Abstract
Patients infected with Helicobacter pylori mount an immune response which fails to clear the infection and may contribute to disease. Mice can be protected by immunization. To further characterize the H. pylori-mouse model, stomachs of unimmunized or intranasally immunized C57BL/6 mice were quantitatively cultured 3 days and 1, 2, 4, 8, 16, 32, and 52 weeks after challenge with H. pylori. At 3 days and 1 week after challenge, colonization was the same in the immunized and unimmunized mice. By 2 weeks after challenge, the immunized mice had a >2-log decrease in bacterial load, and at all later time points, they either were culture negative or had at least a 2-log decrease in bacterial load. Gastritis in the immunized mice peaked at 1 to 2 weeks after challenge and was characterized by a mixed inflammatory infiltrate and epithelial proliferation centered at the transition between corpus and antrum. By 52 weeks postchallenge, the gastric histology in the immunized mice was not different from that in control unchallenged mice. The unimmunized group began to show a reduction in bacterial load as early as 16 weeks after challenge, and by 52 weeks seven of eight unimmunized mice had developed gastritis and reduced bacterial loads. These results indicate that prophylactic immunization does not prevent colonization by H. pylori but enables mice to clear the infection or significantly reduce the number of colonizing bacteria. The reduction in bacterial load is associated with gastric inflammation that subsides over time.
Following the initial association of curved bacteria with gastritis in the early 1980s, Helicobacter pylori has become recognized as an important human pathogen which can cause gastritis and peptic ulcers (44). Eradication of H. pylori reduces ulcer recurrence and accelerates ulcer healing (18, 24, 42). Epidemiologic association of H. pylori infection and gastric cancer has resulted in H. pylori being classified as a type 1-like carcinogen (5, 9, 46). Infection prevalence is estimated to range from 35% in developed countries to 80 or 90% in developing regions. Although most infected individuals do not develop peptic ulcers, all have histologic gastritis, which may increase the risk of cancer (4). Since antimicrobial eradication of H. pylori infection is not feasible for large numbers of people, vaccination may decrease ulcer disease and reduce the incidence of gastric cancer.
Several H. pylori vaccines for humans have been evaluated for safety and immunogenicity (1, 8, 22, 23), but only two have been tested with infected patients (22, 30). Neither vaccine eradicated H. pylori. Although there was a decrease in colonization (CFU/biopsy) in some patients 1 month after vaccination in the study by Michetti et al., gastritis was not decreased, and diarrhea, a side effect attributed to the adjuvant, occurred in 16 of the 24 volunteers (30).
The natural immune response in chronically infected people includes both humoral and local cell-mediated responses, which fail to eliminate the infection and may contribute to disease (2, 7).
The mouse model allows experimentation to determine mechanisms of protection and may provide insights toward the development of an effective vaccine. Mice can be protected by immunization with a variety of H. pylori antigens, adjuvants, and immunization routes (6, 14, 20, 25, 28, 32, 35, 41). Gastric inflammation in mice is mild relative to that in human patients but appears to increase over time (16, 26, 36, 37). The amount of inflammation depends on the mouse strain and may depend on the H. pylori strain (36, 39, 43). The gastritis observed in immunized mice after challenge can be more severe than the gastritis induced by H. pylori in unimmunized mice (17).
The kinetics of infection and protection in mice have not been clearly established. Most published reports on immunization have measured protection at 2 to 4 weeks following challenge. Some evidence in the Helicobacter felis model suggests that immunized mice do become infected before clearing their infection (29, 34). Unimmunized Swiss mice were colonized by H. pylori SS1 by day 3 postinfection, and the level of colonization remained relatively stable through 16 weeks postinfection in spite of increasing levels of anti-H. pylori antibodies in the gastric contents (15).
Our study was designed to determine the kinetics of colonization in immunized and unimmunized mice and examine the associated gastritis. Specifically, we investigated whether prophylactic immunization prevents colonization by H. pylori or promotes clearance of the bacteria, how long protection and gastritis persist, whether colonization remains constant in unimmunized mice, and whether gastritis develops in unimmunized mice.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free 4- to 6-week-old female C57BL/6 mice were purchased from a Helicobacter-free barrier colony at Harlan (Indianapolis, Ind.), housed in autoclaved static microisolator cages and provided with autoclaved water and sterile Teclad chow ad libitum. All procedures involving mice were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University.
Bacteria and bacterial products.
H. pylori Sydney Strain (SS1) which had been passaged through a BALB/c mouse was donated by Steven Danon of Ohio State University, Columbus (26). H. pylori was routinely grown on blood agar plates prepared from Columbia agar base (Becton Dickinson, Cockeysville, Md.) containing 7% (vol/vol) defibrinated horse blood (Cleveland Scientific, Columbus, Ohio) and 2.5 μg of amphotericin B (Sigma, St. Louis, Mo.)/ml and incubated in anaerobic jars (Becton Dickinson) at 37°C in a microaerobic, high-CO2 atmosphere generated by CampyPakPlus packs (Becton Dickinson). When H. pylori was isolated from gastric homogenate, the plates also contained 200 μg of bacitracin (Sigma)/ml, 6 μg of vancomycin (Sigma)/ml, 16 μg of cefsulodin (Sigma)/ml, and 20 μg of trimethoprim (Sigma)/ml to inhibit growth of gastric flora.
The antigen used for immunization was a 5,000 × g supernatant of a sonicate of SS1 prepared from 3- to 6-day cultures on blood agar plates. The supernatant was filtered through a 0.45-μm-pore-size filter, and its protein concentration was determined by a Lowry assay. The antigen was stored at −80°C.
For challenge, H. pylori SS1 was plated from stock stored at −80°C. Growth harvested after 4 days was transferred to T75 flasks containing brucella broth (Difco, Sparks, Md.), 10.5% fetal bovine serum (Gibco BRL, Frederick, Md.), 3 μg of amphotericin B per ml, 7.5 μg of vancomycin per ml, 20 μg of cefsulodin per ml, and 25 μg of trimethoprim per ml and incubated statically at 37°C in an incubator with a high-CO2 (∼12 to 15%) atmosphere for 24 h. The culture was passaged at least once but not more than five times in liquid media. Growth curves have shown that under these conditions an increase in the absorbance at 450 nm of 0.09 to 0.1 above that of uninoculated liquid media gives the highest viable count (2 × 107 to 7 × 107 CFU/ml) in the log growth phase.
A killed whole-cell antigen for enzyme-linked immunosorbent assay (ELISA) was prepared by addition of thimerosal (Sigma) at 0.01% (wt/vol) to liquid cultures. Incubation at 37°C was continued for 7 h, and cultures were harvested by centrifugation at 5,000 × g for 20 min. Pellets were washed three times in phosphate-buffered saline (PBS) (Sigma) containing 0.01% thimerosal. The washed antigen was resuspended in PBS and stored at −80°C. Culture confirmed that the preparation was nonviable. Protein concentration was determined by a Lowry assay.
Experimental design.
Mice were divided into four treatment groups: unimmunized and not challenged (U/NC), immunized and not challenged (I/NC), immunized and challenged (I/C), and unimmunized and challenged (U/C) (Table 1). The I/NC and I/C groups were immunized intranasally four times with weekly doses of 100 μg of H. pylori SS1 sonicate mixed with 5 μg of cholera toxin (List, Campbell, Calif.) administered in a total volume of 20 μl applied in 5-μl aliquots to the external nares of unanesthetized mice. Two weeks after the final immunization, the I/C and U/C groups received 0.5 ml of H. pylori liquid culture containing 2 × 107 CFU by gavage. The dose of the inoculum was confirmed by quantitative culture. The U/NC and I/NC groups received sterile culture media. Mice from each treatment group were sacrificed at 3 days and 1, 2, 4, 8, 16, 32, and 52 weeks after challenge. At least six mice from each treatment group were harvested at each time point (Table 1). Mice were killed by exposure to CO2, and blood was collected by cardiac puncture. A longitudinal strip of glandular stomach approximately 3 to 5 mm wide was cut aseptically from the greater curvature of the stomach and fixed in buffered 10% formalin for histology. The remaining stomach was removed and divided longitudinally along the lesser curvature. The squamous portion was discarded, and one piece of glandular stomach was homogenized to assess colonization, while the other piece was frozen in liquid nitrogen.
TABLE 1.
Experimental design and number of mice per group
| Groupa | Immuni- zationb | Challengec | No. of mice sacrificed at postchallenge week:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.4 | 1 | 2 | 4 | 8 | 16 | 32 | 52 | |||
| U/NC | No | Media | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 7 |
| I/NC | Yes | Media | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 7 |
| I/C | Yes | H. pylori | 6 | 6 | 6 | 6 | 7 | 6 | 6 | 10 |
| U/C | No | H. pylori | 6 | 6d | 6 | 6 | 6 | 6 | 6 | 8 |
Mice were divided into four experimental groups: U, unimmunized; I, immunized; C, challenged; and NC, not challenged.
One hundred micrograms of sonicate and 5 μg of cholera toxin were administered intranasally once per week, four times.
Culture media (0.5 ml) alone or containing 2 × 107 CFU of H. pylori was administered once by gavage.
There are only five quantitative culture results for this group. The sixth animal was culture positive, but quantitation was inaccurate due to spillage.
Quantitative culture.
Stomachs were homogenized in 200 μl of brucella broth containing 10.5% fetal bovine serum by using 1.5-ml disposable polypropylene tissue grinders and pestles (Kontes, Vineland, N.J.). Homogenate (100 μl) was diluted serially 1/10 in Dulbecco's PBS (Gibco BRL) from 1/10 to 1/1,000, and 10 μl of each dilution was plated. The undiluted homogenate was plated in triplicate. Colonies were counted after 5 or 6 days of incubation. Representative colonies were subcloned, gram stained, and tested for urease, oxidase, and catalase activity to confirm their identification. Bacterial load is expressed as log CFU/gram of stomach tissue. Mice that were culture negative were assigned a value of 1 CFU/g in order to calculate the log CFU/gram and geometric means.
Histologic evaluation.
The formalin-fixed longitudinal strips of stomach were processed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. All slides were coded and graded by the same pathologist (C.G.) in a random order in four batches. Graded slides from all time points were then sorted according to score and reviewed to ensure consistency of lesions with the same score. Gastric inflammation was graded on a new 0 to 5 scale instead of the 0 to 10 scale previously reported for H. felis-induced gastritis (31) in order to emphasize the qualitative and quantitative differences in H. pylori-induced gastritis. The worst 10× field was graded, and its location (cardia, body, transition zone, or antrum) was recorded. The scores were as follows: 0, no significant lesions; 0.5, a slight abnormality such as a small focus of inflammatory infiltrate or extensive mucous metaplasia without inflammation; 1, a mild infiltrate of inflammatory cells usually along the base of the glands; 1.5, a mild infiltrate plus slight epithelial hyperplasia or extensive mucous cell metaplasia; 2, a larger focus of inflammation extending between glands and/or in submucosa; 2.5, inflammatory cells between glands and in the submucosa with mucous cell metaplasia and/or mild epithelial cell hyperplasia; 3, a patch of inflammation extending between glands toward the lumen and in the underlying submucosa often accompanied by moderate mucous cell metaplasia and mild to moderate epithelial hyperplasia; 3.5, more intense inflammation than 3.0 with marked epithelial hyperplasia; 4, an area of intense transmucosal inflammatory infiltrate which extends across the 10× field and obscures the normal architecture of the glands, usually accompanied by marked epithelial hyperplasia and extensive mucous cell metaplasia; 4.5, severe inflammation with focal ulceration of the mucosa; 5, extensive mucosal and submucosal inflammation with disruption of glandular architecture and ulceration.
ELISA.
Levels of serum immunoglobulin G (IgG) reactive to whole-cell killed H. pylori SS1 were measured by endpoint titer determination. Ninety-six well Maxisorp plates (Nalge NUNC International, Roskilde, Denmark) were coated overnight at 4°C with 0.5 μg of antigen/well in 0.05 M carbonate-bicarbonate buffer, pH 9.6. Plates were emptied, blocked for at least 2 h at room temperature with PBS (Gibco BRL) containing 1% bovine serum albumin (BSA) (USB, Cleveland, Ohio), and washed three times with PBS containing 0.1% BSA. Sera including positive and negative controls were diluted in PBS with 1% BSA serially from 10−1 to 10−4.5 in 0.5-log steps. Diluted serum, 50 μl/well, was incubated at room temperature for 90 min, and plates were washed three times. IgG was detected by incubation for 60 min with alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology, Birmingham, Ala.) diluted 1/1,500 in PBS containing 1% BSA; plates were washed five times. After the addition of substrate (1 mg of p-nitrophenyl phosphate [Sigma] per ml in a 50 mM glycine-1 mM MgCl2 buffer [pH 9.6]), plates were incubated at room temperature for 60 min and read at 405 nm with a Vmax microplate reader (Molecular Devices, Sunnyvale, Calif.). Endpoint titers were determined by a SOFTmaxPro version 3.1 (Molecular Devices) protocol which uses a four-parameter curve fit and calculates the dilution of the sample which would equal the threshold absorbance. The threshold was 3 standard deviations above the mean absorbance of eight mice that were known to be H. pylori negative.
Statistics.
All experimental groups (the four treatment groups at eight time points) were compared by analysis of variance and Fisher's protected least-significant difference post hoc testing using StatView 4.5 (Abacus Concepts, Berkeley, Calif.). A P value of <0.05 was considered statistically significant.
RESULTS
Colonization of mice with H. pylori.
Study of the long-term effects of H. pylori required a reliable method of colonizing the mice with the bacterium. The literature contains numerous protocols for infecting mice with gastric Helicobacter sp. Many investigators inoculate mice two or more times with high doses of H. pylori. Others report good colonization with a single administration of broth culture (19). We have consistently achieved colonization with one inoculation of H. pylori. In a preliminary experiment comparing five doses of H. pylori and two sampling techniques for each stomach, 100% of the mice became colonized, and the inoculation dose had no effect on colonization level (Table 2). Five groups of C57BL/6 female mice (six per group) were inoculated by gavage with H. pylori SS1 doses ranging from 3 × 103 to 3 × 107 CFU/mouse. A sixth group received 3.0 × 107 CFU of H. pylori on day 0 and 2 × 107 CFU on day 2. The cultures used for this dose response had been passaged in vitro two and three times from frozen stock. Doses were confirmed by quantitative culture of the inoculum. Four weeks after inoculation, colonization was determined by quantitative culture of two samples from the glandular stomach of each mouse. One sample included both body and antrum; the other comprised antrum only. Mice inoculated once with H. pylori had uniformly high levels of colonization detected in the samples containing body plus antrum. The second inoculation did not increase colonization levels. In the samples of antrum only, colonization was variable. Three antral cultures had no growth despite high levels of H. pylori in the corresponding body-plus-antrum samples. Thus, sampling the body and antrum together improves the detection and quantitation of H. pylori infection in mice. In a study in which colonization was graded histologically, Sutton et al. also observed that assessment of the body plus antrum gave higher levels of colonization than antrum alone (40). The absence of a dose response is consistent with Ferrero et al., who reported 100% colonization of Swiss mice given ≥2,000 CFU of H. pylori SS1 (15). Based on these results, a single-dose challenge of 107 CFU was chosen for further experiments.
TABLE 2.
Dose independence of gastric colonization of C57BL/6 mice by H. pylori SS1 following inoculation of low-in vitro-passaged bacteria
| Tissuea | Gastric colonization (log CFU/gram of tissue)b 4 weeks after intragastric inoculation with liquid culture containing the indicated dose(s) (CFU) of H. pylori SS1
|
|||||
|---|---|---|---|---|---|---|
| 3 × 103 | 3 × 104 | 3 × 105 | 3 × 106 | 3 × 107 | 107 (twice)c | |
| Body and antrum | 6.3 ± 0.3 | 6.0 ± 0.2 | 6.5 ± 0.4 | 6.1 ± 0.2 | 6.1 ± 0.3 | 6.3 ± 0.4 |
| Antrum only | 6.0 ± 0.3 | 6.4 ± 0.6 | 4.5 ± 3.5 | 4.9 ± 2.4 | 6.2 ± 0.3 | 5.9 ± 1.4 |
The opened stomach was divided in half longitudinally along the lesser curvature. One side including body and antrum was quantitatively cultured; from the opposite side, the body was removed before culture.
Geometric mean ± SD for six mice per group.
The first dose was 3 × 107; 2 days later a second dose of 2 × 107 CFU was administered. All doses were confirmed by quantitative culture of the inoculum.
Kinetics of colonization in immunized and unimmunized mice.
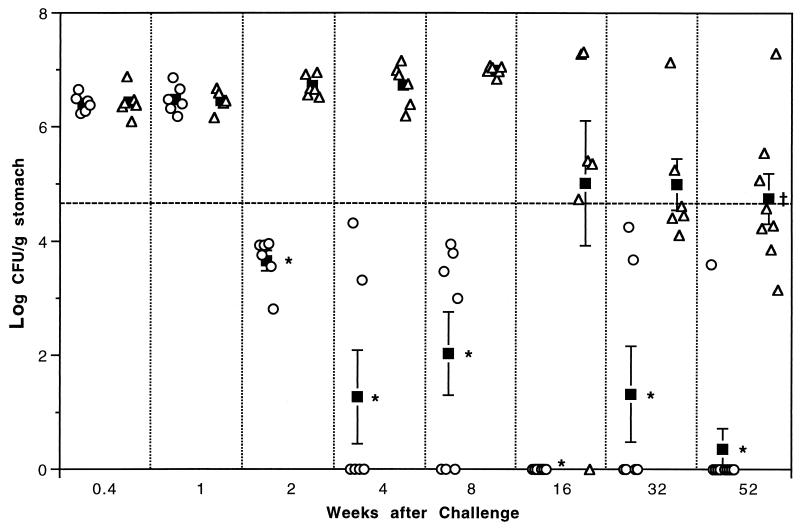
At 3 days and 1 week after challenge there was no difference in the colonization of immunized and unimmunized mice (Fig. 1). By 2 weeks after challenge and at all later time points, there was a significant decrease in the colonization of the immunized mice compared to that of the unimmunized mice at the same time point (P < 0.0001). When protection is defined as a 2-log (99%) decrease in bacterial load from the geometric mean of U/C groups from 3 days to 8 weeks, all immunized mice at 2 weeks and later time points can be considered protected. There was considerable variation in the responses of individual mice to immunization. From 4 weeks to 52 weeks, the majority of the immunized mice (26 of 35) were culture negative, but at each time point except 16 weeks some immunized mice remained colonized at reduced levels. Taken together, these results indicate that prophylactic immunization does not prevent colonization but enables immunized mice to reduce or clear the bacteria.
FIG. 1.
Bacterial colonization in immunized (○) and unimmunized (▵) C57BL/6 mice 3 days to 52 weeks after H. pylori inoculation. ▪, geometric mean ± standard error of the mean. The threshold for protection (- - - -) is defined as a 2-log decrease from the mean of the unimmunized mice from 3 days to 8 weeks. At 2 weeks and later, colonization of immunized mice is significantly less than that of unimmunized mice of the same time point. ∗, P < 0.0001. At 52 weeks, colonization of unimmunized mice is significantly less than that of unimmunized mice at 3 days to 8 weeks.†, P < 0.02.
From 3 days through 8 weeks, all U/C mice were heavily colonized; the increase from 3 days to 8 weeks was not statistically significant. At 16 weeks, one U/C mouse was culture negative. The gastritis score (3.0) and positive α-H. pylori titer (10−3.3) of this mouse indicated that its negative culture result was due to clearance rather than failure to become colonized. Three other U/C mice had reduced colonization at 16 weeks, and the mean of the group was significantly lower than the means of U/C mice at 2 to 8 weeks (P < 0.02). By 32 weeks, five of six U/C mice had reduced bacterial loads, and in four of these the reduction was >2 log from the U/C at 3 days to 8 weeks. Seven of the eight mice harvested at 52 weeks after challenge had reduced colonization (five with >2 log), and the group mean was significantly lower than each of the U/C groups from 3 days to 8 weeks (P < 0.02). Thus, given enough time after challenge, unimmunized mice began to reduce their bacterial loads, although much less efficiently than immunized mice. Again, there was wide variation among individuals, ranging from the four mice that remained heavily colonized at 16 to 52 weeks to the one mouse which became culture negative at 16 weeks.
Gastritis.
Gastric inflammation was graded as described in Materials and Methods. The normal gastric mucosa of the mouse contains more leukocytes than that of other species, and often mild inflammation is seen in untreated mice. Therefore, the negative control (U/NC) stomachs were coded and graded with the other groups, and the mean scores of these mice were >0. There was no significant difference between the gastritis scores of the U/NC mice and the I/NC mice at any time point (data not shown). This confirms that postimmunization gastritis is due to challenge of immunized mice and not immunization alone. Thus, the two unchallenged groups were combined and compared to the I/C and U/C mice (Fig. 2).
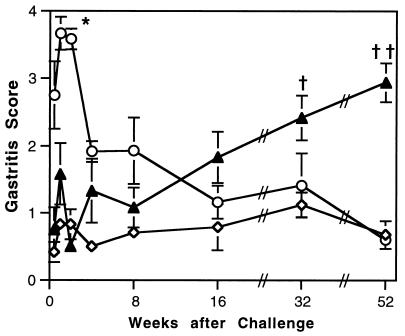
FIG. 2.
Changes in gastric lesions in immunized (○) and unimmunized (▴) C57BL/6 mice after inoculation with H. pylori compared to uninoculated mice (◊). Gastritis was graded on a scale of 0 to 5. Values are means ± standard errors of the means for groups of 6 to 10 inoculated mice and 12 to 14 uninoculated mice. At 1 and 2 weeks after challenge, immunized mice have significantly greater gastritis than unchallenged mice, unimmunized mice at 3 days to 16 weeks after challenge, and immunized mice from 16 to 52 weeks. ∗, P < 0.0001. At 32 and 52 weeks after challenge, unimmunized mice have greater gastritis than immunized mice at 16, 32, and 52 weeks after challenge. †, P < 0.05; ††, P < 0.0005.
Gastritis developed rapidly after challenge in immunized mice. By 3 days after challenge, the mean score of I/C mice (2.75 ± 0.5 [standard error of the mean]) was significantly higher than for the unchallenged mice (0.42 ± 0.15) and the U/C group (0.75 ± 0.33; P < 0.0001). At 1 and 2 weeks after challenge, the gastritis of the I/C mice was significantly higher than in unchallenged mice at all time points and U/C mice from 3 days to 16 weeks (P < 0.0001). The decrease in gastritis in I/C mice at 16 to 52 weeks compared to the peak at 1 and 2 weeks was also significant (P < 0.0001). By 52 weeks, there was no difference in gastric inflammation between the I/C mice and the unchallenged mice, indicating that postchallenge gastritis in immunized mice resolves with time.
Gastritis increased slowly in U/C mice (Fig. 2). By 16 weeks after challenge, mean gastritis in U/C mice (1.83 ± 0.38) was greater than in unchallenged mice (0.79 ± 0.35, P = 0.0077). At 32 and 52 weeks after challenge, the U/C mice had gastritis scores higher than those of both I/C and unchallenged mice (P < 0.03 for 32 weeks, and P < 0.0001 for 52 weeks).
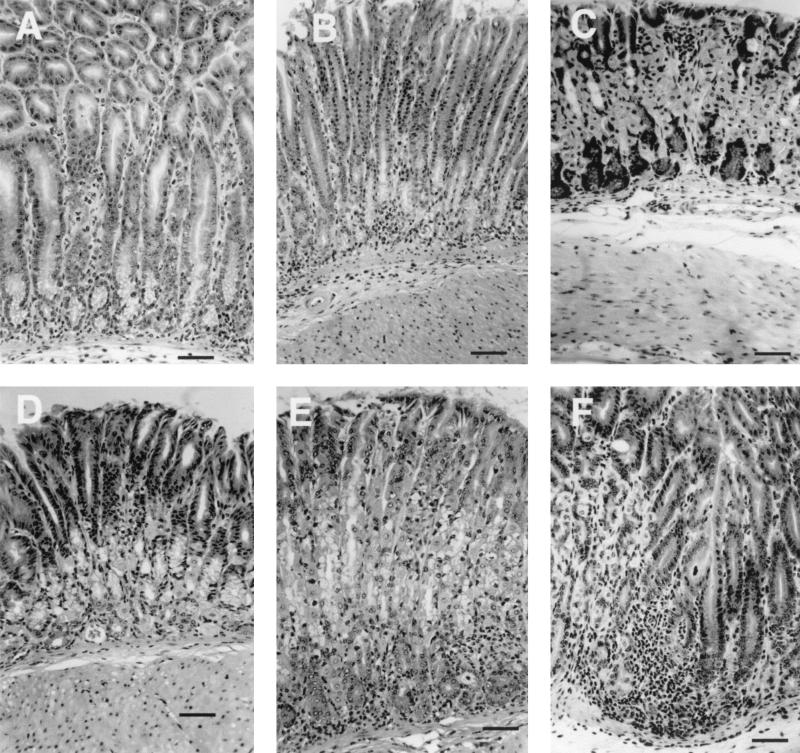
Representative photomicrographs of gastric histology from I/C and U/C mice at 2, 4, and 52 weeks after challenge taken at the same magnification demonstrate the increase in mucosal thickness in mice with gastritis (Fig. 3). At 2 weeks, the I/C mice typically had a mixed inflammatory infiltrate of neutrophils and large mononuclear cells in the lamina propria and submucosa (Fig. 3A). In the lamina propria, the infiltrate separated and displaced the glands. Gland abscesses were common, as was loss of parietal cells, with replacement by mucus-containing cells (mucous cell metaplasia). Gastric pits had lengthened and were lined by less mature flattened epithelial cells with basophilic cytoplasm; mitotic figures were frequent. In the submucosa, edema often accompanied the cellular infiltrate. The worst lesion was usually located at the transition zone between the body and antrum. The sections from U/C mice at 2 weeks were unremarkable and not different from those of unchallenged mice (Fig. 3D). At 4 weeks, three of the U/C mice had scores of 2 or 3, and the lesions were located at the cardia or in the body (Fig. 3E). Gastritis had decreased in the I/C mice by 4 weeks, and only four mice had scores of 2 or 2.5 (Fig. 3B and 4A). Lesions remained at the transition zone, and despite the decrease in inflammatory cell infiltrate, many glands were lined almost entirely by mucus-containing cells (Fig. 4A). At 52 weeks, I/C mice had scores of 0 to 1.0, similar to those of unchallenged mice (Fig. 3C), but seven of eight U/C mice had inflammatory infiltration and epithelial hyperplasia resembling the lesions seen at early time points in I/C mice (Fig. 3F and 4B). The location of the worst field in U/C mice had shifted to the transition zone by 52 weeks.
FIG. 3.
Representative gastric histopathology in C57BL/6 mice following inoculation with H. pylori. (A and D) Two weeks following challenge, immunized mice (A) have marked infiltration of the lamina propria and submucosa by a mixed population of inflammatory cells including numerous neutrophils. Unimmunized mice (D) have at most very mild lesions. (B and E) By 4 weeks post challenge, gastritis is less severe in the immunized mice (B), and some unimmunized mice (E) have developed gastritis. (C and F) By 52 weeks after challenge, most of the unimmunized mice (F) have developed gastritis while the immunized mice (C) are no different from uninoculated mice. Staining was done with hemoxylin and eosin. Bars, 50 μm.
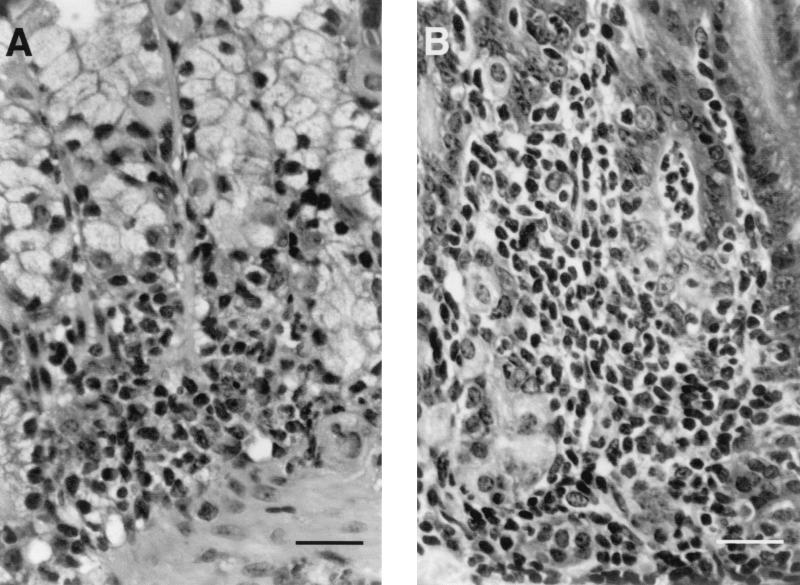
FIG. 4.
Details of gastritis in an I/C mouse at 4 weeks (A) and a U/C mouse at 52 weeks (B) after challenge. The inflammatory infiltrate consists of numerous neutrophils and large mononuclear cells. (A) Glands are lined by tall mucus-containing epithelial cells (clear cytoplasm) with only a few parietal cells remaining. (B) Inflammatory cells have separated and infiltrated the glands. Staining was done with hemoxylin and eosin. Bars, 25 μm.
Association of gastritis and bacterial load.
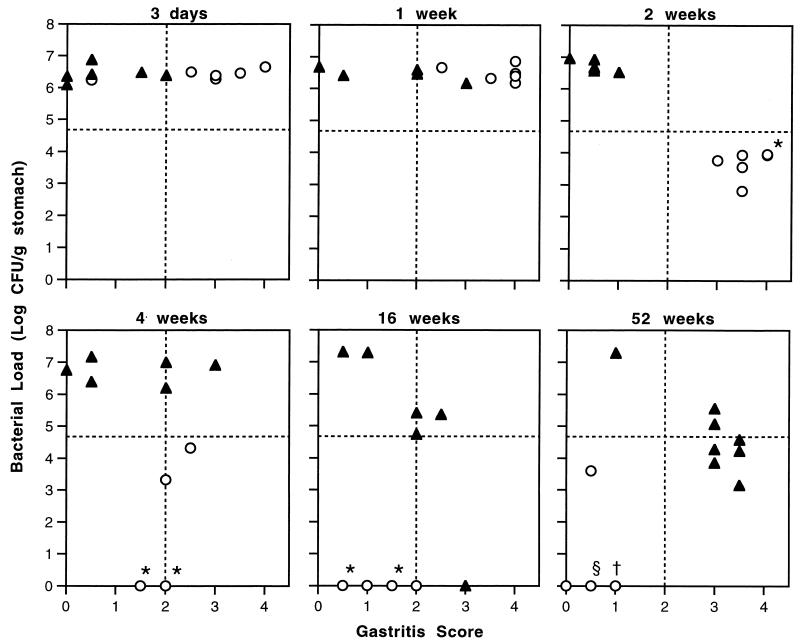
The correlation of gastritis and bacterial clearance is more apparent when colonization is plotted against gastritis scores for individual mice (Fig. 5). I/C mice showed a progression from high colonization with high gastritis scores at 3 days and 1 week to decreased bacterial load while gastritis score remained high at 2 weeks and to further decreases in both colonization and gastritis at later time points. U/C mice were more variable in their responses to H. pylori infection. Some remained heavily colonized with low gastritis throughout the experiment. Others had increased gastritis scores with high bacterial loads at the time they were killed. At 52 weeks, seven of eight U/C mice had reduced bacterial loads and gastritis scores of 3 or 3.5, a pattern resembling that of the I/C mice at 2 weeks postchallenge.
FIG. 5.
Correlation of gastritis and bacterial load in individual immunized (○) and unimmunized (▴) C57BL/6 mice at 3 days and 1, 2, 4, 16, and 52 weeks after challenge with H. pylori. The horizontal dashed line indicates a 2-log decrease in bacterial load compared to unimmunized mice from 3 days to 8 weeks after challenge. Immunized mice quickly develop significant gastritis (scores >2.0). By 2 weeks, immunized mice have high gastritis scores and >2-log decrease in bacterial load. In unimmunized mice increased gastritis scores at 16 and 52 weeks are associated with a decrease in bacterial colonization. ∗, †, and § indicate two, three, and five mice with the same bacterial load and gastritis scores, respectively.
Serum antibody response.
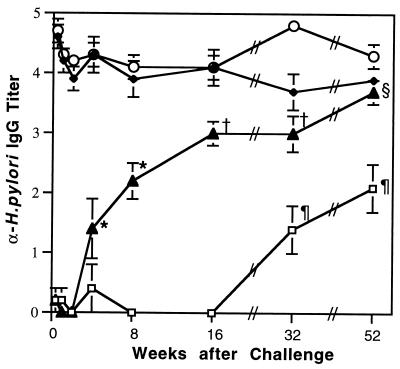
Although antibodies have been shown to be unnecessary in protection from Helicobacter infection (3, 13, 33), we used serum IgG titers to indicate a systemic immune response to H. pylori in this experiment. A significant increase in the titer was seen at 4 weeks in the U/C mice compared to U/NC mice (Fig. 6). The titers in the U/C mice increased at each time point until they were not significantly different from those of I/NC mice at 52 weeks after challenge. Titers in the immunized mice remained high throughout the study regardless of whether they were challenged. The titers in the U/NC mice at 32 and 52 weeks are most likely due to cross-reactivity to another gram-negative motile bacterium. After the termination of the study, we learned that there had been a breakdown in animal husbandry and that mice had been exposed to a Bordetella sp. in improperly autoclaved water. ELISA assays using plates coated with Bordetella demonstrated that the U/NC mice had titers to Bordetella that were 10- to 100-fold higher than the titers to H. pylori at 32 and 52 weeks. All unchallenged mice were culture negative for H. pylori.
FIG. 6.
Serum IgG is reactive against killed H. pylori antigen. Values are geometric means ± standard errors of the means of negative log endpoint titers. Titers in the I/C (○) and I/NC (♦) mice remained elevated. By 4 weeks, titers of U/C mice (▴) were significantly elevated compared to earlier U/C groups and U/NC (□) mice from 3 days to 16 weeks; ∗, P < 0.02 and < 0.0005, respectively. At 16 and 32 weeks, the titers of U/C mice were higher than U/C mice at 3 days to 8 weeks and all groups of U/NC mice; †, P < 0.02 and < 0.005, respectively. At 52 weeks the U/C group had a titer higher than that of all U/C and U/NC groups; §, P < 0.05 and < 0.0001, respectively. Titers in the U/NC groups at 32 and 52 weeks (¶) are most likely due to cross-reactivity (see text).
DISCUSSION
This time course study has added to our knowledge of H. pylori infection in the mouse in several important ways. First, we have shown that prophylactic immunization does not prevent infection in this model but enables immunized mice to reduce the bacterial load significantly and often to eliminate the infection. Transient infection of immunized mice had not been reported previously probably because most H. pylori immunization studies have evaluated protection at a single time point 3 or 4 weeks after challenge, a time at which the heavy colonization that we observed at 3 days and 1 week would have been missed. The use of quantitative culture in evaluating colonization was also important. Some early H. pylori studies based immunization success on finding culture-negative mice. At 2 weeks, we observed that all the immunized mice were still culture positive. Without quantitative culture which showed a >2-log decrease in bacterial load, culture positivity could have been interpreted as an immunization failure if this were the only time point evaluated.
The model we have used involves a single large challenge dose of H. pylori and does not mimic natural acquisition of infection by humans. It is possible to speculate that colonization might not occur in immunized mice if the challenge dose were smaller. To our knowledge, this question has not been addressed for H. pylori SS1. However, using a Type II (nonmotile, CagA-negative) strain of H. pylori, Kleanthous et al. demonstrated a relatively stable 96 to 99% reduction in bacterial load in immunized mice 2 weeks after challenge with 105, 106, or 107 CFU (21). With lower challenge doses, their unimmunized mice did not become colonized. Thus, in spite of decreasing the challenge dose, they did not find a dose at which the unimmunized mice became colonized while the immunized mice did not.
The reduction of bacterial load is antigen specific in I/C mice. Although not included in this kinetic study, immunization with cholera toxin mixed with ovalbumin did not result in a reduction of bacterial load 4 weeks after challenge with H. pylori (C. A. Garhart, F. P. Heinzel, S. J. Czinn, and J. G. Nedrud, unpublished data). Numerous early studies in the H. felis model demonstrated that immunization with Helicobacter antigens in the absence of an adjuvant or administration of adjuvant alone failed to protect mice from infection (25, 27, 45).
A second finding of our study is that clearance or reduction in colonization is associated with gastric inflammation. In fact, the peak gastritis occurred before there was a reduction in bacterial load. The speed with which gastritis developed and its severity were surprising. Immunized mice had significant gastritis 3 days after challenge. Preliminary experiments in our laboratory and the report by Goto et al. had suggested that H. pylori challenge could induce postimmunization gastritis although the severity was less than that seen with H. felis (17). By looking at earlier time points, we have found that H. pylori can induce severe gastritis in immunized mice. In a related model, Eaton et al. reported rapid development of severe gastritis in H. pylori-colonized severe combined immunodeficient (SCID) C57BL/6J[supi]scid/scid mice following adoptive transfer of splenocytes from C57BL/6J donors (11). In the SCID mice, development of gastritis was also accompanied by a rapid decrease in bacterial colonization, and by 45 weeks after transfer, bacteria were not detected in the recipient mice (10).
A third important observation is that gastric inflammation resolves after the bacterial load is reduced or eliminated. While one immunized mouse remained colonized at 52 weeks, its gastritis score was within the range seen in naïve mice. One shortcoming of the mouse model is that tissue can be examined from an individual only once. Thus, there is no way of knowing whether that mouse had had more severe gastritis earlier or perhaps had failed to make as robust a response as the others. Resolution of inflammation was also observed in the SCID-adoptive transfer model when infection was undetectable in the recipient mice (10).
Our results of long-term infection and protection in the H. pylori model appear to differ from a long-term study in the H. felis model by Sutton et al. (38). In the H. felis experiment, colonization of I/C mice, although reduced in comparison to U/C mice, was present throughout the 18-month time course. The authors reported postimmunization gastritis in immunized mice challenged with H. felis at 1 and 3 months postchallenge. By 6 months postchallenge, the gastritis in their I/C mice had decreased to the level observed in the U/C mice, and both groups continued to show similar gastric inflammation levels throughout the study. In contrast, we were unable to detect bacteria in the majority of I/C mice after 8 weeks, and the gastritis scores of our I/C mice, whether colonized or culture negative, were not significantly greater than for unchallenged mice at 16 weeks and beyond. Our differing results may be explained by differences between the two species of Helicobacter, timing, and vaccine efficacy. Vaccination outcomes are very dependent on the antigen preparation, dose of antigen, adjuvant, timing, and route of administration (17, 21, 40, 45). In the long-term H. felis study, Sutton et al. used intragastric immunization, which we found to be less effective than intranasal immunization in a preliminary experiment (Garhart et al., unpublished). Infection by low numbers of bacteria (not detectable histologically) has been implicated as the cause of postimmunization gastritis in the H. felis-mouse model because antibiotic treatment cured the gastritis (12).
It is dangerous to extrapolate directly from mice to humans, but it is encouraging that postchallenge gastritis resolved with time in mice challenged with H. pylori. An important concern about development of a human vaccine against H. pylori is that it may not be possible to induce sterilizing immunity. Long-term mucosal inflammation due to residual bacteria may increase the risk of both peptic ulcers and gastric cancer, which are the risks that vaccination has been proposed to reduce.
Finally, we observed that unimmunized mice began to reduce their bacterial loads at 16 weeks after challenge. Again, there was an association of increased gastritis with reduction in colonization. Intrinsic reduction in H. pylori colonization by C57BL/6 mice has also been reported recently by Eaton et al. (10). It is tempting to speculate that there may be a cause and effect relationship between development of gastritis (a robust local cellular immune response) and the decrease in bacterial load, but it is not a simple relationship. Although we have not observed a decrease in bacterial load without an increase in gastritis, some immunizations result in increased gastritis without a reduction in bacterial load (data not shown).
In summary, our data show that prophylactic intranasal immunization results in a rapid intense gastric mucosal response upon challenge with H. pylori. The inflammatory response precedes reduction in bacterial load but resolves with time after colonization is reduced. Many questions remain such as the mechanism of bacterial clearance and whether the inflammatory response in infected patients can be modulated so that it becomes effective in clearing infection.
Acknowledgments
This work was supported in part by PHS grants CA-73515-02 (C.G.), DK-46461 (S.C. and J.N.), AI-40701 (J.N.), and AI-36359 (J.N. and S.C.).
We thank Rich Masley and LeRoy Brown (HCS, Everson, Wash.) for help in preparing the histologic sections and Steven Emancipator and Abram Stavitsky for many helpful discussions and advice.
Editor: D. L. Burns
REFERENCES
- 1.Angelakopoulos, H., and E. L. Hohmann. 2000. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar Typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 68:2135-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell. Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 4.Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 161:626-633. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, R. Peek, P. Chyou, G. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55:2111-2115. [PubMed] [Google Scholar]
- 6.Czinn, S. J., A. Cai, and J. G. Nedrud. 1993. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine 11:637-642. [DOI] [PubMed] [Google Scholar]
- 7.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 8.DiPetrillo, M. D., T. Tibbetts, H. Kleanthous, K. P. Killeen, and E. L. Hohmann. 2000. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18:449-459. [DOI] [PubMed] [Google Scholar]
- 9.Dobrilla, G., S. Benvenuti, S. Amplatz, and L. Zancanella. 1994. Chronic gastritis, intestinal metaplasia, dysplasia and Helicobacter pylori in gastric cancer: putting the pieces together. Ital. J. Gastroenterol. 26:449-458. [PubMed] [Google Scholar]
- 10.Eaton, K. A., and M. E. Mefford. 2001. Cure of Helicobacter pylori infection and resolution of gastritis by adoptive transfer of splenocytes in mice. Infect. Immun. 69:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton, K. A., S. R. Ringler, and S. J. Danon. 1999. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect. Immun. 67:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermak, T. H., R. Ding, B. Ekstein, J. Hill, G. A. Myers, C. K. Lee, J. Pappo, H. K. Kleanthous, and T. P. Monath. 1997. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology 113:1118-1128. [DOI] [PubMed] [Google Scholar]
- 13.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrero, R. L., J.-M. Thiberge, I. Kansau, N. Wuscher, M. Huerre, and A. Labigne. 1995. The groES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc. Natl. Acad. Sci. USA 92:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrero, R. L., J. M. Thiberge, M. Huerre, and A. Labigne. 1998. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect. Immun. 66:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiara, P., M. Rossi, M. Marchetti, A. Di Tommaso, C. Vindigni, F. Ciampolini, A. Covacci, J. L. Telford, M. T. De Magistris, M. Pizza, R. Rappuoli, and G. Del Giudice. 1997. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect. Immun. 65:4996-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto, T., A. Nishizono, T. Fujioka, J. Ikewaki, K. Mifune, and M. Nasu. 1999. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect. Immun. 67:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham, D. Y., G. M. Lew, P. D. Klein, D. G. Evans, D. J. Evans, Jr., Z. A. Saeed, and H. M. Malaty. 1992. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann. Intern. Med. 116:705-708. [DOI] [PubMed] [Google Scholar]
- 19.Hirayama, F., S. Takagi, Y. Yokoyama, E. Iwao, and Y. Ikeda. 1996. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J. Gastroenterol. 31(Suppl. 9):24-28. [PubMed] [Google Scholar]
- 20.Kleanthous, H., G. A. Myers, K. M. Georgakopoulos, T. J. Tibbitts, J. W. Ingrassia, H. L. Gray, R. Ding, Z. Z. Zhang, W. Lei, R. Nichols, C. K. Lee, T. H. Ermak, and T. P. Monath. 1998. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect. Immun. 66:2879-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleanthous, H., T. J. Tibbitts, H. L. Gray, G. A. Myers, C. K. Lee, T. H. Ermak, and T. P. Monath. 2001. Sterilizing immunity against experimental Helicobacter pylori infection is challenge-strain dependent. Vaccine 19:4883-4895. [DOI] [PubMed] [Google Scholar]
- 22.Kotloff, K. L., M. B. Sztein, S. S. Wasserman, G. A. Losonsky, S. C. DiLorenzo, and R. I. Walker. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 69:3581-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreiss, C., T. Buclin, M. Cosma, I. Corthesy-Theulaz, and P. Michetti. 1996. Safety of oral immunisation with recombinant urease in patients with Helicobacter pylori infection. Lancet 347:1630-1631. [DOI] [PubMed] [Google Scholar]
- 24.Laine, L. 1992. Eradication of Helicobacter pylori reduces gastric and duodenal ulcer recurrence. Gastroenterology 103:1695-1696. [PubMed] [Google Scholar]
- 25.Lee, A., and M. Chen. 1994. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect. Immun. 62:3594-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 27.Lee, C. K., R. Weltzin, W. D. J. Thomas, H. Kleanthous, T. H. Ermak, G. Soman, J. E. Hill, S. K. Ackerman, and T. P. Monath. 1995. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J. Infect. Dis. 172:161-172. [DOI] [PubMed] [Google Scholar]
- 28.Manetti, R., P. Massari, M. Marchetti, C. Magagnoli, S. Nuti, P. Lupetti, P. Ghiara, R. Rappuoli, and J. L. Telford. 1997. Detoxification of the Helicobacter pylori cytotoxin. Infect. Immun. 65:4615-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michetti, P., I. Corthesy-Theulaz, C. Davin, R. Haas, A.-C. Vaney, M. Heitz, J. Bille, J. P. Kraehenbuhl, E. Saraga, and A. L. Blum. 1994. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology 107:1002-1011. [DOI] [PubMed] [Google Scholar]
- 30.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthesy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 31.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. Czinn. 1997. Murine CD4 T cell responses to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113:1848-1857. [DOI] [PubMed] [Google Scholar]
- 32.Pappo, J., W. D. Thomas, Z. Kabok, N. S. Taylor, J. C. Murphy, and J. G. Fox. 1995. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect. Immun. 63:1246-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radcliff, F. J., M. Chen, and A. Lee. 1996. Protective immunization against Helicobacter stimulates long-term immunity. Vaccine 14:780-784. [DOI] [PubMed] [Google Scholar]
- 35.Radcliff, F. J., S. L. Hazell, T. Kolesnikow, C. Doidge, and A. Lee. 1997. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect. Immun. 65:4668-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakagami, T., M. Dixon, J. O'Rourke, R. Howlett, F. Alderuccio, J. Vella, T. Shimoyama, and A. Lee. 1996. Atrophic gastric changes in both H. felis and H. pylori infected mice are host dependent and separate from antral gastritis. Gut 39:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawai, N., M. Kita, T. Kodama, T. Tanahashi, Y. Yamaoka, Y.-I. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect. Immun. 67:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutton, P., S. J. Danon, M. Walker, L. J. Thompson, J. Wilson, T. Kosaka, and A. Lee. 2001. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut 49:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton, P., J. Wilson, R. Genta, D. Torrey, A. Savinainen, J. Pappo, and A. Lee. 1999. A genetic basis for atrophy: dominant non-responsiveness and Helicobacter induced gastritis in F(1) hybrid mice. Gut 45:335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 18:2677-2685. [DOI] [PubMed] [Google Scholar]
- 41.Todoroki, I., T. Joh, K. Watanabe, M. Miyashita, K. Seno, T. Nomura, H. Ohara, Y. Yokoyama, K. Tochikubo, and M. Itoh. 2000. Suppressive effects of DNA vaccines encoding heat shock protein on Helicobacter pylori-induced gastritis in mice. Biochem. Biophys. Res. Commun. 277:159-163. [DOI] [PubMed] [Google Scholar]
- 42.Treiber, G., and J. R. Lambert. 1998. The impact of Helicobacter pylori eradication on peptic ulcer healing. Am. J. Gastroenterol. 93:1080-1084. [DOI] [PubMed] [Google Scholar]
- 43.van Doorn, N. E., F. Namavar, M. Sparrius, J. Stoof, E. P. van Rees, L. J. van Doorn, and C. M. Vandenbroucke-Grauls. 1999. Helicobacter pylori-associated gastritis in mice is host and strain specific. Infect. Immun. 67:3040-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warren, J. R. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i:1273. [PubMed]
- 45.Weltzin, R., H. Kleanthous, F. Guirdkhoo, T. P. Monath, and C. K. Lee. 1997. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine 15:370-376. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. 1994. Infection with Helicobacter pylori, p. 177-241. In Schistosomes, liver flukes and Helicobacter pylori, vol. 61. International Agency for Research on Cancer, Lyon, France.