Abstract
Chlamydia trachomatis serovar E, the leading bacterial agent responsible for sexually transmitted diseases, is required to invade genital epithelial cells for its growth and survival, yet little is known about the adhesin-receptor interactions promoting its entry. In contrast, much has been published on the heparan sulfate receptor for binding C. trachomatis L2 elementary bodies (EBs) prior to entry into HeLa cells. Using a different experimental approach in which a biotinylated apical membrane protein receptor(s) attached to EB at 4°C was stripped off the surface of polarized HEC-1B cells and immunoprecipitated with polyclonal anti-EB antibodies, an ∼55-kDa protein was reproducibly detected by enhanced chemiluminescence and two-dimensional gel electrophoresis. Matrix-assisted laser desorption ionization mass-spectrometry sequence analysis revealed the 55-kDa protein to be protein disulfide isomerase (PDI), a member of the estrogen receptor complex which carries out thiol-disulfide exchange reactions at infected host cell surfaces. Exposure of HEC-1B cells during EB attachment (1.5 to 2 h) to three different inhibitors of PDI reductive reactions—(i) the thiol-alkylating reagent DTNB (5,5′-dithiobis[2-nitrobenzoic acid]), (ii) bacitracin, and (iii) anti-PDI antibodies—resulted in reduced chlamydial infectivity. Since (i) C. trachomatis serovar E attachment to estrogen-dominant primary human endometrial epithelial cells is dramatically enhanced and (ii) productive entry into and infectivity of EB in host cells is dependent on reduction of EB cross-linked outer membrane proteins at the host cell surface, these data provide some preliminary evidence for an intriguing new potential receptor candidate for further analysis of luminal C. trachomatis serovar E entry.
Chlamydia trachomatis serovars D to K are the predominant cause of bacterial sexually transmitted diseases and sequelae in the United States and worldwide (25). These bacteria are thought to be luminal pathogens, entering and exiting the apical surfaces of target columnar epithelial cells lining the genital mucosae. They ascend canalicularly in the female genital tract from the endocervix to the endometrium and, subsequently, to the fallopian tubes. In contrast, the Lymphogranuloma venereum (LGV) serovars L1 to L3 of C. trachomatis are disseminating pathogens, exiting the basal surfaces of epithelial cells lining the endocervix or the urethra. The chlamydiae then spread through the submucosa to regional lymph nodes; an inguinal lymphadenopathy is a common clinical manifestation of this sexually transmitted disease syndrome (25).
Since these bacteria are obligate intracellular pathogens, they must initially attach to and enter the apical surfaces of epithelial host cells. While a few chlamydial envelope components have been proposed as adhesins (2, 6, 12, 19, 21, 27, 29, 32) and even fewer epithelial plasma membrane components have been proposed as receptors (14, 31, 33, 36), the leading candidate adhesin-receptor combination seems to be heparan sulfate, at least in in vitro studies. In a series of studies by Stephens and colleagues (reviewed in reference 27), a chlamydia biosynthetically directed heparan sulfate was proposed as the adhesin for the LGV L2 serovar (18). These data have recently been confirmed, although there may still be some controversy as to the origin of the heparan sulfate, i.e., is it prokaryote or eukaryote derived (33)? While the chlamydial genomes have no open reading frames encoding genes for heparan sulfate, it has been suggested that some of the chlamydial open reading frames coding for the enzymatic machinery may yet be unassigned, or alternatively, the chlamydiae may exploit substrate intermediates supplied by the host (18). Su et al. (31) suggested that heparan sulfate also serves as the receptor for the C. trachomatis mouse pneumonitis serovar by expressing in Escherichia coli a recombinant maltose binding protein-major outer membrane protein (MBP-MOMP) fusion protein which, in turn, binds specifically to heparan sulfate receptors on HeLa cells. However, this same group also found that heparan sulfate had no competitive inhibitory effect on establishment of chlamydial genital infection in mice (30). Perhaps the extended molecules of the high-mannose oligomannose oligosaccharide glycosylated to MOMP (12) serve as an initial adhesin to bring the infectious form of chlamydia—the elementary body (EB)—closer to the epithelial cell for MOMP-heparan sulfate interaction, leading to entry, or alternatively, they use separately mannose-binding receptors.
Little information is available on the receptor for the nondisseminating, non-LGV serovars of C. trachomatis (14). The EB of these D to K serovars are less susceptible to interference of attachment to HeLa cells by heparan sulfate (4, 33). Further, Hayashi et al. (9) demonstrated by immunocytochemistry at both the light- and electron-microscopic levels that heparan sulfate was localized only to the basal surfaces of genital columnar epithelial cells of mouse tissues in vivo. Such basal laminal or interstitial matrix proteins may be redistributed differently, i.e., circumferentially, in nonpolarized epithelial cells cultured in vitro (37). Our laboratory previously examined the endometrial epithelial cell line HEC-1B, grown on coverslips, for the presence of integrins (39). These endometrial cells were moderately to strongly positive for 13 out of 24 major integrins, mostly α, αv, and β. Monoclonal antibodies recognizing seven of these integrins were added to HEC-1B cells, but this maneuver had no effect on blocking serovar E EB attachment, whereas there was markedly decreased attachment of the positive control Yersinia pseudotuberculosis, which binds RGD sequences. Recent chlamydial genomic analyses of the multigene family encoding polymorphic outer membrane proteins (POMPs) suggest the POMPs may have autotransporter function (10). Indeed, the passenger domain of POMPs from several Chlamydia species, including C. trachomatis, contains RDG motifs. While the authors point out that RGD motifs are often associated with proteins predicted to be adhesins, these functional motifs are common and also are seen in proteins that do not require cell adhesion properties.
This study presents a different approach designed to identify receptor components on HEC-1B cells involved in attachment of C. trachomatis serovar E EB. The key strategy was to isolate and identify by matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) chemiluminescence-labeled apical membrane proteins associated with EB during attachment, subsequently immunoprecipitated with EB, and resolved by isoelectric focusing. Our report focuses on one member of the estrogen receptor complex (13) which carries out thiol-disulfide exchange reactions at cell surfaces—a prerequisite for EB infection.
MATERIALS AND METHODS
Chlamydia and host cell lines.
C. trachomatis serovar E (UW5-CX) was cultured in McCoy cells grown on Cytodex microcarrier beads (26) (Pharmacia, Uppsala, Sweden), purified by Percoll (Sigma) gradient centrifugation for EB particle count determination, and stored in 2SPG (0.02 M phosphate buffer, 0.2 M sucrose, 5 mM glutamine [pH 7.2]) at −80°C (4). The titers of aliquots of frozen and thawed stock samples of EB were determined by fluorescent-inclusion count in McCoy cell monolayers, fixed with methanol, stained with a pool of fluorescein-labeled monoclonal antibodies generated against C. trachomatis MOMP (Syva [now Wampole, Carter-Wallace, Inc.], Palatine, Ill.), suspended in Evans blue counterstain, and examined on a Zeiss Axiovert 10 microscope equipped with 495-nm excitation and 520-nm emission filters.
The human endometrial carcinoma subclone 1B (HEC-1B; ATCC HTB-113) epithelial cell line, cultured in Dulbecco's modified Eagle medium (D-MEM; Gibco, Grand Island, N.Y.) with 10% fetal calf serum (FCS) (D-MEM-10) and 2 mM glutamine, was used for apical membrane protein isolation.
Apical membrane protein isolation and identification.
HEC-1B cells were seeded (105 cells/filter) onto each filter of six-well filter inserts (ca 4.7 cm2; Costar Transwell), incubated at 37°C until the confluent monolayer became polarized, and monitored microscopically via a control monolayer on a clear (Costar Transcol) filter insert. A minimum of 12 inserts was used per experimental sample. Two days prior to labeling apical membranes, the concentration of FCS was reduced to 3%.
(i) Labeling of apical membrane proteins.
Labeling of HEC-1B apical membrane proteins was performed by biotinylation (ECL kit; Amersham) using the reagents, unless otherwise specified, and protocol provided by the manufacturer. Briefly, HEC-1B polarized monolayers, washed twice in phosphate-buffered saline (PBS) containing protease inhibitors (Protease Inhibitor Cocktail, Boehringer Mannheim), were exposed to the biotinylation reagent (diethyl formamide-biotinamide-N-OH succinamide ester) in bicarbonate buffer (pH 8.6; 200 μl per filter insert) and placed on a rocking platform for 30 min at 4°C. Subsequently, the biotinylated monolayers were washed two times in PBS containing Ca2+ (0.7 mM) and Mg2+ (0.5 mM).
(ii) Attachment of EB.
Percoll-purified C. trachomatis serovar E EB stock in 2SPG was diluted in Hanks balanced salt solution, pH 7.2, to a multiplicity of infection of 200:1 and inoculated onto the biotinylated HEC-1B monolayers (150 μl/filter); the six-well plates were placed on a rocking platform at 4°C for 1 h to allow EB attachment. Unattached EBs were then removed by washing the monolayers two times in PBS, pH 7.5.
(iii) Apical membrane lift.
Isolation of apical membranes, with or without EB attached, was accomplished by pressing a poly-l-lysine-coated coverslip, gently but firmly, onto the surface of the polarized monolayer and then lifting off the coverslip (24, 38). Two control coverslips were fixed with cold methanol, stained with a pool of fluorescein-labeled monoclonal antibodies generated against C. trachomatis MOMP (Syva) suspended in Evans blue counterstain, and examined microscopically to monitor for efficient EB attachment as well as the absence of whole (contaminating) HEC-1B cells. A minimum of 10 to 12 coverslips containing attached and/or transferred apical membrane were subjected to 0.1% NP-40 (200 μl per coverslip) for 30 min at 4°C to solubilize apical membrane proteins; importantly, this step does not affect EB infectivity. Following two additional washes in lysis buffer and scraping the coverslips with a rubber policeman, the washes were combined, briefly centrifuged to remove large debris, concentrated (from 3.0 to 0.5 ml) in an Amicon concentrator (5,000 rpm for 45 min in a Sorvall SS-34 rotor), and assayed for protein concentration by the BCA microassay (Pierce Chemical Co.).
(iv) Immunoprecipitation.
More reproducible results were obtained if a preclearing step was added prior to immunoprecipitation of lysate supernatants containing EB with an attached, biotinylated apical membrane protein(s). This consisted of adding 30 μl of protein A (Ultralink; Pierce) per 40 μg of lysate protein and incubating for 1 to 2 h at 4°C. The protein A-Sepharose bead slurry was then removed by centrifugation at 13,000 rpm for 1 min (Sorvall MC-12 rotor).
Lysate supernatants were transferred to fresh tubes containing either whole (1 μg of protein/μl) or immunoglobulin G (IgG)-purified (5 μg of protein/μl) anti-EB (rabbit) antiserum (prepared in our laboratory via repeated intravenous injections of live EB over 6 months) or normal, nonimmune rabbit serum (1 μg of protein/μl) and rocked on a platform for 2 to 3 h at 4°C. Protein A-conjugated beads (40 μl) were added to the samples for an additional 2-h incubation. The immunoprecipitated chlamydial EB-protein A beads were sedimented at 2,500 × g for 3 min at 4°C, and the pellets were washed six times in lysis buffer for 5 min at 4°C, washed once in deionized distilled water, and finally subjected to one- and two-dimensional electrophoresis, as previously described (20).
(v) Chemiluminescence.
Resolved proteins were detected by chemiluminescence. Samples were transferred to nitrocellulose membrane (75 mA overnight; Hybond ECL membrane; Amersham International, Little Chalfont, Buckinghamshire, England), blocked with 3% gelatin, exposed for 1 h to neutravidin-conjugated horseradish peroxidase (1:10,000; Pierce), washed five times, exposed to the enhanced chemiluminescence (ECL) detection reagents, and processed 1 to 4 min on blue-light-sensitive autoradiography film (Hyperfilm; Amersham). All experiments were performed a minimum of six times on separate occasions.
(vi) MALDI-MS.
Isoelectric focusing (20) of unlabeled apical membrane proteins was performed in preparation for peptide mass mapping. Unlabeled apical membrane proteins, located via Gel Code Blue staining (Pierce), were excised from three to four two-dimensional gels and mailed to the Protein Core Facility, Howard Hughes Medical Institute, Columbia University, New York, N.Y., for in-gel digestion with endoproteinase Lys-C and MALDI-MS.
Measurement of sulfhydryl blocking reactions.
HEC-1B cells, in D-MEM-10, were grown to subconfluency in four-well plates (Nalge Nunc International, Naperville, Ill.). After washing with PBS, the epithelial host cells were exposed for 30 min at 4 or 35°C to one of the following: (i) the membrane-impermeable, thiol-alkylating reagent DTNB (1 mM; 5,5′-dithiobis[2-nitrobenzoic acid]); (ii) bacitracin (3 mM; Sigma); (iii) an anti-protein disulfide isomerase (anti-PDI) monoclonal antibody (IgG2a; MA3-019, clone RL-90; Affinity Reagents, Golden, Colo.); (iv) an irrelevant mouse IgG control antibody; or (v) D-MEM without FCS. Then, C. trachomatis serovar E EBs, diluted to provide a 50% infection per cell monolayer, were added to each HEC-1B monolayer and incubation was continued for 1.5 to 2 h at 4 or 35°C. The monolayers were then washed, replenished with fresh medium, and incubated for 48 h at 35°C. Quadruplicate coverslips were fixed with cold methanol, stained with the fluorescein-labeled monoclonal antibodies directed against C. trachomatis MOMP (Syva), and examined by fluorescence microscopy for chlamydial infectivity or neutralization thereof by inclusion counting. The fluorescent chlamydial inclusions in 30 grid fields at a magnification of ×40 were counted. All measures of statistical significance were determined using a two-tailed Student's t test. Each experiment was performed a minimum of three times on separate days.
Assembly of data.
Gels and the Western blot were scanned using a Microtek ScanMaker III and assembled using Adobe Photoshop 5.0 and Adobe Pagemaker 6.0 software for the Power Macintosh.
RESULTS AND DISCUSSION
Endometrial epithelial apical membrane proteins associated with attached EB.
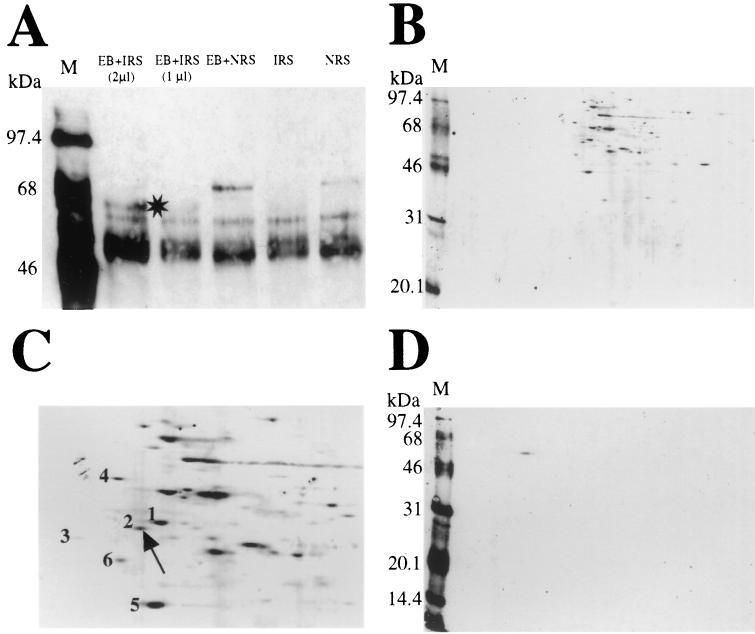
Biotinylated apical membrane proteins, with or without attached C. trachomatis serovar EB (Fig. 1), were analyzed by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (SDS-12.5% PAGE) and detected with neutravidin-horseradish peroxidase and chemiluminescence. A unique protein of ∼55 kDa was reproducibly associated with immunoprecipitated EB using immune rabbit serum (Fig. 1A); proteins of ∼90 and 45 kDa were also frequently detected (data not shown).
FIG. 1.
Electrophoretic analysis of endometrial epithelial apical membrane proteins associated with attached, infectious EB. (A) A 40-μg amount of total protein per lane from biotinylated apical membrane lysates immunoprecipitated with C. trachomatis serovar E EB was resolved by SDS-PAGE (12.5% gel) and visualized by ECL, as described in Materials and Methods. Samples included the following: EB immunoprecipitated with immune rabbit serum (IRS) (2 μl), EB immunoprecipitated with IRS (1 μl), EB immunoprecipitated with normal rabbit serum (NRS) (1 μl), no EB plus IRS (1 μl), and no EB plus NRS (1 μl). The star denotes a unique ∼55-kDa biotinylated apical membrane protein reproducibly associated with immunoprecipitated EB (second and third lanes). (B to D) A 40-μg amount of total protein from apical membrane lysates immunoprecipitated with EB was focused in a pH range from 3 to 10 in the first dimension and separated by SDS-PAGE in the second dimension. Gels were subjected to ECL (B and C) and Western blotting (D). (C) A photographic enlargement of some of the proteins in the acidic range is illustrated. Duplicate unlabeled proteins identified by MALDI-MS were as follows: spot 1, hsp60; spot 2, PDI; spot 3, unidentified (amount too small); spot 4, 78-kDa glucose regulatory protein; spot 5, α actin; spot 6, ATP synthase. Spot 2, identified as PDI, shows immunoreactivity with anti-PDI monoclonal antibody (100 μg/ml; 1/100 dilution) upon Western blotting (D). (A, B, and D) M, molecular mass markers.
Isoelectric focusing of both biotinylated and unlabeled apical membrane proteins, with or without attached and immunoprecipitated EBs, was performed. Control unlabeled apical membrane proteins without associated EBs provided an apical membrane protein profile. Both Gel Code Blue staining of unlabeled proteins and chemiluminescent staining of labeled proteins revealed a number of acidic proteins in the ∼46- to 68-kDa range (Fig. 1B). Six proteins with theoretical pIs ranging between 4.69 and 5.29 (Fig. 1C) were analyzed by MALDI-MS. The ∼55-kDa apical membrane protein repeatedly associated with immunoprecipitated EB (spot 2) was identified as PDI (Swiss-Prot accession no. P07237; p55) (Table 1), a component of the hormone receptor complexes thyrotropin (3) and estrogen (13). Other proteins were reported as follows: spot 1, heat shock protein 60 (hsp60); spot 3, unidentified (amount too small); spot 4, 78-kDa glucose regulatory protein in the hsp70 family; spot 5, α actin; and spot 6, ATP synthase. Confirmation that the ∼55-kDa protein (spot 2) is PDI was obtained by Western blot detection with anti-PDI antibody (Fig. 1D).
TABLE 1.
MS analysis identified isoelectric-focusing acidic spot 2 as PDIa
| Mass (Da) | Position (amino acid no.) | Peptide sequence |
|---|---|---|
| 1,650.829 | 18-31 | DAPEEEDHVLVLRK |
| 2,441.656 | 82-103 | VDATEESDLAQQYGVRGYPTIK |
| 1,881.051 | 115-130 | EYTAGREADDIVNWLK |
| 1,966.244 | 231-247 | HNQLPLVIEFTEQTAPK |
| 2,620.881 | 445-467 | FFPASADRTVIDYNGERTLDGFK |
pI = 4.69; average mass = 55,294.02 (Da).
Thiol-disulfide exchange reactions at the infected eukaryotic cell surface.
While most PDI is located in the endoplasmic reticulum (17), some is clearly recycled to the eukaryotic cell surface, where it is known to carry out thiol-disulfide interchange reactions (5, 11). One example of exploitation of surface PDI-catalyzed disulfide reactions is chain separation of diphtheria toxin, which results in host cell cytotoxicity (15, 22). Similar reactions in the highly disulfide-cross-linked EB envelope are crucial for triggering the transition of EB to reticulate bodies and, perhaps, even earlier conformational rearrangements within kinetically trapped, folded structural proteins for attachment and entry (7, 8, 21). Since thiol exchange reactions occur at a very rapid rate, i.e., once every 10−6 s at 35°C, we used three approaches for examining sulfhydryl blocking and the role of PDI during chlamydial attachment and used chlamydial infectivity as a readout. It should be noted that infectious serovar E EBs attach to host apical surfaces in essentially equal numbers at 35 and 4°C.
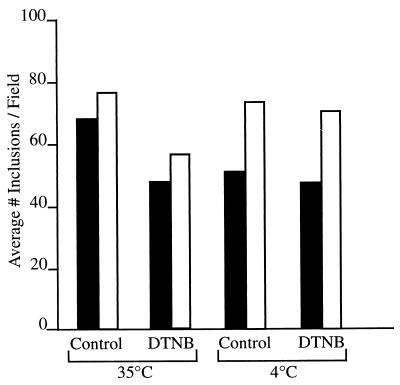
The importance of cross-linking and reduction during the initial stages of EB infection of host epithelial cells was first examined using the non-membrane-permeating, thiol-alkylating reagent DTNB (21). Exposure of chlamydiae to DTNB during the 1- to 2-h attachment phase reduced chlamydial infectivity 30% at 4°C and 10% at 35°C (Fig. 2). These results are virtually identical to those obtained by Abell and Brown (1), who showed that DTNB reduced but did not abolish Sindbis virus entry and infectivity of BHK-1 cells via interfering with viral capsomere protein disulfide cross-linking. Both EB and Sindbis virus are unique in the extent of disulfide proteins in their respective envelopes, which have been described as rigid external matrices with associated lipids as opposed to fluid lipid bilayers with associated proteins (21).
FIG. 2.
The thiol-alkylating reagent DTNB reduces C. trachomatis serovar E EB infectivity in HEC-1B cells. Exposure of HEC-1B cells to DTNB (1 mM) 30 min prior to and during attachment (1 to 2 h) of EB reduced chlamydial inclusion counts by 30% at 35°C and 10% at 4°C, compared to the number of inclusions formed in control, non-DTNB-exposed infected cells. Data from two of the three experiments conducted on separate occasions are represented, one in open-bar format and the other in solid-bar format.
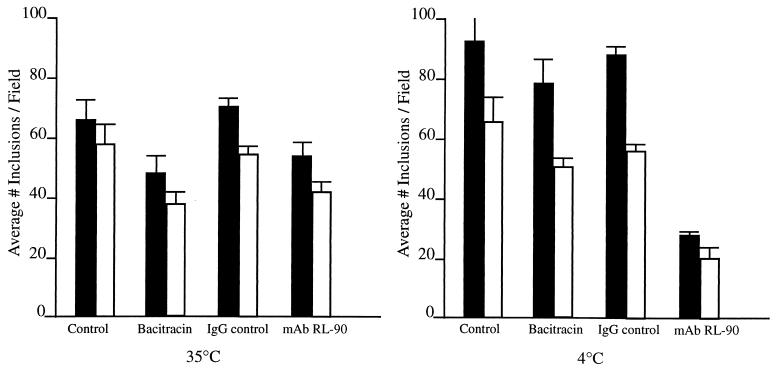
Further emphasis of the importance of disulfide cleavage at infected cell surfaces was obtained by Ryser et al. (23), who demonstrated that, in addition to DTNB, bacitracin, a non-membrane-permeating inhibitor of PDI activity at eukaryotic cell surfaces, as well as anti-PDI antibody, effectively reduced attachment of human immunodeficiency virus, via gp120 protein, and infectivity to human lymphoid cells. In a similar fashion, the presence of bacitracin during EB attachment to HEC-1B cells decreased chlamydial infectivity 27 to 36% at 35°C and 16 to 22% at 4°C (Fig. 3), based on comparison of the number of inclusions in control infected but bacitracin-unexposed monolayers. Addition of anti-PDI monoclonal antibodies to HEC-1B cells during initial chlamydial attachment resulted in a marked reduction (62 to 69%) of chlamydial infectivity at 4°C versus a modest reduction in infectivity at 35°C (Fig. 3). The fact that only partial inhibition of infectivity was observed at 35°C is, again, likely due to the rapid rate of disulfide-exchange reactions that occur within and between closely located cysteine-containing polypeptides.
FIG. 3.
Inhibitors of PDI reductive activity reduce chlamydial infectivity in HEC-1B cells. Exposure of HEC-1B cells to the antibiotic bacitracin (3 mM) or monoclonal antibodies generated against PDI (RL-90) 30 min prior to and during attachment (1 to 2 h) of EB at 35 or 4°C reduced chlamydial inclusion counts. Two of the three experiments conducted on separate occasions are represented. Error bars indicate 1 standard deviation of the mean.
Protein disulfide isomerase, ∼55 kDa, has recently been shown to act in concert with hsp70 as well as accessory proteins of 45 to 48 and 90 kDa to activate the estrogen receptor—but not the progesterone receptor—thereby enhancing the affinity of estrogen binding and then stabilizing the complex for subsequent estrogen-response element-DNA binding (13, 35). Interestingly, two protein disulfide isomerase-encoding genes are identified in the C. trachomatis serovar D genome (28) (CT780 and CT783, contigs 10.1 and 10.2, respectively [http://chlamydia-www.berkeley.edu:4231/], and coordinates 915488 to 915979 and 919539 to 920585 [http://www.stdgen.lanl.gov]). Despite presumed similarity in function, they have little sequence homology to the eukaryotic PDIs (prototype p58, Swiss-Prot accession no. P3010; LALIGN [http://workbench.sdsc.edu]). In a 10-domain alignment comparison, sequence identity averaged 23.8%, and the chlamydial PDIs clearly lack key eukaryotic PDI domains, such as the endoplasmic reticulum retention and nuclear localization signals. Further, the chlamydial PDIs posses only one thioredoxin domain versus two thioredoxin domains in eukaryotic PDIs, and again, there is little sequence homology between the prokaryotic and eukaryotic amino-terminal thioredoxin domains (Prosite accession no. for thioredoxin, PS00194; •. Ndjinn, http://workbench.sdsc.edu). The chlamydial outer membrane proteins, MOMP (OmpA), and the 60-kDa protein (OmcB) are cysteine rich, are highly disulfide cross-linked and have been shown to possess adhesin function (7, 8, 32, 34). One might speculate that the eukaryotic ∼55-kDa PDI, during EB association at the endometrial epithelial apical membrane, serves as “the universal trigger for reduction of the supramolecular cross-linked EB outer membrane complex” (8), thereby exposing OmpA or OmcB adhesin domains and/or, alternatively, activating the chlamydial PDIs, if periplasmic, for further reduction of inter- and intramolecular disulfide cross-links for high-affinity, secondary adhesin binding promoting entry.
In summary, since studies in our laboratory have shown that (i) C. trachomatis serovar E EB attachment and infectivity are dramatically enhanced in estrogen-dominant primary human endometrial epithelial cells (16, 38), (ii) productive entry into and infectivity of EB in host cells is dependent on reduction of EB disulfide-cross-linked outer membrane proteins at the host cell surface (21), (iii) host cell PDI is closely associated with adherent EB, and (iv) PDI interacts with host cell estrogen receptors (13), separate pieces of data seem to be coming together to provide preliminary evidence for a potentially new, interesting receptor for luminal C. trachomatis serovar E. Studies are in progress to verify these findings in estrogen- versus progesterone-dominant primary genital epithelial cells.
Acknowledgments
This study was supported by a Public Health Service grant from the National Institutes of Health (NIAID AI13446).
We especially thank Mary Ann Gawinowicz and the Protein Chemistry Core Facility of the Howard Hughes Medical Institute/Columbia University, New York, N.Y., for MALDI-MS analyses, and Antonio Rusinol, a PDI researcher in the Department of Biochemistry, J. H. Quillen College of Medicine, for helpful discussions on PDI and sequence domain homology analyses.
Editor: D. L. Burns
REFERENCES
- 1.Abell, B. A., and D. Brown. 1993. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J. Virol. 67:5496-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavoil, P. M., R.-C. Hsia, and R. G. Rank. 1996. Prospects for a vaccine against Chlamydia genital disease. I. Microbiology and pathogenesis. Bull. Inst. Pasteur 94:5-54. [Google Scholar]
- 3.Couet, J., S. de Bernard, H. Loosfelt, B. Saunier, E. Milgrom, and M. Misrahi. 1996. Cell surface protein disulfide isomerase is involved in the shedding of human thyrotropin receptor ectodomain. Biochemistry 35:14800-14805. [DOI] [PubMed] [Google Scholar]
- 4.Davis, C. H., and P. B. Wyrick. 1997. Differences in the association of Chlamydia trachomatis serovar E and serovar L2 with epithelial cells in vitro may reflect biological differences in vivo. Infect. Immun. 65:2914-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feener, E. P., W.-C. Shen, and H. J.-P. Ryser. 1990. Cleavage of disulfide bonds in endocytosed macromolecules. A processing not associated with lysosomes or endosomes. J. Biol. Chem. 265:18780-18785. [PubMed] [Google Scholar]
- 6.Hackstadt, T. 1999. Cell biology, p. 101-138. In R. S. Stephens (ed.), Chlamydia: cell biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 7.Hatch, T. P. 1996. Disulfide cross-linked envelope proteins: the functional equivalent of peptidoglycan in chlamydiae? J. Bacteriol. 178:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatch, T. P. 1999. Developmental biology, p. 29-67. In R. S. Stephens (ed.), Chlamydia: cell biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 9.Hayashi, K., M. Hayashi, M. Jalkanen, J. H. Firestone, R. L. Trelstad, and M. Bernfield. 1987. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J. Histochem. Cytochem. 35:1079-1088. [DOI] [PubMed] [Google Scholar]
- 10.Henderson, I. R., and A. C. Lam. 2001. Polymorphic proteins of Chlamydia spp.: autotransporters beyond the proteobacteria. Trends Microbiol. 9:591-596. [DOI] [PubMed] [Google Scholar]
- 11.Klappa, P., H. C. Hawkins, and R. B. Freedman. 1997. Interactions between protein disulfide isomerase and peptides. Eur. J. Biochem. 248:37-42. [DOI] [PubMed] [Google Scholar]
- 12.Kuo. C.-C., N. Takahashi, A. F. Swanson, Y. Ozeki, and S.-I. Hakomori. 1996. An N-linked high mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J. Clin. Investig. 98:2813-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landel, C. C., P. J. Kushner, and G. L. Greene. 1995. Estrogen receptor accessory proteins: effects on receptor-DNA interations. Environ. Health Perspect. 103(Suppl. 7):23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamelak, D., M. Mylvaganam, H. Whetstone, E. Hartmann, W. Lennarz, P. B. Wyrick, J. E. Raulston, H. Han, P. Hoffman, and C. A. Lingwood. 2001. Hsp70s contain a specific sulfogalactolipid binding site. Differential aglycone influence on sulfogalactosyl ceramide binding by recombinant prokaryotic and eukaryotic hsp70 family members. Biochemistry 40:3572-3582. [DOI] [PubMed] [Google Scholar]
- 15.Mandel, R., H. J.-P. Ryser, F. Ghani, M. Wu, and D. Peak. 1993. Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide isomerase. Cell Biol. 90:4112-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maslow, A. S., C. H. Davis, J. Choong, and P. B. Wyrick. 1988. Estrogen enhances attachment of Chlamydia trachomatis to human endometrial epithelial cells in vitro. Am. J. Obstet. Gynecol. 159:1006-1014. [DOI] [PubMed] [Google Scholar]
- 17.Novia, R. 1999. Protein disulfide isomerase: the multifunctional redox chaperone of the endoplasmic reticulum. Cell Dev. Biol. 10:481-493. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen-Lathrop, S. J., K. Koshiyama, N. Phillips, and R. S. Stephens. 2000. Chlamydia-dependent biosynthesis of a heparan sulfate-like compound in eukaryotic cells. Cell. Microbiol. 2:137-144. [DOI] [PubMed] [Google Scholar]
- 19.Raulston, J. E. 1995. Chlamydial envelope components and pathogen-host cell interactions. Mol. Microbiol. 15:607-616. [DOI] [PubMed] [Google Scholar]
- 20.Raulston, J. E., C. H. Davis, D. H. Schmiel, M. W. Morgan, and P. B. Wyrick. 1993. Molecular characterization and outer membrane association of a Chlamydia trachomatis protein related to the hsp70 family of proteins. J. Biol. Chem. 268:23139-23147. [PubMed] [Google Scholar]
- 21.Raulston, J. E., C. H. Davis, T. R. Paul. J. D. Hobbs, and P. B. Wyrick. 2002. Surface accessibility of the 70-kilodalton Chlamydia trachomatis heat shock protein following reduction of outer membrane protein disulfide bonds. Infect. Immun. 70:535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryser, H. J.-P., R. Mandel, and F. G. Ghani. 1991. Cell surface sulfhydryls are required for the cytotoxicity of diphtheria toxin but not ricin in Chinese hamster ovary cells. J. Biol. Chem. 266:18439-18442. [PubMed] [Google Scholar]
- 23.Ryser, H. J.-P., E. M. Levy, R. Mandel, and G. Disciullo. 1994. Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc. Natl. Acad. Sci. USA 91:4559-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambuy, Y., and E. Rodriguez-Boulan. 1998. Isolation and characterization of the apical surface of Madin-Darby canine kidney epithelial cells. Proc. Natl. Acad. Sci. USA 85:1529-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schachter, J. 1999. Infection and disease epidemiology, p. 139-179. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 26.Schachter, J., and P. B. Wyrick. 1994. Culture and isolation of Chlamydia trachomatis. Methods Enzymol. 236:377-390. [DOI] [PubMed] [Google Scholar]
- 27.Stephens, R. S. 1994. Molecular mimicry and Chlamydia trachomatis infection in eukaryotic cells. Trends Microbiol. 2:99-101. [DOI] [PubMed] [Google Scholar]
- 28.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: C.trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 29.Stephens, R. S., K. Koshiyama, E. Lewis, and A. Kubo. 2001. Heparin-binding outer membrane protein of chlamydiae. Mol. Microbiol. 40:691-699. [DOI] [PubMed] [Google Scholar]
- 30.Su, H., and H. D. Caldwell. 1998. Sulfated polysaccharides and a synthetic sulfated polymer are potent inhibitors of Chlamydia trachomatis infectivity in vitro but lack protective effect in an in vivo model of chlamydial genital tract infection. Infect. Immun. 66:1258-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su, H. L. Raymond, D. D. Rockey, E. Fischer, T. Hackstadt, and H. D. Caldwell. 1996. A recombinant Chlamydia trachomatis outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc. Natl. Acad. Sci. USA 93:11143-11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su, H., N. G. Watkins, Y.-X. Zhang, and H. D. Caldwell. 1990. Chlamydia trachomatis-host cell interactions: role of the chlamydial outer membrane protein as an adhesin. Infect. Immun. 58:1017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taraktchoglou, M., A. P. Pacey, J. E. Turnbull, and A. Eley. 2001. Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate. Infect. Immun. 69:968-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ting, L.-M., R.-C. Hsia, C. G. Haidaris, and P. M. Bavoil. 1995. Interaction of outer envelope proteins of Chlamydia psittaci GPIC with the HeLa cell surface. Infect. Immun. 63:3600-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsibris, J. C. M., L. T. Hunt, G. Ballejo, W. C. Barker, J. T. Leighton, and W. N. Spellacy. 1989. Selective inhibition of protein disulfide isomerase by estrogens. J. Biol. Chem. 264:13967-13970. [PubMed] [Google Scholar]
- 36.Vretou, E., P. C. Goswami, and S. K. Bose. 1989. Adherence of multiple serovars of Chlamydia trachomatis to a common receptor on HeLa and McCoy cells is mediated by thermolabile protein(s). J. Gen. Microbiol. 135:3229-3237. [DOI] [PubMed] [Google Scholar]
- 37.Yeaman, C., K. K. Grindstaff, and W. J. Nelson. 1999. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 79:73-98. [DOI] [PubMed] [Google Scholar]
- 38.Wyrick, P. B., J. Choong, C. H. Davis, S. T. Knight, M. O. Royal, A. S. Maslow, and C. R. Bagnell. 1989. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect. Immun. 57:2378-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyrick, P. B., C. H. Davis, and E. A. Wayner. 1994. Chlamydia trachomatis does not bind to αβ1 integrins to colonize a human epithelial cell line cultured in vitro. Microb. Pathog. 17:159-166. [DOI] [PubMed] [Google Scholar]