Abstract
The signaling pathways triggered by adherence of Candida albicans to the host cells or extracellular matrix are poorly understood. We provide here evidence in C. albicans yeasts of a p105 focal adhesion kinase (Fak)-like protein (that we termed CaFak), antigenically related to the vertebrate p125Fak, and its involvement in integrin-like-mediated fungus adhesion to vitronectin (VN) and EA.hy 926 human endothelial cell line. Biochemical analysis with different anti-chicken Fak antibodies identified CaFak as a 105-kDa protein and immunofluorescence and cytofluorimetric analysis on permeabilized cells specifically stain C. albicans yeasts; moreover, confocal microscopy evidences CaFak as a cytosolic protein that colocalizes on the membrane with the integrin-like VN receptors upon yeast adhesion to VN. The protein tyrosine kinase (PTK) inhibitors genistein and herbimycin A strongly inhibited C. albicans yeast adhesion to VN and EA.hy 926 endothelial cells. Moreover, engagement of αvβ3 and αvβ5 integrin-like on C. albicans either by specific monoclonal antibodies or upon adhesion to VN or EA.hy 926 endothelial cells stimulates CaFak tyrosine phosphorylation that is blocked by PTK inhibitor. A role for CaFak in C. albicans yeast adhesion was also supported by the failure of VN to stimulate its tyrosine phosphorylation in a C. albicans mutant showing normal levels of CaFak and VNR-like integrins but displaying reduced adhesiveness to VN and EA.hy 926 endothelial cells. Our results suggest that C. albicans Fak-like protein is involved in the control of yeast cell adhesion to VN and endothelial cells.
Candida albicans is the most prevalent opportunistic fungal pathogen in humans that causes various forms of candidiasis ranging from superficial mucosal infections to life-threatening systemic diseases, all of which have an increased incidence in immunocompromised patients (34). The adherence of C. albicans to host cells such as endothelial and epithelial cells and to extracellular matrix (ECM) components is considered a crucial event in the pathogenesis of candidiasis (6, 12). Multiple adhesins, such as mannoproteins, lectin-like receptors, carbohydrates and integrin-like molecules, can mediate C. albicans-host cell adhesion (6, 12, 23, 24).
Integrins are a large family of highly conserved heterodimers composed of noncovalently linked α and β subunits that mediate cell-matrix and cell-cell interactions in embryogenesis, hemostasis, wound healing, tumor and microorganism invasion, immune response, and inflammation (25, 38). These receptors mediate the tight adhesion of cells to the ECM at sites referred to as focal adhesions. Within focal adhesions, the cytoplasmic domains of the integrin heterodimers provide a site to which cytoskeletal proteins are tethered. In addition to their structural roles, several findings indicate that integrins also play a role in cellular signaling (11, 15, 19). Engagement of integrins with their cognate ligands or with specific antibodies (Abs) induces a number of signaling events within the cell, including changes of pH and intracellular Ca2+ concentration, stimulation of tyrosine phosphorylation of a number of cellular proteins, and induction of gene expression. Since the integrins contain short cytoplasmic domains that exhibit no enzymatic activity, these cellular signals must be elicited through intermediary signaling proteins, namely, protein tyrosine kinases (PTKs). The cytosolic PTKs implicated in integrin-mediated signaling include members of Fak, Src, and Zap70/Syk families (11, 15).
The Fak family consists of two evolutionarily conserved PTKs localized in the focal adhesions, namely, p125Fak and Pyk-2 (8, 36, 43). The cDNA encoding p125Fak has been isolated from different species and shows a high level of homology that reaches 95% identity at the amino acid level. A number of studies have shown that Fak functions as part of a cytoskeletal-associated network of signaling proteins, including p130Cas, Shc, and Grb-2, which act in combination to transduce integrin-generated signals to mitogen-activated protein kinase (MAPK) cascades (44). Tyrosine phosphorylation of the Fak family is regulated by different stimuli which include adhesive events, in that several components of the ECM, such as fibronectin (FN), vitronectin (VN), laminin, and collagen IV, or clustering of β1, β3, β5, and β7 integrins trigger p125Fak tyrosine phosphorylation (5, 15, 29).
Accumulating evidence also indicate that Fak tyrosine phosphorylation is important for cell migration. Indeed, experiments employing Fak null cells, Fak overexpression, or dominant-negative Fak constructs have established the requirement for Fak in integrin-stimulated cell migration (20, 26, 35). Moreover, expression of the protein tyrosine phosphatase PTEN leads to Fak dephosphorylation and the inhibition of cell motility (47).
We have recently reported in C. albicans yeasts the presence of αvβ3 and αvβ5 integrin-like VN receptors (VNRs) that mediate specific adhesion to VN, and the involvement of VN in yeast adhesion to human endothelial cell line (45). Moreover, the existence in C. albicans and in less-pathogenic Candida species of surface proteins antigenically, structurally, and functionally related to the α5β1 integrin FN receptor has been previously demonstrated (39, 40). There is also evidence that C. albicans expresses an INT1 gene encoding a surface protein, InT1p, structurally related to αM and αX subunits of the β2 integrin (18).
The study of signal transduction pathways in virulent fungi is especially important in view of their putative implications in the regulation of pathogenicity. In this regard, a crucial role in the control of Candida adherence, hyphal development, and virulence has been shown for Ras-related GTPase (53), homologues of serine-threonine p21-associated kinase (PAK) (29) and MAPK cascade components (16, 32), phosphoinositide-3 kinase (PI-3K) (4), and for CaHK1 histidine kinase (7).
However, although a number of phosphate-containing proteins have been shown in the C. albicans cell wall (9), and stimulation of tyrosine phosphorylation of several substrates was described in Candida yeast cells adherent to epithelial cells (2), the role of PTKs and tyrosine phosphorylation in the regulation of C. albicans adherence to ECM and to host cells has been poorly elucidated.
The present study sought to determine whether a putative vertebrate p125Fak homologue that we termed CaFak is expressed in C. albicans yeast cells and whether cross-linking of the αvβ3 and αvβ5 integrin-like receptors or adhesion to VN or a human endothelial cell line can regulate its tyrosine phosphorylation.
MATERIALS AND METHODS
Yeast strains and culture conditions.
A C. albicans clinical isolate and an echinocandin-resistant agerminative strain originally derived from a chemically mutagenized culture of C. albicans 3153 (10, 13) were kindly provided by A. Cassone (Istituto Superiore di Sanità, Rome, Italy) and used throughout this study.
Yeasts were cultured into Sabouraud dextrose agar (SDA; Becton Dickinson Microbiology Systems, Cockeysville, Md.), frozen at −80°C in small volumes in Sabouraud dextrose broth containing glycerol 5%, and subcultured monthly in SDA. For assays, a loopful of yeast cells was removed from the agar slant, washed twice with cold phosphate-buffered saline (PBS) by centrifugation at 3,000 rpm, and grown to mid-exponential phase at 24°C in Sabouraud dextrose broth. Growth curves were determined spectrophotometrically by measurement of the optical density at 420 nm and a simultaneous count.
Human EA.hy 926 endothelial cell line culture.
The human EA.hy 926 endothelial cell line (33) was maintained in Dulbecco modified Eagle medium (Gibco-BRL/Life Technologies, Milan, Italy) supplemented with 10% heat-inactivated fetal calf serum (BioWhittaker Europe, Vewiers, Belgium), 2% hypoxanthine-aminopterin-thymidine, 2 mM HEPES, 2 mM l-glutamine, 100 IU of penicillin/ml, and 100 mg of streptomycin/ml. Third- or fourth-passage cells were utilized for adhesion assay.
Abs, adhesive proteins, and reagents.
The following mouse monoclonal Abs (MAbs) were used: anti-NH2 terminus of chicken Fak (immunoglobulin G [IgG]; anti-Fak NH2) was provided by Guido Tarone, University of Turin, Turin, Italy (14); anti-central domain (residues 354 to 533) of chicken Fak (clone 77, IgG1) was purchased from Transduction Laboratories, Lexington, Ky.; anti-human αvβ3 (clone LM609, IgG1) and anti-human αvβ5 (clone P1F6, IgG1; Chemicon International, Inc., Temecula, Calif.); anti-human VN (clone VIT-2, IgG1; Sigma Chemical Co., St. Louis, Mo.); and anti-phosphotyrosine (pTyr) (clone 4G10, IgG2b; Upstate Biotechnology). A MAb to a cell surface glucomannoprotein (GMP) constituent of C. albicans (clone GF2, IgG1) was kindly provided by A. Cassone (48), and anti-rat CD5 (clone OX-19, IgG1) was obtained from Pharmingen, Palo Alto, Calif.
The following rabbit polyclonal Abs were used: antiserum directed to the COOH domain of chicken Fak (anti-Fak COOH), antiserum directed against an intracytoplasmic domain of the human β3 integrin, and antiserum directed to the COOH domain of the human αv integrin (kindly provided by Guido Tarone), antiserum to a COOH-terminal sequence of the human β5 integrin (Bioline Diagnostic, Turin, Italy), and anti-human VN Ab (Gibco-BRL).
Normal rabbit serum from Cappel-Organon Teknika or affinity-purified rabbit anti-mouse IgG (RAM; Zymed Laboratories, Inc., San Francisco, Calif.) was used as a negative control in immunoprecipitation studies. Horseradish peroxidase-conjugated sheep anti-mouse IgG (Amersham Pharmacia Biotech, Uppsala, Sweden) was used in immunoblotting. Purified fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 fragments of anti-mouse (GAM) and anti-rabbit (GAR) IgG were purchased from Cappel-Organon Teknika, Turnhout, Belgium, and Dako, Copenhagen, Denmark, respectively; purified Texas Red-conjugated F(ab′)2 GAM was purchased from Molecular Probes, Inc., Eugene, Oreg.
Human VN was purchased from Gibco-BRL/Life Technologies. Bovine serum albumin (BSA) and poly-l-lysine were purchased from Sigma, and fibrinogen (Fb) was obtained from Calbiochem-Novabiochem, La Jolla, Calif.
The PTK inhibitors herbimycin A and genistein were purchased from Sigma and Calbiochem-Novabiochem, respectively.
Immunofluorescence and flow cytometry.
C. albicans yeast cells were permeabilized, and immunofluorescence and flow cytometric analysis were performed with the anti-Fak COOH rabbit antiserum or the following MAbs: anti-Fak NH2 or anti-Fak clone 77. Normal rabbit serum, isotype-matched (IgG1) anti-rat CD5 or anti-GMP (GF2) mouse MAb were used as negative controls.
Briefly, 106 C. albicans yeast cells/ml suspended in PBS at 1% of saponin from Quillaja Bark (Sigma) and 1% ethanol (EtOH; Baker, B.V. Deventer, Holland) were incubated at 37°C, stirred for 30 min, and then washed twice with cold PBS plus 0.1% saponin (45). Aliquots of 106 C. albicans yeast cells were incubated with 50 μl of the first Ab properly diluted (1:25) for 30 min at 4°C and then washed twice with 2 ml of cold PBS without calcium and magnesium (Euroclone, Ltd.). Cells were then incubated with 20 μl of the FITC-GAM or -GAR secondary Ab (1:20) for 30 min at 4°C, washed twice with 2 ml of cold PBS, and resuspended in 0.5 ml of PBS.
The expression of αvβ3 and αvβ5 integrin-like VNRs on the clinical isolate of C. albicans or the mutagenized C. albicans 3153 yeast cells was evaluated by using anti-αvβ3 and anti-αvβ5 MAbs as previously described (45).
The yeast phase was verified microscopically and was not less than 97%.
The cell populations were analyzed for the percentage of positively stained cells, determined over 10,000 events on a FACScan cytofluorimeter (Becton Dickinson, Mountain View, Calif.). Fluorescence intensity is expressed in arbitrary units on a logarithmic scale.
Confocal scanning microscopy.
A total of 2 × 106 cells of C. albicans grown as described above were pelleted by centrifugation at 3,000 rpm, spread on slides, fixed at 50°C, and dried at room temperature (RT) with 60% EtOH solution and absolute EtOH (3) for 2 and 3 min, respectively. Thereafter, yeasts were incubated for 15 min at 37°C, with 20 μg of proteinase K (Boehringer Mannheim, Mannheim, Germany)/ml, washed in PBS, and covered for 3 min with absolute EtOH. Then, 1% BSA in PBS-Tween 20 was added for 30 min, and cells were incubated for 1 h at RT with 100 μl of the following Abs: anti-Fak (clone 77), anti-Fak NH2, anti-GMP (GF2), or anti-rat CD5 (OX-19) MAb, rabbit anti-Fak COOH polyclonal Ab (1:50), or normal rabbit serum. After incubation, cells were washed three times for 10 min each time and three times for 5 min each time with the same buffer. Finally, yeasts were incubated with 100 μl of FITC-GAR or -GAM (1:100) for 30 min at RT, washed as described above, and then covered with glycerol. Candida permeabilization was checked by pretreating yeast cells with 0.5 mg of propidium iodide (Sigma)/ml for 2 min at RT. In some experiments, 7 × 105 C. albicans yeast cells were allowed to adhere for 30 min at 37°C and 5% CO2 to chamber slides coated with 10 μg of VN/ml. Unattached cells were removed by extensive washing with warm PBS, and adherent C. albicans yeast cells were fixed, permeabilized, and stained with anti-Fak NH2, MAb, or anti-Fak COOH rabbit polyclonal Ab as described above. Moreover, C. albicans yeast cells adherent to VN were fixed and permeabilized as described above and then stained for 1 h at 4°C with 100 μl of anti-Fak NH2 MAb and 100 μl of rabbit anti-αv, anti-β3, or anti-β5 integrin antiserum. Samples were then washed three times for 5 min each time with the same buffer and incubated with 100 μl of Texas Red-GAM (1:100) and FITC-GAR (1:60) for 1 h at 4°C, washed three times, and mounted as described above.
All samples were analyzed by using a Bio-Rad MRC600 confocal laser-scanning microscope attached to a Nikon (Diaphot-TMD) inverted microscope with an 60× NA, 1.4 oil immersion lens with an argon-krypton laser operating at an excitation wavelength of 494 nm and with an emission filter of 520 nm. Pairs of images were collected simultaneously in the green channel for confocal image and in the transmission detector for a phase-contrast image at least twice. For colocalization studies, fluorochromes were excited with the 600 line of the argon-krypton laser and imaged by using a 488- or 588-nm bandpass filter. Serial optical sections 1 μm thick were taken for each cell; only one central optical section is shown. Data were acquired and processed by using Comos Bio-Rad software. Intensities were streched to fill the 256 step of the gray scale. The fluorescent image was superimposed in a suitable pseudocolor upon the transmission image with a software command “merge.”
Cell stimulation.
A total of 108 C. albicans yeast cells diluted in PBS were stimulated with anti-αvβ3, anti-αvβ5, or anti-GMP (GF2) MAb (1 μg/5 × 106 cells) for different times (0, 10, 30, and 60 min) at 37°C or allowed to adhere to VN-, BSA-, Fb-, and poly-l-lysine (10 μg/ml)-coated surfaces.
Binding experiments were performed with C. albicans yeast cells and paraformaldehyde-prefixed EA.hy 926 endothelial cells (yeast/EA.hy 926 cell ratio = 100:1) as previously reported (21). Briefly, EA.hy 926 cells starved for 2 h at 37°C and 5% CO2 in phosphate-free RPMI 1640 medium were pelleted and treated with PBS-1% paraformaldehyde for 30 min at RT in a shaker. EA.hy 926 cells were then washed three times in PBS-1% BSA and resuspended in Dulbecco modified Eagle medium.
In some experiments C. albicans yeast cells cultured in YPD medium (1% yeast extract, 2% Bacto Peptone, 2% dextrose; Difco Laboratories, Detroit, Mich.) were pretreated with 10 μM herbimycin A or vehicle (dimethyl sulfoxide [DMSO]) at the same dose for 16 h at RT and then allowed to adhere to VN at 10 μg/ml or paraformaldehyde-prefixed EA.hy 926 confluent cell monolayers.
Immunoprecipitation and Western blotting.
C. albicans yeast cells grown in SDA as described above described were washed three times in PBS and then resuspended in 0.4 ml of radioimmunoprecipitation assay buffer (0.15 M NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.05 M Tris, 4 mM phenylmethylsulfonyl fluoride, 2 mg of aprotinin/ml, 0.1 mM Na3VO4) to which an equal volume of 500-μm nitric acid-washed glass beads (Sigma) was added. Cell suspensions were then vortexed for 2 min and left for 1 min on ice, and the procedure was repeated 8 to 10 times. Lysates were centrifuged at 14,000 × g for 10 min at 4°C and immunoprecipitated. Briefly, lysates were precleared for 1 h at 4°C with 150 μl of 20% suspension of protein A-Sepharose CL-4B (Pharmacia, Uppsala, Sweden). Precleared lysates were then combined with protein A-Sepharose CL-4B preconjugated with rabbit polyclonal anti-Fak COOH, or normal rabbit serum or RAM used as negative control, and incubated overnight at 4°C. Immunocomplexes were then washed seven times with washing buffer (10 mM Tris; 30 mM NaCl; 1 mM EDTA; 0.03% NaN3; 0.25% Nonidet P-40; 0.1% SDS; 0.1% deoxycholate; 0.1% BSA, pH 7.4) and three times with the same buffer without BSA. Bound proteins were released by boiling them in reducing buffer and then centrifuged at 14,000 × g for 2 min. Samples were analyzed by SDS-polyacrylamide gel electrophoresis on 7.0% polyacrylamide gels and then electrotransferred overnight onto polyvinylidene difluoride membranes (Immobilon-P; Millipore) at 4°C and 20 V with a Trans-Blot Electrophoretic Transfer Cell (Bio-Rad). The membrane was then incubated with a blocking solution (5% BSA-0.05% Tween 20 in PBS) for 60 min at RT and washed four times with washing buffer. Thereafter, the membrane was incubated for 2 h at RT with one of the following mouse MAbs: anti-Fak NH2, anti-Fak (clone 77), or irrelevant anti-rat CD5 at a 1:500 dilution in PBS-Tween.
The membrane was washed three times for 10 min each time and three times for 5 min each time with PBS-Tween at RT and then incubated for 1 h with horseradish peroxidase-conjugated sheep anti-mouse IgG at a 1:10,000 dilution (Amersham Life Sciences). The immunoreactivity was detected by using an enhanced chemiluminescence kit (Amersham Life Sciences) according to the manufacturer's instructions.
Adhesion assay to ECM proteins or to EA.hy 926 human endothelial cell line.
Stock preparations of human VN were diluted in PBS (pH 7.4) at a concentration of 1 μg/ml. Protein solution (100 μl) was distributed in 96-well tissue culture flat-bottom plates (Costar, Cambridge, Mass.). After overnight incubation at 4°C, coated plates were washed three times with cold PBS to remove nonimmobilized protein.
Then, 3 × 104 human EA.hy 926 endothelial cells were grown to confluence for 24 h at 37°C in 5% CO2 in collagen-coated 96-well tissue culture flat-bottom microtiter plates (Costar).
C. albicans yeasts were labeled with [3H]glucose (NET- 807; d-[6-3H]glucose specific activity of 1.29 TBq/mmol, 37 MBq, 35.0 Ci/mmol; Amersham Life Sciences). Briefly, yeast cells (108) were labeled with [3H]glucose (20 mCi) in 1 ml of PBS for 3 h at RT. After labeling, microorganisms were washed twice in cold PBS and resuspended at 5 × 106/ml in PBS.
C. albicans yeast cells were pretreated with different doses of genistein (10, 50, 100, or 200 μM) or vehicle (DMSO) for 1 h at 37°C in PBS or of herbimycin A (1, 5, 10, or 50 μM) or vehicle (DMSO) in YPD medium for 16 h at RT and then radiolabeled as described above. Finally, untreated, genistein-treated, herbimycin A-treated, or vehicle-treated C. albicans yeast cells were allowed to adhere to VN or EA.hy 926 confluent cell monolayers for 30 min or 1 h at 37°C in 5% CO2, respectively.
Adhesion was also verified microscopically; the yeast phase was maintained throughout the adhesion assay, and agglutination was not observed. Unattached cells were removed by extensive washing with warm PBS, and yeast cells adhered to VN or EA.hy 926 endothelial cells were harvested by adding twice 100 μl of bleach for 10 min at RT with a cell scraper. Bound counts per minute (cpm), as well as cpm from nonadherent cells plus washes, were quantitated in a β-scintillation counter. Quadruplicate wells were assayed for each sample.
To rule out the contribution of C. albicans binding to plastic in the adhesion to VN, assays were always performed in the presence of a dose of anti-VN MAb (VIT-2) or a rabbit anti-VN antiserum, which maximally inhibited cell binding to VN, while it did not substantially affect cell binding to the plastic surface, as previously described (45). Cell adhesion was calculated as follows: [adherent cells (in cpm)/total cells (in cpm)] × 100, where the total cpm indicates the sum of nonadherent cells, washes, and adherent cell cpm. This value routinely exceeded 95% of total cell counts. Specific binding to VN was calculated as the difference between the total percent adhesion and the percent adhesion not inhibited by anti-VN Ab (nonspecific adhesion).
Statistical analysis.
Statistical analysis by Student's t test was performed by the STATPAC Computerized Program, and a P value of <0.01 was used as the significance criterion.
RESULTS
C. albicans yeast cell adhesion to VN or the EA.hy 926 human endothelial cell line requires PTK activation.
PTK activation plays a central role in the regulation of adhesive interactions of eukaryotic cells to host cells and ECM components (11, 15). Thus, to analyze whether tyrosine kinase activity is a requisite for C. albicans yeast cell adhesion to VN or a human endothelial cell line, yeast cells were pretreated with tyrosine kinase inhibitors, such as genistein and herbimycin A (1, 49), at concentrations that do not affect yeast cell viability.
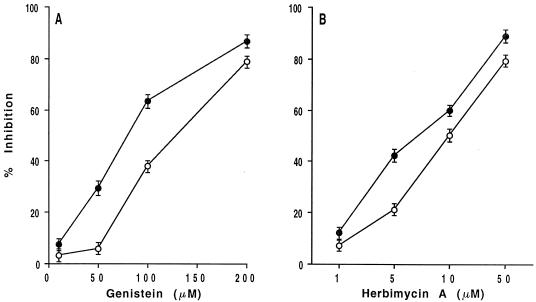
As shown in Fig. 1, both genistein and herbimycin A markedly inhibit yeast cell adhesion to VN and the EA.hy 926 endothelial cell line in a dose-dependent manner, indicating that tyrosine kinase activation is a crucial signaling event required for C. albicans adhesive interactions with VN and the human endothelial cell line.
FIG. 1.
Effect of the PTK inhibitors genistein and herbimycin A on C. albicans yeast cell adhesion to VN and EA.hy 926 human endothelial cell line. Adhesion of [3H]glucose-labeled C. albicans to VN (1 μg/ml) (•) and EA.hy 926 cells (○) evaluated in the absence (control medium) or presence of different doses of genistein (A), herbimycin A (B), or vehicle (DMSO) (data not shown) at 30 min after incubation at 37°C. Results are the mean percent specific inhibition ± the standard deviation (three separate experiments). The mean percent cell yeast adhesions to VN and EA.hy 926 cells were 48 ± 1 and 63 ± 2, respectively. Since no differences were observed between adhesion in the presence of control medium and vehicle, only data in the presence of medium are shown for sake of simplicity.
Expression of a protein antigenically related to the vertebrate p125Fak by C. albicans yeast cells.
Among the PTKs involved in the regulation of cellular adhesion, the prominent role of p125Fak is well established (8, 36, 43). We therefore analyzed whether C. albicans, which expresses several homologues of mammalian signaling machinery, including Ras-related GTPase, MAPK, PAK, and PI-3K (4, 32, 53), could also express a molecule antigenically related to p125Fak.
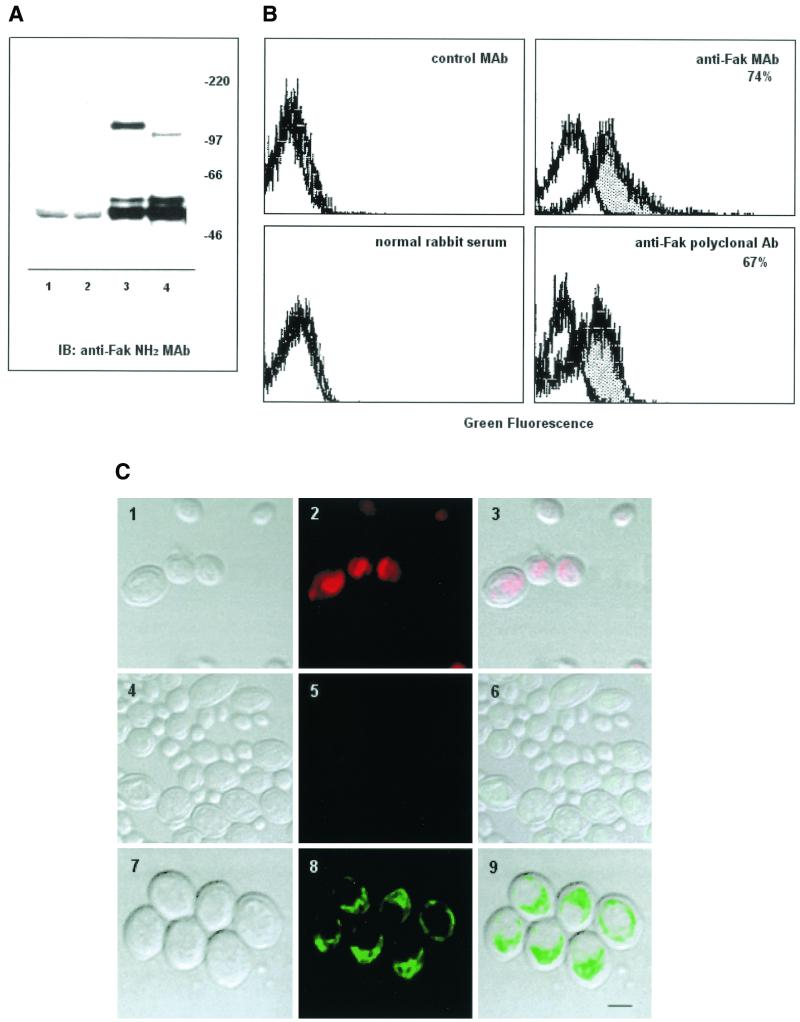
Immunoprecipitation and Western blotting analysis of C. albicans yeast cell lysates with the rabbit anti-Fak COOH polyclonal Ab reveals a protein of 105 kDa and a doublet migrating at 52 to 55 kDa. The higher band at 105 kDa (CAFak) approximates the molecular mass of human Fak (116 kDa) (15) and migrates slightly faster than the Fak protein immunoprecipitated from the human endothelial cell line used as positive control (Fig. 2A ). The identity of the lower bands present in both C. albicans and EA.hy 926 cell immunoprecipitates is currently unknown but likely corresponds to the p125Fak truncated isoform consisting of the COOH-terminal noncatalytic domain, p41/43FRNK (Fak-related nonkinase) (15) or to Fak proteolytic fragments (44).
FIG.2.
C. albicans yeast cells express a protein antigenically related to p125Fak. (A) C. albicans yeast (lane 4) and human endothelial EA.hy 926 (lane 3) cell lysates immunoprecipitated with a rabbit anti-Fak COOH polyclonal Ab and immunoblotted with a mouse anti-Fak NH2 MAb. Lanes 2 and 1 represent normal rabbit serum immunoprecipitates from C. albicans and EA.hy 926 cells used as negative control, respectively. Sizes are indicated in kilodaltons. The results shown are representative of one of three separate experiments. (B) Immunofluorescence and FACS analysis on permeabilized C. albicans yeast cells with mouse anti-Fak NH2 MAb or rabbit anti-Fak COOH polyclonal Ab as primary Ab; an irrelevant isotype-matched anti-rat CD5 MAb or normal rabbit serum was used as control Ab. FITC-conjugated GAM or GAR were used as the second-step Ab. The dotted area represents positive stain. The results are representative of one of three separate experiments. (C) Confocal laser-scanning microscopy analysis on permeabilized C. albicans yeast cells with anti-Fak NH2 MAb as primary Ab and FITC-GAM as a second-step Ab. Panels: 1, 4, and 7, C. albicans yeast cells on a bright field; panel 2, C. albicans yeast cells pretreated with propidium iodide and acquired as confocal image; panel 3, merge of bright field and confocal image; panel 5, C. albicans yeast cells incubated with an isotype-matched irrelevant (anti-rat CD5) MAb and FITC-GAM; panel 6, merge of images in panels 4 and 5; panel 8, C. albicans yeast cells incubated with a mouse anti-Fak NH2 MAb and FITC-GAM; panel 9, merge of images in panels 8 and 9. The results are representative of one of three separate experiments. Bar, 1 μm.
We further evaluated the expression of CaFak in permeabilized C. albicans yeast cells by immunofluorescence and flow cytometry with two different MAbs or a rabbit polyclonal Ab directed against chicken Fak.
As shown in Fig. 2B, both the anti-Fak NH2 MAb and the anti-Fak antiserum positively stained (74 and 67.%, respectively) C. albicans yeast cells. Similar results were obtained by using the other anti-Fak MAb (data not shown), whereas no significant reactivity was observed with an irrelevant isotype-matched MAb or normal rabbit serum used as negative controls.
Finally, confocal microscopy on permeabilized C. albicans yeast cells with the anti-Fak NH2 MAb indicated that CaFak protein is mainly distributed in the cytosol (Fig. 2C). Similar results were obtained with the anti-Fak (clone 77) MAb or the rabbit anti-Fak antiserum (data not shown), whereas no significant reactivity was observed with an irrelevant isotype-matched MAb or normal rabbit serum used as negative controls.
Altogether, these results indicate that C. albicans in the yeast phase expresses a p105Fak-like protein, CaFak, showing an antigenic similarity with the vertebrate p125Fak PTK.
CaFak and integrin-like receptors colocalization is induced in C. albicans yeast cell upon adhesion to VN.
Fak is a cytoplasmic protein kinase that in multicellular organisms localizes to focal adhesions upon cell interaction with ECM proteins. Moreover, in adherent cells also integrins localize into the focal contacts, where they codistribute with cytoskeleton proteins including vinculin, α-actinin, paxillin, tensin, and signaling molecules such as p125Fak (15).
Thus, to support the hypothesis that in C. albicans CaFak may play a role in the intracellular signaling pathways initiated by integrin-like receptors, we evaluated the localization of CaFak and integrin-like receptors in C. albicans yeast cells upon adhesion to VN.
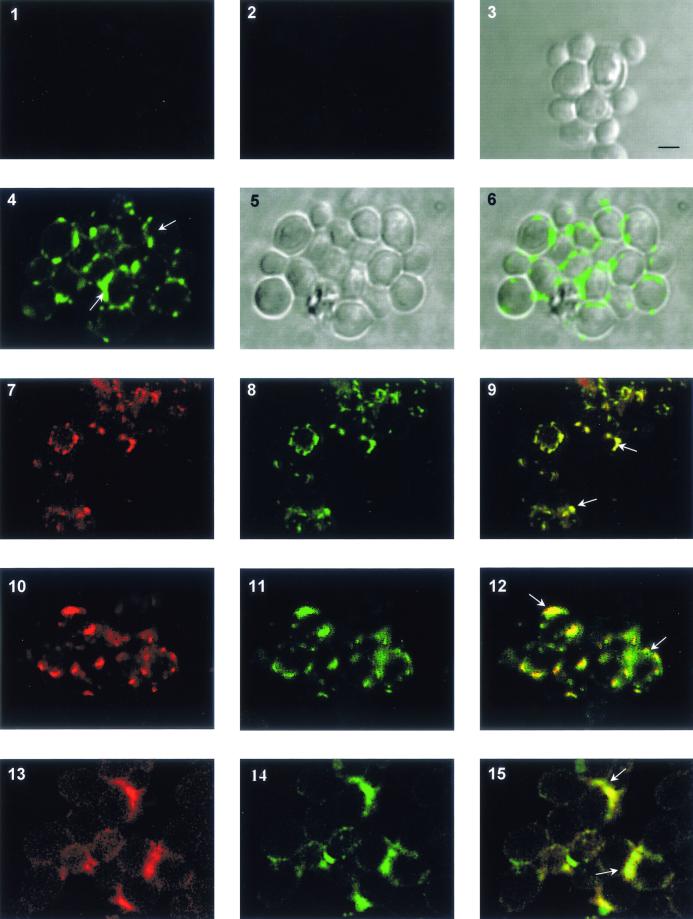
Confocal microscopy analysis on permeabilized adherent C. albicans yeast cells with a rabbit anti-Fak COOH antiserum or the anti-Fak NH2 MAb (data not shown) indicated that, upon adherence of C. albicans yeast cells to VN, CaFak translocates from the cytosol to the membrane and localizes to the focal contacts (Fig. 3). Moreover, we found that upon C. albicans adhesion to VN, CaFak colocalizes with αv, β3, and β5 integrin-like receptors (Fig. 3). No significant reactivity was observed with an irrelevant isotype-matched mouse MAb or normal rabbit serum used as negative controls (data not shown).
FIG. 3.
CaFak and integrin-like receptors colocalize into the focal adhesions in C. albicans yeast cells adherent to VN. Confocal laser-scanning microscopy analysis on permeabilized C. albicans yeast cells with anti-Fak COOH, anti-αv, anti-β3, or anti-β5 integrin rabbit antiserum or anti-Fak NH2 mouse MAb as the primary Ab and FITC-GAR and Texas Red-GAM as second-step Abs. Panels: 3 and 5, C. albicans yeast cells on a bright field; 1 and 2, C. albicans yeast cells incubated with Texas Red-GAM or FITC-GAR, respectively; 4, C. albicans yeast cells stained with anti-Fak COOH and FITC-GAR; 6, merge of images in panels 4 and 5; 7, 10, and 13, C. albicans yeast cells stained with anti-Fak NH2 MAb and Texas Red-GAM; C. albicans yeast cells stained with anti-αv (panel 8), anti-β3 (panel 11), or anti-β5 integrin (panel 14) Ab and FITC-GAR, respectively; 9, 12, and 15, merge of images in panels 7 and 8, 10 and 11, and 13 and 14, respectively. The results are representative of one of three separate experiments. White arrows indicate CaFak localization at focal adhesion sites. Bar, 1 μm.
Tyrosine kinase activation is required for stimulation of CaFak tyrosine phosphorylation upon engagement of αvβ3 and αvβ5 integrin-like VNRs on C. albicans.
A large number of studies indicate that p125Fak is tyrosine phosphorylated in adherent cells, and its tyrosine phosphorylation status increases upon engagement of integrins with their cognate ligands or with specific Abs (5, 8, 22, 29, 36, 42).
We have previously demonstrated that C. albicans yeast cells express αvβ3 and αvβ5 integrin-like VNRs that mediate their adhesion to VN (45).
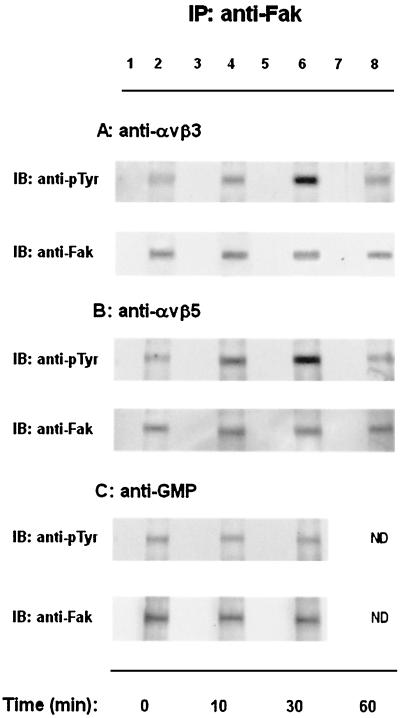
We therefore tested whether ligation of αvβ3 and αvβ5 integrin-like VNRs on C. albicans yeast cells by the natural ligand VN or specific MAb results in CaFak tyrosine phosphorylation. C. albicans yeast cells were left untreated or were treated for different times with anti-αvβ3, anti-αvβ5 MAb, or with anti-GMP MAb used as control. Lysates from stimulated or unstimulated cells were immunoprecipitated with a rabbit anti-Fak COOH polyclonal Ab and blotted with an anti-pTyr or anti-Fak NH2 MAb (Fig. 4). Ab-mediated cross-linking of αvβ3- or αvβ5-like integrins on C. albicans yeasts stimulates CaFak tyrosine phosphorylation that is already evident at 10 min, is maximal at 30 min, and declines at 60 min after stimulation. A basal phosphorylation of this protein was evident in yeast cells, although the levels varied from experiment to experiment. No consistent changes in the tyrosine phosphorylation status of CaFak were observed when C. albicans yeast cells were treated with a control MAb. All immunoprecipitates contained comparable levels of CaFak protein, as evaluated by Western blot analysis. Immunoprecipitates of C. albicans yeast cell lysates with anti-GMP MAb (clone GF2) or an irrelevant rabbit polyclonal Ab, when blotted with anti-pTyr MAb, did not reveal any band (data not shown), indicating that the anti-phosphotyrosine MAb employed in our study specifically reacts with tyrosine-phosphorylated proteins also in Candida.
FIG. 4.
Engagement of αvβ3 and αvβ5 integrin-like VNRs on C. albicans yeast cells stimulates CaFak tyrosine phosphorylation. C. albicans yeast cells were left untreated (time zero) or stimulated for the indicated times at 37°C with mouse anti-αvβ3 (LM609) (A), anti-αvβ5 (P1F6) (B), or anti-GMP (GF2) control MAb (C). Cell lysates were immunoprecipitated with an anti-Fak COOH rabbit polyclonal Ab. The resulting protein complexes were resolved by SDS-7% polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and immunoblotted with anti-pTyr (4G10) (top panel) or anti-Fak NH2 MAb (bottom panel). Lanes 1, 3, 5, and 7 represent normal rabbit serum immunoprecipitates used as negative control. The results shown are representative of one of three separate experiments. As evaluated by densitometric analysis, upon αvβ3 stimulation, a 0.7-fold increase of CaFak tyrosine phosphorylation was observed at 10 min, a 5-fold increase was observed at 30 min, a 0.5-fold increase was observed at 60 min and upon αvβ5 stimulation, a 1.2-fold increase of CaFak tyrosine phosphorylation was observed at 10 min, a 5-fold increase was observed at 30 min, and a 0.3-fold increase was observed at 60 min.
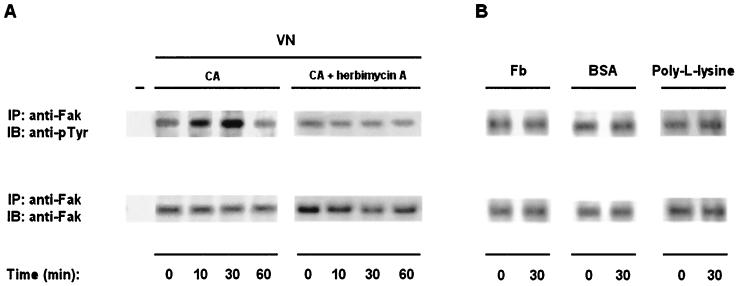
We then tested whether adhesion to VN could also result in CaFak tyrosine phosphorylation. C. albicans yeast cells were allowed to adhere for different times on VN-, Fb-, BSA-, or poly-l-lysine-precoated surfaces, and cell lysates were immunoprecipitated and blotted as described above (Fig. 5). C. albicans yeast cell adhesion to VN results in a marked stimulation of CaFak tyrosine phosphorylation with kinetics similar to those induced by Ab-mediated cross-linking of αvβ3- or αvβ5-like integrins (Fig. 5A). Conversely, no changes in the basal level of CaFak tyrosine phosphorylation were observed upon adhesion to Fb, BSA, or poly-l-lysine (Fig. 5B). All immunoprecipitates contained comparable levels of CaFak protein as evaluated by Western blot analysis. Moreover, pretreatment of C. albicans with the tyrosine kinase inhibitor, herbimycin A, completely inhibited the increased CaFak tyrosine phosphorylation stimulated by yeast cell adhesion to VN.
FIG. 5.
Tyrosine kinase activation is required for stimulation of CaFak tyrosine phosphorylation upon adhesion of C. albicans yeast cells to VN. (A) C. albicans yeast cells preincubated or not with herbimycin A (10 μg/ml) for 16 h at RT in YPD medium were left untreated or were allowed to adhere to VN for the indicated times at 37°C. Cell lysates were analyzed as indicated in Fig. 4. Lane − represents RAM immunoprecipitates used as a negative control. The results shown are representative of one of three separate experiments. As evaluated by densitometric analysis, VN stimulates a twofold increase of CaFak tyrosine phosphorylation at 10 min and a fourfold increase at 30 min. (B) C. albicans yeast cells were left untreated (time zero) or allowed to adhere to Fb, BSA, or poly-l-lysine (10 μg/ml) for the indicated times at 37°C. Cell lysates were analyzed as described above. The results shown are representative of one of three separate experiments.
Collectively, these results indicate that CaFak on C.albicans yeast cells is constitutively tyrosine phosphorylated and that its phosphorylation is markedly enhanced upon ligation of αvβ3 and αvβ5 integrin-like VNRs by specific Abs or the natural ligand VN.
Tyrosine kinase activation is required for stimulation of CaFak tyrosine phosphorylation upon adhesion of C. albicans yeast cells to the EA.hy 926 human endothelial cell line.
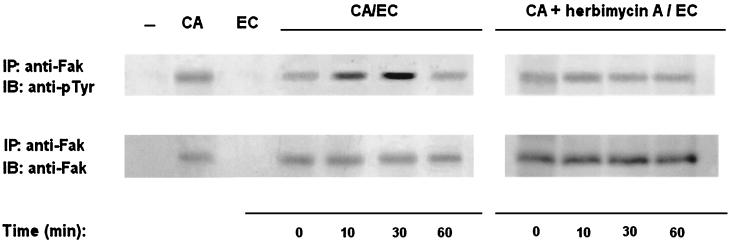
In order to investigate the involvement of CaFak tyrosine phosphorylation in C. albicans yeast cell adhesion to the EA.hy 926 endothelial cell line, yeast cells were stimulated with prefixed endothelial cells for different times at 37°C. Cell lysates were then immunoprecipitated with a rabbit anti-Fak COOH polyclonal Ab and blotted with anti-pTyr or anti-Fak NH2 MAb (Fig. 6). Interaction of C. albicans yeast cells with EA.hy 926 endothelial cells stimulates CaFak tyrosine phosphorylation that is already evident 10 min after binding, reaches maximal levels at 30 min, and declines at 60 min. In addition, pretreatment of C. albicans yeast cells with herbimycin A downmodulates CaFak tyrosine phosphorylation induced by C. albicans interaction with the endothelial cell line.
FIG. 6.
Tyrosine kinase activation is required for stimulation of CaFak tyrosine phosphorylation upon adhesion of C. albicans yeast cells to the EA.hy 926 human endothelial cell line. C. albicans yeast cells pretreated or not pretreated with herbimycin A (10 μg/ml) for 16 at RT in YPD medium were allowed to bind to paraformaldehyde-prefixed EA.hy 926 human endothelial cell line (CA/EC) for the indicated times at 37°C. Cell lysates were analyzed as indicated in Fig. 4. Lane − represents RAM immunoprecipitates used as a negative control. Lanes CA and EC represent anti-Fak immunoprecipitates from C. albicans and prefixed EA.hy 926 cell lysates, respectively. The results shown are representative of one of three separate experiments. As evaluated by densitometric analysis, binding of C. albicans to EA.hy 926 cells stimulates a 2.5-fold increase of CaFak tyrosine phosphorylation at 10 min and a 4-fold increase at 30 min.
CaFak tyrosine phosphorylation is not stimulated in a mutant strain of C. albicans that shows a reduced adhesiveness to VN and to the EA.hy 926 human endothelial cell line.
In an attempt to better understand the significance of CaFak tyrosine phosphorylation in C. albicans adherence and possibly pathogenicity, we investigated the expression and function of this protein in a mutant strain of this fungus, C. albicans 3135, selected primarily for its resistance to echinocandin and showing an inability to form germ tubes, low secretion of proteinases, and virulence potential in systemic infection (10, 13).
We initially tested the expression of integrin-like VNRs and CaFak in this C. albicans mutant strain, as well as its ability to adhere to VN or the EA.hy 926 endothelial cell line (Fig. 7).
FIG. 7.
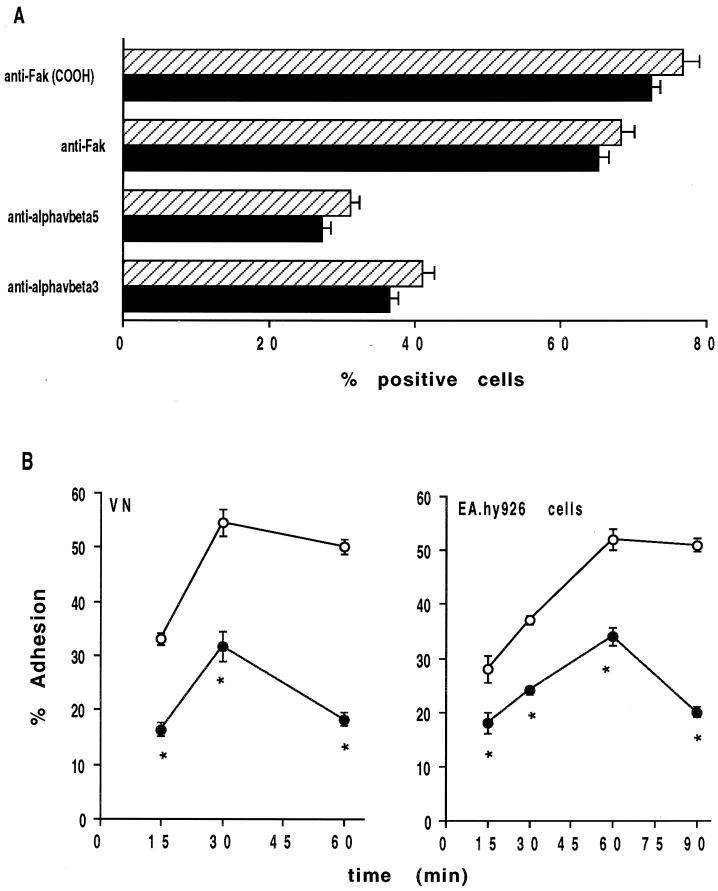
Reduced adhesiveness of yeast cells from C. albicans 3153 mutant strain to VN and EA.hy 926 human endothelial cell line. (A) C. albicans clinical isolate (▪) or 3153 mutant (▨) yeast cells were stained with anti-Fak COOH polyclonal Ab or anti-Fak NH2 MAb after permeabilization, and unpermeabilized cells were stained with anti-αvβ3 (LM609) or anti-αvβ5 (P1F6) MAb and evaluated by FACS analysis. Irrelevant isotype-matched anti-rat CD5 MAb or normal rabbit serum was used as control Ab. FITC-GAM or FITC-GAR was used as secondary Ab. (B) Adhesion of [3H]glucose-labeled C. albicans clinical isolate (○) or 3153 mutant (•) yeast cells to VN (1 μg/ml) or human EA.hy 926 endothelial cell line was tested at different times (15, 30, and 60 min and 15, 30, 60, and 90 min, respectively) at 37°C. The results are the mean percent cell adhesion ± the standard deviation of three separate experiments. ✽, P < 0.01.
As shown in Fig. 7A, the C. albicans mutant strain expresses comparable levels of αvβ3 and αvβ5 integrin-like VNRs and CaFak proteins with respect to the C. albicans clinical isolate, as evaluated by immunofluorescence and fluorescence-activated cell sorting (FACS) analysis. However, the mutant C. albicans strain exhibits a defective adhesiveness to VN or to the endothelial cell line at any time point tested compared to the C. albicans clinical isolate (Fig. 7B).
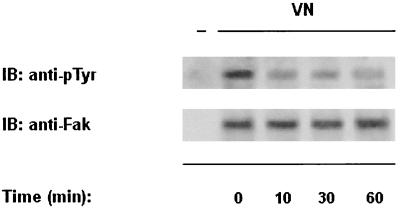
We then examined whether interaction of mutagenized C. albicans yeast cells with VN was capable of stimulating CaFak tyrosine phosphorylation. Yeast cells were allowed to adhere for different times at 37°C on VN-coated plates. Yeast cell lysates were then immunoprecipitated as described above (Fig. 8). Unlike the clinical isolate of C. albicans, adhesion of the mutant strain to VN resulted in a time-dependent tyrosine dephosphorylation of CAFak. All immunoprecipitates contained comparable levels of CaFak protein as assessed by Western blot analysis.
FIG. 8.
Adhesion to VN fails to stimulate CaFak tyrosine phosphorylation in yeast cells from C. albicans 3153 mutant strain. Yeast cells from C. albicans 3153 mutant strain were allowed to adhere to VN (10 μg/ml) for the indicated times at 37°C. Cell lysates were analyzed as indicated in Fig. 4. Lane − represents RAM immunoprecipitates used as negative control.The results shown are representative of one of three separate experiments.
These results suggest a role for CaFak in the control of C. albicans yeast cell adhesion.
DISCUSSION
The pathogenesis of infection by C. albicans, the most frequently isolated fungal pathogen in humans, involves adhesion to epithelial and endothelial cells and to ECM proteins (6, 12, 23); however, the signaling events triggered by Candida adherence to host cell components are only partially understood.
We provide here the first evidence on the presence in C. albicans yeast cells of a p105Fak-like protein, which we termed CaFak, that is antigenically related to the vertebrate focal adhesion kinase p125Fak and on its involvement in the control of integrin-like mediated fungus adhesion to VN and to a human endothelial cell line.
CaFak has been identified by biochemical analysis with a number of different Abs directed against chicken Fak, as a protein of 105 kDa showing a faster electrophoretic mobility compared to p125Fak immunoprecipitated from lysates of EA.hy 926 human endothelial cell line. Immunofluorescence and cytofluorimetric analysis on permeabilized C. albicans yeast cells indicate that Abs that recognize p125Fak, specifically stain the majority (ca. 70%) of C. albicans yeast cells. In addition, confocal laser scanning microscopy analysis demonstrates that CaFak has a cytosolic distribution.
The presence in C. albicans of a Fak-like protein is not surprising in that Fak PTK is evolutionarily conserved and its expression is not restricted to mammalian cells. cDNAs encoding p125Fak have been isolated from human (52), rodent (22), chicken (42), Xenopus (54), and Drosophila (17),and analysis of their sequences shows a high level of homology between the different species.
Tyrosine phosphorylation of cellular proteins is a primary response to integrin stimulation and a role for PTKs belonging to the Fak family in the control of cellular adhesion and migration in vertebrates has been widely documented (8, 11, 15, 19, 36, 43). Moreover, it has been recently reported in Entamoeba histolytica, a protozoan expressing β1 and β2 integrin-like receptors (46, 51), that the adhesion of trophozoites to collagen I induces increased tyrosine phosphorylation of a 110-kDa ppFak-like protein (37). Similarly, in Hartmanella vermiformis, a basally tyrosine phosphorylated pp125Fak-like protein homologue to mammalian p125Fak that undergoes dephosphorylation upon protozoan attachment and invasion of Legionella pneumophila, has been reported (50).
Nothing is known about the involvement of similar PTK in fungus cell adhesion, although phosphotyrosine proteins of fungal origin were identified in β-mercaptoethanol extracts of C. albicans upon adhesion to human buccal epithelial cells (2).
Our study provides evidence that the Fak-like protein migrating at 105 kDa is likely involved in the control of integrin-like VNR-mediated C. albicans yeast cell adhesion to VN and EA.hy 926 endothelial cell line.We demonstrated by using the PTK inhibitors genistein and herbimycin A that tyrosine kinase activation is a primary event required for C. albicans yeast cell adhesion to VN and to the endothelial cell line. Moreover, confocal microscopy analysis on C. albicans yeast cells adherent to VN demonstrates that CaFak redistributes from the cytosol to focal adhesion sites and colocalizes with αv, β3, and β5 integrin-like receptors. Our results also show that engagement of αvβ3 and αvβ5 integrin-like VNRs on C. albicans yeast cells mediated either by specific MAbs or the natural ligand VN stimulates the phosphorylation on tyrosine residues of CaFak, which is basally tyrosine phosphorylated with levels varying from experiment to experiment. Finally, pretreatment of C. albicans with herbimycin A completely nullified Candida adhesion-dependent stimulation of CaFak tyrosine phosphorylation.These results strongly resemble previous evidence in mammalian cells where cell adhesion to VN or clustering of αvβ3 and αvβ5 integrins trigger p125 Fak tyrosine phosphorylation (31, 55). Our findings also demonstrate that adhesion of C. albicans yeast cells to the EA.hy 926 human endothelial cell line specifically increases tyrosine phosphorylation of CaFak, which is completely blocked by herbimycin A pretreatment.
Vascular endothelium plays an important role during the initiation of hematogenous dissemination because blood-borne Candida microorganisms adhere to endothelial basement membrane and/or subendothelial ECM components and penetrate into the endothelial cell lining of the vasculature to gain access to tissue parenchyma (28, 56). We have reported that VN is an important adhesion molecule recognized by C. albicans on the human EA.hy 926 endothelial cell line (45) and that adhesion of C. albicans on germ tube phase to the EA.hy 926 endothelial cells is markedly blocked by anti-β3 integrin subunit MAb, RGD-containing peptides, and heparin (41).
A role of CaFak in C. albicans yeast adhesion is also supported by the failure of VN to stimulate CaFak tyrosine phosphorylation in a mutant strain of C. albicans which shows normal levels of CaFak and VNR-like integrins and reduced adhesiveness to VN and human endothelial cell line, defective germ tube transition, and lower pathogenicity (13). Moreover, our data indicate that adhesion of C. albicans mutant strain to VN results in dephosphorylation of CaFak, suggesting that this adhesive event could activate tyrosine phosphatases.
These findings also suggest that CaFak may be implicated in the control of Candida adherence, hyphal transition, and fungus pathogenicity, and in this regard we have preliminary results showing increased CaFak tyrosine phosphorylation during C. albicans yeast-mycelial transition (G. Santoni et al., unpublished observations).
Our evidence is in accordance with recent reports demonstrating that a number of signaling events are involved in triggering the transition from the budding yeast to a more invasive filamentous form. Thus, disruption or mutations of C. albicans genes encoding Ras-related GTPase (CaRSR1), components of MAPK (HOG1, HST7, and MKC1) and PAK (CaCLA4 and CST20) families, or histidine kinase (CaHK1) result in a defect of hyphal formation, reduced adhesion to human endothelial cells, and impaired systemic dissemination (4, 16, 27, 30, 32, 53). Similarly, homologous deletion of C. albicans PI-3K gene (CaVPS34) generates a mutant strain that exhibits a delayed yeast-to-hyphae transition and a strongly decreased ability to adhere to mouse fibroblasts (4).
Overall, these results indicate that CaFak, the Fak-like protein expressed in C. albicans yeast cells, may be involved in the regulation of Candida adhesiveness to VN and endothelial cells. Our results with the agerminative mutant of C. albicans that exhibits reduced adhesiveness and low virulence in systemic infection also suggest that CaFak tyrosine phosphorylation signals could be involved in the regulation of hyphal transition and, consequently, in the control of invasiveness and pathogenicity of C. albicans yeasts.
Strategies aimed at reducing Fak-like tyrosine phosphorylation by interfering with integrin-like mediated yeast cell adhesion to host components may be an attractive target for the development of antifungal therapies.
Acknowledgments
We thank A. Cassone, Istituto Superiore di Sanitá, Rome, Italy, for kindly providing C. albicans clinical isolate and strain CA-2 and the anti-GMP Ab, and G. Tarone, University of Turin, Turin, Italy, for providing the anti-αv and anti-β3 integrins and the anti-Fak Abs.
This research was supported by a grant obtained from MURST 2000 from the University of Camerino.
Editor: T. R. Kozel
REFERENCES
- 1.Akiyama, T., and H. Ogawara. 1998. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 201:553-561. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, A., E. Wadsworth, and R. Calderone. 1995. Adherence of Candida albicans to human buccal epithelial cells: host-induced protein synthesis and signaling events. Infect. Immun. 63:569-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard, M. M., and V. Nowotny. 1994. High throughput rapid yeast DNA extraction: application to yeast artificial chromosomes as polymerase chain reaction templates. Genet. Anal. Tech. Appl. 11:7-11. [DOI] [PubMed] [Google Scholar]
- 4.Bruckmann, A., W. Kunkel, A. Hartl, R. Wetzker, and R. Eck. 2000. A phosphatidylinositol 3-kinase of Candida albicans influences adhesion, filamentous growth, and virulence. Microbiology 146:2755-2764. [DOI] [PubMed] [Google Scholar]
- 5.Burridge, C. A., C. Turner, and L. Romer. 1992. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J. Cell Biol. 119:893-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderone, R. A., and P. C. Braun. 1991. Adherence and receptor relationships in Candida albicans. Microbiol. Rev. 55:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calera, J. A., X. J. Zhao, F. De Bernardis, M. Sheridan, and R. Calderone. 1999. Avirulence of Candida albicans CaHK1 mutants in a murine model of hematogenously disseminated candidiasis. Infect. Immun. 67:4280-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cary, L. A., and J. L. Guan. 1999. Focal adhesion kinase in integrin-mediated signaling. Front. Biosci. 4:102-113. [DOI] [PubMed] [Google Scholar]
- 9.Casanova, M., and W. L. Chaffin. 1991. Phosphate-containing proteins and glycoproteins of the cell wall of Candida albicans. Infect. Immun. 59:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassone, A., E. Mason, and D. Kerridge. 1981. Lysis of growing yeast-form cells of Candida albicans by echinocandin: a cytological study. Sabouraudia 19:97-110. [PubMed] [Google Scholar]
- 11.Clark, E. A., and J. S. Brugge. 1995. Integrins and signal transduction pathways: the road taken. Science 268:233-239. [DOI] [PubMed] [Google Scholar]
- 12.Cutler, J. E. 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45:187-218. [DOI] [PubMed] [Google Scholar]
- 13.De Bernardis, F., D. Adriani, R. Lorenzini, E. Pontieri, G. Carruba, and A. Cassone. 1993. Filamentous growth and elevated vaginopathic potential of a nongerminative variant of Candida albicans expressing low virulence in systemic infection. Infect. Immun. 61:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defilippi, P., C. Bozzo, G. Volpe, G. Romano, M. Venturino, L. Silengo, and G. Tarone. 1994. Integrin-mediated signal transduction in human endothelial cells: analysis of tyrosine phosphorylation events. Cell Adhesion Commun. 2:75-86. [DOI] [PubMed] [Google Scholar]
- 15.Defilippi, P., A. Gismondi, A. Santoni, and G. Tarone. 1997. Activation of tyrosine kinases, p. 29-59. In S. Ghosh (ed.), Signal transduction by integrins. Springer-Verlag, Heiderberg, Germany.
- 16.Diez-Orejas, R., G. Molero, F. Navarro-Garcia, J. Pla, C. Nombela, and M. Sanchez-Perez. 1997. Reduced virulence of Candida albicans MKC1 mutants: a role for mitogen-activated protein kinase in pathogenesis. Infect. Immun. 65:833-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox, G. L., I. Rebay, and R. O. Hynes. 1999. Expression of DFak56, a Drosophila homolog of vertebrate focal adhesion kinase, supports a role in cell migration in vivo. Proc. Natl. Acad. Sci. USA 96:14978-14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gale, C., D. Finkel, N. Tao, M. Meinke, M. McClellan, J. Olson, K. Kendrick, and M. K. Hostetter. 1996. Cloning and expression of a gene encoding an integrin-like protein in Candida albicans. Proc. Natl. Acad. Sci. USA 93:357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giancotti, T., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore, A. P., and L. H. Romer. 1996. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesion decreases cell motility and proliferation. Mol. Biol. Cell 7:1209-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gismondi, A., J. Jacobelli, F. Mainiero, R. Paolini, M. Piccoli, L. Frati, and A. Santoni. 2000. Functional role for proline-rich tyrosine kinase 2 in NK cell-mediated natural cytotoxicity. J. Immunol. 164:2272-2276. [DOI] [PubMed] [Google Scholar]
- 22.Hanks, S. K., M.B Calalb, M. C. Harper, and S. K. Patel. 1992. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. USA 89:8487-8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hostetter, M. K. 1994. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin. Microbiol. Rev. 7:29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hostetter, M. K. 2000. RGD-mediated adhesion in fungal pathogens of humans, plants, and insects. Curr. Opin. Microbiol. 3:344-348. [DOI] [PubMed] [Google Scholar]
- 25.Hynes, R. O. 1993. Integrins: versatility, modulation and signalling in cell adhesion. Cell 69:11-25. [DOI] [PubMed] [Google Scholar]
- 26.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, T. Yamamoto, and S. Aizawa. 1995. Reduced cell motility and enhanced focal contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- 27.Jong, A. Y., M. F. Stings, S. H. Huang, S. H. M. Chen, and K. S. Kim. 2001. Traversal of Candida albicans accross human blood-brain barrier in vitro. Infect. Immun. 69:4536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klotz, S. A., D. J. Drutz, J. L. Harrison, and M. Hupper. 1983. Adherence and penetration of vascular endothelium by Candida yeasts. Infect. Immun. 42:374-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornberg, L., H. S. Earp, J. T. Parsons, M. Schaller, and R. L. Juliano. 1992. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J. Biol. Chem. 267:23439-23442. [PubMed] [Google Scholar]
- 30.Leberer, E., K. Ziegelbauer, A. Schimdt, D. Harcus, D. Dignard, J. Ash, L. Johnson, and D. Y. Thomas. 1997. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr. Biol. 7:539-546. [DOI] [PubMed] [Google Scholar]
- 31.Lewis, J. M., D. A. Cheresh, and M. A. Schwartz. 1996. Protein kinase C regulates αvβ5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J. Cell Biol. 134:1323-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhani, H. D., and G. R. Fink. 1998. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 8:348-353. [DOI] [PubMed] [Google Scholar]
- 33.Meri, S., P. Matila, and R. Renkonen. 1993. Regulation of CD59 expression on the human endothelial cell line EA.hy 926. Eur. J. Immunol. 23:2511-2516. [DOI] [PubMed] [Google Scholar]
- 34.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Balliere Tindall, London, England.
- 35.Owen, J. D., P. J. Ruest, D. W. Fry, and S. K. Hanks. 1999. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 19:4806-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons, J. T., K. H. Martin, J. K. Slack, J. M. Taylor, and S. A. Weed. 2000. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 19:5606-5613. [DOI] [PubMed] [Google Scholar]
- 37.Perez, E., M. L. Munoz, and A. Ortega. 1996. Entamoeba histolytica: involvement of pp125FAK in collagen-induced signal transduction. Exp. Parasitol. 82:164-170. [DOI] [PubMed] [Google Scholar]
- 38.Ruoslahti, E. 1991. Integrins. J. Clin. Investig. 87:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santoni, G., A. Gismondi, L. Jin Hong, A. Puntirieri, A. Santoni, L. Frati, M. Piccoli, and J. Y. Djeu. 1994. Candida albicans expresses a fibronectin receptor antigenically related to α5β1 integrin. Microbiology 140:2971-2979. [DOI] [PubMed] [Google Scholar]
- 40.Santoni, G., P. Birarelli, L. Jin Hong, A. Gamero, J. Y. Djeu, and M. Piccoli. 1995. An α5β1-like integrin receptor mediates the binding of less pathogenic Candida species to fibronectin. J. Med. Microbiol. 43:360-367. [DOI] [PubMed] [Google Scholar]
- 41.Santoni, G., E. Spreghini, R. Lucciarini, C. Amantini, and M. Piccoli. 2001. Involvement of αvβ3 integrin-like receptor and glycosaminoglycans in Candida albicans germ tube adhesion to vitronectin and human endothelial cell line. Microb. Pathog. 31:159-172. [DOI] [PubMed] [Google Scholar]
- 42.Schaller, M. D., C. A. Borgman, B. S. Cobb, R. R. Vines, A. B. Reynolds, and J. T. Parsons. 1992. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA 89:5192-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlaepfer, D. D., and T. Hunter. 1998. Integrin signalling and tyrosine phosphorylation: just the FAK? Trends Cell Biol. 8:151-157. [DOI] [PubMed] [Google Scholar]
- 44.Schlaepfer, D. D., C. R. Hauck, and D. J. Sieg. 1999. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71:435-478. [DOI] [PubMed] [Google Scholar]
- 45.Spreghini, E., A. Gismondi, M. Piccoli, and G. Santoni. 1999. Evidence for αvβ3 and αvβ5 integrin-like vitronectin (VN) receptors in Candida albicans and their involvement in yeast cell adhesion to VN. J. Infect. Dis. 180:156-166. [DOI] [PubMed] [Google Scholar]
- 46.Talamas-Rohana, P., V. I. Hernandez-Ramirez, J. N. Perez-Garcia, and J. Ventura-Juarez. 1998. Entamoeba histolytica contains a β1 integrin-like molecule similar to fibronectin receptors from eukaryotic cells. J. Eukaryot. Microbiol. 45:356-360. [DOI] [PubMed] [Google Scholar]
- 47.Tamura, M. J., J. Gu, K. Matsumoto, S. Aota, R. Parsons, and K. M. Yamada. 1998. Inhibition of cell migration, spreading and focal adhesion by tumor suppressor PTEN. Science 280:1614-1617. [DOI] [PubMed] [Google Scholar]
- 48.Torosantucci, A., M. Boccanera, I. Casalinuovo, G. Pellegrini, and A. Cassone. 1990. Differences in the antigenic expression of immunomodulatory mannoprotein constituens on yeast and mycelial forms of Candida albicans. J. Gen. Microbiol. 136:1421-1428. [DOI] [PubMed] [Google Scholar]
- 49.Uehara, Y., and H. Fuzawaka. 1991. Use and selectivity of hermimycin A as inhibitor of protein-tyrosine kinases. Methods Enzymol. 201:563-571. [DOI] [PubMed] [Google Scholar]
- 50.Venkataraman, C., L. Y. Gao, S. Bondada, and Y. A. Kwaik. 1998. Identification of putative cytoskeletal protein homologues in the protozoan host Hartmanella vermiformis as substrates for induced tyrosine phosphatase activity upon attachment to the Legionnaires' Disease bacterium, Legionnaires pneumophila. J. Exp. Med. 188:505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vines, R. R., G. Ramakrishnan, J. B. Rogers, L. A. Lockhart, B. J. Mann, and W. A. Petri, Jr. 1998. Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a β2 integrin motif. Mol. Biol. Cell 9:2069-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiner, T. M., E. T. Liu, R. J. Craven, and W. G. Cance. 1993. Expression of focal adhesion kinase gene and invasive cancer. Lancet 342:1024-1025. [DOI] [PubMed] [Google Scholar]
- 53.Yaar, L., M. Mevarech, and Y. Koltin. 1997. A Candida albicans RAS-related gene (CaRSR1) is involved in budding, cell morphogenesis and hypha development. Microbiology 143:3033-3044. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, X., C. V. Wright, and S. K. Hanks. 1995. Cloning of a Xenopus laevis cDNA encoding focal adhesion kinase (FAK) and expression during early development. Gene 160:219-222. [DOI] [PubMed] [Google Scholar]
- 55.Zheng, D. Q., A. S. Woodard, G. Tallini, and L. R. Languino. 2000. Substrate specificity of αvβ3 integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J. Biol. Chem. 275:24565-24574. [DOI] [PubMed] [Google Scholar]
- 56.Zink, S., T. Nass, P. Rosen, and J. F. Ernst. 1996. Migration of fungal pathogen Candida albicans across endothelial monolayers. Infect. Immun. 64:5085-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]