These unique immunostimulatory activities may explain why the reactivation of murine latent tuberculosis infections was essentially prevented by immunization with the hsp60 DNA vaccine. Alternatively, the disparate results could be explained by the different reactivation models used in the two studies. While both groups used a modification of the Cornell reactivation model, the specific antibiotic regimens, reactivation schedules, and bacterial strains varied. In a recent report in which various modifications of the Cornell latency model were evaluated, Scanga et al. concluded that the model was technically difficult and problematic for studying the immunologic basis of latent and reactivated tuberculosis (22). Further experiments are clearly needed to examine how applicable these findings from a mouse tuberculosis reactivation model are to the therapy of human latent tuberculosis infections with DNA vaccines.
In sum, these studies have illustrated how difficult the generation of an effective postexposure tuberculosis vaccine is likely to be. A DNA vaccine combination that protects against tuberculosis infection as effectively as BCG in a primary aerogenic challenge model does not prevent reactivation of latent infections. Moreover, this vaccine preparation is only modestly effective in reducing the growth of a secondary challenge in a previously exposed animal. Interestingly, we observed during our reinfection experiments that the secondary challenge grew substantially in the lungs of the mice with active disease. Therefore, even a persistent tuberculosis infection does not completely protect mice against a subsequent tuberculosis challenge. Based on these results, it is clear that the development of an immunotherapeutic vaccine to prevent tuberculous disease in the vast human population with latent tuberculosis infections is an important but daunting task that will require new and extremely innovative strategies.
Editor: J. D. Clements
REFERENCES
- 1.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. M. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates, J. H., W. W. Stead, and T. A. Rado. 1976. Phage type of tubercle bacilli isolated from patients with two or more sites of involvement. Am. Rev. Respir. Dis. 114:353-358. [DOI] [PubMed] [Google Scholar]
- 3.Braden, C. R., G. P. Morlock, C. L. Woodley, K. R. Johnson, A. C. Colombel, M. D. Cave, Z. Yang, S. E. Valway, I. M. Onorato, and J. T. Crawford. 2001. Simultaneous infections with multiple strains of Mycobacterium tuberculosis. Clin. Infect. Dis. 33:42-47. [DOI] [PubMed] [Google Scholar]
- 4.Caminero, J. A., M. J. Pena, M. I. Campos-Herrero, J. C. Rodriguez, O. Afonso, C. Martin, J. M. Pavon, M. J. Torres, M. Burgos, P. Cabrera, P. M. Small, and D. N. Enarson. 2001. Exogenous reinfection with tuberculosis on a European island with a moderate incidence of disease. Am. J. Respir. Crit. Care Med. 163:717-720. [DOI] [PubMed] [Google Scholar]
- 5.Chaves, F., F. Dronda, M. Alonso-Sanz, and A. R. Noriega. 1999. Evidence of exogenous reinfection and mixed infection with more than one strain of Mycobacterium tuberculosis among Spanish HIV-infected inmates. AIDS 13:615-620. [DOI] [PubMed] [Google Scholar]
- 6.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Bordich, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis: meta analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 7.Delogu, G., A. Howard, F. M. Collins, and S. L. Morris. 2000. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect. Immun. 68:3097-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delogu, G., A. Li, C. Repique, F. Collins, and S. L. Morris. 2002. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect. Immun. 70:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhillon, J., and D. A. Mitchison. 1994. Effect of vaccines in a murine model of dormant tuberculosis. Tuber. Lung Dis. 75:61-64. [DOI] [PubMed] [Google Scholar]
- 10.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 11.Fine, P. E. M., and P. M. Small. 1999. Exogenous reinfection in tuberculosis. N. Engl. J. Med. 341:1226-1227. [DOI] [PubMed] [Google Scholar]
- 12.Grosset, J., C. Truffot, J. Fermanian, and H. Lecoeur. 1982. Sterilizing activity of the main drugs on the mouse experimental tuberculosis. Pathol. Biol. (Paris) 30:444-448. [PubMed] [Google Scholar]
- 13.Li, Z., A. Howard, C. Kelley, G. Delogu, F. M. Collins, and S. L. Morris. 1999. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect. Immun. 67:4780-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. Lima, L. H. Faccoli, S. Stravropoulus, M. J. Colston, R. G. Hewinson, K. Moelling, and C. L. Silva. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 15.McCune, R. M., F. M. Feldman, H. P. Lambert, and W. McDermott. 1966. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J. Exp. Med. 123:445-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCune, R. M., F. M. Feldman, and W. McDermott. 1966. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J. Exp. Med. 123:469-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan, V. P., C. A. Scanga, K. Yu, H. M. Scott, K. E. Tanaka, E. Tsang, M. M. Tsai, J. L. Flynn, and J. Chan. 2001. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect. Immun. 69:1847-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris, S. L., C. Kelley, A. Howard, Z. Li, and F. M. Collins. 2000. The immunogenicity of single and combination tuberculosis DNA vaccines. Vaccine 18:2155-2163. [DOI] [PubMed] [Google Scholar]
- 19.Olsen, A. W., L. A. H. van Pinxteren, L. M. Okkels, P. B. Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 21.Rhoades, E. R., A. A. Frank, and I. M. Orme. 1997. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber. Lung Dis. 78:57-66. [DOI] [PubMed] [Google Scholar]
- 22.Scanga, C. A., V. P. Mohan, H. Joseph, K. Yu, J. Chan, and J. L. Flynn. 1999. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect. Immun. 67:4531-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon-γ and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shafer, R. W., S. P. Sigh, C. Larkin, and P. M. Small. 1995. Exogenous reinfection with multidrug-resistant tuberculosis in an immunocompetent patient. Tuber. Lung Dis. 76:575-577. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava, P. K., and R. J. Amato. 2001. Heat shock proteins: the "Swiss Army Knife' vaccines against cancers and infectious agents. Vaccine 19:2590-2597. [DOI] [PubMed] [Google Scholar]
- 26.Turner, J., E. R. Rhoades, M. Keen, J. T. Belisle, A. A. Frank, and I. M. Orme. 2000. Effective preexposure tuberculosis vaccines fail to protect when they are given in an immunotherapeutic mode. Infect. Immun. 68:1706-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Pinxteren, L. A. H., J. P. Cassidy, B. H. C. Smedegaard, E. M. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 30:3689-3698. [DOI] [PubMed] [Google Scholar]
- 28.van Rie, A., R. Warren, M. Richardson, T. C. Victor, R. P. Gie, D. A. Enarson, N. Beyers, and P. D. van Helden. 1999. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 341:1174-1179. [DOI] [PubMed] [Google Scholar]
- 29.Wells, A. D., and M. Malkovsky. 2000. Heat shock proteins, tumor immunogenicity, and antigen presentation: an integrated view. Immunol. Today 21:129-132. [DOI] [PubMed] [Google Scholar]